Abstract
Background
Human immunodeficiency virus (HIV) and enteric parasite co-infection not only aggravates the clinical symptoms of parasites but also accelerates acquired immunodeficiency syndrome (AIDS) progression. However, co-infection research on men who have sex with men (MSM), the predominant high-risk population of HIV/AIDS in China, is still limited. In this study, we investigated the epidemiology of enteric parasites, risk factors, and associations with clinical significance in an MSM HIV/AIDS population in Heilongjiang Province, northeast China.
Methods
We recruited 308 MSMs HIV/AIDS patients and 199 HIV-negative individuals in two designated AIDS hospitals in Heilongjiang between April 2016 and July 2017. Fresh stool samples were collected. DNA extraction, molecular identification, and genotyping of Cryptosporidium species, Entamoeba histolytica, Cyclospora cayetanensis, Enterocytozoon bieneusi, and Blastocystis hominis were performed. Fourteen diarrhea-related pathogens were examined to exclude the influence of other bacterial pathogens on diarrhea incidence.
Results
31.5% of MSM HIV/AIDS participants were infected with at least one parasite species, a significantly higher proportion than that found in the HIV-negative individuals (2.5%). E. bieneusi presented the highest prevalence, followed by B. hominis, E. histolytica, Cryptosporidium spp., and C. cayetanensis. Warm seasons were the risk factor for parasitic infections in this population [odds ratio (OR) = 2.6, 95% CI: 1.47–4.57]. In addition, these individuals showed a higher proportion (35.8%) of present diarrhea (PD) compared with men who have sex with women (MSW) with HIV/AIDS (16.7%). The infection proportions of both Cryptosporidium spp. and E. histolytica were significantly higher in the PD. E. bieneusi infection was more prevalent in the historic diarrhea (HD) group. CD4+ T cell counts in the MSM patients with the above three parasites were significantly lower. New species and genotypes were found, and MSM patients had a wider range of species or genotypes.
Conclusions
Enteric parasitic infection was prevalent in the MSM HIV/AIDS population, especially in patients with present diarrhea during warm seasons. E. histolytica and B. hominis should also be considered high-risk parasites for opportunistic infections in AIDS patients in addition to Cryptosporidium spp.
Author summary
Human immunodeficiency virus (HIV) and enteric parasite co-infection not only aggravates the clinical symptoms of parasites but also accelerates acquired immunodeficiency syndrome (AIDS) progression. In China, despite a large number of HIV-infected people, the data regarding co-infection with enteric intracellular parasites in this population is still sparse. In the present study, we investigated the epidemiology of Cryptosporidium spp., Enterocytozoon bieneusi, Blastocystis hominis, Entamoeba histolytica and Cyclospora cayetanensis in 384 HIV/AIDS including 308 MSM HIV/AIDS populations in two designated AIDS hospitals in Heilongjiang Province, North-East China between April 2016 and July 2017 by polymerase chain reaction. We also try to track the possible sources, risk factors, and any associations, with clinical significance, of human parasitic infections. Such knowledge will provide insights into prognosis, treatment, and preventive strategies against infections with such pathogens. The findings in this study have important implications for public health, the control of AIDS progression and control of parasitic infections.
Introduction
Human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS), caused by HIV infection, remain significant public health challenges. HIV infection leads to severe dysfunction of both adaptive and innate immune responses and results in severe opportunistic infections [1]. HIV-positive hosts and AIDS patients (HIV/AIDS) are at a higher risk for opportunistic infections, including enteric parasites, particularly when combined with impaired or deficient T cell function [2]. Moreover, HIV infection may negatively impact the natural evolution of certain parasitic infections, lead to more serious clinical symptoms, and complicate the treatment of both diseases [3]. Conversely, the mucosal damage and changes in the immunological mini-environment caused by some parasitic infections may increase susceptibility to HIV infections, facilitate HIV replication, and accelerate disease progression [4]. This field has gradually attracted more attention due to the adverse impacts of co-infections in HIV/AIDS individuals. However, the epidemiological characteristics of co-infection remain poorly understood, as there are many parasite species, and the prevalence varies in different parts of the world [5, 6].
China has a large number of HIV-infected individuals, and HIV transmission patterns have changed dramatically in recent years. By the end of 2020, China had approximately 1053 thousands people with HIV/AIDS, and there were an estimated 34,512 new HIV infections in 2017, and 25.5% of these were men who have sex with men (MSM) [7,8]. In addition, it was reported that the number of university students infected with HIV increased by 35% annually from 2011 to 2015, possibly due to an increase in MSM students [9]. Growing numbers of MSM individuals in China and the rise of HIV infection in the population have become a significant challenge for HIV/AIDS prevention [7].
As a high-risk population of HIV, MSM individuals also have an increased risk of contracting sexually transmissible enteric infections (STEIs), and the widespread distribution of STEI outbreaks in MSM warrants public health attention [10]. Investigators have found that the prevalence of enteric parasites reached 52–59% in the MSM populations of Scotland, Australia, and the USA [11–13]. Of the enteric parasites found in HIV/AIDS individuals, some genera of protozoa, such as Cryptosporidium, Cyclospora, Isospora, Microsporidia (phylogenetically related to fungi), and Blastocystis, are prevalent worldwide [5, 6].
In China, despite a large number of HIV-infected people, the data regarding co-infection with enteric intracellular parasites in this population is still sparse. To date, several reports have focused on HIV and intestinal parasites in middle and southern China [5, 14–16]. However, molecular detection methods were used in no more than two of those reports [16, 17]. Furthermore, concerning parasitic intestinal infections in the MSM population of mainland China, only one study has been published, and the seroprevalence of E. histolytica was found to be 41.1% in Beijing and Tianjin [18].
Epidemiological investigations have shown that some enteric pathogens of humans (including Cryptosporidium spp. Enterocytozoon bieneusi and Blastocystis spp.) are widespread in nature and can infect multiple animals, including farm, household, and wild animals. In addition, there are multiple zoonotic species/genotypes of these pathogens [19–21]. Therefore, we propose that the scale of infection by enteric intracellular parasites in HIV/AIDS individuals has been underestimated in China. More importantly, as enteric parasitic infections are associated with either severe diarrhea or worsening severity of other symptoms in HIV/AIDS individuals, co-infection in this population poses a significant threat if awareness of these dynamics continues to be neglected.
In Heilongjiang Province, northeast China, MSM HIV/AIDS individuals account for >75% of all those infected with HIV [22] and where enteric parasites in local animals are widely distributed (S1–S3 Tables). Nevertheless, the co-infection of enteric intracellular parasites with the human immunodeficiency virus (HIV) among homosexuals in northeast China remains poorly defined. Therefore, the present study aimed to investigate the epidemiology of five enteric parasites and evaluate risk factors and associations with clinical significance in an HIV/AIDS population, especially for MSM in Heilongjiang Province, northeast China.
Materials and methods
Ethics approval and consent to participate
Approvals for these studies were obtained from the Ethics Committees of the Fourth Affiliated Hospital of Harbin Medical University and the Harbin Infectious Disease Hospital (SCILLSC-2017-01). Written informed consent was signed by each participant (for the 17-year-old participants, the consent forms were obtained from their parents or guardian) after they or their parents or guardian were informed of the purposes and procedures of the study.
Study area
The study was conducted in two public designated AIDS hospitals in northeast China: the Fourth Affiliated Hospital of Harbin Medical University and the Harbin Infectious Disease Hospital. These hospitals are located in the Xiangfang and Nangang districts of Harbin City, respectively (Fig 1).
Fig 1. Map of the two hospitals where samples were collected location in Heilongjiang Province, China.
The map was originally designed using the software ArcGIS 10.4. The original vector diagram was adopted from National Geomatics Center of China (http://www.ngcc.cn). The map has been modified and assembled according to permission and attribution guidelines using Microsoft PowerPoint 2003 and Adobe Photoshop CS6.
Study population
The study population consisted of patients with HIV/AIDS, who underwent outpatient examinations or hospitalization at the two designated AIDS treatment hospitals in Heilongjiang Province. In addition, the age-matched, HIV-negative, healthy individuals were recruited from the medical examination center of the Fourth Affiliated Hospital of Harbin Medical University.
Sampling method and data collection
Between April 2016 and August 2017, fecal samples were collected from the patients with HIV/AIDS. The patients or their parents/guardians who provided consent on behalf of their children were trained in the relevant guidelines by the staff and were provided with a labeled plastic fecal collector marked with the date of collection and patient identity information (age and sex). After collection, the samples were stored at 4°C.
Each HIV-positive participant was interviewed using a standardized questionnaire to collect information regarding sociodemographic characteristics, living habits, animal exposure history, medication history (including the use of antiretroviral drugs and antibiotics), and recent clinical manifestations. Present diarrhea (PD) was defined as more than three episodes of watery stools or loose stools per day at the sampling time. Historic diarrhea (HD) was defined as having a history of diarrhea (two or more instances of watery stools or loose stools per day, more than five days within the month before sampling) but no diarrhea at the time of sampling. The HIV infection and AIDS stages of participants were determined according to World Health Organization (WHO) guidelines [23].
Moreover, we collected the CD4+ T cell counts, HIV viral load (VL), and routine blood parameters, including white blood cell (WBC), neutrophil (NEUT), lymphocyte (LYMPH), red blood cell (RBC), hemoglobin (HGB), monocyte (MONO), eosinophil (EOSO) and basophil (BASO) counts, obtained from the sampling hospital to evaluate changes in the parameters in the presence of parasitic infections.
Sample size determination
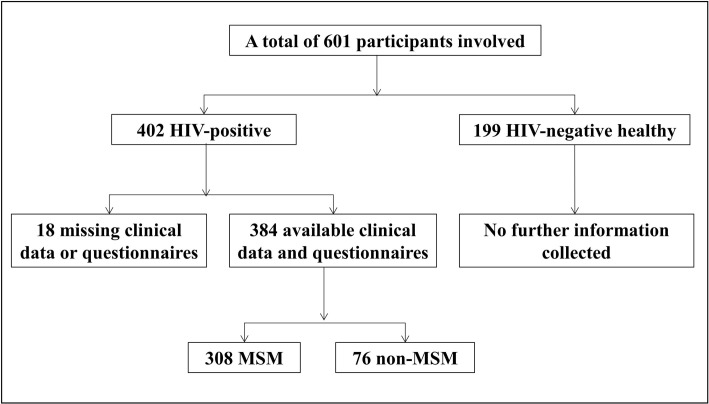
A total of 601 fecal samples were collected from humans in the two hospitals in Heilongjiang Province, China, including 402 HIV-positive participants drawn from various regions of Heilongjiang Province and diagnosed in two designated AIDS hospitals in Harbin, the Fourth Affiliated Hospital of Harbin Medical University and the Harbin Infectious Disease Hospital and 199 HIV-negative healthy individuals recruited from the medical examination center of the Fourth Affiliated Hospital of Harbin Medical University.
Inclusion/exclusion criteria
Doctors communicated and provided consulting services for voluntary participation in the survey and signed written informed consent forms. The participants had not undergone an antiparasitic treatment. Fecal samples of the HIV-positive participants were used for parasitological analysis only if they were coupled with the questionnaire and appropriate clinical data.
DNA extraction and PCR amplification
Genomic DNA was extracted from 180–200 mg of the fecal specimen using a QIAamp DNA Stool Mini Kit (QIAgen, Hilden, Germany), following the manufacturer’s guidelines. DNA was finally eluted in 200 μL of AE buffer and stored at –20°C until further use in PCR analysis. The presence of Cryptosporidium, E. histolytica, Cyclospora, E. bieneusi, and Blastocystis was examined using nested PCR amplification based on the methods described elsewhere [24–28]. To further identify the genotypes of E. bieneusi isolates, a 389-bp fragment of rDNA, including part of the small subunit (SSU), internal transcribed spacer (ITS) region, and part of the large subunit (LSU), was amplified using a new nested PCR [29]. Subtypes of Cryptosporidium isolates were identified using an approximately 800–850-bp fragment of the gp60 gene [30]. S4 and S5 Tables present the primers used to identify the parasites and the genotypes of E. bieneusi and subtypes of Cryptosporidium and the conditions of the PCR experiment, respectively (S4 and S5 Tables). TaKaRa Taq DNA Polymerase (TaKaRa Bio Inc., Tokyo, Japan) was used in PCR amplification. A negative control without DNA was included in all the tests. Each specimen was analyzed twice, with 2 μL of DNA used as a template for each PCR. All the secondary PCR products were subjected to electrophoresis on a 1.5% agarose gel and visualized by staining the gel with Goldenview.
The presence of 14 diarrhea-associated pathogens, including Staphylococcus aureus, Salmonella, Enterobacter sakazakii, Yersinia enterocolitica, Aeromonas hydrophila, Bacillus cereus, Listeria monocytogenes, Escherichia coli O157, Shigella castellani, Vibrio cholerae, Campylobacter coli, Vibrio parahaemolyticus, Escherichia coli, and Campylobacter jejuni in the stool samples was examined using a Multiplex PCR diagnostic kit for rapid identification of foodborne pathogenic bacteria (Beijing Applied Biological Technologies Co., Ltd.; Beijing, China).
Nucleotide sequencing and molecular analysis
All PCR products of the expected size were purified and then sequenced with their respective primers by Sinogeno-max Biotechnology Co. Ltd. (Beijing, China), using a Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA) on an ABI PRISM 3730 XL DNA Analyzer. The accuracy of nucleotide sequences was confirmed by sequencing from both ends of the product and by sequencing additional PCR products when mutations were detected.
All the sequences obtained in this study were subjected to BLAST searches (http://www.ncbi.nlm.nih.gov/blast/) and aligned with reference sequences to determine the species and genotypes/subtypes using Clustal X 1.83 (http://www.clustal.org/). Novel genotypes of E. bieneusi were identified if the sequences were not identical to known genotypes in the GenBank database, according to the established nomenclature system [31].
Statistical analysis
Data entry and analysis were performed using Statistical Package for Social Sciences (SPSS) 19.0 software. The statistical significance of differences in infection proportions was generally evaluated by Pearson’s chi-squared test, while the Fisher’s exact test was used when more than 20% of cells in contingency tables had the expected frequencies of <5. In addition, bivariate and multivariate logistic regression analyses were used to assess the possible risk factors associated with parasitic infections in HIV-positive participants.
The data contained 9 variables, including routine blood parameters (WBC, NEUT, LYMPH, MONO, EOSO, BASO, RBC, and HGB) and CD4+ T cell counts, as well as associated parasitic infection status and the AIDS stage of all MSM participants. Outlier values were removed to reduce their influence on the analyses. To generate the heat map of the data, hierarchical clustering was performed with average linkage and Pearson’s correlation. Next, the cluster groups were determined using the dynamic tree cut algorithm [32]. Following clustering, the relationship between the parasitic infection status and the cluster groups was tested using the chi-squared test or Fisher’s exact test. The above data analyses were carried out using R software, version 3.4.1.
The significant level of all the tests was established with a p-value threshold of 0.05. Then, the 95% confidence intervals (95% CI) for prevalence were calculated based on the Poisson distribution.
Results
Samples and patient data
Altogether, 384 out of the 402 HIV/AIDS individuals had available clinical data and questionnaires (Fig 2). The average age of HIV/AIDS individuals was 36.9 years, ranging from 17 to 76 years. Among them, 96.1% were male, 66.7% were non-farmers, 66.7% drank boiled water, 30% had a history of contact with animals, 41.1% had an antiretroviral therapy (ART), 65.1% had an antibiotic treatment history, and 46.4% had diarrhea (S6 Table). Of all the subjects, 80.2% were MSM. According to WHO clinical staging guidelines, 118 MSM HIV/AIDS individuals were at stages I and II (I/II), referred to as early-stage, and 190 were at stages III and IV (III/IV), referred to as late-stage. Meanwhile, 150 and 234 the samples were collected in May to October (warm season) and November to April (cold season), respectively (S7 Table). For the 199 HIV-negative individuals, no information was collected except for age; their average age was 35.8 years and ranged from 20 to 62 years.
Fig 2. Number of study participants at various stages of recruitment.
The prevalence of enteric parasites in healthy and HIV-infected individuals
Among the 199 HIV-negative individuals, the overall prevalence of B. hominis and E. bieneusi was 1.5% (3/199) and 1.0% (2/199), respectively. No other parasites were found in the healthy individuals (Table 1). On the contrary, 31.5% (121/384) of the HIV/AIDS individuals were co-infected with at least one species of parasites. Of the 384 HIV/AIDS patients, E. bieneusi infection (14.6%) was the most predominant, followed by B. hominis (8.1%), E. histolytica (6.3%), and Cryptosporidium spp. (3.4%), while C. cayetanensis was the least prevalent (1.8%) (Table 1). Mixed infections with two species of parasite were found in 10 patients (2.6%). Among these 10 individuals, co-infection with B. hominis and E. bieneusi was the most common occurrence (S8 Table).
Table 1. Prevalence of five enteric intracellular parasites among HIV-negative and HIV-positive participants with different transmission routes.
| Parasites | HIV-negative (n = 199) | HIV-positive (n = 384) | Routes of HIV infection | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MSM (n = 308) | MSW (n = 31) | Others (n = 45) | ||||||||
| n | % | n | % | n | % | n | % | n | % | |
| Any of parasites | 5 | 2.5 | 121 | 31.5 | 92 | 29.9 | 12 | 35.5 | 18 | 40.0 |
| E. histolytica | 0 | / | 24 | 6.3 | 19 | 6.2 | 3 | 9.7 | 2 | 4.0 |
| E. bieneusi | 2 | 1.0 | 56 | 14.6 | 45 | 14.6 | 5 | 16.1 | 6 | 13.3 |
| Cryptosporidium spp. | 0 | / | 13 | 3.4 | 9 | 2.9 | 2 | 6.5 | 2 | 4.0 |
| C. cayetanensis | 0 | / | 7 | 1.8 | 5 | 1.6 | 1 | 3.2 | 1 | 2.0 |
| B. hominis | 3 | 1.5 | 31 | 8.1 | 19 | 6.2 | 5 | 16.1 | 7 | 15.6 |
MSM = men having sex with men. MSW = men having sex with women. Others = sexually transmitted women, non-sexually transmitted individuals, and unknown transmission routes were included
For MSM HIV/AIDS, the overall prevalence of all five parasites was 29.9% (92/308). E. bieneusi still showed the highest prevalence (14.6%), followed by B. hominis (6.2%), E. histolytica (6.2%), Cryptosporidium spp. (2.9%), and C. cayetanensis (1.6%) (Table 1). Surprisingly, the prevalence of B. hominis in the MSM population (6.2%) was significantly lower than in the men having sex with women MSW group (16.1%) and others (women, non-sexually transmitted individuals and unknown transmission routes) (15.6%) (Table 1).
Risk factors of parasitic infection in HIV-infected individuals
Proportion of any parasite was not significantly different between age groups. The total positivity rate for intestinal protozoa was lower in males than females with HIV infection, but the difference was not significant (p = 0.661). Positivity rates for different parasite species differed between male and female patients, with a significantly higher rate of B. hominis positivity in females than in males (p = 0.027). The total positivity rates for intestinal protozoa (p = 0.002), E. bieneusi (p = 0.002) and B. hominis (p = 0.029) in farmers, were significantly higher than those in non-farmers. (S9 Table).
The total positivity rate for protozoa in the group drinking raw water was slightly higher than that in the group drinking boiled water (p = 0.187). The positivity rate for Cryptosporidium in the group drinking raw water was significantly higher than that in the group drinking boiled water (p = 0.028); other intestinal protozoan infections were not related to drinking water. (S9 Table).
The total intestinal protozoan infections in these individuals, as well as infections with protozoa other than C. cayetanensis, were higher than in those without animal contact, although the differences were not significant. (S9 Table).
The total protozoa positivity rate was significantly higher (p < 0.00) for samples collected during the warm season (May to October) than for those collected during the cool season (November to April of the next year) (p < 0.001). Except for Cryptosporidium, the other four protozoa positivity rates were higher in the warm season than in the cold season, with significant differences between E. histolytica (P = 0.001), E. bieneusi (p < 0.001), and B. hominis (p = 0.005). (S9 Table).
The presence or absence of antibiotics did not significantly affect the overall infection with protozoa; however, the positivity rate for B. hominis appeared to be somewhat higher in patients taking antibiotics (p = 0.058). (S8 Table).
Except for E. bieneusi, the other four protozoa were positively correlated with the stage of AIDS disease. The rate of positivity for all intestinal protozoa was significantly higher in the III/IV patients than in the I/II patients (p < 0.05) except for E. bieneusi, but the rate of positivity for E. bieneusi was slightly higher in stage I/II patients (p = 0.07). (S9 Table).
There was no difference in the overall positivity rate for intestinal protozoa between the different HIV transmission route groups, and the positivity rate for B. hominis was significantly lower in the MSM population than in the other two groups (p = 0.02). (S9 Table)
The risk factor of parasitic infection in MSM individuals
Concerning MSM HIV/AIDS, E. histolytica (p < 0.001), C. cayetanensis (p = 0.006), and B. hominis (p < 0.002) were more prevalent in the warm season than in the cold season (Table 2). The infection proportion of any parasite at the late stage (36.4%) was significantly higher than at the early stage (22.6%) (p = 0.004) (Table 2). E. histolytica and B. hominis were more common in late-stage patients than in early-stage patients (9.5% vs. 0.8%, p = 0.005; 8.9% vs. 1.7%, p = 0.020, respectively) (Table 2). However, the prevalence of E. bieneusi in early-stage patients (19.5%) was slightly higher than that in late-stage patients (11.6%) (p = 0.081; Table 2). The univariate analysis showed that, apart from seasonality and AIDS stages, CD4+ counts were significant predictors (p < 0.05) (Table 3). However, multivariable analysis showed that the warm season was the only risk factor for parasitic infection [odds ratio (OR) = 2.6, 95% CI: 1.47–4.57] (Table 3).
Table 2. Risk factors associated with enteric parasite infection in MSM HIV-positive participants.
| Characteristics | No. | Any of parasites | E. histolytica | E. bieneusi | Cryptosporidium | C. cayetanensis | B. hominis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | p value | n (%) | p value | n (%) | p value | n (%) | p value | n (%) | p value | n (%) | p value | |||
| Age | ||||||||||||||
| ≤30 | 131 | 36 (27.5) | 0.509 | 8 (6.1) | 0.653 | 19 (14.5) | 0.519 | 3 (2.3) | 0.108 | 2 (1.5) | 0.230 | 6 (4.6) | 0.285 | |
| 31–50 | 142 | 47 (33.1) | 10 (7.0) | 23 (16.2) | 3 (2.1) | 2 (0.7) | 12 (8.5) | |||||||
| >50 | 35 | 9 (25.7) | 1 (2.9) | 3 (8.6) | 3 (8.6) | 1 (2.9) | 1 (2.9) | |||||||
| Occupation | ||||||||||||||
| Farmer | 86 | 30 (34.9) | 3 (3.5) | 16 (18.6) | 4 (4.7) | 0 | 7 (8.1) | |||||||
| No-farmer | 222 | 62 (27.9) | 0.231 | 16 (7.2) | 0.341 | 29 (13.1) | 0.217 | 5 (2.3) | 0.457 | 5 (2.3) | 0.327* | 12 (5.4) | 0.371 | |
| Drinking boiled water | ||||||||||||||
| Yes | 207 | 58 (28.0) | 14 (6.8) | 29 (14.0) | 4 (1.9) | 2 (1.0) | 13 (6.3) | |||||||
| No | 101 | 34 (33.7) | 0.310 | 5 (5.0) | 0.535 | 16 (15.8) | 0.669 | 5 (5.0) | 0.264 | 3 (3.0) | 0.409 | 6 (5.9) | 0.907 | |
| Contact with animal | ||||||||||||||
| Yes | 97 | 33 (34.0) | 7 (7.2) | 14 (14.4) | 4 (4.1) | 0 | 9 (9.3) | |||||||
| No | 211 | 59 (28.0) | 0.281 | 12 (5.7) | 0.604 | 31 (14.7) | 0.952 | 5 (2.4) | 0.628 | 5 (2.4) | 0.330* | 10 (4.7) | 0.124 | |
| Season (the samples and the patient data collected dates) | ||||||||||||||
| Nov.-Apr | 197 | 43 (21.8) | 5 (2.5) | 27 (13.7) | 7 (3.6) | 0 | 6 (3.0) | |||||||
| May.-Oct | 111 | 49 (44.1) | 0.000 | 14 (12.6) | 0.000 | 18 (16.2) | 0.549 | 2 (1.8) | 0.600 | 5 (4.5) | 0.006 | 13 (11.7) | 0.002 | |
| Antibiotics | ||||||||||||||
| Yes | 190 | 58 (30.5) | 0.750 | 13 (6.8) | 0.533 | 26 (13.7) | 0.559 | 6 (3.2) | 1.000 | 2 (1.1) | 0.588 | 15 (7.9) | 0.176 | |
| No | 118 | 34 (28.8) | 6 (5.1) | 19 (16.1) | 3 (2.5) | 3 (2.5) | 4 (3.4) | |||||||
| AIDS stages | ||||||||||||||
| I-II | 118 | 27 (22.6) | 0.035 | 1 (0.8) | 0.005 | 23 (19.5) | 0.081 | 1 (0.8) | 0.161 | 0 | 0.161* | 2 (1.7) | 0.020 | |
| III-IV | 190 | 65 (36.4) | 18 (9.5) | 22 (11.6) | 8 (4.2) | 5 (2.6) | 17 (8.9) | |||||||
| Total | 308 | 92 (29.9) | 19 (6.2) | 45 (14.6) | 9 (2.9) | 5 (1.6) | 19 (6.2) | |||||||
*Fisher’s Exact Test. Bold = the values statistically significant higher than that in the same group were shown in bold.
Table 3. Univariate and Multivariable analysis of the potential risk factors of parasites infection in MSM HIV/AIDS participants.
| Characteristics | n | Infection n (%) |
Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|---|---|
| p value | Crude OR (95% CI) | p value | Adjusted OR (95% CI) | |||
| Age | ||||||
| ≤30 | 131 | 36 (27.5) | 0.84 | 1.1 (0.47–2.56) | 0.59 | 1.3 (0.51–3.29) |
| 31–50 | 142 | 47 (33.1) | 0.40 | 1.4 (0.62–3.29) | 0.37 | 1.5 (0.61–3.83) |
| >50 | 35 | 9 (25.7) | 1.00 | 1.00 | ||
| Residence | ||||||
| Farmer | 86 | 30 (34.9) | 0.23 | 1.4 (0.81–2.35) | 0.204 | 1.5 (0.81–2.68) |
| Non-farmer | 222 | 62 (27.9) | 1.00 | 1.00 | ||
| Drinking boiled water | ||||||
| Yes | 207 | 58 (28.0) | 0.31 | 1.3 (0.78–2.18) | 0.73 | 1.1 (0.63–1.95) |
| No | 101 | 34 (33.7) | 1.00 | 1.00 | ||
| Contact with animals | ||||||
| Yes | 97 | 33 (34.0) | 0.28 | 1.3 (0.79–2.22) | 0.99 | 1.0 (0.56–1.77) |
| No | 211 | 59 (28.0) | 1.00 | 1.00 | ||
| Season | ||||||
| May-Oct. | 111 | 49 (45.1) | 0.000 | 2.8 (1.71–4.69) | 0.001 | 2.6 (1.47–4.57) |
| Nov.-Apr. | 197 | 43 (21.8) | 1.00 | 1.00 | ||
| Antibiotics | ||||||
| Yes | 190 | 58 (30.5) | 0.75 | 1.1 (0.66–1.80) | 0.75 | 0.91 (0.51–1.61) |
| No | 118 | 34 (28.2) | 1.00 | 1.00 | ||
| AIDS stages | ||||||
| I-II | 118 | 27 (22.9) | 0.041 | 0.6 (0.34–0.96) | 0.65 | 0.8 (0.36–1.92) |
| III-IV | 190 | 65 (34.2) | 1.00 | 1.00 | ||
| CD4+ counts | ||||||
| <150 | 121 | 47 (38.8) | 0.031 | 2.0 (1.1–3.6) | 0.30 | 1.5 (0.68–3.47) |
| 150–350 | 97 | 23 (23.7) | 0.91 | 1.0 (0.5–1.9) | 0.55 | 0.8 (0.38–1.66) |
| >350 | 90 | 22 (24.4) | 1.00 | 1.00 | ||
Bold = the values statistically significant higher than that in the same group were shown in bold. OR = odds ratio.
Parasitic infection and diarrhea
To exclude the influence of bacterial pathogens on diarrhea, we examined 14 diarrhea-associated pathogens. We found that three parasite-infected patients were co-infected with Yersinia enterocolitica, and one was co-infected with Vibrio parahaemolyticus. These four cases were removed from the diarrhea group in the following analysis of the association of the infection with diarrhea. In MSM HIV/AIDS population, the overall prevalence of parasites in diarrhea patients was higher than that in non-diarrhea patients (ND) (38.8% vs. 18.9%, p < 0.001), and both PD and HD were higher than that in ND (44.4% vs 18.9%, p < 0.001 and 35.7% vs 18.9%, p = 0.003, respectively) (Table 4). Meanwhile, there is statistical different of the overall prevalence of parasites among the PD, HD and ND groups (44.4% vs 35.7% vs 18.9%, p < 0.001) (Table 4). In particular, the infection proportion of E. bieneusi, E. histolytica, and Cryptosporidium spp. in diarrhea patients was higher than in non-diarrhea subjects, with only the difference for E bieneusi being statistically significant (17.8% vs. 9.5%, p = 0.034) (Table 4). Furthermore, the infection proportions of both Cryptosporidium spp. and E. histolytica were slightly higher in PD individuals than in HD individuals (8.9% and 15.5% vs. 0.6% and 3.6%, respectively) (Table 4). On the other hand, E. bieneusi infection was significantly more prevalent in the HD group than in the PD group (21.4% vs. 11.1%) (Table 4). B. hominis and C. cayetanensis did not show any difference in the infection proportion between diarrhea and non-diarrhea groups (Tables 4 and S10).
Table 4. Parasite infection proportion in MSM HIV-positive participants with different diarrhea status.
| Diarrhea status # | n | Any of parasites | E. histolytica | E. bieneusi | Cryptosporidium | C. cayetanensis | B. hominis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | p value | n (%) | p value | n (%) | p value | n (%) | p value | n (%) | p value | n (%) | p value | |||
| Yes | PDa | 45 | 20 (44.4) | 0.000 | 7 (15.5) | 0.003 | 5 (11.1) | 0.742 | 4 (8.9) | 0.007 | 1 (2.2) | 0.752 | 3 (6.7) | 1.000 |
| HDb | 84 | 30 (35.7) | 0.003 | 4 (4.8) | 0.902 | 18 (21.4) | 0.009 | 1 (1.2) | 1.000 | 1 (1.2) | 0.309 | 6 (7.1) | 1.000 | |
| Subtotalc | 129 | 50 (38.8) | 0.332 | 11 (8.5) | 0.078 | 23 (17.8) | 0.145 | 5 (3.9) | 0.093 | 2 (1.6) | 1.000 | 9 (7.0) | 1.000 | |
| No | NDd | 169 | 32 (18.9) | 0.000 | 6 (3.6) | 0.066 | 16 (9.5) | 0.034 | 1 (0.6) | 0.113 | 2 (1.2) | 1.000 | 7 (4.1) | 0.282 |
| Totale | 308 | 92 (29.9) | 0.000 | 19 (6.2) | 0.008 | 45 (14.6) | 0.026 | 9 (2.9) | 0.006 | 5 (1.6) | 1.000 | 19 (6.2) | 0.591 | |
PD = present diarrhea. HD = historic diarrhea, as described in the methods. ND = non-diarrhea. Bold = the values statistically significant higher than that in the same group were shown in bold.
#Parasite infection identified as a single infection.
ap value = PD vs ND.
bp value = HD vs ND.
cp value = PD vs HD.
dp value = Yes vs No.
ep value = PD vs HD vs ND.
Parasitic infection and hemogram parameters
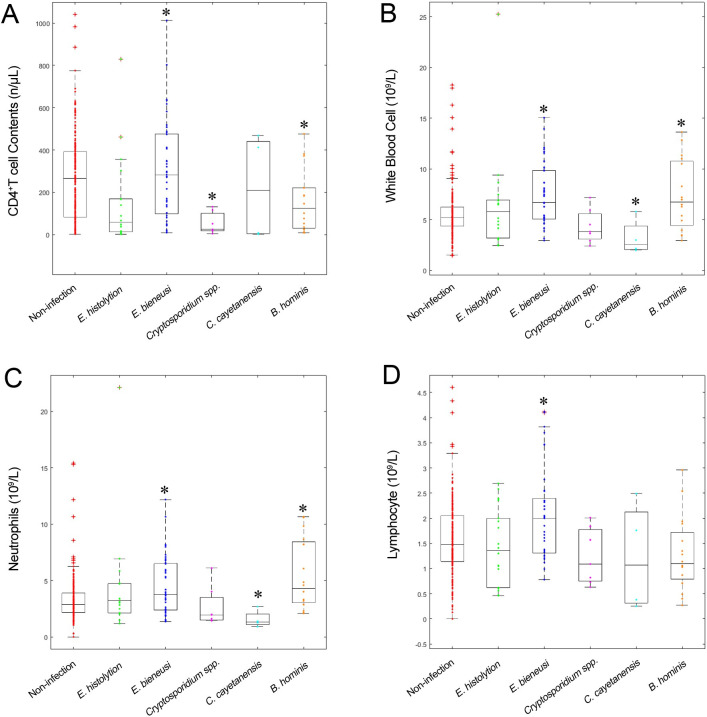
To eliminate the influence of mixed infections with different parasites, we included only cases of single infection (n = 302). CD4+ T cell, WBC, NEUT, and LYMPH counts were significantly different between individuals with and without enteric parasite infections (Table 5 and Fig 3). CD4+ T cell counts were lower in patients infected with Cryptosporidium spp. (52.1 ± 50.9), E. histolytica (153.1 ± 215.8), or B. hominis (157.4 ± 146.6) than in the non-infected group (266.1 ± 201.3) (p < 0.001, p = 0.002, and p = 0.006, respectively). Furthermore, the CD4+ T cell count was below 150 cells/μL in all nine Cryptosporidium-infected MSM HIV/AIDS individuals (S11 Table). There were no significant differences in routine hemogram parameters between Cryptosporidium-infected and non-infected MSM HIV/AIDS individuals. WBC and most of its components, except basophil (BASO), were elevated in E. bieneusi-infected patients.
Table 5. The hemogram parameters and CD4+ T cell counts of MSM HIV-positive participants with parasitic infection (Mean ± SD).
| Parameters | Non-infection | Cryptosporidium | E. histolytica | C. cayetanensis | E. bieneusi | B. hominis | p-value* |
|---|---|---|---|---|---|---|---|
| CD4 (cells/μL) | 266.1 ± 201.3 | 52.1 ± 50.9† | 153.1 ± 215.8† | 222.3 ± 252.5 | 303.7 ± 235.5 | 157.4 ± 146.6† | 0.000 |
| WBC (cells ×109/L) | 5.6 ± 2.5 | 4.4 ± 1.7 | 6.5 ± 5.1 | 3.2 ± 1.8† | 7.3 ± 3.0† | 7.4 ± 3.5† | 0.001 |
| NEUT (cells ×109/L) | 3.3 ± 2.1 | 2.7 ± 1.8 | 4.4 ± 4.7 | 1.6 ± 0.8† | 4.6 ± 2.6† | 5.4 ± 3.1† | 0.000 |
| LYMPH (cells ×109/L) | 1.6 ± 0.8 | 1.2 ± 0.6 | 1.4 ± 0.8 | 1.2 ± 1.1 | 2.0 ± 0.9† | 1.3 ± 0.7 | 0.012 |
| MONO (cells ×109/L) | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.5 ± 0.3 | 0.4 ± 0.2 | 0.5 ± 0.2† | 0.5 ± 0.2 | 0.102 |
| EOSO (cells ×109/L) | 0.1 ± 0.2 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.02 | 0.2 ± 0.2† | 0.2 ± 0.4† | 0.291 |
| BASO (cells ×109/L) | 0.01 ± 0.02 | 0.01 ± 0.01 | 0.02 ± 0.02† | 0.01 ± 0.01 | 0.01 ± 0.02 | 0.02 ± 0.06 | 0.095 |
| RBC (cells ×1012/L) | 4.6 ± 0.8 | 4.4 ± 0.7 | 4.3 ± 0.9 | 3.2 ± 1.8† | 4.7 ± 0.7 | 4.4 ± 0.7 | 0.054 |
| HGB (g/L) | 138.8 ± 23.5 | 129.9 ± 27.0 | 130.3 ± 25.4 | 96.5 ± 55.8† | 142.6 ± 19.4 | 132.6 ± 18.8 | 0.066 |
*p-values indicated the comparison among different infection conditions using Kruskal-Wallis test.
†Statistically different compared with non-infection, using Kruskal-Wallis test.
Fig 3. CD4+ T cell counts and hemogram parameters in different parasite-infection and non-infection groups of the MSM HIV/AIDS population.
CD4+ T cell counts (A), WBC (B), NEUT (C) and LYMPH (D) in different parasite-infection and non-infection groups.* indicates statistical significant difference between the population with parasite infections versus the one without it.
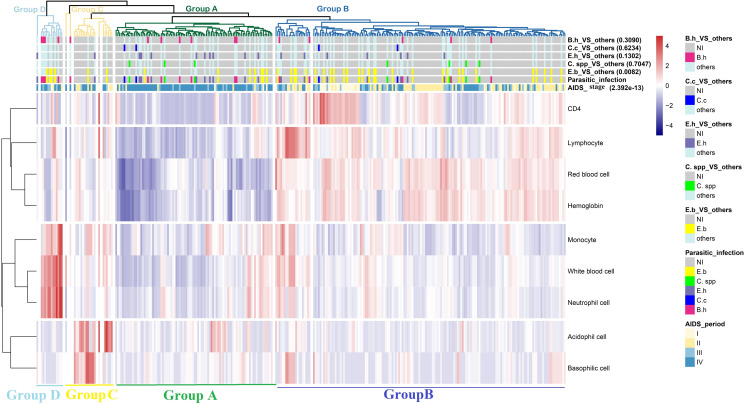
CD4+ T cell, LYMPH, RBC, and HGB were clustered in a separate branch showing similar patterns in the heat map (Fig 4). WBC, MONO, and NEUT were clustered together, while EOSO and BASO were clustered in another branch. A total of 302 MSM HIV/AIDS individuals that were singly infected with a parasite were divided into four groups. Individuals in groups A and B, accounting for 90% of all individuals, displayed their own unique hemogram patterns.
Fig 4. Clustering heatmap of the infection data.
Among the 7 color bars, the bottom two are parasitic infection statuses and AIDS periods, and different colors represent different kinds of parasitic infections or different AIDS stages. The remaining 5 color bars are derived from the parasitic infection variable. For instance, in the group of “E.b._VS_other”, samples with parasitic infection of E.b. make up the group of interest, and parts 2 to 5 are separated into another group, while the uninfected group is comprised of samples with no parasitic infection. The other group and the non-infected group are labeled with cyan and gray colors, respectively. The group of interest is marked with a distinct color corresponding to the color in the parasitic infection status (NI, not infected; E.b., E. bieneusi; C. spp., Cryptosporidium spp.; E.h., E. histolytica; C.c., C. cayetanensis; B.h., B. hominis).
E. bieneusi-infected patients were more numerous in group B (p = 0.008) (S12 Table), with higher CD4+ T cell, LYMPH, RBC, and HGB counts and lower MONO, WBC, NEUT, EOSO, and BASO counts. However, individuals infected with the four other parasites could not be distinguished by these hemogram parameters. We also found that individuals at AIDS stages III/IV were the most numerous in group A (p < 0.001), with lower cell counts in the hemogram parameters. In contrast, individuals in early AIDS stages (I/II) were dominant in group B, consistent with the association of E. bieneusi and the AIDS stage above.
Species, genotypes/subtypes of enteric parasites
There were three species of Cryptosporidium, including C. hominis (1.8%), C. meleagridis (1.3%), and C. cuniculus (0.3%) (Table 6). C. hominis and C. meleagridis were the dominant species accounting for 92.3% of Cryptosporidium isolates from all HIV/AIDS individuals. In addition, we found three novel subtypes of C. meleagridis, IIIeA15G2R1 (GenBank accession number MG917667), IIIbA23G1R1d (GenBank accession number MG917668), and IIIgA26G1R1 (GenBank accession number MG917669). This is the first time C. cuniculus infection is reported in HIV-infected individuals.
Table 6. The species, genotypes, and subtypes of Cryptosporidium, E. bieneusi, and B. hominis in HIV-positive participants.
| Routes of HIV infection (n) | Cryptosporidium | E. bieneusi | B. hominis | ||||
|---|---|---|---|---|---|---|---|
| n (%) | Species (n, %) | Subtype (n) | n (%) | Genotype (n) | n (%) | ST subtype (n) | |
| MSM (308) | 9 (2.9) | C. hominis (6, 1.9) | IbA20G2 (5); NA (1) | 45 (14.6) | D (33); EbpC (1); CHN-H1 (4); CHN-H2 (1); CHN-H3 (1); NA (5) | 19 (6.2) | ST1 (3); ST3 (15); ST14 (1) |
| C. meleagridis (2, 0.6) | IIIbA23G1R1 (1); NA (1) | ||||||
| C. culuculus (1, 0.3) | NA (1) | ||||||
| MSW & Others (76) | 4 (5.3) | C. hominis (1, 1.3) | Ia18R4 (1) | 11 (14.5) | D (4); NA (7) | 12 (15.8) | ST3 (12) |
| C. meleagridis (3, 3.9) | IIIeA15G2R1 (1); IIIgA26G1R1 (2) | ||||||
MSM = men having sex with men. MSW = men having sex with women. Others = sexually transmitted women, non-sexually transmitted individuals, and unknown transmission routes were included. NA = not available.
Five genotypes of E. bieneusi were found among all the HIV/AIDS patients studied (Table 6), including two known genotypes (D and EbpC) and three novel genotypes (CHN-H1, CHN2, and CHN-H3). Genotype D was dominant, being present in 84.1% of all isolates. Genotypes CHN-H1 (GenBank accession number MG255733) and CHN-H2 (GenBank accession number MG255734) showed 98.8% and 98.4% homology with genotype KIN-1 (GenBank accession number KR815514), respectively. Indeed, the genotype CHN-H3 (GenBank accession number MG255735) showed 97.9% homology with genotype EbpC (GenBank accession number KX905207). Phylogenetic analysis indicated that all five genotypes belonged to zoonotic E. bieneusi group 1 (S1 Fig).
Three ST subtypes of Blastocystis were found, including ST1 (9.7%; 3/31), ST3 (87.1%; 27/31), and ST14 (3.2%; 1/31) (Table 6). This is the first time that subtype ST14 is reported in humans.
We found some unique features in MSM HIV/AIDS individuals. For instance, these individuals were infected with a wider range of species or genotypes of Cryptosporidium, E. bieneusi, and B. hominis than non-MSM subjects. The MSM group had more C. hominis-infected individuals, and the newly identified C. culuculus was also found in this group. MSM HIV/AIDS individuals had five genotypes of E. bieneusi, while the non-MSM group had only one genotype (D). ST1 and ST14 of B. hominis were only found in MSM individuals (Table 6).
Discussion
To the best of our knowledge, this study is the first systematic investigation of the epidemiology of five enteric parasites, including E. bieneusi, B. hominis, E. histolytica, Cryptosporidium spp., and C. cayetanensis in HIV/AIDS and MSM HIV/AIDS populations in China. The prevalence of five parasitic infections in HIV/AIDS and MSM HIV/AIDS populations was much higher than in healthy individuals. Considering the lack of information about such infections in the MSM HIV/AIDS population, we could not compare data from the present study with other areas of China. Moreover, to date, only two publications have reported on the epidemic of enteric parasites using molecular tools and indicated that the prevalence of E. bieneusi was 5.1% in Henan Province and 11.6% in Guangxi Province [16, 17], while that of Cryptosporidium spp. was 3.5% in Henan [17]. The prevalence of E. bieneusi in Heilongjiang Province is higher than that in Guangxi and Henan Province, while that of Cryptosporidium spp. is similar to that in Henan. Some reports also revealed various infection rates of B. hominis, E. histolytica, and Cryptosporidium spp. using morphology or serology methods [5, 14, 15]. It has been well established that these methods may not be adequate to reflect the true epidemic [33].
Epidemiological data suggest that water quality, personal hygiene, animal contact, educational status, ambient temperature, and season are environmental factors associated with parasitic infections [34,35]. However, these factors significantly vary between geographical regions and within different populations [36]. Multivariate logistic regression showed that the warm season was the only statistically significant risk factor for parasitic infection. This risk factor is almost applicable to all parasitic infections except Cryptosporidium spp., which might be mainly related to immunodeficiency conditions. Therefore, clinicians and AIDS patients should be aware of the risk of parasitic infections during this season.
In AIDS patients, the prevalence of sexually transmitted infections (STIs) and the severity of other infections are associated with CD4+ T cell counts [10]. However, to date, only a limited number of parasites, such as Cryptosporidium spp., E. bieneusi, and E. histolytica, have been defined as AIDS-associated, and such infections are correlated with low CD4+ T cell counts [36–38]. Contradictory findings with E. histolytica and E. bieneusi infection were also reported [39, 40]. We found that the low CD4+ T cell counts significantly correlated with infection with Cryptosporidium spp., E. histolytica, and B. hominis but not with E. bieneusi in HIV/AIDS and MSM HIV/AIDS populations. Therefore, we suggest adding E. histolytica and B. hominis as risk parasites for opportunistic infections in AIDS patients and advise examining these infections.
In addition, we also analyzed the haematologic elements of MSM HIV/AIDS individuals and their correlation to parasitic infection. These hemogram parameters can be divided into three branches; LYMPH, RBC, and HGB were clustered in the same branch with CD4+ T cell count. These four parameters showed higher counts in the early stages and lower counts in the late stages of AIDS, while the other parameters were generally lower in MSM HIV/AIDS patients. Thus, we suggest that LYMPH, RBC, and HGB can predict the immune status of HIV/AIDS patients together with CD4+ T cell count. Parinitha et al. also found that the haematologic manifestations of HIV infection are common and more frequent with disease progression and that there is a significant increase in the number of cases of anemia and lymphopenia with decreasing CD4+ T cell counts [41]. This may highlight the importance of simultaneously treating AIDS patients for haematologic manifestations to reduce morbidity. E. bieneusi and the other four parasites could well be distinguished by CD4+ T cell, LYMPH, RBC, and HGB counts. Meanwhile, the severity of AIDS does not increase the infection of E. bieneusi, the tendency of which is quite different from the four other parasites. We do not know the mechanism of this phenomenon, although there is a similar report [17]. Thus, we need to pay more attention to the high prevalence of E. bieneusi among AIDS populations and ought to examine HIV-infected individuals with diarrhea even if they are in the early stages of AIDS.
Of the five parasites, E. histolytica, Cryptosporidium spp., E. bieneusi, and C. cayetanensis have been found to cause diarrhea associated with HIV, particularly in the context of very low CD4+ T cell counts. The influence of B. hominis infection is still debated [20]. However, when we tried to correlate parasitic infections and diarrhea, we had to consider parasitic infection itself and minimize the influence of non-parasite factors as much as possible. We examined and excluded bacterial co-infection in this study and evaluated the correlation between diarrhea and parasitic infection. The fact that the proportion of PD in the MSM HIV/AIDS group was higher than in the MSW group suggests that MSM may produce a more severe diarrhea situation. Furthermore, the infection proportions of both Cryptosporidium spp. and E. histolytica are slightly higher in PD individuals than in HD individuals. The facts above imply that E. histolytica or Cryptosporidium spp. infection is related to more severe diarrhea in MSM HIV/AIDS individuals. In addition, co-infection of Cryptosporidium spp. and Yersinia enterocolitica resulted in more severe diarrhea. Furthermore, E. bieneusi infection was also diarrhea-related but was more significantly associated with HD (moderate cases). B. hominis was not found to be a diarrhea-related parasite, consistent with other findings [42].
The application of molecular methods for identification and characterization has led to more reliable results, revealed the true prevalence of infections, improved source tracking, calculated host range, and correlated the pathogenic potential of many parasite types [33]. We also analyzed genotypes and the phylogeny of parasites and attempted to track possible sources for high-risk infection routes in HIV/AIDS individuals. In HIV/AIDS individuals, C. hominis was dominant and possibly correlated with latent infection in local human beings, as it is a species with the human-to-human transmission, and no infection was found in local horses (the sensitive animal) [43, 44]. C. cuniculus may be derived from local rabbits, while novel subtypes of C. meleagridis are from animals or humans (S2 Table and S2 Fig). Genotype EbpC was dominant in local children, and D was found in tumor patients [45, 46]. Indeed, genotype D is dominant in various farm animals, while EbpC is dominant in household animals. Therefore, the sources of these two genotypes vary and may be from humans or animals (S1 Table and S1 Fig). Three ST subtypes of B. hominis were found in HIV/AIDS individuals. ST1 and ST3 are common in humans worldwide. Meanwhile, ST1 and ST3 have been found in local sheep and cattle, and ST3 has been identified in local tumor patients [46, 47]. ST14 has never been identified in humans before. Thus, local cattle and sheep may act as a source of infection for the newly identified ST14, while ST1 and ST3 may be derived from humans, sheep, or cattle (S3 Table). The above findings not only extend the range of zoonotic enteric parasites in humans but also imply the apparent underestimated latent infections by those pathogens and the important source of animals. Extensive investigation of zoonotic enteric parasitic infections, source tracking, and proper prevention strategies should be considered to reduce AIDS-related morbidity and mortality.
Regarding each parasite, we may claim that: 1) E. bieneusi is highly prevalent in the HIV-infected population and infected individuals with low CD4+ T cell counts and diarrhea, especially mild diarrhea, distinguished from the four other parasites. 2) E. histolytica is related to low CD4+ T cell counts and diarrhea, especially severe diarrhea, and is more prevalent at the late stages of AIDS. 3) Cryptosporidium spp., like E. histolytica, are more closely associated with low CD4+ T cell counts. 4) B. hominis infection is associated with low CD4+ T cell counts and is more prevalent during the late stages of AIDS but does not occur with diarrhea. Unexpectedly, the positive rate of B. hominis in MSM was significantly lower than in the MSW population. It is likely that the lower proportion is associated with a high proportion of MSM among the whole HIV/AIDS population because the proportion in females was very high in the present study (26.7%) (S9 Table;), as has been seen elsewhere [48].
This study had some limitations that should be mentioned. 1) There was a significant difference between the number of males and females recruited for the study. 2) The study collected only one stool specimen instead of multiple samples, which could improve the detection rates of different parasites. 3) HIV-negative MSM individuals were not recruited in this study. Although we do not know the prevalence of parasitic infections in the whole MSM population, the main purpose of improving knowledge of co-infection in MSM HIV/AIDS individuals has already been achieved. 4) Most of the enrolled participants had just started ART treatment. Thus, this factor was not taken into the analysis of the influence on parasitic infection. We will conduct continuous follow-ups of these patients to determine the relationship between ART and parasitic infections in the future. 5) The present study was confined to research on MSM HIV/AIDS with enteric pathogens in cold areas; the situation in the tropics may be different. Our study does not represent the entire MSM HIV/AIDS population worldwide.
Currently, studies that reflect actual human infections with enteric parasites are still insufficient. The findings of this study have important implications for public health, the control of AIDS progression, and the control of parasitic infections. First, the presence of asymptomatic carriers of the parasites, the high burden among local animals, and the prevalence in AIDS patients emphasize their importance in the transmission cycle. Therefore, latent infection with enteric parasites in humans and the burden in local animals should be extensively investigated and emphasized to public health workers and patients to prevent and control these infections and decrease mortality in immunosuppressed individuals. Second, source tracking by molecular tools of human infections can more clearly identify possible sources. This is beneficial for adequately managing the local stock breeding industry and blocking transmission routes. The identification of new types or subtypes in humans suggests the evolution of enteric intracellular parasites and a new threat to humans and animals. Third, parasitic infections and diarrhea correlated by eliminating the influence of bacterial pathogens, providing clearer clinical significance for these parasitic infections in the HIV/AIDS population.
In conclusion, there is a common occurrence of the co-infection of enteric intracellular parasites with HIV among MSM from northeast China, and a higher infection proportion was shown in the warm season. The infection of E. histolytica and Cryptosporidium spp. is likely related to more severe diarrhea, while that of E. bieneusi is related to mild diarrhea. The MSM HIV/AIDS population has a wider range of species or genotypes of Cryptosporidium, E. bieneusi, and B. hominis. In addition, the possible sources of parasitic infections in these individuals are either humans or animals. Therefore, AIDS patients should be educated and informed to minimize the risk of transmission of parasites from humans and animals.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We are grateful to members of the Ling laboratory for helpful comments and all the staff at the participating departments for contributing to patient care and disease control and helping provide the data that this manuscript is based on.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81772190 to HL, 81672003 to FXW, 81871654 to MZ); Natural Science Foundation of Heilongjiang Province of China (LH2021H007 to MZ) and Harbin Medical University Scientific Research Innovation Fund (2016JCZX42 to LY). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chastain DB, Henao-Martínez AF, Franco-Paredes C. Opportunistic Invasive Mycoses in AIDS: Cryptococcosis, Histoplasmosis, Coccidiodomycosis, and Talaromycosis. Curr Infect Dis Rep. 2017; 19: 36. doi: 10.1007/s11908-017-0592-7 [DOI] [PubMed] [Google Scholar]
- 2.Iribarren JA, Rubio R, Aguirrebengoa K, Arribas JR, Baraia-Etxaburu J, Gutiérrez F, et al. Prevention and treatment of opportunistic infections and other coinfections in HIV-infected patients: May 2015. Enferm Infecc Microbiol Clin. 2016; 34: 516.e1-516.e18. [DOI] [PubMed] [Google Scholar]
- 3.Ford N, Ball A, Baggaley R, Vitoria M, Low-Beer D, Penazzato M, et al. The WHO public health approach to HIV treatment and care: looking back and looking ahead. Lancet Infect Dis. 2018; 18: e76–e86. doi: 10.1016/S1473-3099(17)30482-6 [DOI] [PubMed] [Google Scholar]
- 4.Andreani G, Lodge R, Richard D, Tremblay MJ. Mechanisms of interaction between protozoan parasites and HIV. Curr Opin HIV AIDS. 2012; 7: 276–82. doi: 10.1097/COH.0b013e32835211e9 [DOI] [PubMed] [Google Scholar]
- 5.Wang ZD, Liu Q, Liu HH, Li S, Zhang L, Zhao YK, et al. Prevalence of Cryptosporidium, microsporidia and Isospora infection in HIV-infected people: a global systematic review and meta-analysis. Parasit Vectors. 2018; 11: 28. doi: 10.1186/s13071-017-2558-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcos LA, Gotuzzo E. Intestinal protozoan infections in the immunocompromised host. Curr Opin Infect Dis. 2013; 26: 295–301. doi: 10.1097/QCO.0b013e3283630be3 [DOI] [PubMed] [Google Scholar]
- 7.New progress in HIV/AIDS epidemiology research in China. The Chinese Journal of Disease Control. 2021,25(12):1365–1368+1480. (In Chinese). [Google Scholar]
- 8.NCAIDS, NCSTD, China CDC. Update on the AIDS/STD epidemic in China in December 2017. Chinese Journal of AIDS & STD. 2018; 02. 01. (In Chinese). [Google Scholar]
- 9.Zou H, Tucker JD, Fan S, Xu J, Yu M, Luo Z, et al. Learning about HIV the hard way: HIV among Chinese MSM attending university. Lancet Infect Dis. 2018; 18: 16–18. doi: 10.1016/S1473-3099(17)30711-9 [DOI] [PubMed] [Google Scholar]
- 10.Mitchell H, Hughes G. Recent epidemiology of sexually transmissible enteric infections in men who have sex with men. Curr Opin Infect Dis. 2018; 31: 50–56. doi: 10.1097/QCO.0000000000000423 [DOI] [PubMed] [Google Scholar]
- 11.Peters CS, Sable R, Janda WM, Chittom AL, Kocka FE. Prevalence of enteric parasites in homosexual patients attending an outpatient clinic. Journal of Clinical Microbiology. 1986; 24: 684–685. doi: 10.1128/jcm.24.4.684-685.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pakianathan MR, McMillan A. Intestinal protozoa in homosexual men in Edinburgh. International Journal of STD & AIDS. 1999; 10: 780–784. doi: 10.1258/0956462991913547 [DOI] [PubMed] [Google Scholar]
- 13.Stark D, Fotedar R, van Hal S, Beebe N, Marriott D, Ellis JT, et al. Prevalence of enteric protozoa in human immunodeficiency virus (HIV)-positive and HIV-negative men who have sex with men from Sydney, Australia. American Journal of Tropical Medicine and Hygiene. 2007; 76: 549–552. [PubMed] [Google Scholar]
- 14.Tian LG, Chen JX, Wang TP, Cheng GJ, Steinmann P, Wang FF, et al. Co-infection of HIV and intestinal parasites in rural area of China. Parasit Vectors. 2012; 5: 36. doi: 10.1186/1756-3305-5-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian LG, Wang TP, Lv S, Wang FF, Guo J, Yin XM, et al. HIV and intestinal parasite co-infections among a Chinese population: an immunological profile. Infect Dis Poverty. 2013; 2: 18. doi: 10.1186/2049-9957-2-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Zhang H, Zhao X, Zhang L, Zhang G, Guo M, et al. Zoonotic Cryptosporidium species and Enterocytozoon bieneusi genotypes in HIV-positive patients on antiretroviral therapy. J Clin Microbiol. 2013; 51: 557–63. doi: 10.1128/JCM.02758-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Jiang Z, Yuan Z, Yin J, Wang Z, Yu B, et al. Infection by and genotype characteristics of Enterocytozoon bieneusi in HIV/AIDS individuals from Guangxi Zhuang autonomous region, China. BMC Infect Dis. 2017; 17: 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F, Li M, Li X, Yang Y, Gao C, Jin Q, et al. Seroprevalence of Entamoeba histolytica infection among Chinese men who have sex with men. PLoS Negl Trop Dis. 2013; 7: e2232. doi: 10.1371/journal.pntd.0002232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan U, Zahedi A, Paparini A. Cryptosporidium in humans and animals-a one health approach to prophylaxis. Parasite Immunol. 2016; 38: 535–47. doi: 10.1111/pim.12350 [DOI] [PubMed] [Google Scholar]
- 20.Stensvold CR, Clark CG. Current status of Blastocystis: A personal view. Parasitol Int. 2016; 65: 763–771. doi: 10.1016/j.parint.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 21.Fayer R, Santin-Duran M. Epidemiology of microsporidia in human infections. In: Weiss LM., Becnel JJ. (Eds.), Microsporidia: Pathogens of Opportunity, first ed. John Wiley & Sons, Inc., Chichester, UK. 2014; doi: 10.1002/9781118395264.ch3 [DOI] [Google Scholar]
- 22.Shao B, Li Y, Yu L, Wang K, Chang M, Wang B, et al. The HIV/AIDS epidemic characteristics in a northeast province of China—men who have sex with men have made a tremendous contribution to the growth of the HIV epidemic. J Infect. 2014; 68: 273–80. doi: 10.1016/j.jinf.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 23.Organization WH. Interim WHO clinical staging of HVI/AIDS and HIV/AIDS case definitions for surveillance: African region. World Health Organization, 2005. [Google Scholar]
- 24.Xiao L, Escalante L, Yang C, Sulaiman I, Escalante AA, Montali RJ, et al. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl Environ Microbiol. 1999; 65: 1578–83. doi: 10.1128/AEM.65.4.1578-1583.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paglia MG, Visca P. An improved PCR-based method for detection and differentiation of Entamoeba histolytica and Entamoeba dispar in formalin-fixed stools. Acta Trop. 2004; 92: 273–7. doi: 10.1016/j.actatropica.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 26.Li G, Xiao S, Zhou R, Li W, Wadeh H. Molecular characterization of Cyclospora-like organism from dairy cattle. Parasitol Res. 2007; 100: 955–61. doi: 10.1007/s00436-006-0380-z [DOI] [PubMed] [Google Scholar]
- 27.Zhao W, Zhang W, Yang F, Cao J, Liu H, Yang D, et al. High prevalence of Enterocytozoon bieneusi in asymptomatic pigs and assessment of zoonotic risk at the genotype level. Appl Environ Microbiol. 2014; 80: 3699–3707. doi: 10.1128/AEM.00807-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scicluna SM, Tawari B, Clark CG. DNA barcoding of blastocystis. Protist. 2006; 157: 77–85. doi: 10.1016/j.protis.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 29.Buckholt MA, Lee JH, Tzipori S. Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughterhouse in Massachusetts. Appl Environ Microbiol. 2002; 68: 2595–9. doi: 10.1128/AEM.68.5.2595-2599.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alves M, Xiao L, Sulaiman I, Lal AA, Matos O, Antunes F. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J Clin Microbiol. 2003; 41: 2744–7. doi: 10.1128/JCM.41.6.2744-2747.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santín M, Fayer R. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: a consensus. J Eukaryot Microbiol. 2009; 56: 34–8. doi: 10.1111/j.1550-7408.2008.00380.x [DOI] [PubMed] [Google Scholar]
- 32.Langfelder P, Zhang B, Horvath S. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics. 2008; 24:719–20. doi: 10.1093/bioinformatics/btm563 [DOI] [PubMed] [Google Scholar]
- 33.Verkerke HP, Sobuz SU, Petri WA Jr. Molecular diagnosis of infectious diarrhea: focus on enteric protozoa. Expert Rev Mol Diagn. 2014; 14: 935–46. doi: 10.1586/14737159.2014.951035 [DOI] [PubMed] [Google Scholar]
- 34.Nkenfou CN, Tchameni SM, Nkenfou CN, Djataou P, Simo UF, Nkoum AB, et al. Intestinal Parasitic Infections in Human Immunodeficiency Virus-Infected and Noninfected Persons in a High Human Immunodeficiency Virus Prevalence Region of Cameroon. Am J Trop Med Hyg. 2017; 97: 777–781. doi: 10.4269/ajtmh.16-0900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarkari B, Hosseini G, Motazedian MH, Fararouei M, Moshfe A. Prevalence and risk factors of intestinal protozoan infections: a population-based study in rural areas of Boyer-Ahmad district, Southwestern Iran. BMC Infect Dis. 2016; 16: 703. doi: 10.1186/s12879-016-2047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Checkley W, White AC Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis. 2015; 15: 85–94. doi: 10.1016/S1473-3099(14)70772-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matos O, Lobo ML, Xiao L. Epidemiology of Enterocytozoon bieneusi Infection in Humans. J Parasitol Res. 2012; 2012: 981424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung CC, Chang SY, Ji DD. Entamoeba histolytica infection in men who have sex with men. Lancet Infect Dis. 2012; 12: 729–36. doi: 10.1016/S1473-3099(12)70147-0 [DOI] [PubMed] [Google Scholar]
- 39.Akinbo FO, Okaka CE, Omoregie R. Prevalence of intestinal parasitic infections among HIV patients in Benin City, Nigeria. Libyan J Med. 2010; 5. doi: 10.3402/ljm.v5i0.5506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nkinin SW, Asonganyi T, Didier ES, Kaneshiro ES. Microsporidian infection is prevalent in healthy people in Cameroon. J Clin Microbiol. 2007; 45: 2841–6. doi: 10.1128/JCM.00328-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parinitha S, Kulkarni M. Haematological changes in HIV infection with correlation to CD4 cell count. Australas Med J. 2012; 5: 157–62. doi: 10.4066/AMJ.20121008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forsell J, Bengtsson-Palme J, Angelin M, Johansson A, Evengård B4, Granlund M. The relation between Blastocystis and the intestinal microbiota in Swedish travellers. BMC Microbiol. 2017; 17: 231. doi: 10.1186/s12866-017-1139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jian F, Liu A, Wang R, Zhang S, Qi M, Zhao W, et al. Common occurrence of Cryptosporidium hominis in horses and donkeys. Infect Genet Evol. 2016; 43: 261–6. doi: 10.1016/j.meegid.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 44.Liu A, Zhang J, Zhao J, Zhao W, Wang R, Zhang L. The first report of Cryptosporidium andersoni in horses with diarrhea and multilocus subtype analysis. Parasit Vectors. 2015; 8: 483. doi: 10.1186/s13071-015-1102-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J, Song M, Wan Q, Li Y, Lu Y, Jiang Y, et al. Enterocytozoon bieneusi genotypes in children in Northeast China and assessment of risk of zoonotic transmission. J Clin Microbiol. 2014; 52: 4363–7. doi: 10.1128/JCM.02295-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang W, Ren G, Zhao W, Yang Z, Shen Y, Sun Y, et al. Genotyping of Enterocytozoon bieneusi and Subtyping of Blastocystis in Cancer Patients: Relationship to Diarrhea and Assessment of Zoonotic Transmission. Front Microbiol. 2017; 8: 1835. doi: 10.3389/fmicb.2017.01835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Gong B, Yang F, Zhang W, Zheng Y, Liu A. Subtype distribution and genetic characterizations of Blastocystis in pigs, cattle, sheep and goats in northeastern China’s Heilongjiang Province. Infect Genet Evol. 2018; 57: 171–176. doi: 10.1016/j.meegid.2017.11.026 [DOI] [PubMed] [Google Scholar]
- 48.Tian LG, Chen JX, Cheng GJ, Wang FF, Guo J, Yin XM, et al. Survey on Blastocystis hominis infection in HIV positive individuals in Fuyang City, Anhui Province. Zhong guo Xue Xi Chong Bing Fang Zhi Za Zhi. 2012; 24: 303–6 (In Chinese). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.