ABSTRACT
Purpose:
Spontaneous intracerebral hemorrhage (ICH) is still a major public health problem, with high mortality and disability. Ulinastatin (UTI) was purified from human urine and has been reported to be anti-inflammatory, organ protective, and antioxidative stress. However, the neuroprotection of UTI in ICH has not been confirmed, and the potential mechanism is unclear. In the present study, we aimed to investigate the neuroprotection and potential molecular mechanisms of UTI in ICH-induced early brain injury in a C57BL/6 mouse model.
Methods:
The neurological score, brain water content, neuroinflammatory cytokine levels, oxidative stress levels, and neuronal damage were evaluated.
Results:
UTI treatment markedly increased the neurological score, alleviated brain edema, decreased the levels of the inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and NF-κB, decreased the levels of reactive oxygen species (ROS) and malondialdehyde (MDA), and upregulated the levels of glutathione (GSH), superoxide dismutase (SOD), and Nrf2. This finding indicated that UTI-mediated inhibition of neuroinflammation and oxidative stress alleviated neuronal damage after ICH. The neuroprotective capacity of UTI is partly dependent on the ROS/MAPK/Nrf2 signaling pathway.
Conclusions:
UTI improves neurological outcomes in mice and reduces neuronal death by protecting against neural neuroinflammation and oxidative stress.
Key words: Cerebral Hemorrhage, Brain Injuries, Oxidative Stress, Neuroinflammatory Diseases, Mice
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a common critical disease with high mortality and disability that accounts for 15-20% of all strokes, especially in elderly patients1-4. Large intracranial hematoma can lead to primary brain injury through the destruction of brain tissue and high intracranial pressure (ICP)5,6. In previous studies, craniotomies to remove hematomas were found to limit primary brain damage and decrease ICP after ICH6-8. However, hematoma evacuation shows no clinical benefit to patients and rarely affects neurological recovery9. Increasing evidence shows that red blood cell debris and its degradation products trigger secondary brain injury following ICH, and the mechanisms include neuroinflammation, oxidative stress, blood-brain barrier (BBB) damage, and neuron death10-14. According to previous studies15-17, treatment or drugs can improve neurological function after reducing mitochondrial apoptosis and decrease cerebral edema in mice after ICH.
Ulinastatin (UTI) is a serine protease inhibitor purified from human urine. UTI acts primarily against inflammation, regulates immunity, and protects organs18,19. He et al.20 reported that UTI plays an important role in pulmonary protective effects after cardiac surgery by meta-analysis. UTI can also alleviate cerebral ischemia-reperfusion injury and BBB permeability in animals21-23. However, no clear evidence has shown that UTI has an effect on early brain injury (EBI) during acute ICH, and its association with the levels of apoptotic molecules and oxidative stress remains to be elucidated.
In ICH, reactive oxygen species (ROS)-induced oxidative stress and glutamate-induced excitotoxicity can cause neuronal death rapidly in the brain24,25. The release of ROS after an extracellular or intracellular stimulus is crucial to the injury of neurons and tissues6. The transcription factor nuclear factor erythroid 2-related factor 2 (Nfe2L2, commonly referred to as Nrf2) regulates the expression of more than 250 genes and it is marked by its binding site, antioxidant response element (ARE)26. In acute central nervous system disease, Nrf2 can also modulate the cellular antioxidant response and mitigate electrophilic or oxidative stress, inflammation, proteostasis, xenobiotic/drug metabolism, iron/heme metabolism, carbohydrate, and lipid metabolism6. Due to cerebral tissue’s higher oxygen consumption than most other organs, excessive ROS are generated after hemorrhage as a result of oxidative stress27. It would be valuable to investigate new potential drug goals by targeting the ROS/MAPK/Nrf2 signaling pathway in oxidative stress and neuroinflammation.
In the present study, we constructed a mouse ICH model to study the effects of UTI on EBI and explored the crosstalk between oxidative stress and neuroinflammation. We also explored the mechanism by which the ROS/MAPK/Nrf2 signaling pathway may regulate this process.
Methods
All animal experiments performed in this study complied with the National Institutes of Health guidelines for the handling of laboratory animals and were approved by the Ethics Committee of the Wuxi Medical College of Anhui Medical University (YXLL-2021-A15). A total of 60 healthy adult male C57BL/6J mice (n = 20/group; age 8-10 weeks old; Anhui Medical University, Hefei, China) weighing between 22-25 g were used when conducting all the experiments for the current study. The mice were housed in animal care facilities on a 12-h light/dark cycle and had free access to food and water.
Animal intracerebral hemorrhage model
The ICH mouse model was generated based on a previously described protocol involving autologous blood injection28. Briefly, male C57BL6/J mice were anesthetized by intraperitoneal (i.p.) injection of 50 mg/kg pentobarbital sodium and placed in a prone position with a stereotactic head frame. The rectal temperature was kept at 37 ± 0.5°C during the operation using a heating pad. An artificial tear ointment was used to protect the eye from injury during surgery. A midline scalp incision was made, and a cranial burr hole with a 1-mm diameter was made at the following coordinates relative to bregma: 0.2 mm posterior, 2.2 mm lateral to bregma, and 3.5 mm below the dura. A total of 30 μL of autologous blood without anticoagulation was collected from the caudal artery and rapidly injected into the basal ganglia through the burr hole via the 26-gauge needle of a 10-μL Hamilton syringe. First, 5 μL of arterial blood was injected at a depth of 2.8 mm from the dura (injection speed: 3 μL/min). Five minutes later, the other 25 μL of blood was injected at a depth of 3.5 mm (injection speed: 3 μL/min). After the injection of autologous blood, the needle was kept in the brain for 10 min to prevent blood backflow along the needle tract. Finally, the hole was covered with medical bone wax. The animals in the sham group received similar surgical procedures, but they were injected at the same site with an equal volume of sterile saline instead of blood.
Drug administration
UTI (Techpool Biochem, Guangdong, China) was stored at 4°C and dissolved in 0.9% normal saline when used. UTI (104 U/kg) was administered by intraperitoneal injection before the onset of ICH29.
Neurobehavioral assessment
Neurobehavioral assessment at 72 hours after ICH was performed according to a previous study30. The neurological scores were summed to calculate the neurological scores, which ranged from 0 to 18 points. Neurological dysfunction was represented by a higher score. An independent observer was blinded in this study.
Brain water content measurement
Brain water content measurement was detected using the standard wet-dry method, according to previous studies31-33. After mice were sacrificed, the entire brain was harvested and separated into different parts, and the wet weight was acquired by precision balance and then dehydrated at 105°C for 24 hours to acquire the dry weight (Eq. 1).
| (1) |
Evans blue extravasation
The method of Evans blue extravasation was performed according to a previous study34. Briefly, mice were anesthetized 72 hours after ICH. Evans blue dye (2%, 5 mL/kg; Sigma–Aldrich, St. Louis, MO, United States of America) was injected into the left femoral vein and circulated for 60 min. Then, phosphate-buffered saline (PBS) was intracardially perfused after the mice were sacrificed. The brains were removed, weighed, homogenized in saline, and centrifuged at 15,000 × g for 30 min. Subsequently, the resultant supernatant was added to an equal volume of trichloroacetic acid, incubated overnight at 4°C, and centrifuged at 15,000 × g for 30 min. Next, the resultant supernatant was collected and spectrophotometrically quantified at 610 nm for Evans blue dye.
Analysis of reactive oxygen species
The nonfluorescent diacetylated 2',7'-dichlorofluorescein (DCF-DA) probe (Sigma–Aldrich), which becomes highly fluorescent upon oxidation, was used to evaluate intracellular ROS production according to the manufacturer’s instructions35.
Analysis of lipid peroxidation
Malondialdehyde (MDA), GSH, and superoxide dismutase (SOD) levels were detected with a corresponding assay kit (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions36.
Cytokine measurements of ipsilateral cortex tissue
The levels of interleukin-1β (IL-1β) (cat. no. ab197742; Abcam), IL-6 (cat. no. ab222503; Abcam), tumor necrosis factor-α (TNF-α) (cat. no. ab208348; Abcam), and NF-κB (cat. no. ab176663; Abcam) were measured by enzyme-linked immunoassay (ELISA) according to the manufacturer’s instructions37.
TUNEL staining
TUNEL staining was used to evaluate neuronal death in the hippocampus according to a previous study32. Brain specimens were immersed in a TUNEL mixture for 2 h at 37.5°C, followed by staining with DAPI. The procedure was performed with a TUNEL staining kit according to the manufacturer’s instructions.
Western blot analysis
Western blot analyses were performed as previously described31. Briefly, cerebral cortex samples were collected, homogenized, and extracted in RIPA lysis buffer containing a 1% (v/v) protease and phosphatase inhibitor cocktail. The protein samples were separated by SDS–PAGE and transferred to PVDF. After blocking with 5% (w/v) nonfat milk, the membranes were incubated with the following primary antibodies overnight at 4°C: rabbit anti-β-actin (1:1,000, rabbit polyclonal, Abcam, ab8227), rabbit ZO-1 (1:1,000, rabbit polyclonal, Abcam, ab 96587), phospho-p38 (CST, #4551, 1:2,000), and rabbit anti-Nrf2 (1:1,000, rabbit polyclonal, Abcam, ab92946). Afterwards, the membranes were incubated with HRP-conjugated goat anti-rabbit IgG or goat anti-mouse IgG secondary antibodies (1:5,000) for 1 h at room temperature and then scanned with a Bio-Rad (California, United States of America) gel imaging system.
Quantitative real-time polymerase chain reaction
Quantitative real-time (qRT) polymerase chain reaction (PCR) analysis was performed according to a previous study38. Total RNA was extracted using TRIzol Reagent (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, United States of America) according to the manufacturer’s instructions. Then, a miScript cDNA synthesis kit (K1622; Thermo Fisher Scientific Inc., Rockford, IL, United States of America) was used for the reverse transcription reaction. For qRT-PCR, a 7,500 real-time PCR thermocycler (Applied Biosystem) was used. Real-time RT-PCR was performed in a total volume of 10 μL containing 1 μL of cDNA, 0.6 μL of primers, and 8.4 μL of SYBR Green Master Mix (Toyobo Co., Ltd., Osaka, Japan). The program steps were 30 s at 95°C, 40 cycles of 5 s at 95°C, and 30 s at 60°C, followed by melt curve analysis. Gene expression was quantified with standard samples and normalized to GAPDH. The target genes and the specific primers are as follows:
Nrf2 (forward, 5’- ATCACGAGCCCTGAAACCAA-3’; reverse, 5’- GGCTGCAAAATGCTGGAAAA-3’);
MAPK (forward, 5’- TGTGTTCACCCCTGCCAAGT-3’; reverse, 5’- GCCCCCGAAGAATCTGGTAT-3’);
GAPDH (forward, 5’- ATGGGTGTGAACCACGAGA-3’ and reverse, 5’- CAGGGATGATGTTCTGGGCA-3’).
Statistical analysis
All data are presented as the means ± standard deviations. All statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA, United States of America). After checking for normal distribution, differences between two groups were analyzed with Student’s t-test (two-tailed), and data were analyzed by one-way analysis of variance (ANOVA) with post hoc Tukey’s test or Dunnett’s test applied to assess multiple comparisons. Nonparametric data were analyzed using Kruskal-Wallis H analysis followed by a Mann-Whitney U test, and p < 0.05 was considered statistically significant.
Results
Ulinastatin alleviates early brain injury after intracerebral hemorrhage
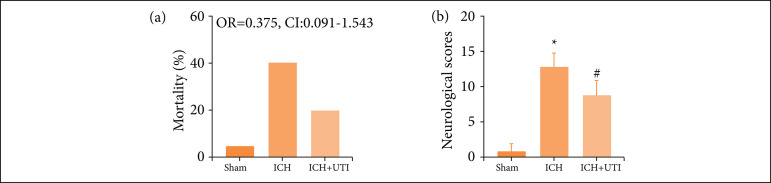
The results showed that ICH significantly increased the mortality rates (Fig. 1a). This effect was alleviated by UTI treatment, while there was no significant difference among the three groups. We also found that neurological scores increased significantly after ICH, and UTI administration significantly improved neurological function (Fig. 1b).
Figure 1. UTI alleviates neurological deficits and mortality after ICH. (a) Comparison of the mortality between the three groups. (b) Neurological scores of mice in the sham group, ICH group, and ICH group treatedwith UTI at 72 h (n = 10, analysis of variance; means ± standard error of mean).
ICH: intracerebral hemorrhage; UTI: Ulinastatin; OR: odds ratio; CI: confidence interval; *P < 0.05 vs. sham; #P < 0.05 vs. ICH.
Ulinastatin alleviates brain edema and blood-brain barrier permeability after intracerebral hemorrhage
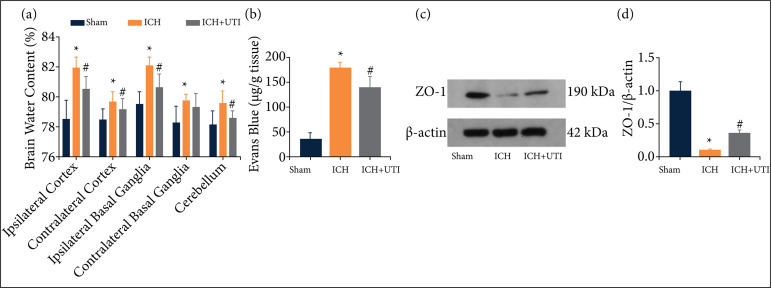
The results showed that ICH significantly increased the brain water content, which was alleviated after UTI treatment (Fig. 2a). BBB permeability was increased significantly after ICH, and UTI administration significantly alleviated this effect (Fig. 2b). The expression levels of Zonula occludens-1 (ZO-1) were detected by Western blotting (Fig. 2c). The results showed that ZO-1 (Fig. 2d) was downregulated significantly after ICH, while UTI treatment markedly alleviated this decrease.
Figure 2. UTI alleviates brain edema and BBB permeability after ICH. (a) UTI alleviates brain water content after ICH.(b) UTI alleviates BBB permeability after ICH. (c) Expression of ZO-1 was determined by Western blotting. (d) Quantification of ZO-1 to β-actin loading control (n = 5; analysis of variance; means ± standard error of mean).
ICH: intracerebral hemorrhage; UTI: Ulinastatin; BBB: blood-brain barrier; *P < 0.05 vs. sham; #P < 0.05 vs. ICH.
Ulinastatin alleviates neuronal apoptosis after intracerebral hemorrhage
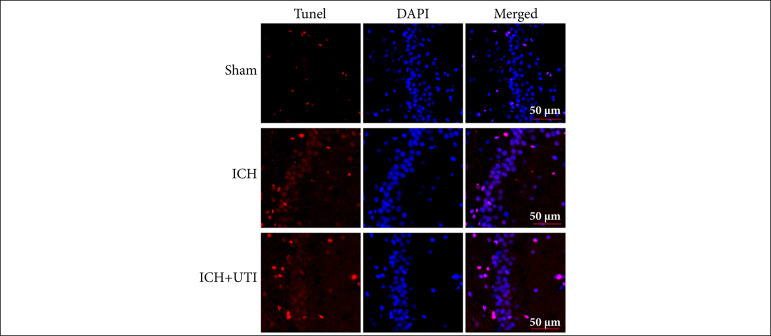
The TUNEL assay results showed more hippocampal neuronal damage after ICH, which was reversed after UTI administration (Fig. 3). Based on these results, UTI can alleviate hippocampal neuronal damage after ICH.
Figure 3. UTI alleviates neuronal apoptosis after ICH. TUNEL staining showed that UTI alleviated neuronal apoptosis in the hippocampus at 72 h after ICH, and representative images of apoptotic neurons are shown. Scale bar = 50 μm.
ICH: intracerebral hemorrhage; UTI: Ulinastatin.
Ulinastatin alleviates neuroinflammation after intracerebral hemorrhage
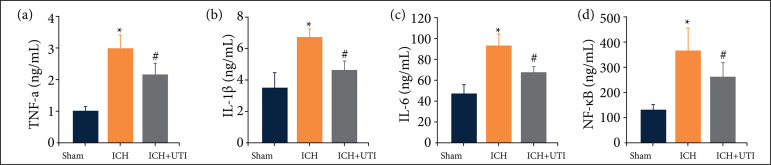
As previous studies have identified a vital role for neuroinflammation in EBI after ICH, increased neuroinflammation aggravates EBI. The inflammatory complex induces the secretion of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, and the subsequent activation of pro-inflammatory signaling through NF-κB initiates pyroptosis. Therefore, we measured the hippocampal levels of IL-1β, IL-6, TNF-α, and NF-κB using ELISA. The levels of proinflammatory cytokines increased significantly after ICH, while the levels of proinflammatory cytokines decreased significantly after UTI treatment (Figs. 4a-4d). Hence, these results suggested that UTI exhibited potent anti-inflammatory activity against ICH-induced neuroinflammation.
Figure 4. UTI alleviates neuroinflammation after ICH. UTI significantly reduced hippocampal (a) TNF-α, (b) interleukin-1β (IL-1β), (c) IL-6, and (d) NF-κB levels at 72 h after ICH (n = 5, analysis of variance; means ± standard error of mean).
ICH: intracerebral hemorrhage; UTI: Ulinastatin; TNF-α: tumor necrosis factor-α; IL: interleukin; *P<0.05 vs. sham; #P<0.05 vs. ICH
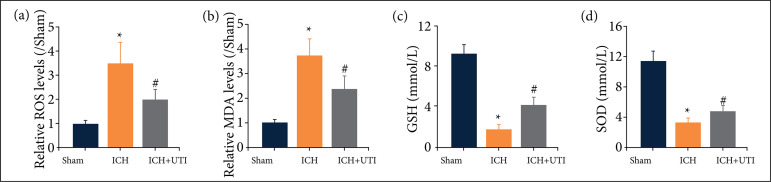
Ulinastatin inhibits intracerebral hemorrhage-induced oxidative stress in the hippocampus
To understand the neuroprotective mechanism of UTI, we focused on its antioxidation effect. We detected the levels of ROS, GSH, SOD, and MDA (Figs. 5a-5d). The levels of both ROS and MDA increased after ICH, but they decreased significantly after UTI treatment. The levels of GSH and SOD decreased after ICH, but they increased significantly after UTI treatment. Hence, these data showed that UTI can inhibit oxidative stress activation after ICH.
Figure 5. UTI inhibits ICH-induced oxidative stress in the hippocampus. (a) The levels of ROS by the DCF-DA method. (b-d) The levels of MDA, GSH, and SOD were quantified by using commercial kits (n = 5, mean ± standard error of mean).
ICH: intracerebral hemorrhage; UTI: Ulinastatin; ROS: reactive oxygen species; MDA: malondialdehyde; GSH: glutathione; SOD: superoxide dismutase; *P<0.05 vs. sham; #P<0.05 vs. ICH
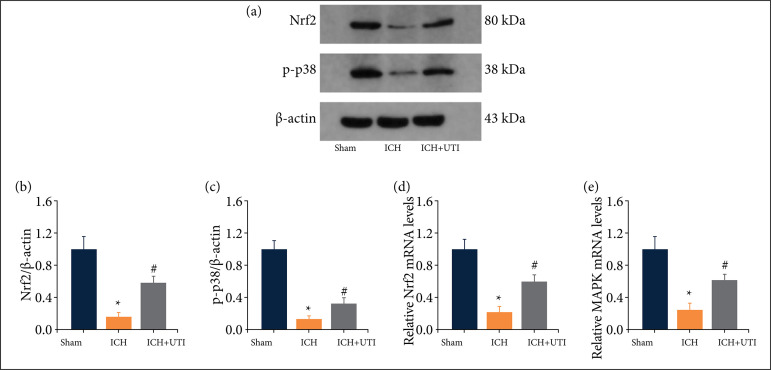
Ulinastatin regulates oxidative stress and neuroinflammation via ROS/MAPK/Nrf2 signaling pathway
ROS/MAPK/Nrf2 is a core signaling pathway of oxidative stress and neuroinflammation. According to previous studies39,40, activation of MAPK/Nrf2 signaling pathway is partially dependent on ROS production. We detected the levels of MAPK and Nrf2 proteins by performing Western blotting (Fig. 6a). The levels of MAPK and Nrf2 decreased significantly in the ICH group and increased after UTI administration (Figs. 6b-6c). Additionally, real-time PCR also demonstrated a similar result (Figs. 6d-6e). Thus, these results showed that UTI may exert neuroprotection by regulating the MAPK/Nrf2 signaling pathway.
Figure 6. UTI regulates oxidative stress and neuroinflammation by modulating the ROS/MAPK/Nrf2 signaling pathway after ICH.(a) Levels of Nrf2 and MAPK after ICH were determined using Western blotting. (b) Quantification of Nrf2 levels to β-actin, the loading control. (c) Quantification of MAPK (p-p38) levels to β-actin. (d) Levels of Nrf2 mRNA were measured by real-timePCR. (e) Levels of TMAPK mRNA were measured by real-time PCR (n=5, mean ± standard error of mean).
ICH: intracerebral hemorrhage; UTI: Ulinastatin; *P < 0.05 vs. sham; #P < 0.05 vs. ICH; PCR: polymerase chain reaction.
Discussion
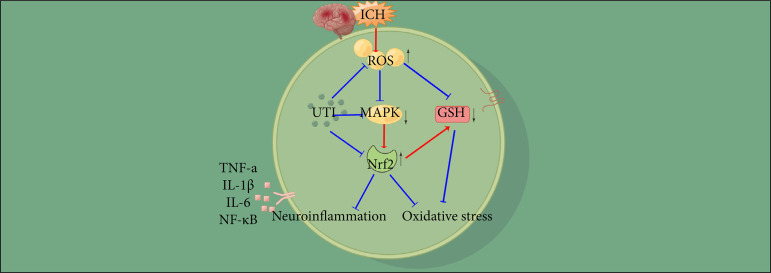
Here, we evaluated the therapeutic potential of UTI to alleviate EBI in a mouse model of ICH. As shown in the present study, UTI is a neuroprotective agent that attenuates early brain injury following ICH. We found that UTI improves neurological dysfunction after ICH, alleviates brain damage in a mouse ICH model, relieves neuroinflammation after ICH and then decreases inflammatory damage in the brain, prevents oxidative stress after ICH and alleviates neuronal death, and the antiapoptotic and antioxidative stress effects of UTI may be related to the ROS/MAPK/Nrf2 signaling pathway (Fig. 7).
Figure 7. A diagram of the proposed model explaining the observations of ROS/MAPK/Nrf2-mediated regulation of oxidative stress and neuroinflammation after ICH and potential mechanisms underlying the effect of UTI intervention.
ICH: intracerebral hemorrhage; ROS: reactive oxygen species; UTI: Ulinastatin; GSH: glutathione; TNF-α: tumor necrosis factor-α; IL: interleukin.
UTI is a 67 kDa glycoprotein purified from the urine of healthy humans that is a nonspecific protease inhibitor and a urinary trypsin inhibitor used to treat acute inflammatory disorders, sepsis, toxic shock, and hemorrhagic shock41,42. Recent studies have demonstrated that UTI can alleviate cerebral ischemia-reperfusion injury by regulating inflammation and oxidative stress21,43. However, the neuroprotective effect of UTI in ICH is unclear and lacks related clinical studies. Liu et al.19 reported that UTI can decrease the brain water content and BBB permeability significantly after ICH, possibly through7 decreased activation of astrocytes and ET-1, inhibiting the expression of proinflammatory VEGF and MMP-9. Another study also reported that UTI can attenuate brain edema after ICH in male Sprague-Dawley rats. The preliminary molecular mechanism may be through a decrease in the expression level of aquaporin-4 (AQP4) and proinflammatory cytokines, including IL-1β and TNF-α, as well as the activity of NF-κB44. In the present study, we also demonstrated that UTI can alleviate brain edema, improve neurological function, relieve the neuroinflammatory response, and decrease hippocampal neuronal damage and oxidative stress.
The mechanism of neuroprotection of UTI is multiple and complicated18,19. Cui et al.21 reported that UTI regulates inflammation and oxidative stress in ischemia-reperfusion (I/R) injury, possibly through the Nrf-2/HO-1 pathway. Li et al.22 also demonstrated that UTI can alleviate ischemic injury by improving BBB permeability by decreasing the expression of MMP-9 and increasing ZO-1. Koga et al.45 showed that UTI can inhibit ROS generation, prevent oxidative stress, and alleviate early inflammation in an ischemia/reperfusion model. Additionally, UTI can also improve hippocampal endoplasmic reticulum stress and apoptosis46. In central nervous system disease, the neuroprotection of UTI is partly dependent on the inhibition of apoptosis, neuroinflammation, and oxidative stress43,47,48.
The molecular mechanisms of oxidative stress and neuroinflammation are complex. The present study showed that UTI decreases the levels of ROS production, subsequently upregulating the expression levels of MAPK and Nrf2 and alleviating the activation of oxidative stress and neuroinflammation. ROS play a vital role in neuronal or other tissue injury and are generated by extracellular or intracellular stimuli after oxidative stress6. UTI can increase the nuclear translocation of Nrf2, stimulate Nrf2 DNA binding activity, and then upregulate the expression levels of HO-1, decrease airway inflammation, alleviate tissue injury, reduce oxidative stress, and enhance antioxidant enzyme activities in allergic inflammatory diseases49. Li et al.50 reported that UTI pretreatment can significantly suppress LPS-induced ROS production by activating the nuclear translocation of Nrf2 via promotion of p62-associated Keap1 degradation and then inhibiting inflammation and oxidation. In a cerebral ischemia model, UTI enhances Nrf2-ARE signals and inhibits products of oxidative stress in the hippocampus, which can improve neurological deficiencies51.
Additionally, activated p38 MAPK could facilitate the disassociation of Nrf2 from Keap1 to initiate the transcription of several anti-apoptotic, anti-ferroptosis, and antioxidant genes6,52. Li et al.53 demonstrated that UTI can alleviate brain injury by regulating the expression of TLR4 and NF-κB. Cui e Zhu44 also concluded that UTI therapy in traumatic brain injury decreased the activities of IL-1, TNF-, and NF-B.
In the present study, we observed that UTI can alleviate EBI after ICH by regulating crosstalk between oxidative stress and neuroinflammation by mediating ROS generation and via the MAPK/Nrf2 signaling pathway. The specific mechanism remains unclear. Additionally, this experiment was conducted in mice, and the effectiveness of the treatment in humans remains debated. Further exploration of the clinical effects of UTI on ICH patients will be conducted in the future.
Conclusions
Oxidative stress and neuroinflammation are mediated by ROS and play an important role in EBI after ICH. UTI-mediated regulation of oxidative stress and neuroinflammation is partly dependent on the ROS/MAPK/Nrf2 pathway and provided a new idea to explore the biological effects and mechanisms underlying the antioxidative stress, anti-inflammatory, and neuroprotective properties of UTI.
Acknowledgments
Not applicable.
Footnotes
Data availability statement: Data will be available upon request.
Funding: Not applicable.
Research performed at Department of Neurosurgery, Wuxi Clinical College, Anhui Medical University, 904th Hospital of Joint Logistic Support Force of PLA, Wuxi, China.
References
- 1.Zhang Y, Zhang X, Wei Q, Leng S, Li C, Han B, Bai Y, Zhang H, Yao H. Activation of sigma-1 receptor enhanced pericyte survival via the interplay between apoptosis and autophagy: implications for blood-brain barrier integrity in stroke. Transl Stroke Res. 2020;11(2):267–287. doi: 10.1007/s12975-019-00711-0. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Cho S, Rehni AK, Quero HN, Dave KR, Zhao W. Automated assessment of hematoma volume of rodents subjected to experimental intracerebral hemorrhagic stroke by bayes segmentation approach. Transl Stroke Res. 2020;11(4):789–798. doi: 10.1007/s12975-019-00754-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross BA, Jankowitz BT, Friedlander RM. Cerebral intraparenchymal hemorrhage: a review. Jama. 2019;321(13):1295–1303. doi: 10.1001/jama.2019.2413. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Zhu J, He J, Wang Y, Chen L, Zhang C, Zhou J, Yang L. Ultra-early microsurgical treatment within 24 h of SAH improves prognosis of poor-grade aneurysm combined with intracerebral hematoma. Oncol Lett. 2016;11(5):3173–3178. doi: 10.3892/ol.2016.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, Mayo SW, Bistran-Hall AJ, Gandhi D, Mould WA, Ullman N, Ali H, Carhuapoma JR, Kase CS, Lees KR, Dawson J, Wilson A, Betz JF, Sugar EA, Hao Y, Avadhani R, Caron JL, Harrigan MR, Carlson AP, Bulters D, LeDoux D, Huang J, Cobb C, Gupta G, Kitagawa R, Chicoine MR, Patel H, Dodd R, Camarata PJ, Wolfe S, Stadnik A, Money PL, Mitchell P, Sarabia R, Harnof S, Barzo P, Unterberg A, Teitelbaum JS, Wang W, Anderson CS, Mendelow AD, Gregson B, Janis S, Vespa P, Ziai W, Zuccarello M, Awad IA. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393(10175):1021–1032. doi: 10.1016/s0140-6736(19)30195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Wang Y, Wu J, Yang J, Li M, Chen Q. The potential value of targeting ferroptosis in early brain injury after acute CNS disease. Front Mol Neurosci. 2020;13:110–110. doi: 10.3389/fnmol.2020.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nat Rev Neurol. 2010;6(11):593–601. doi: 10.1038/nrneurol.2010.146. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Luo J, Liu H, Cui W, Guo K, Zhao L, Bai H, Guo W, Guo H, Feng D, Qu Y. Recombinant adiponectin peptide ameliorates brain injury following intracerebral hemorrhage by suppressing astrocyte-derived inflammation via the inhibition of Drp1-mediated mitochondrial fission. Transl Stroke Res. 2020;11(5):924–939. doi: 10.1007/s12975-019-00768-x. [DOI] [PubMed] [Google Scholar]
- 9.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382(9890):397–408. doi: 10.1016/s0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao WD, Zhou XT, Zhou LT, Wang F, Yin X, Lu Y, Zhu LQ, Liu D. Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model. Aging Cell. 2020;19(11):e13235. doi: 10.1111/acel.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JH, Yang LK, Chen L, Wang YH, Wu Y, Jiang BJ, Zhu J, Li PP. Atorvastatin ameliorates early brain injury after subarachnoid hemorrhage via inhibition of AQP4 expression in rabbits. Int J Mol Med. 2016;37(4):1059–1066. doi: 10.3892/ijmm.2016.2506. [DOI] [PubMed] [Google Scholar]
- 12.Gautam J, Xu L, Nirwane A, Nguyen B, Yao Y. Loss of mural cell-derived laminin aggravates hemorrhagic brain injury. J Neuroinflammation. 2020;17(1):103–103. doi: 10.1186/s12974-020-01788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Lu C, Yao W, Xu L, Zhou J, Zheng B. Aging. 21. Vol. 12. Albany NY: 2020. Dexmedetomidine inhibits inflammatory response and autophagy through the circLrp1b/miR-27a-3p/Dram2 pathway in a rat model of traumatic brain injury; pp. 21687–21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao X, Xiong Y, Li Q, Han M, Shan D, Yang G, Zhang S, Xin D, Zhao R, Wang Z, Xue H, Li G. Extracellular vesicle-mediated transfer of miR-21-5p from mesenchymal stromal cells to neurons alleviates early brain injury to improve cognitive function via the PTEN/Akt pathway after subarachnoid hemorrhage. Cell Death Dis. 2020;11(5):363–363. doi: 10.1038/s41419-020-2530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zrzavy T, Schwaiger C, Wimmer I, Berger T, Bauer J, Butovsky O, Schwab JM, Lassmann H, Höftberger R. Acute and non-resolving inflammation associate with oxidative injury after human spinal cord injury. Brain. 2021;144(1):144–161. doi: 10.1093/brain/awaa360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, Shao C, Zhang L, Siedlak SL, Meabon JS, Peskind ER, Lu Y, Wang W, Perry G, Cook DG, Zhu X. Antioxidants. 6. Vol. 10. Basel: 2021. Oxidative stress signaling in blast TBI-induced Tau phosphorylation; pp. 955–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montivero AJ, Ghersi MS, Silvero CM, Artur de la Villarmois E, Catalan-Figueroa J, Herrera M, Becerra MC, Hereñú CB, Pérez MF. Early IGF-1 gene therapy prevented oxidative stress and cognitive deficits induced by traumatic brain injury. Front Pharmacol. 2021;12:672392. doi: 10.3389/fphar.2021.672392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakanishi K, Takeda S, Sakamoto A, Kitamura A. Effects of ulinastatin treatment on the cardiopulmonary bypass-induced hemodynamic instability and pulmonary dysfunction. Crit Care Med. 2006;34(5):1351–1357. doi: 10.1097/01.Ccm.0000215110.55899.Ae. [DOI] [PubMed] [Google Scholar]
- 19.Liu T, Liao XZ, Zhou MT. Ulinastatin alleviates traumatic brain injury by reducing endothelin-1. Transl Neurosci. 2021;12(1):1–8. doi: 10.1515/tnsci-2021-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He QL, Zhong F, Ye F, Wei M, Liu WF, Li MN, Li QB, Huang WQ, Sun LB, Shu HH. Does intraoperative ulinastatin improve postoperative clinical outcomes in patients undergoing cardiac surgery: a meta-analysis of randomized controlled trials. Biomed Res Int. 2014;2014:630835. doi: 10.1155/2014/630835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui L, Cao W, Xia Y, Li X. Ulinastatin alleviates cerebral ischemia-reperfusion injury in rats by activating the Nrf-2/HO-1 signaling pathway. Ann Transl Med. 2020;8(18):1136–1136. doi: 10.21037/atm-20-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li XF, Zhang XJ, Zhang C, Wang LN, Li YR, Zhang Y, He TT, Zhu XY, Cui LL, Gao BL. Ulinastatin protects brain against cerebral ischemia/reperfusion injury through inhibiting MMP-9 and alleviating loss of ZO-1 and occludin proteins in mice. Exp Neurol. 2018;302:68–74. doi: 10.1016/j.expneurol.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Liu M, Shen J, Zou F, Zhao Y, Li B, Fan M. Effect of ulinastatin on the permeability of the blood-brain barrier on rats with global cerebral ischemia/reperfusion injury as assessed by MRI. Biomed Pharmacother. 2017;85:412–417. doi: 10.1016/j.biopha.2016.11.044. [DOI] [PubMed] [Google Scholar]
- 24.Yan XL, Xu FY, Ji JJ, Song P, Pei YQ, He MJ, Wang ZC, You SJ, Hua ZC, Cheng J, Jia J. Activation of UCP2 by anethole trithione suppresses neuroinflammation after intracerebral hemorrhage. Acta Pharmacol Sin. 2022;43(4):811–828. doi: 10.1038/s41401-021-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu F, Zi L, Yang P, Wei Y, Zhong R, Wang Y, You C, Li Y, Tian M, Gu Z. Efficient iron and ROS nanoscavengers for brain protection after intracerebral hemorrhage. ACS Appl Mater Interfaces. 2021;13(8):9729–9738. doi: 10.1021/acsami.1c00491. [DOI] [PubMed] [Google Scholar]
- 26.Hayes JD, Dinkova-Kostova AT. Epigenetic control of NRF2-directed cellular antioxidant status in dictating life-death decisions. Mol Cell. 2017;68(1):5–7. doi: 10.1016/j.molcel.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Sun XG, Zhang MM, Liu SY, Chu XH, Xue GQ, Zhang BC, Zhu JB, Godje Godje, Zhu LJ, Hu HY, Hai W, Shen YJ, Wang GQ. Role of TREM-1 in the development of early brain injury after subarachnoid hemorrhage. Exp Neurol. 2021;341:113692. doi: 10.1016/j.expneurol.2021.113692. [DOI] [PubMed] [Google Scholar]
- 28.Deng S, Sherchan P, Jin P, Huang L, Travis Z, Zhang JH, Gong Y, Tang J. Recombinant CCL17 enhances hematoma resolution and activation of CCR4/ERK/Nrf2/CD163 signaling pathway after intracerebral hemorrhage in mice. Neurotherapeutics. 2020;17(4):1940–1953. doi: 10.1007/s13311-020-00908-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji J, Hong X, Su L, Liu Z. Proteomic identification of hippocalcin and its protective role in heatstroke-induced hypothalamic injury in mice. J Cell Physiol. 2019;234(4):3775–3789. doi: 10.1002/jcp.27143. [DOI] [PubMed] [Google Scholar]
- 30.Tang C, Shan Y, Hu Y, Fang Z, Tong Y, Chen M, Wei X, Fu X, Xu X. FGF2 attenuates neural cell death via suppressing autophagy after rat mild traumatic brain injury. Stem Cells Int. 2017;2017:2923182. doi: 10.1155/2017/2923182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen JH, Wu T, Xia WY, Shi ZH, Zhang CL, Chen L, Chen QX, Wang YH. An early neuroprotective effect of atorvastatin against subarachnoid hemorrhage. Neural Regen Res. 2020;15(10):1947–1954. doi: 10.4103/1673-5374.280326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Xuan Y, Chen Y, Wu T, Chen L, Guan H, Yang S, He J, Shi D, Wang Y. Netrin-1 alleviates subarachnoid haemorrhage-induced brain injury via the PPAR gamma/NF-KB signalling pathway. J Cell Mol Med. 2019;23(3):2256–2262. doi: 10.1111/jcmm.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Zhang C, Yan T, Yang L, Wang Y, Shi Z, Li M, Chen Q. Atorvastatin ameliorates early brain injury after subarachnoid hemorrhage via inhibition of pyroptosis and neuroinflammation. J Cell Physiol. 2021;236(10):6920–6931. doi: 10.1002/jcp.30351. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Dong Y, Liu D, Zou Z, Hao G, Gao X, Pan P, Liang G. NEK7 coordinates rapid neuroinflammation after subarachnoid hemorrhage in mice. Front Neurol. 2020;11:551–551. doi: 10.3389/fneur.2020.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Y, Feng X, Chen J, Wu Y, Shen L. Hydrogen-rich saline alleviates early brain injury through inhibition of necroptosis and neuroinflammation via the ROS/HO-1 signaling pathway after traumatic brain injury. Exp Ther Med. 2022;23(2):126–126. doi: 10.3892/etm.2021.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das S, Chattopadhyay D, Chatterjee SK, Mondal SA, Majumdar SS, Mukhopadhyay S, Saha N, Velayutham R, Bhattacharya S, Mukherjee S. Increase in PPAR? inhibitory phosphorylation by Fetuin-A through the activation of Ras-MEK-ERK pathway causes insulin resistance. Biochim Biophys Acta Mol Basis Dis. 2021;1867(4):166050–166050. doi: 10.1016/j.bbadis.2020.166050. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Zhang C, Yan T, Yang L, Wang Y, Shi Z, Li M, Chen Q. Atorvastatin ameliorates early brain injury after subarachnoid hemorrhage via inhibition of pyroptosis and neuroinflammation. J Cell Physiol. 2021;236(10):6920–6931. doi: 10.1002/jcp.30351. [DOI] [PubMed] [Google Scholar]
- 38.Chen J-H, Wu T, Yang L-K, Chen L, Zhu J, Li P-P, Hu X, Wang Y-H. Protective effects of atorvastatin on cerebral vessel autoregulation in an experimental rabbit model of subarachnoid hemorrhage. Mol Med Rep. 2018;17(1):1651–1659. doi: 10.3892/mmr.2017.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang CC, Hsiao LD, Lin HH, Tseng HC, Situmorang JH, Leu YL, Yang CM. Induction of HO-1 by 5, 8-Dihydroxy-4’,7-Dimethoxyflavone via activation of ROS/p38 MAPK/Nrf2 attenuates thrombin-induced connective tissue growth factor expression in human cardiac fibroblasts. Oxid Med Cell Longev. 2020;2020:1080168. doi: 10.1155/2020/1080168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin CH, So YK, Han SN, Kim JB. Biomol Ther. 5. Vol. 24. Seoul: 2016. Isoegomaketone upregulates heme oxygenase-1 in RAW264.7 cells via ROS/p38 MAPK/Nrf2 pathway; pp. 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S, Xu J, Gao Y, Shen P, Xia S, Li Z, Zhang M. Multi-organ protection of ulinastatin in traumatic cardiac arrest model. World J Emerg Surg. 2018;13:51–51. doi: 10.1186/s13017-018-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karnad DR, Bhadade R, Verma PK, Moulick ND, Daga MK, Chafekar ND, Iyer S. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: a multicenter randomized controlled study. Intensive Care Med. 2014;40(6):830–838. doi: 10.1007/s00134-014-3278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lv B, Jiang XM, Wang DW, Chen J, Han DF, Liu XL. Protective effects and mechanisms of action of ulinastatin against cerebral ischemia-reperfusion injury. Curr Pharm Des. 2020;26(27):3332–3340. doi: 10.2174/1381612826666200303114955. [DOI] [PubMed] [Google Scholar]
- 44.Cui T, Zhu G. Ulinastatin attenuates brain edema after traumatic brain injury in rats. Cell Biochem Biophys. 2015;71(2):595–600. doi: 10.1007/s12013-014-0239-3. [DOI] [PubMed] [Google Scholar]
- 45.Koga Y, Fujita M, Tsuruta R, Koda Y, Nakahara T, Yagi T, Aoki T, Kobayashi C, Izumi T, Kasaoka S, Yuasa M, Maekawa T. Urinary trypsin inhibitor suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Neurol Res. 2010;32(9):925–932. doi: 10.1179/016164110x12645013515133. [DOI] [PubMed] [Google Scholar]
- 46.Li HF, Zhao SX, Xing BP, Sun ML. Ulinastatin suppresses endoplasmic reticulum stress and apoptosis in the hippocampus of rats with acute paraquat poisoning. Neural Regen Res. 2015;10(3):467–472. doi: 10.4103/1673-5374.153698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho YS, Shin MS, Ko IG, Kim SE, Kim CJ, Sung YH, Yoon HS, Lee BJ. Ulinastatin inhibits cerebral ischemia-induced apoptosis in the hippocampus of gerbils. Mol Med Rep. 2015;12(2):1796–1802. doi: 10.3892/mmr.2015.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang W, Huang W, Chen S, Li Z, Wang W, Wang M. Changes of tumor necrosis factor-alpha and the effects of ulinastatin injection during cardiopulmonary cerebral resuscitation. J Huazhong Univ Sci Technolog Med Sci. 2004;24(3):269–271. doi: 10.1007/bf02832009. [DOI] [PubMed] [Google Scholar]
- 49.Song D, Song G, Niu Y, Song W, Wang J, Yu L, Yang J, Lv X, Steinberg H, Liu SF, Wang B. Ulinastatin activates haem oxygenase 1 antioxidant pathway and attenuates allergic inflammation. Br J Pharmacol. 2014;171(19):4399–4412. doi: 10.1111/bph.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li ST, Dai Q, Zhang SX, Liu YJ, Yu QQ, Tan F, Lu SH, Wang Q, Chen JW, Huang HQ, Liu PQ, Li M. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-κB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol Sin. 2018;39(8):1294–1304. doi: 10.1038/aps.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu HX, Xu DH, Ju WN, Ma C, Wang X, Liu XL. Neuroprotection of ulinastatin on transient cerebral ischemia via antioxidative mechanisms. J Biol Regul Homeost Agents. 2018;32(2):283–288. [PubMed] [Google Scholar]
- 52.Kim YM, Kim HJ, Chang KC. Glycyrrhizin reduces HMGB1 secretion in lipopolysaccharide-activated RAW 264.7 cells and endotoxemic mice by p38/Nrf2-dependent induction of HO-1. Int Immunopharmacol. 2015;26(1):112–118. doi: 10.1016/j.intimp.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Su L, Zhang X, Zhang C, Wang L, Li Y, Zhang Y, He T, Zhu X, Cui L. Ulinastatin downregulates TLR4 and NF-kB expression and protects mouse brains against ischemia/reperfusion injury. Neurol Res. 2017;39(4):367–373. doi: 10.1080/01616412.2017.1286541. [DOI] [PubMed] [Google Scholar]