Abstract
Darkly pigmented individuals are at the greatest risk of hypovitaminosis D, which may result in microvascular endothelial dysfunction via reduced nitric oxide (NO) bioavailability and/or increased oxidative stress and inflammation. We investigated the associations among skin pigmentation (M-index; skin reflectance spectrophotometry), serum vitamin D concentration [25(OH)D], circulating inflammatory cytokine (TNF-α, IL-6, and IL-10) concentrations, and the NO contribution to local heating-induced cutaneous vasodilation (%NO-mediated vasodilation) in a diversely pigmented cohort of young adults. An intradermal microdialysis fiber was placed in the forearms of 33 healthy adults (14 men/19 women; 18–27 yr; M-index, 30–81 AU) for local delivery of pharmacological agents. Lactated Ringer’s solution was perfused through the fiber during local heating-induced (39°C) cutaneous vasodilation. After attaining stable elevated blood flow, 15 mM NG-nitro-l-arginine methyl ester (l-NAME; NO synthase inhibiter) was infused to quantify %NO-mediated vasodilation. Red cell flux was measured (laser-Doppler flowmetry; LDF) and cutaneous vascular conductance (CVC = LDF/MAP) was normalized to maximal (%CVCmax; 28 mM sodium nitroprusside + 43°C). Serum [25(OH)D] and circulating cytokines were analyzed by ELISA and multiplex assay, respectively. M-index was negatively associated with [25(OH)D] (r = −0.57, P < 0.0001) and %NO-mediated vasodilation (r = −0.42, P = 0.02). Serum[25(OH)D] was positively related to %NO (r = 0.41, P = 0.02). Controlling for [25(OH)D] weakened the association between M-index and %NO-mediated dilation (P = 0.16, r = −0.26). There was a negative curvilinear relation between [25(OH)D] and circulating IL-6 (r = −0.56, P < 0.001), but not TNF-α or IL-10 (P ≥ 0.14). IL-6 was not associated with %NO-mediated vasodilation (P = 0.44). These data suggest that vitamin D insufficiency/deficiency may contribute to reduced microvascular endothelial function in healthy, darkly pigmented young adults.
NEW & NOTEWORTHY Endothelial dysfunction, an antecedent to hypertension and overt CVD, is commonly observed in otherwise healthy Black adults, although the underlying causes remain unclear. We show that reduced vitamin D availability with increasing degrees of skin pigmentation is associated with reduced microvascular endothelial function, independent of race or ethnicity, in healthy young adults. Greater prevalence of vitamin D deficiency in more darkly pigmented individuals may predispose them to increased risk of endothelial dysfunction.
Keywords: inflammation, melanin, nitric oxide, race, skin blood flow
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in the United States, and the Black population experiences a disproportionate burden compared with other groups (1) (note: through the use of “Black” or “White,” we are using standard National Institutes of Health population descriptors; however, for the purposes of this study, participants were primarily described by skin pigmentation). Furthermore, hypertension, one of the primary risk factors for CVD, is more prevalent and develops at a younger age in the Black population. Although the causes of hypertension and CVD are multifaceted, vascular endothelial dysfunction has been implicated in their development (2, 3). Reduced endothelial function in conduit arteries (flow-mediated dilation; FMD) and the microvasculature (local skin heating) have been demonstrated in otherwise healthy Black adults (4–7), at least in part because of elevated oxidative stress and/or reduced nitric oxide (NO) production and bioavailability. Although less is known about other minority populations in the United States, some evidence suggests an increasing prevalence of cardiovascular dysfunction and disease in the Hispanic-American, Asian-American, and Native-American populations (8–12). The focus on “racial” differences in vascular function (4, 5, 13, 14) often ignores the underlying biological factors, as well as disparate social and environmental conditions, that precipitate physiological dysfunction (15).
The biologically active metabolite of vitamin D, calcitriol, may play an important role in endothelial function by signaling for the transcription of endothelial NO synthase (eNOS) (16), by reducing oxidative stress via increased expression of superoxide dismutase (SOD) (17) and/or by suppressing nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) (18–20). In addition, vitamin D may improve endothelial function by reducing inflammation; vitamin D deficiency in humans is associated with increased concentrations of circulating inflammatory cytokines (21, 22) including interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) and activation of the proinflammatory transcription factor nuclear factor-κB (NF-κB) (23). Approximately 90% of the human vitamin D requirement {serum [25(OH)D] of 30–60 ng/mL} is met by skin exposure to sunlight (24, 25). Mechanistically, ultraviolet radiation (UVR, specifically UVB in the context of vitamin D production) is absorbed by epidermal and dermal stores of 7-dehydrocholesterol (7-DHC), resulting in formation of previtamin D3, which is rapidly converted to vitamin D3. Because eumelanin in keratinocytes of the skin absorbs UVR, increased skin melanization (i.e., a darker skin pigmentation) decreases UVB-induced cutaneous vitamin D synthesis (26), resulting in greater risk of vitamin D insufficiency (20–30 ng/mL) or deficiency (<20 ng/mL) in more darkly pigmented adults (27).
Recent work from our laboratory demonstrated lower serum [25(OH)D] concentrations in young, healthy Black compared with White subjects (7). Furthermore, we and others (4, 5) have reported that the magnitude of the cutaneous vasodilation response during local heating, as well as the NO contribution to that response (%NO-mediated vasodilation), was lower in Black compared with White participants. Four weeks of vitamin D supplementation improved serum [25(OH)D] and the magnitude of the cutaneous microvascular response to local heating, as well as the %NO-mediated dilation, in Black but not White subjects, suggesting that ensuring sufficient [25(OH)D] in Black adults may be an effective intervention to mitigate the development of vascular endothelial dysfunction. However, only two distinctly divergent samples of Black (who identified specifically as African American) and White (who identified specifically as Caucasian or European American) participants were included in those studies. Those studies have provided important insights into the mechanisms underlying disparities in the development of CVD between those two distinct groups; however, it remains unclear to what degree skin pigmentation per se and vitamin D status influence vascular endothelial function across a diverse population with a wide range of skin pigmentation.
Therefore, the purpose of the present study was to investigate the associations between 1) skin pigmentation and [25(OH)D], 2) skin pigmentation and microvascular function (measured as the %NO contribution to local heating-induced vasodilation), and 3) [25(OH)D] and the %NO contribution to the local heating response (39°C protocol). A secondary aim was to examine the relation between [25(OH)D] and microvascular function with select circulating biomarkers of inflammation (TNF-α, IL-6, and IL-10). We hypothesized that darker skin pigmentation would be associated with lower [25(OH)D] and microvascular function and that higher [25(OH)D] would be associated with better microvascular function. We further hypothesized that higher [25(OH)D] would be associated with lower circulating cytokine concentrations and that lower circulating cytokine concentrations would be associated with better microvascular function.
METHODS
Subjects
All experimental protocols were approved by the Institutional Review Board at The Pennsylvania State University (No. 12598). Written and verbal consent were obtained voluntarily from all subjects before participation, in accordance with the guidelines set forth by the Declaration of Helsinki. Healthy men and women aged 18–27, with normal blood pressure (SBP < 130, and DBP < 85 mmHg), low-density lipoprotein cholesterol < 150 mg/dL, and hemoglobin A1C (HbA1C) ≤ 5.7% were included. Subjects were recruited without regard to race or ethnicity and were normally active, healthy nonsmokers who were free of cardiovascular, kidney, or skin diseases, disorders of pigmentation, or skin allergies, and were not taking any prescription medications with primary or secondary vascular effects. Subjects were excluded if they were taking a vitamin D supplement or other supplements that may influence vitamin D status. All women were premenopausal; thus, a urine pregnancy test confirmed the absence of pregnancy before experimental visits. The phase of menstrual cycle was not controlled for among women participants (28). All subjects underwent an initial screening that included physical examination, lipid profile, and blood chemistry (Quest Diagnostics, Pittsburgh, PA). All data were collected between the months of October and March, during which time UVB exposure in the northeastern region of the United States does not produce vitamin D even in lightly pigmented skin, thus eliminating seasonality as a potential source of variation (29).
With an anticipated slope of 0.6, based on preliminary data collected in our laboratory before the start of the current study, an a priori sample size analysis (α = 0.05 and 1−β = 0.8) suggested that 27 participants would be sufficient to detect a significant relation between [25(OH)D] and the %NO contribution to the cutaneous vasodilation response during local heating.
Assessment of Skin Pigmentation
Skin pigmentation was measured by reflectance spectrophotometry (DermaSpectrometer; Cortex Technology, Hadsund, Denmark), as previously described (7, 30, 31), to determine the melanin index (M-index) of the skin on the subject’s inner aspect of the upper arm. The M-index was measured in this region because of its ease of access and because it represents constitutive skin pigmentation due to its relatively low sun exposure (32). Lower and higher M-indices are related to lighter and darker skin pigmentation, respectively.
Experimental Procedures
All protocols were performed in a thermoneutral laboratory with the subject lying in a semisupine position. A single intradermal microdialysis fiber (CMA Linear 31 probe, 55 kDa, Harvard Apparatus, Holliston, MA) was placed into the dermal layer of the ventral aspect of the left forearm for local delivery of pharmacological agents (33). Pharmacological agents were mixed just before use, dissolved in lactated Ringer’s solution, sterilized using syringe microfilters (Acrodisc; Pall, Port Washington, NY), and wrapped in foil to prevent degradation due to light exposure. All solutions were perfused through microdialysis fibers at a rate of 2 µL/min (Bee Hive controller and Baby Bee microinfusion pumps; Bioanalytical Systems) (33). Local red blood cell flux was measured directly over each microdialysis site throughout local heating with an integrated laser-Doppler flowmetry probe placed in a local heating unit (Moor Instruments SHO2, Moor Instruments, Inc., Wilmington, DE).
After placement of microdialysis fibers, an ∼60-min period was allowed for hyperemia associated with fiber placement to resolve. Baseline data were then collected (∼20 min) before beginning a local heating (39°C) protocol as previously described (4, 34), during which lactated Ringer’s solution was perfused through the microdialysis fiber. This heating protocol elicits an initial axon-reflex-mediated peak skin blood flow response, followed by a brief nadir, after which there is a gradual rise and eventual (after ∼40 min) blood flow plateau. After the observation of a stable local heating plateau, 15 mM of NG-nitro-l-arginine methyl ester (l-NAME; NO synthase inhibitor) was perfused, allowing for quantification of NO-dependent vasodilation (%NO) (35, 36). After the observation of a stable l-NAME plateau, 28 mM sodium nitroprusside (SNP; USP, Rockland, MD) was perfused and local temperature was increased to 43°C to elicit maximal vasodilation (CVCmax) (37, 38).
Serum Vitamin D and Inflammatory Cytokine Analyses
Blood samples were collected in serum separator tubes during experimental visits. Serum was isolated by centrifugation and stored at −80°C for later analysis. Serum concentrations of 25(OH)D, the primary circulating metabolite of vitamin D, were quantified in duplicate using an ELISA kit according to manufacturer’s instructions (CrystalChem, Elk Grove Village, IL; CV < 10%). Serum concentrations of IL-6, IL-10, and TNF-α were quantified using a Meso Scale Discovery (MSD, Rockville, MD; CV < 20%) multiplex assay.
Data Acquisition and Analysis
Data were recorded at 40 Hz and stored for off-line analysis (PowerLab/LabChart, ADInstruments, Bella Vista, NSW, Australia; WINDAQ, DATAQ Instruments, Akron, OH). Mean arterial pressure (MAP) was calculated for each phase of the protocol using blood pressure taken from an automated blood pressure monitor (CardioCap; GE Healthcare, Chicago, IL). Cutaneous vascular conductance was calculated as red blood cell flux divided by MAP and expressed as a percentage of CVCmax (%CVCmax) for each phase of the local heating protocol (38, 39). The NO contribution to cutaneous vasodilation was calculated as the difference between the local heating and l-NAME plateau responses.
Normality of all data was confirmed through visual inspection with the use of quantile-quantile (Q-Q) plots. Simple linear regression analyses (GraphPad Software, San Diego, CA) were used to assess correlations between 1) M-index and [25(OH)D], 2) M-index and the %NO contribution to cutaneous vasodilation during local heating, and 3) [25(OH)D] and the %NO contribution to the local heating response. Partial correlation analysis was used to determine the relation between M-index and %NO-mediated dilation while controlling for serum [25(OH)D] (SPSS, version 9.4, SAS Institute, Inc., Cary, NC). Additional regression analyses were used to assess correlations between [25(OH)D] and circulating inflammatory cytokine concentrations and between cytokine concentrations and the %NO contribution to the cutaneous vasodilation response. The relation between [25(OH)D] and IL-6 concentration was better described by a nonlinear model; thus, a nonlinear curve was fit to describe the data. Separate one-way ANOVAs were performed to detect differences in %NO contribution to the local heating response and circulating IL-6 concentrations among groups who were vitamin D deficient (<20 ng/mL), insufficient (21–30 ng/mL), and sufficient (>30 ng/mL) based on clinical vitamin D categories set forth by The Endocrine Society (40–42). Tukey’s correction was used for post hoc pairwise comparisons. Hedges’ g effect sizes were calculated and reported for group comparisons. All analyses regarding %NO-mediated dilation were also conducted using the absolute magnitude of the vasodilation response, as %CVCmax; because the magnitude of the response to 39°C local heating is almost entirely mediated by NO (34), these analyses did not alter the results or interpretation. Thus, we opted to report the results using only the %NO contribution to the response.
Significance was accepted at α < 0.05 for all analyses. All summary data are presented as means ± SD.
RESULTS
Subject characteristics are presented in Table 1. Anthropometric characteristics and blood biochemistry values were within normal ranges for this age group. There were no associations between M-index and body mass index, MAP, HbA1c, or total or LDL cholesterol (all P > 0.07). The self-reported race of participants are as follows: White (n = 11), Black/African American (n = 9), Asian/Indian (n = 7), Hispanic/Latino (n = 2), and Mixed (n = 4). The range of M-indices represents the range from lightly (Fitzpatrick type 1) to darkly pigmented (Fitzpatrick type 6) skin (43).
Table 1.
Subject characteristics
| Characteristics | Means ± SD | Ranges |
|---|---|---|
| Age, yr | 22 ± 2 | 18–27 |
| BMI, kg/m2 | 24 ± 3 | 19–32 |
| Systolic BP, mmHg | 113 ± 7 | 100–124 |
| Diastolic BP, mmHg | 71 ± 8 | 50–82 |
| Heart rate, beats/min | 64 ± 7 | 51–76 |
| M-index, AU | 46 ± 14 | 30–81 |
| Blood biochemistry | ||
| HbA1c, % | 5.1 ± 0.3 | 4.5–5.7 |
| Total cholesterol, mg/dL | 160 ± 29 | 104–237 |
| HDL, mg/dL | 58 ± 13 | 35–92 |
| LDL, mg/dL | 87 ± 23 | 26–135 |
Values are means ± SD and ranges; n = 33 (14 men, 19 women). AU, arbitrary units; BMI, body mass index; BP, blood pressure; HbA1c, hemoglobin A1C; HDL, high-density lipoprotein; LDL, low-density lipoprotein; M-index, a skin-reflectance measure of melanization.
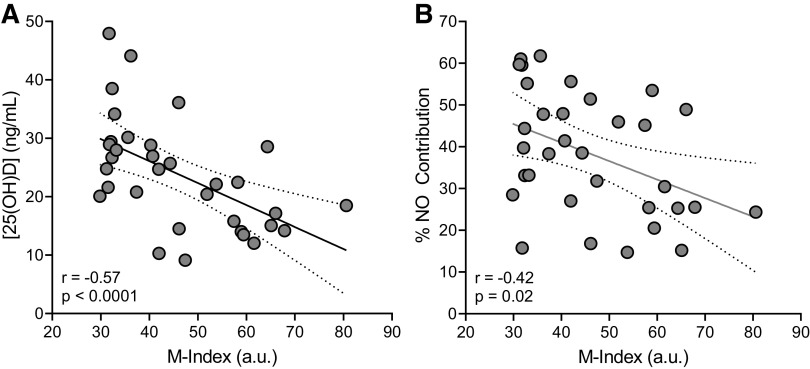
There was a negative relation (P < 0.0001, r = −0.57, β = −0.38) between M-index and serum [25(OH)D] (Fig. 1A), such that those with higher M-indices (more darkly pigmented) had lower circulating [25(OH)D]. Similarly, M-index was negatively related to the %NO contribution to the local heating response (Fig. 1B; P = 0.02, r = −0.42, β = −0.44). Importantly, there was no relation between skin pigmentation (P = 0.48) or serum [25(OH)D] (P = 0.42) and CVCmax.
Figure 1.
Associations between the melanin index (M-index) and serum vitamin D concentrations ([25(OH)D] (A) and M-index and the nitric oxide contribution (%NO) (B) to the cutaneous vasodilation response to local heating. Data were analyzed using simple linear regression analysis. For A and B, n = 33 (14 men, 19 women).
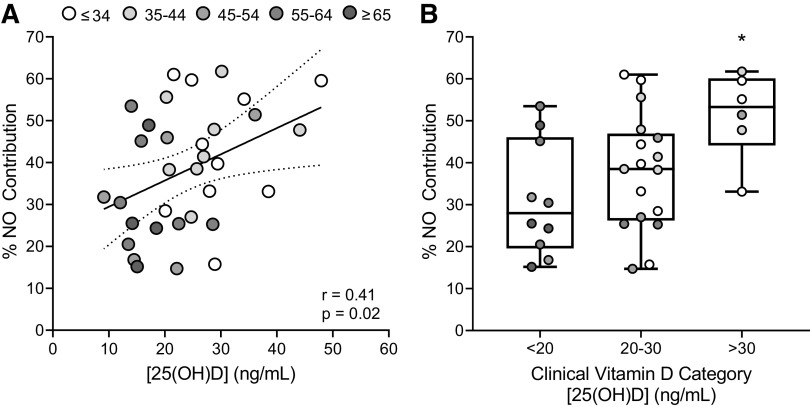
Serum [25(OH)D] was directly related to the %NO contribution to the local heating response (Fig. 2A; P = 0.02, r = 0.41, β = 0.26). When separating participants into clinical classifications based on serum [25(OH)D] (Fig. 2B), %NO-mediated dilation was significantly greater in those with vitamin D sufficiency (51.46 ± 10.35%) than with those who were deficient (31.21 ± 13.59%, P = 0.007; g = 1.61), but not the intermediate insufficient classification (37.79 ± 13.96%, P = 0.09; g = 1.04). There was no difference between groups with vitamin D deficiency and insufficiency (P = 0.44; g = 0.48).
Figure 2.
A: association between serum vitamin D concentrations [25(OH)D] and the nitric oxide contribution (%NO) to the cutaneous vasodilation response to local heating (n = 33; 14 men, 19 women). Data were analyzed using simple linear regression analysis. Grayscale of individual data points indicates graded increases in M-index (AU), as shown in the legend. B: group comparisons in the %NO contribution to the local heating response when participants were separated into groups based on clinical vitamin D categories (deficient, <20 ng/mL; insufficient, 20–30 ng/mL; and sufficient, >30 ng/mL). Grayscale of individual data points indicates graded increases in M-index, as shown in the legend in A. Group comparisons were made using one-way ANOVA with Tukey’s post hoc pairwise comparisons. *P < 0.05 compared with deficient.
To more directly determine if vitamin D status mediates the relation between skin pigmentation and microvascular function, we examined the association between M-index and %NO-mediated dilation while controlling for serum [25(OH)D]. Controlling for serum [25(OH)D] weakened the association between M-index and %NO-mediated dilation such that they were no longer significantly related (P = 0.16, r = −0.26).
There was a negative, curvilinear relation (P < 0.001, r = −0.56, β = −1.06) between serum [25(OH)D] and circulating IL-6 concentration (Fig. 3A). When participants were separated into clinical vitamin D categories (Fig. 3B), circulating IL-6 concentrations were significantly lower in those sufficient in vitamin D (0.30 ± 0.21 pg/mL) than in those who were vitamin D deficient (0.71 ± 0.42 pg/mL, P = 0.03; g = 1.13), but not the insufficient group (0.43 ± 0.22 pg/mL, P = 0.64; g = 0.60). However, there was no association between circulating IL-6 concentrations and the %NO contribution to the local heating response (Fig. 3C; P = 0.44, r = 0.14, β = −6.11). There were no associations between serum [25(OH)D] and circulating TNF-α (P = 0.14, r = −0.26, β = −0.01) or IL-10 (P = 0.25, r = −0.21, β = −0.006) concentrations, nor between circulating TNF-α (P = 0.29, r = −0.19, β = −7.49) or IL-10 (P = 0.87, r = −0.03, β = −1.72) concentrations and the %NO contribution to the local heating response.
Figure 3.
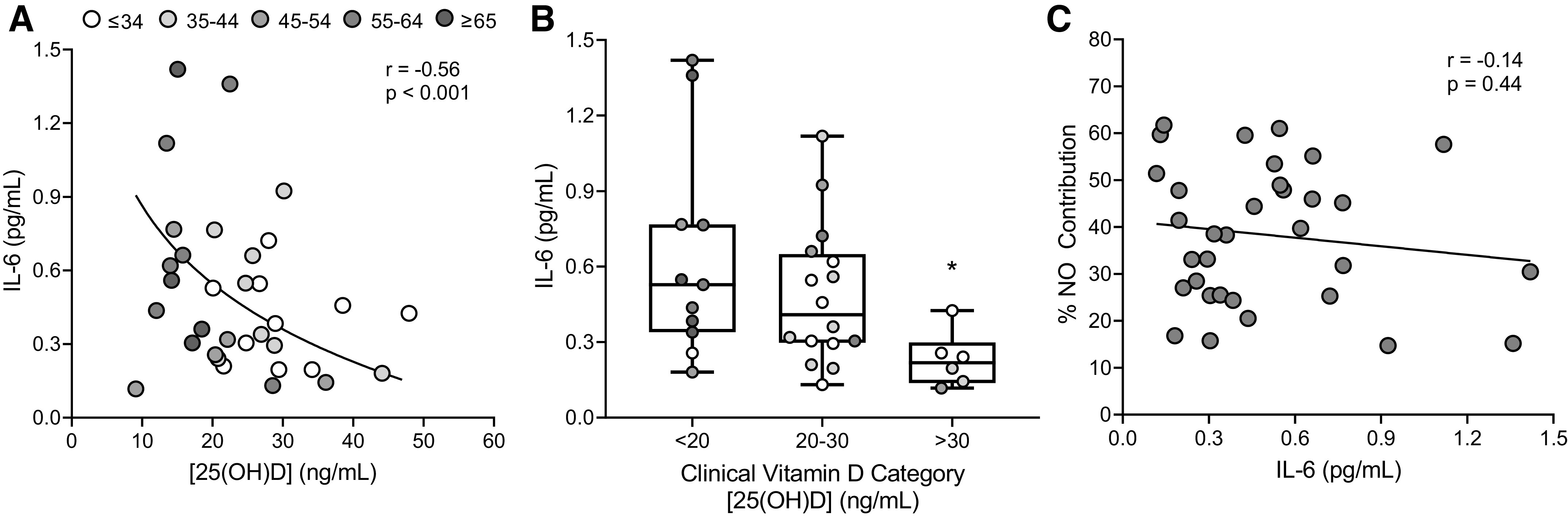
A: association between serum vitamin D concentrations [25(OH)D] and circulating interleukin-6 (IL-6) concentrations (n = 33; 14 men, 19 women). Grayscale of individual data points indicates graded increases in M-index (AU), as shown in the legend. Data were analyzed using nonlinear regression analysis. B: group comparisons in circulating IL-6 when participants were separated into groups based on clinical vitamin D categories (deficient, <20 ng/mL; insufficient, 20–30 ng/mL; and sufficient, >30 ng/mL). Grayscale of individual data points indicates graded increases in M-index, as shown in legend in A. Group comparisons were made using one-way ANOVA with Tukey’s post hoc pairwise comparisons. *P < 0.05 compared with deficient. C: association between circulating IL-6 concentrations and the nitric oxide contribution (%NO) to the cutaneous vasodilation response to local heating. Data were analyzed using simple linear regression analysis.
DISCUSSION
The primary findings of this study were that 1) skin pigmentation was negatively related to both serum [25(OH)D] and the NO contribution to cutaneous vasodilation during local heating and 2) serum [25(OH)D] was directly related to the NO contribution to the local heating response. Notably, controlling for serum [25(OH)D] weakened the association between skin pigmentation and %NO-mediated dilation, suggesting that vitamin D status mediates the relation between skin pigmentation and NO-dependent microvascular function. In addition, there was a negative curvilinear relation between serum [25(OH)D] and circulating concentrations of IL-6, although circulating IL-6 concentration was not related to NO-dependent cutaneous vasodilation. Finally, there were no associations between serum [25(OH)D] and TNF-α or IL-10. These findings suggest that reduced vitamin D availability in those with more darkly pigmented skin, independent of race or ethnicity, may contribute to reduced microvascular endothelial function. These data further suggest that although circulating IL-6 was elevated in those with lower vitamin D availability, differences in NO-dependent dilation of the cutaneous microvasculature among young adults with differently pigmented skin are not explained by circulating pro- or anti-inflammatory cytokine concentrations.
Hypertension and CVD are more prevalent and develop at a younger age in the Black population in the United States (1, 5). Reduced vascular endothelial function is an early manifestation in the pathogenesis of CVD development (2, 3, 44, 45) and is prevalent in young, otherwise healthy Black adults even in the absence of comorbidities such as hypertension or hypercholesterolemia (5, 7, 46, 47). The development of vascular endothelial dysfunction, hypertension, and overt CVD is multifactorial, likely resulting from social, environmental, and biological determinants (15). One biological factor that may contribute to reduced endothelial function in the Black population is vitamin D deficiency (7, 27). Eumelanin in keratinocytes of the skin absorbs UVR; thus, increased skin melanization (i.e., a darker skin pigmentation) decreases UVB-induced cutaneous vitamin D synthesis, resulting in a greater risk of vitamin D deficiency in more darkly pigmented populations living in the United States (26, 27, 48).
We previously demonstrated reduced serum [25(OH)D] and NO-dependent cutaneous microvascular responses to local skin heating in otherwise healthy, young Black adults (7). Four weeks of vitamin D3 supplementation significantly improved both serum [25(OH)D] and NO-dependent cutaneous microvascular function. Together, those findings support the notion that reduced vitamin D availability contributes to reduced endothelial function in the Black population. The present study extends those findings to a diverse cohort of healthy young adults with a wide range of skin pigmentation, demonstrating reduced [25(OH)D] with increasing degrees of skin pigmentation and increased NO-dependent microvascular responses with increasing serum [25(OH)D]. It is worth noting that the sample of participants in the present study was primarily American; thus, although we expect that our findings would apply to darkly pigmented individuals from other regions (e.g., Africa, South Asia, Melanesia, etc.) who move to areas of relatively low UVR exposure, this remains speculative and future investigation is warranted to examine these effects of skin pigmentation on vitamin D status and endothelial function in populations of various geographical origin.
In addition to examining the associations between serum [25(OH)D] and NO-dependent dilation in the cutaneous microvasculature, we separated participants into distinct groups based on clinical vitamin D categories: deficient (<20 ng/mL), insufficient (21–30 ng/mL), and sufficient (>30 ng/mL) (40, 42). That analysis demonstrated that those who were vitamin D sufficient had significantly greater NO-dependent dilation than the deficient group, with the insufficient group intermediate. In a previous study, brachial artery FMD responses were higher in middle-aged and older adults who were clinically sufficient for vitamin D relative to those who were deficient or insufficient, with no difference between the deficient and insufficient groups (49). Although we found no difference between the sufficient and insufficient groups in our study, the magnitude of the difference in the NO contribution to the cutaneous vasodilation response between those groups (51.46 vs. 37.73% in sufficient and insufficient groups, respectively) closely reflected the previously reported difference in FMD (49). Thus, the lack of a significant difference between those two groups in our study is likely due to the relatively small number (n = 6) of participants categorized as sufficient, despite a large effect size (g = 1.04). Regardless, our data support those of Jablonski et al. (49), suggesting that vascular endothelial function is healthiest in those who are clinically sufficient for vitamin D.
Vitamin D inhibits the proinflammatory transcription factor NF-κB (20, 23, 49). Downstream circulating proinflammatory biomarkers such as IL-6 and TNF-α are associated with increased risk of cardiovascular events (50) and reduced endothelial function (51, 52). IL-10 may also be regulated by vitamin D (53–55) and is typically considered to have anti-inflammatory and antioxidant properties (56, 57), potentially supporting healthy endothelial function (58, 59). We found a negative, curvilinear relation between serum [25(OH)D] and circulating IL-6 concentration, but no relations between [25(OH)D] and TNF-α or IL-10. Furthermore, there was no association between circulating IL-6 and NO-dependent cutaneous vasodilation. Increased expression of NF-κB and/or downstream proinflammatory cytokine protein expression in biopsied endothelial cells is associated with reduced endothelium-dependent vasodilation (49, 60, 61), independent of circulating factors. Furthermore, increasing serum concentrations of [25(OH)D] were associated with reduced endothelial cell NF-κB and IL-6 protein expression in middle-aged and older adults (49). Thus, although there were no associations between circulating inflammatory/anti-inflammatory markers and microvascular endothelial function in the current study, we cannot rule out the possibility of increased endothelial cell expression of proinflammatory cytokines in participants with lower serum [25(OH)D], which in turn may contribute to inflammation- and oxidative stress-mediated vascular endothelial function. Furthermore, the full expression of IL-6 effects on the endothelium requires classic (membrane-bound receptors) and trans (solubilized receptors) signaling (62, 63); thus, it is unsurprising that circulating IL-6 alone is not related to endothelial function.
In populations living at northern latitudes, dietary vitamin D intake is associated with vitamin D status, although hypovitaminosis D is still highly prevalent (64, 65). In the Toronto area, skin pigmentation was inversely related to serum [25(OH)D)], but only participants of European ancestry met the recommended adequate intake for vitamin D of 200 IU/day; thus, vitamin D intake may have influenced the association between skin pigmentation and vitamin D status in that study (66). However, even in participants, who met or exceeded the recommended 200 IU/day vitamin D intake, 84.4% of the participants had serum [25(OH)D] < 75 nmol/L (30 ng/mL), and 40.6% of the participants had [25(OH)D] < 50 nmol/L (20 ng/mL), suggesting that additional vitamin D supplementation may be warranted even in lightly pigmented individuals living at that latitude (∼44°N). Although we excluded participants who were taking any supplement that may influence vitamin D status, we did not conduct a dietary assessment to determine vitamin D intake through food sources in the present study. Approximately 90% of the human vitamin D requirement is met by skin exposure to sunlight in most people (24, 25), however; we, therefore, do not expect that accounting for dietary vitamin D intake would appreciably alter our findings.
We recognize that the development of vascular endothelial dysfunction, hypertension, and overt CVD is likely affected by myriad factors including social and environmental determinants of health [i.e., socioeconomic status, as well as the conditions in which people live, learn, work, etc., that affect a wide range of health and quality-of-life risks and outcomes (67)] in addition to biological conditions (15). The current study aimed to characterize the independent role of skin pigmentation/vitamin D bioavailability on microvascular endothelial function in a diverse cohort of young adults. In this context, we previously demonstrated that although NO-mediated cutaneous microvascular function and self-reported socioeconomic status were both lower in healthy, young Black compared with White adults on average, there was no relation between microvascular function and socioeconomic status (7). However, because these studies are conducted in young, healthy adults, it is unclear to what extent lifelong exposures to vitamin D deficiency, lower socioeconomic status, and other environmental conditions contribute to the pathogenesis of CVD. Findings from epidemiological studies have been equivocal regarding the influence of vitamin D status on CVD endpoints among racial and ethnic groups (68–70), potentially suggesting that the relative contributions of the various factors involved in the development of vascular dysfunction and CVD evolve throughout the aging process. Future work is critical to better understand these and other contributors to the pathogenesis of CVD throughout the lifespan and how best to mitigate the impact of those contributors.
In summary, this is the first study to our knowledge to demonstrate associations between skin pigmentation and both serum vitamin D concentrations and microvascular endothelial function in a diversely pigmented, multiracial cohort of healthy young adults. Our data further demonstrate that vitamin D status is directly related to NO-mediated cutaneous microvascular function, such that those who are vitamin D sufficient have the healthiest microvascular function. Moreover, controlling for serum vitamin D concentrations weakened the association between skin pigmentation and the NO contribution to the local heating response, suggesting that vitamin D status mediates, at least in part, the relation between skin pigmentation and NO-dependent microvascular function. Finally, reduced NO-dependent microvascular function in participants with lower vitamin D concentrations was not explained by increased circulating proinflammatory cytokines. Collectively, these findings have important implications regarding the factors underlying race differences in the pathogenesis of hypertension and CVD, suggesting that vitamin D status may be an important modulator of vascular endothelial function, particularly in those with more darkly pigmented skin living in areas with relatively low UVR exposure and/or high seasonality.
DATA AVAILABILITY
All supporting data are included within the main article. Additional information is available from the corresponding author upon request.
GRANTS
This research was supported by The Penn State Center for Human Evolution and Diversity Research Endowment Grant (to S.T.W.), National Institutes of Health (NIH) Grant 5T32AG049676-03 (to S.T.W. and G.A.D.), The Penn State College of Health and Human Development Limited Endowed Funds for Dissertation Research, and NIH Grant UL1 TR002014.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.T.W., L.M.A., N.G.J., and W.L.K. conceived and designed research; S.T.W. and G.A.D. performed experiments; S.T.W. and G.A.D. analyzed data; S.T.W. and W.L.K. interpreted results of experiments; S.T.W. prepared figures; S.T.W. drafted manuscript; S.T.W., G.A.D., L.M.A., N.G.J., and W.L.K. edited and revised manuscript; S.T.W., L.M.A., N.G.J., and W.L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful for the subjects’ participation and the expert assistance of Susan Slimak, RN.
REFERENCES
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135: e146–e603, 2017. [Erratum in Circulation 135: e646, 2017; and in Circulation 136: e196, 2017]. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noon JP, Walker BR, Webb DJ, Shore AC, Holton DW, Edwards HV, Watt GC. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest 99: 1873–1879, 1997. doi: 10.1172/JCI119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taddei S, Virdis A, Mattei P, Arzilli F, Salvetti A. Endothelium-dependent forearm vasodilation is reduced in normotensive subjects with familial history of hypertension. J Cardiovasc Pharmacol 20: S193–S195, 1992. doi: 10.1097/00005344-199204002-00054. [DOI] [PubMed] [Google Scholar]
- 4. Hurr C, Patik JC, Kim K, Christmas KM, Brothers RM. Tempol augments the blunted cutaneous microvascular thermal reactivity in healthy young African Americans. Exp Physiol 103: 343–349, 2018. doi: 10.1113/EP086776. [DOI] [PubMed] [Google Scholar]
- 5. Patik JC, Curtis BM, Nasirian A, Vranish JR, Fadel PJ, Brothers RM. Sex differences in the mechanisms mediating blunted cutaneous microvascular function in young black men and women. Am J Physiol Heart Circ Physiol 315: H1063–H1071, 2018. doi: 10.1152/ajpheart.00142.2018. [DOI] [PubMed] [Google Scholar]
- 6. Perregaux D, Chaudhuri A, Rao S, Airen A, Wilson M, Sung BH, Dandona P. Brachial vascular reactivity in blacks. Hypertension 36: 866–871, 2000. doi: 10.1161/01.HYP.36.5.866. [DOI] [PubMed] [Google Scholar]
- 7. Wolf ST, Jablonski NG, Ferguson SB, Alexander LM, Kenney WL. Four weeks of vitamin D supplementation improves nitric oxide-mediated microvascular function in college-aged African Americans. Am J Physiol Heart Circ Physiol 319: H906–H914, 2020. doi: 10.1152/ajpheart.00631.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alshehri MM, Alqahtani AS, Alenazi AM, Aldhahi M, Alothman S, Gray C, Alqahtani B, Khunti K, Kluding P. Associations between ankle-brachial index, diabetes, and sleep apnea in the Hispanic community health study/study of Latinos (HCHS/SOL) database. BMC Cardiovasc Disord 20: 118, 2020. doi: 10.1186/s12872-020-01402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deen JF, Adams AK, Fretts A, Jolly S, Navas-Acien A, Devereux RB, Buchwald D, Howard BV. Cardiovascular disease in American Indian and Alaska Native youth: unique risk factors and areas of scholarly need. J Am Heart Assoc 6: e007576, 2017. doi: 10.1161/JAHA.117.007576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, Cucchiara AJ, Savage PJ, Howard BV. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol 132: 1141–1155, 1990. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 11. North KE, Howard BV, Welty TK, Best LG, Lee ET, Yeh JL, Fabsitz RR, Roman MJ, MacCluer JW. Genetic and environmental contributions to cardiovascular disease risk in American Indians: the strong heart family study. Am J Epidemiol 157: 303–314, 2003. doi: 10.1093/aje/kwf208. [DOI] [PubMed] [Google Scholar]
- 12. Shariff AI, Kumar N, Yancy WS Jr, Corsino L. Type 2 diabetes and atherosclerotic cardiovascular disease in south asians: a unique population with a growing challenge. Curr Diab Rep 20: 4, 2020. doi: 10.1007/s11892-020-1291-6. [DOI] [PubMed] [Google Scholar]
- 13. Brothers RM, Fadel PJ, Keller DM. Racial disparities in cardiovascular disease risk: mechanisms of vascular dysfunction. Am J Physiol Heart Circ Physiol 317: H777–H789, 2019. doi: 10.1152/ajpheart.00126.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vranish JR, Holwerda SW, Young BE, Credeur DP, Patik JC, Barbosa TC, Keller DM, Fadel PJ. Exaggerated vasoconstriction to spontaneous bursts of muscle sympathetic nerve activity in healthy young Black men. Hypertension 71: 192–198, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wolf ST, Jablonski NG, Kenney WL. Examining “race” in physiology. Am J Physiol Heart Circ Physiol 319: H1409–H1413, 2020. doi: 10.1152/ajpheart.00698.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andrukhova O, Slavic S, Zeitz U, Riesen SC, Heppelmann MS, Ambrisko TD, Markovic M, Kuebler WM, Erben RG. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol Endocrinol 28: 53–64, 2014. doi: 10.1210/me.2013-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhong W, Gu B, Gu Y, Groome LJ, Sun J, Wang Y. Activation of vitamin D receptor promotes VEGF and CuZn-SOD expression in endothelial cells. J Steroid Biochem Mol Biol 140: 56–62, 2014. doi: 10.1016/j.jsbmb.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujii H, Kono K, Nakai K, Nishi S, Goto S, Fukagawa M, Kitazawa R, Kitazawa S, Hirata M, Shinohara M. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats. Am J Hypertens 27: 586–595, 2014. doi: 10.1093/ajh/hpt160. [DOI] [PubMed] [Google Scholar]
- 19. Lau CW, Wong SL, Yao X, Huang Y, Dong J, Zhang L, Lee HK, Ng CF, Chen ZY, Vanhoutte PM. Calcitriol protects renovascular function in hypertension by down-regulating angiotensin II type 1 receptors and reducing oxidative stress. Eur Heart J 33: 2980–2990, 2012. doi: 10.1093/eurheartj/ehr459. [DOI] [PubMed] [Google Scholar]
- 20. Wolf ST, Kenney WL. The vitamin D-folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiol 317: R491–R501, 2019. doi: 10.1152/ajpregu.00136.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med 168: 1340–1349, 2008. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 22. Peterson CA, Heffernan M. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25 (OH) D concentrations in healthy women. J Inflamm (Lond) 5: 10, 2008. doi: 10.1186/1476-9255-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suzuki Y, Ichiyama T, Ohsaki A, Hasegawa S, Shiraishi M, Furukawa S. Anti-inflammatory effect of 1α,25-dihydroxyvitamin D3 in human coronary arterial endothelial cells: implication for the treatment of Kawasaki disease. J Steroid Biochem Mol Biol 113: 134–138, 2009. doi: 10.1016/j.jsbmb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 24. Holick MF. Vitamin D: a millenium perspective. J Cell Biochem 88: 296–307, 2003. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 25. Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 6: 621–630, 2009. doi: 10.1038/nrcardio.2009.135. [DOI] [PubMed] [Google Scholar]
- 26. Young AR, Morgan KA, Ho TW, Ojimba N, Harrison GI, Lawrence KP, Jakharia-Shah N, Wulf HC, Cruickshank JK, Philipsen PA. Melanin has a small inhibitory effect on cutaneous vitamin D synthesis: a comparison of extreme phenotypes. J Invest Dermatol 140: 1418–1426.e1, 2020. doi: 10.1016/j.jid.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 27. Parva NR, Tadepalli S, Singh P, Qian A, Joshi R, Kandala H, Nookala VK, Cheriyath P. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus 10: e2741, 2018. doi: 10.7759/cureus.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stanhewicz AE, Wong BJ. Counterpoint: Investigators should not control for menstrual cycle phase when performing studies of vascular control that include women. J App Physiol (1985) 129: 1117–1119, 2020. doi: 10.1152/japplphysiol.00427.2020. [DOI] [PubMed] [Google Scholar]
- 29. Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 67: 373–378, 1988. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 30. Wolf ST, Berry CW, Stanhewicz AE, Kenney LE, Ferguson SB, Kenney WL. Sunscreen or simulated sweat minimizes the impact of acute ultraviolet radiation on cutaneous microvascular function in healthy humans. Exp Physiol 104: 1136–1146, 2019. doi: 10.1113/EP087688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolf ST, Stanhewicz AE, Jablonski NG, Kenney WL. Acute ultraviolet radiation exposure attenuates nitric oxide-mediated vasodilation in the cutaneous microvasculature of healthy humans. J App Physiol (1985) 125: 1232–1237, 2018. doi: 10.1152/japplphysiol.00501.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parra EJ, Kittles RA, Shriver MD. Implications of correlations between skin color and genetic ancestry for biomedical research. Nat Genet 36: S54–S60, 2004. doi: 10.1038/ng1440. [DOI] [PubMed] [Google Scholar]
- 33. Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J App Physiol (1985) 112: 2019–2026, 2012. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi PJ, Brunt VE, Fujii N, Minson CT. New approach to measure cutaneous microvascular function: an improved test of NO-mediated vasodilation by thermal hyperemia. J App Physiol (1985) 117: 277–283, 2014. doi: 10.1152/japplphysiol.01397.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alexander LM, Kutz JL, Kenney WL. Tetrahydrobiopterin increases NO-dependent vasodilation in hypercholesterolemic human skin through eNOS-coupling mechanisms. Am J Physiol Regul Integr Comp Physiol 304: R164–R169, 2013. doi: 10.1152/ajpregu.00448.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stanhewicz AE, Greaney JL, Kenney WL, Alexander LM. Sex- and limb-specific differences in the nitric oxide-dependent cutaneous vasodilation in response to local heating. Am J Physiol Regul Integr Comp Physiol 307: R914–R919, 2014. doi: 10.1152/ajpregu.00269.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnson JM, O’Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol 6: 337–346, 1986. doi: 10.1111/j.1475-097x.1986.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 38. Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985) 91: 1619–1626, 2001. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 39. Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol (1985) 109: 1239–1246, 2010. doi: 10.1152/japplphysiol.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holick MF. Vitamin D deficiency. N Engl J Med 357: 266–281, 2007. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 41. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 96: 1911–1930, 2011. [Erratum in J Clin Endocrinol Metab 96: 3908, 2011]. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 42. Pludowski P, Holick MF, Grant WB, Konstantynowicz J, Mascarenhas MR, Haq A, Povoroznyuk V, Balatska N, Barbosa AP, Karonova T, Rudenka E, Misiorowski W, Zakharova I, Rudenka A, Łukaszkiewicz J, Marcinowska-Suchowierska E, Łaszcz N, Abramowicz P, Bhattoa HP, Wimalawansa SJ. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol 175: 125–135, 2018. doi: 10.1016/j.jsbmb.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 43. Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 124: 869–871, 1988. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 44. Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 105: 370–372, 2008. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 45. Kenney WL, Edward F. Adolph distinguished lecture: skin-deep insights into vascular aging. J Appl Physiol (1985) 123: 1024–1038, 2017. doi: 10.1152/japplphysiol.00589.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. D’Agata MN, Hoopes EK, Berube FR, Hirt AE, Kuczmarski AV, Ranadive SM, Wenner MM, Witman MA. Evidence of reduced peripheral microvascular function in young Black women across the menstrual cycle. J Appl Physiol (1985) 131: 1783–1791, 2021. doi: 10.1152/japplphysiol.00452.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim K, Hurr C, Patik JC, Matthew Brothers R. Attenuated cutaneous microvascular function in healthy young African Americans: role of intradermal l-arginine supplementation. Microvasc Res 118: 1–6, 2018. doi: 10.1016/j.mvr.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 48. Ames BN, Grant WB, Willett WC. Does the high prevalence of vitamin D deficiency in African Americans contribute to health disparities? Nutrients 13: 499, 2021. doi: 10.3390/nu13020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension 57: 63–69, 2011. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moriya J. Critical roles of inflammation in atherosclerosis. J Cardiol 73: 22–27, 2019. doi: 10.1016/j.jjcc.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 51. Esteve E, Castro A, López-Bermejo A, Vendrell J, Ricart W, Fernández-Real J-M. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care 30: 939–945, 2007. doi: 10.2337/dc06-1793. [DOI] [PubMed] [Google Scholar]
- 52. Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol 103: 398–406, 2008. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kelly P, Suibhne TN, O’Morain C, O’Sullivan M. Vitamin D status and cytokine levels in patients with Crohn’s disease. Int J Vitam Nutr Res. 81: 205–210, 2011. doi: 10.1024/0300-9831/a000066. [DOI] [PubMed] [Google Scholar]
- 54. Liu W, Zhang L, Xu H-J, Li Y, Hu C-M, Yang J-Y, Sun M-Y. The anti-inflammatory effects of vitamin D in tumorigenesis. Int J Mol Sci 19: 2736, 2018. doi: 10.3390/ijms19092736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shab-Bidar S, Neyestani TR, Djazayery A, Eshraghian M-R, Houshiarrad A, Kalayi A, Shariatzadeh N, Khalaji N, Gharavi A. Improvement of vitamin D status resulted in amelioration of biomarkers of systemic inflammation in the subjects with type 2 diabetes. Diabetes Metab Res Rev 28: 424–430, 2012. doi: 10.1002/dmrr.2290. [DOI] [PubMed] [Google Scholar]
- 56. Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol 32: 23–63, 2012. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Steen EH, Wang X, Balaji S, Butte MJ, Bollyky PL, Keswani S. The role of the anti-inflammatory cytokine interleukin-10 in tissue fibrosis. Adv Wound Care (New Rochelle) 9: 184–198, 2020. doi: 10.1089/wound.2019.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gunnett CA, Heistad DD, Berg DJ, Faraci FM. IL-10 deficiency increases superoxide and endothelial dysfunction during inflammation. Am J Physiol Heart Circ Physiol 279: H1555–H1562, 2000. doi: 10.1152/ajpheart.2000.279.4.H1555. [DOI] [PubMed] [Google Scholar]
- 59. Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4+CD25+ natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol 31: 2534–2542, 2011. doi: 10.1161/ATVBAHA.111.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-κB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 119: 1284–1292, 2009. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Walker AE, Kaplon RE, Pierce GL, Nowlan MJ, Seals D. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor κB. Cin Sci (Lond) 127: 645–654, 2014. doi: 10.1042/CS20140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kang S, Kishimoto T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp Mol Med 53: 1116–1123, 2021. doi: 10.1038/s12276-021-00649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Montgomery A, Tam F, Gursche C, Cheneval C, Besler K, Enns W, Manku S, Rey K, Hanson PJ, Rose-John S, McManus BM, Choy JC. Overlapping and distinct biological effects of IL-6 classic and trans-signaling in vascular endothelial cells. Am J Physiol Cell Physiol 320: C554–C565, 2021. doi: 10.1152/ajpcell.00323.2020. [DOI] [PubMed] [Google Scholar]
- 64. Dalgård C, Petersen MS, Schmedes AV, Brandslund I, Weihe P, Grandjean P. High latitude and marine diet: vitamin D status in elderly Faroese. Br J Nutr 104: 914–918, 2010. doi: 10.1017/S0007114510001509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Macdonald HM, Mavroeidi A, Fraser WD, Darling AL, Black AJ, Aucott L, O’Neill F, Hart K, Berry JL, Lanham-New SA, Reid DM. Sunlight and dietary contributions to the seasonal vitamin D status of cohorts of healthy postmenopausal women living at northerly latitudes: a major cause for concern? Osteoporos Int 22: 2461–2472, 2011. [Erratum in Osteoporos Int 22: 2473–2474, 2011]. doi: 10.1007/s00198-010-1467-z. [DOI] [PubMed] [Google Scholar]
- 66. Gozdzik A, Barta JL, Wu H, Wagner D, Cole DE, Vieth R, Whiting S, Parra EJ. Low wintertime vitamin D levels in a sample of healthy young adults of diverse ancestry living in the Toronto area: associations with vitamin D intake and skin pigmentation. BMC Public Health 8: 336, 2008. doi: 10.1186/1471-2458-8-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Koh HK, Piotrowski JJ, Kumanyika S, Fielding J. Healthy people: a 2020 vision for the social determinants approach. Health Educ Behav 38: 551–557, 2011. doi: 10.1177/1090198111428646. [DOI] [PubMed] [Google Scholar]
- 68. Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol 102: 1540–1544, 2008. doi: 10.1016/j.amjcard.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 69. Robinson-Cohen C, Hoofnagle AN, Ix JH, Sachs MC, Tracy RP, Siscovick DS, Kestenbaum BR, de Boer IH. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA 310: 179–188, 2013. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens 20: 713–719, 2007. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All supporting data are included within the main article. Additional information is available from the corresponding author upon request.