Abstract
The risks of heart diseases are significantly modulated by age and sex, but how these factors influence baseline cardiac gene expression remains incompletely understood. Here, we used RNA sequencing and mass spectrometry to compare gene expression in female and male young adult (4 mo) and early aging (20 mo) mouse hearts, identifying thousands of age- and sex-dependent gene expression signatures. Sexually dimorphic cardiac genes are broadly distributed, functioning in mitochondrial metabolism, translation, and other processes. In parallel, we found over 800 genes with differential aging response between male and female, including genes in cAMP and PKA signaling. Analysis of the sex-adjusted aging cardiac transcriptome revealed a widespread remodeling of exon usage patterns that is largely independent from differential gene expression, concomitant with upstream changes in RNA-binding protein and splice factor transcripts. To evaluate the impact of the splicing events on cardiac proteoform composition, we applied an RNA-guided proteomics computational pipeline to analyze the mass spectrometry data and detected hundreds of putative splice variant proteins that have the potential to rewire the cardiac proteome. Taken together, the results here suggest that cardiac aging is associated with 1) widespread sex-biased aging genes and 2) a rewiring of RNA splicing programs, including sex- and age-dependent changes in exon usages and splice patterns that have the potential to influence cardiac protein structure and function. These changes contribute to the emerging evidence for considerable sexual dimorphism in the cardiac aging process that should be considered in the search for disease mechanisms.
NEW & NOTEWORTHY Han et al. used proteogenomics to compare male and female mouse hearts at 4 and 20 mo. Sex-biased cardiac genes function in mitochondrial metabolism, translation, autophagy, and other processes. Hundreds of cardiac genes show sex-by-age interactions, that is, sex-biased aging genes. Cardiac aging is accompanied with a remodeling of exon usage in functionally coordinated genes, concomitant with differential expression of RNA-binding proteins and splice factors. These features represent an underinvestigated aspect of cardiac aging that may be relevant to the search for disease mechanisms.
Keywords: aging, alternative splicing, proteoforms, proteogenomics, sex difference
INTRODUCTION
There are considerable sex differences in the risk, prevalence, and prognosis of heart diseases (1). The effect of sex is age dependent; whereas premenopausal adult women are relatively protected from cardiovascular diseases compared with age-matched men, older women at postmenopausal ages experience higher risks for adverse cardiac remodeling and higher incidences of heart failure with preserved ejection fraction (2–4). Recent work has centered on the sexual dimorphism in aging between men and women that underlies clinical observations, where biological sex exerts a modulating influence on the fundamental biology of cardiac aging processes (5). Among other molecular and phenotypic differences, aging women experience a greater increase in left ventricular wall thickness and concentric remodeling (3, 6), increased collagen deposition, and diastolic dysfunction (4, 5) compared with men. The limited efficacy of hormone replacement therapy in remediating sex-dependent risks is consistent with multifactorial underlying causes that should be considered based on intrinsic differences of female and male hearts. Targeting cardiac aging-related gene regulatory networks has been proposed as a promising therapeutic approach against heart diseases (5, 7), but progress will likely require more comprehensive understanding of the interacting effects of both biological age and sex on cardiac gene expression.
In rodents, female and male hearts show intrinsic differences in cell proportion, cell morphology, and myocyte contractility (8, 9). Several hundreds of cardiac genes have previously been found to display sexual dimorphism in expression levels (10). Differences between male and female hearts may be attributable to gonadal hormonal differences, as well as developmental differences driven by sex chromosomes (8, 11, 12). Paralleling humans, young female mice are generally protected and have better survivals in multiple cardiac remodeling and failure models, whereas older females experience steeper functional decline during cardiac aging (13). Here, we investigated the effect of age and sex associations on gene expression in the mouse heart, by performing a proteogenomic characterization of 4-mo- versus 20-mo-old female and male C57BL/6J mice, with the latter age group corresponding roughly to the seventh decade of life in human age (14). With this study design, we compared baseline sex differences and sex-adjusted effects of aging, as well as genes showing age-by-sex interactions. The results demonstrate that both sex and age exert significant effects on the expression levels of thousands of cardiac genes, and moreover there are significant sex-biased effects on age-associated changes in gene expression involving cAMP signaling and cell–cycle regulation pathways. Latent space representation of the sex-adjusted age comparisons revealed gene regulatory modules in cardiac aging involving RNA-binding proteins, concomitant with a widespread remodeling of exon usage patterns, including splice events that can be linked to proteoform composition changes at the protein level. These findings expand the current understanding on the molecular underpinnings of sex differences in cardiac phenotypes and portray a complex proteogenomic landscape of sex-dependent changes in chronological aging in mice.
METHODS
Animal Models
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) guidelines at the University of Colorado Denver/Anschutz Medical Campus. Wild-type C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in a temperature-controlled environment on a 12-h:12-h light/dark cycle and fed with normal diet and water ad libitum under National Institutes of Health (NIH) “Guidelines for the Care and Use of Laboratory Animals.” Young adult (4 mo) versus early aging (20 mo) mice (males and females, n = 5) were examined by echocardiographic analysis and euthanized. Body weight, heart weight, and tibia length were measured. Heart ventricles were collected and kept frozen at –80°C.
Echocardiographic Analysis
Transthoracic echocardiograms were performed using the VisualSonics Vevo2100 system (VisualSonics, Toronto, Canada). Animals were anesthetized in an induction chamber at 2% isoflurane. Once appropriately sedated, mice were transferred to the imaging platform. Chest hair was removed with a depilatory lotion, and the mice was secured by taping all four limbs to the platform. Body temperature was maintained at 37°C. The body temperature of the mouse was monitored through a rectal probe connected to the Advanced Physiological Monitoring Unit, which also displays and records the animal’s ECG and respiration rate. Isoflurane was delivered through a nose cone at 1.5% throughout the imaging session. Mice were under anesthesia for no longer than 15 min. Parasternal long-axis (PSLAX) and parasternal short-axis (SAX) views of the left ventricle were acquired using B-mode imaging. SAX views of the LV at the papillary muscle level were used to acquire M-mode images. Left ventricular anterior wall (LVAW) thickness, left ventricular posterior wall (LVPW) thickness, and left ventricular internal diameter (LVID) in systole and diastole were measured using the M-mode images to measure changes in wall thickness and systolic function (Supplemental Table S1). Heart rate was measured in each M-mode image and averaged. All measurements were averaged from at least three cardiac cycles during the exhale phase and taken at body temperatures between 37°C and 38°C. All echocardiographic measurements and analyses were performed blinded to animal and group labels.
RNA Extraction and Sequencing
To extract total RNA, frozen cardiac tissues were weighed, thawed, and excised into small cubes (2 to 3 mm3) on weighing boats on ice. Tissue pieces were transferred to 2.0 mL RNase-free Eppendorf tubes. Cold TRIzol (Invitrogen Cat. No. 15596026) was added to tissues at a ratio of 75:1 (75 µL TRIzol for 1 mg of tissue). Samples were homogenized with a handheld homogenizer (OMNI International TH) for 10 s, followed by 1 min cooling on ice, repeated for a total of three rounds of homogenization. Following homogenization, the samples were centrifuged at 14,000 g for 15 min at 4°C, and the supernatants were transferred to a new tube and proceed to RNA extraction using Direct-zol RNA kit (ZYMO No. R2072) following manufacturer’s instructions. RNA concentration and quality were measured on a Nanodrop instrument (Thermo No. ND-2000). PolyA+ mRNA was selected using oligo-dT beads, and libraries were constructed with NEBNext Ultra II RNA Library Prep (unstranded) (Illumina). Paired-end sequencing (150-bp reads) was performed on a NovaSeq 6000 Sequencing System (Illumina). Untrimmed raw reads (fastq, 150 nt PE) were mapped using STAR v.2.7.6a (15) against the GRCm39 genome assembly and GENCODE M26 (16) annotations. Aligned reads were assembled into transcripts using Stringtie v.2.1.1 to output the gene and transcript count matrices. From the gene count matrix files, we performed differential gene expression analysis using DESeq2 v.1.30.1 (17) in R v.4.0.4 with the formula “∼sex + age + age:sex” for the linear model to account for the effects of sex and age and their interaction term, followed by fold-change shrinkage using apeglm (18) to estimate logFC and false discovery rate (FDR)-adjusted s-value (false sign or small) for |logFC| ≥ 0.1.
Protein Extraction and Mass Spectrometry
Frozen cardiac tissues were weighed, thawed, and cut into small pieces (2 to 3 mm3) on weigh boats on ice. Tissue pieces were transferred to 2.0-mL eppendorf tubes. Cold RIPA lysis buffer (Thermo No. 89901) with protease inhibitors (Pierce Halt) was added to tissues at a ratio of 20:1 (20 µL RIPA per 1 mg of tissue), and samples were homogenized as above and then sonicated with a handheld sonicator (Fisher No. FB120110) at 40% amplitude for 15 cycles (each cycle is 1 s of sonication, followed by 5 s pause, for three rounds). The samples were centrifuged at 14,000 g for 15 min at 4°C, and the supernatants were collected. Soluble protein concentration was measured with the BCA protein assay kit following manufacturer instruction; 100 µg of proteins were digested using a filter-assisted protocol as described (19, 20) with a 3-h LysC digestion and 12-h trypsin digestion. Peptide digests were tagged with tandem mass tags (TMT; 10-plex reagent, Thermo No. 90110) following manufacturer’s instructions, using a random number generator to assign balanced randomized samples across TMT blocks and channels. Tagged digests within the same block are then mixed and fractionated with the high-pH reversed-phase peptide fractionation kit (Thermo No. 84868). Fractionated peptides are dried and resuspended in 0.1% formic acid (LC-MS grade, Thermo No. 28905) at a final concentration of 0.5 µg/µL.
Each peptide fraction (∼1.5 µg) was further separated with online low-pH reversed-phase LC (PepMap C18 column, 3-μm particle, 100-Å pore; 75 μm × 150 mm; Thermo Fisher Scientific) via the EASYnLC 1200 system coupled to the Easy-Spray ion source (Thermo Fisher Scientific) at 300 nL/min with a 120-min gradient: 0–105 min: 0 to 40% B; 105–110 min: 40 to 70% B; 110–115 min: 70% to 100% B; 115–120 min: 100% B (solvent A: 0.1% vol/vol formic acid; solvent B: 80% vol/vol acetonitrile; column temperature: 50°C). Mass spectra were acquired on a Thermo Scientific Q-Exactive HF Orbitrap mass spectrometer with the following settings: polarity, positive; data-dependent acquisition (DDA), top 15 ions; MS resolution, 60,000; mass range, 300–1,650 m/z; precursor dynamic exclusion, 30 s; maximum ion injection time, 20 ms; MS automatic gain control (AGC) target, 3e6; isolation window, 1.4 m/z; stepped normalized collision energy (NCE), 28, 30, 32; MS2 resolution, 60,000; MS2 maximum ion injection time, 100 ms; and MS2 AGC target, 2e5. Raw mass spectrometry data were converted to mzML format using ThermoRawFileParser v.1.2.0 (21) and then searched against UniProt SwissProt (22) “Mus musculus” sequences (v2021_02) using Comet v.2020_013 (23). Database search results were postprocessed using Percolator v.3 (Crux v.4.0 distribution) (24) requiring 1% FDR for identification of UniProt sequences. TMT intensities were extracted from MS2 spectra using a custom Python script (25) built on the pymzml library (26) and then corrected for isotope contamination and normalized across blocks as previously described (27).
Pathway Enrichment Evaluation and Gene Function Analysis
Pathway overrepresentation analysis was performed using Fisher’s exact test against Reactome (28) pathways using ReactomePA (29) and gene set enrichment analysis (GSEA) using the fgsea package (30) against MSigDB Canonical Pathways (31). To perform gene set variance analysis (GSVA) (32), for each gene set, we used the gsva package to calculate an enrichment score in each sample, which was then used to perform differential enrichment analysis by limma. An integer normalized count matrix was created from the DESeq2 data, after which mouse Ensembl was queried with biomaRt (33) to retrieve the gene name from the Ensembl Gene ID. We treated human and mouse genes sharing identical gene names as orthologs and used the gene names to retrieve human Entrez Gene ID from the human Ensembl Biomart. We then used the Broad MSigDB ontology gene set collection v7.4 to construct the GSVA data set. To perform pathway lever information extractor (PLIER) (34) analysis, starting with the DESeq2 data set again, we retrieved the VST transformed data or the normalized counts. Because of the small sample size, we used a transfer learning framework to generalize the PLIER method by allowing the pathway loading matrix to be first trained in a large collection of RNA sequencing data containing gene expression latent structural information and then transferred to the collected data set (35). To do so, we used the Genotype-Tissue Expression GTEx v8 (36) RNA sequencing data, containing 17,382 RNA sequencing runs from 54 tissues or cell types from 948 donors, to learn the latent variable-pathway mapping and train a PLIER model, employing the built-in regularizations to favor latent variables that map to only a few human-interpretable pathways (34). Differential regulation of the gene sets and latent variables were then assessed using limma v.3.50.1 (37) with age and sex as factors in the linear model.
Exon Usage Analysis and RNA-Guided Protein Sequence Databases
Differential exon usage analysis was performed with the aid of DEXSeq v.1.40 (38). The flattened annotation file was generated with the DEXSeq Python scripts using GENCODE M26 gtf annotation. Differential exon usage was estimated using a ∼sample + exon + age:exon + sex:exon + age:sex:exon formula in the DEXSeq linear model.
To generate custom protein sequence databases containing sample-specific splice junctions, we used a proteogenomics pipeline and Python software, JCAST, we previously developed (39, 40). Briefly, we first used rMATS v.4.1.0 (41) with read-length 150 option and the GENCODE M26 gtf annotation to model alternative splicing events in all gathered RNA sequencing data. From the resulting 31,899 splice junctions belonging to 8,712 genes, we used JCAST to perform one frame in silico translation using Ensembl annotated translation start sites and frames to produce custom protein sequences corresponding to likely translatable splice junctions. In total, we translated 6,295 noncanonical sequences that did not experience frameshift (jcast_t1) and can be joined from the upstream and downstream exons back to the canonical Uniprot sequence via 10 residue flanks, among which 5,412 are not found in the mouse SwissProt canonical + isoform databases. Translations that experience frameshift regardless of whether they encounter premature termination codons (jcast_t2–t4) and those that cannot be merged back to SwissProt canonical sequences (orphans) were discarded (39, 42). We then appended the translated noncanonical jcast_t1 sequences to canonical mouse entries and searched the labeled tandem mass spectrometry experiments on the mouse hearts.
Structure Prediction and Additional Analyses
To predict isoform structure, folded 3 D protein models were generated from .fasta files with the “AlphaFold2_advanced” Google Colab notebook (43, 44). The resulting highest-ranked .pdb files were used as input to the DeepFRI web server (45) to predict protein function. The resulting functional salience maps were used to estimate functional importance of individual residues. Isoforms were visualized overlaid to their canonical forms using UCSF ChimeraX (46).
Other Analyses and Statistics
Additional statistical analysis and visualization was performed in R v.4.1.3 with the aid of the ggpubr (47) and ggsankey packages. Network visualization was performed with the aid of Cytoscape v.3.9.1 (48) with the ReactomeFIVIz plugin (49). Single-cell RNA-sequencing data were retrieved from the Tabula Muris data set (50) mouse heart samples, containing Smart-seq2 sequencing data of 4,365 FACS-sorted cells from four male and three female mice and processed using Seurat v.4.1.0 (51) to identify cell clusters. Bulk RNA sequencing data were deconvolved using MuSiC v.0.2.0 (52).
Data and Code Availability
The generated RNA sequencing data (total 1.47 billion reads, 150 nt paired end) have been deposited to NCBI Gene Expression Omnibus (GEO) under the accession GSE202384. Mass spectrometry data (total 5 TMT blocks, 40 raw files) have been deposited to ProteomeXchange (53) via jPOST (54) under the accession PXD033719. JCAST is available on GitHub at https://github.com/ed-lau/jcast (40).
RESULTS
Sex- and Age-Associated Differential Gene Expression in the Mouse Heart
We performed echocardiographic and proteogenomic analysis of male and female 20-mo versus 4-mo C57BL6/J mice (n = 3–5 per group) (Fig. 1A; Supplemental Tables S1 and S2). The age groups are consistent with previous aging studies in the mouse, with the former thought to correspond to the seventh decade of human life based on cumulative mortality (14). In our hands, aged animals at 20 mo showed a significant increase in left ventricular mass divided by tibia length, as well as increases in systolic and diastolic left ventricular anterior and posterior wall thickness (Fig. 1B), consistent with age-associated left ventricular hypertrophy. Echocardiography shows no significant difference in systolic function (measured by fractional shortening and ejection fraction) in the aged animals but a difference in diastolic function (measured by E′/A′), especially in aging male animals (Fig. 1, C and D).
Figure 1.
Study design. A: animal groups and schema of proteogenomics workflow. B: body mass, heart mass, and anterior wall thicknesses in each group (n = 5 biological replicates/group). P values: Student’s t test. C and D: echocardiographic measures associated with systolic (C) and diastolic (D) function.
To compare age and sex differences in the cardiac transcriptomes, we performed deep polyA+ RNA sequencing from 12 of the mouse heart samples (3 males and 3 females at 4 mo; 3 males and 3 females at 20 mo; total ∼1.5 billion reads). Principal component analysis (PCA) of the RNA sequencing data showed clear separation of transcriptome profiles by sex and by age, with the first principal component of the data largely separating the samples by sex (28% variance) and the second by age (23% variance) (Fig. 2A). From the data, we first interrogated the contribution of sex factor to gene expression profiles that are consistent in young adult and early aging hearts. This analysis identified 2,666 sexually dimorphic genes (1,651 higher in male; 1,015 higher in female) at 10% false discovery rate (FDR) (Supplemental Data S1). At a more conservative 5% FDR cutoff, 1,777 sex-dependent genes were found with 1,124 higher in male and 654 higher in female. An inspection of the top sex-associated genes show that they include genes residing on sex chromosomes including Xist, Eif2s3y, and Ddx3y, as well as participating in known sex-determination processes (Fig. 2B).
Figure 2.
Age- and sex-associated cardiac genes. A: scatter plot of first two principal components of total transcriptome profiles showing linear separation of samples by age and sex groupings (n = 3 per group). B: point ranges of log2 fold changes and standard errors (y-axis) in male (M) vs. female (F) C57BL/6J hearts, showing the top 50 significant genes (x-axis). Point glyphs denote sex chromosome/autosome of the gene in mice. Color: log2 fold changes (FC). C: point ranges of log2 fold changes and standard errors (y-axis) in sex-adjusted 20-mo vs. 4-mo C57BL/6J hearts, showing the top 50 significant genes (x-axis). D: overrepresentation analysis against Reactome pathways (y-axis) among differentially expressed genes (10% FDR of |log2FC| ≥ 10%) in male (M) vs. female (F) hearts. Red dashed line, 10% FDR; point color, –log10-adjusted P; point size, number of quantified genes in a pathway. Red pathways are significant within 10% FDR. E: pathway diagram for Reactome DAG and IP3 signaling pathway genes. Associated dot plots show the normalized read counts of sexually dimorphic genes. P values are from individual t test for reference; refer to Supplemental Data S1 for DESeq2 model P values. Genes that are more highly expressed in female are in blue; male, in red. F: overrepresentation against Reactome pathways (y-axis) among differentially expressed genes (10% FDR of |log2FC| ≥ 10%) in 20-mo vs. 4-mo hearts.
Upon accounting for baseline sex differences in the DESeq2 model (see methods), we further identified 2,519 genes that are significantly differentially expressed in 20-mo versus 4-mo mouse hearts, with at least |log2FC| ≥ 10% at a conventional 10% FDR, including 1,464 upregulated genes and 1,055 downregulated genes. At a more conservative 5% FDR of |log2FC| ≥ 10% threshold, 1,738 genes were differentially expressed including 1,052 upregulated and 686 downregulated genes (Supplemental Data S2). We note that almost 40% fewer genes are found to be age regulated when sex differences at the baseline are not considered (1,611 vs. 2,519 at 10% FDR), which is consistent with significant sexual dimorphism in gene expression in both young and aged hearts and underlines the importance of taking sex into account in aging comparisons (see Supplemental Data S1 for the list of sexually dimorphic genes). The differentially expressed genes include 45 of 98 previously reported proteins found in aging hearts using microarrays (55) including immune genes Cfb and Cd209d, whereas the most significantly changed genes also included several poorly characterized genes that are associated with age-associated diseases in other tissues (e.g., Ric3, Parkinson’s disease; Prkcz, cancer) (Fig. 2C; Supplemental Fig. S1B).
On a pathway level, we found that sex-differential genes appear to primarily cluster around metabolic pathways over a background of all quantified cardiac genes, including genes involved in pyruvate metabolism and TCA cycle, (P.adj: 7.8e–8), mitochondrial translation (P.adj: 5.0e4), gluconeogenesis (P.adj: 2.3e–2); as well as baseline differences in DAG and IP3 signaling (P.adj 4.8e–3) and calmodulin-induced events (P.adj 1.0e–2), ion channel transport, cardiac conduction, autophagy, and other pathways (Fig. 2D; Supplemental Fig. S1A). To examine the individual genes that drive these pathway-level differences, we inspected two particular pathways in more detail. In the first case, the pyruvate metabolism and TCA cycle term is associated with individual gene level changes of a majority of pyruvate metabolism genes, including Pdhx, Pdhb, Pdha1, and Dlat, which are higher in male than female. On the other hand, Slc16a3, which codes for a monocarboxylate transporter that mediates the transport of pyruvate across the plasma membrane, is higher in female, suggesting baseline differences in pyruvate metabolism gene wiring between male and female (Supplemental Fig. S2). Second, we found a difference in sex-biased usage in two related terms of DAG and IP3 signaling (P.adj 4.8e–3) and calmodulin-induced events (P.adj 1.0e–2) (Fig. 2, D and E). Upon inspection, the statistical enrichment of DAG and IP3 signaling pathways and calmodulin-induced event terms is not linked to differential expression of calmodulin directly but to associated genes. There is a higher male expression of Prkca, Pde1c, three members of the adenylate cyclase family (Adcy1, Adcy5, and Adcy9), and four members of the protein kinase A (PKA) complex (Prkar1a, Prkar2a, Prkaca, and Prkcacb), and higher female expression of phosphodiesterases Pde1a and Pde1b, as well as Adcy4. Cyclic AMP and PKA pathways lie downstream of important G protein-coupled receptor signaling and are strongly linked to cardiac aging and failure (56–58). The results here are suggestive of potential baseline differences in cyclic AMP and PKA signaling between female and male hearts driven by gene expression dimorphism, which would be in a position to effectuate functional differences.
Intriguingly, a similar conventional overrepresentation analysis of age-associated genes using Fisher’s exact test against Reactome annotations found only two pathways to be enriched within the 1,738 (2,519) aging-differential genes (R-MMU-112316 Neuronal System and R-MMU-112315 Transmission Across Chemical Synapses), which have no obvious connection to cardiac aging processes (Fig. 2F; Supplemental Fig. S1A). Similarly, we performed gene set enrichment analysis (GSEA) against MSigDB canonical pathways but likewise found few easily interpretable pathways (Supplemental Fig. S1B), together suggesting that although a sizeable number of age-differential genes are found in the model, they do not readily map to known processes through conventional enrichment comparisons (see Latent Space Representations Reveal the Involvement of RNA Processing in Aging).
Sex-Biased Aging Genes Are Enriched in cAMP Signaling
To discover gene signatures of sex-biased differences in aging, we next performed differential gene expression analysis on the age-sex interaction term in the DESeq2 linear model and found 853 genes with sex-biased response to aging processes using the male:aged versus female:aged contrast (336 higher in male:aged, 517 lower) at 10% FDR (553 genes with 234 higher in male:aged and 319 lower at 5% FDR) (Fig. 3A; Supplemental Data S3). To illustrate the effect of sex-by-age interactions, Figure 3B shows example of cardiac genes with positive sex-by-age interactions (Sprr1a and Cd72) and negative sex-by-age interactions (Tfrc and Cpxm2). In the positive interaction cases, Sprr1a (small proline-rich protein 1a) is induced in aging more highly in males than in females; Cd72, which encodes a protein functioning in B cell proliferation, is induced in aging in males but suppressed in females. Both genes have a positive sex-by-age interaction as the difference in gene expression in 20-mo over 4-mo in male and that in female is positive. In the negative interaction cases, Tfrc, which mediates iron uptake, is elevated in females in aging but reduced in males, and Cpxm2, which encodes a carboxypeptidase, is induced more prominent in aging in females than in males; both genes have a negative sex-by-age interaction despite the different direction of changes in aging versus young male. A functional analysis using Fisher’s exact test for genes differentially regulated at 10% FDR against Reactome annotation showed significant enrichment of two clusters of terms. The first cluster again includes Reactome pathways DAG and IP3 signaling (R-MMU-1489509; P.adjust 0.0013), calmodulin-induced events (R-MMU-111933; P.adjust 0.0030), and PKA activation (R-MMU-163615; P.adjust 0.0073), which are mediated by Adcy1, Adcy5, Adcy9, Prkar1a, and other genes (Fig. 3C), suggesting the sex difference in cAMP- and PKA-related gene expression may also play a relevant role in sex-biased aging processes. The second cluster contains an enrichment in the Reactome mTOR signaling term (R-MMU-165159; P.adjust 0.032), although this enrichment is mediated by the translation initiation factors Eif4g1 and Eif4e, the AMPK subunit Prkaa2, and other genes not part of the mTORC complex (Fig. 3C).
Figure 3.
Cardiac genes and pathways with significant age-by-sex interaction. A: heat maps showing the top 60 sexually dimorphic aging genes that show significant differences in aging response across sexes (DESeq2 age:sex 10% s-value). Colors denote standardized expression (Z score of normalized read counts). B: normalized read counts of four genes from panel a were visualized across groups individually. P values represent individual t tests in M vs. F comparison in 4-mo and 20-mo groups for reference; refer to Supplemental Data S3 for DESeq2 model P values. C: gene-concept network of 2 groups of significantly enriched Reactome terms among genes with significant age-by-sex interaction. Brown nodes are Reactome terms, linked through edges to their annotated genes in the foreground of the functional enrichment analysis. Gene node colors, log2 FC in male aged/adult vs. female aged/adult; Reactome term node size, gene count; edge color, Reactome terms. D: gene-concept network involving top enriched term in gene set enrichment analysis of the ordered gene list (by log2FC, male:aged vs. female:aged), linked to their annotated genes as in C.
A complementary gene set enrichment analysis, which does not rely on arbitrary significance cutoffs of the differential gene expression analysis (such as those presented in Supplementary Data S3), further reveals an enrichment in cell cycle (R-MMU-69278, P.adjust 0.013), mitochondrial translation (R-MMU-5368287, P.adjust 0.041), and neddylation (R-MMU-8951664; P.adjust 0.067)-related genes (Fig. 3D). These changes reveal transcriptome-wide bias of whether the genes in the gene lists are more likely to be biased toward negative interaction than randomly distributed, rather than individual gene-level significant changes, although they can also include genes that are significantly changed in the individual gene levels. For example, the cell-cycle term includes Cenpf (male:aged interaction log2FC –1.23, s-value 0.0034) and the mitochondrial translation term includes Mrpl19 (male:aged interaction log2FC –0.32, s-value 0.018) (Supplemental Data S3).
Therefore, our analysis revealed age-by-sex interactions in cardiac gene expression both among genes that show significant sexual dimorphism in aging changes in the individual gene level (i.e., FDR < 10% in DESeq age:sex interaction term), as well as transcriptome-wide trends (i.e., GSEA analysis). In the former case (significant genes), we found there is a negative male:old interactions in cAMP and PKA signaling genes, which suggests a negative relative change in aging in male versus female. We note that this interaction can denote biologically distinct changes in different genes dependent on the main effect of age and sex on their expression, as seen in the examples of Fig. 3B. In a number of genes in the cAMP and PKA signaling pathways (Adcy1, Adcy9, Pde1c, etc.), as well as for Rxra and Pdpr in pyruvate metabolism (Supplemental Fig. S2), we found that that sexually dimorphic genes overlap with sex-by-age interactions, suggesting that baseline sexual dimorphism in gene expression may precipitate sex-biased aging changes. In the second case (transcriptome-wide trend), we find a negative interaction term (i.e., more severe decline or less induction) among genes associated with cell-cycle and mitochondrial translation.
To examine whether cell-type heterogeneity influences age- and sex-dependent gene expression, we further deconvolved the bulk RNA sequencing data using mouse heart single-cell sequencing data from the Tabula Muris data set (50). We did not find evidence for significant differences in the estimated cell-type proportions between female and male, young and aged hearts that would suggest the observed gene expression differences are driven primarily by cell population changes (Supplemental Fig. S3). Among the significant sex-biased aging genes are genes that are highly enriched within particular cell clusters in the single-cell sequencing data including putative cardiomyocyte, macrophage, and myofibroblast clusters, suggesting age-by-sex interactions broadly affect multiple cell types in the heart (Supplemental Table S3).
Proteogenomics Comparison of Cardiac Gene Expression by Age and Sex
We next analyzed matching cardiac samples with mass spectrometry to acquire proteomics data. The proteomics data are expected to diverge from RNA sequencing data in several important ways: on one hand, at present a typical mass spectrometry dataset has less depth than RNA sequencing and also carries distinct sources of technical variations than transcriptomics data. On the other hand, protein-level data provide unique information to reveal posttranscriptional regulation that likely lies closer to overt phenotypes. We collected both left and right ventricular samples for mass spectrometry and performed a general comparison of protein levels across age, sex, and chamber factor. Blood protein contamination is more severe at the protein level than the transcript level, which may be due to the higher dynamic range of concentration of proteins.
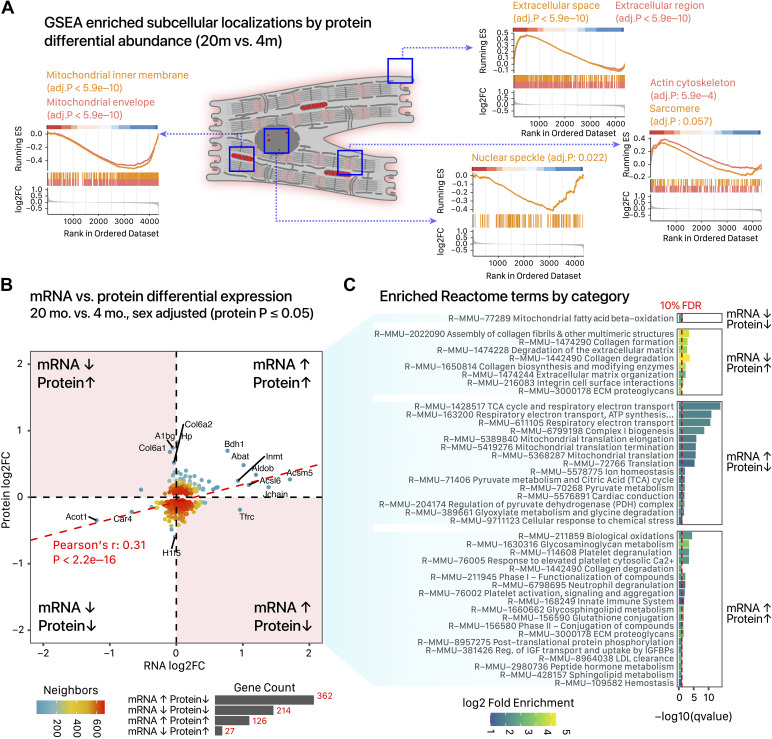
In total, we quantified 4,334 unique proteins identified at 1% FDR by UniProt accessions from five separate blocks of TMT experiments. Following normalization, the proteomics expression profiles segregate largely by age and sex groups rather than experimental batch, and the left ventricular proteomes of 4-mo versus 20-mo males and those of 4-mo versus 20-mo females were linearly separable in the first two principal components despite residual variability (Supplemental Fig. S4, A and B). In total, 764 proteins show suggestive evidence of differential regulations in aging hearts after adjusting for sex and chamber (limma sex-adjusted P value ≤ 0.05; 733 cross-referenced to gene names), 249 of which was significant at 10% FDR (Supplemental Fig. S4C; Supplemental Data S4). We likewise identified proteins that are sexually dimorphic including proteins that are nominally found in the plasma but sampled in the cardiac samples, e.g., the male versus female comparison found higher levels of murinoglobulin, which is known to circulate at higher levels in male mouse plasma (59) (Supplemental Fig. S4D; Supplemental Data S5). In contrast to the mRNA data, protein-level information on cardiac aging primarily revealed differential regulation along organellar configurations in the heart, with GSEA enrichment plots showing significant running enrichment scores (i.e., nonrandom distribution in the ranked gene list) for mitochondria (adjusted P ≤ 5.9e–10), extracellular space (adjusted P ≤ 5.9e–10), actin cytoskeleton (adjusted P = 5.9e–4), and RNA-binding protein (RBP)-enriched nuclear speckle (adjusted P = 0.022) (Fig. 4A). At an individual protein level, when contrasting 20-mo and 4-mo mouse hearts, 249 unique proteins consistently changed across age after adjusting for sex and chamber difference at 10% FDR, and 64 protein groups were consistently changed across sex. To understand the effect of aging on cardiac proteome, we further performed a subgroup analysis where 20-mo male compared with 4-mo male and 20-mo females were compared with 4-mo female without adjusting for age-sex interactions. We found 645 unique proteins to be differentially regulated at 10% in early aging male versus young adult male, but only 31 proteins in the female comparisons. Of interest, one of the top-regulated targets at the protein level is β-hydroxybutyrate dehydrogenase 1 (BDH1), a key enzyme in ketone body metabolism (60). BDH1 catalyzes the oxidation of β-hydroxybutyrate, which is thought to serve as an alternative fuel for stressed and failing hearts. An increased expression of BDH1 has been reported in failing hearts in the literature (61); the increased expression found here may suggest a parallel metabolic switch in cardiac aging.
Figure 4.
Comparisons of proteomics and transcriptomics data. A: running enrichment score (ES) plot of significant subcellular localizations in gene set enrichment analysis (GSEA) of the quantitative mass spectrometry comparisons of 20-mo vs. 4-mo hearts. Proteins were ordered from highest to lowest log2 fold change (log2FC). Left to right: age-associated proteins are enriched in extracellular (trending upregulated), mitochondrial (downregulated), nuclear speckle (downregulated), and actin cytoskeletal (upregulated) localizations. B: scatter plot of log2FC among differentially regulated proteins (limma P ≤ 0.05) and the corresponding log2FC of their corresponding mRNA. Data are divided into 4 quadrants based on whether they show concordant or discordant protein and mRNA changes as labeled. Point colors, data density; bars, mRNA/protein counts in each quadrant. C: bar charts showing significantly enriched Reactome terms (at 10% FDR) among proteins in each quadrant; x-axis, –log10 FDR-adjusted P value, Fisher’s exact test; bar color, log2 fold enrichment against all quantified proteins.
Several observations were made upon contrasting the mRNA-level and protein-level changes. First, there was minimal overlap between candidate differential proteins (limma P value ≤ 0.05) with significantly changed mRNA in the DESeq results (Jaccard index 0.08). Nevertheless, among these proteins we found a moderate but significant correlation between RNA and protein fold change (Pearson’s r = 0.31, P < 2.2e–16; Spearman’s ρ 0.17, P = 5.4e–6) consistent with previous reports (Fig. 4B). In the sex-adjusted 20-mo versus 4-mo comparison, as well as both subgroup analyses, we found myotilin, a protein involved in muscular dystrophy, to be significantly elevated with age (logFC 0.41 in both sexes, adj.P 0.022; logFC 0.53 in female, logFC 0.30 in male). Concordantly, myotilin is suggestively upregulated in the RNA level data (logFC 0.14, s-value = 0.17) and is also significantly upregulated from a previously published gene microarray dataset that found 98 differentially regulated genes in 24-mo versus 6-mo C57BL/6N mouse hearts (55).
Not unexpectedly, we observed a compression in the magnitude of fold changes of protein abundance compared with fold changes of transcript abundance, which are likely due to a combination of technical reasons (62) and biological buffering effects such as the higher cellular protein concentrations relative to mRNA (63–65). A scatterplot of mRNA versus protein fold changes show that the commonly quantified mRNAs and proteins primarily occupied three quadrants based on their respective fold changes (Fig. 4B); whereas downregulation of RNA levels largely led to decreased protein levels, increase in mRNA can lead to either a corresponding increase of proteins or a paradoxical decrease of protein levels. Notably, the largest cluster of proteins involved contradirectional changes of increased mRNA but decreased proteins. As this quadrant also contains the highest mean protein abundance compared with other quadrants (Supplemental Data S4), the discordant mRNA and protein changes are not likely to be due to less reliable protein quantification. Functional enrichment analysis of the quadrant categories shows the nondiagonal quadrants (i.e., increased RNA but decreased proteins and vice versa) to be enriched in large multiprotein complexes. Multiprotein complexes are known to show lower correlation between their mRNA and protein levels in part because of complex stoichiometry, that is, an increase in one gene at the mRNA level would create an excess of a supernumerary subunit that does not necessarily lead to higher total number of functional complexes, thus creating a buffering effect that reduces the transmission of transcript-level regulation (Fig. 4C). The results are consistent with an uncoupling of genetic regulations (induction of transcription) from the biochemical state (proteins) in the aging hearts. Moreover, they suggest that additional confirmation may be warranted for any observed increase in age-associated mRNAs-encoding large complexes to corroborate their concordant changes at the protein level and hence potential downstream functional significance.
Latent Space Representations Reveal the Involvement of RNA Processing in Aging
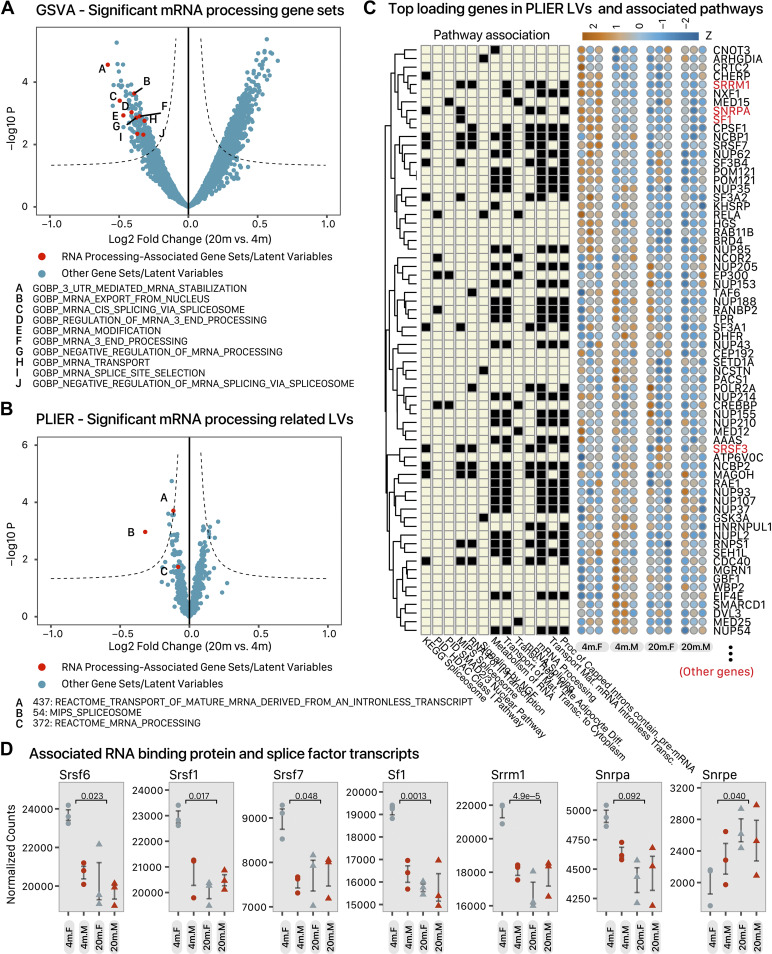
As the overrepresentation and gene set enrichment analysis unexpectedly returned few interpretable results in the sex-adjusted age comparison, we performed additional analyses to discern potential regulatory principles that are altered in the aging heart. To achieve this goal, we employed two complementary strategies to directly assess the concerted changes of extracted gene sets and modules. First, we performed gene set variance analysis (GSVA) (32), an unsupervised method that first calculates a gene set enrichment score for each sample and from there performs direct statistic testing of cross-sample score differences in multifactorial experimental designs (i.e., ∼age + sex + age:sex). Using this approach, we found that mRNA processing and splicing pathways are heavily implicated in cardiac aging, with a total of 10 gene ontology (GO) biological process (BP) terms enriched at 10% FDR, including BP: mRNA cis-splicing via spliceosome (P = 3.9e–4) and BP: mRNA splice site selection (P = 4.9e–3) (Fig. 5A). Other significantly enriched terms include BP: mRNA modification, BP: mRNA splice site selection, altogether suggesting the preferential involvement of module of mRNA processing and splicing-related genes in sex-adjusted aging that evaded overrepresentation analysis. To corroborate the GSVA results, we further used a transfer learning strategy, where we first trained a model to learn the latent structure of mammalian gene expression using human transcriptome data from over 17,000 RNA sequencing runs across 54 tissues and cell types in the Genotype-Tissue Expression (GTEx) v8 (36) data set. We employed the matrix decomposition algorithm, pathway level information extractor (PLIER) (34), to embed the gene-by-sample data matrix in a latent variable space, for which the algorithm learned the latent variables using the input gene expression data, as well as a gene-pathway membership matrix. The pathway loading matrix learned from the large data set was then mapped to the current dataset by assuming conservation of latent gene expression structure (35) to assess the differential regulation of related gene modules in adult versus early aging mouse hearts. Among the differential latent variables, we found two (latent variables 54 and 437) that were differentially abundant in aging at 10% FDR (P = 1.1e–3 and 1.9e–4, respectively) and another related variable (latent variable 372) that was suggestively changed in the aging heart (P = 1.8e–2) to be related to pre-mRNA splicing and mRNA processing (Fig. 5B). The top gene loadings associated with these latent variables confirm they map to various annotated mRNA splicing-related terms including Reactome mRNA processing, mRNA splicing, metabolism of RNA, and spliceosome (latent variable 54); reactome mRNA processing (latent variable 372); and processing of capped intron containing pre-mRNA (latent variable 437) (Fig. 5C). On an individual gene level, a number of transcripts encoding spliceosome and RNA-binding proteins were altered in aged hearts at 10% FDR including Srrm1, Sf1, Snrpa, Srsf1, Srsf6, and Srsf7 (Fig. 5D). The serine/arginine-rich splicing factor (SRSF) family encodes multiple cardiac RBPs (66), among which Srsf1, also known as ASF/SF2, is known to regulate CaMKII splicing and whose deletion results in cardiomyopathy phenotypes in the mouse (67). Other known RBP-coding transcripts that are altered in aging hearts include Rbm10 (logFC –0.18, s-value = 9.9e–3), Rbm28 (logFC –0.18, s-value = 0.040), and Ptbp2 (logFC –0.19, s-value = 0.023) Taken together, these gene expression and functional analysis results indicate that chronological aging exerts broad effects on mRNA splicing and processing pathways in the mouse heart.
Figure 5.
mRNA-processing and splicing-related pathways in sex-adjusted aging hearts. A: volcano plot showing the limma –log10 P value (y-axis) vs. log2 fold change (x-axis) of gene set variance analysis (GSVA)-extracted gene sets. Gene sets related to mRNA splicing are labeled and colored in red and labeled with letters corresponding to the annotated pathways printed. B: volcano plot showing the limma –log10 P value (y-axis) vs. log2 fold change (x-axis) of PLIER-learned latent variables. Latent variables related to mRNA splicing are labeled and colored in red and labeled with letters corresponding to the annotated pathways printed. C: heat maps showing a subset of genes with highest feature scores in the mRNA splicing associated latent variables that are differentially regulated in aged hearts, i.e., genes belonging to mRNA processing and splicing pathways that are represented in latent variables 437 and 54 in B. The left matrix denotes the membership of the gene in the PLIER prior knowledge matrix (annotated pathways); colors in the right heat maps denote relative read counts in 20-mo vs. 4-mo female (F) and male (M) hearts. D: individual plots of normalized gene counts across each group among selected significant transcripts encoding RNA-binding proteins and splice factors. Numbers represent DESeq2 model s-value in 20-mo and 4-mo heart comparisons after adjusting for sex. See Supplemental Data S2 for details.
Widespread Reorganization of Exon Usage Patterns in Aging Hearts
As the functional analysis implicates mRNA processing to be involved in aging, we next asked whether the changes in splicing-related genes are associated with changes in alternative splicing patterns in the mouse heart. First, we used the statistical model in DEXSeq (38) to assess the effects of individual exons within a gene and their interaction with age and sex factors on individual exon read counts. Differential exon usage can therefore be distinguished from differential gene expression by considering differential read counts between young adult and early aging hearts only in specific exons, which are first preprocessed into disjoint (nonoverlapping) exonic parts for calculations in the DEXSeq model.
In total, we tested the exon usage of 228,932 exonic parts across age and 209,838 exonic parts across sex. We found that age is associated with significant differences in exon usage of 7,520 exonic parts corresponding to 3,617 genes at 10% FDR. At a more conservative 5% FDR, there are significant exon usage differences in 4,238 exonic parts in the cardiac transcriptome belonging to 2,315 genes (Supplemental Data S6). For example, a significant preferential decrease in the read counts of exonic parts 31–33 (adjusted P values = 1.2e–18, 8.9e–10, and 3.4e–4) in 20-mo hearts versus 4-mo hearts can be seen in F-box only protein 11 (Fbxo11), a member of the F-box protein family whose members form parts of the RING-domain containing E3-ubiquitin ligase complexes (Fig. 6A). The DEXSeq exonic parts 31–33 map through their genomic coordinates to the exon 1 of the Ensembl full-length Fbxo11-201 canonical transcript. Inference of the differential exon usage regions suggest the aging differences correspond to the Fbxo11-201- and Fbxo11-203-annotated protein coding transcripts, which are translated into UniProt accession Q7PD1-1 and Q7PD1-3, respectively, with the latter isoform omitting the majority of exon 1 and containing an alternative translation start site. The exon usage data are therefore consistent with a decrease in the shorter isoform of the FBXO11 protein in aging hearts. Another example is found in the polyhomeotic homolog 2 (Phc2), which is part of the large chromatin associated polycomb group multiprotein PRC1-like complex. Phc2 shows significant decrease in exon usage in exonic parts 19–21 and 28–29 (Fig. 6B).The DEXSeq exonic parts 19–21 map to intron 9–10 in the Ensembl full-length Phc2-201 canonical transcript, and to the untranslated exon 1 of the shorter Phc2-202 isoform. Exonic parts 28–29 map to exon 10 of the full-length Phc2-201 isoform on Ensembl, and exon 2 of the shorter Phc2-202 isoform. Subsequent exonic parts mapping to other annotated exons (e.g., the DEXSeq disjoint exonic parts 32–35 mapping to the full-length Phc2-201 exon 11 and 12) were unchanged or slightly upregulated in aging hearts. Therefore, although the RNA sequencing data strongly point to changes in exon usage patterns in the Phc2 gene, it is not possible from this analysis of collapsed short-read data to resolve whether the changes in exonic parts 19–21 and 28–29 correspond to combinatorial differences in multiple-annotated isoforms, or unannotated age-specific events such as involving a cassette exon 10 or a partially retained intron. Nevertheless, the DEXSeq results strongly indicate sweeping effects of exon membership on read counts suggesting a reorganization of transcript isoform patterns.
Figure 6.
Differential exon usages in aging hearts. A–C: exon usages in sex-adjusted comparisons between 20-mo (blue) and 4-mo (red) mouse hearts. Y-axis shows normalized read counts across disjoint exonic parts in DEXSeq after adjusting for gene-level differential expression. The disjoint exonic parts in DEXSeq are mapped to annotated exons in the Ensembl gene model below with the genomic coordinates labeled. Exonic parts with significant differential usage in 20-mo vs. 4-mo mouse hearts are colored in purple. D: intersection between genes with differential exon usage (DEU) at 20 mo vs. 4 mo (DEU_AGE) at 10% FDR with genes with differential gene expression (DGE) across age and sex comparisons. E: dot plot showing the enrichment ratio and adjusted P values of overrepresented Reactome pathways among genes with age-associated exon usage difference. Exon usage changes are enriched along RNA metabolism, ribosomal, and mitochondrial metabolic pathways. F: gene concept network showing top 15-enriched Reactome terms and their associated genes with significant differences in exon usage in 20-mo vs. 4-mo hearts.
Sarcomeric genes are known to be under heavy influence of alternative splicing regulation, and we find complex and pervasive differential exon usage, including in Myh7, Obscn, Myom1, Ttn, and others (Supplemental Fig. S5, A and B and Supplemental Data S6). Other cardiac genes with known functional significance showing differential exon usages in the aging heart include Akt1 with lower coverage in aging in exonic parts 27, 28, 30, and 31 corresponding to a 5' untranslated region of the full-length Akt1-201 canonical transcript in Ensembl (Fig. 6C); as well as Sirt1 with lower coverage of DEXSeq exon part 16 in aging corresponding to the first translated exon (exon 6) of the canonical Sirt1-203 transcripts on Ensembl.
Notably, integrated analysis of mRNA abundance and exon usage reveals that a greater number of genes are consistently affected by aging via exon usage pattern and splicing than those that are differentially expressed across identical significance thresholds. Moreover, genes that exhibit differential exon usage across age appear to overlap only minimally than differentially expressed genes, suggesting differential exon usage is able to exert independent effects from differential gene-level expression to aging hearts (Fig. 6D). Overrepresentation and network analyses suggest that genes with differential exon usage in aging hearts are clustered along coordinated functional categories including RNA metabolism, ribosome, nonsense mediated decay (NMD), as well as mitochondrial metabolism pathways (Fig. 6, E and F). Within the RNA metabolism category, multiple splicing-related genes show evidence of differential exon usage including Hnrnpd, Hnrnpa2b1, Srsf1, Prpf8, Prpf19, Srsf10, and others. An example of a splicing-related gene showing differential exon usage is Prpf8, which codes for PRP8, a core conserved component of the spliceosome, where in aged hearts there is an increased usage of exonic parts 4 to 5 (Supplemental Fig. S5C) that codes for an NH2-terminal disordered region of the PRP8 protein. The differential exon usage of these genes and the enrichment of RNA metabolism-related terms is consistent with the existence of a positive feedback in transcript processing in aging where initial changes in RNA-binding proteins and splicing factors cause further changes in transcripts involved in post-transcriptional regulation processes. Taken together, these results are consistent with previous observation in the reanalysis of GTEx transcriptome data in multiple human tissue (68) that transcript splicing may be more prominently altered in aging than gene-level differential expression.
We likewise observed differences in exon usage across sex, affecting 1,537 exon parts in 1,056 genes by sex, and 1,868 exon parts in 1,168 genes by age-sex interactions (Supplemental Data S7 and S8). Genes with sex differential exon usages included the 5' exons in Obscn and Pdlim5 (Supplemental Fig. S6, A and B). The first two 5′ exons in Mt1 display a complex age-sex interaction, with exon usage increasing during female aging hearts but decreasing in male aging hearts. Again, we observed a poor overlap between differential exon usage genes and differential expression genes in sex and age-sex interaction comparisons (171 genes or 16% in sex comparison, 48 genes or 4% in age-sex interaction), further highlighting that exon usage constitute an independent feature of sex-biased gene regulation. Exonic parts with differential usages appeared to be unevenly distributed among coding regions with an enrichment for 5′ FDR overlapping exons (Supplemental Fig. S6, C and D). Taken together, the results from differential expression and exon usage analyses corroborate the hypothesis that changes in RNA processing and alternative splicing present a salient feature in cardiac aging processes.
Detectability of Alternative Transcripts at the Protein Level
Finally, we asked whether any differential exon usage might show discernible proteome-level consequences, which would be important in establishing their molecular and potential functional significance. The extent to which alternative transcripts influence the proteome remains poorly understood. Alternative splicing can influence proteome compositions in at least two ways: first, by diverting total transcript usage away from productive, canonical isoforms toward unproductive transcripts, which has the net effect of decreasing total canonical protein levels; and second, by the production of noncanonical transcript isoforms encoding splice variant protein sequences (splice variant proteoforms). Not every alternative isoform carries protein translation potential. Large-scale analysis of human transcriptomics data across multiple cell types and tissues have generally found prevalent splicing noise and isoforms that contain premature termination codons likely targeted by nonsense-mediated decay (69–71), whereas cotranslational protein quality control mechanisms conserved from yeast to humans are known to degrade up to 30% of nascent peptides (72–75). Hence, the extent to which alternative splicing events may influence cardiac functions await further elucidation at the protein level.
Alternative protein isoform identification in shotgun proteomics remains highly challenging for a number of reasons, including the low abundance of noncanonical proteins, the current poor annotation of isoforms in protein databases (76), the requirement to detect a specific isoform-typic peptide not shared from the canonical sequence (39), and the prevalence of tryptic lysine residue around splice junctions (77, 78). To examine whether the identified splice events may translate to stable proteins at the proteome level, we used a proteogenomics approach based on RNA sequencing-derived protein sequence database to reanalyze the acquired mouse heart proteomics data. Because alternative splice isoforms remain incompletely annotated in common mouse proteomics databases (e.g., UniProt SwissProt mouse contains 8,318 noncanonical sequences), this workflow allows potential undocumented isoforms to be salvaged from mass spectrometry data while avoiding runaway database size inflation and the accompanying false positive identifications.
To do so, we used a computational pipeline we previously developed, JCAST, to filter detected splice junctions in the RNA sequencing data and write likely detectable alternative protein sequences in custom protein sequence FASTA files (39, 79). We first modeled the potential alternative splicing events that can explain the exon usage in the RNA sequencing data through exon splice junction spanning reads using rMATS v.4.1.0. This allowed splice junction spanning reads to be identified to model splice events including skipped exons (SE), mutually exclusive exons (MXE), alternative splice donor/acceptor sites (A5SS/A3SS), and retained intron (RI) events. A comparison of exon inclusion levels from these splicing events performed on two-sample aged versus young adult male hearts and aged versus young adult female hearts separately confirmed that splice event changes are prevalent, with 2,862 splice events in both comparisons belonging to a total of 2,911 genes to show significant changes in aging hearts at 10% FDR, among which 977 overlapped with genes with significant differential exon usages.
Second, this analysis also confirmed that a majority of alternative splicing events are likely undetectable as protein, both due to the very low transcript count and because they introduce frameshift leading to premature termination, although a substantial number of potentially translatable splice isoforms remain (Fig. 7A). In the former case, we note that the majority of modeled splicing events in the cardiac transcriptome can be categorized as cassette exons (skipped exons). A large number of cassette exons have very low read counts and are thought to arise from splicing noise (80), and thus we reason they are unlikely to be translated into functional protein products or even if translated would not exist at a level detectable by mass spectrometry-based proteomics. The splice junction read count distribution can be modeled using a Gamma-Gaussian mixture model (Fig. 7A) in RNA sequencing data to separate the splice junctions into low and high read populations, which allows the opportunity to compare translation proportion or alternatively to remove primarily low count-skipped exons that fall into a distinct distribution of read counts from the majority of other exon junctions when generating RNA-guided protein sequence databases (see methods).
Figure 7.
Protein-level consequences of exon usage changes. A: Sankey diagram illustrating the fate of all modeled splice junctions in the RNA-guided protein database methods. Node sets from left to right denote the modeled splice type of the exon junctions, whether the skipped junction count is above a threshold modeled from total junction read counts, translation status (see methods), and identification status. Inset: modeled read count cutoff. B, top: peptide spectrum match (PSM) of a noncanonical peptide belonging to titin from a database search using the RNA-guided protein database in A. Matched peptide fragment b- and y-ions are labeled and colored in red. PEP, Percolator PSM posterior error probability. B, bottom: best-hit candidate sequence matched to the identical spectrum (top) when queried against UniProt SwissProt canonical and isoform entries. C: differential exon usage of Acsl1 in 20-mo vs. 4-mo mouse heart. A disjoint exonic part E030 (green) shows higher usage in aged hearts and maps to an exon absent in the canonical transcript (Acsl1-201). D: peptide-spectrum match of a noncanonical peptide corresponding to a cassette exon matching to E030 identified in the quantitative mass spectrometry data using the RNA-guided protein database. E: predicted protein structure of the predicted full-length alternative ACSL1 containing the translated alternative region from which the noncanonical peptide was identified, joined to the full-length UniProt canonical sequence via a 10-amino acid joint in the upstream and downstream exons. E, left: predicted protein structure with the alternative region highlighted. E, right: predicted structure of the alternative region overlaid on the predicted structure of the canonical protein.
From the custom databases, we identified 460 peptide sequences at 5% Percolator FDR that are uniquely mappable within the FASTA database to noncanonical protein isoforms, 349 of which are not found among UniProt SwissProt mouse isoform sequences at the time of writing, and 130 of which are not in TrEMBL at the time of writing. In addition, 103 potential noncanonical isoform peptides are potentially identifiable as part of a unique protein group that does not include a canonical sequence, 82 of which are not found among UniProt SwissProt mouse isoform sequences at the time of writing (Supplemental Data S9). The identification of noncanonical peptides demands caution as there are potential alternative explanations of peptide spectrum matches such as from unaccounted canonical peptides or modifications. We manually examined a subset of identification and found that at least a portion of noncanonical identifications may represent veritable detectable splice variant proteins. For instance, we identified a titin isoform peptide that is modeled to originate from a mutually exclusive exon and that is not mappable to UniProt SwissProt or TrEMBL sequences at the time of writing (Fig. 7B), which suggests novel protein isoforms remain to be found in the cardiac proteome, although safeguard measures will be needed to exclude common sources of spurious identification (see examples below).
From the labeled quantitative proteomics data, we further attempted to quantify the differential abundance of noncanonical sequences across age and sex groups. There are multiple challenges associated with quantifying isoform peptides, including the difficulty of identifying these sequences confidently and the high burden of multiple testing. Moreover, the current workflow does not incorporate match between runs (MBR) across fractions or TMT blocks, as the false discovery rates of MBR remain unclear and can compound the caveats of noncanonical sequences. Accordingly, we were able to gather age and sex quantitative information on 115 translated noncanonical isoform groups in the heart belonging to 86 genes, which were identifiable at 1% Percolator posterior error probability (PEP) in at least 1 of 5 blocks of TMT experiments, and for which sufficient samples remained for 20-mo versus 4-mo statistical comparisons in male and female hearts (Fig. 7C). The identified alternative isoforms map to multiple cellular compartments, suggesting alternative splicing has the potential to exert far reaching consequences on cardiac proteome makeup and function. We then compared age associated expression in old versus young males and old versus young females. When comparing the TMT channel intensity data, we found 15 unique isoform peptides to show suggestive evidence of differential expression in aged hearts at limma P ≤ 0.05 in male and 11 in female, although only 8 in male and 2 in female reached significance following multiple testing corrections at 10% FDR (Supplemental Data S10). The potential differential isoform peptides include those belonging to known RBM target genes, including Ldb3 (An SE peptide, logFC 0.39, adjP 0.046 in male) and Mlip (logFC 0.34, adj P 0.051 in male). We examine here two potential cases where the transcripts with differential exon usage may be linked to protein-level changes.
In the first example, we identified a putative isoform peptide from Acsl1. Acsl1 codes for the long-chain fatty acid-CoA ligase 1 that participates in beta oxidation and is found in the mitochondria and the peroxisome. At the transcript level, exon usage analysis shows a complex pattern of age versus young patterns across disjoint exon parts suggestive of complex splicing pattern. We focused on exon part 30 that shows a suggestive increase in exon usage in aging hearts after adjusting for sex (Fig. 7C). Splice junction modeling predicts a skipped exon event where a cassette exon (chr8:46974447–46974524) is optionally included in between the upstream (chr8:46974305–46974382) and downstream (chr8:46977980–46978114) exons. The exon triads correspond to Ensembl canonical transcript (Acsl-201) exons 11 to x-12, with the cassette exons not found in the canonical transcript but part of an APPRIS alternative transcript (Acsl-202) in the gene model where the triad corresponds to exons x-10 to 11. From the proteomics data, we tentatively identified a peptide that corresponds to the in-frame translation product of a transcript linking the upstream Acsl-201 exon 11 with the cassette exon corresponding to alternative Acsl-202 exon 10, resulting in a peptide not found in TrEMBL. This peptide sequence is 33-residue long and semi-tryptic A[229.16]TESAFIASTDDVLISFLPLAHM[15.99]FETVVESALT with moderate spectral evidence (Fig. 7D); hence, there is cause for consideration of false discovery identification of the peptide, although we note that the peptide sequence was identified at least 12 times across 5 blocks of TMT quantitative proteomics data at percolator PEP ≤ 0.01 (median percolator PEP of 0.00028; max 7.6e–3; min 7.6e–6), with a number of diagnostic fragments (y1, y2, y3, y4, y6, y7, y9, y12, y20, y1_, y2_, y3_, y10_, y30_) that distinguish the variant portion of the sequence from its canonical-invariant portion, i.e., the part of the sequence that is shared with the canonical Acsl1 protein (ATESAFIASTDDVLISFLPLAHMFETVVE[…]) (Supplemental Fig. S7, A and B). In the UniProt SwissProt canonical/isoform database search the top scoring spectrum was nonidentifiable to a forward target sequence and was assigned to a decoy sequence (Supplemental Fig. S7C), suggesting the isoform database afforded substantial increase in power to explain the spectrum by peptide spectrum match (PSM). To explore whether the spectrum may arise from unknown modifications, we further performed an open mass window search using MSFragger v.3.2 (81) followed by Percolator v.3.0. The spectrum was likewise unidentifiable and was assigned to n[229.1629]EATYGERVVAFAAVEGIFFSGSFASIFWLK[229.1629] sp | P11157|RIR2_MOUSE with a nonsignificant Percolator PEP (0.299), with none of the top five MSFragger PSM candidates matched to ACSL1. In the quantitative proteomics data, the putative protein isoform had a corresponding increase in aging (logFC 0.43, P value = 1.7e–3, P.adjust 0.078). Taken together, we consider that there are multiple lines of tentative evidence that suggest the differential exon usage at a minor exon in Acsl1 has protein-level consequences. Intriguingly, the alternative peptide overlaps with the main AMP-binding motif in the ACSL1 protein. The alternative sequence is not predicted to be intrinsically disordered in either the deep learning algorithm Metapredict v.2.0 (82) or the biophysics-based algorithms IUPred3/ANCHOR2 (83) (Supplemental Fig. S7, D and E). To corroborate the sequence analysis, we performed structural predictions on the modeled alternative full-length protein isoform using AlphaFold2 (43), after the translated exon triads were joined back to the canonical SwissProt isoform sequence. The predicted protein folding suggests that the isoform-variant sequence belongs to a pocket of unstructured loop within the AMP-binding domain that does not alter overall protein structure when overlaid on the canonical sequence structural model (Fig. 7E). Structure-based function prediction suggests the alternative region overlaps with predicted ion binding and ligase activity.
In a second example, we considered the exon usage patterns of aspartyl β-hydroxylase (Asph). In mouse and in human, the Asph locus is known to contain multiple genes through alternative transcript processing or splicing: the 24-exon BAH, the 14-exon catalytically inactive humbug, and the 5-exon junctin (84, 85), as well as multiple isoforms of cardiac junctate (86), each with distinct molecular function and regulation. In the DEXSeq results, we saw multiple contiguous disjoint exonic parts (E028–E030) that were suppressed in aged hearts. Splice junction modeling revealed multiple MXE and SE events in the region that involved the optional inclusion for exonic part E030 corresponding to chr4:9624359–9624401 (Supplemental Fig. S8A). This exonic part corresponds to an exon between exons 3 and 4 of the APPRIS canonical Asph-202 transcript that codes for full-length ASPH/BAH but does not constitute part of the canonical transcript and instead forms a constitutive peptide of Asph-206/Trembl Q9CR06 (Junctin) and other transcripts in the Asph locus. From the mass spectrometry data, we identified two corresponding peptides LLEGPGGLAK and VLLEGPGGLAK (PSM Percolator PEP ≥ 7.4e–5) (Supplemental Fig. S8, B and C) that span exonic parts E030 and E035. The peptides are part of SwissProt isoform but not in SwissProt canonical sequences (Supplemental Fig. S8D). In the quantitative proteomics data, the isoform is significantly downregulated in aged hearts consistent with the transcript data (protein logFC –0.22, limma P value = 4.5e–6, P.adjust 9.7e–4). Due to the nature of short-read sequencing and bottom-up proteomics data, it is not possible to discern which of the known full-length transcripts is differentially regulated. Nevertheless, the data are consistent with a rerouting of exon usage in aging hearts from the full length ASPH/BAH isoforms toward exons shared by junctin/junctate through splicing changes, which is likely transmitted to the protein level.
DISCUSSION
Recent work has pointed to the intrinsic sexual dimorphism in cardiac aging processes in rodents and in humans as a modulating factor of cardiovascular risk. It has been well established that many cardiac genes have sex-differential expression patterns. Previous studies have found tens to hundreds of sexually dimorphic genes in the mouse heart (87–89), rat (10, 90), and in human hearts (87, 90–95) that involve both sex chromosome and autosomal genes participating in diverse processes particularly metabolism and ion transport pathways (90, 91). Moreover, sex-biased gene expression may show sex-by-environment interactions with pathophysiological factors, including cardiac hypertrophy, cardiomyopathies, and heart failure (89, 95).
In the present work, we characterized cardiac gene expression across sexes and two age groups in C57BL6/J mice using a combination of RNA sequencing and mass spectrometry approaches. The results revealed one of the most comprehensive lists of sexually dimorphic genes in the mouse heart and moreover suggest that sex also exerts an influence on age-associated differential expression of multiple genes. It is increasingly recognized that there are important sex differences in cardiovascular aging mechanisms and manifestations, where females have increased sensitivity to cardiometabolic risks and diastolic dysfunction (5). The results in mice here suggest that these functional differences may be underpinned in part by sex-biased aging changes in gene expression, including in mitochondrial metabolism and cAMP and PKA signaling pathways, the latter of which is known to play important roles in cardiac failure. Moreover, these sex-biased aging changes overlap partially with sexually dimorphic gene expression, suggesting they may be driven by intrinsic differences in gene expression between female and male hearts.
Intriguingly, we also find that gene expression changes may represent only part of the gene regulation program in aging, as more genes were found with differential exon usage than those with differential gene expression and further these processes appeared to regulate nonoverlapping sets of genes. Differential exon usage encompasses changes in transcript start sites, polyadenylation, and more typically in the case of inner exons, alternative splicing. Cardiac genes are prominently regulated through alternative splicing (96). Cardiac development and maturation involve successive changes in splice patterns modulated by the RBFOX, RBM, MNBL, and other families of splice factors, whereas the adult splicing program is reverted to the fetal/neonatal splicing pattern during pathological cardiac remodeling (96, 97). In parallel, recent work has implicated changes in alternative splicing in the blood, the brain, and other tissues during organismal aging processes (68, 98–101). Despite progress, how alternative splicing in the heart varies by the interaction of age and sex remains incompletely understood and may shed light on established sex biases in the risks of age-associated heart diseases. The analysis here of sex-adjusted aging changes points to RBPs and splicing factors as principally different in transcript levels in sex-adjusted aging hearts. These changes occur concomitantly with broad exon usage differences in thousands of genes in the cardiac genome, including functionally coordinated sets of genes pertaining to posttranscriptional regulations, protein translation, and mitochondrial metabolism that are commonly implicated in heart diseases. It is not necessarily predictable that splicing changes should coordinately affect functionally related sets of genes, given the “splicing code” that controls alternative exon selection remains poorly understood. A principle of alternative splicing regulation has been proposed where RBPs and splice factors are themselves subjected to splicing changes, leading to differences in multivalent protein-RNA interactions that further direct splicing regime (102). The results on aging differential exon usage here are suggestive of such a splicing regulatory network that could regulate transcript compositions in the aging hearts.
An outstanding challenge of alternative splicing research concerns examining the protein-level effects of exon usage, whereas current knowledge on which alternative transcript isoforms are translated into splice variant polypeptides and their potential molecular functions remains limited. Here, we used an RNA-guided protein sequence database proteogenomics approach to identify alternative splicing-derived proteoforms in mass spectrometry data. With this approach, we were able to identify hundreds of noncanonical peptides that may be detectable from mass spectrometry data and that correspond to splice junctions modeled from the transcriptome profiles of young adult and early aging mouse hearts, including instances where exon usage changes at the transcript level could be connected to downstream protein-level consequences. Continued work to elucidate how the interactions between RBPs, alternative splicing, and proteoforms orchestrate to alter cardiac proteome compositions and function will likely play an important role in advancing the understanding of cardiac aging processes.
In summary, this study shows that cardiac aging is associated with hundreds of sex-biased aging gene expression, as well as splicing changes in functionally coordinated genes. There are several limitations of the current work. First, the sample size is small (n = 3–5) and only one inbred mouse strain (C57BL/6J) and one aged group (20 mo) was examined. The use of bulk RNA sequencing is unable to directly assess cell-type heterogeneity of aging processes. These may be addressed in future work as multiomics approaches continue to advance. Second, our analysis on splice variant proteins also corroborates the present challenges in linking transcript level consequences of splicing to proteoform level changes. Further progress in this area will likely require continued advances in analytical and informatics approaches including more sensitive mass spectrometry, long-read sequencing (103), and top-down/middle-down proteomics methods (104). We anticipate future work might also interrogate overlaps between the global remodeling of splicing patterns in cardiac aging and those in pathological cardiac hypertrophy and failure to identify potential correlates of age-associated risks of heart diseases.
SUPPLEMENTAL DATA
Supplemental Figures, Tables, and Data are available at https://doi.org/10.6084/m9.figshare.19790161.
GRANTS
This study was supported in part by National Institutes of Health Grants F32-HL149191 (to Y.H.), R03-OD032666 and R00-HL144829 (to E.L.), and R00-HL127302, R01-HL141278, and R21-HL150456 (to M.P.Y.L.); University of Colorado Undergraduate Research Opportunities Program mini-grant (to J.W.); and University of Colorado Consortium for Fibrosis Research and Translation funding (to M.P.Y.L.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.H. and M.P.Y.L. conceived and designed research; Y.H., S.A.W., J.M.W., and M.P.Y.L. performed experiments; Y.H., S.A.W., R.W.L., E.L., and M.P.Y.L. analyzed data; Y.H., S.A.W., R.W.L., E.L., and M.P.Y.L. interpreted results of experiments; Y.H., E.L., and M.P.Y.L. prepared figures; E.L. and M.P.Y.L. drafted manuscript; E.L. and M.P.Y.L. edited and revised manuscript; Y.H., S.A.W., J.M.W., R.W.L., E.L., and M.P.Y.L. approved final version of manuscript.
REFERENCES
- 1. Leinwand LA. Sex is a potent modifier of the cardiovascular system. J Clin Invest 112: 302–307, 2003. doi: 10.1172/JCI19429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barton M, Meyer MR. Heart failure with preserved ejection fraction in women: new clues to causes and treatment. JACC Basic Transl Sci 5: 296–299, 2020. doi: 10.1016/j.jacbts.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation 122: 570–578, 2010. doi: 10.1161/CIRCULATIONAHA.110.937821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sotomi Y, Hikoso S, Nakatani D, Mizuno H, Okada K, Dohi T, Kitamura T, Sunaga A, Kida H, Oeun B, Sato T, Komukai S, Tamaki S, Yano M, Hayashi T, Nakagawa A, Nakagawa Y, Yasumura Y, Yamada T, Sakata Y; PURSUIT‐HFpEF Investigators. Sex differences in heart failure with preserved ejection fraction. J Am Heart Assoc 10: e018574, 2021. doi: 10.1161/JAHA.120.018574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ji H, Kwan AC, Chen MT, Ouyang D, Ebinger JE, Bell SP, Niiranen TJ, Bello NA, Cheng S. Sex differences in myocardial and vascular aging. Circ Res 130: 566–577, 2022. doi: 10.1161/CIRCRESAHA.121.319902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gebhard C, Stähli BE, Gebhard CE, Tasnady H, Zihler D, Wischnewsky MB, Jenni R, Tanner FC. Age- and gender-dependent left ventricular remodeling. Echocardiogr Mt Kisco N 30: 1143–1150, 2013. doi: 10.1111/echo.12264. [DOI] [PubMed] [Google Scholar]
- 7. Chen MS, Lee RT, Garbern JC. Senescence mechanisms and targets in the heart. Cardiovasc Res 118: 1173–1187, 2022. doi: 10.1093/cvr/cvab161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Squiers GT, McLellan MA, Ilinykh A, Branca J, Rosenthal NA, Pinto AR. Cardiac cellularity is dependent upon biological sex and is regulated by gonadal hormones. Cardiovasc Res 117: 2252–2262, 2021. doi: 10.1093/cvr/cvaa265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walker CJ, Schroeder ME, Aguado BA, Anseth KS, Leinwand LA. Matters of the heart: cellular sex differences. J Mol Cell Cardiol 160: 42–55, 2021. doi: 10.1016/j.yjmcc.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trexler CL, Odell AT, Jeong MY, Dowell RD, Leinwand LA. Transcriptome and functional profile of cardiac myocytes is influenced by biological sex. Circ Cardiovasc Genet 10: e001770, 2017. doi: 10.1161/CIRCGENETICS.117.001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeLeon-Pennell KY, Lindsey ML. Somewhere over the sex differences rainbow of myocardial infarction remodeling: hormones, chromosomes, inflammasome, oh my. Expert Rev Proteomics 16: 933–940, 2019. doi: 10.1080/14789450.2019.1664293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi W, Sheng X, Dorr KM, Hutton JE, Emerson JI, Davies HA, Andrade TD, Wasson LK, Greco TM, Hashimoto Y, Federspiel JD, Robbe ZL, Chen X, Arnold AP, Cristea IM, Conlon FL. Cardiac proteomics reveals sex chromosome-dependent differences between males and females that arise prior to gonad formation. Dev Cell 56: 3019–3034.e7, 2021. doi: 10.1016/j.devcel.2021.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DuPont JJ, Kim SK, Kenney RM, Jaffe IZ. Sex differences in the time course and mechanisms of vascular and cardiac aging in mice: role of the smooth muscle cell mineralocorticoid receptor. Am J Physiol Heart Circ Physiol 320: H169–H180, 2021. doi: 10.1152/ajpheart.00262.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flurkey K, Mcurrer J, Harrison D. Mouse models in aging research. In: The Mouse in Biomedical Research, edited by Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL.. Cambridge, MA: Academic Press, 2007, p. 637–672. [Google Scholar]
- 15. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frankish A, Diekhans M, Jungreis I, Lagarde J, Loveland JE, Mudge JM , et al. GENCODE 2021. Nucleic Acids Res 49: D916–D923, 2021. doi: 10.1093/nar/gkaa1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu A, Ibrahim JG, Love MI. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics 35: 2084–2092, 2019. doi: 10.1093/bioinformatics/bty895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manza LL, Stamer SL, Ham AJL, Codreanu SG, Liebler DC. Sample preparation and digestion for proteomic analyses using spin filters. Proteomics 5: 1742–1745, 2005. doi: 10.1002/pmic.200401063. [DOI] [PubMed] [Google Scholar]
- 20. Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods 6: 359–262, 2009. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 21. Hulstaert N, Shofstahl J, Sachsenberg T, Walzer M, Barsnes H, Martens L, Perez-Riverol Y. ThermoRawFileParser: modular, scalable, and cross-platform RAW file conversion. J Proteome Res 19: 537–542, 2020. doi: 10.1021/acs.jproteome.9b00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.UniProt Consortium. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res 49: D480–D489, 2021. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eng JK, Hoopmann MR, Jahan TA, Egertson JD, Noble WS, MacCoss MJ. A deeper look into Comet–implementation and features. J Am Soc Mass Spectrom 26: 1865–1874, 2015. doi: 10.1007/s13361-015-1179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. The M, MacCoss MJ, Noble WS, Käll L. Fast and accurate protein false discovery rates on large-scale proteomics data sets with percolator 3.0. J Am Soc Mass Spectrom 27: 1719–1727, 2016. doi: 10.1007/s13361-016-1460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dostal V, Wood SD, Thomas CT, Han Y, Lau E, Lam MPY. Proteomic signatures of acute oxidative stress response to paraquat in the mouse heart. Sci Rep 10: 18440, 2020. doi: 10.1038/s41598-020-75505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kösters M, Leufken J, Schulze S, Sugimoto K, Klein J, Zahedi RP, Hippler M, Leidel SA, Fufezan C. pymzML v2.0: introducing a highly compressed and seekable gzip format. Bioinformatics 34: 2513–2514, 2018. doi: 10.1093/bioinformatics/bty046. [DOI] [PubMed] [Google Scholar]
- 27. Han Y, Li LZ, Kastury NL, Thomas CT, Lam MPY, Lau E. Transcriptome features of striated muscle aging and predictability of protein level changes. Mol Omics 17: 796–808, 2021. doi: 10.1039/d1mo00178g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gillespie M, Jassal B, Stephan R, Milacic M, Rothfels K, Senff-Ribeiro A, Griss J, Sevilla C, Matthews L, Gong C, Deng C, Varusai T, Ragueneau E, Haider Y, May B, Shamovsky V, Weiser J, Brunson T, Sanati N, Beckman L, Shao X, Fabregat A, Sidiropoulos K, Murillo J, Viteri G, Cook J, Shorser S, Bader G, Demir E, Sander C, Haw R, Wu G, Stein L, Hermjakob H, D'Eustachio P. The reactome pathway knowledgebase 2022. Nucleic Acids Res 50: D687–D692, 2022. doi: 10.1093/nar/gkab1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu G, He Q-Y. ReactomePA: an R/Bioconductor package for reactome pathway analysis and visualization. Mol Biosyst 12: 477–479, 2016. doi: 10.1039/C5MB00663e. [DOI] [PubMed] [Google Scholar]
- 30. Korotkevich G, Sukhov V, Budin N, Shpak B, Artyomov MN, Sergushichev A. Fast gene set enrichment analysis (Preprint; bioRxiv 060012). bioRxiv, 2016. doi: 10.1101/060012. [DOI]
- 31. Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst 1: 417–425, 2015. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-Seq data. BMC Bioinformatics 14: 7, 2013. doi: 10.1186/1471-2105-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc 4: 1184–1191, 2009. doi: 10.1038/nprot.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mao W, Zaslavsky E, Hartmann BM, Sealfon SC, Chikina M. Pathway-level information extractor (PLIER) for gene expression data. Nat Methods 16: 607–610, 2019. doi: 10.1038/s41592-019-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taroni JN, Grayson PC, Hu Q, Eddy S, Kretzler M, Merkel PA, Greene CS. MultiPLIER: a transfer learning framework for transcriptomics reveals systemic features of rare disease. Cell Syst 8: 380–394.e4, 2019. doi: 10.1016/j.cels.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369: 1318–1330, 2020. doi: 10.1126/science.aaz1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47, 2015. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anders S, Reyes A, Huber W. Detecting differential usage of exons from RNA-seq data. Genome Res 22: 2008–2017, 2012. doi: 10.1101/gr.133744.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lau E, Han Y, Williams DR, Thomas CT, Shrestha R, Wu JC, Lam MPY. Splice-junction-based mapping of alternative isoforms in the human proteome. Cell Rep 29: 3751–3765.e5, 2019. doi: 10.1016/j.celrep.2019.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ludwig RW, Lau E. JCAST: sample-specific protein isoform databases for mass spectrometry-based proteomics experiments. Softw Impacts 10: 100163, 2021. doi: 10.1016/j.simpa.2021.100163. [DOI] [Google Scholar]
- 41. Shen S, Park JW, Lu Z, Lin L, Henry MD, Wu YN, Zhou Q, Xing Y. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci USA 111: E5593–E5601, 2014. doi: 10.1073/pnas.1419161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han Y, Wright JM, Lau E, Lam MPY. Determining Alternative Protein Isoform Expression Using RNA Sequencing and Mass Spectrometry. STAR Protoc 1: 100138, 2020. doi: 10.1016/j.xpro.2020.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature 596: 583–589, 2021. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M. ColabFold—making protein folding accessible to all. Nat Methods 19: 679–682, 2022. doi: 10.1038/s41592-022-01488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gligorijevic V, Renfrew PD, Kosciolek T, Leman JK, Berenberg D, Vatanen T, Chandler C, Taylor BC, Fisk IM, Vlamakis H, Xavier RJ, Knight R, Cho K, Bonneau R. Structure-based protein function prediction using graph convolutional networks. Nat Commun 12: 3168, 2021. doi: 10.1038/s41467-021-23303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, Morris JH, Ferrin TE. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci 30: 70–82, 2021. doi: 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kassambara A. ggpubr: ‘ggplot2’ Based Publication Ready Plots (Online). https://www.rdocumentation.org/packages/ggpubr/versions/0.4.0 [2021 Nov 9].
- 48. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13: 2498–2504, 2003. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu G, Dawson E, Duong A, Haw R, Stein L. ReactomeFIViz: a Cytoscape app for pathway and network-based data analysis. F1000Res 3: 146, 2014. doi: 10.12688/f1000research.4431.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The Tabula Muris Consortium, Overall coordination. Logistical coordination, Organ collection and processing, Library preparation and sequencing, Computational data analysis, Cell type annotation, Writing group, Supplemental text writing group, Principal investigators. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562: 367–372, 2018. doi: 10.1038/s41586-018-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, Hoffman P, Stoeckius M, Papalexi E, Mimitou EP, Jain J, Srivastava A, Stuart T, Fleming LM, Yeung B, Rogers AJ, McElrath JM, Blish CA, Gottardo R, Smibert P, Satija R. Integrated analysis of multimodal single-cell data. Cell 184: 3573–3587.e29, 2021. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang X, Park J, Susztak K, Zhang NR, Li M. Bulk tissue cell type deconvolution with multi-subject single-cell expression reference. Nat Commun 10: 380, 2019. doi: 10.1038/s41467-018-08023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Deutsch EW, Bandeira N, Sharma V, Perez-Riverol Y, Carver JJ, Kundu DJ, García-Seisdedos D, Jarnuczak AF, Hewapathirana S, Pullman BS, Wertz J, Sun Z, Kawano S, Okuda S, Watanabe Y, Hermjakob H, MacLean B, MacCoss MJ, Zhu Y, Ishihama Y, Vizcaíno JA. The ProteomeXchange consortium in 2020: enabling “big data” approaches in proteomics. Nucleic Acids Res 48: D1145–D1152, 2020. doi: 10.1093/nar/gkz984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Okuda S, Watanabe Y, Moriya Y, Kawano S, Yamamoto T, Matsumoto M, Takami T, Kobayashi D, Araki N, Yoshizawa AC, Tabata T, Sugiyama N, Goto S, Ishihama Y. jPOSTrepo: an international standard data repository for proteomes. Nucleic Acids Res 45: D1107–D1111, 2017. doi: 10.1093/nar/gkw1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bartling B, Niemann K, Pliquett RU, Treede H, Simm A. Altered gene expression pattern indicates the differential regulation of the immune response system as an important factor in cardiac aging. Exp Gerontol 117: 13–20, 2019. doi: 10.1016/j.exger.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 56. Dorn GW, Molkentin JD. Manipulating cardiac contractility in heart failure: data from mice and men. Circulation 109: 150–158, 2004. doi: 10.1161/01.CIR.0000111581.15521.F5. [DOI] [PubMed] [Google Scholar]
- 57. Lompré A-M, Hajjar RJ, Harding SE, Kranias EG, Lohse MJ, Marks AR. Ca2+ cycling and new therapeutic approaches for heart failure. Circulation 121: 822–830, 2010. doi: 10.1161/CIRCULATIONAHA.109.890954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lucia C. D, Eguchi A, Koch WJ. New insights in cardiac β-adrenergic signaling during heart failure and aging. Front Pharmacol 9: 904, 2018. doi: 10.3389/fphar.2018.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamamoto K, Tsujino Y, Saito A, Sinohara H. Concentrations of murinoglobulin and alpha-macroglobulin in the mouse serum: variations with age, sex, strain, and experimental inflammation. Biochem Int 10: 463–469, 1985. [PubMed] [Google Scholar]
- 60. Chu Y, Zhang C, Xie M. Beta-hydroxybutyrate, friend or foe for stressed hearts. Front Aging 2: 681513, 2021. doi: 10.3389/fragi.2021.681513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, Recchia FA, Kelly DP. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 4: e124079, 2019. doi: 10.1172/jci.insight.124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rauniyar N, Yates JR. Isobaric labeling-based relative quantification in shotgun proteomics. J Proteome Res 13: 5293–5309, 2014. doi: 10.1021/pr500880b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kusnadi EP, Timpone C, Topisirovic I, Larsson O, Furic L. Regulation of gene expression via translational buffering. Biochim Biophys Acta Mol Cell Res 1869: 119140, 2022. doi: 10.1016/j.bbamcr.2021.119140. [DOI] [PubMed] [Google Scholar]
- 64. Liu Y, Beyer A, Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell 165: 535–550, 2016. doi: 10.1016/j.cell.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 65. Perl K, Ushakov K, Pozniak Y, Yizhar-Barnea O, Bhonker Y, Shivatzki S, Geiger T, Avraham KB, Shamir R. Reduced changes in protein compared to mRNA levels across non-proliferating tissues. BMC Genomics 18: 305, 2017. doi: 10.1186/s12864-017-3683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liao Y, Castello A, Fischer B, Leicht S, Föehr S, Frese CK, Ragan C, Kurscheid S, Pagler E, Yang H, Krijgsveld J, Hentze MW, Preiss T. The cardiomyocyte RNA-binding proteome: links to intermediary metabolism and heart disease. Cell Rep 16: 1456–1469, 2016. doi: 10.1016/j.celrep.2016.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res 98: 342–350, 2006. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 68. Wang K, Wu D, Zhang H, Das A, Basu M, Malin J, Cao K, Hannenhalli S. Comprehensive map of age-associated splicing changes across human tissues and their contributions to age-associated diseases. Sci Rep 8: 10929, 2018. doi: 10.1038/s41598-018-29086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lewis BP, Green RE, Brenner SE. Evidence for the widespread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proc Natl Acad Sci USA 100: 189–192, 2003. doi: 10.1073/pnas.0136770100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pickrell JK, Pai AA, Gilad Y, Pritchard JK. Noisy splicing drives mRNA isoform diversity in human cells. PLoS Genet 6: e1001236, 2010. doi: 10.1371/journal.pgen.1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tapial J, Ha KCH, Sterne-Weiler T, Gohr A, Braunschweig U, Hermoso-Pulido A, Quesnel-Vallières M, Permanyer J, Sodaei R, Marquez Y, Cozzuto L, Wang X, Gómez-Velázquez M, Rayon T, Manzanares M, Ponomarenko J, Blencowe BJ, Irimia M. An atlas of alternative splicing profiles and functional associations reveals new regulatory programs and genes that simultaneously express multiple major isoforms. Genome Res 27: 1759–1768, 2017. doi: 10.1101/gr.220962.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chouaib R, Safieddine A, Pichon X, Imbert A, Kwon OS, Samacoits A, Traboulsi A-M, Robert M-C, Tsanov N, Coleno E, Poser I, Zimmer C, Hyman A, Le Hir H, Zibara K, Peter M, Mueller F, Walter T, Bertrand E. A dual protein-mRNA localization screen reveals compartmentalized translation and widespread co-translational RNA targeting. Dev Cell 54: 773–791.e5, 2020. doi: 10.1016/j.devcel.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 73. Joazeiro CAP. Mechanisms and functions of ribosome-associated protein quality control. Nat Rev Mol Cell Biol 20: 368–383, 2019. doi: 10.1038/s41580-019-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shao S, von der Malsburg K, Hegde RS. Listerin-dependent nascent protein ubiquitination relies on ribosome subunit dissociation. Mol Cell 50: 637–648, 2013. doi: 10.1016/j.molcel.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang F, Durfee LA, Huibregtse JM. A cotranslational ubiquitination pathway for quality control of misfolded proteins. Mol Cell 50: 368–378, 2013. doi: 10.1016/j.molcel.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Blencowe BJ. The relationship between alternative splicing and proteomic complexity. Trends Biochem Sci 42: 407–408, 2017. doi: 10.1016/j.tibs.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 77. Smithers B, Oates ME, Gough J. Splice junctions are constrained by protein disorder. Nucleic Acids Res 43: 4814–4822, 2015. doi: 10.1093/nar/gkv407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang X, Codreanu SG, Wen B, Li K, Chambers MC, Liebler DC, Zhang B. Detection of proteome diversity resulted from alternative splicing is limited by trypsin cleavage specificity. Mol Cell Proteomics 17: 422–430, 2018. doi: 10.1074/mcp.RA117.000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Han Y, Wood SD, Wright JM, Dostal V, Lau E, Lam MPY. Computation-assisted targeted proteomics of alternative splicing protein isoforms in the human heart. J Mol Cell Cardiol 154: 92–96, 2021. doi: 10.1016/j.yjmcc.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wan Y, Larson DR. Splicing heterogeneity: separating signal from noise. Genome Biol 19: 86, 2018. doi: 10.1186/s13059-018-1467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kong AT, Leprevost FV, Avtonomov DM, Mellacheruvu D, Nesvizhskii AI. MSFragger: ultrafast and comprehensive peptide identification in mass spectrometry-based proteomics. Nat Methods 14: 513–520, 2017. doi: 10.1038/nmeth.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Emenecker RJ, Griffith D, Holehouse AS. Metapredict: a fast, accurate, and easy-to-use predictor of consensus disorder and structure. Biophys J 120: 4312–4319, 2021. doi: 10.1016/j.bpj.2021.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Erdős G, Pajkos M, Dosztányi Z. IUPred3: prediction of protein disorder enhanced with unambiguous experimental annotation and visualization of evolutionary conservation. Nucleic Acids Res 49: W297–W303, 2021. doi: 10.1093/nar/gkab408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dinchuk JE, Henderson NL, Burn TC, Huber R, Ho SP, Link J, O'Neil KT, Focht RJ, Scully MS, Hollis JM, Hollis GF, Friedman PA. Aspartyl β-hydroxylase (Asph) and an evolutionarily conserved isoform of Asph missing the catalytic domain share exons with junctin. J Biol Chem 275: 39543–39554, 2000. doi: 10.1074/jbc.M006753200. [DOI] [PubMed] [Google Scholar]
- 85. Dinchuk JE, Focht RJ, Kelley JA, Henderson NL, Zolotarjova NI, Wynn R, Neff NT, Link J, Huber RM, Burn TC, Rupar MJ, Cunningham MR, Selling BH, Ma J, Stern AA, Hollis GF, Stein RB, Friedman PA. Absence of post-translational aspartyl β-hydroxylation of epidermal growth factor domains in mice leads to developmental defects and an increased incidence of intestinal neoplasia. J Biol Chem 277: 12970–12977, 2002. doi: 10.1074/jbc.M110389200. [DOI] [PubMed] [Google Scholar]
- 86. Treves S, Feriotto G, Moccagatta L, Gambari R, Zorzato F. Molecular cloning, expression, functional characterization, chromosomal localization, and gene structure of junctate, a novel integral calcium binding protein of sarco(endo)plasmic reticulum membrane. J Biol Chem 275: 39555–39568, 2000. doi: 10.1074/jbc.M005473200. [DOI] [PubMed] [Google Scholar]
- 87. Isensee J, Witt H, Pregla R, Hetzer R, Regitz-Zagrosek V, Noppinger PR. Sexually dimorphic gene expression in the heart of mice and men. J Mol Med (Berl) 86: 61–74, 2008. doi: 10.1007/s00109-007-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li B, Qing T, Zhu J, Wen Z, Yu Y, Fukumura R, Zheng Y, Gondo Y, Shi L. A comprehensive mouse transcriptomic BodyMap across 17 tissues by RNA-seq. Sci Rep 7: 4200, 2017. doi: 10.1038/s41598-017-04520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tsuji M, Kawasaki T, Matsuda T, Arai T, Gojo S, Takeuchi JK. Sexual dimorphisms of mRNA and miRNA in human/murine heart disease. PLoS One 12: e0177988, 2017. [Erratum in PLoS One 15: e0229750, 2020]. doi: 10.1371/journal.pone.0177988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Naqvi S, Godfrey AK, Hughes JF, Goodheart ML, Mitchell RN, Page DC. Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science 365: eaaw7317, 2019. doi: 10.1126/science.aaw7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fermin DR, Barac A, Lee S, Polster SP, Hannenhalli S, Bergemann TL, Grindle S, Dyke DB, Pagani F, Miller LW, Tan S, dos Remedios C, Cappola TP, Margulies KB, Hall JL. Sex and age dimorphism of myocardial gene expression in nonischemic human heart failure. Circ Cardiovasc Genet 1: 117–125, 2008. doi: 10.1161/CIRCGENETICS.108.802652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gershoni M, Pietrokovski S. The landscape of sex-differential transcriptome and its consequent selection in human adults. BMC Biol 15: 7, 2017. doi: 10.1186/s12915-017-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lopes-Ramos CM, Chen C-Y, Kuijjer ML, Paulson JN, Sonawane AR, Fagny M, Platig J, Glass K, Quackenbush J, DeMeo DL. Sex differences in gene expression and regulatory networks across 29 human tissues. Cell Rep 31: 107795, 2020. doi: 10.1016/j.celrep.2020.107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mayne BT, Bianco-Miotto T, Buckberry S, Breen J, Clifton V, Shoubridge C, Roberts CT. Large scale gene expression meta-analysis reveals tissue-specific, sex-biased gene expression in humans. Front Genet 7: 183, 2016. doi: 10.3389/fgene.2016.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Newman MS, Nguyen T, Watson MJ, Hull RW, Yu H-G. Transcriptome profiling reveals novel BMI- and sex-specific gene expression signatures for human cardiac hypertrophy. Physiol Genomics 49: 355–367, 2017. doi: 10.1152/physiolgenomics.00122.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Baralle FE, Giudice J. Alternative splicing as a regulator of development and tissue identity. Nat Rev Mol Cell Biol 18: 437–451, 2017. doi: 10.1038/nrm.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gao C, Wang Y. mRNA metabolism in cardiac development and disease: life after transcription. Physiol Rev 100: 673–694, 2020. doi: 10.1152/physrev.00007.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Balliu B, Durrant M, Goede O. D, Abell N, Li X, Liu B, Gloudemans MJ, Cook NL, Smith KS, Knowles DA, Pala M, Cucca F, Schlessinger D, Jaiswal S, Sabatti C, Lind L, Ingelsson E, Montgomery SB. Genetic regulation of gene expression and splicing during a 10-year period of human aging. Genome Biol 20: 230, 2019. doi: 10.1186/s13059-019-1840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Deschênes M, Chabot B. The emerging role of alternative splicing in senescence and aging. Aging Cell 16: 918–933, 2017. doi: 10.1111/acel.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rodríguez SA, Grochová D, McKenna T, Borate B, Trivedi NS, Erdos MR, Eriksson M. Global genome splicing analysis reveals an increased number of alternatively spliced genes with aging. Aging Cell 15: 267–278, 2016. doi: 10.1111/acel.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Stegeman R, Weake VM. Transcriptional signatures of aging. J Mol Biol 429: 2427–2437, 2017. doi: 10.1016/j.jmb.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ule J, Blencowe BJ. Alternative splicing regulatory networks: functions, mechanisms, and evolution. Mol Cell 76: 329–345, 2019. doi: 10.1016/j.molcel.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 103. Miller RM, Jordan BT, Mehlferber MM, Jeffery ED, Chatzipantsiou C, Kaur S, Millikin RJ, Dai Y, Tiberi S, Castaldi PJ, Shortreed MR, Luckey CJ, Conesa A, Smith LM, Deslattes Mays A, Sheynkman GM. Enhanced protein isoform characterization through long-read proteogenomics. Genome Biol 23: 69, 2022. doi: 10.1186/s13059-022-02624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Melby JA, Roberts DS, Larson EJ, Brown KA, Bayne EF, Jin S, Ge Y. Novel strategies to address the challenges in top-down proteomics. J Am Soc Mass Spectrom 32: 1278–1294, 2021. doi: 10.1021/jasms.1c00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figures, Tables, and Data are available at https://doi.org/10.6084/m9.figshare.19790161.
Data Availability Statement
The generated RNA sequencing data (total 1.47 billion reads, 150 nt paired end) have been deposited to NCBI Gene Expression Omnibus (GEO) under the accession GSE202384. Mass spectrometry data (total 5 TMT blocks, 40 raw files) have been deposited to ProteomeXchange (53) via jPOST (54) under the accession PXD033719. JCAST is available on GitHub at https://github.com/ed-lau/jcast (40).