Abstract
Chronic obstructive pulmonary disease (COPD) and lung cancer are common global causes of morbidity and mortality. Because both diseases share several predisposing risks, the 2 diseases may occur concurrently in susceptible individuals. The diagnosis of COPD has important implications for the diagnostic approach and treatment options if lesions concerning for lung cancer are identified during screening. Importantly, the presence of COPD has significant implications on prognosis and management of patients with lung cancer. In this monograph, we review the mechanistic linkage between lung cancer and COPD, the impact of lung cancer screening on patients at risk, and the implications of the presence of COPD on the approach to the diagnosis and treatment of lung cancer. This manuscript succinctly reviews the epidemiology and common pathogenetic factors for the concurrence of COPD and lung cancer. Importantly for the clinician, it summarizes the indications, benefits, and complications of lung cancer screening in patients with COPD, and the assessment of risk factors for patients with COPD undergoing consideration of various treatment options for lung cancer.
Keywords: copd, chronic obstructive pulmonary disease, lung cancer
Introduction
Chronic obstructive pulmonary disease (COPD) and lung cancer are common global causes of morbidity and mortality. COPD has a worldwide prevalence of 7% to 19% and is the 3rd leading cause of death. Over 65 million people suffer from COPD worldwide; COPD caused 3.23 million deaths in 2019.1 Lung cancer is one of the most frequently diagnosed cancers worldwide (11.6% of all cancers)2 and is the most common cancer diagnosed in men and third most common cancer diagnosed in women (Table 1). Lung cancer is the leading cause of all cancer deaths at 1.74 million (18.4%), a number expected to reach 2.45 million patients worldwide by 2030, a 39% increase since 2018.2 Tobacco smoking causes lung cancer in 80% of cases, but exposures to biomass fuels, radon, and asbestos also contribute.2
Because COPD and lung cancer share similar risks, both diseases may concur in susceptible patients. Lung cancer is an important comorbidity of COPD that contributes to increased mortality.3,4 Conversely, COPD is associated with reduced overall survival in patients with lung cancer and COPD compared to those without COPD, especially in those with an emphysematous predominant component.5 Smokers with COPD have a 6-fold risk of lung cancer compared to smokers without airflow limitation6 and lung cancer incidence increases as forced expiratory volume in 1 second (FEV1) declines, regardless of cigarette smoke exposure.7,8 Emphysema also increases lung cancer risk.9 In the Danish Lung Cancer Screening Trial, patients with airflow limitation, emphysema, age > 70 years and ≥ 35 pack-years smoking had a 2-fold greater lung cancer risk.10
The diagnosis and severity of COPD has important diagnostic and therapeutic implications for the population undergoing low-dose computed tomography (LDCT) for lung cancer screening. This monograph reviews the mechanistic linkage between lung cancer and COPD, the impact of lung cancer screening, and the implications of COPD on the diagnosis and treatment of lung cancer.
Pathobiological Factors Linking Lung Cancer and COPD
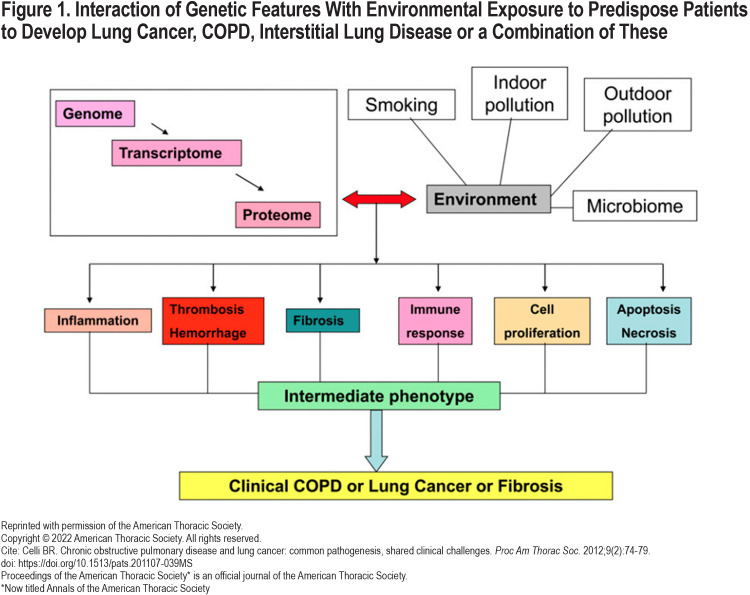
Lung cancer is caused by mutations in oncogenes leading to an uncontrolled proliferation of cells and tumor formation.11 Pathophysiological links between COPD and lung cancer have been elusive due to heterogeneity in responses to chronic inflammation and lung reparative processes.12 Possible common pathobiological processes include: chronic inflammation, genetic predisposition, epigenetic changes, telomere shortening, protease and anti-protease imbalance, mitochondrial dysfunction, premature aging, and aberrant reparative processes. (Figure 1)13
Inflammation and cancer are closely linked; most cancerous tissue shows inflammation.14-16 Tobacco smoking, a shared risk between lung cancer and COPD, is a major factor contributing to lung carcinogenesis since smoking-related inflammation is superimposed upon the presence of tobacco smoke carcinogens.17 It is also feasible that chronic lung inflammation in COPD predisposes to lung cancer.18 A reduction in mucociliary clearance may enable carcinogens to reside longer in the lung.8 The COPD lung microbiome differs from healthy individuals and may induce inflammatory changes that promote lung cancer development.19,20 Cigarette smoke may also induce release of vascular endothelial growth factor from epithelial cells causing angiogenesis which facilitates the progression, invasion, and metastasis of lung cancer.21
Immune cell composition and function is important in non-small cell lung cancer (NSCLC) as well as COPD. COPD severity has been related to CD4+ T cell content and differentiation status (T-helper type-1cells, TH17, regulator t cells) with increases in CD4+ TH1 as the disease progresses.22 Interleukin17 (IL-17) drives protumor inflammatory responses and facilitates tumor growth in animal models.22,23 COPD has increased sensitivity of CD8+ tumor-infiltrating T lymphocytes to tumor-mediated immune escape mechanisms suggesting higher sensitivity to PD-1 blockade.22 In NSCLC, immune cell composition is heterogenous and varies between adenocarcinomas and squamous cell carcinomas.12 In stage I non-squamous NSCLC, a more favorable gene signature for survival (FAIM3) is predominantly expressed in tumor-infiltrating leukocytes.24 Patients with COPD and lung cancer are reported to have a decline in IgG-secreting plasma cell levels but not in other cell types compared to patients with lung cancer but without COPD.25
Aging of the lung may represent a common thread between lung cancer and COPD.26 The failure of organs to repair DNA damage, caused by oxidative stress and telomere shortening, drives aging and occurs in COPD.27 Cigarette smoking decreases telomere length; lung cancer and COPD are both associated with shortened telomere length.28-30
Oxidative stress plays a role in lung cancer and COPD through DNA damage and leads to carcinogenic mutations.11,31,32 In COPD, nitration of histone deacetylase leads to its inactivation and enhances further inflammation. Oxidants inactivate other proteins making them auto-antigenic and thereby, immunoinflammatory.33
Extensive exposure to nicotine is also common to patients with COPD and those who develop lung cancer. Nicotine promotes tumor growth by increasing proliferation, angiogenesis, migration, and epithelial to mesenchymal transition, and stimulates tumor growth.34 It is also the principal compound that drives smoking addiction.
Genetic predisposition to lung cancer and COPD35-37 has been localized to chromosome 6. Genome Wide Association Study studies have implicated loci at cholinergic receptor nicotinic alpha subunit (CHRNA) 3 and CHRNA5 single nucleotide polymorphisms and regions at 4q31, 4q24 and 5q.38 Genetic polymorphism of IL-10 in peripheral blood mononuclear cells was found to be associated with increased rates of COPD and lung cancer.14,39
Epigenetic changes (DNA methylation, micro-RNA expression, covalent histone modifications and nucleosome remodeling) may play roles in COPD and lung cancer.40,41 COPD patients with lung cancer have hypermethylation of tumor suppressor and other gene promoters than COPD patients without lung cancer.11
Clinical Features of COPD That Increase Lung Cancer Risk
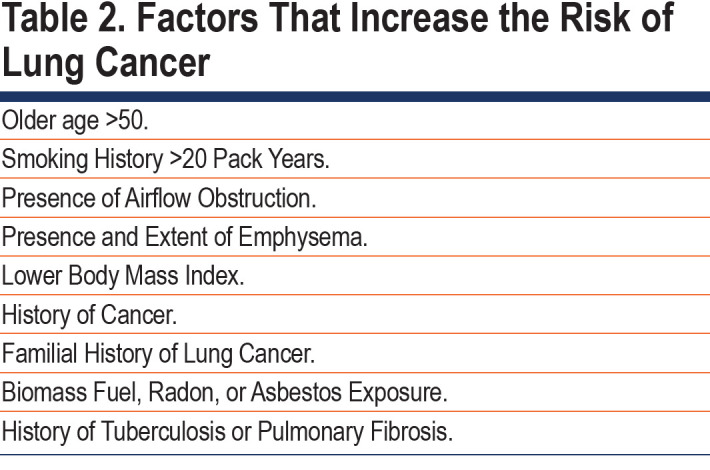
The presence and severity of airflow limitation and/or emphysema (diagnosed using computerized chest tomography [CT] or diffusing capacity for carbon monoxide [DLCO]) are important risks for lung cancer development.7,9,42-44 Studies in smokers and nonsmokers report a relationship between severity of airflow limitation and lung cancer risk.7,42,45 (Table 2) In a post hoc subset analysis of the National Lung Screening Trial (NLST), participants with COPD had a 2-fold increase in lung cancer incidence.46 Others, however, report the opposite relationship, that severity of airflow limitation is inversely related to lung cancer risk.47,48 In 2517 patients with COPD followed over 60 months, lung cancer occurred in those with less severe airflow limitation (Global initiative for chronic Obstructive Lung Disease [GOLD] stages 1 and 2), lower body mass index and DLCO < 80%.49 Others report emphysema may be a greater risk factor for lung cancer compared to airflow limitation.9,43 The Pamplona International Early Lung Cancer Detection Program and the Pittsburgh Lung Screening Study databases showed50 that emphysema was independently associated with increased lung cancer risk using a risk stratification score (range 0-10 points). In both cohorts, the risk of lung cancer was 3.5-fold higher in the high (7-10) versus the low (0-6) risk group. Severity of emphysema was related to greater likelihood of developing and dying from lung cancer even after adjustment for age and smoking history.51 Others report no impact of emphysema severity on lung cancer risk.45
The histology and localization of lung cancer is linked to the regional presence and degree of emphysema. Squamous cell carcinoma is more common when COPD and emphysema are present.49,52 A link exists between lung cancer location and degree of emphysema.53 A lower emphysema burden is found with central tumors while a greater emphysema burden is associated with peripheral lesions.51
The aggressiveness of tumors is associated with the extent of emphysema and presence of COPD. Patients with COPD who develop adenocarcinoma have less invasive characteristics, while lung cancers arising in emphysematous tissue are more aggressive.54,55 Smokers with impaired lung function have shorter doubling times and less indolent lung cancer.56-58 Epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) rearrangements are less prevalent in patients with COPD-associated lung cancers and EGFR mutation is inversely related to the severity of airflow limitation.59,60
Between 10% to 39% of COPD patients are never smokers and some evidence exists for an association between COPD and lung cancer in never smokers. In a population-based cohort of 338,548 Korean citizens, lung cancer incidence in never smokers with COPD was increased compared to never smokers without COPD.61 The highest risk of lung cancer was in patients who had COPD and had smoked with a 6-fold risk of developing lung cancer compared with never smokers without COPD.61
Inhaled corticosteroids (ICSs) are recommended in select patients with COPD and their impact on lung cancer development has conflicting reports. Data from a British Columbia database62 suggested a 30% reduction in lung cancer risk with ICSs, however, the study lacked key inclusion variables including severity of airflow limitation, presence of emphysema, family history of lung cancer, and degree of tobacco exposure.63 Others report a reduction in lung cancer risk in COPD patients prescribed ICSs.64-68 A more pronounced protective effect of ICSs was reported in former compared to current smokers,65 those with a concurrent diagnosis of asthma,68 or those prescribed a higher ICS dose.66 A systematic review reported a protective effect of a higher dose of ICS in observational studies but no benefit in randomized trials.69 An analysis designed to avoid immortal time bias found no effect of ICSs on reducing lung cancer risk.70 Similarly, a large observational study reported no effect of ICS use on lung cancer incidence.11 One study reported increased lung cancer risk in patients prescribed ICSs.10
Large prospective controlled trials conducted in patients with moderate to severe COPD focused on lung function decline, exacerbation reduction, or mortality, reported no difference in cancer deaths in patients randomized to ICS versus non-ICS use.12,14-18
Conflicting results between observational and randomized controlled trials may be due to different patient populations, characterization of lung cancer risk, follow-up time, and whether an annual LDCT was used to screen for lung cancer. Based on available data, there is no clear evidence that ICS use increases or decreases lung cancer risk.
Other pulmonary diseases may increase lung cancer risk such as a history of tuberculosis, chronic bronchitis, or emphysema.71 Patients with combined pulmonary emphysema and fibrosis have a higher incidence of lung cancer.72 Pulmonary fibrosis also increases lung cancer risk.73
Lung Cancer Screening
Prognosis in lung cancer is tightly linked to tumor stage at time of diagnosis, typically too late to allow for surgical treatment.74,75 The Mayo Lung Project randomized 4600 male smokers to either chest x-rays (CXRs) and sputum cytology tests every 4 months for 6 years or annually.76 Twice as many lung cancers were diagnosed, more surgical procedures were performed, and lung cancer 5-year survival was better in intensively screened patients, however, overall mortality was similar. Other CXR screening studies confirmed lack of mortality benefit.77,78
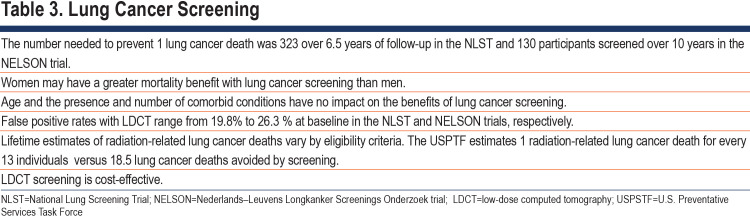
Several studies have demonstrated that lung cancer screening with LDCT reduces mortality by detecting lung cancer at earlier stages.10,79-91 (Table 3) The beneficial effect on survival is balanced by false positives that increases radiation exposure, morbidity, and mortality from unnecessary diagnostic procedures, patient distress, and medical costs. Many studies, although consistent in showing greater detection of lung cancer, were not powered to show a mortality benefit.84,85,88,91
The NLST and the Dutch–Belgian lung-cancer screening trial (Nederlands–Leuvens Longkanker Screenings Onderzoek [NELSON]) were population- based, multicentered trials with adequate power to examine the impact of LDCT on lung cancer-specific and all-cause mortality.79,80
The NLST enrolled 53,454 patients between 55–74 years of age with a history of cigarette smoking > 30 pack years, or, if former smokers, within 15 years of quitting.79 Participants in the intervention arm underwent 3 annual LDCT screenings and the controls had single view posteroanterior CXRs. Study adherence was greater than 90%; the rate of positive screening tests was 24.2 % with LDCT and 6.9% with CXRs over the 3 rounds. Lung cancer incidence was 645 cases per 100,000 person- years in the LDCT group and 572 cases per 100,000 person-years in the CXR group. With LDCT there were 247 lung cancer deaths per 100,000 person-years compared to 309 deaths per person-years with CXR: a relative mortality reduction from lung cancer of 20% with LDCT. Mortality from any cause was reduced by 6.7% with LDCT compared to the CXR group.
NELSON was powered to show a reduction in lung cancer mortality of ≥ 25% with volume-based LDCT screenings in high-risk male participants at 10 years follow-up.80 A total of 13,195 men and 2594 women between 50 and 74 years old were randomized to LDCT at baseline and years 1, 3, and 5.5 versus no screening. At the 10 year-follow-up among men, the incidence of lung cancer was 5.58 cases per 100 person years with LDCT and 4.91 cases per 1000 person years in controls; lung cancer mortality was 2.50 versus 3.30 deaths per 1000 person years, respectively. The cumulative rate ratio for lung cancer death at 10 years was 0.76 (95% confidence interval [CI], 0.61 to 0.94; p=0.01) with LDCT compared to controls. In women at 10 years follow-up, the rate ratio was 0.67 (95% CI, 0.38 to 1.14).
Patient populations in the above 2 trials were predominately White (91% in the NLST), <5% were African American and 2% were Hispanic. The trials differed in positive screen definitions, number of screening rounds, screening intervals, mean age, and baseline smoking status. Participants numbers ranged79,91 from 2472 to 53,542 and follow-up periods from 5.2 to 10 years.80,92 Male predominance existed in both studies (range 56%– 84%).80,84 A unique aspect of NELSON was volumetric measurements of nodules and calculations of volume doubling.80
The number needed to screen to prevent 1 cancer death was 323 over 6.5 years of follow-up in the NLST79 and 130 participants screened over 10 years of follow-up in NELSON.80
Influence of Age, Sex, Smoking Status, and Comorbid Conditions on Computed Tomography Screening Benefits
Age, sex, smoking status, comorbidities, and other pulmonary conditions may impact prevention of lung cancer death.93 Sixty-four percent of NLST participants had no pulmonary conditions at baseline, 24.7% had 1 pulmonary condition, and 10.8% had 2 or more conditions.79 There was no difference in the efficacy of screening according to the number of coexisting pulmonary conditions.
A trend of greater benefit was found in the NLST women participants compared to men and the same benefit was found in NELSON. The German Lung Cancer Screening Intervention88 also found women had a significant reduction in lung cancer mortality compared to men. Sub-analyses showed that age or smoking status did not impact LDCT to reduce lung cancer mortality.
False Positive Rates
False positive rates varied across studies due to definitions of positive results, thresholds for nodule size, and use of volume doubling time.83
The NLST reported false positive rates of 26.3% at baseline, and 27.2% and 15.9% at rounds 2 and 3, respectively.79 NELSON reported false positive rates of 19.8% at baseline and 7.1% at year 1, 9.0% at year 3 (males), and 3.9 % at year 5.5 (males).80 Needle biopsies for false positive rates in several studies56,79,81,88,94-103 ranged from 0.09%–0.56% and surgical resections from 0.1%–0.5%. Invasive procedures were performed in 1.7% of screened participants in the NLST; number needed79 to harm, n=59. Use of Lung Reporting and Data System (Lung-RADS) criteria may avoid 23.4% of invasive procedures for false positive results.104
Radiation Risk with Low-Dose Computed Tomography
Precise risks of developing cancer from cumulative radiation from lung cancer screenings are unknown. Estimates of radiation exposure after 25 years of annual screenings yields 20.8–32.5 mSv.105,106 Estimates of lifetime cancer risk from radiation exposure following 10 annual LDCTs was 0.26–0.81 major cancers for 1000 individuals screened.105 The 2021 U.S. Preventative Services Task Force (USPSTF) recommendation estimates a higher rate of radiation-related lung cancer deaths (29.0 to 42.5 versus 20.6 per 100,000) than the 2013 recommendation but is outweighed by increases in lung cancer deaths prevented and life-years gained for women.107
Cost-effectiveness
Some critics consider lung cancer screenings to be less cost-effective than smoking cessation.108 The U.K. LDCT trial compared screening to usual care in 4055 individuals81 and estimated the cost-effectiveness of screening to be £8466 per quality adjusted life-year gained. Annual screenings might be most cost-effective when eligibility is restricted to high-risk groups.109
Screening Intervals
The Multicentric Italian Lung Detection trial randomized 2376 screening participants to annual (n=1190) or biennial (n=1186) LDCTs for median screening periods of 6.2 years and 23,083 person-years follow-up.82 Biennial LDCTs showed similar overall mortality and lung cancer specific mortality at 10 years compared with annual LDCTs. Biennial screenings saved 44% of follow-up LDCTs in individuals with a negative baseline LDCT and 38% of LDCTs in patients without increased occurrence of Stage II–IV lung cancer.
Impact of Lung Cancer Screenings on Smoking Cessation
Several studies found no impact of LDCT screenings on smoking cessation, abstinence, smoking relapse, or smoking intensity.110-118
Psychosocial Harms of Lung Cancer Screenings
Several studies report81,119-123 no worsening in quality of life, anxiety, or other measures of distress in screened patients compared to controls. Although participants in NELSON reported short-term recipients of indeterminant results had increased lung cancer distress, quality of life improved following negative scans.122
U.S. Preventative Services Task Force Updated Lung Cancer Screening Recommendations
In 2021, the USPSTF updated its recommendation124 based on a systematic review of the accuracy, benefits, and harms associated with lung cancer screening. It assessed if screening benefits vary by subgroup (e.g., race or sex), or number or frequency of LDCT scans, and whether harms associated with screening and nodule evaluation differs when using the International Early Lung Cancer Action Program, Lung-RADs, or other approaches to reduce false positive rates. USPSTF also commissioned modeling studies from the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network (CISNET) to provide optimal ages to begin and end screening. USPSTF now recommends annual LDCT screening in adults aged 50-80 years with a 20-pack-year smoking history who currently smoke or quit smoking < 15 years. They recommended stopping screening once a person has not smoked for 15 years or develops a health problem that limits life expectancy or ability to undergo lung surgery. The 2021 recommendations advise LDCT screening at a younger age with less smoking burden, based on results from NELSON.80,124 Additionally, CISNET analyses supports screening at a younger age with lower smoking burden to address racial disparities.124-128
Implementation of Lung Cancer Screening in Clinical Practice
Most cited trials were conducted at large academic medical centers. The transition to community practices, especially those serving minority populations, may be different.79,129
Screening solely based on NLST criteria could miss a significant number of lung cancer cases. A retrospective analysis combining emphysema detected by high-resolution computed tomography (HRCT) with NLST criteria detected a higher number of lung cancers.130
Implementation of lung cancer screening has been proposed in high-risk populations such as COPD patients. Combining a screening program in ever smokers with ≥ 10 pack years and aged 55–74 years with their annual COPD review found a positive LDCT scan in 5% of patients.131 Although detected cancers were at an earlier stage, because of lower lung function and more comorbidities, the rates of surgical resections were lower, and stereotactic body radiotherapy (SBRT) higher.
Implementation of lung cancer screening is challenging in low- and middle-income countries (LMICs). More than 70% of global smoking-related deaths occur in LMICs where > 80% of 1.3 billion smokers reside.132 Upper middle-income countries have the highest incidence of cancer and mortality; lung cancer incidence increased by 465% in China over the past 30 years.133 Common barriers in LMICs include inadequate transportation and infrastructure134 and lack of awareness of screening guidelines and the need for shared decision making.135
Strategies to improve lung cancer screening in LMICs includes more restricted eligibility criteria (additional inclusion of family history of lung cancer or COPD), biennial screening, intensive smoking cessation, private-public implementation efforts, digital technologies for remote locations, and adequate funding.133
Detection of Comorbidities During Lung Cancer Screening
Malignancies, cardiovascular diseases, and COPD share common risks: smoking, obesity, physical inactivity, and alcohol abuse are responsible for >75% of deaths from non-communicable diseases.136 Patients with comorbid conditions may present with lung cancer at an earlier age.137-139 The “surveillance hypothesis” purports that patients with coexisting diseases have increased medical visits and more opportunities for cancer detection.140 The detection of comorbid conditions during an annual lung cancer screening, however, has received limited attention.
In 8637 heavy smokers screened in the Pomeranian Pilot Lung Cancer Screening Program, 52% had cardiovascular disease (33 %), diabetes (26%), and COPD (21%).141 A study that performed a “lung health check” during a lung cancer screening reported COPD in 57% of patients; 67% did not have a prior diagnosis.142
Studies that incorporate spirometry into screening have similarly reported high incidences of previously undiagnosed COPD. In 2525 screened individuals of which 99.4% performed spirometry, 37.4% had airflow limitation and 49.7% had no prior COPD diagnosis.143 The detection of undiagnosed COPD was more likely in males, individuals of younger age, lower smoking duration and fewer cigarettes per day, and asymptomatic individuals. Screening- detected lung cancer was higher in those with an airflow limitation with prior diagnosis of COPD (OR 2.80; p=0.002).
Special Considerations Using Lung Cancer Screening in COPD Patients
Lung cancer screening has benefits and risks that should be discussed during the shared decision-making process especially in patients with COPD and limited lung reserve. Patients with COPD are at increased risk for morbidity and mortality during evaluation of false positive lesions or with lung cancer treatments.
Most patients with COPD are, or have been, smokers and represent the age of patients enrolled into the NLST and NELSON and meet 2021 USPSTF recommendations for lung cancer screening. COPD patients should undergo annual lung cancer screening like any other individual based on the screening criteria recommended by the USPSTF. LDCT in COPD patients can identify structural abnormalities that characterize COPD such as emphysema, bronchial inflammation, or mucous plugging. These individuals merit lung function testing to confirm or exclude the diagnosis of COPD and therefore, begin treatment.
A secondary analysis of NLST data assessed impact of COPD on patient outcome.144 Among 24,453 individuals who underwent screening, 30.5% underwent a diagnostic study and 4.2% an invasive procedure of which 0.9% experienced a procedure-related complication. Patients with COPD were more likely to undergo an invasive procedure and have a serious complication (OR 1.78, p=0.01).
Because of reduced lung function and comorbid conditions, some suggest that screening COPD patients has limitations due to “competing causes of death.”145 In an analysis of NLST data, a mortality benefit of lung cancer screening was found in COPD patients with mild to moderate but not severe or very severe disease.145
The impact of newer diagnostic techniques to investigate indeterminate lung lesions identified by LDCT is unknown. Positive emission tomography may help characterize solid lesions for malignant potential and decrease the number of false positive lesions undergoing unnecessary invasive procedures.146 Navigational bronchoscopy coupled with cone-beam CT imaging and augmented fluoroscopy may help increase the proportion of diagnosed lesions with less morbidity and mortality.147
Assessment of Treatment Risks for Patients with COPD and Non-small Cell Lung Cancer
Note: The discussion below focuses only on the assessment of risk and treatment of NSCLC. The treatment of small cell lung cancer and other types of malignant lung diseases is outside the purview of this focused review.
Pulmonary Risk
Standard treatment for Stage I NSCLC is lobectomy with systematic mediastinal lymph node examination.148 (Table 4) Approximately 25% of patients are not candidates for curative lobectomy due to frailty (e.g., the presence of fatigue, low activity, weakness, weight loss, and slowness of gait) and pulmonary or non-pulmonary comorbidities. The presence of COPD is associated with an increased need for tracheostomy, pneumonia, and decreased disease-free and overall survival in patients undergoing lobectomy with lymph node dissection in Stage IA lung cancer.149
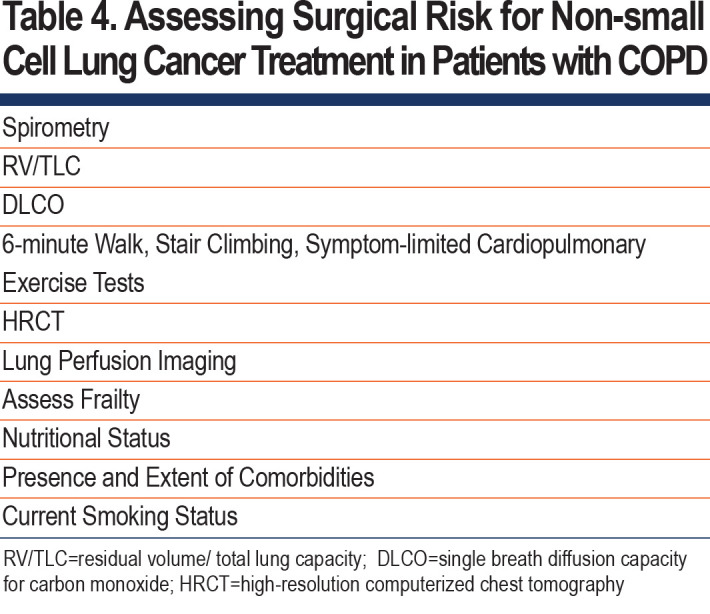
Preoperative evaluation should include spirometry to measure FEV1, and the measurement of DLCO if there is diffuse disease or dyspnea disproportionate to the level of FEV1 reduction150 (Table 4). If FEV1 or DLCO is less than 80%, then an estimation of postoperative pulmonary reserve should be done by either the anatomic method (e.g., number of segments or lobes to be removed) or lung perfusion scanning.151 An estimated FEV1 or DLCO ≤ 40% is associated with increased perioperative complications including death. Further assessment using cardiopulmonary exercise testing is recommended; VO2 max<15 ml/kg/min indicates an increased risk of perioperative complications. Alternative types of testing using stair climbing, shuttle walk, or 6-minute walk may be used if exercise testing is not available.150-154
HRCT imaging can estimate perioperative risk. Regression based forced vital capacity (FVC) and FEV1 derived from HRCT data correlated with physiologically measured FVC and FEV1, suggesting that HRCT could be used to estimate preoperative pulmonary function in patients unable to perform spirometry.155
Non-pulmonary Risk
The high-risk group for lung cancer surgery has been defined by male sex, older age, lower FEV1, lower DLCO, poor performance status, obesity, renal disease, diabetes, malnutrition, frailty, steroid use, and coronary heart disease.156-158 In patients ≥ 75 years of age who underwent lobectomy, performance status, coronary heart disease, history of stroke, restrictive lung disease, male sex, and interstitial pneumonia were associated with increased postoperative complications.159-163 Air trapping measured by residual volume//total lung capacity (TLC), prolongs operative hospitalization.164
Nutritional status influences postoperative reoccurrence and death, especially in those with more severe airflow limitation.165 Sarcopenia predicts postoperative complications and survival following lung cancer surgery.162
Current smoking may adversely affect surgical outcomes (e.g., prolonged air leak, pneumonia, tracheostomy, and atelectasis) with reduced relapse free survival in GOLD stage 2/3 patients.166
Lung Cancer Surgical Therapies and Reduced Lung Function
Anatomical lung resection can be performed in selected high-risk patients based on preoperative lung function without increased morbidity and mortality167 (Table 5). Assessment of the COPD patient’s fitness for surgery should be thoroughly discussed in multidisciplinary fashion. In some patients, the operative risk of death exceeds the risk of lung cancer death.167

The frequent occurrence of lung cancer in older COPD patients with other pulmonary and comorbid conditions has prompted exploration of therapies other than curative resection.168
Lobectomy via video-assisted thoracoscopic surgery in high-risk patients (e.g., age> 75 years, FEV1 < 50% predicted, DLCO < 50% predicted, history of coronary heart disease) has a low, but not negligible incidence of major complications.169 Survival benefits may not be greater in patients > 71 years of age compared to palliative resection.170
The most common surgical approaches for limited resection are segmentectomy or wedge resection. Segmentectomy includes lymph node dissection, whereas wedge resection consists of lung tumor removal with surrounding normal lung parenchyma. Segmentectomy has been reported to be superior to wedge resection in overall survival and lung cancer-specific survival in patients with Stage IA NSCLC,171-174 especially when resected tumors are ≤ 2 cm in size and lymphadenectomy is performed. Perioperative complications are lower with sublobar resection175-179 compared to lobectomy in older patients and patients with FEV1 < 85%. In tumors 2-5 cm in size, sublobar resection is inferior to lobectomy,180 and sublobar resection may be inferior to lobectomy even for Stage IA tumors.181 Differences in patient populations, the extent of lymph node dissection, and the margin size around the resected tumor may all affect outcomes.182,183 Reduced lymphadenectomy during sublobar resection results in inferior survival outcomes compared with lobectomy; an increased number of lymph nodes resected may be more important than the extent of lung resection.184,185
Only using a calculation of postoperative FEV1 to predict postoperative lung function may be misleading in patients with emphysema. In patients with predicted postoperative FEV1 < 40% who underwent lobectomy for NSCLC in an emphysematous lobe, no significant reduction in postoperative FEV1 was observed.186
Thermal Ablation
Thermal ablation is an alternative local therapy for NSCLC.187-194 Radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation (CRYO) have been used in patients with NSCLC.187,191-200 Tumor location has bearing on ablation choice. In the middle and outer thirds of the lung, CYRO, MWA, or RFA are all possible considerations, while in the central lung zone, CRYO is preferred for lesions abutting airways or along the pleura or chest wall. Most literature reports percutaneous image guided RFA. In 51 patients with inoperable Stage IA NSCLC, overall survival rate was 86.3% year 1 and 69.8% year 2. Local control and recurrence free rates were 68.9% and 59.8% at years 1 and 2, respectively.187 Recurrence rate was worse for tumors > 2 cm in size. Several studies have reported minimal to no significant decline in lung function at 1 and 3 months post ablation.187,191,198,199 Reduced lung function post RFA treatment has been infrequently attributed to pleuritis or ablation volume.199 Follow-ups at 1 and 2 years post RFA reported no decline in lung function or DLCO.187,198 FVC increased in some individuals possibly due to remodeled emphysematous tissue and decreased hyperinflation.187 Most studies report success with tumor sizes <3 cm, preferably <2 cm. Pneumothorax, pleural effusion, and tumor track seeding may be complications.189,198 MWA ablation produces larger ablation zones with reduced time compared to RFA and MWA. CRYO is safer to use in patients with pacemakers. Bronchoscopic approaches with thermal ablation are undergoing feasibility trials.201
Stereotactic Body Radiation Therapy
SBRT or stereotactic ablative radiation therapy delivers high doses of precisely focused radiation therapy to malignancies. It is standard care for patients who either refuse or have contraindications to definitive surgery.168 Treatment of tumor sizes of up to 5 cm has become routine.202 Optimally, tumors should be > 1cm from the chest wall although this is not an absolute contraindication. Central (within 2 cm of the proximal bronchial tree and/or abutting the mediastinal pleural) and ultra-central tumors (abutting the proximal bronchial tree) were considered high risk, subsequent studies demonstrate no increased toxicities using 5 fraction treatment regimens.203
SBRT in early-stage NSCLC has shown favorable outcomes in quality of life, high local control rates, and reduced treatment-related complications. SBRT has increased more than 2-fold from 2008 to 2013 (6.7% to 16.3%).204 A study in patients treated with inoperable NSCLC reported a 55.8%, 3-year overall survival with 90.6% local control rate.205 Higher maximum doses further improved local control and overall survival.206
Studies comparing local control rate and overall survival between surgery and SBRT have shown surgery to be equivalent or superior to SBRT. A review that compared SBRT to sublobar resection in high-risk patients for lobectomy reported similar 1-year survivals. However, overall, 3-year survival was higher with sublobar resection compared to SBRT.207 A metanalysis of 11,540 high-risk elderly patients with Stage I NSCLC reported that sublobar resection compared to conventional fraction radiation therapy or SBRT significantly improved survival without differences in treatment failure or complications.208 Fatigue, pneumonitis, and chest wall pain were reported with SBRT but mortality at 30 days was 0%. Sublobar resection207 had morbidity between 7.3%–33.7% with a 30-day mortality of 1%–2.6%. Multiple attempts to perform multicentered randomized trials to evaluate surgical resection and SBRT have been aborted due to low patient accrual.209,210 Several trials comparing SBRT with surgical resection are currently ongoing.209
SBRT has also been used for salvage after prior surgery or radiation therapy. Median survivals of 23 (95% CI 15–31) and 50 months (95% CI 35–65) and overall, 5-year survivals of 26.2% and 42.4% were reported for patients with prior radiation therapy and surgery, respectively.
COPD and Treatment Outcomes in Advanced Non-Small Cell Lung Cancer
The identification of several driver mutations has led to the development of targeted therapies and immune checkpoint inhibitors that provide viable options to traditional chemotherapy. Although for some patients without driver mutations, the combination of chemotherapy and a checkpoint inhibitor is considered standard care, patients with high PD-L1 expressing tumors may be treated with a single agent checkpoint inhibitor. Given the clinical efficacy of targeted therapies and immunotherapy, emphasis has been placed on offering treatment even to patients with multiple comorbidities.
The impact of COPD on treatment response with chemotherapeutic agents has received limited attention. COPD has been reported to negatively impact overall survival in stage IV NSCLC and current smokers treated with conventional chemotherapy.211 However, others have shown no negative impact of COPD in patients treated with platinum-based chemotherapy or tyrosine kinase inhibitors.212 COPD patients have reduced ventilatory reserve and may have comorbidities such as congestive heart failure (CHF) or renal failure.213 The use of cisplatin in patients with renal dysfunction is problematic and generally avoided. Pemetrexed, a drug commonly used as a component of a platinum doublet chemotherapy backbone in patients with adenocarcinoma of lung, requires creatinine clearances ≥45 ml/min. Chemotherapies such as etoposide and vinorelbine can cause cardiotoxicity and may aggravate CHF. Etoposide is used to treat small cell lung cancer and less commonly in NSCLC.
Molecular testing of NSCLC tumors allows identification of patients with driver mutations that could be treated with targeted agents. The likelihood of finding molecular changes is higher in never smokers, however, available data clearly indicates that smokers could also have a targetable alteration. Targeted therapies exist for a number of genetic alterations such as EGFR mutations, c-ROS oncogene 1 fusions, ALK translocations, and other subtypes.214,215 Drugs used in these settings are largely well tolerated oral agents with fewer side effects than conventional chemotherapies. Rash and diarrhea are among the most common adverse events. Pneumonitis and interstitial lung disease have been reported in some patients treated with these agents and with EGFR tyrosine kinase inhibitors.
Immune checkpoint inhibitors have transformed NSLC treatment and are used as sole first- line agents, or in combination with other chemotherapeutic agents. Although checkpoint inhibitors are more effective and less toxic compared to conventional chemotherapy, side effects may develop secondary to their mechanisms of action. Immune check point inhibitors may promote T-cell attack on normal cells expressing self-antigens in the skin, thyroid, digestive tract, lungs, and joints. Some data suggest a higher sensitivity to immune checkpoint inhibition in COPD patients with NSCLC.22,216 COPD was associated with significantly longer overall and progression-free survival in patients treated with palliative pembrolizumab.217
Pneumonitis is a common side effect of checkpoint inhibition therapy followed by sarcoid-like granulomatosis, or tuberculous or other infections.218 Pneumonitis occurs in <0.5% to 10% of all patients when immune checkpoint therapy is combined with chemotherapy or nivolumab and cytotoxic T-lymphocyte-associated protein 4 combinations.219 In an analysis of 11,921 NSCLC patients receiving immune checkpoint inhibition, deaths related to adverse respiratory events was about 0.2%; pneumonitis was the cause in 0.1% of deaths.218
Although patients with a diagnosis of lung cancer and COPD are commonly encountered in everyday clinical practice, the treatment options for lung cancer are rarely significantly altered due to the presence of COPD.
Summary and Conclusions
COPD and lung cancer are highly prevalent causes of morbidity and mortality, worldwide. Their combined presence poses important challenges to diagnosis, treatment, and prognosis. Shared risks and mechanistic factors may play roles in the higher association of lung cancer in patients with COPD and provide opportunities for novel target identification for the prevention and treatment of lung cancer. LDCT is a major advancement for earlier diagnosis and treatment of lung cancer, however, its use requires special considerations in patients with reduced lung function due to COPD because of false positive or indeterminate lesions that may require invasive procedures. Lung cancer screening provides an opportunity to assess patients for the presence of COPD that may allow identification and earlier treatment of patients not yet diagnosed. Finally, the presence of COPD has important implications for the management of lung cancer.
Abbreviations
Abbreviations: chronic obstructive pulmonary disease, COPD; forced expiratory volume in 1 second, FEV1; low-dose computed tomography, LCDT; non-small cell lung cancer, NSCLC; cholinergic receptor nicotinic alpha, CHRNA; computed tomography, CT; diffusing capacity for carbon monoxide, DLCO; the National Lung Screening Trial, NLST; epidermal growth factor receptor, EGFR; anaplastic lymphoma kinase, ALK; inhaled corticosteroid, ICS; chest x-ray, CXR; Nederlands–Leuvens Longkanker Screenings Onderzoek, trial, NELSON; confidence interval, CI; Lung Imaging Reporting and Data System, Lung-RADS; U.S. Preventative Services Task Force, USPSTF; Cancer Interventional Surveillance Modeling Network, CISNET; high-resolution computed tomography, HRCT; stereotactic body radiotherapy, SBRT; low-and middle-income countries, LMICs; forced vital capacity, FVC; total lung capacity, TLC; radiofrequency ablation, RFA; microwave ablation, MWA; cryoablation, CRYO; programmed death-ligand, PD-L1; congestive heart failure, CHF
Funding Statement
Not applicable
References
- 1.World Health Organization (WHO). Chronic obstructive pulmonary disease (COPD). WHO website. Published 2021. Updated 2022. Accessed 2021. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) [Google Scholar]
- 2.CHEST. World lung cancer day 2020 fact sheet. CHEST website. Published 2020. Accessed February 2022. https://www.chestnet.org/newsroom/chest-news/2020/07/world-lung-cancer-day-2020-fact-sheet [Google Scholar]
- 3.Divo M,Cote C,de Torres JP,et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155-161. doi: https://doi.org/10.1164/rccm.201201-0034OC [DOI] [PubMed] [Google Scholar]
- 4.Anthonisen NR,Skeans MA,Wise RA,et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142(4):233-239. doi: https://doi.org/10.7326/0003-4819-142-4-200502150-00005 [DOI] [PubMed] [Google Scholar]
- 5.Gao YH,Guan WJ,Liu Q,et al. Impact of COPD and emphysema on survival of patients with lung cancer: a meta-analysis of observational studies. Respirology. 2016;21(2):269-279. doi: https://doi.org/10.1111/resp.12661 [DOI] [PubMed] [Google Scholar]
- 6.Amirahmadi R,Kumar AJ,Cowan M,Deepak J. Lung cancer screening in patients with COPD-A case report. Medicina (Kaunas). 2019;55(7):364. doi: https://doi.org/10.3390/medicina55070364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tockman MS,Anthonisen NR,Wright EC,Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med. 1987;106(4):512-518. doi: https://doi.org/10.7326/0003-4819-106-4-512 [DOI] [PubMed] [Google Scholar]
- 8.Skillrud DM,Offord KP,Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease. A prospective, matched, controlled study. Ann Intern Med. 1986;105(4):503-507. doi: https://doi.org/10.7326/0003-4819-105-4-503 [DOI] [PubMed] [Google Scholar]
- 9.de Torres JP,Bastarrika G,Wisnivesky JP,et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132(6):1932-1938. doi: https://doi.org/10.1378/chest.07-1490 [DOI] [PubMed] [Google Scholar]
- 10.Wille MM,Dirksen A,Ashraf H,et al. Results of the randomized danish lung cancer screening trial with focus on high-risk profiling. Am J Respir Crit Care Med. 2016;193(5):542-551. doi: https://doi.org/10.1164/rccm.201505-1040OC [DOI] [PubMed] [Google Scholar]
- 11.Durham AL,Adcock IM. The relationship between COPD and lung cancer. Lung Cancer. 2015;90(2):121-127. https://doi.org/10.1016/j.lungcan.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houghton AM,Mouded M,Shapiro SD. Common origins of lung cancer and COPD. Nat Med. 2008;14:1023-1024. doi: https://doi.org/10.1038/nm1008-1023 [DOI] [PubMed] [Google Scholar]
- 13.Celli BR. Chronic obstructive pulmonary disease and lung cancer: common pathogenesis, shared clinical challenges. Proc Am Thorac Soc. 2012;9(2):74-79. doi: https://doi.org/10.1513/pats.201107-039MS [DOI] [PubMed] [Google Scholar]
- 14.Schetter AJ,Heegaard NH,Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31(1):37-49. doi: https://doi.org/10.1093/carcin/bgp272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi PH,Zeldis JB. Molecular biology of viral hepatitis and hepatocellular carcinoma. Compr Ther. 1993;19(5):188-196. https://europepmc.org/article/med/8275664 [PubMed] [Google Scholar]
- 16.Pera M,Trastek VF,Pairolero PC,Cardesa A,Allen MS,Deschamps C. Barrett's disease: pathophysiology of metaplasia and adenocarcinoma. Ann Thorac Surg. 1993;56:1191-1197. doi: https://doi.org/10.1016/0003-4975(95)90050-0 [DOI] [PubMed] [Google Scholar]
- 17.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131(12):2724-2732. doi: https://doi.org/10.1002/ijc.27816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballaz S,Mulshine JL. The potential contributions of chronic inflammation to lung carcinogenesis. Clin Lung Cancer. 2003;5(1):46-62. doi: https://doi.org/10.3816/CLC.2003.n.021 [DOI] [PubMed] [Google Scholar]
- 19.Chalela R,Gea J,Barreiro E. Immune phenotypes in lung cancer patients with COPD: potential implications for immunotherapy. J Thorac Dis. 2018;10(Suppl 18):S2186-S2189. doi: https://doi.org/10.21037/jtd.2018.06.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rangelov K,Sethi S. Role of infections. Clin Chest Med. 2014;35(1):87-100. doi: https://doi.org/10.1016/j.ccm.2013.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao H,Rahman I. Current concepts on the role of inflammation in COPD and lung cancer. Curr Opin Pharmacol. 2009;9(4):375-383. doi: https://doi.org/10.1016/j.coph.2009.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biton J,Ouakrim H,Dechartres A,et al. Impaired tumor-infiltrating T cells in patients with chronic obstructive pulmonary disease impact lung cancer response to PD-1 blockade. Am J Respir Crit Care Med. 2018;198(7):928-940. doi: https://doi.org/10.1164/rccm.201706-1110OC [DOI] [PubMed] [Google Scholar]
- 23.Chang SH,Mirabolfathinejad SG,Katta H,et al. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci U S A. 2014;111(15):5664-5669. doi: https://doi.org/10.1073/pnas.1319051111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gentles AJ,Bratman SV,Lee LJ,et al. Integrating tumor and stromal gene expression signatures with clinical indices for survival stratification of early-stage non-small cell lung cancer. J Natl Cancer Inst. 2015;107(10):djv211. doi: https://doi.org/10.1093/jnci/djv211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang J,Ramis-Cabrer D,Curull V,et al. Immune cell subtypes and cytokines in lung tumor microenvironment: influence of COPD. Cancers (Basel). 2020;12(5):1217. doi: https://doi.org/10.3390/cancers12051217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Divo MJ,Celli BR,Poblador-Plou B,et al. Chronic obstructive pulmonary disease (COPD) as a disease of early aging: evidence from the EpiChron cohort. PLoS One. 2018;13(2):e0193143. doi: https://doi.org/10.1371/journal.pone.0193143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caramori G,Adcock IM,Casolari P,et al. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax. 2011;66(6):521-527. doi: https://doi.org/10.1136/thx.2010.156448 [DOI] [PubMed] [Google Scholar]
- 28.Morla M,Busquets X,Pons J,Sauleda J,MacNee W,Agusti AG. Telomere shortening in smokers with and without COPD. Eur Respir J. 2006;27(3):525-528. doi: https://doi.org/10.1183/09031936.06.00087005 [DOI] [PubMed] [Google Scholar]
- 29.Hosgood HD,Cawthon R,He X,Chanock S,Lan Q. Genetic variation in telomere maintenance genes, telomere length, and lung cancer susceptibility. Lung Cancer. 2009;66(2):157-161. doi: https://doi.org/10.1016/j.lungcan.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang JS,Choi YY,Lee WK,et al. Telomere length and the risk of lung cancer. Cancer Sci. 2008;99(7):1385-1389. doi: https://doi.org/10.1111/j.1349-7006.2008.00831.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mambo E,Chatterjee A,de Souza-Pinto NC,et al. Oxidized guanine lesions and hOgg1 activity in lung cancer. Oncogene. 2005;24:4496-4508. doi: https://doi.org/10.1038/sj.onc.1208669 [DOI] [PubMed] [Google Scholar]
- 32.Vulimiri SV,Wu X,Baer-Dubowska W,et al. Analysis of aromatic DNA adducts and 7,8-dihydro-8-oxo-2' deoxyguanosine in lymphocyte DNA from a case-control study of lung cancer involving minority populations. Mol Carcinog. 2000;27(4):330. doi: https://doi.org/10.1002/(SICI)1098-2744(200004)27:4<330::AID-MC11>3.0.CO;2-E [DOI] [PubMed] [Google Scholar]
- 33.Osoata GO,Yamamura S,Ito M,et al. Nitration of distinct tyrosine residues causes inactivation of histone deacetylase 2. Biochem Biophys Res Commun. 2009;384(3):366-371. doi: https://doi.org/10.1016/j.bbrc.2009.04.128 [DOI] [PubMed] [Google Scholar]
- 34.Warren GW,Singh AK. Nicotine and lung cancer. J Carcinog. 2013;12(1). doi: https://doi.org/10.4103/1477-3163.106680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz AG,Ruckdeschel JC. Familial lung cancer: genetic susceptibility and relationship to chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(1):16-22. doi: https://doi.org/10.1164/rccm.200502-235PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joost O,Wilk JB,Cupples LA,et al. Genetic loci influencing lung function: a genome-wide scan in the Framingham Study. Am J Respir Crit Care Med. 2002;165(6):795-799. doi: https://doi.org/10.1164/ajrccm.165.6.2102057 [DOI] [PubMed] [Google Scholar]
- 37.Silverman EK,Mosley JD,Palmer LJ,et al. Genome-wide linkage analysis of severe, early-onset chronic obstructive pulmonary disease: airflow obstruction and chronic bronchitis phenotypes. Hum Mol Genet. 2002;11(6):623-632. doi: https://doi.org/10.1093/hmg/11.6.623 [DOI] [PubMed] [Google Scholar]
- 38.Yang IA,Holloway JW,Fong KM. Genetic susceptibility to lung cancer and co-morbidities. J Thorac Dis. 2013;5(Suppl 5):S454-462. https://jtd.amegroups.com/article/view/1561/html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Dyke AL,Cote ML,Wenzlaff AS,et al. Cytokine and cytokine receptor single-nucleotide polymorphisms predict risk for non-small cell lung cancer among women. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1829-1840. doi: https://doi.org/10.1158/1055-9965.EPI-08-0962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franco R,Schoneveld O,Georgakilas AG,Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266(1):6-11. doi: https://doi.org/10.1016/j.canlet.2008.02.026 [DOI] [PubMed] [Google Scholar]
- 41.Kabesch M,Adcock IM. Epigenetics in asthma and COPD. Biochimie. 2012;94(11):2231-2241. doi: https://doi.org/10.1016/j.biochi.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 42.Mannino DM,Aguayo SM,Petty TL,Redd SC. Low lung function and incident lung cancer in the United States: data From the First National Health and Nutrition Examination Survey follow-up. Arch Intern Med. 2003;163(12):1475-1480. doi: https://doi.org/10.1001/archinte.163.12.1475 [DOI] [PubMed] [Google Scholar]
- 43.Wilson DO,Weissfeld JL,Balkan A,et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178(7):738-744. doi: https://doi.org/10.1164/rccm.200803-435OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de-Torres JP,Marín JM,Casanova C,et al. Identification of COPD patients at high risk for lung cancer mortality using the COPD-LUCSS-DLCO. Chest. 2016;149(4):936-942. doi: https://doi.org/10.1378/chest.15-1868 [DOI] [PubMed] [Google Scholar]
- 45.Maldonado F,Bartholmai BJ,Swensen SJ,Midthun DE,Decker PA,Jett JR. Are airflow obstruction and radiographic evidence of emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest. 2010;138(6):1295-1302. doi: https://doi.org/10.1378/chest.09-2567 [DOI] [PubMed] [Google Scholar]
- 46.Young RP,Duan F,Chiles C,et al. Airflow limitation and histology shift in the national lung screening trial. The NLST-ACRIN Cohort Substudy. Am J Respir Crit Care Med. 2015;192(9):1060-1067. doi: https://doi.org/10.1164/rccm.201505-0894OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caplin M,Festenstein F. Relation between lung cancer, chronic bronchitis, and airways obstruction. Br Med J. 1975;3:678-680. doi: https://doi.org/10.1136/bmj.3.5985.678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van den Eeden SK,Friedman GD. Forced expiratory volume (1 second) and lung cancer incidence and mortality. Epidemiology. 1992;3(3):253-257. doi: https://doi.org/10.1097/00001648-199205000-00011 [DOI] [PubMed] [Google Scholar]
- 49.de Torres JP,Marin JM,Casanova C,et al. Lung cancer in patients with chronic obstructive pulmonary disease -- incidence and predicting factors. Am J Respir Crit Care Med. 2011;184(8):913-919. doi: https://doi.org/10.1164/rccm.201103-0430OC [DOI] [PubMed] [Google Scholar]
- 50.de-Torres JP,Wilson DO,Sanchez-Salcedo P,et al. Lung cancer in patients with chronic obstructive pulmonary disease. Development and validation of the COPD Lung Cancer Screening Score. Am J Respir Crit Care Med. 2015;191(3):285-291. doi: https://doi.org/10.1164/rccm.201407-1210OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zulueta JJ,Wisnivesky JP,Henschke CI,et al. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141(5):1216-1223. doi: https://doi.org/10.1378/chest.11-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith BM,Schwartzman K,Kovacina B,et al. Lung cancer histologies associated with emphysema on computed tomography. Lung Cancer. 2012;76(1):61-66. doi: https://doi.org/10.1016/j.lungcan.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 53.Hohberger LA,Schroeder DR,Bartholmai BJ,et al. Correlation of regional emphysema and lung cancer: a lung tissue research consortium-based study. J Thorac Oncol. 2014;9(5):639-645. doi: https://doi.org/10.1097/JTO.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schiavon M,Marulli G,Nannini N,et al. COPD-related adenocarcinoma presents low aggressiveness morphological and molecular features compared to smoker tumours. Lung Cancer. 2014;86(3):311-317. doi: https://doi.org/10.1016/j.lungcan.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 55.Murakami J,Ueda K,Sano F,Hayashi M,Nishimoto A,Hamano K. Pulmonary emphysema and tumor microenvironment in primary lung cancer. J Surg Res. 2016;200(2):690-697. doi: https://doi.org/10.1016/j.jss.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 56.Veronesi G,Maisonneuve P,Bellomi M,et al. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2012;157(11):776-784. doi: https://doi.org/10.7326/0003-4819-157-11-201212040-00005 [DOI] [PubMed] [Google Scholar]
- 57.Young RP,Hopkins RJ. Estimating overdiagnosis of lung cancer. Ann Intern Med. 2013;158(8):635. doi: https://doi.org/10.7326/0003-4819-158-8-201304160-00013 [DOI] [PubMed] [Google Scholar]
- 58.Maisonneuve P,Veronesi G,Bertolotti R. Estimating overdiagnosis of lung cancer--reply. Ann Intern Med. 2013;158(8):635-636. doi: https://doi.org/10.7326/0003-4819-158-8-201304160-00014 [DOI] [PubMed] [Google Scholar]
- 59.Lim JU,Yeo CD,Rhee CK,et al. Chronic obstructive pulmonary disease-related non-small-cell lung cancer exhibits a low prevalence of EGFR and ALK driver mutations. PLoS One. 2015;10(11):e0142306. doi: https://doi.org/10.1371/journal.pone.0142306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hashimoto N,Matsuzaki A,Okada Y,et al. Clinical impact of prevalence and severity of COPD on the decision-making process for therapeutic management of lung cancer patients. BMC Pulm Med. 2014;14:14. doi: https://doi.org/10.1186/1471-2466-14-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park HY,Kang D,Shin SH,et al. Chronic obstructive pulmonary disease and lung cancer incidence in never smokers: a cohort study. Thorax. 2020;75(6):506-509. doi: https://doi.org/10.1136/thoraxjnl-2019-213732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raymakers AJN ,Sadatsafavi M,Sin DD,FitzGerald JM,Marra CA,Lynd LD. Inhaled corticosteroids and the risk of lung cancer in COPD: a population-based cohort study. Eur Respir J. 2019;53(6):1801257. doi: https://doi.org/10.1183/13993003.01257-2018 [DOI] [PubMed] [Google Scholar]
- 63.Seijo LM,Soriano JB,Peces-Barba G. New evidence on the chemoprevention of inhaled steroids and the risk of lung cancer in COPD. Eur Respir J. 2019;53(6):1900717. doi: https://doi.org/10.1183/13993003.00717-2019 [DOI] [PubMed] [Google Scholar]
- 64.Ge F,Feng Y,Huo Z,et al. Inhaled corticosteroids and risk of lung cancer among chronic obstructive pulmonary disease patients: a comprehensive analysis of nine prospective cohorts. Transl Lung Cancer Res. 2021;10(3):1266-1276. doi: https://doi.org/10.21037/tlcr-20-1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee YM,Kim SJ,Lee JH,Ha E. Inhaled corticosteroids in COPD and the risk of lung cancer. Int J Cancer. 2018;143(9):2311-2318. doi: https://doi.org/10.1002/ijc.31632 [DOI] [PubMed] [Google Scholar]
- 66.Parimon T,Chien JW,Bryson CL,McDonell MB,Udris EM,Au DH. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(7):712-719. doi: https://doi.org/10.1164/rccm.200608-1125OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiri VA,Fabbri LM,Davis KJ,Soriano JB. Inhaled corticosteroids and risk of lung cancer among COPD patients who quit smoking. Respir Med. 2009;103(1):85-90. doi: https://doi.org/10.1016/j.rmed.2008.07.024 [DOI] [PubMed] [Google Scholar]
- 68.Sandelin M,Mindus S,Thuresson M,et al. Factors associated with lung cancer in COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:1833-1839. doi: https://doi.org/10.2147/COPD.S162484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raymakers AJ,McCormick N,Marra CA,Fitzgerald JM,Sin D,Lynd LD. Do inhaled corticosteroids protect against lung cancer in patients with COPD? A systematic review. Respirology. 2017;22(1):61-70. doi: https://doi.org/10.1111/resp.12919 [DOI] [PubMed] [Google Scholar]
- 70.Suissa S,Kezouh A,Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010;123(11):1001-1006. doi: https://doi.org/10.1016/j.amjmed.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 71.Brenner AV,Wang Z,Kleinerman RA,et al. Previous pulmonary diseases and risk of lung cancer in Gansu Province, China. Int. J Epidemiol. 2001;30(1):118-124. doi: https://doi.org/10.1093/ije/30.1.118 [DOI] [PubMed] [Google Scholar]
- 72.Kwak N,Park CM,Lee J,et al. Lung cancer risk among patients with combined pulmonary fibrosis and emphysema. Respir Med. 2014;108(3):524-530. doi: https://doi.org/10.1016/j.rmed.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 73.Karampitsakos T,Tzilas V,Tringidou R,et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2017;45:1-10. doi: https://doi.org/10.1016/j.pupt.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 74.Bray F,Ferlay J,Soerjomataram I,Siegel RL,Torre LA,Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: https://doi.org/10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 75.Ferlay J,Colombet M,Soerjomataram I,et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-1953. doi: https://doi.org/10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 76.Fontana RS,Sanderson DR,Woolner LB,Taylor WF,Miller WE,Muhm JR. Lung cancer screening: the Mayo program. J Occup Med. 1986;28(8):746-750. doi: https://doi.org/10.1097/00043764-198608000-00038 [DOI] [PubMed] [Google Scholar]
- 77.Melamed M,Flehinger B,Zaman M. Impact of ealry detection on the clinical course of lung cancer. Surg Clin North Am. 1987;67(5):909-924. doi: https://doi.org/10.1016/S0039-6109(16)44329-X [DOI] [PubMed] [Google Scholar]
- 78.Tockman M. Survival and mortality from lung cancer in a screened population. Chest. 1986;89(4):324S-325S. doi: https://doi.org/10.1378/chest.89.4_Supplement.324S-a3956302 [Google Scholar]
- 79.Aberle DR,Adams AM,Berg CD,et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi: https://doi.org/10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Koning HJ,van der Aalst CM,de Jong PA,et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi: https://doi.org/10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 81.Field JK,Duffy SW,Baldwin DR,et al. The UK lung cancer screening trial: a pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technol Assess. 2016;20(40):1-146. doi: https://doi.org/10.3310/hta20400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pastorino U,Sverzellati N,Sestini S,et al. Ten-year results of the Multicentric Italian Lung Detection trial demonstrate the safety and efficacy of biennial lung cancer screening. Eur J Cancer. 2019;118:142-148. doi: https://doi.org/10.1016/j.ejca.2019.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jonas DE,Reuland DS,Reddy SM,et al. Screening for lung cancer with low-dose computed tomography: updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;325(10):971-987. doi: https://doi.org/10.1001/jama.2021.0377 [DOI] [PubMed] [Google Scholar]
- 84.Saghir Z,Dirksen A,Ashraf H,et al. CT screening for lung cancer brings forward early disease. The randomised Danish lung cancer screening trial: status after five annual screening rounds with low-dose CT. Thorax. 2012;67(4):296-301. doi: https://doi.org/10.1136/thoraxjnl-2011-200736 [DOI] [PubMed] [Google Scholar]
- 85.Paci E,Puliti D,Lopes Pegna A,et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax. 2017;72(9):825-831. doi: https://doi.org/10.1136/thoraxjnl-2016-209825 [DOI] [PubMed] [Google Scholar]
- 86.Becker N,Motsch E,Gross ML,et al. Randomized study on early detection of lung cancer with MSCT in Germany: study design and results of the first screening round. J Cancer Res Clin Oncol. 2012;138:1475-1486. doi: https://doi.org/10.1007/s00432-012-1228-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Becker N,Motsch E,Gross ML,et al. Randomized study on early detection of lung cancer with MSCT in Germany: results of the first 3 years of follow-up after randomization. J Thorac Oncol. 2015;10(6):890-896. doi: https://doi.org/10.1097/JTO.0000000000000530 [DOI] [PubMed] [Google Scholar]
- 88.Becker N,Motsch E,Trotter A,et al. Lung cancer mortality reduction by LDCT screening-results from the randomized German LUSI trial. Int J Cancer. 2020;146(6):1503-1513. doi: https://doi.org/10.1002/ijc.32486 [DOI] [PubMed] [Google Scholar]
- 89.Infante M,Lutman FR,Cavuto S,et al. Lung cancer screening with spiral CT: baseline results of the randomized DANTE trial. Lung Cancer. 2008;59(3):355-363. doi: https://doi.org/10.1016/j.lungcan.2007.08.040 [DOI] [PubMed] [Google Scholar]
- 90.Infante M,Cavuto S,Lutman FR,et al. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180(5):445-453. doi: https://doi.org/10.1164/rccm.200901-0076OC [DOI] [PubMed] [Google Scholar]
- 91.Infante M,Cavuto S,Lutman FR,et al. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med. 2015;191(10):1166-1175. doi: https://doi.org/10.1164/rccm.201408-1475OC [DOI] [PubMed] [Google Scholar]
- 92.Gohagan JK,Marcus PM,Fagerstrom RM,et al. Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer. 2005;47(1):9-15. doi: https://doi.org/10.1016/j.lungcan.2004.06.007 [DOI] [PubMed] [Google Scholar]
- 93.Kovalchik SA,Tammemagi M,Berg CD,et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369(3):245-254. doi: https://doi.org/10.1056/NEJMoa1301851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Church TR,Black WC,Aberle DR,et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi: https://doi.org/10.1056/NEJMoa1209120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pinsky PF,Gierada DS,Hocking W,Patz EF,Kramer BS. National lung screening trial findings by age: medicare-eligible versus under-65 population. Ann Intern Med. 2014;161(9):627-633. doi: https://doi.org/10.7326/M14-1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lopes Pegna A,Picozzi G,Falaschi F,et al. Four-year results of low-dose CT screening and nodule management in the ITALUNG trial. J Thorac Oncol. 2013;8(7):866-875. doi: https://doi.org/10.1097/JTO.0b013e31828f68d6 [DOI] [PubMed] [Google Scholar]
- 97.Croswell JM,Baker SG,Marcus PM,Clapp JD,Kramer BS. Cumulative incidence of false-positive test results in lung cancer screening: a randomized trial. Ann Intern Med. 2010;152(8):505-512. doi: https://doi.org/10.7326/0003-4819-152-8-201004200-00007 [DOI] [PubMed] [Google Scholar]
- 98.Wagnetz U,Menezes RJ,Boerner S,et al. CT screening for lung cancer: implication of lung biopsy recommendations. Am J Roentgenol. 2012;198(2):351-358. doi: https://doi.org/10.2214/AJR.11.6726 [DOI] [PubMed] [Google Scholar]
- 99.Menezes RJ,Roberts HC,Paul NS,et al. Lung cancer screening using low-dose computed tomography in at-risk individuals: the Toronto experience. Lung Cancer. 2010;67(2):177-183. doi: https://doi.org/10.1016/j.lungcan.2009.03.030 [DOI] [PubMed] [Google Scholar]
- 100.Veronesi G,Bellomi M,Mulshine JL,et al. Lung cancer screening with low-dose computed tomography: a non-invasive diagnostic protocol for baseline lung nodules. Lung Cancer. 2008;61(3):340-349. doi: https://doi.org/10.1016/j.lungcan.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 101.Swensen SJ,Jett JR,Hartman TE,et al. CT screening for lung cancer: five-year prospective experience. Radiology. 2005;235(1):259-265. doi: https://doi.org/10.1148/radiol.2351041662 [DOI] [PubMed] [Google Scholar]
- 102.Walker BL,Williamson C,Regis SM,et al. Surgical outcomes in a large, clinical, low-dose computed tomographic lung cancer screening program. Ann Thorac Surg. 2015;100(4):1218-1223. doi: https://doi.org/10.1016/j.athoracsur.2015.04.112 [DOI] [PubMed] [Google Scholar]
- 103.Infante M,Chiesa G,Solomon D,et al. Surgical procedures in the DANTE trial, a randomized study of lung cancer early detection with spiral computed tomography: comparative analysis in the screening and control arm. J Thorac Oncol. 2011;6(2):327-335. doi: https://doi.org/10.1097/JTO.0b013e318200f523 [DOI] [PubMed] [Google Scholar]
- 104.Pinsky PF,Gierada DS,Black W,et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015;162(7):485-491. doi: https://doi.org/10.7326/M14-2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rampinelli C,De Marco P,Origgi D,et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347. doi: https://doi.org/10.1136/bmj.j347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mascalchi M,Belli G,Zappa M,et al. Risk-benefit analysis of x-ray exposure associated with lung cancer screening in the Italung-CT trial. Am J Roentgenol. 2006;187(2):421-429. doi: https://doi.org/10.2214/AJR.05.0088 [DOI] [PubMed] [Google Scholar]
- 107.Meza R,Jeon J,Toumazis I,et al. Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: a collaborative modeling study for the U.S. preventive services task force. JAMA. 2021;325(10):988-997. doi: https://doi.org/10.1001/jama.2021.1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoogendoorn M,Feenstra TL,Hoogenveen RT,Rutten-van Mölken MP. Long-term effectiveness and cost-effectiveness of smoking cessation interventions in patients with COPD. Thorax. 2010;65(8):711-718. doi: https://doi.org/10.1136/thx.2009.131631 [DOI] [PubMed] [Google Scholar]
- 109.Ten Haaf K,Tammemägi MC,Bondy SJ,et al. Performance and cost-effectiveness of computed tomography lung cancer screening scenarios in a population-based setting: a microsimulation modeling analysis in Ontario, Canada. PLoS Med. 2017;14(2):e1002225. doi: https://doi.org/10.1371/journal.pmed.1002225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taylor KL,Cox LS,Zincke N,Mehta L,McGuire C,Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer. 2007;56(1):125-134. doi: https://doi.org/10.1016/j.lungcan.2006.11.015 [DOI] [PubMed] [Google Scholar]
- 111.Cox LS,Clark MM,Jett JR,et al. Change in smoking status after spiral chest computed tomography scan screening. Cancer. 2003;98(11):2495-2501. doi: https://doi.org/10.1002/cncr.11813 [DOI] [PubMed] [Google Scholar]
- 112.van der Aalst CM,van Klaveren RJ,van den Bergh KA,Willemsen MC,de Koning HJ. The impact of a lung cancer computed tomography screening result on smoking abstinence. Eur Respir J. 2011;37(6):1466-1473. doi: https://doi.org/10.1183/09031936.00035410 [DOI] [PubMed] [Google Scholar]
- 113.Townsend CO,Clark MM,Jett JR,et al. Relation between smoking cessation and receiving results from three annual spiral chest computed tomography scans for lung carcinoma screening. Cancer. 2005;103(10):2154-2162. doi: https://doi.org/10.1002/cncr.21045 [DOI] [PubMed] [Google Scholar]
- 114.Styn MA,Land SR,Perkins KA,Wilson DO,Romkes M,Weissfeld JL. Smoking behavior 1 year after computed tomography screening for lung cancer: effect of physician referral for abnormal CT findings. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3484-3489. doi: https://doi.org/10.1158/1055-9965.EPI-09-0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anderson CM,Yip R,Henschke CI,Yankelevitz DF,Ostroff JS,Burns DM. Smoking cessation and relapse during a lung cancer screening program. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3476-3483. doi: https://doi.org/10.1158/1055-9965.EPI-09-0176 [DOI] [PubMed] [Google Scholar]
- 116.Clark MA,Gorelick JJ,Sicks JD,et al. The relations between false positive and negative screens and smoking cessation and relapse in the national lung screening trial: implications for public health. Nicotine Tob Res. 2016;18(1):17-24. doi: https://doi.org/10.1093/ntr/ntv037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ashraf H,Tønnesen P,Holst Pedersen J,Dirksen A,Thorsen H,Døssing M. Effect of CT screening on smoking habits at 1-year follow-up in the Danish Lung Cancer Screening Trial (DLCST). Thorax. 2009;64(5):388-392. doi: https://doi.org/10.1136/thx.2008.102475 [DOI] [PubMed] [Google Scholar]
- 118.Ashraf H,Saghir Z,Dirksen A,et al. Smoking habits in the randomised Danish Lung Cancer Screening Trial with low-dose CT: final results after a 5-year screening programme. Thorax. 2014;69(6):574-579. doi: https://doi.org/10.1136/thoraxjnl-2013-203849 [DOI] [PubMed] [Google Scholar]
- 119.Gareen IF,Duan F,Greco EM,et al. Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer. 2014;120(21):3401-3409. doi: https://doi.org/10.1002/cncr.28833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaerlev L,Iachina M,Pedersen JH,Green A,Nørgård BM. CT-Screening for lung cancer does not increase the use of anxiolytic or antidepressant medication. BMC Cancer. 2012;12:188. doi: https://doi.org/10.1186/1471-2407-12-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rasmussen JF,Siersma V,Pedersen JH,Brodersen J. Psychosocial consequences in the Danish randomised controlled lung cancer screening trial (DLCST). Lung Cancer. 2015;87(1):65-72. doi: https://doi.org/10.1016/j.lungcan.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 122.van den Bergh KA,Essink-Bot ML,Borsboom GJ,Scholten ET,van Klaveren RJ,de Koning HJ. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. Eur Respir J. 2011;38(1):154-161. doi: https://doi.org/10.1183/09031936.00123410 [DOI] [PubMed] [Google Scholar]
- 123.Aggestrup LM,Hestbech MS,Siersma V,Pedersen JH,Brodersen J. Psychosocial consequences of allocation to lung cancer screening: a randomised controlled trial. BMJ Open. 2012;2(2):e000663. doi: https://doi.org/10.1136/bmjopen-2011-000663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krist AH,Davidson KW,Mangione CM,et al. Screening for lung cancer: US preventive services task force recommendation statement. JAMA. 2021;325(10):962-970. doi: https://doi.org/10.1001/jama.2021.1117 [DOI] [PubMed] [Google Scholar]
- 125.Haiman CA,Stram DO,Wilkens LR,et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333-342. doi: https://doi.org/10.1056/NEJMoa033250 [DOI] [PubMed] [Google Scholar]
- 126.Aldrich MC,Mercaldo SF,Sandler KL,Blot WJ,Grogan EL,Blume JD. Evaluation of USPSTF lung cancer screening guidelines among African American adult smokers. JAMA Oncol. 2019;5(9):1318-1324. doi: https://doi.org/10.1001/jamaoncol.2019.1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaplan RC,Bangdiwala SI,Barnhart JM,et al. Smoking among U.S. Hispanic/Latino adults: the Hispanic community health study/study of Latinos. Am J Prev Med. 2014;46(5):496-506. doi: https://doi.org/10.1016/j.amepre.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bandiera FC,Assari S,Livaudais-Toman J,Pérez-Stable EJ. Latino and Black smokers in the Health and Retirement Study are more likely to quit: the role of light smoking. Tob Induc Dis. 2016;14:23. doi: https://doi.org/10.1186/s12971-016-0090-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bach PB,Cramer LD,Schrag D,Downey RJ,Gelfand SE,Begg CB. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345(3):181-188. doi: https://doi.org/10.1056/NEJM200107193450306 [DOI] [PubMed] [Google Scholar]
- 130.Sanchez-Salcedo P,Wilson DO,de-Torres JP,et al. Improving selection criteria for lung cancer screening. The potential role of emphysema. Am J Respir Crit Care Med. 2015;191(8):924-931. doi: https://doi.org/10.1164/rccm.201410-1848OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grover H,Ross T,Fuller E. Implementation of targeted screening for lung cancer in a high-risk population within routine NHS practice using low-dose computed tomography. Thorax. 2020;75(4):348-350. doi: https://doi.org/10.1136/thoraxjnl-2019-214303 [DOI] [PubMed] [Google Scholar]
- 132.Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer. 2009;9:655-664. doi: https://doi.org/10.1038/nrc2703 [DOI] [PubMed] [Google Scholar]
- 133.Saul EE,Guerra RB,Saul ME,et al. The challenges of implementing low-dose computed tomography for lung cancer screening in low- and middle-income countries. Nat Cancer. 2020;1:1140-1152. doi: https://doi.org/10.1038/s43018-020-00142-z [DOI] [PubMed] [Google Scholar]
- 134.Li J,Shi L,Liang H,Ding G,Xu L. Urban-rural disparities in health care utilization among Chinese adults from 1993 to 2011. BMC Health Serv Res. 2018;18:102. doi: https://doi.org/10.1186/s12913-018-2905-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Abbasi A,Siddiqi R,Owais A,et al. Prevalence and barriers to lung cancer screening in Karachi, Pakistan: a cross-sectional survey of smokers and physicians. Cureus. 2017;9(5):e1248. doi: https://doi.org/10.7759/cureus.1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Institute for Health Metrics and Evaluation (IHME). Global burden of disease. IHME website. Published 2021. Accessed September 7, 2021. http://www.healthdata.org/gbd/2019 [Google Scholar]
- 137.McCarthy EP,Ngo LH,Chirikos TN,et al. Cancer stage at diagnosis and survival among persons with Social Security Disability Insurance on Medicare. Health Serv Res. 2007;42(2):611-628. doi: https://doi.org/10.1111/j.1475-6773.2006.00619.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin J,Carter CA,McGlynn KA,et al. A prognostic model to predict mortality among non-small-cell lung cancer patients in the U.S. military health system. J Thorac Oncol. 2015;10(12):1694-1702. doi: https://doi.org/10.1097/JTO.0000000000000691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin J,McGlynn KA,Carter CA,et al. The impact of preexisting mental health disorders on the diagnosis, treatment, and survival among lung cancer patients in the U.S. military health system. Cancer Epidemiol Biomarkers Prev. 2016;25(12):1564-1571. doi: https://doi.org/10.1158/1055-9965.EPI-16-0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schapira MM,McAuliffe TL,Nattinger AB. Underutilization of mammography in older breast cancer survivors. Med Care. 2000;38(3):281-289. doi: https://doi.org/10.1097/00005650-200003000-00005 [DOI] [PubMed] [Google Scholar]
- 141.Ostrowski M,Marczyk M,Dziedzic R,et al. Lung cancer survival and comorbidities in lung cancer screening participants of the Gdańsk screening cohort. Eur J Public Health. 2019;29(6):1114-1117. doi: https://doi.org/10.1093/eurpub/ckz052 [DOI] [PubMed] [Google Scholar]
- 142.Vogelmeier CF,Criner GJ,Martinez FJ,et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease, 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;49:1700214. doi: https://doi.org/10.1183/13993003.00214-2017 [DOI] [PubMed] [Google Scholar]
- 143.Balata H,Harvey J,Barber PV,et al. Spirometry performed as part of the Manchester community-based lung cancer screening programme detects a high prevalence of airflow obstruction in individuals without a prior diagnosis of COPD. Thorax. 2020;75(8):655-660. doi: https://doi.org/10.1136/thoraxjnl-2019-213584 [DOI] [PubMed] [Google Scholar]
- 144.Iaccarino JM,Silvestri GA,Wiener RS. Patient-level trajectories and outcomes after low-dose CT screening in the national lung screening trial. Chest. 2019;156(5):965-971. doi: https://doi.org/10.1016/j.chest.2019.06.016 [DOI] [PubMed] [Google Scholar]
- 145.Young RP,Hopkins RJ. Chronic obstructive pulmonary disease (COPD) and lung cancer screening. Transl Lung Cancer Res. 2018;7(3):347-360. doi: https://doi.org/10.21037/tlcr.2018.05.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hadique S,Jain P,Hadi Y,Baig A,Parker JE. Utility of FDG PET/CT for assessment of lung nodules identified during low dose computed tomography screening. BMC Med Imaging. 2020;20:69. doi: https://doi.org/10.1186/s12880-020-00469-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pritchett MA,Schampaert S,de Groot JAH ,Schirmer CC,van der Bom I. Cone-beam CT with augmented fluoroscopy combined with electromagnetic navigation bronchoscopy for biopsy of pulmonary nodules. J Bronchology Interv Pulmonol. 2018;25(4):274-282. doi: https://doi.org/10.1097/LBR.0000000000000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Donington J,Ferguson M,Mazzone P,et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest. 2012;142(6):1620-1635. doi: https://doi.org/10.1378/chest.12-0790 [DOI] [PubMed] [Google Scholar]
- 149.Sekine Y,Yamada Y,Chiyo M,et al. Association of chronic obstructive pulmonary disease and tumor recurrence in patients with stage IA lung cancer after complete resection. Ann Thorac Surg. 2007;84(3):946-950. doi: https://doi.org/10.1016/j.athoracsur.2007.04.038 [DOI] [PubMed] [Google Scholar]
- 150.Colice GL,Shafazand S,Griffin JP,Keenan R,Bolliger CT. Physicians ACOC. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest. 2007;132(3):161S-177S. doi: https://doi.org/10.1378/chest.07-1359 [DOI] [PubMed] [Google Scholar]
- 151.Marjanski T,Wnuk D,Bosakowski D,Szmuda T,Sawicka W,Rzyman W. Patients who do not reach a distance of 500 m during the 6-min walk test have an increased risk of postoperative complications and prolonged hospital stay after lobectomy. Eur J Cardiothorac Surg. 2015;47(5):e213-219. doi: https://doi.org/10.1093/ejcts/ezv049 [DOI] [PubMed] [Google Scholar]
- 152.Nakagawa T,Tomioka Y,Toyazaki T,Gotoh M. Association between values of preoperative 6-min walk test and surgical outcomes in lung cancer patients with decreased predicted postoperative pulmonary function. Gen Thorac Cardiovasc Surg. 2018;66:220-224. doi: https://doi.org/10.1007/s11748-018-0888-z [DOI] [PubMed] [Google Scholar]
- 153.Nikolić I,Majerić-Kogler V,Plavec D,Maloca I,Slobodnjak Z. Stairs climbing test with pulse oximetry as predictor of early postoperative complications in functionally impaired patients with lung cancer and elective lung surgery: prospective trial of consecutive series of patients. Croat Med J. 2008;49(1):50-57. doi: https://doi.org/10.3325/cmj.2008.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Makey I,Berger RL,Cabral HJ,Celli B,Folch E,Whyte RI. Maximal oxygen uptake--risk predictor of NSCLC resection in patients with comorbid emphysema: lessons from NETT. Semin Thorac Cardiovasc Surg. 2015;27(2):225-231. doi: https://doi.org/10.1053/j.semtcvs.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 155.Ueda K,Murakami J,Tanaka T,Hayashi M,Okabe K,Hamano K. Preoperative risk assessment with computed tomography in patients undergoing lung cancer surgery. J Thorac Dis. 2018;10(7):4101-4108. doi: https://doi.org/10.21037/jtd.2018.06.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang CY,Lin YS,Tzao C,et al. Comparison of Charlson comorbidity index and Kaplan-Feinstein index in patients with stage I lung cancer after surgical resection. Eur J Cardiothorac Surg. 2007;32(6):877-881. doi: https://doi.org/10.1016/j.ejcts.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 157.Moro-Sibilot D,Aubert A,Diab S,et al. Comorbidities and Charlson score in resected stage I nonsmall cell lung cancer. Eur Respir J. 2005;26(3):480-486. doi: https://doi.org/10.1183/09031936.05.00146004 [DOI] [PubMed] [Google Scholar]
- 158.Otake S,Ohtsuka T,Asakura K,Kamiyama I,Kohno M. Impact of comorbidity index on morbidity and survival in non-small cell lung cancer. Asian Cardiovasc Thorac Ann. 2016;24(1):30-33. doi: https://doi.org/10.1177/0218492315617834 [DOI] [PubMed] [Google Scholar]
- 159.Kawaguchi Y,Hanaoka J,Ohshio Y,et al. A risk score to predict postoperative complications after lobectomy in elderly lung cancer patients. Gen Thorac Cardiovasc Surg. 2018;66:537-542. doi: https://doi.org/10.1007/s11748-018-0960-8 [DOI] [PubMed] [Google Scholar]
- 160.Puri V,Crabtree TD,Bell JM,et al. National cooperative group trials of "high-risk" patients with lung cancer: are they truly "high-risk"? Ann Thorac Surg. 2014;97(5):1678-1683. doi: https://doi.org/10.1016/j.athoracsur.2013.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Husain ZA,Kim AW,Yu JB,Decker RH,Corso CD. Defining the high-risk population for mortality after resection of early-stage NSCLC. Clin Lung Cancer. 2015;16(6):e183-187. doi: https://doi.org/10.1016/j.cllc.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 162.Kawaguchi Y,Hanaoka J,Ohshio Y,et al. Sarcopenia predicts poor postoperative outcome in elderly patients with lung cancer. Gen Thorac Cardiovasc Surg. 2019;67:949-954. doi: https://doi.org/10.1007/s11748-019-01125-3 [DOI] [PubMed] [Google Scholar]
- 163.Kaneda H,Nakano T,Murakawa T. The predictive value of preoperative risk assessments and frailty for surgical complications in lung cancer patients. Surg Today. 2021;51:86-93. doi: https://doi.org/10.1007/s00595-020-02058-8 [DOI] [PubMed] [Google Scholar]
- 164.Hu XL,Xu ST,Wang XC,et al. Development and validation of nomogram estimating post-surgery hospital stay of lung cancer patients: relevance for predictive, preventive, and personalized healthcare strategies. EPMA J. 2019;10:173-183. doi: https://doi.org/10.1007/s13167-019-00168-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shoji F,Haratake N,Akamine T,et al. The preoperative controlling nutritional status score predicts survival after curative surgery in patients with pathological stage I non-small cell lung cancer. Anticancer Res. 2017;37(2):741-747. doi: https://doi.org/10.21873/anticanres.11372 [DOI] [PubMed] [Google Scholar]
- 166.Yoshida Y,Kage H,Murakawa T,Sato Y,Ota S,Fukayama M,Nakajima J. Worse prognosis for stage IA lung cancer patients with smoking history and more severe chronic obstructive pulmonary disease. Ann Thorac Cardiovasc Surg. 2015;21(3):194-200. doi: https://doi.org/10.5761/atcs.oa.14-00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wilson H,Gammon D,Routledge T,Harrison-Phipps K. Clinical and quality of life outcomes following anatomical lung resection for lung cancer in high-risk patients. Ann Thorac Med. 2017;12(2):83-87. doi: https://doi.org/10.4103/atm.ATM_385_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chua GWY ,Chua KLM . Which patients benefit most from stereotactic body radiotherapy or surgery in medically operable non-small cell lung cancer? An in-depth look at patient characteristics on both sides of the debate. Thorac Cancer. 2019;10(10):1857-1867. doi: https://doi.org/10.1111/1759-7714.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sandri A,Papagiannopoulos K,Milton R,et al. High-risk patients and postoperative complications following video-assisted thoracic surgery lobectomy: a case-matched comparison with lower-risk counterparts†. Interact Cardiovasc Thorac Surg. 2015;21(Suppl 1):761-765. doi: https://doi.org/10.1093/icvts/ivv204.146 [DOI] [PubMed] [Google Scholar]
- 170.Mery CM,Pappas AN,Bueno R,Colson YL,et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest. 2005;128(1):237-245. doi: https://doi.org/10.1378/chest.128.1.237 [DOI] [PubMed] [Google Scholar]
- 171.Zhang H,Liu C,Tan Z,Zhang T. Segmentectomy versus wedge resection for Stage I non-small cell lung cancer: a meta-analysis. J Surg Res. 2019;243:371-379. doi: https://doi.org/10.1016/j.jss.2019.05.058 [DOI] [PubMed] [Google Scholar]
- 172.Zhao M,Lu T,Huang Y,et al. Survival and long-term cause-specific mortality associated with stage IA lung adenocarcinoma after wedge resection vs. segmentectomy: a population-based propensity score matching and competing risk analysis. Front Oncol. 2019;9:593. doi: https://doi.org/10.3389/fonc.2019.00593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Smith CB,Swanson SJ,Mhango G,Wisnivesky JP. Survival after segmentectomy and wedge resection in stage I non-small-cell lung cancer. J Thorac Oncol. 2013;8(1):73-78. doi: https://doi.org/10.1097/JTO.0b013e31827451c4 [DOI] [PubMed] [Google Scholar]
- 174.Hou B,Deng XF,Zhou D,Liu QX,Dai JG. Segmentectomy versus wedge resection for the treatment of high-risk operable patients with stage I non-small cell lung cancer: a meta-analysis. Ther Adv Respir Dis. 2016;10(5):435-443. doi: https://doi.org/10.1177/1753465816667121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tsutani Y,Tsubokawa N,Ito M,et al. Postoperative complications and prognosis after lobar resection versus sublobar resection in elderly patients with clinical Stage I non-small-cell lung cancer. Eur J Cardiothorac Surg. 2018;53(2):366-371. doi: https://doi.org/10.1093/ejcts/ezx296 [DOI] [PubMed] [Google Scholar]
- 176.Zhang Z,Feng H,Zhao H,et al. Sublobar resection is associated with better perioperative outcomes in elderly patients with clinical stage I non-small cell lung cancer: a multicenter retrospective cohort study. J Thorac Dis. 2019;11(5):1838-1848. doi: https://doi.org/10.21037/jtd.2019.05.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Linden PA,D'Amico TA,Perry Y,et al. Quantifying the safety benefits of wedge resection: a society of thoracic surgery database propensity-matched analysis. Ann Thorac Surg. 2014;98(5):1705-1711. doi: https://doi.org/10.1016/j.athoracsur.2014.06.017 [DOI] [PubMed] [Google Scholar]
- 178.Razi SS,John MM,Sainathan S,Stavropoulos C. Sublobar resection is equivalent to lobectomy for T1a non-small cell lung cancer in the elderly: a surveillance, epidemiology, and end results database analysis. J Surg Res. 2016;200(2):683-689. doi: https://doi.org/10.1016/j.jss.2015.08.045 [DOI] [PubMed] [Google Scholar]
- 179.Wang W,Sun Y,Li H,et al. Surgical modality for stage IA non-small cell lung cancer among the elderly: analysis of the surveillance, epidemiology, and end results database. J Thorac Dis. 2020;12(11):6731-6742. doi: https://doi.org/10.21037/jtd-20-2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stiles BM,Mao J,Harrison S,et al. Sublobar resection for node-negative lung cancer 2-5 cm in size. Eur J Cardiothorac Surg. 2019;56(5):858-866. doi: https://doi.org/10.1093/ejcts/ezz146 [DOI] [PubMed] [Google Scholar]
- 181.Speicher PJ,Gu L,Gulack BC,et al. Sublobar resection for clinical stage IA non-small-cell lung cancer in the United States. Clin Lung Cancer. 2016;17(1):47-55. doi: https://doi.org/10.1016/j.cllc.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Subramanian M,McMurry T,Meyers BF,Puri V,Kozower BD. Long-term results for clinical stage IA lung cancer: comparing lobectomy and sublobar resection. Ann Thorac Surg. 2018;106(2):375-381. doi: https://doi.org/10.1016/j.athoracsur.2018.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mohiuddin K,Haneuse S,Sofer T,et al. Relationship between margin distance and local recurrence among patients undergoing wedge resection for small (≤2 cm) non-small cell lung cancer. J Thorac Cardiovasc Surg. 2014;147(4):1169-1175. doi: https://doi.org/10.1016/j.jtcvs.2013.11.056 [DOI] [PubMed] [Google Scholar]
- 184.Stiles BM,Mao J,Harrison S,et al. Extent of lymphadenectomy is associated with oncological efficacy of sublobar resection for lung cancer ≤2 cm. J Thorac Cardiovasc Surg. 2019;157(6):2454-2465. e2451. doi: https://doi.org/10.1016/j.jtcvs.2019.01.136 [DOI] [PubMed] [Google Scholar]
- 185.Yendamuri S,Dhillon SS,Groman A,et al. Effect of the number of lymph nodes examined on the survival of patients with stage I non-small cell lung cancer who undergo sublobar resection. J Thorac Cardiovasc Surg. 2018;156(1):394-402. doi: https://doi.org/10.1016/j.jtcvs.2018.03.113 [DOI] [PubMed] [Google Scholar]
- 186.Edwards JG,Duthie DJ,Waller DA. Lobar volume reduction surgery: a method of increasing the lung cancer resection rate in patients with emphysema. Thorax. 2001;56(10):791-795. doi: https://doi.org/10.1136/thorax.56.10.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dupuy DE,Fernando HC,Hillman S,et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. 2015;121(19):3491-3498. doi: https://doi.org/10.1002/cncr.29507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kodama H,Yamakado K,Takaki H,et al. Lung radiofrequency ablation for the treatment of unresectable recurrent non-small-cell lung cancer after surgical intervention. Cardiovasc Intervent Radiol. 2012;35:563-569. doi: https://doi.org/10.1007/s00270-011-0220-0 [DOI] [PubMed] [Google Scholar]
- 189.Huang L,Han Y,Zhao J,et al. Is radiofrequency thermal ablation a safe and effective procedure in the treatment of pulmonary malignancies? Eur J Cardiothorac Surg. 2011;3(3)9:348-351. doi: https://doi.org/10.1016/j.ejcts.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 190.Huang BY,Li XM,Song XY,et al. Long-term results of CT-guided percutaneous radiofrequency ablation of inoperable patients with stage Ia non-small cell lung cancer: a retrospective cohort study. Int J Surg. 2018;53:143-150. doi: https://doi.org/10.1016/j.ijsu.2018.03.034 [DOI] [PubMed] [Google Scholar]
- 191.Palussiere J,Lagarde P,Aupérin A,Deschamps F,Chomy F,de Baere T. Percutaneous lung thermal ablation of non-surgical clinical N0 non-small cell lung cancer: results of eight years' experience in 87 patients from two centers. Cardiovasc Intervent Radiol. 2015;38:160-166. doi: https://doi.org/10.1007/s00270-014-0999-6 [DOI] [PubMed] [Google Scholar]
- 192.Palussière J,Chomy F,Savina M,et al. Radiofrequency ablation of stage IA non-small cell lung cancer in patients ineligible for surgery: results of a prospective multicenter phase II trial. J Cardiothorac Surg. 2018;13:91. doi: https://doi.org/10.1186/s13019-018-0773-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Beland MD,Wasser EJ,Mayo-Smith WW,Dupuy DE. Primary non-small cell lung cancer: review of frequency, location, and time of recurrence after radiofrequency ablation. Radiology. 2010;254(1):301-307. doi: https://doi.org/10.1148/radiol.00000090174 [DOI] [PubMed] [Google Scholar]
- 194.Hiraki T,Gobara H,Mimura H,Matsui Y,Toyooka S,Kanazawa S. Percutaneous radiofrequency ablation of clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2011;142(1):24-30. doi: https://doi.org/10.1016/j.jtcvs.2011.02.036 [DOI] [PubMed] [Google Scholar]
- 195.Liu H,Steinke K. High-powered percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: a preliminary study. J Med Imaging Radiat Oncol. 2013;57(4):466-474. doi: https://doi.org/10.1111/1754-9485.12068 [DOI] [PubMed] [Google Scholar]
- 196.Albert RK,Connett J,Bailey WC,et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689-698. doi: https://doi.org/10.1056/NEJMoa1104623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yang X,Ye X,Zheng A,et al. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol. 2014;110(6):758-763. doi: https://doi.org/10.1002/jso.23701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lencioni R,Crocetti L,Cioni R,et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol. 2008;9(7):621-628. doi: https://doi.org/10.1016/S1470-2045(08)70155-4 [DOI] [PubMed] [Google Scholar]
- 199.Tada A,Hiraki T,Iguchi T,et al. Influence of radiofrequency ablation of lung cancer on pulmonary function. Cardiovasc Intervent Radiol. 2012;35:860-867. doi: https://doi.org/10.1007/s00270-011-0221-z [DOI] [PubMed] [Google Scholar]
- 200.Moore W,Talati R,Bhattacharji P,Bilfinger T. Five-year survival after cryoablation of stage I non-small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol. 2015;26(3):312-319. doi: https://doi.org/10.1016/j.jvir.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 201.Sabath BF,Casal RF. Bronchoscopic ablation of peripheral lung tumors. J Thorac Dis. 2019;11(6):2628-2638. doi: https://doi.org/10.21037/jtd.2019.01.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ricardi U,Filippi AR,Guarneri A,et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer. 2010;68(1):72-77. doi: https://doi.org/10.1016/j.lungcan.2009.05.007 [DOI] [PubMed] [Google Scholar]
- 203.Bezjak A,Paulus R,Gaspar LE,et al. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non-small-cell lung cancer: NRG Oncology/RTOG 0813 Trial. J Clin Oncol. 2019;37(15):1316-1325. doi: https://doi.org/10.1200/JCO.18.00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tandberg DJ,Tong BC,Ackerson BG,Kelsey CR. Surgery versus stereotactic body radiation therapy for stage I non-small cell lung cancer: a comprehensive review. Cancer. 2018;124(4):667-678. doi: https://doi.org/10.1002/cncr.31196 [DOI] [PubMed] [Google Scholar]
- 205.Timmerman R,Paulus R,Galvin J,et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070-1076. doi: https://doi.org/10.1001/jama.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tateishi Y,Takeda A,Horita N,et al. Stereotactic body radiation therapy with a high maximum dose improves local control, cancer-specific death, and overall survival in peripheral early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2021;111(1):143-151. doi: https://doi.org/10.1016/j.ijrobp.2021.04.014 [DOI] [PubMed] [Google Scholar]
- 207.Mahmood S,Bilal H,Faivre-Finn C,Shah R. Is stereotactic ablative radiotherapy equivalent to sublobar resection in high-risk surgical patients with stage I non-small-cell lung cancer? Interact Cardiovasc Thorac Surg. 2013;17(5):845-853. doi: https://doi.org/10.1093/icvts/ivt262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang H-H ,Zhang C-Z ,Zhang B-L ,et al. Sublobar resection is associated with improved outcomes over radiotherapy in the managment of high-risk elderly patients with Stage I non-small lung cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(4):6033-6042. doi: https://doi.org/10.18632/oncotarget.14010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chang JY,Senan S,Paul MA,et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630-637. doi: https://doi.org/10.1016/S1470-2045(15)70168-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fernando HC,Timmerman R. American College of Surgeons Oncology Group Z4099/Radiation Therapy Oncology Group 1021: a randomized study of sublobar resection compared with stereotactic body radiotherapy for high-risk stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2012;144(3):S35-38. doi: https://doi.org/10.1016/j.jtcvs.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lim JU,Yeo CD,Rhee CK,et al. Overall survival of driver mutation-negative non-small cell lung cancer patients with COPD under chemotherapy compared to non-COPD non-small cell lung cancer patients. Int J Chron Obstruct Pulmon Dis. 2018;13:2139-2146. doi: https://doi.org/10.2147/COPD.S167372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Izquierdo JL,Resano P,El Hachem A,Graziani D,Almonacid C,Sánchez IM. Impact of COPD in patients with lung cancer and advanced disease treated with chemotherapy and/or tyrosine kinase inhibitors. Int J Chron Obstruct Pulmon Dis. 2014;9:1053-1058. doi: https://doi.org/10.2147/COPD.S68766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Leduc C,Antoni D,Charloux A,Falcoz PE,Quoix E. Comorbidities in the management of patients with lung cancer. Eur Respir J. 2017;49(3):1601721. doi: https://doi.org/10.1183/13993003.01721-2016 [DOI] [PubMed] [Google Scholar]
- 214.Lim SM,Kim EY,Kim HR,et al. Genomic profiling of lung adenocarcinoma patients reveals therapeutic targets and confers clinical benefit when standard molecular testing is negative. Oncotarget. 2016;7(17):24172-24178. doi: https://doi.org/10.18632/oncotarget.8138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sholl LM. Biomarkers in lung adenocarcinoma: a decade of progress. Arch Pathol Lab Med. 2015;139(4):469-480. doi: https://doi.org/10.5858/arpa.2014-0128-RA [DOI] [PubMed] [Google Scholar]
- 216.Mark NM,Kargl J,Busch SE,et al. Chronic obstructive pulmonary disease alters immune cell composition and immune checkpoint inhibitor efficacy in non-small cell lung cancer. Am J Respir Crit Care Med. 2018;197(3):325-336. doi: https://doi.org/10.1164/rccm.201704-0795OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shin SH,Park HY,Im Y,et al. Improved treatment outcome of pembrolizumab in patients with nonsmall cell lung cancer and chronic obstructive pulmonary disease. Int J Cancer. 2019;145(9):2433-2439. doi: https://doi.org/10.1002/ijc.32235 [DOI] [PubMed] [Google Scholar]
- 218.Cadranel J,Canellas A,Matton L,et al. Pulmonary complications of immune checkpoint inhibitors in patients with nonsmall cell lung cancer. Eur Respir Rev. 2019;28(153):190058. doi: https://doi.org/10.1183/16000617.0058-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Borghaei H,Paz-Ares L,Horn L,et al. Nivolumab versus Docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: https://doi.org/10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]