ABSTRACT
Schistosomiasis is considered one of the Neglected Tropical Diseases (NTDs), which affects around 240 million people worldwide. In Brazil, Schistosomiasis mansoni has been registered in 19 states, predominantly in rural areas. This study aimed to analyze the spatial distribution of Schistosomiasis mansoni cases in the Maranhao State from 2007 to 2016, as well as the temporal trend over this period. The data were obtained from secondary sources: Schistosomiasis Control Program of Maranhao (PCE-MA) and Information System for Notifiable Diseases (SINAN). The State Health Regions (HRs) were considered analysis units. Maranhao had a positivity rate of 3.8 for the period. The Regions that presented the highest percentages of positivity in the state were Pinheiro (7.92), Ze Doca (3.30), and Viana (3.10). Municipalities such as Bacuri, Serrano do Maranhao, and Bequimao, located in the Pinheiro HR, showed positivity rates of 16.56, 13.31, and 11.01 respectively. The spatial analysis of schistosomiasis cases showed that Maranhao has two main centers for the spread of the disease, both located in the northern portion of the state, namely the Baixada Maranhense and the east coast. This study concluded that the positivity of Schistosomiasis mansoni in Maranhao was stable over the analyzed period. The state still maintains the Baixada Maranhense micro-region as an important area for the spread of the disease reaching socially vulnerable population groups.
Keywords: Neglected diseases, Epidemiology, Transmission, Spatial distribution
INTRODUCTION
Schistosomiasis, a parasitic disease caused by trematode worms of the genus Schistosoma Weinland, 1858, is the second most prevalent worldwide, being one of the 20 Neglected Tropical Diseases. This endemic disease is highly relevant in the global health scenario and it predominantly affects the poorest populations living in rural or in vulnerable urban areas. Such environments bring together factors that contribute to the successful establishment of schistosomiasis, such as the absence of or poor basic sanitation, abundant water collections with the presence of intermediate hosts and a lack of information about the disease or how to prevent it 1 .
As schistosomiasis is a mandatory water-borne disease, for the completeness of the Schistosoma mansoni Sambon 1907 biological cycle and the establishment of parasitic transmission in a certain area, three conditions must be met: existence of parasitized people; existence of water collections contaminated by feces and colonized by snails of the genus Biomphalaria Preston, 1910; and human contact with these water streams 2,3 .
The areas where these conditions are most observed are the rural regions and the peri-urban areas of the municipalities. The lack of basic sanitation, inadequate water supply, and disinformation are the factors that contribute most to this situation 3-5 .
The endemic affects more than 240 million people worldwide. Its transmission has been recorded in 78 countries, mainly in Africa and the Eastern Mediterranean. However, preventive treatment is only required in 51 countries where moderate to high transmission is reported. Estimates show that at least 236.6 million people needed this type of treatment for schistosomiasis in 2019, whereas only 105.4 million were reported to have been treated 1,6 .
Brazil is considered the largest endemic area in the Americas. According to data from the Ministry of Health, it is estimated that approximately 1.5 million people are infected with S. mansoni in Brazil. The occurrence of schistosomiasis is registered in 18 states and the Federal District, spread over all regions of the country. However, most cases are concentrated in the Northeastern and Southeastern regions, mainly in coastal areas 2,7-11 .
In the Maranhao State, schistosomiasis has been known since 1920 12,13 . According to the 2011 epidemiological situation report, the disease was considered endemic in 20 municipalities and focal in 29 out of the 217 existing municipalities. The oldest foci with the highest prevalence rates were found in the North Coast Zones and in the Baixada Maranhense, a region of wetlands. In 2010, the prevalence of the state was 5.27% of 69,005 people examined. From 2005 to 2010, there was an annual average of six hospitalizations and eight deaths, with a reduction in the mortality rate per 100,000 inhabitants from 0.20 in 2005 to 0.02 in 2010 14 .
In 2007, 5,363 cases of schistosomiasis were reported in the endemic area of the state, a number that gradually regressed to a total of 920 cases in 2016. For the non-endemic area, 3 cases were registered in 2007, which increased to 79 cases in 2010, and progressively decreased to 9 cases in 2016 15 .
Some peripherical neighborhoods in the capital of Maranhao have foci of schistosomiasis transmission, as demonstrated by Oliveira et al. 16 , and other regions of the Maranhao State still demonstrate a maintenance of the disease prevalence 13,17 .
Given the scarcity of epidemiological studies on schistosomiasis in the Maranhao State, as well as studies that analyze the spatial distribution of the disease and the factors that interfere with it, it is extremely important to complete an in-depth verification of how the disease has been established in the state over the last years. This study aimed to analyze the temporal trend and the spatial distribution of the positivity of Schistosomiasis mansoni in the state of Maranhao from 2007 to 2016.
MATERIALS AND METHODS
Study design
An analytical observational study was carried out with an ecological design in which secondary data sources were used. The area considered for this study was the Maranhao State (MA), Brazil. With an area of 331,937,450 km 2 and with 217 municipalities, it is the eighth largest state in Brazil and has a population of 7,153,262 inhabitants. The state is divided into 19 Health Regions (HRs) which constituted the unit of analysis for this study.
The population included in the analysis was the “population surveyed”, a designation given by the Schistosomiasis Control Program (Programa de Controle da Esquistossomose [PCE-MA]), which consists of the number of people who are considered for the coproscopic survey. This population is reached by active search carried out by the program. The data were collected from records of the State Health Department (Secretaria de Estado da Saude [SES-MA]), between 2007 and 2016, which were made available through the Information System of the Schistosomiasis Control Program (Sistema de Informacao do Programa de Controle da Esquistossomose [SIS-PCE]) 18 .
The positivity of schistosomiasis was considered the dependent variable, which is the percentage of positive cases recorded over a period of time and is obtained by the following calculation:
The positivity of each HR corresponds to the ratio number of cases/ number of tests performed in the municipalities belonging to this collection area. Municipalities that did not present a positivity index were excluded from the study. The years of study were considered as an independent variable.
Data analysis
Thematic maps were constructed using the software Quantum GIS (version 2.18, QGIS Geographic Information System, QGIS Association) for the positivity of the disease in the HRs during the period considered for the analysis of the parasitosis spatial distribution in the state. The positivity in each HR was stratified at two levels: low (< 5%) and medium (5 to 15%). For this study, the high positivity level (≥ 15%) was disregarded, as the state does not present positivity of the disease at this level. The white areas on the map represent HRs with no registered transmission of schistosomiasis, with no data available or those that were disregarded in this study.
The Kernel density estimation method was also used (at municipalities level) with the averages of the positivity rate, in order to identify the disease density patterns. Through this method, events are analyzed on a continuous surface (without political-administrative divisions). It describes how much the density at a point can influence its neighborhood, in the case of this study, for neighboring municipalities, and it also estimates the probability of the occurrence of an event in each cell of a regular grid, so that the nearest events receive higher weights, and the most distant, lower weights, creating a gradual scale of values 19 .
The data on the number of people surveyed and people with eggs were entered into Microsoft Excel® spreadsheets (version 2201, Microsoft, USA), where the positivity was calculated.
For the analysis of the trend in the positivity of schistosomiasis cases over the period, the annual percentage change (APC) was calculated with a 95% confidence interval (CI), using the Joinpoint method for modeling, where the positivity was considered as a response variable and the years as a regressive variable. This model allows for the analysis of temporal trends (incidence, mortality, survival, or prevalence rates) checking for changes (joinpoints) in the observed trend pattern. The Monte Carlo permutation tests are used to compare the various models tested, and then one finds the best model, that is, the one that fits the data best 20 . Only models from those HRs that had data for the complete series of years were obtained. The schistosomiasis trend analysis tests were performed using the Joinpoint Regression Program (version 4.7.0.0, National Cancer Institute, Calverton, MD, USA).
The data used in this research were obtained from secondary and public domain sources, in which there is no nominal identification of patients, thus respecting ethical aspects of Resolution N° 466/2012 of the National Health Council.
RESULTS
The PCE-MA registered 1,024,413 individuals as population surveyed from 2007 to 2016. In this period, 899,672 coproscopic examinations were performed (corresponding to 87.82% of this population). 34,806 positive people were diagnosed with S. mansoni in the state, obtaining an average positivity percentage of 3.87. The highest positivity rate (4.97) was recorded in 2010 and the lowest (2.54) in 2016. The total number of tests performed decreased by 65.4%, dropping from 132,301 to 45,747 by the end of the period. There was also a variation in the number of municipalities surveyed, reducing from 45 to 32. During the period, there was an average of 87.37% of these registered positive cases for S. mansoni. The treatment coverage for schistosomiasis in the state was 91.3% (Table 1).
Table 1. Operational indicators of the Schistosomiasis Control Program, Maranhao State, Brazil, 2007 to 2016.
| Indicators | Year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | Total | |
| Municipalities covered | 45 | 36 | 41 | 40 | 40 | 35 | 32 | 32 | 33 | 32 | - |
| Endemic municipalities | 41 | 31 | 35 | 38 | 37 | 30 | 26 | 28 | 29 | 26 | - |
| Population surveyed | 149.313 | 103.558 | 129.163 | 117.220 | 107.301 | 100.984 | 94.190 | 93.375 | 75.892 | 53.417 | 1.024.413 |
| Performed exams | 132.301 | 90.640 | 111.879 | 102.260 | 93.640 | 93.263 | 80.635 | 81.946 | 67.388 | 45.747 | 899.699 |
| Positive exams | 5.363 | 3.904 | 5.200 | 5.083 | 3.381 | 2.543 | 2.857 | 3.133 | 2.180 | 1.162 | 34.806 |
| Positivity (%)* | 4.05 | 4.31 | 4.65 | 4.97 | 3.61 | 2.73 | 3.54 | 3.82 | 3.23 | 2.54 | 3.87 |
| Proportion of municipalities worked/endemic (%) | 91.11 | 86.11 | 85.37 | 95.00 | 92.50 | 85.71 | 81.25 | 87.50 | 87.88 | 81.25 | - |
| People to treat | 6.696 | 3.904 | 5.224 | 5.085 | 3.831 | 2.543 | 2.888 | 3.134 | 2.180 | 1.236 | 36.721 |
| People treated | 5.235 | 3.817 | 5.050 | 5.019 | 3.313 | 2.487 | 2.568 | 2.939 | 2.011 | 1.097 | 33.536 |
*number of people positive for S. mansoni / number of people examined X 100.
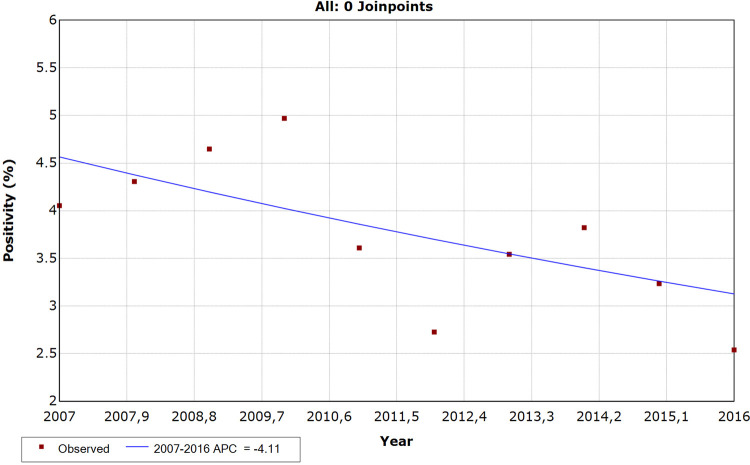
The Maranhao State showed a stable trend in the positivity rate of Schistosomiasis mansoni (APC = -4.11; CI = -8.5 to 0.5; p > 0.05), according to the analysis of the historical series by segmented regression (joinpoint regression) (Figure 1). Maranhao has always been below Brazil’s positivity, except in 2015, when it was observed that the state positivity (3.23) was slightly above the national (3.16).
Figure 1. Historical series of the positivity rate of schistosomiasis mansoni in the Maranhao State, Brazil, 2007 to 2016.
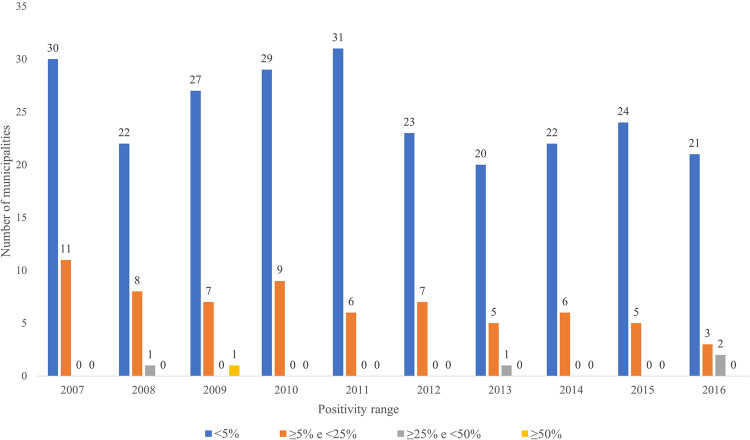
The distribution of Maranhao municipalities according to the positivity range for the disease shows that 22.9% of them are located in endemic regions (> 5%), while 77.71% registered a positivity rate of < 5% (Figure 2). For this study, the high positivity level (> 15%) was disregarded as the state does not present positivity of the disease at this level.
Figure 2. Distribution of municipalities according to the positivity range in the Maranhao State, Brazil, 2007 to 2016.
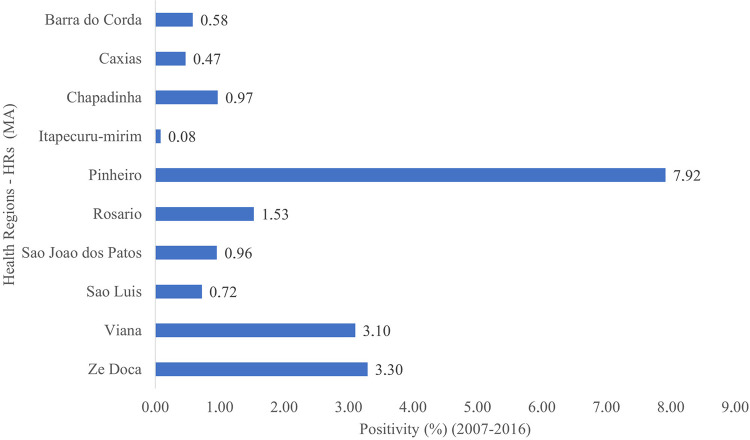
During the period under study, some HRs showed expressive positivity rates such as the Pinheiro HR, followed by the HRs of Viana and Ze Doca. The HRs of Caxias and Itapecuru had the lowest positivity rates (Figure 3). The HRs of Acailandia, Bacabal, Balsas, Codo, Imperatriz, Pedreiras, Presidente Dutra, Santa Ines, and Timon were excluded from the study because they presented incomplete or non-computed data.
Figure 3. Positivity of Schistosomiasis mansoni by Health Region in the Maranhao State, Brazil, 2007 to 2016.
When the positivity was analyzed by HR, two of them showed a statistically significant decrease in cases (p < 0.05). The Sao Luis HR showed the greatest downward trend (APC = -20.11; CI = -30.7 to -7.8; p < 0.05), followed by the Ze Doca HR (APC = -16.72; CI = -22.0 to -11.1; p < 0.05).
The HRs of Chapadinha, Pinheiro, Sao Joao dos Patos and Viana have a stable trend, indicating that these HRs present a stationary series. The annual rates of change were: Chapadinha (APC = -6.01; CI = -15.9 to 5.0; p > 0.05); Pinheiro (APC = -4.16; CI = -8.6 to 0.5; p > 0.05); Sao Joao dos Patos (APC = -4.75; CI = -10.5 to 1.4; p > 0.05); Viana (APC = -2.77; CI = -7.9 to 2.7; p > 0.05).
For the remaining Regions (Barra do Corda, Caxias, Itapecuru-Mirim, Rosario) it was not possible to generate a temporal trend due to insufficient data for the period.
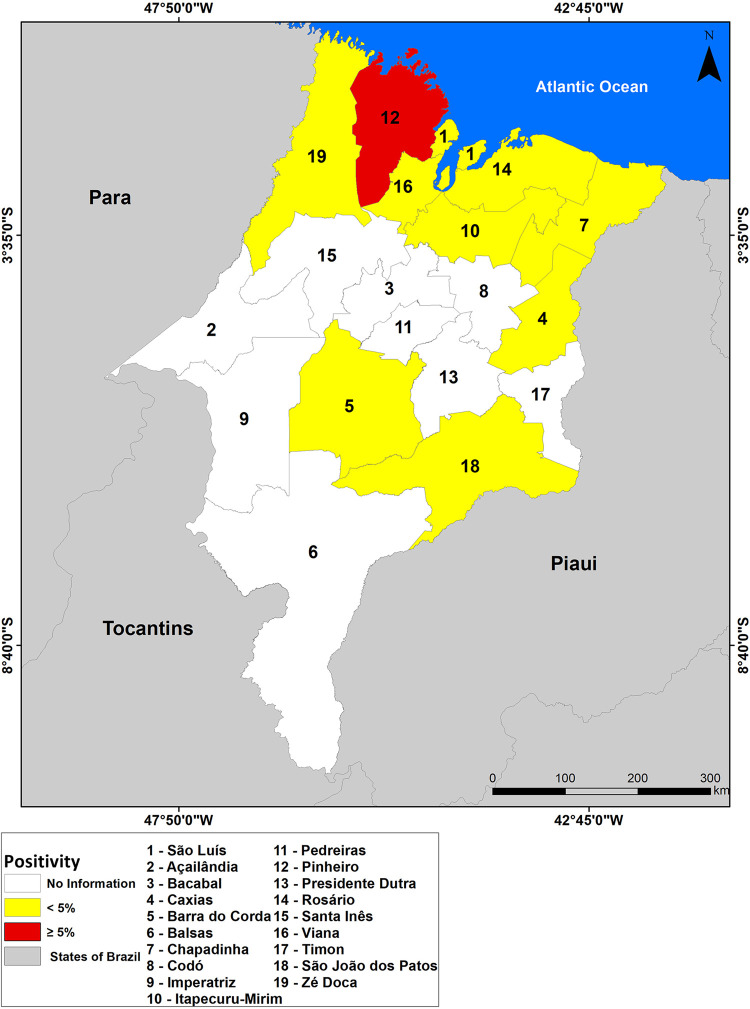
Schistosomiasis was registered in 44 municipalities in the Maranhao State over the period, distributed across ten HRs. This means that the endemic disease reached 20.3% of Maranhao’s municipalities, and 52.6% of the HRs (Figure 4).
Figure 4. Spatial distribution of the positivity of schistosomiasis by Health Region in the Maranhao State, Brazil, 2007 to 2016.
Through the map in Figure 4, it can be observed that only the HR of Pinheiro registered positivity above 5%, being located in the medium positivity range. The remaining Regions that had reported cases (Barra do Corda, Caxias, Chapadinha, Itapecuru-mirim, Rosario, Sao Joao dos Patos, Sao Luis, Viana and Ze Doca) remained in the low positivity range (< 5%). The HRs of Acailandia, Bacabal, Balsas, Codo, Imperatriz, Pedreiras, Presidente Dutra, Santa Ines, and Timon are shown in white because they do not have data available (or have incomplete data).
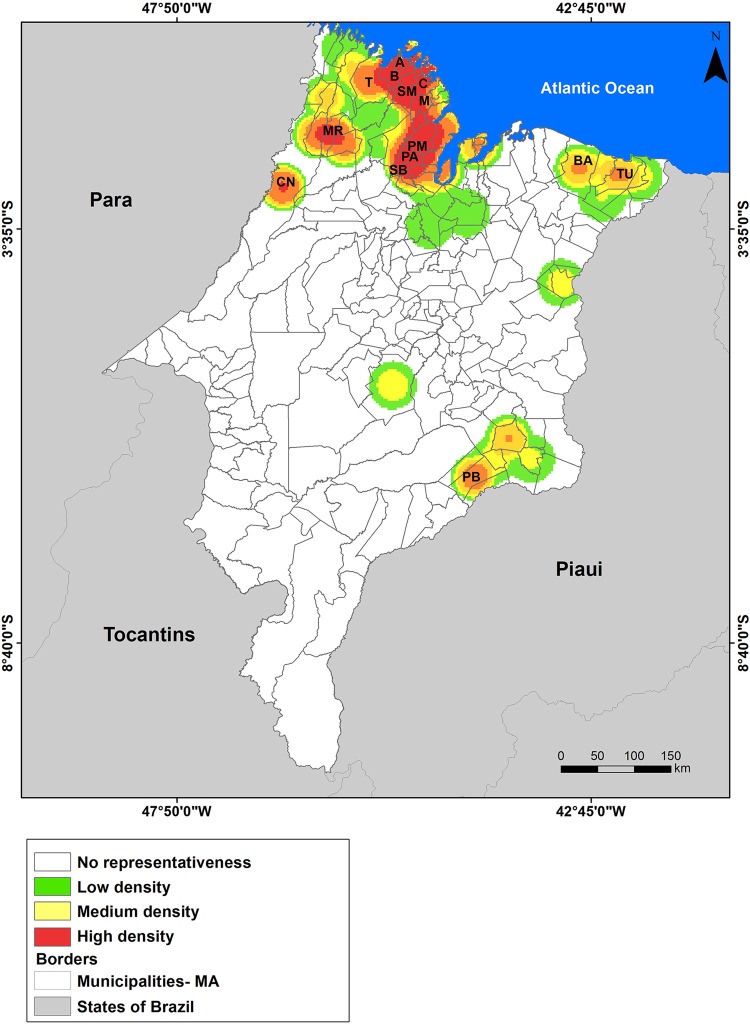
The Kernel Map, through interpolation, showed that there is a high density (hot spots) of higher rates of occurrence of schistosomiasis cases located in the northern and western mesoregions of Maranhao (Figure 5).
Figure 5. Kernel analysis map on the density of schistosomiasis cases by municipality in the Maranhao State, Brazil, 2007 to 2016.
In the northern mesoregion, where the Baixada Maranhense micro-region and the Pinheiro and Viana HRs are located, the municipalities with the highest positivity rates in the state were (B) Bacuri (16.56), (SM) Serrano do Maranhao (13.31), (A) Apicum-acu (11.00), (C) Cururupu (8.31), (SB) Sao Bento (5.61) and (PM) Peri-mirim (5.12), being located in the range of medium endemicity. Altogether, these six municipalities represented 69.9% of schistosomiasis cases in the Maranhao State between the years 2007 and 2016 (Figure 5).
The municipalities of (T) Turiacu and (M) Mirinzal (Pinheiro HR) and (PA) Palmeirandia (Viana HR), showed positivity below 5%, but they also represent important foci of schistosomiasis in the Baixada Maranhense region. In the western mesoregion, the municipalities of (CN) Centro Novo do Maranhao and (MR) Maranhaozinho proved to be important areas for the occurrence of schistosomiasis with a positivity rate of 4.34 and 4.38 respectively.
Other points that deserve attention on the map are the municipalities located on the east coast of Maranhao, such as (TU) Tutoia and (BA) Barreirinhas, and in the eastern mesoregion, (PB) Pastos Bons, near to the Boa Esperanca Dam, which constitute hot spots of positivity of cases (although moderate).
DISCUSSION
In the period considered for this study, the mean positivity of schistosomiasis for the Maranhao State (3.87) remained below the Brazilian average (4.77), being similar in 2010 and 2015. The temporal analysis showed that, although there is a tendency to reduce positivity in Maranhao, this is not significant, which leads us to conclude that there is a maintenance of the rate of schistosomiasis in the state. Only the Sao Luis and Ze Doca HRs showed a trend of significant reduction in their positivity rates.
Cantanhede et al. 17 , analyzing the trend of prevalence in the Health Regions of Maranhao between 1997 and 2003, found divergent results from the present study. In the analysis, the Presidente Dutra and Bacabal Regions had the highest rates, with 12.8% and 9%, respectively, while Balsas and Pinheiro Regions had the lowest percentages.
In the Pernambuco State, the average positivity for schistosomiasis between 2005 and 2010 was 9.2%, above the national average of 5.9%. The Health Region III (Palmares) had the highest positive average (13.8%), followed by HRs II (Limoeiro: 9.9%) and I (Recife: 7.8%) 21 . For the Sergipe State, between 2005 and 2014, the average prevalence was 8.8% 11 . There was a significant downward trend in the number of cases in the analyzed periods for both states.
Despite the decrease in prevalence, Pernambuco was the Brazilian state with the highest proportion of deaths due to schistosomiasis between 1999 and 2014, 33.4% (2,578 deaths), and stood out among other historically endemic states, such as Alagoas (1,103), Minas Gerais (857) and Bahia (768). Maranhao was the tenth state regarding number of deaths, with 96 records 22 .
The first inquiry carried out by Pellon and Teixeira (1947-1952), which comprised the states of Maranhao, Piaui, Ceara, Rio Grande do Norte, Paraiba, Pernambuco, Alagoas, Sergipe, Bahia, Espirito Santo and Minas Gerais, found a prevalence of 10%. After this, two other inquiries followed. The second was carried out in the context of the implementation of the Special Program for the Control of Schistosomiasis - PECE (1975-1979) by the Superintendence of Public Health Campaigns - SUCAM, from the Ministry of Health. In this survey, 447,768 students were examined, resulting in 30,068 positive tests, with a prevalence of 6.7%. The results of this survey helped the PECE to define the limits of its area of operation and guide the application of control measures 23 .
The third and most current Schistosomiasis and Geo-helminth Prevalence Survey was the first with coverage in all states of the Federation, in which 197,564 schoolchildren aged 7 to 17 years old, of both genders, residing in 521 municipalities were examined. The survey results showed that the Northeastern and Southeastern regions of the country had the highest rates of positivity, 1.27% and 2.35%, respectively. The states with the highest prevalence rates were Sergipe (10.67%), Pernambuco (3.77%), Alagoas (3.35%), Minas Gerais (5.81%) and Bahia (2.91%) 23 . Comparing the rates revealed by the three schistosomiasis surveys conducted, it can be seen that there was in fact a reduction in the rate of positivity of the disease in the country, although the Northeastern and Southeastern regions still remain important areas for the spread of the endemic disease. In the South Region, there is no evidence of infection by S. mansoni.
The results of the spatial analysis performed in this study suggest that the distribution of schistosomiasis in the Maranhao State is configured in a way to form clusters, which we call “hot areas”. In general, it can be noted that the occurrence of the disease is more intense in the northern portion of the state and less intense in the central and southern regions.
The southern, central-eastern and northeastern regions of Maranhao are part of the biome Cerrado and have a higher relief. Such environmental conditions provide a less humid environment, so that they do not have large water courses. In addition, these regions have municipalities with a higher Municipal Human Development Index - MHDI when compared to other regions of the state, demonstrating that these municipalities have better income, education and longevity 24 .
The northwestern portion of Maranhao has a lower relief, where most of the water courses converge, and has high rainfall, when compared to other regions of the state. For example, the Baixada Maranhense is a region of lowlands and flood plains, which are supplied by several rivers and streams from other parts of the state and have high precipitation rates (above 2,000 mm per year), with more than nine humid months and a period of drought which is not strict 25 . Such environmental conditions allow for the establishment of the intermediate host snails of schistosomiasis and the establishment of the disease transmission cycle.
Through spatial analysis, a study showed that in Sergipe the distribution of the endemic disease affects 68% of the municipalities, with the highest prevalence rates concentrated mainly in the eastern and coastal regions of the state 11 . According to the Maranhao Epidemiological Situation Report for the year 2011, schistosomiasis was considered endemic in 20 municipalities and focal in 29 of the 217 existing municipalities, with the North Coast and Baixada Maranhense Zones concentrating the oldest foci, with the highest prevalence rates when compared to other regions of the state 14 . This study found a scenario similar to that described in the document.
When observing the rates of positivity by Health Regions, it appears that schistosomiasis has two main endemic nuclei in Maranhao. The first, and epidemiologically most important, is the microregion of the Baixada Ocidental Maranhense, an area considered endemic and comprising the Pinheiro, Viana, and Ze Doca Regions (partially), with positivity rates of 7.69, 3.07, and 3.18 respectively. The second area of dissemination of the disease that deserves to be highlighted are the HRs of Rosario and Chapadinha, located in the northern mesoregion of Maranhao, with positive rates of 1.53 and 0.95 respectively.
When considering the positivity rate of schistosomiasis at a regional level, we must pay attention to which municipalities are most responsible for this number. For example, the positivity of the HRs of Rosario and Chapadinha is strongly influenced by the municipalities of Barreirinhas and Tutoia, which present a positivity of 1.58 and 1.52, respectively. Both municipalities are located on the east coast of the state. In an epidemiological survey carried out in 2008, Santos and Melo 26 found a prevalence of 3.2% in the village of Bom Gosto, in the municipality of Tutoia.
Pinheiro, Viana, and Ze Doca HRs also have municipalities with high rates when compared to the others. This is the case of Bacuri, Serrano do Maranhao and Bequimao, located in the Pinheiro HR, with positivity rates of 16.56, 13.31 and 11.01 respectively. In the Viana HR, the municipalities of Sao Bento (5.61), Palmeirandia (3.24) and Bacurituba (2.16) stand out. In the Ze Doca HR, the municipalities of Maranhaozinho and Centro Novo do Maranhao have the highest rates (4.38 and 4.34, respectively). In the Sao Luis HR, the municipality with the highest positivity rate was Alcantara (2.21), the only one of the four in this region located on the continent, adjacent to the Pinheiro HR, and it may be suffering from its influence.
In the Pernambuco State, Zona da Mata, adjacent to the coastal region of the state and consisting of 43 municipalities, is a traditional endemic area for schistosomiasis, being the region of the state with the largest number of children infected by parasites and the populations most intensely infected by S. mansoni 27,28 . This area has favorable environmental conditions for the phenomenon, an abundance of water collections, and the presence of breeding grounds for host snails. Such factors enable the parasite to survive outside the human organism. In addition, the high poverty rate, low levels of hygiene, and low coverage of basic sanitation contribute to sustaining the disease cycle in this region 21 . Other studies have shown that the coastal region has presented risk factors that contribute to the spread of the disease in this area 29,30 .
This scenario that brings together biotic, social and economic factors is similar to that found in the Baixada Maranhense Region, in the northwest of the Maranhao State. The environmental characteristics of this territory, added to the work activities performed there, constitute a favorable scenario for the establishment of several diseases. For example, Schistosomiasis has been registered in the region due to the presence of intermediate hosts that live there, snails B. glabrata and B. straminea, species of great epidemiological importance for the disease. The existence of flooded areas for a long period of the year contributes to the proliferation of these mollusks, which end up transmitting the disease to humans. Ramos et al. 31 concluded that floods can contribute to the expansion of schistosomiasis by providing conditions for the installation of new outbreaks.
In general, it was found that the highest percentages of positivity for schistosomiasis were observed not in the main city of each HR, but in the smaller municipalities covered by the latter. This can be explained by the fact that the host cities concentrate a greater offer of health services available to their population, while the others do not have them or depend largely on the services offered by the main city.
The use of data from secondary sources, such as SISPCE, has some limitations due to the way they are organized for exposure to the public. This can happen at different stages of the organization and availability process of the data. There may be flaws in the data collection stage such as underreporting of cases, insufficient or representative coproscopic survey coverage, lack or typo of data, non-transfer of data to the superior office instance and the way to making the data available. As it is the responsibility of the municipalities, they carry out the disease control actions within their respective realities and possibilities which do not always correspond to a satisfactory and adequate level of work. Even so, SISPCE constitutes an important source of data due to the great extent in which the Control Program operates, reflecting the epidemiological situation of the disease in the state even if it’s in an inaccurate way.
CONCLUSION
Through this study, it is concluded that the Maranhao State presented a tendency towards stability in the positivity rate of schistosomiasis. In terms of geographic distribution, Maranhao has two main areas for the dissemination of the disease in the state: the Baixada Maranhense micro-region and the east coast. The Health Regions that include the municipalities of Baixada Maranhense have the highest positivity rates, a fact that can be explained by the precarious environmental conditions associated with low socioeconomic indicators of the population living in these cities.
ACKNOWLEDGMENTS
We wish to thank the Fundacao de Amparo a Pesquisa e ao Desenvolvimento Cientifico e Tecnologico do Maranhao (FAPEMA), for the scholarship provided to RJAM and the Secretaria Estadual de Saude do Estado do Maranhao for providing the data for this research.
REFERENCES
- 1.World Health Organization Schistosomiasis. [cited 2022 Jul 6]. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
- 2.Martins-Melo FR, Pinheiro MC, Ramos AN, Jr, Alencar CH, Bezerra FS, Heukelbach J. Spatiotemporal patterns of schistosomiasis-related deaths, Brazil, 2000–2011. Emerg Infect Dis. 2015;21:1820–1823. doi: 10.3201/eid2110.141438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbosa CS, Favre TC, Quinino LR, Gomes EC, Domingues AL, Pieri OS. Guia para vigilância e controle da esquistossomose: práticas de laboratório e campo. Recife: Editora Universitária da Universidade Federal de Pernambuco; 2008. Os moluscos transmissores; pp. 27–37. [Google Scholar]
- 4.Saucha CV, Silva JA, Amorim LB. Condições de saneamento básico em áreas hiperendêmicas para esquistossomose no estado de Pernambuco em 2012. Epidemiol Serv Saude. 2015;24:497–506. [Google Scholar]
- 5.Freeman MC, Garn JV, Sclar GD, Boisson S, Medlicott K, Alexander KT, et al. The impact of sanitation on infectious disease and nutritional status: a systematic review and meta-analysis. Int J Hyg Environ Health. 2017;220:928–949. doi: 10.1016/j.ijheh.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization [cited 2022 Jul 6];Schistosomiasis: status of schistosomiasis endemic countries, 2020. https://apps.who.int/neglected_diseases/ntddata/sch/sch.html
- 7.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Coordenação-Geral de Desenvolvimento da Epidemiologia em Serviços . Guia de vigilância em saúde. 3. Brasília: Ministério da Saúde; 2019. [cited 2022 Jul 6]. https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_3ed.pdf [Google Scholar]
- 8.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde Doenças tropicais negligenciadas: 30 de janeiro – dia mundial de combate às doenças tropicais negligenciadas. [cited 2022 Jul 6];Bol Epidemiol. 2021 (Esp):1–74. https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/boletins-epidemiologicos/especiais/2021/boletim_especial_doencas_negligenciadas.pdf [Google Scholar]
- 9.Silva BM, Ferreira AF, Silva JA, Amorim RG, Domingues AL, Pinheiro MC, et al. Persistence of schistosomiasis-related morbidity in Northeast Brazil: an integrated spatio-temporal analysis. Trop Med Infect Dis. 2021;6:193. doi: 10.3390/tropicalmed6040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinheiro MC, Ferreira AF, Silva JD, Filho, Lima MS, Martins-Melo FR, Bezerra FS, et al. Burden of schistosomiasis-related mortality in Brazil: epidemiological patterns and spatial–temporal distribution, 2003–2018. Trop Med Int Health. 2020;25:1395–1407. doi: 10.1111/tmi.13483. [DOI] [PubMed] [Google Scholar]
- 11.Santos AD, Lima AC, Santos MB, Alves JA, Góes MA, Nunes MA, et al. Spatial analysis for the identification of risk areas for schistosomiasis mansoni in the State of Sergipe, Brazil, 2005-2014. Rev Soc Bras Med Trop. 2016;49:608–615. doi: 10.1590/0037-8682-0137-2016. [DOI] [PubMed] [Google Scholar]
- 12.Alvim MC. A esquistossomose mansônica no Maranhão. Hiléia Med. 1980;2:151–157. [Google Scholar]
- 13.Lira MG, Miranda GS, Rodrigues JG, Nogueira RA, Gomes GC, Silva-Souza N. Ocorrência de Schistosoma mansoni no município de São Bento, Baixada Ocidental Maranhense, estado do Maranhão, Brasil. Rev Pan-Amaz Saude. 2017;8:45–51. [Google Scholar]
- 14.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde . Sistema nacional de vigilância em saúde: relatório de situação: Maranhão. 5. Brasília: Ministério da Saúde; 2011. [cited 2022 Jul 6]. https://bvsms.saude.gov.br/bvs/publicacoes/sistema_nacional_vigilancia_saude_ma_5ed.pdf [Google Scholar]
- 15.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica [cited 2022 Jul 6];PCE - Programa de Controle da Esquistossomose. http://tabnet.datasus.gov.br/cgi/sinan/pce/PCE-nota-tecnica.pdf
- 16.Oliveira DS, Nunes GS, Mendes RJ, França CR, Pereira AA, Filho, Tavares CP, et al. Inquérito malacológico para identificar a célula de expansão da esquistossomose mansônica na Vila Embratel, um bairro de periferia de São Luís do Maranhão. Cad Pesq. 2013;20(Esp):16–19. [Google Scholar]
- 17.Cantanhede SP, Ferreira AP, Mattos IE. Esquistossomose mansônica no Estado do Maranhão, Brasil, 1997-2003. Cad Saude Publica. 2011;27:811–816. doi: 10.1590/s0102-311x2011000400020. [DOI] [PubMed] [Google Scholar]
- 18.Brasil. Ministério da Saúde. DATASUS [cited 2022 Jul 6];Programa de Controle da Esquistossomose (PCE) https://datasus.saude.gov.br/acesso-a-informacao/programa-de-controle-da-esquistossomose-pce/
- 19.Carvalho MS, Souza-Santos R. Análise de dados espaciais em saúde pública: métodos, problemas, perspectivas. Cad Saude Pública. 2005;21:361–378. doi: 10.1590/s0102-311x2005000200003. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with application to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.Barreto AV, Melo ND, Ventura JV, Santiago RT, Silva MB. Análise da positividade da esquistossomose mansoni em Regionais de Saúde endêmicas em Pernambuco, 2005 a 2010. Epidemiol Serv Saude. 2015;24:87–96. [Google Scholar]
- 22.Barbosa CS, Gomes EC, Campos JV, Oliveira FJ, Mesquita MC, Oliveira EC, et al. Morbidity of mansoni schistosomiasis in Pernambuco - Brazil: analysis on the temporal evolution of deaths, hospital admissions and severe clinical forms (1999–2014) Acta Trop. 2016;164:10–16. doi: 10.1016/j.actatropica.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Katz N. Inquérito nacional de prevalência da esquistossomose mansoni e geo-helmintoses. Belo Horizonte: Fiocruz; 2018. [Google Scholar]
- 24.Instituto Brasileiro de Geografia e Estatística. Coordenação de População e Indicadores Sociais . Indicadores sociais municipais: uma análise dos resultados do universo do censo demográfico 2010. Rio de Janeiro: IBGE; 2011. [cited 2022 Jul 6]. https://biblioteca.ibge.gov.br/biblioteca-catalogo?id=254598&view=detalhes [Google Scholar]
- 25.Farias MS., Filhoorganizador . O espaço geográfico da Baixada Maranhense. 2. São Luís: EDUFMA; 2013. [Google Scholar]
- 26.Santos AM, Melo AC. Prevalência da esquistossomose num povoado do Município de Tutóia, Estado do Maranhão. Rev Soc Bras Med Trop. 2011;44:97–99. doi: 10.1590/s0037-86822011000100021. [DOI] [PubMed] [Google Scholar]
- 27.Barbosa CS, Favre TC, Wanderley TN, Callou AC, Pieri OS. Assessment of schistosomiasis, through school surveys, in the Forest Zone of Pernambuco, Brazil. Mem Inst Oswaldo Cruz. 2006;101(Suppl):55–62. doi: 10.1590/s0074-02762006000900009. [DOI] [PubMed] [Google Scholar]
- 28.Barbosa VS, Araújo KC, Leal OB, Neto, Barbosa CS. Spatial distribution of schistosomiasis and geohelminthiasis cases. Rev Soc Bras Med Trop. 2012;45:633–638. doi: 10.1590/s0037-86822012000500017. [DOI] [PubMed] [Google Scholar]
- 29.Barbosa CS, Pieri OS, Silva CB, Simões FB. Ecoepidemiologia da esquistossomose urbana na ilha de Itamaracá, Estado de Pernambuco. Rev Saude Publica. 2000;34:337–341. doi: 10.1590/s0034-89102000000400004. [DOI] [PubMed] [Google Scholar]
- 30.Leal OB, Neto, Gomes EC, Oliveira FJ, Junior, Andrade R, Reis DL, Souza-Santos R, et al. Biological and environmental factors associated with risk of schistosomiasis mansoni transmission in Porto de Galinhas, Pernambuco State, Brazil. Cad Saude Publica. 2013;29:357–367. doi: 10.1590/s0102-311x2013000200022. [DOI] [PubMed] [Google Scholar]
- 31.Ramos AS, Piza JT, Fróes E. A importância das inundações na expansão da esquistossomose mansoni. Rev Saude Publica. 1970;4:1–5. [PubMed] [Google Scholar]