Keywords: gastric, dysmotility, motility, pylorospasm
Abstract
Delayed gastric emptying may result from diverse pathophysiological mechanisms including antral hypomotility and pylorospasm. With increasing use of gastric peroral endoscopic myotomy and preliminary evidence of efficacy, our aim was to assess the motor functions of the distal antrum and pylorus in patients with symptoms of gastroparesis using high-resolution antropyloroduodenal manometry (HR-ADM). Sixteen patients with symptoms suggestive of gastroparesis underwent HR-ADM with 13 sensors, 1 cm apart, placed across the antropyloroduodenal (APD) junction and 2 sensors, 10 cm apart, in descending and distal duodenum. The 1-h postprandial motility was quantitated as contraction frequency/minute, average amplitude, and motility index (MI). Six healthy volunteers served as controls. In the patient group, the HR-ADM identified postprandial antral hypomotility, isolated pyloric pressure waves, and tonic elevation of baseline pressure in pylorus. Patients had significantly reduced frequency of the full-hour postprandial antral contractions/minute compared with healthy volunteers [1.52 (0.97, 1.67) vs. 2.04 (1.70, 2.67), P = 0.005], as well as reduced MI [9.65 (8.29, 10.31) vs. 11.04 (10.65, 11.63), P = 0.002]. The average contraction amplitude was numerically, but not significantly reduced [51.9 (21.9, 74.9) vs. 73.0 (59.8, 82.7), P = 0.14]. Bland–Altman plots showed similar distribution of antral contraction frequency and MI during the first and second postprandial 30-min periods for both patients and controls. High-resolution ADM can characterize a variety of postprandial antral contractile and pyloric motility dysfunctions. This technique shows promise to provide guidance for the selection of optimal treatment of patients with gastroparesis.
NEW & NOTEWORTHY Current selection of different treatments for patients with gastroparesis is empiric or based on trial and error, though pyloric distensibility and diameter may predict response to pyloric interventions. High-resolution antropyloroduodenal manometry (HR-ADM) can characterize a variety of postprandial antral contractile and pyloric motility dysfunctions in patients with suspected gastroparesis. HR-ADM shows promise to provide guidance for selection and individualization of treatments such as prokinetic agents or pyloric interventions for patients with gastroparesis based on documented pathophysiology.
INTRODUCTION
Delayed gastric emptying is an essential diagnostic criterion for gastroparesis (1), and increased gastric emptying is a measurement associated with treatment efficacy (2). However, measurement of gastric emptying does not discriminate between antral hypomotility, or pylorospasm as the cause of the delayed gastric emptying. Moreover, it does not differentiate neuropathic from myopathic disorders and does not lead to individualization of treatment in an attempt to optimize treatment strategies. With the limited clinical efficacy of the approved prokinetic agent (metoclopramide), pyloric botulinum toxin injection based on some though not all clinical trials (3–5), and gastric electrical stimulation (approved under humanitarian device exemption) (6–8), there has been change in practice to promote pyloric interventions such as gastric peroral endoscopic myotomy (G-POEM) for patients with delayed gastric emptying (9). Although recent evidence suggests 12-mo clinical efficacy of 56%, predictive parameters for the success of G-POEM remain unclear (10, 11). A recent randomized sham-controlled study has for the first time documented the benefit at 6 mo follow-up in patients with moderate to severe gastroparesis (12). Transpyloric EndoFLIP has been introduced to assess the diameter or cross-sectional area of the pylorus, as well as the distensibility index (DI) (13). However, it is still unclear which patients with gastroparesis defined by upper gastrointestinal symptoms with delayed gastric emptying are ideal candidates for this procedure. It has been proposed (14) that exclusion of antral hypomotility and documentation of spasm or reduced pyloric DI may help individualize treatment of gastroparesis, but it must be acknowledged that a study did not identify predictors of benefit with G-POEM based on EndoFLIP or antroduodenal manometry, with the latter performed using high-resolution solid-state antroduodenal recordings and quantitating antral function over 2 h postprandially (15).
In the past, gastroduodenal manometry based on three or five manometric sensors separated by 1 cm with or without a transpyloric Dent sleeve had been used to appraise contractile activities of the distal gastric antrum and duodenum, as well as the pyloric motor function (16–18). However, this is operator dependent due to the limitations of the number of sensors available (3 or 5) and the tendency for the manometric catheter to slide proximally or distally during the study period despite assistance through the measurement of transmucosal potential difference at the gastroduodenal junction with some devices. However, this method has been used predominantly in research rather than clinical studies. In addition, the acute angulation of the gastroduodenal junction renders measurements with multiple solid-state transducers more difficult, with significant propensity to damage the expensive catheter device. Over the years, significant upgrades have been introduced to mitigate these limitations.
We have used high-resolution, water-perfused gastroduodenal motility catheters comprising 13 sensors that are 1 cm apart, and 2 sensors 10 cm and 20 cm beyond the last of the 13 closely placed sensors (19). With this catheter, we had shown that the ghrelin receptor agonist, relamorelin, stimulated antral contractions in healthy human volunteers (19). Such a catheter can be placed endoscopically or transorally without endoscopy and the sensor placement is confirmed fluoroscopically across the antropyloroduodenal (APD) junction for continuous manometric monitoring of fasting and postprandial motility with a particular focus on the postprandial function. These features of continuous recording over prolonged periods contrast with the EndoFLIP approach that measures pyloric motility almost exclusively during fasting and during endoscopy under significant sedation and opioid analgesia. The latter treatments may alter the measured motility.
Our aim was to assess the first hour postprandial motor function of the distal antrum and pylorus in patients with symptoms of gastroparesis using high-resolution antropyloroduodenal manometry (HR-ADM) and to compare results with those of healthy adult controls. A second aim was to determine whether the recordings during the first and second 30 min of the first postprandial hour were consistent within the same patient.
METHODS
Data Source and Participants
We obtained data through review of the electronic medical records of 16 patients with symptoms of gastroparesis who were evaluated by a staff gastroenterologist at the Mayo Clinic, Rochester, MN and had undergone gastric emptying by scintigraphy. The HR-ADM was performed for clinical indications, that is, suspected gastroparesis or chronic intestinal dysmotility. We used data from a previously published randomized, crossover controlled trial in healthy volunteers of the pharmacological effects of the ghrelin receptor agonist, relamorelin, conducted in the laboratory of the same investigator (M.C.). For this cohort, only the data from the placebo treatment arm were used (19). Table 1 summarizes the demographic features of the 16 patients with symptoms of gastroparesis and the six normal healthy controls.
Table 1.
Demographics
| Patients (n = 16) | Healthy Controls (n = 6) | P Value | |
|---|---|---|---|
| Age, yr, means (SD) | 41.2 ± 14.9 | 46.5 ± 18.2 | 0.286 |
| Women, n (%) | 13 (81) | 4 (67) | 0.585 |
| Body mass index, kg/m2, SD | 22.8 ± 3.9 | 25.6 ± 3.6 | 0.0138 |
| Race | White: 15; Black 1 | White: 6 |
All participants underwent clinical evaluations including physical examination and review of their medical records to be sure there were no additional confounders that could alter their HR-ADM results such as opioid use or concomitant treatment with agents that could retard gastric emptying. Additional details regarding the clinical manifestations of the healthy participants are included in the previously published article (19).
Regulatory
Written informed consent was obtained from all healthy participants. This study was approved by the Mayo Clinic Institutional Review Board (IRB No. 21-006494). The medical records of any patients who had denied authorization of use of their medical (including research) records for research purposes were not used in the analysis in the current study.
Gastric Emptying Study
Procedure.
Gastric emptying was measured by an established and validated scintigraphic method (20). Patients ate a 99mTc-labeled meal of two scrambled eggs, one slice of whole wheat bread, and one glass of skim milk (320 kcal, 30% fat) after fasting overnight for at least 8 h. Anterior and posterior abdominal images were obtained with a γ-camera, each for a duration of 2 min at 1, 2, and 4 h. Study participants were not taking any prescription or over-the-counter medications that could interfere with gastric emptying for at least 48 h before and during their tests.
Analysis and quantitation of emptying.
We measured transit based on 99mTc counts measured within a 140 keV (±20%) window. We used a variable region of interest program to quantify the counts in the stomach. Gastric emptying half-time (T1/2) was estimated from a plot that linearly interpolated the imaging data obtained during the 4 h after eating the radiolabeled meal. We also quantitated the gastric emptying results by the percent (%) emptied from the stomach at 1, 2, and 4 h after the radiolabeled meal was eaten. This was consistent with previous publication that such data provide clinically relevant information (21), providing the basis for the consensus recommendations of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine (1). The normal values for proportion emptied at 1, 2, 3, and 4 h and T1/2 from our prior studies using the same test meal in 319 healthy volunteers are shown in Table 2 (20).
Table 2.
Clinical features and gastric emptying in 16 patients with suspected gastroparesis
| Data Show n (%) | |
|---|---|
| Symptoms | |
| Nausea/vomiting | 13 (81) |
| Abdominal pain | 9 (56) |
| Anorexia/early satiation | 3 (19) |
| Bloating/distention | 6 (38) |
| Weight loss | 6 (38) |
| Comorbidities | |
| Diabetes | 1 |
| Constipation | 6 |
| Pelvic floor dyssynergy | 4 |
| GERD | 7 |
| POTS/autonomic dysfunction | 3 |
| Anxiety/depression | 3 |
| Treatment | |
| Antiacids (PPI, H2 blocker) | 6 |
| Antiemetics | 8 |
| Prokinetics (including macrolides) | 14 |
| Neuromodulators | 3 |
| Laxatives | 6 |
| Tube feeding | 6 |
| Parenteral feeding | 2 |
| Gastric Interventions | 3 |
| Gastric emptying, median [IQR] | |
| 1 h (%) [Normal median 10–90%ile 17 (7, 31)] | 12.0 [4.0, 24.0] |
| 2 h (%) [Normal median 10–90%ile 50 (32–73)] | 32.0 [7.0, 50.0] |
| 4 h (%) [Normal median 10–90%ile 96 (82, 100)] | 74.0 [25.5, 94.5] |
| T1/2 (min) [Normal median 10–90%ile 120 (88–163)] | 173.0 [127.8, 436.7] |
Normal values for proportion emptied at 1, 2, 3, and 4 h and half time (T1/2) from 319 healthy volunteers (20). GERD, gastroesophageal reflux disease; POTS, postural orthostatic tachycardia syndrome; IQR, interquartile range.
High-Resolution Antropyloroduodenal Manometry
Procedure.
HR-ADM was performed using a custom-built (MUI Scientific, Inc., Mississauga, ON, Canada), 15-lumen polyvinyl chloride manometric tube with an outer diameter of 4 mm and inner lumen diameter of 0.3 mm for each manometric tube. The tube has 13 sensors that are 1 cm apart, with two sensors that are 10 and 20 cm beyond the last of the closely placed sensors (Fig. 1, left). Each channel was perfused with distilled water via a pneumohydraulic pump (perfusion rate 0.5 mL/min, perfusion pressure 14 psi) and attached to a strain-gauge transducer (Model PX-MK099; Edwards Lifesciences, Irvine, CA).
Figure 1.
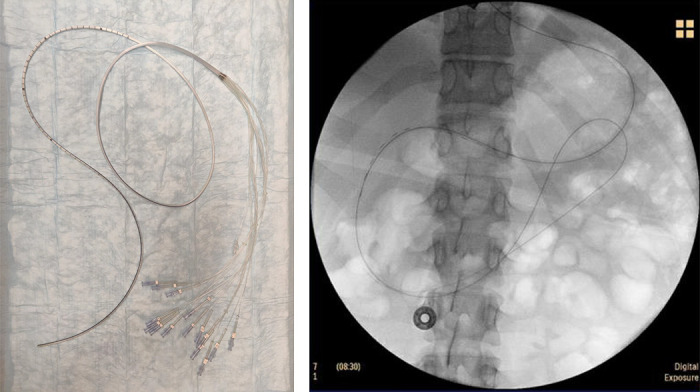
Left: water-perfused 15-luminal high-resolution antropyloroduodenal manometry catheter has 13 sensors that are 1 cm apart, with two sensors that are 10 and 20 cm beyond the last of the closely placed sensors. Right: fluoroscopy demonstrating placement of high-resolution antropyloroduodenal manometry catheter across the antropyloroduodenal junction with at least five sensors proximal to the pylorus.
After overnight (8 h) fast, patients with symptoms suggestive of gastroparesis undergoing HR-ADM for clinical purpose underwent an endoscopic placement of the 15-lumen manometric tube under midazolam sedation with or without 20% benzocaine local anesthetic spray on the back of the throat. The position of the manometry tube was verified using fluoroscopy to ensure that at least five manometric sensors that are 1 cm apart were located across the APD junction (Fig. 1, right). For healthy controls, the same manometric tube was placed transnasally by a trained technologist (D.B.) and staff physician (M.C.) without sedation and with participant in the sitting position (19). After recording fasting motility for at least 30 min in the healthy volunteers and 3 h fasting recording in the patients, participants ingested a 450–550 kcal solid-liquid meal (chicken, potato, butter, pudding, and 190 mL of water) consistent with patient’s food tolerance; motility was monitored for at least 60 min after the meal. All participants included in this study were able to ingest the average 500 kcal meal. During the entire test, the manometric recording was monitored by a nurse or technologist to keep the manometric sensors in the APD junction to ensure consistent measurement of distal antral and pyloric motor functions (Fig. 2). If the catheter migrated proximally or distally during the measurement period, repositioning was promptly performed to recapture the APD junction. At the end of the recordings, the manometric tube was withdrawn by gentle traction.
Figure 2.
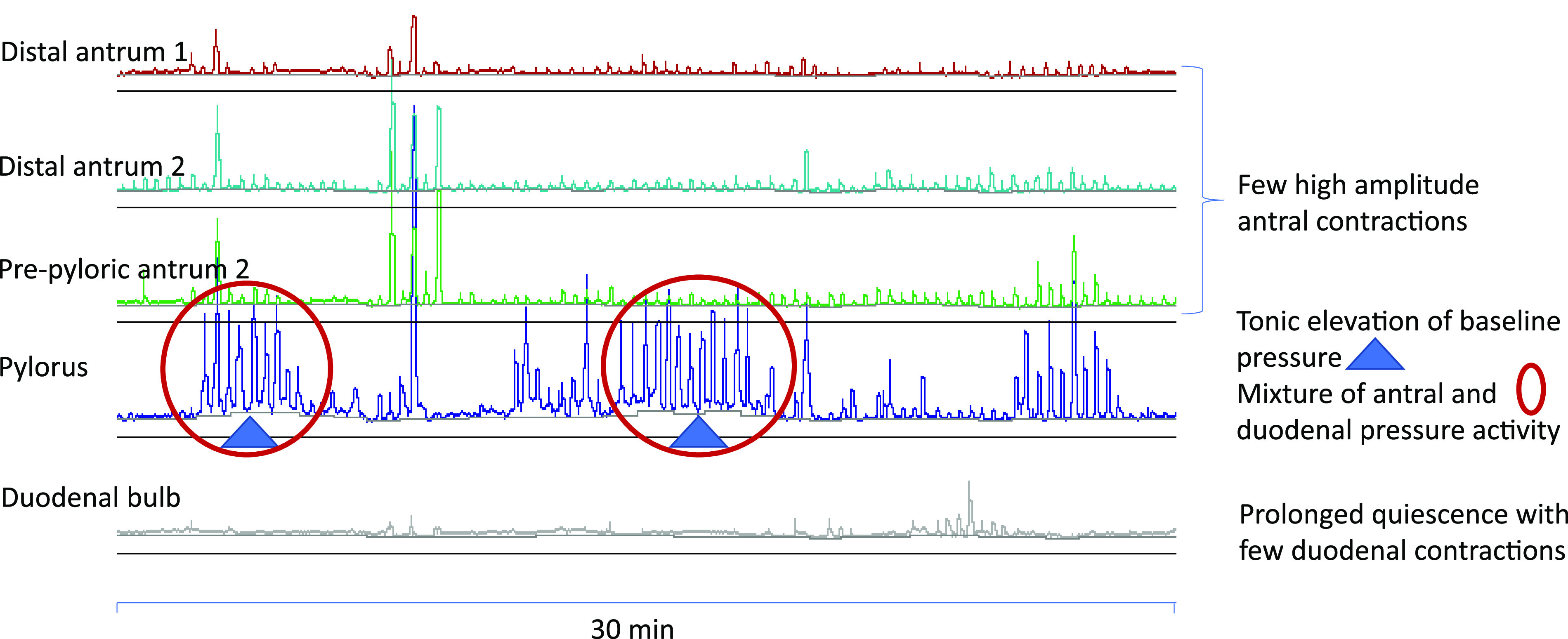
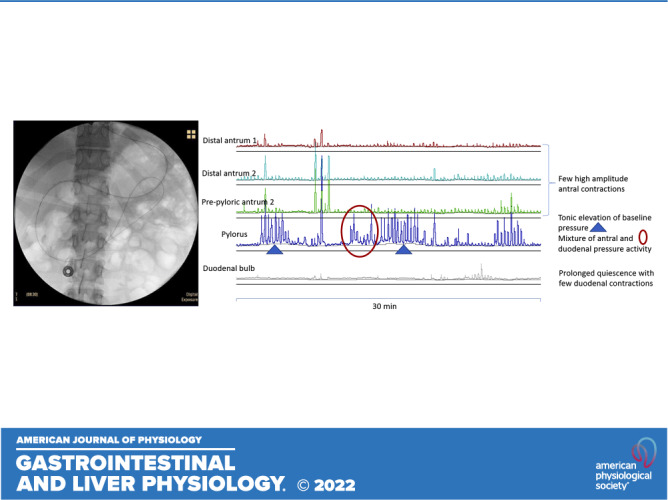
Manometric characteristics of distal antrum, pylorus, and the duodenal bulb. Pylorus is identified by at least two of the four distinct patterns: 1) baseline elevation of ≥3 mmHg (tonic pattern); 2) antral-type phasic pressure activity mixed with duodenal phasic activity (phasic pattern); 3) phasic pattern superimposed on tonic activity (combined tonic-phasic pattern); or 4) by the contractile activity in a sensor proximal to duodenal bulb quiescence.
Analysis.
HR-ADM was assessed with a combination of manual assessment and automated quantitation. Sensors corresponding to the antrum, pylorus, and duodenum were identified by the motility expert (M.C.) with decades of experience in reading gastroduodenal manometric studies, based on the following manometric characteristics for the distal antrum and pylorus.
Antral contractions were recognized by the flat baseline, with duration lasting >5 s, and frequency up to 3 per minute; duodenal contractions were recognized by the shorter duration (<5 s), and frequencies of up to 11 contractions per minute. Phasic pressure activity in the distal antrum was quantified in the three manometric recordings proximal to the manometrically identified pyloric recordings.
The pylorus was identified by at least two of the four distinct patterns: 1) baseline elevation of ≥3 mmHg for ≥1 min (tonic phase characteristic of a sphincter); 2) antral-type phasic pressure activity mixed with duodenal phasic activity (phasic pattern); 3) phasic pattern superimposed on tonic activity (combined tonic-phasic pattern) (18); or 4) by the contractile activity with duodenal bulb quiescence 1 cm distal to a recording showing antral contractions (22). These motor patterns are illustrated in Fig. 2.
The mean amplitude and the number of distal antral contractions were measured using computer software developed by Medical Measurement and Systems (Medical Measurement Systems B.V., Enschede, The Netherlands); to obtain a representative assessment of the distal antral phasic pressure activity, we analyzed statistically the average of the three most distal antral recordings; in addition, we provided the information for the pressure recording immediately proximal to the manometrically identified pylorus.
For the preprandial 30-min period and for the entire 1-h postprandial period, a motility index (MI) was calculated using the formula:
Previous studies also showed relationship with gastric emptying, and impaired antral contractility in neuropathic diseases such as diabetic and autonomic neuropathies (23–25).
In addition to automated measurement of the antral contractile frequency, amplitude, and MI, the motility expert (M.C.) appraised the recording to identify other characteristic manometric patterns, such as pylorospasm and postprandial migrating motor complex (MMC)-like phasic pressure activities in the duodenum.
Endpoints for Analysis
Primary endpoints for analysis between patients with gastroparetic symptoms and normal healthy controls was the full 1-h postprandial distal antral motility index studied by HR-ADM. Secondary endpoints were distal antral motility index during the first and second 30-min postprandial periods, and the frequency and amplitude of antral activity during first and second 30-min period, as well as the full hour postprandial period.
Statistical Analysis
Participant demographics and clinical features were summarized using descriptive statistics for both patients with symptoms of gastroparesis and healthy volunteers. Manometric endpoints (MI, frequency, and amplitude), as well as gastric emptying endpoints (half-time, percent emptied at 1, 2, and 4 h, respectively) were summarized as median, interquartile range (IQR). Antral hypomotility in patients was defined as postprandial first-hour antral contraction frequency below 10th percentile of the healthy volunteers. Wilcoxon–Mann–Whitney test was used to compare each manometric end point (postprandial antral contraction frequency, amplitude, and motility index) between patients with gastroparesis and healthy volunteers. Bland–Altman plots (26) were used to assess visually the intrasubject variations in antral contraction frequency and MI, respectively, in the first and second 30-min postprandial periods. Analysis was performed using SigmaPlot.
RESULTS
Demographics and Clinical Features
Table 1 summarizes the demographic features of the 16 patients with symptoms of gastroparesis, and six normal healthy controls. There is no significant difference in the age and gender between the groups. The patient group has significantly lower body mass index compared with healthy controls, although most individuals in both groups are within or close to normal range.
The clinical features and gastric emptying rates by scintigraphic measurements are summarized in Table 2. Nausea and vomiting, observed in 81% of the patients, were the most common symptoms, followed by abdominal pain (56%), bloating/distention (38%), weight loss (38%), and anorexia/early satiation (19%). Constipation with (4/16) or without pelvic floor dyssynergy (6/16) and gastroesophageal reflux disease (GERD) (7/16) were the most common relevant comorbidities. Autonomic dysfunction (3/16), anxiety/depression (3/16), and type 1 diabetes (1/16) were also identified. Two patients had previously undergone colectomy for severe constipation. One patient each had fundoplication, duodenoplasty, and superior mesenteric artery stenting and arterial bypass, respectively.
Among the pharmacological agents received, prokinetics were the most common (14/16) in this cohort of patients. Other agents included antiemetics (8/16), antiacid acid secretory agents (6/16), laxatives (6/16), and central neuromodulators (3/16). Three patients were on Δ9tetrahydrocannabinol (THC) products, and only one patient in this cohort had received opioid treatment.
A significant number (6/16) of patients were currently receiving or had been receiving enteric tube feeding (1 nasojejunal tube, 3 percutaneous gastrojejunostomy, and 2 percutaneous jejunostomy), and two patients had previously received total parenteral nutrition. Three patients had undergone gastric interventions, including gastric electrical stimulator (1/3), pyloric botulinum toxin injection (2/3), and G-POEM (1/3).
Gastric Emptying
Among patients with symptoms of gastroparesis, two had gastric emptying scintigraphy performed at an outside facility where one reported percent emptied at 1, 2, and 4 h but no T1/2, and the other reported T1/2 without percent emptied at the hourly time points. After these two patients were excluded, the gastric emptying half-time, T1/2 was 173.0 [127.8, 436.7] min for the patient cohort. Percent gastric emptying at 1, 2, and 4 h were 12.0 [4.0, 24.0], 32.0 [7.0, 50.0], and 74.0 [25.5, 94.5], respectively (Table 2). Based on the 10th or 90th percentile of emptying in 319 healthy volunteers based on the identical meal and scintigraphic method (20), 50.0% (7/14) of the patients had evidence of delayed gastric emptying at 2 h, 71.4% (10/14) delayed gastric emptying at 4 h, and 64.3% (9/14) had prolonged T1/2.
Pictorial Examples of HR-ADM
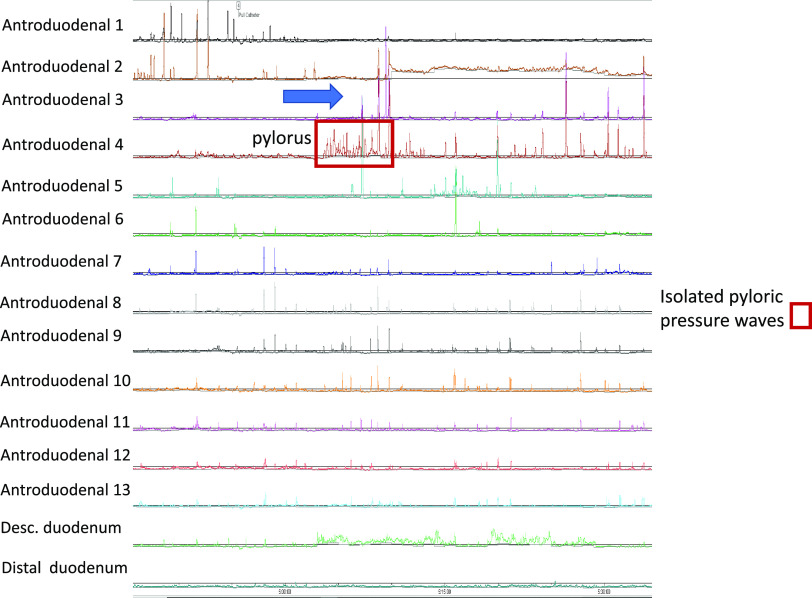
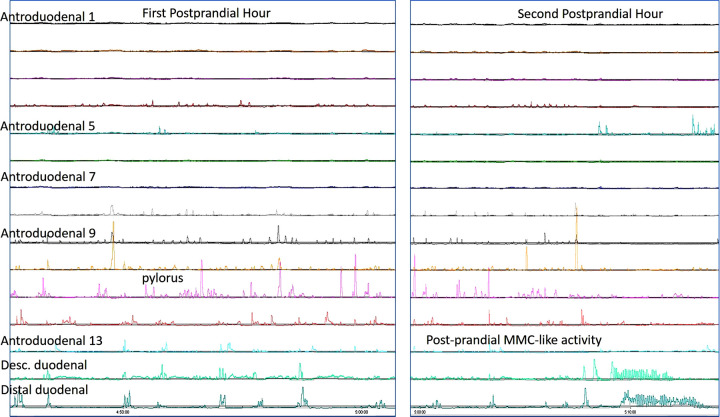
Inspection of manometric tracings reveals distinct postprandial APD findings, including postprandial antral hypomotility (Figs. 3, 4, and 5), isolated pyloric pressure waves (Fig. 4), and postprandial duodenal migrating motor complex (MMC)-like activity (Fig. 5). Because of the availability of 13 sensors placed across the APD junction, the pylorus was identified throughout the first postprandial hour in all patients and healthy volunteers.
Figure 3.
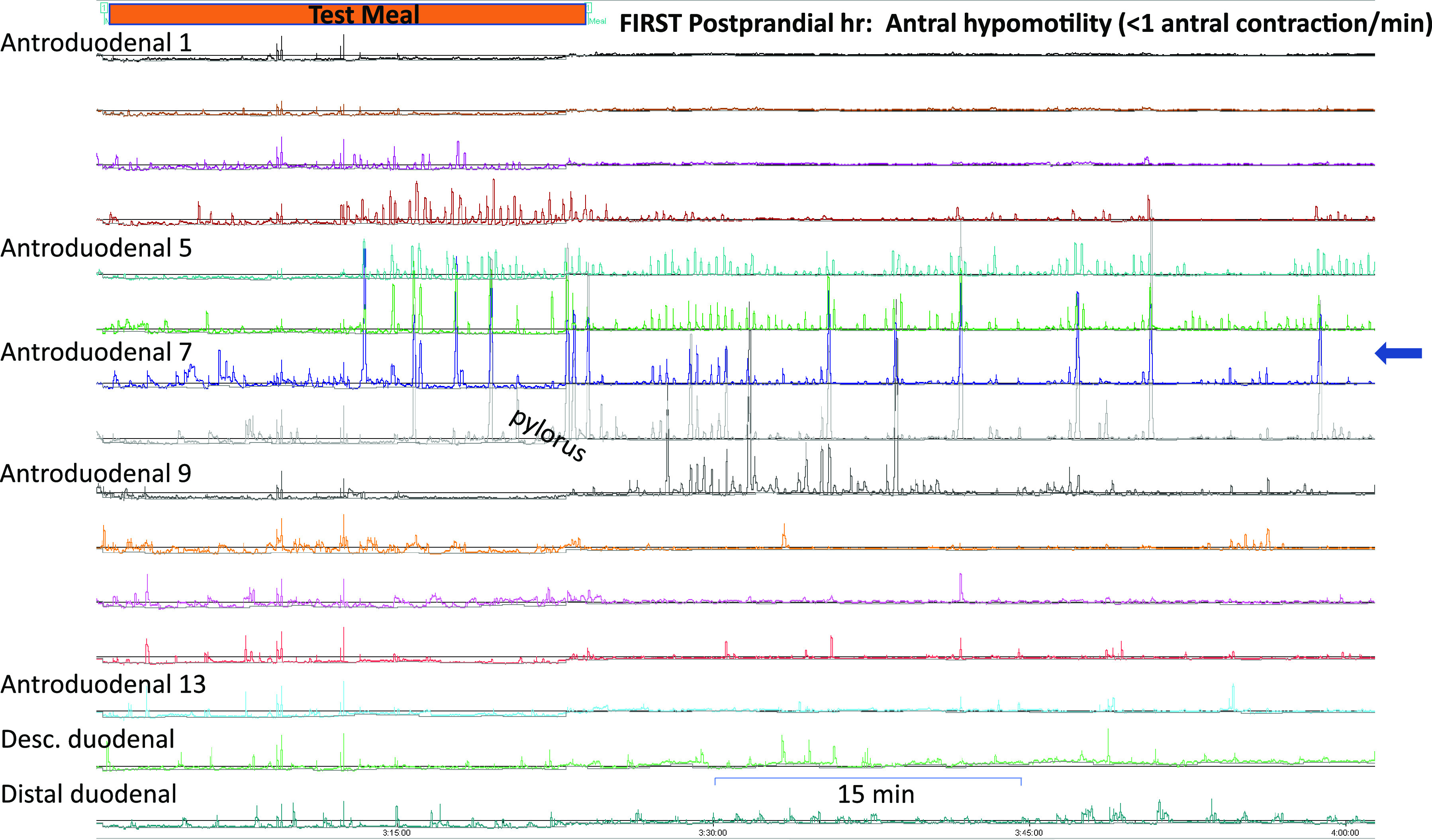
High-resolution antropyloroduodenal manometry tracing during the first postprandial hour. Orange bar: test meal. Antral hypomotility (<1 contraction/min) can be observed at sensors proximal to the pylorus (arrow).
Figure 4.
Episode of isolated pyloric pressure waves (pylorospasm) in a patient with symptoms of gastroparesis during the first postprandial hour. Also notable is hypomotility (<1 contraction/min) at the distal antrum (arrow).
Figure 5.
During the first postprandial hour, high-resolution antropyloroduodenal manometry demonstrates decreased antral contraction (left); patient also had duodenal migrating motor complex-like activity during the second postprandial hour, suggest possible neuropathic dysmotility.
Clinical Report of Antropyloroduodenal Contractile Abnormalities
The clinical reports of HR-ADM were reviewed and summarized in Table 3. Of 16 patients with suspected gastroparesis, 11/16 had postprandial antral hypomotility, which was the most common observation in this cohort. Two patients had additional pylorospasm, characterized by isolated pyloric pressure waves. The small intestinal contractile activity was qualitatively assessed and dysmotility was found in three patients; one patient showed a postprandial MMC-like activity suggestive of neuropathic dysmotility unassociated with any underlying primary neurological disorder. In addition, 2/16 were deemed to have a reduced frequency but normal amplitude of postprandial contractions during the first postprandial hour, which may have been attributable to delayed gastric emptying. The overall manometric pattern suggested neuropathic etiology of dysmotility in 15 patients, and myopathic dysmotility (based on amplitude <20 mmHg in the small bowel) in one patient.
Table 3.
Clinical report of antropyloroduodenal contractile abnormalities in 16 patients with suspected gastroparesis
| Manometric Abnormalities at the APD Junction | Data Show n (%) |
|---|---|
| Antral hypomotility | 11 (69) |
| Pylorospasm | 2 (13) |
| Duodenal dysmotility | 3 (19) |
| Neuropathic pattern | 15 (94) |
| Myopathic pattern | 1 (6) |
Distal Antral Motility
Postprandial antral contraction frequency was reduced in patients with symptoms of gastroparesis compared with healthy controls in the first 30 min, second 30 min, and full hour (Table 4). The difference is significant at the first 30 min and over the full hour. Similarly, patients had reduced postprandial motility index in the first 30 min, second 30 min, and full hour, respectively, compared with healthy controls (Table 4). Postprandial antral contraction amplitude was not statistically significant between patients and healthy volunteers (Table 4). Similar differences and statistical significance were observed when only patients with confirmed gastroparesis (10/16, based on scintigraphic evidence of delayed gastric emptying conducted using Mayo Clinic’s protocol) were compared with healthy volunteers (Table 4). Within the patient group, 9/16 patients had antral hypomotility alone and 2/16 had pylorospasm in addition to antral hypomotility. In this cohort, we did not observe any patients with pylorospasm alone. Notably, the patient who had received gastric electrical stimulation and G-POEM had no signs of antral hypomotility or pylorospasm, and his gastric emptying scintigraphy was normal, though he remained symptomatic. Among two patients with history botulinum toxin injection to the pylorus at least 3 mo before the HR-ADM study, one had normal antral and pyloric motility, and the other had antral hypomotility without pylorospasm.
Table 4.
High resolution antropyloroduodenal manometry results in patients and healthy controls (median [IQR])
| All Patients | Delayed Gastric Emptying Only | Healthy Controls | P* | P** | |
|---|---|---|---|---|---|
| Postprandial antral contraction, no./min | |||||
| First 30 min | 1.40 [1.06, 1.73] | 1.47 [1.16, 1.78] | 2.37 [1.89, 2.64] | 0.002 | 0.005 |
| Second 30 min | 1.34 [0.80, 1.74]^ | 1.47 [1.17, 1.73] | 1.92 [1.37, 2.83]^^ | 0.129 | 0.175 |
| Full hour | 1.52 [0.97, 1.67] | 1.53 [ 1.13, 1.65] | 2.04 [1.70, 2.67] | 0.005 | 0.006 |
| Amplitude, mmHg | |||||
| First 30 min | 46.3 [ 22.8, 83.5] | 61.3 [30.8, 94.6] | 67.0 [59.9, 83.0] | 0.357 | 0.704 |
| Second 30 min | 26.0 [18.5, 80.0] | 27.5 [20.3, 76.6] | 71.8 [56.6, 99.6] | 0.073 | 0.082 |
| Full hour | 51.9 [21.9, 74.9] | 55.5 [24.8, 79.4] | 73.0 [ 59.8, 82.7] | 0.140 | 0.175 |
| Motility index | |||||
| First 30 min | 9.67 [ 8.69, 10.63] | 9.98 [9.11, 10.75] | 11.38 [11.00, 11.51] | <0.001 | 0.003 |
| Second 30 min | 9.29 [8.73, 10.34] | 9.55 [ 8.97, 10.42] | 10.87 [10.18, 11.83] | 0.009 | 0.020 |
| Full hour | 9.65 [8.29, 10.31] | 9.74 [ 9.16, 10.45] | 11.04 [10.65, 11.63] | 0.002 | 0.006 |
IQR, interquartile range. *P value of all patients vs. healthy controls; **P value of only patients with delayed gastric emptying vs. healthy controls; ^P = 0.679 in comparison of first and second 30 minutes; ^^P = 0.313 in comparison of first and second 30 min.
Consistency of Measured Antral Motility Indices during the Two Postprandial Periods
Bland–Altman plots showed that in patients with gastroparetic symptoms, the postprandial antral contraction frequency and motility index during the first 30 and second 30 min were similarly distributed across mean frequency of 0.2–2.5 contractions/min, and motility index of 5–11, respectively (Fig. 6, top). For normal healthy controls, the mean postprandial antral contractions ranged 1.6–3.0/min, and the mean motility index ranged from 10.4 to 12. There was no significant difference between the first and second 30 min (Fig. 6, bottom).
Figure 6.
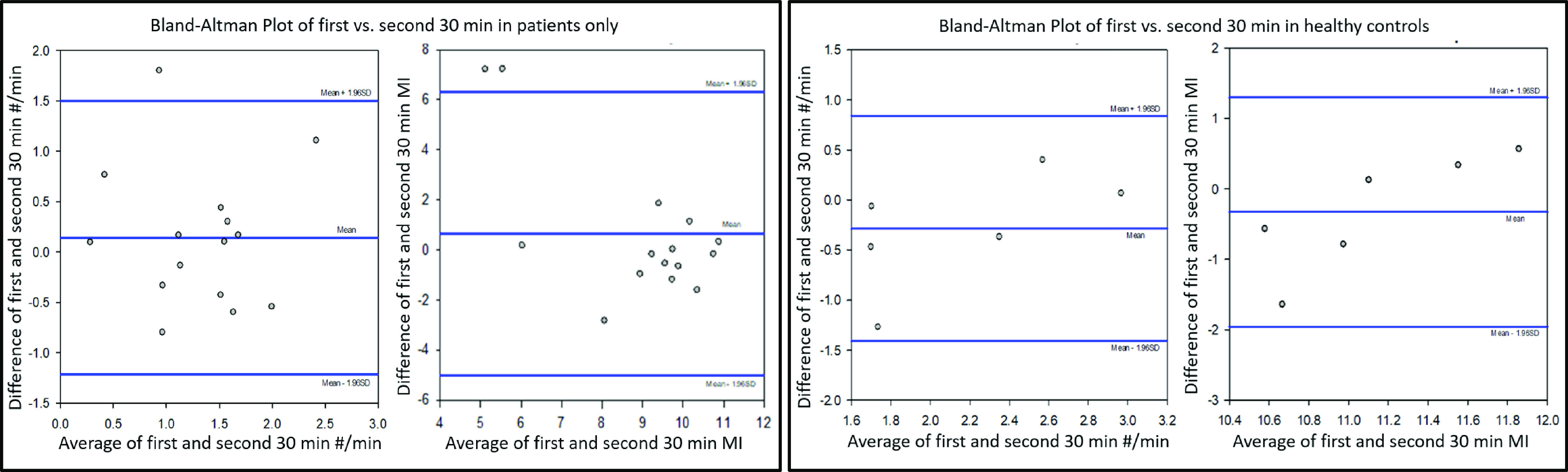
Bland–Altman plots showed similar distribution of first and second 30-min postprandial antral contraction frequency and motility index cross their respective means for both patients (left) and healthy controls (right).
DISCUSSION
Whereas delayed gastric emptying is a common motility disorder and a diagnostic criterion for gastroparesis, its underlying pathophysiological processes are not discriminated with scintigraphy, stable isotope breath test, or wireless motility capsule. In this study, we showed HR-ADM tracings of postprandial antral hypomotility, postprandial duodenal MMC-like activity, and isolated pyloric pressure waves. We also provided quantitative information on the reduced antral motility index, based predominantly on the reduced frequency and motility index of distal antral contractions, during the first postprandial hour. These findings were consistent with previously published observations (18). However, the previously documented observations of the pathophysiology have not received widespread application, and one impediment reflected the technical difficulties with obtaining consistent recordings of antropyloroduodenal motility with the typical three or five sensors placed across that region. In fact, therapeutic approaches such as botulinum toxin injection and G-POEM have been applied independent of detailed information about antral motility. In recent years, EndoFLIP has been applied for quantitative measurements of pyloric diameter and distensibility index, with the implication that this will assist in selecting the best candidates for pyloric interventions such as botulinum toxin injection or G-POEM. Although it is plausible that EndoFLIP may serve as a complementary test to HR-ADM, comparison of pyloric assessment using HR-ADM and EndoFLIP should be conducted in future studies. To date, the pyloric EndoFLIP measurements have been performed in fasting, sedated, or anesthetized patients and the postprandial function of the pylorus (which is relevant to the emptying of food from the stomach) is totally unknown based on those EndoFLIP investigations. By incorporating 13 sensors placed across the antropyloroduodenal junction, we have been able to systematically quantify antral motility in the crucial first postprandial hour. Our study has documented qualitative and quantitative analysis of antral motility that provides evidence of a mechanism of gastric dysmotility, that may be associated with different outcomes of pylorus-targeted therapies such as G-POEM.
For patients with symptoms of gastroparesis, postprandial antral contraction frequency and motility index was reduced during the first 30 min, second 30 min, and full hour compared with healthy controls. Characterization of frequency and amplitude of distal antral contractions in response to a 500-kcal solid liquid meal has been previously shown to facilitate differentiation of myopathic versus neuropathic disorders (16). The presence of reduced contractions of normal amplitude or antral hypomotility would be an indication for prokinetic medications such as 5-HT4 receptor agonists, ghrelin receptor agonists, or motilin receptor agonists including macrolides. In our study, there was no significant difference in the amplitude of postprandial antral contraction between patients and controls, and this is consistent with the lack of patients (only 1 patient had myopathic pattern) with an underlying disease suggestive of myopathic etiology (e.g., scleroderma, amyloidosis), which is less common compared with the neuropathic type such as idiopathic and diabetic gastroparesis. Importantly, three patients had previously undergone gastric interventions. One patient had received gastric electrical stimulator with inadequate symptom relief and underwent G-POEM before presenting for second opinion. His HR-ADM showed normal antral motility and no pylorospasm, and gastric emptying by scintigraphy showed normal gastric emptying, thus suggesting his current symptoms are functional in nature. Two patients had pyloric botulinum toxin injection at least 3 mo before the HR-ADM study. Although neither patient had pylorospasm, one continued to have antral hypomotility and delayed gastric emptying. These findings, revealed by HR-ADM, highlight the importance of differentiating antral hypomotility from pylorospasm as the underlying cause of delayed gastric emptying, as well as defining delayed gastric emptying in gastrointestinal (GI) motility disorders, so that treatment options (prokinetics, neuromodulators, botulinum toxin injection, and G-POEM) can be offered based on the underlying pathophysiology.
Bland–Altman plots suggest that HR-ADM measurements are precise and consistent over the first hour postprandially, as shown by similar distribution of the postprandial antral contraction frequency and motility index during the first 30 and second 30 min. This pattern was seen in both patients and healthy controls.
Although transpyloric EndoFLIP is currently regarded as the most relevant measurement to characterize pyloric distensibility and cross-sectional area, HR-ADM can identify different patterns of motor function including increased tone and increased isolated pyloric phasic contractions. It is hypothesized that isolated pyloric contractions of spasm documented by persistent elevation of pyloric tone on HR-ADM may facilitate selection of patients for G-POEM, as has been proposed if there is reduced pyloric diameter or reduced distensibility index documented on EndoFLIP (13). When antral hypomotility is observed by HR-ADM, however, patients may benefit from additional prokinetics with or without G-POEM. These questions require formal prospective, sham-controlled studies in the future.
The strength of this study includes patient and control groups with well-characterized phenotypes. HR-ADM with 13 sensors allowing for adequate length of antropyloroduodenal measurements, performed by an experienced team, and analyzed with a combination of manual and automated efforts, ensuring almost 100% recognition and quantification of distal antral and pyloric activity. Some limitations include the small sample size and the lack of patients with confirmed myopathy. Although most (10/16) in the patient cohort had confirmed delay in gastric emptying, the sample size was too small to detect myopathic disorders or abnormalities of small bowel motility in patients with confirmed gastroparesis. Some of the patients had received central neuromodulators or opioid medications in the past. Although all these medications as well as prokinetic agents were stopped at least 48 h before the HR-ADM procedure and presumably circulating levels were reduced, it is conceivable that the tissue levels may have persisted and led to some residual effects of these agents and may therefore have impacted the measured antral and pyloric contractions.
It is important to note that the HR-ADM in its current state is not capable of quantitating duodenal and jejunal motility, as its distal two sensors are 10 cm apart from each other. Prior studies demonstrated that, in the presence of normal antral motility measured manometrically, small intestinal dysmotility may be associated with prolonged gastric emptying (e.g., T1/2) even though the lag phase of gastric emptying of solids (reflecting antral trituration and dependent on normal distal antral motility) is normal. Those data were consistent with intestinal dysmotility inducing resistance to flow of triturated food from the stomach and overall delay in gastric emptying (23). A recent study has also applied water-perfusion high-resolution manometry to quantitate small intestinal motility (27). The application of high-resolution manometry at both APD and more distal small intestinal segments has the potential to enhance the physiological measurements of gastrointestinal functions in health and disease.
Conclusions
High-resolution antropyloroduodenal manometry can mechanistically characterize a variety of postprandial antral contractile and pyloric dysfunctions and may, with further validation, provide guidance to the selection of optimal treatment options, including G-POEM and/or prokinetics, for patients with gastroparesis. Having established that the method is feasible and provides detailed antral and pyloric measurements in patients with suspected gastroparesis, future research is required to prospectively evaluate whether the findings impacted the choice and outcomes of treatment such as with prokinetic agents for patients with antral hypomotility or pyloric interventions such as G-POEM for patients with pyloric motor dysfunctions. Future research should also explore the results of pyloric motor recordings obtained with manometry or solid-state transducers and measurements of pyloric diameter and distensibility index obtained with EndoFLIP during fasting in unsedated patients, as has recently been demonstrated in healthy human volunteers (28).
GRANTS
M. Camilleri receives funding from National Institutes of Health Grants R01-DK122280 and R01-DK125680 for research work on gastroparesis.
DISCLOSURES
In the past 24 months, M. Camilleri has received a research grant from Takeda for the study of felcisetrag in gastroparesis. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
T.Z. and M.C. conceived and designed research; T.Z. performed experiments; T.Z., J.B., W.S., D.D.B., and M.C. analyzed data; T.Z. and D.D.B. interpreted results of experiments; T.Z. and M.C. prepared figures; T.Z. and M.C. drafted manuscript; T.Z., J.B., W.S., D.J.E., D.D.B., and M.C. edited and revised manuscript; T.Z., J.B., W.S., D.J.E., D.D.B., and M.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Mayo Clinic staff gastroenterologists who referred patients and nursing staff for assistance with performance of motility measurements, and Cindy Stanislav for excellent secretarial support.
REFERENCES
- 1. Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, McCallum RW, Nowak T, Nusynowitz ML, Parkman HP, Shreve P, Szarka LA, Snape WJ Jr, Ziessman HA; American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol 103: 753–763, 2008. doi: 10.1111/j.1572-0241.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 2. Vijayvargiya P, Camilleri M, Chedid V, Mandawat A, Erwin PJ, Murad MH. Effects of promotility agents on gastric emptying and symptoms: a systematic review and meta-analysis. Gastroenterology 156: 1650–1660, 2019. doi: 10.1053/j.gastro.2019.01.249. [DOI] [PubMed] [Google Scholar]
- 3. Desprez C, Melchior C, Wuestenberghs F, Zalar A, Jacques J, Leroi AM, Gourcerol G. Pyloric distensibility measurement predicts symptomatic response to intrapyloric botulinum toxin injection. Gastrointest Endosc 90: 754–760.e1, 2019. doi: 10.1016/j.gie.2019.04.228. [DOI] [PubMed] [Google Scholar]
- 4. Arts J, Holvoet L, Caenepeel P, Bisschops R, Sifrim D, Verbeke K, Janssens J, Tack J. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther 26: 1251–1258, 2007. doi: 10.1111/j.1365-2036.2007.03467.x. [DOI] [PubMed] [Google Scholar]
- 5. Friedenberg FK, Palit A, Parkman HP, Hanlon A, Nelson DB. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol 103: 416–423, 2008. doi: 10.1111/j.1572-0241.2007.01676.x. [DOI] [PubMed] [Google Scholar]
- 6. Abell TL, Yamada G, McCallum RW, Van Natta ML, Tonascia J, Parkman HP, Koch KL, Sarosiek I, Farrugia G, Grover M, Hasler W, Nguyen L, Snape W, Kuo B, Shulman R, Hamilton FA, Pasricha PJ. Effectiveness of gastric electrical stimulation in gastroparesis: results from a large prospectively collected database of national gastroparesis registries. Neurogastroenterol Motil 31: e13714, 2019. doi: 10.1111/nmo.13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ducrotte P, Coffin B, Bonaz B, Fontaine S, Bruley Des Varannes S, Zerbib F, Caiazzo R, Grimaud JC, Mion F, Hadjadj S, Valensi PE, Vuitton L, Charpentier G, Ropert A, Altwegg R, Pouderoux P, Dorval E, Dapoigny M, Duboc H, Benhamou PY, Schmidt A, Donnadieu N, Gourcerol G, Guerci B; ENTERRA Research Group. Gastric electrical stimulation reduces refractory vomiting in a randomized crossover trial. Gastroenterology 158: 506–514.e2, 2020. doi: 10.1053/j.gastro.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 8. Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 108: 18–37, 2013. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aghaie Meybodi M, Qumseya BJ, Shakoor D, Lobner K, Vosoughi K, Ichkhanian Y, Khashab MA. Efficacy and feasibility of G-POEM in management of patients with refractory gastroparesis: a systematic review and meta-analysis. Endosc Int Open 7: E322–E329, 2019. doi: 10.1055/a-0812-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vosoughi K, Ichkhanian Y, Benias P, Miller L, Aadam AA, Triggs JR, Law R, Hasler W, Bowers N, Chaves D, Ponte-Neto AM, Draganov P, Yang D, El Halabi M, Sanaei O, Brewer Gutierrez OI, Bulat RS, Pandolfino J, Khashab M. Gastric per-oral endoscopic myotomy (G-POEM) for refractory gastroparesis: results from an international prospective trial. Gut 71: 25–33, 2022. doi: 10.1136/gutjnl-2020-322756. [DOI] [PubMed] [Google Scholar]
- 11. Zheng T, Camilleri M. Selecting optimal patients with gastroparesis for G-POEM procedure. Gut 71: 659–660, 2022. doi: 10.1136/gutjnl-2021-324631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinek J, Hustak R, Mares J, Vackova Z, Spicak J, Kieslichova E, Buncova M, Pohl D, Amin S, Tack J. Endoscopic pyloromyotomy for the treatment of severe and refractory gastroparesis: a pilot, randomized, sham-controlled trial. Gut. In press. doi: 10.1136/gutjnl-2022-326904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vosoughi K, Ichkhanian Y, Jacques J, Aadam AA, Benias PC, Law R, Hasler WL, Canakis A, Ragi O, Triggs J, Bowers N, Brewer Gutierrez OI, Kumbhari V, Kalloo AN, Bulat RS, Pandolfino JE, Khashab MA. Role of endoscopic functional luminal imaging probe in predicting the outcome of gastric peroral endoscopic pyloromyotomy (with video). Gastrointest Endosc 91: 1289–1299, 2020. doi: 10.1016/j.gie.2020.01.044. [DOI] [PubMed] [Google Scholar]
- 14. Camilleri M. Relationship of motor mechanisms to gastroparesis symptoms: toward individualized treatment. Am J Physiol Gastrointest Liver Physiol 320: G558–G563, 2021. doi: 10.1152/ajpgi.00006.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conchillo JM, Straathof JWA, Mujagic Z, Brouns JH, Bouvy ND, Keszthelyi D, Masclee AAM. Gastric peroral endoscopic pyloromyotomy for decompensated gastroparesis: comprehensive motility analysis in relation to treatment outcomes. Endosc Int Open 9: E137–E144, 2021. doi: 10.1055/a-1311-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thumshirn M, Bruninga K, Camilleri M. Simplifying the evaluation of postprandial antral motor function in patients with suspected gastroparesis. Am J Gastroenterol 92: 1496–1500, 1997. [PubMed] [Google Scholar]
- 17. Stanghellini V, Camilleri M, Malagelada JR. Chronic idiopathic intestinal pseudo-obstruction—clinical and intestinal manometric findings. Gut 28: 5–12, 1987. doi: 10.1136/gut.28.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology 90: 1919–1925, 1986. doi: 10.1016/0016-5085(86)90262-3. [DOI] [PubMed] [Google Scholar]
- 19. Nelson AD, Camilleri M, Acosta A, Busciglio I, Linker Nord S, Boldingh A, Rhoten D, Ryks M, Burton D. Effects of ghrelin receptor agonist, relamorelin, on gastric motor functions and satiation in healthy volunteers. Neurogastroenterol Motil 28: 1705–1713, 2016. doi: 10.1111/nmo.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Camilleri M, Iturrino J, Bharucha AE, Burton D, Shin A, Jeong I-D, Zinsmeister AR. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil 24: 1076-e562, 2012. doi: 10.1111/j.1365-2982.2012.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tougas G, Eaker EY, Abell TL, Abrahamsson H, Boivin M, Chen J, Hocking MP, Quigley EM, Koch KL, Tokayer AZ, Stanghellini V, Chen Y, Huizinga JD, Rydén J, Bourgeois I, McCallum RW. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterology 95: 1456–1462, 2000. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 22. Camilleri M, Malagelada JR, Stanghellini V, Zinsmeister AR, Kao PC, Li CH. Dose-related effects of synthetic human β-endorphin and naloxone on fed gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol 251: G147–G154, 1986. doi: 10.1152/ajpgi.1986.251.1.G147. [DOI] [PubMed] [Google Scholar]
- 23. Camilleri M, Brown ML, Malagelada JR. Relationship between impaired gastric-emptying and abnormal gastrointestinal motility. Gastroenterology 91: 94–99, 1986. doi: 10.1016/0016-5085(86)90444-0. [DOI] [PubMed] [Google Scholar]
- 24. Camilleri M, Malagelada JR, Brown ML, Becker G, Zinsmeister AR. Relation between antral motility and gastric-emptying of solids and liquids in humans. Am J Physiol Gastrointest Liver Physiol 249: G580–G585, 1985. doi: 10.1152/ajpgi.1985.249.5.G580. [DOI] [PubMed] [Google Scholar]
- 25. Camilleri M, Malagelada JR, Stanghellini V, Fealey RD, Sheps SG. Gastrointestinal motility disturbances in patients with orthostatic hypotension. Gastroenterology 88: 1852–1859, 1985. doi: 10.1016/0016-5085(85)90010-1. [DOI] [PubMed] [Google Scholar]
- 26. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327: 307–310, 1986. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 27. Alcala-Gonzalez LG, Malagelada C, Galan C, Nieto A, Accarino A, Azpiroz F. Propagation patterns of jejunal motor activity measured by high-resolution water-perfused manometry. Neurogastroenterol Motil 33: e14240, 2021. doi: 10.1111/nmo.14240. [DOI] [PubMed] [Google Scholar]
- 28. Zheng T, Vosoughi K, Busciglio I, Tebay L, Burton D, Camilleri M. Fasting pyloric diameter and distensibility by functional endoluminal imaging probe in unsedated healthy volunteers. Neurogastroenterol Motil e14386, 2022. doi: 10.1111/nmo.14386. [DOI] [PMC free article] [PubMed] [Google Scholar]