Abstract
The SARS-CoV-2 pandemic has shown how serious the problem of re-emerging zoonotic infections is for our existence. Migrations of animals, which are natural reservoirs of a particular virus, play a colossal role in the spread of pathogens to new territories. Examples are the migrations of both land animals (carnivores, rodents, and ungulates) and many marine mammals (pinnipeds and cetaceans). Yet the most interesting from the point of view of the speed and range of the spread of viral infections are migrations associated with flights. In nature, these can be migrations of insects, bats, and, of course, birds. Unfortunately, there are very few studies on the migration of these animals in Russia. Considering the problems related to climate change and other environmental factors, it is important to obtain up-to-date data on the changing animal migration routes and, as a consequence, to develop domestic equipment, particularly transmitters, to fix them.
Keywords: zoonotic infections, migrations, viruses, natural reservoir
Global biological threats pose a serious danger not only to the development but also to the very existence of humankind. These threats include environmental ones, that is, associated with disturbing habitats and even making them completely unsuitable for life; climate change, a sharp degradation of existing ecosystems; and bioterrorism-associated dangers. Viral and bacterial pathogens that can cause massive epizootics, epidemics, and even pandemics also endanger modern civilization, especially in the context of huge megalopolises and active year-round movement of people and foods and other goods around the globe [1–4].
It is estimated that at least 10 000 different viruses currently identified in animals can potentially infect humans [5]. Over the last few decades alone, such dangerous pathogens as filoviruses (Marburg virus and Ebola virus), orthomyxoviruses (new highly pathogenic influenza viruses of the H5N1, H5N8, H9N2, and H7N9 subtypes), and new coronaviruses (SARS, MERS, and COVID-19) have been detected and described [6]. Undoubtedly, humanity will more than once encounter new dangerous viruses, which can lead to numerous victims and cause serious damage to the planet’s population and economy.
Most of the newly detected dangerous viral infections of the 21st century originated and spread in China: SARS in 2002, highly pathogenic avian influenza viruses of various subtypes (H5N1 in 2005, H7N9 in 2013, and H9N2 in 1997‒2009), and coronavirus infection COVID-19 (2019). Some of them, such as the H5N1 influenza virus and COVID-19, subsequently became widespread, covering almost all countries of the world [7], and, importantly, these outbreaks and epidemics took humanity by surprise.
This is evident by the example of the SARS-CoV-2 coronavirus, which caused the COVID-19 pandemic, forcing us to restructure seriously the antiepidemic measures of the healthcare systems of most countries, some sectors of the economy, and the very everyday life of many millions of people. Let us emphasize once again: we all have become aware of how serious the problem of newly emerging zoonotic infections is for our very existence and how fragile the world in which we lived until 2020 was. It turned out that, despite the active development of science in recent decades, we faced a lack of available fundamental knowledge about ecological and epidemic processes in wildlife at the current stage of civilization development and their response to anthropogenic impacts, climate change, and catastrophic natural phenomena.
To reduce current and potential epidemiological and other biological risks and prevent negative scenarios for the development of epizootics and pandemics of new infections, including zoonotic ones, it is necessary to enhance fundamental knowledge about the features of the ecology of viral pathogens and related natural reservoirs and the system of functional relationships ensuring circulation of such pathogens in natural reservoirs and changes in the host under certain conditions.
An important, sometimes dominant, role in the spread of new viral infections is played by the migration of wild animals, which are natural vectors of a particular pathogen. An example is the avian influenza virus. Thus, the highly pathogenic H5N1 influenza virus, which emerged in northwestern China in April 2005 during the spring and autumn migration of birds, spread to the entire territory of Northeastern Eurasia and later (in the fall of 2005) to all of Eurasia and the African continent [8].
Most emerging human viral diseases (Ebola virus disease, Zika, Lassa fever, Crimean‒Congo hemorrhagic fever, severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and the 2019 coronavirus infection (COVID-19) are of zoonotic origin [9, 10]. As a rule, pathogenic viruses are associated with certain types of hosts and vectors, and the outbreaks or sporadic cases of diseases they cause in humans are recorded within certain areas of the ranges of reservoir species. At the same time, some viruses quickly and effectively adapt to human-to-human transmission, which allows infections to expand beyond the natural focus and in some cases leads to a large-scale spread of infection to other continents (Ebola, Zaire, and Zika viruses and MERS-CoV and SARS-CoV-2).
One thing is clear—the colossal role of the migrations of animals that are the natural reservoir of a virus, especially potentially dangerous to humans, in spreading to new territories. The study of such migrations is of great scientific and practical interest. The importance of the problem is also due to the fact that climate change and anthropogenic impact have changed traditional, known ways of animal migration, which can lead and already leads to the introduction of viral pathogens, previously absent in these places [11].
To protect a territory against biological threats, it is necessary to solve the following tasks:
• identifying and assessing the risk of emerging viral pathogens;
• studying the ecology of the natural hosts of these pathogens;
• determining the migratory potential of natural hosts;
• studying the anthropogenic and climate change impacts on the migration routes of the vectors of viral pathogens.
A few words about terminology. Zoonoses are infections that are common to humans and animals in natural conditions [12, 13]. In the domestic literature, zoonoses are infectious and invasive human diseases, in which various species of domestic and wild mammals and birds serve as a reservoir and source of infection. Of more than 1400 pathogens dangerous to humans, 61–75% are zoonoses [14, 15].
In the 20th century and the first two decades of the 21st century alone, we have encountered more than twenty new zoonotic viruses, which together have killed about 100 million people. Table 1 presents a list of zoonotic infections detected in humans in the 20th–21st centuries that is far from complete [4, 16]. The most famous of them are the Spanish influenza virus H1N1 (1918), the Ebola virus (1976), the human immunodeficiency virus (1983), the highly pathogenic H5 influenza virus (1997), the swine influenza virus H1N1pdm09 (2009), the SARS-CoV coronavirus (2003), the MERS-CoV coronavirus (2012), and the SARS-CoV-2 coronavirus (2019) [16]. These infections for a long time remained (SARS-CoV-2 still remains) in the focus of the press and, accordingly, health authorities and sanitary and veterinary supervision bodies.
Table 1.
New viruses of zoonotic nature detected in humans in the 20th‒21st centuries
| Name | Year of detection, country |
|---|---|
| Spanish flu virus | 1918 |
| Japanese encephalitis virus | 1935–1937 (isolation), Soviet Union |
| West Nile virus | 1937, Uganda |
| Crimean‒Congo hemorrhagic fever virus | 1944−1945 (isolation), Soviet Union |
| Zika virus | 1947, Uganda |
| Machupo virus | 1952, Bolivia |
| Junin virus | 1958, Argentina |
| Hepatitis B virus | 1965, Australia |
| Lassa virus | 1969, Nigeria |
| Ebola virus | 1976, Sudan, Zaire (the Democratic Republic of the Congo) |
| Hepatitis E virus | 1978, India |
| HIV | 1983, United States, first described in 1981 |
| Hendra virus | 1994, Australia |
| Influenza virus “highly pathogenic H5” | 1997, Hong Kong |
| Nipah virus | 1999, Malaysia, Singapore |
| Swine influenza virus | 2009, Mexico, United States |
| Coronavirus SARS-CoV | 2003, China |
| Coronavirus MERS-CoV | 2012, Saudi Arabia |
| Coronavirus SARS-CoV-2 | 2019, China |
Especially noteworthy are viruses of the Coronaviridae family (genus Betacoronavirus). In the past 20 years alone, they have caused high-fatality diseases in humans three times, the last member of this genus, the SARS-CoV-2 virus, causing a pandemic that continues to this day. Chronologically, these are the following:
(1) atypical pneumonia, or severe acute respiratory syndrome (SARS); its natural source is animals of the viverrid family; mortality in humans is 9.6% [17];
(2) the Middle East respiratory syndrome virus (MERS, MERS-CoV); its natural reservoir is bats; lethality in humans is up to 17.6% [18];
(3) SARS-CoV-2 coronavirus (COVID-19); the alleged reservoir is bats, pangolins; lethality is 2 to 4% [19].
In analyzing the possibility and danger for people to contract infection from wild animals, we should note that the process is also reversible. We, too, are a source of infection for many domestic and farm animals, as demonstrated by the SARS-CoV-2 virus. The scientific literature describes many cases of this infection in domestic cats and dogs caused by this virus caught from their owners with COVID-19 [20–22].
A few words about migration processes in the animal world. Migration of animals in nature is a natural process, necessary for the existence of one species or another due to various reasons. One of the main ones is the search for new places for reproduction and rearing offspring. (We will not consider human-induced migration here since this process can be controlled quite easily, although its role in the spread of viruses and other pathogens should not be ignored [23].)
Classical migrations are divided into seasonal, periodic (in both cases, the movement of animals occurs within the range), and nonperiodic (outside the range). An example is the migration of land animals such as carnivorous rodents and ungulates. These animals, moving within their habitat, can be a source of many dangerous infections: rabies, tick-borne encephalitis, hemorrhagic fever with renal syndrome, and others [11]. Thus, the main natural reservoir of African swine fever is wild boars, which can travel hundreds of kilometers and spread this infection, infecting domestic pigs.
Huge distances during migrations are covered by many marine mammals, although it is believed that the epidemic and epizootic significance of their migrations is low. This is probably because the role of marine animals in the emergence and spread of new viral infections is practically unstudied; only a few cases of human infection in dolphinariums, oceanariums, and hunting have been described [24–27]. However, we should bear in mind that marine mammals are natural vectors (reservoirs) of a colossal number of different viral pathogens, including those dangerous to humans. For example, viruses belonging to the following genera have been identified in pinnipeds (earless seals): Poxvirus, Adenovirus, Herpesvirus, Morbillivirus, Influenzavirus, Calicivirus, Coronavirus, Retrovirus, and Rabies [28, 29]. Cetaceans (dolphins) are reservoirs of viruses such as Poxvirus, Papillomavirus, Herpesvirus, Morbillivirus, Influenzavirus, Calicivirus, Hepandavirus, and Rhabdovirus; and cetaceans (whales), Poxvirus, Papillomavirus, Adenovirus, Herpesvirus, Morbillivirus, Influenzavirus, and Calicivirus [28, 29].
The most interesting from the point of view of the speed and range of the spread of viral infections are migrations associated with flights. In nature, it is insects, bats, and, of course, birds that make flights.
As for flying insects (dragonflies, butterflies, and others), their role as a natural reservoir of viruses dangerous to humans is practically unknown. Most often, mosquitoes are examined as virus carriers [30]. Long-distance migrations of flying insects are also very little studied. Note that even the available data indicate that insects can cover distances of several thousand kilometers (Fig. 1).
Fig. 1.
Seasonal migrations of the globe skimmer (Pantala flavescens) in the Afro-Asian region. The dots indicate gathering sites of the dragonflies; the arrows indicate spring and autumn migrations. Source: [51].
Chiropterans (bats) serve as a natural reservoir for over 200 viruses, many of which cause serious, often life-threatening, diseases in humans, livestock, and wild animals. The Ebola virus is the best known among them, but bats can also carry the rabies virus, paramyxoviruses, coronaviruses, and others, belonging to 30 different families of viruses, which indicates their great diversity. Less than 7% of bats are known or suspected migrants [31]. In recent years, special attention has been paid to the role of bats in the spread and maintenance of foci of lyssaviruses (including the classical rabies virus), coronaviruses, and para- and orthomyxoviruses [32–35].
In Russia, there are about 40 species of bats belonging to two families. The greatest species diversity is in the southern border areas (the North Caucasus, South Siberia, Primorski krai) [36]. Unfortunately, there are very few studies on the migration of these animals in Russia. Throughout the 20th century, study of the migration routes of bats was carried out mainly in the European part of the country.
Data on the migration of chiropterans in Siberia are scarce; long-distance migrations were noted for three species of bats—from Western and Eastern Siberia to Europe, Kazakhstan, and back [37–39]. There are practically no data on the migration of bats in the Far East [40]. Considering the existence of common species of bats in this region and Northern China, one can assume the existence of migration routes connecting the two regions.
Birds are very important natural reservoirs and vectors of viruses dangerous to humans (the influenza virus, the West Nile virus, the Saint Louis virus, and many others) [4, 41]. In addition, they can act as hosts and vectors of various ticks, which, in turn, can be carriers of dangerous viruses, such as the Crimean‒Congo hemorrhagic fever virus, the Dhori virus, and others, an incomplete list of which is given in Table 2 [43, 44].
Table 2.
Natural reservoirs and vectors of various viruses dangerous to humans
| Natural reservoirs | Viruses dangerous to humans |
|---|---|
| Birds | Influenza A virus, West Nile virus, St. Louis encephalitis virus, tick-borne encephalitis virus, Powassan virus, Japanese encephalitis virus, Rocio encephalitis virus, Murray Valley encephalitis virus, Uukuniemi, Rift Valley virus, Sindbis virus, Western equine encephalitis virus, Eastern equine encephalitis virus, Ross River virus (Togaviridae), Batai virus, California group viruses |
| Ticks, Hyalomma | Crimean‒Congo hemorrhagic fever virus and Dhori virus |
| Ticks, Argasidae | Paramushir virus, Rukutama virus, Sakhalin virus (Bunyaviridae, Nairovirus), Baku virus, Chenuda virus, Kemerovo virus, Okhotsk virus, Tribeč virus (Reoviridae, Orbivirus). |
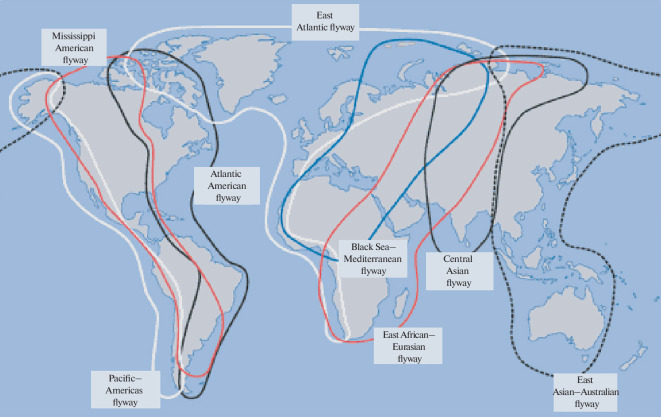
Importantly, a great number of birds are long-distance migrants, making flights over long distances from north to south and back twice a year. In total, there are 8 main migration routes that cross all continents, and the territory of Russia is crossed by 6 of these routes, connecting our country with Europe, Africa, Australia, Asia, and North America (Fig. 2). Our analysis of the return of the rings of birds marked in Novosibirsk and Omsk oblasts shows that the southern part of Western Siberia is visited by birds from all of the above continents during migration, which creates prerequisites for the introduction of numerous viruses from Australia, Africa, Europe, and Asia into this territory [45] (Fig. 3).
Fig. 2.
Main world routes of migratory birds. Source: International Wader Study Group.
Fig. 3.
Scattering of near-water birds to nesting and wintering places from the southern part of Western Siberia. The scheme is based on data from [45].
An analysis of the avifauna carried out by ISEA RAS SB specialists (according to the data of the ring returns database) showed that out of more than 700 bird species living in Siberia, about 600 bird species are long-distance migrants (215 species migrate to Europe; 172 species, to Asia; 201, to Africa; 15, to America; and 12, to Australia). This impressive mobility makes birds ideal carriers of viral pathogens over long distances and within short periods of time.
In terms of health care and veterinary medicine, the most interesting and important virus from the standpoint under consideration is the influenza virus. Birds, serving as a reservoir for almost all variants of this virus, can act as a source of infection for wild and domestic animals and humans. The diversity of variants of the influenza virus is very large. Since the generally accepted formula of the influenza A virus, the main reservoir and carrier of which are birds, is HxNx, where H is hemagglutinin and N is neuraminidase, then, given that 18 variants of hemagglutinin and 11 variants of neuraminidase have now been identified, 198 subtypes of the avian flu virus are theoretically possible [46] (more than 80 have already been described in the scientific literature). What properties the variants that have not yet been detected may have, we cannot even guess yet.
As an example, consider the emergence and spread of the highly pathogenic H5N1 subtype influenza virus, which was first identified and described in Hong Kong in 1997, where it caused several human deaths and mass mortality of poultry. By 2005, this variant of the influenza virus had penetrated the population of wild birds and during the spring migration of the same year was introduced to the territory of the southern part of Western Siberia [47]. During the autumn migration, it was spread by birds across Asia, Europe, and Africa. The spread of the H5N1 virus across three continents resulted in the death of about 1000 people and caused enormous damage to the poultry industry [48, 49]. This case demonstrates the important role of birds in the transmission of a new highly pathogenic virus over great distances and spread over vast territories. Continuing to mutate (H5N1‒H5N8, H5N5, H5N6), the virus is still actively circulating in wild bird populations, causing extensive poultry epizootics and sporadic deaths.
Currently, information is being accumulated on the spread of variants of the avian influenza virus, highly pathogenic for poultry and potentially dangerous to humans: H7N9, H6N1, H10N8, H5N8, H5N6, H5N5, H7N7, and H7N1. Each of them can lead to an epidemic or pandemic in humans, with the potential lethality of many of these influenza virus variants reaching 60%. In addition, these viruses are already causing enormous economic damage to the poultry industry. According to the World Organization of Animal Health (OIE), more than 246 million poultry died from the avian influenza virus alone from 2005 to 2020. The OIE 2022 report for the period from January 13, 2022, to February 16, 2022, noted the death of more than 5 mln poultry from the influenza virus [50]. The growth of the disaster to panzootic proportions could lead to starvation in many regions of the world, especially in densely populated countries in Asia, where poultry and eggs form the basis of the protein diet.
In the context of this danger and the possibility of the emergence of other dangerous variants of the influenza virus, of interest is a fact that we have established over many years of observations of the influenza virus in wild birds in the Asian part of Russia. According to our estimates, up to 10% of wild birds are carriers of one variant of the influenza virus or another.
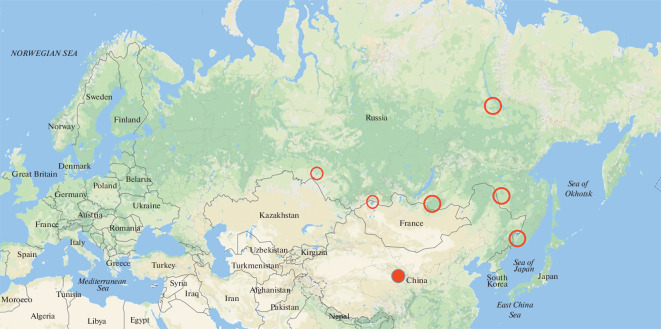
The second important result of our twenty years of work on monitoring the influenza virus in wild birds is the identification of key points for observation and collection of material in the Asian part of Russia: these are the largest lakes along the border with China and several places in the north of Siberia (Fig. 4).
Fig. 4.
Key points for observation and collection of material within the framework of monitoring the influenza virus in wild birds in the Asian part of Russia (marked with circles). The key point in China is Qinghai Lake.
Now a few words about problems in the study of bird migrations. The classical method of such study is the ringing method. The last mass ringing using this method was carried out in the south of Siberia in the 1970s–1980s. At present, the most accurate and objective method for studying bird migrations is based on the use of transmitters.
Considering the problems associated with climate change and other environmental factors, it is necessary to obtain fresh data on the migration routes of birds and to identify the species that are most important for the transmission of viruses dangerous to humans and farm animals. Unfortunately, Russia currently lacks the necessary domestic equipment, and we have to use devices manufactured abroad. However, first, good transmitters manufactured in the United States and Europe are very expensive (up to $5000) and, second, information reading systems are on the territories of the countries that produce these devices; as a result, we receive only the information that they make available to us.
All the above demonstrates the urgent need to solve the following problems facing our area of science.
(1) Study of changes in animal migration routes due to the changing climatic, environmental, and anthropogenic factors.
(2) Development of domestic equipment for fixing the migratory routes of birds and other animals.
(3) Widespread use of modern methods of fixing the route (transmitters, isotopic analysis). Here we observe a real lag behind China, the United States, South Korea, and the countries of Western and Eastern Europe.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Footnotes
Aleksandr Mikhailovich Shestopalov, Dr. Sci. (Biol.), is Director of the Research Institute of Virology at the Federal Research Center for Fundamental and Translational Medicine (FRC FTM). Aleksandr Yur’evich Alekseev, Cand. Sci. (Biol.), is a Laboratory Head at the FRC FTM Research Institute of Virology. RAS Corresponding Member Viktor Vyacheslavovich Glupov is Director of the Institute of Systematics and Ecology of Animals, RAS Siberian Branch (ISEA RAS SB). RAS Academician Mikhail Ivanovich Voevoda is Director of FRC FTM.
Translated by B. Alekseev
Contributor Information
A. M. Shestopalov, Email: shestopalov2@mail.ru
A. Yu. Alekseev, Email: ayalekseev@centercem.ru
V. V. Glupov, Email: skif@eco.nsc.ru
M. I. Voevoda, Email: director@frcftm.ru
REFERENCES
- 1.T. Allen, K. A. Murray, C. Zambrana-Torrelio, et al., “Global hotspots and correlates of emerging zoonotic diseases,” Nature Communications. 8 (1), Article no. 1124 (2017). [DOI] [PMC free article] [PubMed]
- 2.Morens D. M., Folkers G. K., Fauci A. S. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geoghegan J. L., Holmes E. C. Evolutionary virology at 40. Genetics. 2018;210:1151–1162. doi: 10.1534/genetics.118.301556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolhouse M., Gaunt E. Ecological origins of novel human pathogens. Crit. Rev. Microbiol. 2007;33:231–242. doi: 10.1080/10408410701647560. [DOI] [PubMed] [Google Scholar]
- 5.Carlson C. J., Zipfel C. M., Garnier R., Bansal S. Global estimates of mammalian viral diversity accounting for host sharing. Nat. Ecol. Evol. 2019;3:1070–1075. doi: 10.1038/s41559-019-0910-6. [DOI] [PubMed] [Google Scholar]
- 6.Mercer A. Protection against severe infectious disease in the past. Pathog. Glob. Health. 2021;115:151–167. doi: 10.1080/20477724.2021.1878443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buowari D. Y., Ogundipe H. D. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection: An epidemiological review. Ann. Ib. Postgrad. Med. 2021;19:S68–S76. [PMC free article] [PubMed] [Google Scholar]
- 8.G. G. Onishchenko, S. P. Berezhnov, A. M. Shestopalov, et al., “Molecular-biologic analysis of avian virus isolates, which caused epizootics on the south of west Siberia and in Crimea,” Zh. Mikrobiol., Epidemiol. Immunobiol., No. 5, 28−32 (2007). [PubMed]
- 9.Wolfe N. D., Dunavan C. P., Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams P. C., Bartlett A. W., Howard-Jones A. Impact of climate change and biodiversity collapse on the global emergence and spread of infectious diseases. J. Paediatr. Child Health. 2021;57:1811–1818. doi: 10.1111/jpc.15681. [DOI] [PubMed] [Google Scholar]
- 11.Dash S. P., Dipankar P., Burange P. S. Climate change: How it impacts the emergence, transmission, resistance and consequences of viral infections in animals and plants. Crit. Rev. Microbiol. 2021;47:307–322. doi: 10.1080/1040841X.2021.1879006. [DOI] [PubMed] [Google Scholar]
- 12.“Zoonoses,” in Big Medical Encyclopedia. https://бмэ.орг/index.php/ЗООНОЗЫ. Cited March 23, 2022.
- 13.WHO Zoonoses. https://www.who.int/news-room/fact-sheets/detail/zoonoses
- 14.Taylor L. H., Latham S. M., Woolhouse M. E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.J. Recht, V. J. Schuenemann, and M. R. Sánchez-Villagra, “Host diversity and origin of zoonoses: The ancient and the new,” Animals 10 (9), Article no. 1672 (2020). [DOI] [PMC free article] [PubMed]
- 16.Mohsin H., Asif A., Fatima M., Rehman Y. Potential role of viral metagenomics as a surveillance tool for the early detection of emerging novel pathogens. Arch. Microbiol. 2021;203:865–872. doi: 10.1007/s00203-020-02105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Severe syndrome (SARS)-multi-country outbreak—Update 60. https://www.who.int/emergencies/disease-outbreak-news/item/2003_05_20-en. Cited March 23, 2022.
- 18.Middle East respiratory syndrome coronavirus (MERS-CoV) updates. https://www.euro.who.int/ru/ health-topics/communicable-diseases/influenza/middle-east-respiratory-syndrome-coronavirus-mers-cov/ middle-east-respiratory-syndrome-coronavirus-mers-cov-updates. Cited March 23, 2022.
- 19.EuroMOMO Bulletin, Week 11, 2022. https://www.euromomo.eu/. Cited March 23, 2022.
- 20.T. S. Salajegheh, D. P. Magalhães, P. Rahimi, et al., “Transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to animals: an updated review,” J. Transl. Med. 18 (1), Article no. 358 (2020). [DOI] [PMC free article] [PubMed]
- 21.Sailleau C., Dumarest M., Vanhomwegen J. First detection and genome sequencing of SARS-CoV-2 in an infected cat in France. Transbound. Emerg. Dis. 2020;67:2324–2328. doi: 10.1111/tbed.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tewari D., Boger L., Brady S. Transmission of SARS-CoV-2 from humans to a 16-year-old domestic cat with comorbidities in Pennsylvania, USA. Vet. Med. Sci. 2022;8:899–906. doi: 10.1002/vms3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D. H. Braam, F. L. Jephcott, and J. L. N. Wood, “Identifying the research gap of zoonotic disease in displacement: A systematic review,” Glob. Health Res. Policy 6 (1), Article no. 25 (2021). [DOI] [PMC free article] [PubMed]
- 24.Hunt T. D., Ziccardi M. H., Gulland F. M. Health risks for marine mammal workers. Dis. Aquat. Org. 2008;81:81–92. doi: 10.3354/dao01942. [DOI] [PubMed] [Google Scholar]
- 25.Webster R. G., Geraci J., Petursson G., Skirnisson K. Conjunctivitis in human beings caused by influenza A virus of seals. N. Engl. J. Med. 1981;304:911. doi: 10.1056/NEJM198104093041515. [DOI] [PubMed] [Google Scholar]
- 26.Kiers A., Klarenbeek A., Mendelts B. Transmission of mycobacterium pinnipedii to humans in a zoo with marine mammals. Int. J. Tuberc. Lung Dis. 2008;12:1469–1473. [PubMed] [Google Scholar]
- 27.Smith A. W., Skilling D. E., Cherry N. Calicivirus emergence from ocean reservoirs: Zoonotic and interspecies movements. Emerg. Infect. Dis. 1998;4:13–20. doi: 10.3201/eid0401.980103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulland F. M., Hall A. J. Is marine mammal health deteriorating? Trends in the global reporting of marine mammal disease. EcoHealth. 2007;4:135–150. doi: 10.1007/s10393-007-0097-1. [DOI] [Google Scholar]
- 29.M. A. Gulyaeva, A. Yu. Alekseev, K. A. Sharshov, et al., “Orthomyxo- and paramyxoviruses in marine mammals,” South Russ.: Ecol., Dev. 13 (1), 154−165 (2018).
- 30.E. Atoni, L. Zhao, S. Karungu, et al., “The discovery and global distribution of novel mosquito-associated viruses in the last decade (2007−2017),” Rev. Med. Virol. 29, e2079 (2019). 10.1002/rmv.2079 [DOI] [PubMed]
- 31.Krauel J. J., McCracken G. F. Bat Evolution, Ecology, and Conservation. New York: Springer; 2013. [Google Scholar]
- 32.V. V. Makarov and D. A. Lozovoi, “Bats are a little-known reservoir of especially dangerous infections,” Veterinariya, No. 9, 3−9 (2017).
- 33.A. M. Porshakov, Yu. V. Kononova, V. B. Loktev, and M. I. Boiro, “Bats as a possible reservoir of viruses dangerous to humans on the territory of the Republic of Guinea. Part 1,” Probl. Osobo Opasn. Infekt., No. 3, 32−39 (2018).
- 34.A. D. Luis, D. T. Hayman, T. J. O’Shea, et al., “A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special?,” Proc. Biol. Sci. 280 (1756), 20122753 (2013). [DOI] [PMC free article] [PubMed]
- 35.Bat conservation international. https://www.batcon.org/about-bats/bats-101/. Cited March 23, 2022.
- 36.List of mammals of Russia. https://ru.wikipedia.org/ wiki/Список_млекопитающих_России. Cited March 23, 2022.
- 37.V. G. Lyalin, “On the study of Chiroptera migrations in the South-East of western Siberia,” in The Mammals of the USSR: III Congress of the All-Union Therological Society (Moscow, 1982), Vol. 2 [in Russian].
- 38.K. A. Bernikov, Fauna and ecology of bats (Chiroptera) in the plain taiga of Western Siberia (The case of the Khanty-Mansi Autonomous Okrug), Extended Abstract of a Candidate’s Dissertation in Biology (Novosibirsk, 2009).
- 39.Zhigalin A. V., Gadzhiev A. A., Daudova M. G. Ecology of chiroptera bats in Altai-Sayan region of southern Siberia. South Russ.: Ecol., Dev. 2019;14:9–25. [Google Scholar]
- 40.Gorobeiko U. V. About study of bats history for the last century In Vladivostok. Reg. Probl. 2018;21:33–38. [Google Scholar]
- 41.Reed K. D., Meece J. K., Henkel J. S., Shukla S. K. Birds, migration, and emerging zoonoses: West nile virus, lyme disease, influenza A, and enteropathogens. Clin. Med. Res. 2003;1:5–12. doi: 10.3121/cmr.1.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morse S. S., Mazet J. A., Woolhouse M. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sparagano O., George D., Giangaspero A., Špitalská E. Arthropods and associated arthropod-borne diseases transmitted by migrating birds: The case of ticks and tick-borne pathogens. Vet. Parasitol. 2015;213:61–66. doi: 10.1016/j.vetpar.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 44.A. M. Buczek, W. Buczek, A. Buczek, and K. Bartosik, “The potential role of migratory birds in the rapid spread of ticks and tick-borne pathogens in the changing climatic and environmental conditions in Europe,” Int. J. Environ. Res. Public Health 17 (6), Article no. 2117 (2020). [DOI] [PMC free article] [PubMed]
- 45.A. K. Tyrlov, V. M. Chernyshev, and A. P. Yanovskii, “New information on migration routes and wintering areas of some bird species from the southern part of Western Siberia,” Mat. Rasprostr. Ptits Ural., Priural. Zapad. Sib., No. 3, 189−192 (1998).
- 46.J. S. Long, B. Mistry, S. M. Haslam, and W. S. Barclay, “Host and viral determinants of influenza A virus species specificity,” Nat. Rev. Microbiol. 17 (2), 67−81 (2019). Erratum in: Nat. Rev. Microbiol. (2018). [DOI] [PubMed]
- 47.Shestopalov A. M., Durimanov A. G., Evseenko V. A. H5N1 influenza virus, domestic birds, western Siberia, Russia. Emerg. Infect. Dis. 2006;12:1167–1169. doi: 10.3201/eid1207.051338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webster R. G., Govorkova E. A. H5N1 influenza—Continuing evolution and spread. N. Engl. J. Med. 2006;355:2174–2177. doi: 10.1056/NEJMp068205. [DOI] [PubMed] [Google Scholar]
- 49.Global spread of H5N1. https://en.wikipedia.org/wiki/Global_spread_of_H5N1. Cited March 23, 2022.
- 50.High pathogenicity avian influenza (HPAI): Situation report Feb. 24 (2022). https://www.oie.int/app/uploads/2022/03/hpai-situation-report-20220224.pdf. Cited March 23, 2022.
- 51.S. N. Borisov, I. K. Iakovlev, A. S. Borisov, et al., “Seasonal migrations of Pantala flavescens (Odonata: Libellulidae) in Middle Asia and understanding of the migration model in the Afro–Asian region using stable isotopes of hydrogen,” Insects, No. 11, Article no. 890 (2020). [DOI] [PMC free article] [PubMed]