Abstract
Background: Eosinophils are involved in the development of chronic obstructive pulmonary disease (COPD) and inhaled corticosteroid responsiveness. We evaluated clinical predictors of high sputum eosinophil levels in a COPD cohort in China.
Methods: We conducted an observational, prospective, population-based, cross-sectional study. Participants were tested for COPD and underwent spirometry, computed tomography scans, and a blood test. Participants also produced induced sputum and responded to an information-gathering questionnaire. High sputum eosinophils were defined as ≥3.0%. Multivariate logistic regression was used to identify predictors of high sputum eosinophil levels.
Results: We recruited 895 patients with complete and quality control data. The median percentage of sputum eosinophil abundance was 2.00% (interquartile range: 0.75–5.00) and the prevalence of COPD with high sputum eosinophils was 38.0%. Covariance analysis indicated that the high sputum eosinophil group had lower lung function, more severe emphysema, and air trapping. Multivariate logistic regression indicated that high blood eosinophil levels, severe respiratory symptoms, being a former smoker, and a family history of respiratory diseases were associated with high sputum eosinophil levels.
Conclusion: High blood eosinophil levels, severe respiratory symptoms, being a former smoker, and a family history of respiratory diseases may be predictors of high sputum eosinophil levels in Chinese COPD patients. High sputum eosinophils were associated with lower lung function, more emphysema, and gas trapping.
Keywords: copd, chronic obstructive pulmonary disease, high sputum eosinophils
Introduction
This article contains supplemental material.
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation and respiratory symptoms caused by airway or alveolar abnormalities induced by smoking or exposure to noxious particles.1,2 COPD has become a global public health challenge because of its high prevalence and associated disability and mortality.1,3 The multiplicity of risk factors involved results in heterogeneity of clinical and pathophysiological traits,4-6 underscoring the importance of individualized management and precision therapy.5
Eosinophilic airway inflammation is typically observed in asthma and not COPD patients, but studies have shown that the eosinophilic inflammation phenotype is also present in the airways of COPD patients.6,7 Importantly, these patients respond well to inhaled corticosteroid (ICS) treatment.7,8 Eosinophilic inflammation in COPD patients is a good biomarker of ICS responsiveness,6,9 and eosinophil levels provide guidance in the use of ICSs, especially in the prevention of some exacerbations.9 ICS trials have suggested that the threshold of a blood eosinophil count ≥300cells/µL identifies the top of the continuous relationship with ICSs,7,8,10 and severe exacerbations have been reported to be reduced by lowering eosinophil levels.11 Increases in sputum eosinophils have been related to improvements in lung function after short-term prednisone treatment.12 Therefore, it is important to identify patients who would benefit most from target cortical steroid therapy by eosinophilic airway inflammation. However, previous studies on eosinophilic airway inflammation are mostly hospital-derived patients, which are affected by diseases or drugs and lack of community-based data,13 and the prevalence and clinical characteristics of eosinophilic inflammation in Chinese COPD patients are still unclear. We, therefore, conducted a cross-sectional study of clinical predictors of high sputum levels of eosinophils. This study provided evidences from community-based patients.
Materials and Methods
Study Design
Our participants were mainly recruited from the population-based cohort study of patients with chronic airway diseases (ChiCTR 1900024643) conducted in the Guangzhou, Shao Guan, and He Yuan communities of Guangdong Province, China, between July 2019 and December 2020.14 Participants aged 40–80 years were recruited, and patients with other respiratory-related diseases, such as asthma, active tuberculosis, bronchiectasis, lung cancer, and pulmonary fibrosis, were strictly excluded. All participants were tested for COPD using an epidemiological questionnaire, spirometry, chest computed tomography (CT), blood parameters, and induced sputum. This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2018-53), and written informed consent was obtained from all patients.
Epidemiological Questionnaire
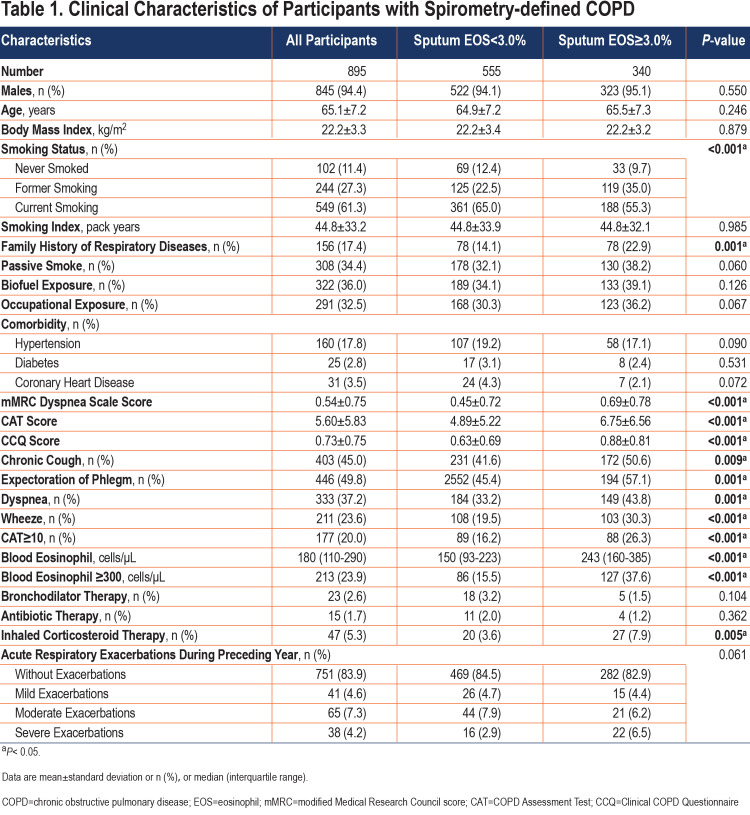
The questionnaire used in this study was revised from the International Chronic Obstructive Pulmonary Epidemiology Research Questionnaire15,16 and the Chinese Chronic Obstructive Pulmonary Epidemiology Research Questionnaire.17 The technicians who were responsible for administering this questionnaire had been strictly trained and also passed a training test. The information collected mainly included demographic variables, respiratory symptoms/disease history, comorbidities, smoking status and other potential risk factors for COPD, the modified Medical Research Council score, the COPD Assessment Test score,18 the Clinical COPD Questionnaire score, and drug therapy. Smoking status was described as “never-smoker,” “former smoker,” or “current –smoker.” Participants who had smoked for at least 6 months or had smoked at least 100 cigarettes in their lifetime were defined as “current smokers”; otherwise, they were considered “never-smokers.” “Former smoker” was defined as an individual who had quit smoking for at least 6 months.19 Smoking index was calculated by multiplying regular smoking years by daily smoking packs. Family history of respiratory disease was defined as any parent or sibling diagnosed with chronic bronchitis, emphysema, asthma, or COPD. Clinical COPD Assessment Test scores ≥10 points were considered high-symptom categories.20,21 History of biofuel exposure was defined as using firewood or coal for cooking purposes for more than 1 year. Occupational exposure to dust, fumes, or gases was defined as an exposure lasting more than 1 year. Passive smoking was defined as exposure to cigarette smoke at home or at work for more than 1 year.22 The criteria of ICS therapy were defined as a patient who had a long-term regular therapy history of ICS or ICS+ long-acting muscarinic antagonist (LAMA)/ long-acting beta2-agonist (LABA) combo at the time of enrollment or during the preceding year. A high level of blood eosinophil counts was defined as those over 300cells/µL. Chronic cough or chronic phlegm was defined as cough or phlegm not occurring during a “cold” and on most days for as much as 3 months each year for 2 years. Wheezing was the presence of “episodes of wheezing or whistling in the chest associated with a feeling of shortness of breath, in the past 1 year not occurring during a cold.” Dyspnea was defined as “troubled by shortness of breath when hurrying on the level or walking up a slight hill.” Acute exacerbation was defined as worsening of at least 2 major symptoms (cough, sputum purulence, sputum volume, dyspnea, or wheezing) that persisted for at least 48 hours, after excluding other diseases (ventricular dysfunction, pulmonary embolism, pneumothorax, pleural effusion, and arrhythmia).23,24 A mild event was one that resulted in domiciliary management with COPD medications alone. A moderate event was one that resulted in an outpatient or emergency department visit and the modification of regimen, including antibiotic agents, oral glucocorticoids, or both. A severe event was one that resulted in hospitalization.24
Spirometry
Portable spirometers (Master Screen Pneumo PC spirometer; Care Fusion, Yorba Linda, California ) were used by trained and certified technicians. The method of operation followed spirometry guidelines set by the American Thoracic Society and European Respiratory Society.25 Quality control checks for the measurement results were based on criteria from these entities and conducted by an expert panel of physicians and senior technicians. After spirometry testing, participants were instructed to inhale 400mL of salbutamol from 500-mL storage tanks. After 20 min, the pulmonary ventilation tests were repeated. Participants with COPD were defined as those having a forced expiratory volume in 1 second (FEV1) to forced vital capacity ratio (FVC) <0.7 after bronchodilator use, and COPD was classified by the percentage of predicted FEV1. A positive bronchial reversibility test was defined26 as an increase of 12% in FEV1 after inhalation of bronchodilator and improvement in absolute value of more than 200mL.
Imaging
Detailed CT protocols adopted in the study followed the COPD Genetic Epidemiology study.27 Quantitative analysis of emphysema severity and gas trapping was performed on segmented images using software programs (3D Slicer).28 Low-attenuation area of the lung with attenuation values below −950 Hounsfield units (LAA−950) in inspiratory was used as a quantitative evaluation index of emphysema.29 Low-attenuation area of the lung with attenuation values below −856 Hounsfield units (LAA−856) in expiratory was used as a quantitative evaluation index of air trapping. We converted data of emphysema and air trapping into natural logarithm (Ln) because those CT variables were not normally distributed. Covariance analysis was used to determine associations between high sputum eosinophils with emphysema and gas trapping.
Peripheral Blood
Peripheral blood was collected by well-trained staff using an ethylenediamine tetraacetic anticoagulant tube to collect 3mL of blood and then gently inverted 3–5 times. The collected blood samples were stored at room temperature for at least 30 min, and then sent to the clinical laboratory to complete the blood routine indicators.
Induced Sputum
Induced sputum examination and processing were performed by professional personnel in accordance with a published method.30,31 Each participant inhaled nebulized saline (3%) in 3 sequential 7-min inhalation periods from an ultrasonic nebulizer with an output of 1.5mL/min. Staff patted each participant’s back at intervals to promote expectoration. Participants were then asked to rinse their mouths and throats and expectorate sputum into a Petri dish, and the quality of sputum was evaluated under light microscopy at 10× magnification. The standard for qualified sputum specimens was that the percentage of squamous cells in the cell count classification32 was <20%. Qualified sputum plugs were then selected and weighed. Sputum was processed within 3 hours of sampling for optimum cell viability. A volume of phosphate-buffered solution containing 0.1% dithiothreitol was used to digest the sputum. Portions were agitated by vortexing, placed on ice for 15 minutes, rocked on a bench rocker for 5 minutes, filtered through 48-mm nylon mesh into a conical tube, and centrifuged at 3000 rpm and 4°C for 10 minutes. The sputum supernatant was discarded. The cell pellet was resuspended in 1000 μL of phosphate-buffered saline. Thirty microliters of cell suspension were used to make a cell smear, and sputum cells were stained with hematoxylin and eosin. On average, 2 slides were prepared for each patient. The slides were placed in an oven at 65°C for 20 minutes and then sealed with resin. At least 400 non-squamous cells were counted under light microscopy, including neutrophils, eosinophils, macrophages, lymphocytes, and epithelial cells. The absolute count and percentages of each cell type were recorded. For airway inflammatory phenotypes, ≥3.0% of sputum eosinophils were considered to represent high eosinophil airway inflammation33 and participants were divided into 2 groups using this cut-off point.
Statistical Analysis
All data were analyzed using SPSS version 26 (IBM SPSS, Armonk, New York). Continuous variables are presented as mean ± standard deviation or median (interquartile range [IQR]), and categorical variables are expressed as frequencies and percentages. The difference between cohorts for each variable was evaluated using Student’s t-test or the Mann-Whitney U test for continuous variables and the Chi-squared test or Fisher’s exact test for categorical variables. Covariance analysis was used to calculate emphysema and air trapping parameter differences between the 2 groups and adjusted for potential confounders. Multivariate logistic regression was used to determine predictors of high sputum eosinophils, and odds ratios (ORs) were calculated after adjustment for age, sex, body mass index, smoking status, pack years of smoking, family history of respiratory diseases, biofuel exposure, exposure to occupational irritants, passive smoke, the history of inhaled corticosteroid therapy, level of blood eosinophil, and respiratory symptoms. We used variance inflation factor to test the collinearity between variables. Variables with a p value <0.05 were considered statistically significant.
Results
Participant Demographics
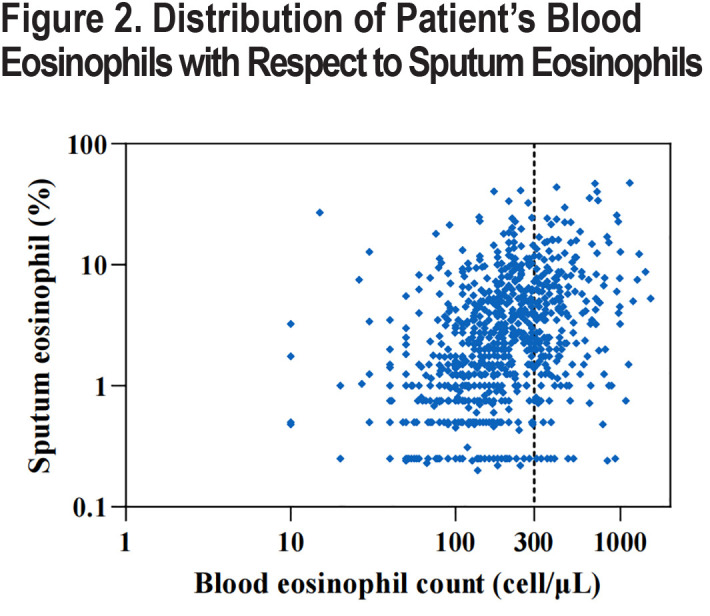
We included a total of 895 spirometry-defined COPD patients with complete data and qualified pulmonary function quality control, with a mean age of 65.1 years (standard deviation: 7.2) years in our study. A total of 845 (94.4%) of the patients were male, 549 (61.3%) were current smokers, and 244 (27.3%) were former smokers (Table 1). Overall, eosinophil levels in sputum exhibited a skewed distribution, with a median eosinophil abundance of 2.00% (IQR: 0.75–5.00) in spirometry-defined COPD patients, a mean of 4.03% (Figure 1B), and a median blood eosinophil count of 180cells/µL (IQR: 110–290; Figure 1A). Importantly, sputum eosinophil proportion was significantly positively correlated with blood eosinophil count (p=0.431, p<0.001) (Figure 2)
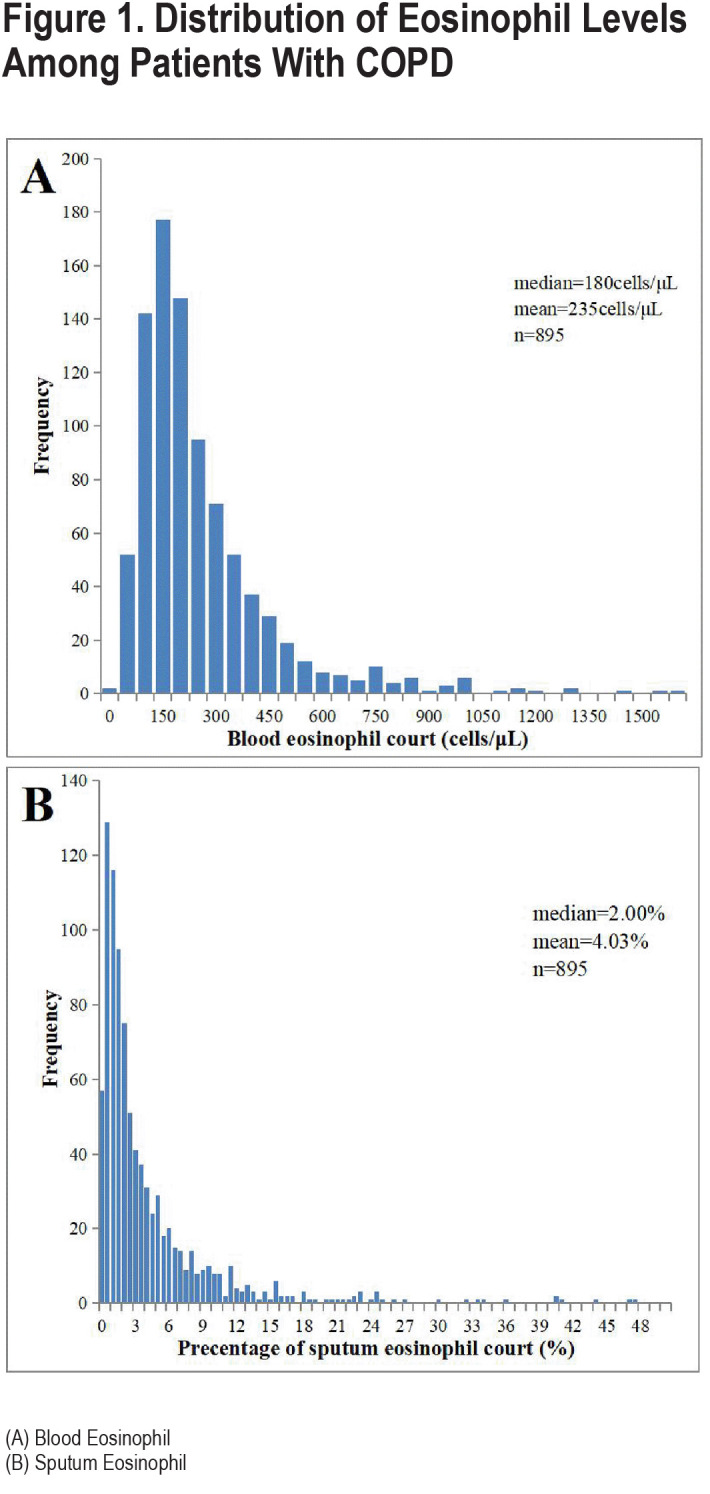
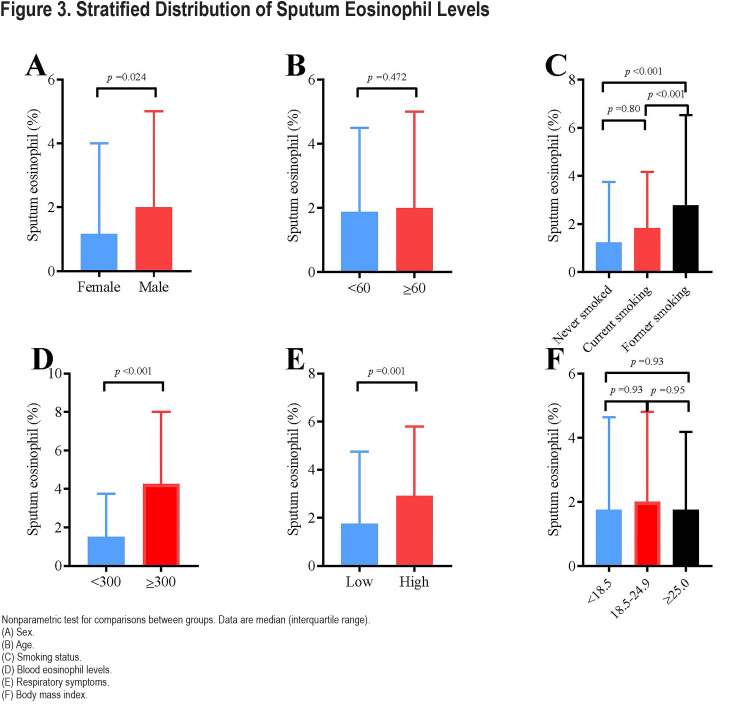
The median sputum eosinophil abundance was 1.17% (IQR: 0.25–4.00) in women and 2.00% (IQR: 0.75–5.00) in men. There was no significant difference in sputum eosinophil level between patients aged over 60 years old and those under 60 years old (2.00% [IQR: 0.75–5.00] versus 1.87% [IQR: 0.75–4.50], p=0.472). Sputum eosinophil levels in COPD patients who had previously smoked were higher than in participants who never smoked (2.78% [IQR: 0.90–6.54] versus 1.24% [IQR: 0.25–3.75], p<0.001) and those current smoking (2.78% [IQR: 0.90–6.54] versus 1.82% [IQR: 0.75–4.16], p<0.001; Figure 3). Sputum eosinophil levels in participants with high respiratory symptoms were higher than in participants without (2.92% [IQR: 1.15–5.80] versus 1.75 [IQR: 0.75–4.75], p=0.001). Figure 3 shows the detail characteristics of the study population.
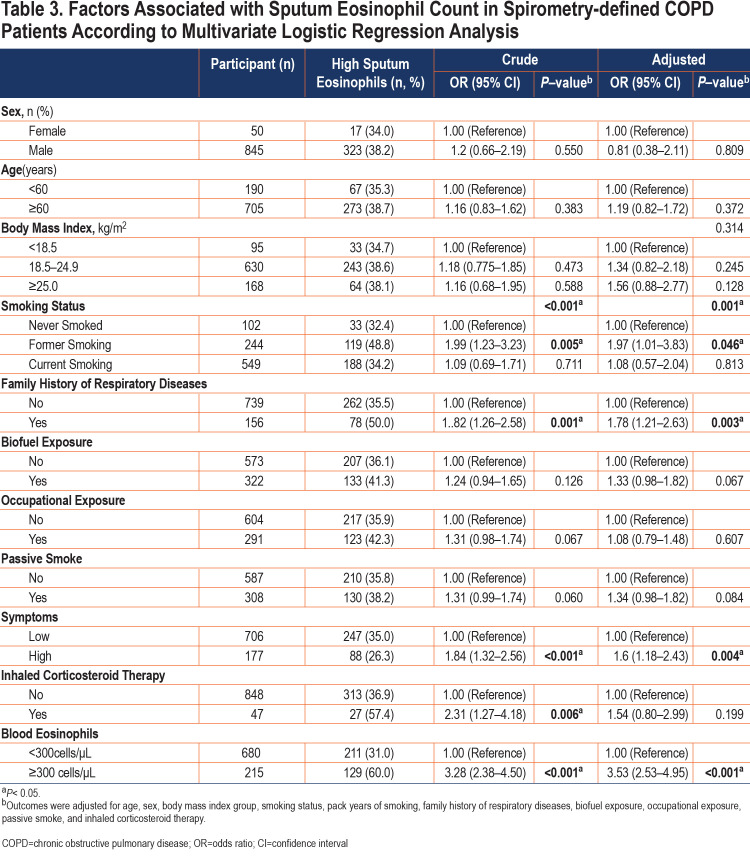
Table 1 lists the demographic characteristics of the study participants stratified by sputum eosinophil levels (<3.0% or ≥3.0%). There was no significant difference in age, gender, body mass index, and comorbidities between the 2 groups. But the participants in the high-level group had a higher proportion of former smokers (35.0% versus 22.5%, p<0.001), more family history of respiratory diseases (22.9% versus 14.1%, p=0.001), a higher level of blood eosinophils (243 [160–385]cells/µL versus 150 [93–223]cells/µL, p<0.001) and a higher proportion of inhaled corticosteroid therapy history (7.9% versus 3.6%, p=0.005) than participants in the low-level group. In addition, participants in the high sputum eosinophil group exhibited more respiratory symptoms such as chronic cough, chronic expectoration, wheezing, and dyspnea, as well as a worst quality of life.
Spirometry and Imaging
Adjusted for potential confounding factors, patents with high sputum eosinophils had lower pre-bronchodilator FEV1 (1.23±0.06 versus 1.38± 0.06, p<0.001), FEV1/FVC (49.06±0.99 versus 52.68±1.00, p<0.001) and FEV1%predicted (59.38±2.08 versus 64.69±2.08, p<0.001) compared with low sputum eosinophils (Table 2). After using a bronchodilator, patients with high sputum eosinophils had lower FEV1 (1.35±0.06 versus 1.50±0.06, p<0.001), FEV1/FVC (50.28±0.97 versus 53.68±0.96, p<0.001) and FEV1%predicted (65.14±2.02 versus 70.15±2.02, p<0.001) compared with patients with low sputum eosinophils.
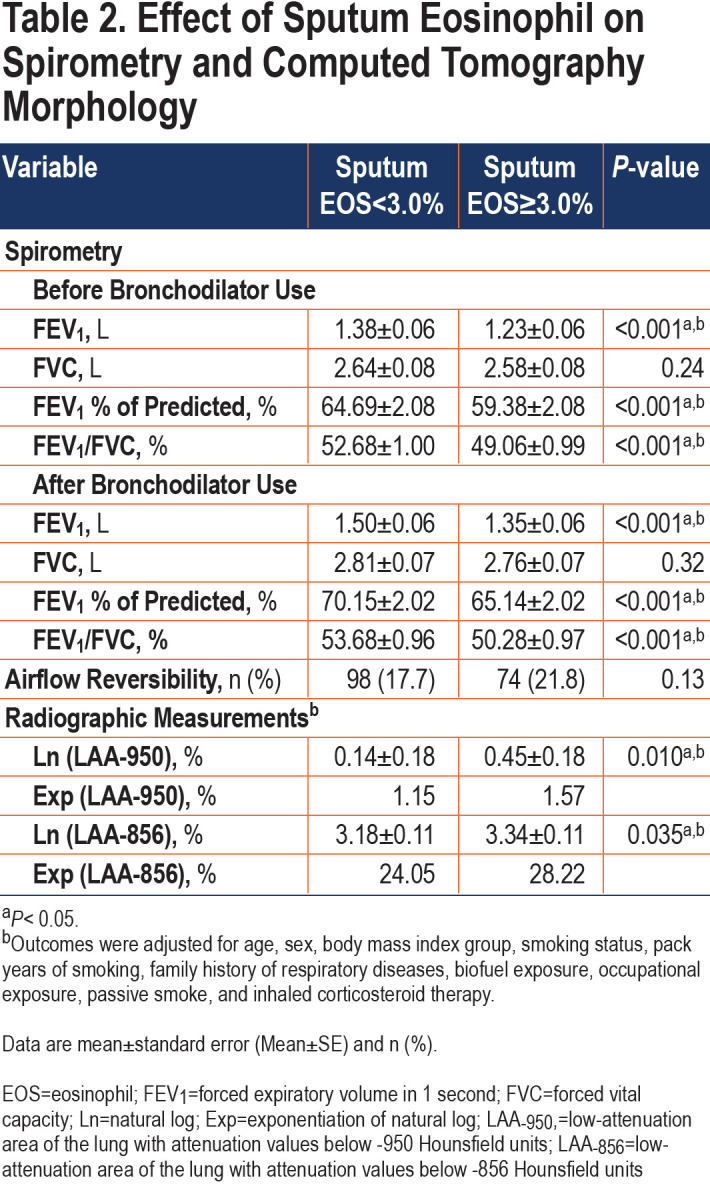
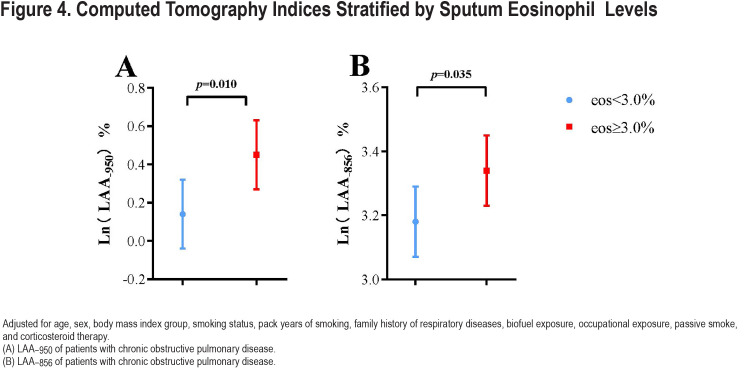
There was no difference in airflow reversibility between the 2 groups (p=0.13). Indices of emphysema (LAA−950) and air trapping (LAA−856) differed statistically between the 2 groups (Table 2). The high sputum eosinophils (≥3.0%) group had significantly more Ln (LAA−950) (0.45±0.18 versus 0.14±0.18, p=0.010) and Ln (LAA−856) (3.34±0.11 versus 3.18±0.11, p=0.035) than the low-level group (<3.0%) (Figure 4). We got a similar conclusion after excluding the influence of ICS therapy (Table S1 in the online supplement).
Clinical Predictors of High Sputum Eosinophils
Variables related to COPD according to previous literature included age, sex, body mass index, smoking status, family history of respiratory diseases, biofuel exposure, exposure to occupational irritants, passive smoke, the history of inhaled corticosteroid therapy, blood eosinophils level, and respiratory symptoms and were entered into the forced-entry multivariate logistic model. Smoking status, a family history of respiratory disease, high respiratory symptoms, and high level of blood eosinophils were associated with high sputum eosinophils (Table 3). Since previous ICS medications may affect the level of eosinophils in patients, we excluded patients with a history of ICS therapy and got a similar result (Table S2 in the online supplement).
Discussion
To our knowledge, this is the first study to investigate the factors of high sputum eosinophils in Chinese COPD patients. Three important findings were found in this study. First, in this cohort, the median value of sputum eosinophils was 2.00% (IQR: 0.75–5.00), and the prevalence of participants with high sputum eosinophils was 38.0% when using a threshold of 3.00%. Second, the quality-of-life score of participants in the high sputum eosinophils group was worst, and this group had lower lung function, more severe emphysema, and air trapping. Third, high blood eosinophil levels, severe respiratory symptoms, being a former smoker, and a family history of respiratory diseases may be predictors of high levels of sputum eosinophils.
Clinical studies have shown that high eosinophilic COPD may be a specific clinical phenotype.7,9,34 ICS treatment has a good effect on patients with COPD with the eosinophilic airway inflammatory phenotype, and a value of 300 eosinophils/µL peripheral blood is often used as the application indication for ICSs. However, blood eosinophil levels can fluctuate greatly and are susceptible to multiple factors,35 requiring further assessment of eosinophilic airway inflammation. Sputum eosinophils have been reported to be confined to the distal airway, and their levels may more directly reflect the status of pulmonary eosinophilic airway inflammation.36,37 Elevated blood eosinophils were associated with COPD exacerbations only in combination with elevated sputum eosinophils,38 underscoring the importance of monitoring sputum eosinophil levels. Therefore, exploring sputum eosinophils can better identify airway eosinophilic inflammatory phenotype, so as to better guide the use of ICSs.
Current research on COPD and eosinophilic inflammation is mainly based on clinical features and phenotypes. Age has been suggested to be related to COPD with the eosinophilic inflammatory phenotype,6,39 but this indication was obtained through univariate analysis. Our study didn’t confirm an association between age and eosinophilic airway inflammation using multivariate analysis, we found that age was not associated with sputum eosinophil levels. This may be because the COPD patients we included in the study were older. Another study reported that sex was closely associated with eosinophilic airway inflammation and that men were more likely to have higher levels of eosinophils,40 contrary to our findings. As the proportion of women in our cohort was smaller, there may have been a certain population bias. The differences also may be related to factors such as ethnicity. Therefore, the relationship between sex and eosinophilic airway inflammation warrants further study.
We found that cigarette smoking was also associated with high sputum eosinophils, consistent with a former report.41 Smoking can induce acute lung injury by recruiting and activating eosinophils in some patients.41 We found higher counts of sputum eosinophils in former smokers than in never-smokers, but no difference in sputum eosinophils between current smokers and never-smokers. This might be attributable to disease severity, which led participants to quit smoking. The relationship between blood eosinophils and sputum eosinophils has been reported in many studies, whether eosinophils in patients with stable COPD can predict sputum eosinophils remains controversial.38,42,43 Our study confirmed that high levels of blood eosinophils were effective predictors of sputum eosinophils. It was demonstrated that the concentrations of many cytokines were increased in the bronchial mucosa of COPD patients.44 Eosinophilic inflammation has been associated with a decline in FEV1 and a susceptibility to more asthma symptoms.45 We found that the degree of emphysema and gas trapping in the high eosinophil levels group was more pronounced, perhaps because of aggregation of eosinophils in the distal airway and the resultant lung injury, consistent with previous findings.38 This suggested that patients with high levels of sputum eosinophils should pay more attention. A total of 47 (5.3%) patients we included had a long history of ICS therapy. Because long-term use of ICSs is strongly associated with eosinophil levels in patients, we did subgroup analysis to exclude the impact of ICS therapy in our study. The research results are consistent with previous ones.
Some potential limitations in our study should be mentioned. First, there might exist a selection bias in our study because some participants were not able to successfully induce sputum, especially nonsmoking female participants. There was no significant difference in other aspects between successful and unsuccessful induction groups. However, our sampling was conducted in strict accordance with the standard protocol30 and the success rate of induced sputum was 80.6% (Table S3 in the online supplement), which is higher than in other studies.31,46 Second, we excluded patients with previously diagnosed asthma; it is possible that asthma is underdiagnosed in China because of limited medical resources. However, we have reason to believe that low asthma rates in the community do not affect our main findings.47 Parasitic infection is also a common cause of high sputum eosinophils, but we did not investigate an infectious state. However, the prevalence of parasitic infection is low in China because of improvements in national prevention and control strategies.48,49 The median blood eosinophil level in our study population was 180cells/µL (IQR: 210–290), similar to values recorded in other countries with low parasitic infection rates.50 Third, patients with COPD in our study were diagnosed only from a single spirometry, and these readings may fluctuate or even reverse in some participants.51,52 Therefore, overdiagnosis or underdiagnosis of COPD may be a concern in our study.
Conclusion
In summary, we found that high blood eosinophil levels, severe respiratory symptoms, being a former smoker, and a family history of respiratory diseases may be predictors of high levels of sputum eosinophils in Chinese COPD patients. High sputum eosinophils were associated with lower lung function, more emphysema, and gas trapping. Our findings may assist clinicians in identifying airway inflammation phenotypes to achieve individualized and precision therapy.
Abbreviations
Abbreviations: chronic obstructive pulmonary disease, COPD; inhaled corticosteroid, ICS; computed tomography, CT; long-acting muscarinic antagonist, LAMA; long-acting beta2-agonist, LABA; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; low attenuation area of the lung with attenuation values below -950 Hounsfield units, LAA-950; low attenuation area of the lung with attenuation values below -856 Hounsfield units, LAA-856; natural logarithm, Ln; interquartile range, IQR; odds ratio, OR; eosinophil, EOS; modified Medical Research Council score, mMRC; COPD Assessment Test, CAT; Clinical COPD Questionnaire, CCQ
Funding Statement
This work was supported by the National Key Research and Development Program (2016YFC1304101), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01S155), the National Natural Science Foundation of China (81970045), and Zhongnanshan Medical Foundation of Guangdong Province (ZNSA-2020003, ZNSA-2020012, and ZNSA-2020013).
References
- 1.Wang C,Xu J,Yang L,et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706-1717. doi: https://doi.org/10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
- 2.Lareau SC,Fahy B,Meek P,Wang A. Chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med. 2019;199(1):1-2. doi: https://doi.org/10.1164/rccm.1991P1 [DOI] [PubMed] [Google Scholar]
- 3.Lozano R,Naghavi M,Foreman K,et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095-2128. doi: https://doi.org/10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postma DS,Bush A,van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385(9971):899-909. doi: https://doi.org/10.1016/S0140-6736(14)60446-3 [DOI] [PubMed] [Google Scholar]
- 5.Bafadhel M,McKenna S,Terry S,et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662-671. doi: https://doi.org/10.1164/rccm.201104-0597OC [DOI] [PubMed] [Google Scholar]
- 6.Singh D,Kolsum U,Brightling CE,Locantore N,Agusti A,Tal-Singer R. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697-1700. doi: https://doi.org/10.1183/09031936.00162414 [DOI] [PubMed] [Google Scholar]
- 7.Pascoe S,Barnes N,Brusselle G,et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019;7(9):745-756. doi: https://doi.org/10.1016/S2213-2600(19)30190-0 [DOI] [PubMed] [Google Scholar]
- 8.Lipson DA,Barnhart F,Brealey N,et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671-1680. doi: https://doi.org/10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 9.Stockley RA,Halpin DMG ,Celli BR,Singh D. Chronic obstructive pulmonary disease biomarkers and their interpretation. Am J Respir Crit Care Med. 2019;199(10):1195-1204. doi: https://doi.org/10.1164/rccm.201810-1860SO [DOI] [PubMed] [Google Scholar]
- 10.Oh YM,Lee KS,Hong Y,et al. Blood eosinophil count as a prognostic biomarker in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3589-3596. doi: https://doi.org/10.2147/COPD.S179734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green RH,Brightling CE,McKenna S,et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715-1721. doi: https://doi.org/10.1016/S0140-6736(02)11679-5 [DOI] [PubMed] [Google Scholar]
- 12.Brightling CE,McKenna S,Hargadon B,et al. Sputum eosinophilia and the short-term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60(3):193-198. doi: https://doi.org/10.1136/thx.2004.032516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Sullivan JA,Bochner BS. Eosinophils and eosinophil-associated diseases: an update. J Allergy Clin Immunol. 2018;141(2):505-517. doi: https://doi.org/10.1016/j.jaci.2017.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu F,Zhou Y,Peng J,et al. Rationale and design of the Early Chronic Obstructive Pulmonary Disease (ECOPD) study in Guangdong, China: a prospective observational cohort study. J Thorac Dis. 2021;13(12):6924-6935. doi: https://doi.org/10.21037/jtd-21-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buist AS,McBurnie MA,Vollmer WM,et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741-750. doi: https://doi.org/10.1016/S0140-6736(07)61377-4 [DOI] [PubMed] [Google Scholar]
- 16.Buist AS,Vollmer WM,Sullivan SD,et al. The burden of obstructive lung disease initiative (BOLD): rationale and design. COPD. 2005;2(2):277-283. doi: https://doi.org/10.1081/COPD-57610 [PubMed] [Google Scholar]
- 17.Zhou Y,Hu G,Wang D,et al. Community based integrated intervention for prevention and management of chronic obstructive pulmonary disease (COPD) in Guangdong, China: cluster randomised controlled trial. BMJ. 2010;341:c6387. doi: https://doi.org/10.1136/bmj.c6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones PW,Harding G,Berry P,Wiklund I,Chen WH,Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654. doi: https://doi.org/10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 19.Yang G,Fan L,Tan J,et al. Smoking in China: findings of the 1996 National Prevalence Survey. JAMA. 1999;282(13):1247-1253. doi: https://doi.org/10.1001/jama.282.13.1247 [DOI] [PubMed] [Google Scholar]
- 20.Karloh M,Fleig Mayer A,Maurici R,Pizzichini MMM ,Jones PW,Pizzichini E. The COPD assessment test: what do we know so far?: A systematic review and meta-analysis about clinical outcomes prediction and classification of patients into GOLD stages. Chest. 2016;149(2):413-425. doi: https://doi.org/10.1378/chest.15-1752 [DOI] [PubMed] [Google Scholar]
- 21.Gupta N,Pinto LM,Morogan A,Bourbeau J. The COPD assessment test: a systematic review. Eur Respir J. 2014;44(4):873-884. doi: https://doi.org/10.1183/09031936.00025214 [DOI] [PubMed] [Google Scholar]
- 22.Zhong N,Wang C,Yao W,et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176(8):753-760. doi: https://doi.org/10.1164/rccm.200612-1749OC [DOI] [PubMed] [Google Scholar]
- 23.Tan WC,Bourbeau J,Hernandez P,et al. Exacerbation-like respiratory symptoms in individuals without chronic obstructive pulmonary disease: results from a population-based study. Thorax. 2014;69(8):709-717. doi: https://doi.org/10.1136/thoraxjnl-2013-205048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anthonisen NR,Manfreda J,Warren CP,Hershfield ES,Harding GK,Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196-204. doi: https://doi.org/10.7326/0003-4819-106-2-196 [DOI] [PubMed] [Google Scholar]
- 25.Miller MR,Hankinson J,Brusasco V,et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338. doi: https://doi.org/10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 26.Singh D,Agusti A,Anzueto A,et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi: https://doi.org/10.1183/13993003.00164-2019 [DOI] [PubMed] [Google Scholar]
- 27.Schroeder JD,McKenzie AS,Zach JA,et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol. 2013;201(3):W460-W470. doi: https://doi.org/10.2214/AJR.12.10102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fedorov A,Beichel R,Kalpathy-Cramer J,et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. 2012;30(9):1323-1341. doi: https://doi.org/10.1016/j.mri.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Regan EA,Lynch DA,Curran-Everett D,et al. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175(9):1539-1549. doi: https://doi.org/10.1001/jamainternmed.2015.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Efthimiadis A,Spanevello A,Hamid Q,et al. Methods of sputum processing for cell counts, immunocytochemistry and in situ hybridisation. Eur Respir J Suppl. 2002;20(37 Suppl):19s-23s. doi: https://doi.org/10.1183/09031936.02.00001902 [DOI] [PubMed] [Google Scholar]
- 31.Guiot J,Demarche S,Henket M,et al. Methodology for sputum induction and laboratory processing. J Vis Exp. 2017;130:e56612. doi: https://doi.org/10.3791/56612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson WJ,The S,Leigh R. Establishing a normal range for induced sputum cell counts in Western Canada. Can Respir J. 2013;20(6):424-425. doi: https://doi.org/10.1155/2013/547309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh D,Bassi M,Balzano D,et al. COPD patients with chronic bronchitis and higher sputum eosinophil counts show increased type-2 and PDE4 gene expression in sputum. J Cell Mol Med. 2021;25(2):905-918. doi: https://doi.org/10.1111/jcmm.16146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benson VS,Hartl S,Barnes N,Galwey N,Van Dyke MK,Kwon N. Blood eosinophil counts in the general population and airways disease: a comprehensive review and meta-analysis. Eur Respir J. 2021;59(1):2004590. doi: https://doi.org/10.1183/13993003.04590-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landis S,Suruki R,Maskell J,Bonar K,Hilton E,Compton C. Demographic and clinical characteristics of COPD patients at different blood eosinophil levels in the UK clinical practice research datalink. COPD. 2018;15(2):177-184. doi: https://doi.org/10.1080/15412555.2018.1441275 [DOI] [PubMed] [Google Scholar]
- 36.Berry M,Hargadon B,Morgan A,et al. Alveolar nitric oxide in adults with asthma: evidence of distal lung inflammation in refractory asthma. Eur Respir J. 2005;25(6):986-991. doi: https://doi.org/10.1183/09031936.05.00132404 [DOI] [PubMed] [Google Scholar]
- 37.Hogg JC,Chu F,Utokaparch S,et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645-2653. doi: https://doi.org/10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 38.Hastie AT,Martinez FJ,Curtis JL,et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956-967. doi: https://doi.org/10.1016/S2213-2600(17)30432-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiSantostefano RL,Hinds D,Le HV,Barnes NC. Relationship between blood eosinophils and clinical characteristics in a cross-sectional study of a US population-based COPD cohort. Respir Med. 2016;112:88-96. doi: https://doi.org/10.1016/j.rmed.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 40.Yang M,Yang T,Li X,et al. Clinical predictors of high blood eosinophils in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2021;16:2467-2474. doi: https://doi.org/10.2147/COPD.S324511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar A,Cherian SV,Vassallo R,Yi ES,Ryu JH. Current concepts in pathogenesis, diagnosis, and management of smoking-related interstitial lung diseases. Chest. 2018;154(2):394-408. doi: https://doi.org/10.1016/j.chest.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 42.Antus B,Paska C,Barta I. Predictive value of exhaled nitric oxide and blood eosinophil count in the assessment of airway eosinophilia in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:2025-2035. doi: https://doi.org/10.2147/COPD.S257965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartjes FJ,Vonk JM,Faiz A,et al. Predictive value of eosinophils and neutrophils on clinical effects of ICS in COPD. Respirology. 2018;23(11):1023-1031. doi: https://doi.org/10.1111/resp.13312 [DOI] [PubMed] [Google Scholar]
- 44.Gorska K,Nejman-Gryz P,Paplinska-Goryca M,Korczynski P,Prochorec-Sobieszek M,Krenke R. Comparative study of IL-33 and IL-6 levels in different respiratory samples in mild-to-moderate asthma and COPD. COPD. 2018;15(1):36-45. doi: https://doi.org/10.1080/15412555.2017.1416074 [DOI] [PubMed] [Google Scholar]
- 45.Tan WC,Bourbeau J,Nadeau G,et al. High eosinophil counts predict decline in FEV(1): results from the CanCOLD study. Eur Respir J. 2021;57(5):2000838. doi: https://doi.org/10.1183/13993003.00838-2020 [DOI] [PubMed] [Google Scholar]
- 46.Kulkarni N,Pierse N,Rushton L,Grigg J. Carbon in airway macrophages and lung function in children. N Engl J Med. 2006;355:21-30. doi: https://doi.org/10.1056/NEJMoa052972 [DOI] [PubMed] [Google Scholar]
- 47.Huang K,Yang T,Xu J,et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. 2019;394(10196):407-418. doi: https://doi.org/10.1016/S0140-6736(19)31147-X [DOI] [PubMed] [Google Scholar]
- 48.Wang R-B ,Hong Y-T ,Zhou X-N . Seventy years' achievements of international cooperation by the National Institute of Parasitic Diseases at the Chinese Center for Disease Control and Prevention. Infect Dis Poverty. 2020;9:164. doi: https://doi.org/10.1186/s40249-020-00783-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J-H ,Wang H,Chen J-X ,et al. Frontiers of parasitology research in the People's Republic of China: infection, diagnosis, protection and surveillance. Parasit Vectors. 2012;5:221. doi: https://doi.org/10.1186/1756-3305-5-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vedel-Krogh S,Nielsen SF,Lange P,Vestbo J,Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The copenhagen general population study. Am J Respir Crit Care Med. 2016;193:965-974. doi: https://doi.org/10.1164/rccm.201509-1869OC [DOI] [PubMed] [Google Scholar]
- 51.Mirza S,Clay RD,Koslow MA,Scanlon PD. COPD guidelines: a review of the 2018 GOLD report. Mayo Clin Proc. 2018;93(10):1488-1502. doi: https://doi.org/10.1016/j.mayocp.2018.05.026 [DOI] [PubMed] [Google Scholar]
- 52.Aaron SD,Tan WC,Bourbeau J,et al. Diagnostic instability and reversals of chronic obstructive pulmonary disease diagnosis in individuals with mild to moderate airflow obstruction. Am J Respir Crit Care Med. 2017;196(3):306-314. doi: https://doi.org/10.1164/rccm.201612-2531OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplemental material.