Abstract
For optimal drug delivery, dry powder inhalers (DPIs) depend on the patient’s peak inspiratory flow (PIF) and the internal resistance of the device to create turbulent energy and disaggregate the powder. A suboptimal PIF may lead to ineffective drug inhalation into the lungs. Our objective was to report the prevalence of suboptimal PIF in patients with COPD hospitalized for any reason using 1 or more DPIs. In this real-world, observational, single‑site, retrospective study, PIF was measured for each DPI using the In-Check™ DIAL set to match the resistance of the DPI used by each patient. PIFs <60 and <30L/min were considered suboptimal for low to medium-high- and high-resistance DPIs, respectively. At initial hospitalization, the prevalence of suboptimal PIF was 44.6% in 829 patients (mean age, 71.7 years; 56.8% female); 21.2% were measured during admission for a COPD exacerbation. Suboptimal PIF percentages were 61.0% (38.1±9.5L/min [mean±standard deviation (SD)]) across low to medium-high-resistance DPIs and 17.2% (20.7±4.2L/min) for high-resistance DPIs. Overall, 190/829 patients had 1 or more 30-day all-cause readmission with 253 corresponding PIF measurements. For readmissions, suboptimal PIFs were observed in 49.5% (94/190) of patients. Suboptimal PIF percentages were 65.4% (38.4±9.2L/min) for low to medium-high-resistance DPIs and 19.8% (22.4±3.3L/min) for high-resistance DPIs. As the overall prevalence of suboptimal PIFs in hospitalized patients with COPD varied according to the specific internal resistance of the DPI, these findings may have clinical implications for inhaler selection.
Keywords: chronic obstructive pulmonary disease, dry powder inhaler, peak inspiratory flow
Introduction
This article contains supplemental material.
Inhaled bronchodilators are the mainstay of pharmacological management of chronic obstructive pulmonary disease (COPD) and are delivered using metered-dose inhalers, dry powder inhalers (DPIs), the soft mist inhaler, and nebulizers.1,2 Successful delivery of medication to the lungs is dependent upon the drug formulation, delivery system, and various patient‑related factors.3 One important patient factor is correct inhalation flow,2 which is particularly critical when using DPIs because of their structural design and formulation characteristics. With DPIs, patients’ inspiratory flow and the resistance within the DPI are important for the creation of turbulent energy, which is needed for drug-carrier particle disaggregation, optimal drug release, and drug delivery into the airways.4,5 Therefore, a low inspiratory flow may result in poor drug-carrier particle disaggregation within the DPI and suboptimal drug delivery into the lungs. Hard and fast inhalation is generally recommended for the optimal delivery of medications with DPIs.6
Peak inspiratory flow (PIF) is the maximal airflow generated during an inspiratory cycle.5 Although minimal and optimal PIFs depend on the specific DPI, a PIF of ≥60L/min is generally considered optimal for low to medium-high-resistance DPIs,5,7-15 whereas a PIF of ≥30L/min is generally considered optimal for high-resistance DPIs.3,15 However, some patients with COPD may not be able to achieve the optimal PIF to derive optimal drug delivery from their prescribed DPI.4,16
The prevalence of suboptimal PIFs among patients with COPD has been reported in cohorts of stable outpatients with COPD (19%–100%) and in patients hospitalized for an acute exacerbation of COPD (AECOPD; 32%–52%).16-24 However, in these in-patient studies, PIF was measured only against 1 resistance, regardless of the DPI used by the patient.16 Furthermore, data are lacking for broad populations of patients with COPD in real-world settings.
The objective of this observational real-world study was to report the prevalence of suboptimal PIF measured against the simulated resistance of the DPI(s) used prior to admission in hospitalized patients with COPD. Testing was performed at a single medical center with patients admitted for an exacerbation or for a non‑exacerbation diagnosis when clinically stable. Furthermore, PIF was measured in this cohort of patients who had a subsequent 30-day all-cause readmission.
Methods
Study Design and Patients
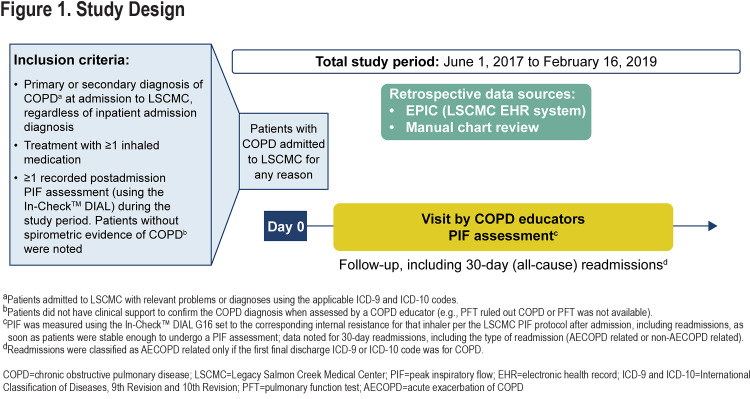
This observational study was conducted using data from patients diagnosed with COPD admitted to Legacy Salmon Creek Medical Center (LSCMC) for all-cause admission from June 1, 2017, to February 16, 2019 (Figure 1). LSCMC (Vancouver, Washington) serves the southwest Washington state area. A retrospective chart review and collection of patient health information and other data from Epic (the LSCMC electronic health record system) were approved by the Legacy Health Institutional Review Board (#FWA00001280). The study was conducted in accordance with the principles of the Declaration of Helsinki,25 the International Council for Harmonisation Good Clinical Practice guidelines,26 and applicable regulatory requirements.
Patients aged ≥40 years with an International Classification of Diseases, 9th Revision (ICD-9) or 10th Revision (ICD-10) code corresponding to a primary or secondary diagnosis of COPD at admission, using ≥1 DPI at the time of admission, and having ≥1 postadmission PIF reading during the study period, were included.
Peak Inspiratory Flow Assessments and Outcomes
PIFs were measured 7 days/week and for 8 hours/day as part of a quality improvement project at LSCMC to optimize therapy for patients admitted with a COPD exacerbation. One measure of PIF was taken during the session. PIFs were measured using the In-Check™ DIAL G16 (Alliance Tech Medical Granbury, Texas)27 following standard instructions28 set to match the resistance of the DPI(s) being used at admission, including readmissions, namely, Neohaler® (low); Diskus®, Ellipta® (medium-low); RespiClick®, Aerolizer®, Flexhaler®, Pressair® (medium); Twisthaler® (medium-high); or HandiHaler® (high) as soon as the patients were clinically stable, as assessed by a COPD educator (Supplementary Table S1 in the online supplement). If a patient had been using more than 1 DPI, PIF was measured against the simulated resistance of each DPI.
Therefore, the number of PIFs available for analyses was greater than the number of patients. A PIF of <60 and <30L/min was considered suboptimal for low to medium-high resistance DPIs and the high-resistance DPI, respectively.5,7-15 Additional information on PIF assessment is presented in Supplementary Information and Supplementary Figure S1 in the online supplement.
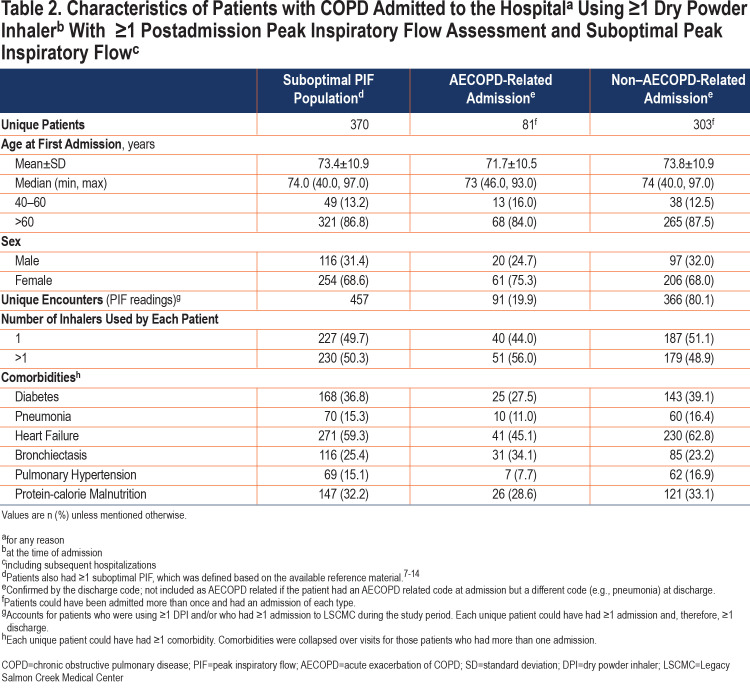
The primary outcome was the prevalence of suboptimal PIFs in the overall study population and in those readmitted within 30 days of discharge. Suboptimal PIF values were stratified by the DPI internal resistance category. Patient demographics and clinical characteristics included age at first encounter, sex, type of admission (AECOPD related or non-AECOPD related), and the most commonly observed comorbidities in patients with COPD admitted to LSCMC (i.e., diabetes, pneumonia, heart failure, bronchiectasis, pulmonary hypertension, and protein-calorie malnutrition). If a patient had >1 admission during the study period, their age at first admission was used in analyses. Exploratory analyses included the percentage of patients with more than or equal to one 30-day readmission stratified by the PIF category (suboptimal or optimal), DPI internal resistance category, and type of readmission.
Data Abstraction and Analysis
De-identified data (PIF readings and corresponding patient information) were abstracted from Epic. Comorbidities and 30-day readmission data were abstracted manually from Epic by study personnel. Readmissions were classified as AECOPD related only if COPD was listed as the primary encounter diagnosis (ICD-9 or ICD-10 code). Otherwise, readmissions were classified as non-AECOPD related. Because of the descriptive nature of the study, no statistical hypothesis testing or modeling was planned.
Results
Patient Disposition
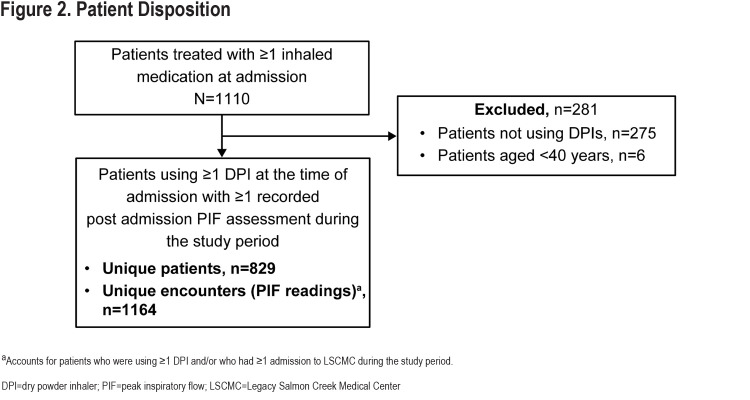
Overall, 829 patients were admitted to LSCMC for any reason, had COPD as a primary or secondary diagnosis at admission, and met the inclusion criteria during the study period. A total of 1164 corresponding PIF readings were obtained (Figure 2).
Cohort and Dataset Characteristics (Overall Population)
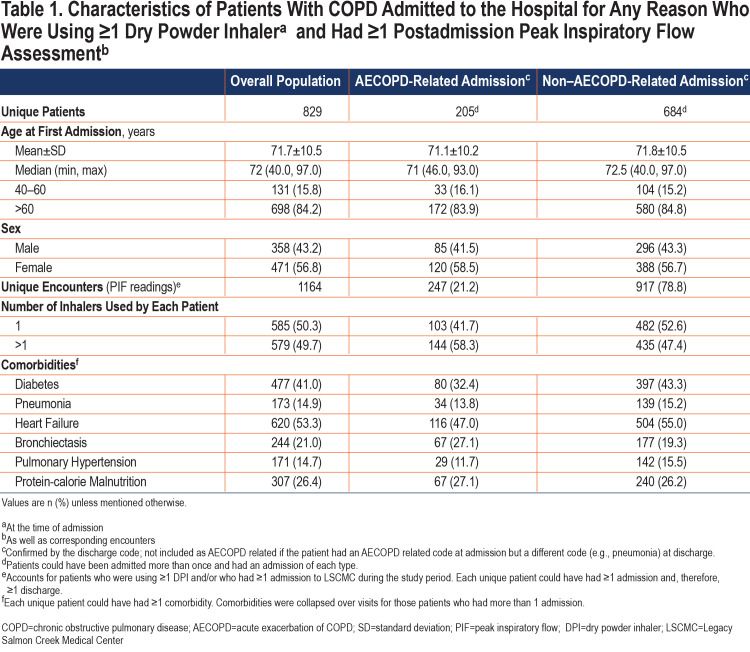
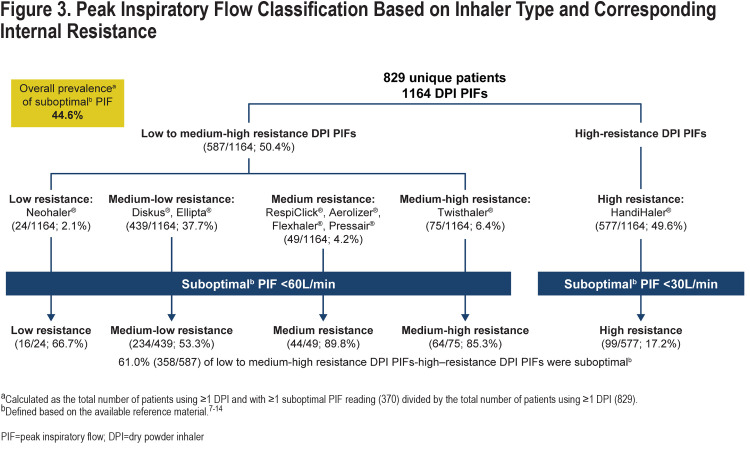
Age at first encounter (mean±standard deviation [SD]) of the 829 patients included in the study was 71.7±10.5 years. The overall study population consisted of more women than men (56.8% versus 43.2%) (Table 1). Most of the corresponding 1164 PIF readings (collected over the duration of the study) were measured against medium-low resistance (Diskus®, Ellipta®; 439 [37.7%]) or high resistance (HandiHaler®; 577 [49.6%]) DPIs (Figure 3) and during non–AECOPD-related admissions (917 [78.8%]; Table 1). Heart failure (53.3%) and diabetes (41.0%) were the most common comorbidities among those evaluated and were higher in the non-AECOPD group than in the AECOPD group (Table 1).
Suboptimal Peak Inspiratory Flow Prevalence
The prevalence of suboptimal PIF was 44.6% (370/829) in the overall study population (Figure 3). The prevalence of suboptimal PIF in patients who never had an AECOPD-related admission was 42.5% (265/624) and for patients who had 1 or more AECOPD-related admissions was 51.2% (105/205). Percentages (mean±SD L/min) of suboptimal PIF by inhaler resistance category were 61.0% (PIF: 38.1±9.5L/min) against low to medium-high resistance and 17.2% (PIF: 20.7±4.2L/min) against high resistance.
Characteristics of the Suboptimal Peak Inspiratory Flow Population
Age at first encounter of the 370 patients with 1 or more suboptimal PIF readings was 73.4±10.9 years. This group included 68.6% of women and 31.4% of men (Table 2). Similar to observations from the overall population, most of the 457 corresponding PIF recordings were measured against medium-low resistance (Diskus®, Ellipta®; 234) or high resistance (HandiHaler®; 99) (Figure 3) DPIs and during non–AECOPD-related admissions (366 [80.1%]) (Table 2). Heart failure (59.3%) and diabetes (36.8%) were the most common comorbidities among those evaluated.
Thirty-Day All-Cause Readmissions
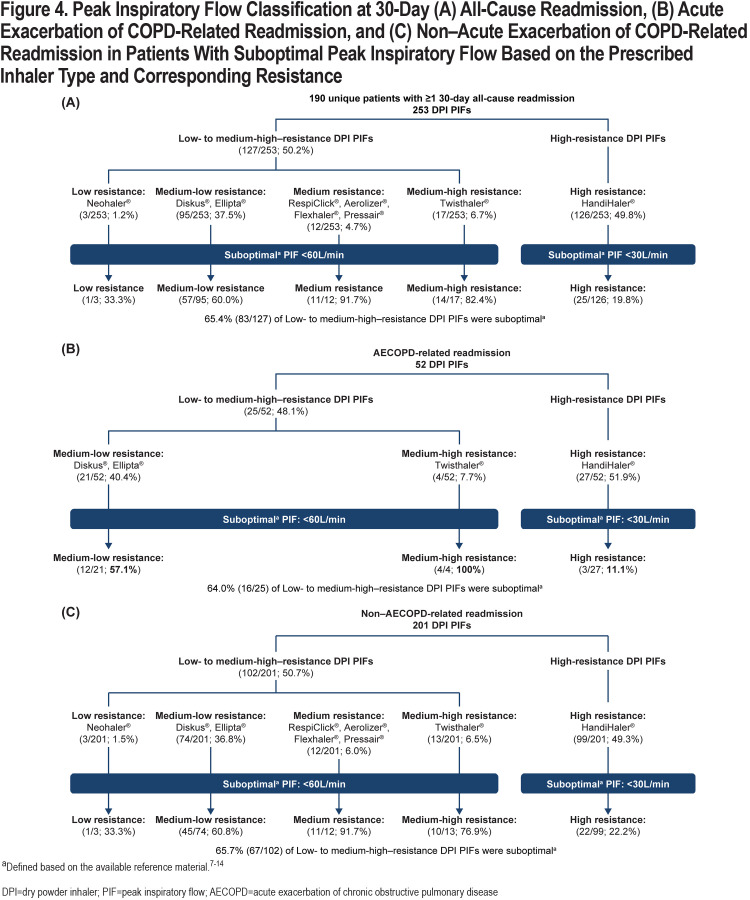
Overall, 190 of 829 (22.9%) patients had 1 or more 30-day all-cause readmissions, with 253 corresponding PIF readings taken during readmissions. Notably, 41 patients had 49 AECOPD-related readmissions, with 52 corresponding PIF readings; 160 patients had 192 non–AECOPD-related readmissions, with 201 corresponding PIF readings (patients could have >1 readmission with a different cause each time; therefore, the number of patients is >190 [41+160]). Of the 190 unique patients who had 1 or more 30-day readmissions, 94 had ≥1 suboptimal PIF reading during the readmission. The prevalence of suboptimal PIF in patients with 30-day all-cause readmissions was 49.5%. Percentages (mean±SD) of suboptimal PIFs at the time of readmission were 65.4% (38.4±9.2L/min) against low to medium-high resistance and 19.8% (22.4±3.3L/min) against high-resistance DPIs (Figure 4A). Of the 52 PIFs recorded during AECOPD-related readmissions, 25 and 27 were suboptimal against low to medium-high-resistance and high-resistance DPIs, respectively (Figure 4B). Of the 201 PIFs assessed during non–AECOPD-related readmissions, 102 and 99 were suboptimal against low to medium-high-resistance and high-resistance DPIs, respectively (Figure 4C).
Discussion
To our knowledge, these data represent the largest real-world study in which PIF was measured against a full range of simulated internal DPI resistance levels in hospitalized patients with COPD. The major findings are that: (1) the overall prevalence of suboptimal PIF was 44.6% among 829 DPI users, (2) most (80.1%) of the suboptimal PIFs were measured during non–AECOPD-related admissions, and (3) the prevalence of suboptimal PIF was 49.5% among 190 patients with 1 or more 30‑day all-cause readmissions.
The results of our study provide detailed demographic and clinical characteristics of patients with COPD as well as PIF values that correspond to each DPI’s internal resistance category (Table 1 and Table 2). The overall study population consisted of more women than men (56.8% versus 43.2%), and this difference was more pronounced in those with a suboptimal PIF (women: 68.6% versus men: 31.4%). This observation is in line with previously reported studies in which female patients with COPD were more likely to have suboptimal PIFs than male patients with COPD.16,23 This can be explained, in part, by lower predicted values for lung function in women than men.29
Our study incorporated 2 unique features. First, PIFs were measured in patients with COPD regardless of the cause of hospital admission. Second, PIFs were measured against the simulated resistance of the DPI(s) that patients were actually using at the time of admission. In previous studies of patients hospitalized for an exacerbation of COPD, there was a range of 32% to 100% for the prevalence of a suboptimal PIF prior to discharge.16,22,24,30,31 The variability in the prevalence of suboptimal PIF may reflect differences in patient populations, as well as differences in methodology, such as the time that PIF was measured relative to discharge and/or the specific instructions given to patients to perform the PIF maneuver.
A unique feature of our study was measurement of PIF in patients readmitted to the hospital within 30 days of their initial enrollment. Overall, 190 of 829 (22.9%) patients had 1 or more 30-day all-cause readmissions, with 253 corresponding PIF readings taken during the readmission. The prevalence of suboptimal PIF was 49.5% among these patients with 1 or more 30-day all-cause readmissions. Our observational study was not designed to identify hospital readmissions in patients based on their initial PIF status.
The findings of this study have clinical implications for both health care professionals (HCPs) and patients with COPD. When prescribing inhaled therapy, HCPs select a medication(s) as well as the delivery system based on individual patient factors. However, an inhaled medication(s) in a DPI may not provide the intended efficacy in patients with a suboptimal PIF. In 2 prospective clinical trials, in which an inclusion criterion was a suboptimal PIF, there were greater increases in lung function with nebulized therapy compared with a similar class of bronchodilator delivered via a DPI.32,33 These findings suggest that patients with a PIF <60L/min against a medium-low-resistance DPI may not achieve optimal inhalation of the powder medication into the lower respiratory tract. An in vitro study by Borgström and colleagues34 supports this hypothesis. These investigators showed that deposition of radiolabeled budesonide within the Turbuhaler® DPI (Astra Draco AB) nearly doubled (from 15% to 28%) when inspiratory flow was increased from 36 to 58L/min.33
For optimal use of a DPI, the patient needs to inhale “hard and fast” to create turbulent energy within the device. PIF can be measured to assess the patient’s inspiratory ability to disaggregate the powder into fine particles that can be inhaled deep into the lungs. PIF is considered as a predictive therapeutic biomarker to determine whether the patient is likely, or unlikely, to achieve optimal drug delivery when using a DPI and obtain clinical benefit.35 The optimal/suboptimal thresholds for PIF are generally based on in vitro studies using lung models to assess dry powder drug delivery. Although information is limited on clinical correlations with a suboptimal PIF, Mahler and colleagues36 reported that 184 patients with COPD with a suboptimal PIF had a significantly greater symptom burden as measured on the COPD Assessment Test and had significantly more shortness of breath on the modified Medical Research Council scale compared with 219 patients who had an optimal PIF.
The impact of a suboptimal PIF on hospital readmissions is an important consideration for development of discharge pathways/programs aimed to reduce readmission rates. Three studies evaluated readmissions in patients with COPD hospitalized for an exacerbation and had a suboptimal PIF prior to discharge.16,22,30 Loh and colleagues22 demonstrated a higher rate of 90-day COPD readmissions and fewer number of days before all-cause readmissions in 64 patients with a suboptimal PIF compared with 59 who had an optimal PIF. In contrast, Samarghandi et al30 and Sharma et al16 found similar readmission rates up to 90 days and 180 days, respectively, between suboptimal and optimal PIF groups. However, in a pay-for-performance management program involving 383 outpatients with COPD in Taiwan, Chen and colleagues37 reported a significant reduction in severe exacerbations among patients in whom inhaled therapy was guided by PIF measurement using the In-Check™ DIAL G16 compared with any previous inhaler education provided to patients before the PIF-guided approach was instituted.
There are several limitations of this study. First, the study was performed at a single site and the results may be influenced by prescribing behaviors and may not be generalizable to other institutions. Second, PIF was measured when patients were considered stable enough by a COPD educator to perform the maneuver and not at a specific time period prior to discharge as in some other studies. Third, although the class of medication prescribed at discharge was available, the type of inhaler used to deliver the medication and whether patients obtained and/or used their prescribed medication after discharge were not documented. Fourth, PIF values were grouped for low to medium-high DPI resistances because a suboptimal PIF threshold <60L/min applies to these different internal resistances. This clustering may have introduced a bias as factors such as disease severity were not included in the analysis. Finally, the study was descriptive by design; therefore, analytical comparisons across internal inhaler resistance categories were not conducted.
The strengths of this retrospective observational study include the large, real-world patient population and corresponding number of PIF readings. The overall prevalence of suboptimal PIF was 44.6% in patients with COPD who were hospitalized for any cause and were using at least 1 DPI at admission. These findings may have implications for inhaler selection as a DPI may not provide the expected clinical efficacy in a patient with suboptimal PIF. Other patient factors, including cognitive function and manual dexterity, should also be considered by HCPs when selecting the right medication in the right delivery device for an individual patient with COPD.
Abbreviations
Abbreviations: dry powder inhaler, DPIs; peak inspiratory flow, PIF; standard deviation, SD; chronic obstructive pulmonary disease, COPD; acute exacerbation of COPD, AECOPD; Legacy Salmon Creek Medical Center, LSCMC; electronic health record, EHR; International Classification of Diseases, 9th and 10th Revisions, ICD-9, ICD-10; health care professionals, HCPs
Funding Statement
This study was supported by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property consideration.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, 2021 report. GOLD website. Published 2021. Accessed December 4, 2020. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf [Google Scholar]
- 2.Bonini M Usmani OS. The importance of inhaler devices in the treatment of COPD. COPD Res Pract. 2015;1:9. doi: https://doi.org/10.1186/s40749-015-0011-0 [Google Scholar]
- 3.Ibrahim M Verma R Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices (Auckl). 2015;8:131-139. doi: https://doi.org/10.2147/MDER.S48888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahler DA. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(7):1103-1107. doi: https://doi.org/10.1513/AnnalsATS.201702-156PS [DOI] [PubMed] [Google Scholar]
- 5.Ghosh S Ohar JA Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30(6):381-387. doi: https://doi.org/10.1089/jamp.2017.1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahler DA. The role of inspiratory flow in selection and use of inhaled therapy for patients with chronic obstructive pulmonary disease. Respir Med. 2020;161:105857. doi: https://doi.org/10.1016/j.rmed.2019.105857 [DOI] [PubMed] [Google Scholar]
- 7.Advair Diskus . [package insert]. Research Triangle Park, NC: GlaxoSmithKline. 2020. Accessed May 13, 2021. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Advair_Diskus/pdf/ADVAIR-DISKUS-PI-PIL-IFU.PDF [Google Scholar]
- 8.Anoro Ellipta. [package insert]. Research Triangle Park, NC: GlaxoSmithKline. 2020. Accessed May 13, 2021. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Anoro_Ellipta/pdf/ANORO-ELLIPTA-PI-PIL-IFU.PDF [Google Scholar]
- 9.Trelegy Ellipta. [package insert]. Research Triangle Park, NC: GlaxoSmithKline. 2019. Accessed May 13, 2021. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Trelegy/pdf/TRELEGY-ELLIPTA-PI-PIL-IFU.PDF [Google Scholar]
- 10.Airduo® Respiclick®. [package insert]. Teva Respiratory, LLC. 2020. Accessed May 13, 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9c122b91-a11f-49ca-80bb-3eec5112db87 [Google Scholar]
- 11.Foradil® Aerolizer®. [package insert]. Novartis Pharma AG. 2012. Accessed May 13, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020831s028lbl.pdf [Google Scholar]
- 12.Pulmicort Flexhaler™. [package insert]. Wilmington, DE: AstraZeneca LP. 2010. Accessed May 13, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021949s006lbl.pdf [Google Scholar]
- 13.Tudorza® Pressair®. [package insert]. Circassia Pharmaceuticals Inc. 2019. Accessed May 13, 2021. https://www.dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4cefdf4c 9e01-4b98-804b- c520f1b9716e [Google Scholar]
- 14.Asmanex® Twisthaler®. [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; . Published December 2019. https://www.merck.com/product/usa/pi_circulars/a/asmanex/asmanex_pi.pdf [Google Scholar]
- 15.Ghosh S Pleasants RA Ohar JA et al. Prevalence and factors associated with suboptimal peak inspiratory flow rates in COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:585-595. doi: https://doi.org/10.2147/COPD.S195438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma G Mahler DA Mayorga VM et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217-224. doi: https://doi.org/10.15326/jcopdf.4.3.2017.0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Showair RAM Tarsin WY Assi KH et al. Can all patients with COPD use the correct inhalation flow with all inhalers and does training help? . 2007;101(11):2395-2401. doi: https://doi.org/10.1016/j.rmed.2007.06.008 [DOI] [PubMed] [Google Scholar]
- 18.Azouz W Chetcuti P Hosker H et al. Inhalation characteristics of asthma patients, COPD patients and healthy volunteers with the Spiromax® and Turbuhaler® devices: a randomised, cross-over study. BMC Pulm Med. 2015;15:47. doi: https://doi.org/10.1186/s12890-015-0043-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chodosh S Flanders JS Kesten S et al. Effective delivery of particles with the HandiHaler dry powder inhalation system over a range of chronic obstructive pulmonary disease severity. J Aerosol Med. 2001;14(3):309-315. doi: https://doi.org/10.1089/089426801316970268 [DOI] [PubMed] [Google Scholar]
- 20.Duarte AG Tung L Zhang W et al. Spirometry measurement of peak inspiratory flow identifies suboptimal use of dry powder inhalers in ambulatory patients with COPD. Chronic Obstr Pulm Dis. 2019;6(3):246-255. doi: https://doi.org/10.15326/jcopdf.6.3.2018.0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssens W VandenBrande P Hardeman E et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78-83. doi: https://doi.org/10.1183/09031936.00024807 [DOI] [PubMed] [Google Scholar]
- 22.Loh CH Peters SP Lovings TM et al. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all-cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305-1311. doi: https://doi.org/10.1513/AnnalsATS.201611-903OC [DOI] [PubMed] [Google Scholar]
- 23.Mahler DA Waterman LA Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174-179. doi: https://doi.org/10.1089/jamp.2012.0987 [DOI] [PubMed] [Google Scholar]
- 24.Broeders MEAC Molema J Hop WCJ et al. The course of inhalation profiles during an exacerbation of obstructive lung disease. Respir Med. 2004;98(12):1173-1179. doi: https://doi.org/10.1016/j.rmed.2004.04.010 [DOI] [PubMed] [Google Scholar]
- 25.World Medical Association (WMA). World Medical Association Declaration of Helsinki - Ethical principles for medical research involving human subjects. WMA website. Published July 9, 2018. Accessed May 13, 2021. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ [Google Scholar]
- 26.United States Food and Drug Administration (FDA). E6(R2) good clinical practice: integrated addendum to ICH E6(R1): guidance for industry. FDA website. Published March 2018. Accessed May 13, 2021. https://www.fda.gov/media/93884/download [Google Scholar]
- 27.Alliance Tech Medical. InCheck™ DIAL G16 Inhaler Technique Training and Assessment Tool. Alliance Tech Medical website. Published 2016. Accessed May 13, 2021. https://alliancetechmedical.com/check-dial-training-device/ [Google Scholar]
- 28.Sanders MJ. Guiding inspiratory flow: development of the In-Check DIAL G16, a tool for improving inhaler technique. Pulm Med. 2017;2017:1495867. doi: https://doi.org/10.1155/2017/1495867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma G Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1(3):253-260. https://www.dovepress.com/effect-of-aging-on-respiratory-system-physiology-and-immunology-peer-reviewed-fulltext-article-CIA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samarghandi A Ioachimescu OC Qayyum R. Association between peak inspiratory flow rate and hand grip muscle strength in hospitalized patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS One. 2020;15(1):e0227737. doi: https://doi.org/10.1371/journal.pone.0227737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harb HS Laz NI Rabea H Abdelrahim MEA. Prevalence and predictors of suboptimal peak inspiratory flow rate in COPD patients. Eur J Pharm Sci. 2020;147:105298. doi: https://doi.org/10.1016/j.ejps.2020.105298 [DOI] [PubMed] [Google Scholar]
- 32.Mahler DA Ohar JA Barnes CN et al. Nebulized versus dry powder long-acting muscarinic antagonist bronchodilators in patients with COPD and suboptimal peak inspiratory flow rate. Chronic Obstr Pulm Dis. 2019;6(4):321-331. doi: https://doi.org/10.15326/jcopdf.6.4.2019.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahler DA Waterman LA Ward J Gifford AH. Comparison of dry powder versus nebulized beta-agonist in patients with COPD who have suboptimal peak inspiratory flow rate. J Aerosol Med Pulm Drug Deliv. 2014;27(2):103-109. doi: https://doi.org/10.1089/jamp.2013.1038 [DOI] [PubMed] [Google Scholar]
- 34.Borgström L Bondesson E Morén F et al. Lung deposition of budesonide inhaled via Turbuhaler: a comparison with terbutaline sulphate in normal subjects. Eur Respir J. 1994;7(1):69-73. doi: https://doi.org/10.1183/09031936.94.07010069 [DOI] [PubMed] [Google Scholar]
- 35.Mahler DA Halpin DMG. Peak inspiratory flow as a predictive therapeutic biomarker in COPD. Chest. 2021;160(2):491-498. doi: https://doi.org/10.1016/j.chest.2021.03.049 [DOI] [PubMed] [Google Scholar]
- 36.Mahler DA Niu X Deering KL Dembek C. Prospective evaluation of exacerbations associated with suboptimal peak inspiratory flow among stable outpatients with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:559-568. doi: https://doi.org/10.2147/COPD.S353441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen SY Huang CK Peng HC et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12:704316. doi: https://doi.org/10.3389/fphar.2021.704316 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplemental material.