Keywords: enterocyte, intestinal transcriptome, necrotizing enterocolitis, RNAseq, Toll-like receptor 4
Abstract
Necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in premature infants and is steadily rising in frequency. Patients who develop NEC have a very high mortality, illustrating the importance of developing novel prevention or treatment approaches. We and others have shown that NEC arises in part from exaggerated signaling via the bacterial receptor, Toll-like receptor 4 (TLR4) on the intestinal epithelium, leading to widespread intestinal inflammation and intestinal ischemia. Strategies that limit the extent of TLR4 signaling, including the administration of amniotic fluid, can reduce NEC development in mouse and piglet models. We now seek to test the hypothesis that a secretome derived from amnion-derived cells can prevent or treat NEC in preclinical models of this disease via a process involving TLR4 inhibition. In support of this hypothesis, we show that the administration of this secretome, named ST266, to mice or piglets can prevent and treat experimental NEC. The protective effects of ST266 occurred in the presence of marked TLR4 inhibition in the intestinal epithelium of cultured epithelial cells, intestinal organoids, and human intestinal samples ex vivo, independent of epidermal growth factor. Strikingly, RNA-seq analysis of the intestinal epithelium in mice reveals that the ST266 upregulates critical genes associated with gut remodeling, intestinal immunity, gut differentiation. and energy metabolism. These findings show that the amnion-derived secretome ST266 can prevent and treat NEC, suggesting the possibility of novel therapeutic approaches for patients with this devastating disease.
NEW & NOTEWORTHY This work provides hope for children who develop NEC, a devastating disease of premature infants that is often fatal, by revealing that the secreted product of amniotic progenitor cells (called ST266) can prevent or treat NEC in mice, piglet, and “NEC-in-a-dish” models of this disease. Mechanistically, ST266 prevented bacterial signaling, and a detailed transcriptomic analysis revealed effects on gut differentiation, immunity, and metabolism. Thus, an amniotic secretome may offer novel approaches for NEC.
INTRODUCTION
Necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in premature infants (1). Seven percent of all very low birth weight infants in the neonatal intensive care unit (NICU) are likely to develop NEC (2), and the Centers for Disease Control’s 2021 National Vital Statistics Reports show that the infant mortality rate increased by 19.0% for NEC in newborn infants (3). This disease typically develops at approximately 3–4 wk of age in premature infants, most often after the administration of a diet that contains infant formula as opposed to breast milk (4). NEC can develop gradually over several days, or its onset can be dramatic, with the abrupt development of abdominal distention, bile-stained emesis, and the passage of bloody stools (5). Current treatment for NEC involves the cessation of oral feeds, administration of broad-spectrum antibiotics, and surgical removal of dead and dying intestine (6). Patients who develop NEC have a very high mortality (7), raising the importance of developing novel prevention or treatment approaches for this devastating disease.
To develop new approaches for the prevention or treatment of NEC, we and others have sought to develop a unifying construct that explains its pathogenesis in premature infants (8). To this end, we have shown that an abnormal intestinal microbiome activates the bacterial receptor, Toll-like receptor 4 (TLR4) on the intestinal epithelium, leading to widespread intestinal inflammation and intestinal ischemia (9–11). Mice lacking TLR4 are protected from NEC development (12–15), whereas activating mutations in the TLR4 pathway are linked to increased NEC development in humans (16). The expression of TLR4 is significantly elevated in the premature intestine as compared with the full-term infant intestine (17, 18), which represents in part the nonimmune role for TLR4 in the regulation of normal gut development (13). Accordingly, strategies that limit the extent of TLR4 signaling can reduce NEC development in mouse and piglet models (19–23). For instance, we have recently shown that breast milk can limit NEC development in part through TLR4 inhibition (24).
In seeking to identify novel approaches for the prevention or treatment of NEC, we have shown that the administration of amniotic fluid, which normally bathes the gut as it develops in utero, can limit the development of NEC in pr-clinical models by inhibiting TLR4 signaling on the intestinal epithelium (25). Although useful in principle, the administration of amniotic fluid to premature infants may be impractical, given the practical difficulties of obtaining a sterile and reliable source for clinical use. By contrast, the biological products that are secreted from cultured amnion progenitor cells may possess anti-NEC properties without these practical limitations.
We therefore now seek to test the hypothesis that a secretome derived from proprietary-selected amnion-derived cells can prevent or treat NEC in preclinical models of this disease. In support of this hypothesis, we show that the administration of this secretome, named ST266 (26), to mice or piglets can prevent and treat experimental NEC. We further show that the protective effects of ST266 occurred in the presence of marked TLR4 inhibition in the intestinal epithelium of cultured epithelial cells, intestinal organoids, and human intestinal samples ex vivo, consistent with the known pathophysiological processes that underlie this disease. Finally, we report a detailed transcriptomic analysis of the intestinal epithelium in the presence of ST266 in mice, providing additional mechanistic insights into how ST266 prevents NEC.
METHODS
Animal Study Approval
All mice experiments described in this study were carried out following the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by Johns Hopkins University protocol MO20M276, according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) Guidelines by National Centre for the Replacement & Reduction of Animal In Research (NC3Rs). C57BL/6J mice were purchased from the Jackson Laboratory and bred in the pathogen-free facility at Johns Hopkins University for multiple generations to stabilize intestinal microbiota. All mice were given ad libitum access to water, food, and housed in a temperature-controlled room (22°C) with 12 h of light and dark cycles. All mice used in the study were euthanized humanely using isoflurane anesthesia by inhalation (∼3%–4% isoflurane) followed by cervical dislocation.
Induction of NEC in Neonatal Mice
Neonatal mouse pups 7 to 8 days old (∼3.2 g body wt) of both sexes were used to induce experimental NEC as described previously (27). In brief, neonatal mice were separated from multiple dams and randomly allocated into NEC or NEC + ST266 treatment groups. To induce NEC, mice were gavage fed (40 µL/g) five times a day (7 AM to 7 PM) with formula supplemented with bacterial stock that had been cultured from the stool of an infant with severe NEC (12.5-μL stool slurry in 1-mL formula). The stool mixed formula (50 µL/g of mouse body weight, all feeds were supplemented with the stool) was administered using a 24-French angiocatheter placed into the mouse esophagus. Mice were exposed to hypoxia (5% O2-95% N2) for 10 min in a chamber (Billups-Rothenberg, Inc., Del Mar, CA), twice daily for 4 days. The breast-fed (control) animals remained with their mothers and received breast milk ad libitum.
Induction of NEC in Piglets
All piglet studies were approved by Johns Hopkins University Swine protocol SW21M162. To induce NEC in piglets, timed-pregnant White Yorkshire (Yorkshire X Landrace) sows were obtained from Oak Hill Genetics, and piglets were delivered prematurely via cesarean section at ∼95% gestation as we have described previously (20). NEC was induced in piglets of either sex, which were randomly divided into NEC and NEC + ST266 treatment groups. NEC was induced by feeding formula (20 mL/kg every 3 h, 7 AM–7 PM) for 4 days of the following composition (per liter): Pepdite Junior (93.9 g; Nutricia), MCT Oil (38.3 g, USP grade; Now Foods), whey protein isolate (56 g, Now Foods), and 837 g of water, which was supplemented with enteric bacteria made from a specimen obtained from an infant with surgical NEC. The cause of any mortality in the piglet model was determined by immediate necropsy when possible; for those piglets who died before necropsy could be performed, a most likely cause of death was attributed based upon clinical parameters.
ST266 Treatments in NEC
ST266 (Noveome Biotherapeutics, Pittsburgh, PA) (28) was either added to the NEC formula (20 µL/mL/mice/day) or administrated via intraperitoneal route (20 µL/mice/day). ST266 is a secretome that is released by a subset of amnion epithelial cells that are called amnion multipoint progenitor (AMP) cells, as described (26, 29). For NEC prevention models, ST266 were given from the start to end of the NEC model. For NEC treatment models, ST266 was given from day 3 to end of the NEC model. For piglet NEC models, ST266 was given via the intraperitoneal route (0.5 mL or 1.0 mL/piglet/day) at 10 AM from day 1 to 4 of the NEC model. In anti-epidermal growth factor receptor (EGFR) NEC studies, mice were administered intraperitoneal injections of Cetuximab (Selleck Chemicals, LLC, 20 µL/mice/day) for the first 4 days of the NEC model.
NEC Severity Assessment
NEC severity was determined based on a validated scoring system applied to deidentified, paraformaldehyde (PFA)-fixed/paraffin-embedded/hematoxylin and eosin (H&E)-stained intestinal sections from distal small intestine (ileum) in consultation with a pediatric pathologist blinded to the group allocation. Histological NEC severity score were assigned as described previously, 0 (no injury), 1 (minor-submucosal, lamina propria separation), 2 (moderate separation of submucosa, lamina propria, and edema in submucosal and muscular layers), and 3 (severe separation of submucosa, lamina propria, severe edema, and villous sloughing or loss of villi), as described (30).
ST266 Treatments in LPS-Induced Endotoxemia
C57/Bl6 neonatal pups (p11, ∼4.5–5 g) of sexes from multiple dams were randomly divided into control, lipopolysaccharide (LPS), or LPS + ST266 treatment groups. LPS (5 mg/kg) was administrated via intraperitoneal injections. ST266 cotreatments were given via either oral route (20 µL/mice) or intraperitoneal route (20 µL/mice). In anti-EGFR studies, mice were 30-min pretreated with cetuximab (20 µL/mice via the intraperitoneal route). To assess the anti-inflammatory effects of ST266 cotreatments, distal small intestine (ileal) tissue was harvested 6 h posttreatment for analysis of proinflammatory cytokines gene expression.
IEC6 Cell Culture and Treatments
The small intestinal epithelial cell (IEC)-6 was obtained from American Type Culture Collection (ATCC, Manassas, VA) and maintained in culture, in growth medium consisting of DMEM (Dulbecco’s modified Eagle medium, Gibco BRL) supplemented with 10% fetal bovine serum (Atlanta biosciences) and 4 µg/mL insulin (insulin, human recombinant, zinc solution, Gibco Cat. No. 12585014) in a humidified incubator maintained at 37°C, 5% CO2. For experimental treatments, IEC6 cells were cultured overnight in 12-well plates with or without coverslips (∼70%–80% confluent) and treated either with LPS (50 µg/mL) without or with 30-min pretreatment with 20 µL/mL ST266 or 20 µL/mL cetuximab. For the evaluation of cytokine gene expression by qRT-PCR, cells were treated for an additional 6 h and harvested for RNA isolation and qPCRs. For evaluation of NF-κB translocation, IEC-6 cells were treated for additional 45 min and were washed in cold PBS, fixed for 10 min with 4% fresh paraformaldehyde (PFA), blocked with 5% goat serum/0.5% bovine serum albumin (BSA), then immunostained for NF-κB p65 subunit, and examined by confocal microscopy. Nuclear NF-κB p65 staining was then quantified using ImageJ software in which a threshold limit was set and a corresponding nuclear region was defined by stenciling a circular region using the wand tracing tool, which automatically draws the nuclear area of interest. The average integrated pixel density pertaining to the corresponding NF-κB p65 staining within the nuclear regions was then determined for more than 200 cells per treatment group in at least 3 replicates. Data were plotted on a scatter graph depicting values of each individual cell, and statistical analysis was performed using Prism 9.0 software, as we have performed previously (20).
Mouse Ileal Enteroids Culture and Treatments
Primary intestinal crypt cultures (enteroids) were generated from the ileum of neonatal (p7–p11) as described previously (21) and maintained in Matrigel (Corning). The enteroids were digested and passaged using TrypLE Express (Gibco) weekly and used between passages 3 and 10 for all experiments. For LPS studies, the enteroids were pretreated with ST266 (20 µL/mL) and then treated with LPS (50 μg/mL) for 6 h. For NEC-in-a-dish studies, enteroids were treated with NEC bacteria and 1%–2% hypoxia in the presence or absence of ST266 (20 µL/mL) or cetuximab (20 µL/mL) for 6 h in antibiotic-free media, as recently described (21). Each experiment was repeated three times with 2–3 wells/treatment group.
Human Ileal Sample Collection and Explant Culture
Deidentified human ileal samples were collected during surgery for NEC or at the time of stoma closure. Tissue collection occurred with the approval of the Institutional Review Board (IRB) at Johns Hopkins University, where informed consent was waived (IRB00094036). For explant cultures, fresh human ileum samples were washed with sterile phosphate-buffered saline containing gentamycin (5 μg/mL), minced into 2- to 4-mm diameter pieces, and then cultured in Dulbecco’s modified Eagle growth medium supplemented with 10% fetal bovine serum. Human ilea explant cultures were then cotreated with 50 μg/LPS with or without 20 µL/mL ST266 for 6 h in a humidified incubator at 37°C and then processed for total RNA isolation followed by qRT-PCR. Each experiment was repeated 2–3 times with 2–3 wells/treatment group.
Immunohistochemistry and Hematoxylin and Eosin Staining
Immunofluorescent staining of ileal tissues was performed on 4% paraformaldehyde-fixed 5 μM thick paraffin sections. The sections were first warmed to 56°C in a vacuum incubator (Isotemp Vacuum Oven, Fisher Scientific) then washed immediately in xylene, gradually redehydrated in ethanol (100%, 95%, 70%, water), and then processed for antigen retrieval in citrate buffer (10 mM pH 6.0)/microwave (1,000 W, 6 min). Samples were then washed with PBS, blocked with 1% BSA/5% donkey-serum (1 h, room temperature), then incubated overnight at 4°C with primary antibodies (1:200 dilutions in 0.5% BSA), washed three times with PBS, incubated with appropriate fluorescent-labeled secondary antibodies (1:1,000 dilution in 0.5% BSA, Life Technologies, Inc.) and the nuclear marker, 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI, Cat. No. D9542, Sigma, St. Louis, MO). The primary antibodies used were anti-3′-nitrotyrosine (3′-NT, Cat. No. ab61392, Abcam), anti-Ki67 (Cat. No. ab15580-100, Abcam), anti-ZO-1 (Cat. No. 40–2200, ZYMED), anti-NF-κB p65 (Cat. No. sc-8008, Santa Cruz), and anti-occludin (Cat. No. 33–150, Invitrogen). For goblet cell staining, paraffin sections were stained with Alcian blue solution and counterstained with nuclear fast red solution (Sigma-Aldrich, St. Louis, MO). All immunohistochemistry and H&E-stained slides were mounted using Gelvatol solution before imaging using a Nikon confocal microscope (Nikon, Inc.). Images were quantified using ImageJ2 software (v. 2.3.0/1.5f). All confocal imaging was performed at the same exposure between groups. An area of investigation for each high-power field (hpf) was subjected to a threshold value that was applied to each group. The area of tissue used for quantification corresponded to a region encompassing 10–15 crypt-villus units for each sample. For immunohistochemistry quantification, the stromal area was excluded from analysis. A minimum of 3–4 hpf images were quantified for each animal, and an average value was obtained per specimen (n = 1).
Quantitative Real-Time Polymerase Reaction
Total RNA was isolated from the snap-frozen full-thickness small intestine (ileum) using RNeasy kit (Cat. No. 74106, Qiagen, Germantown, MD), checked for RNA purity, and concentrated on SpectraMax microplate reader (Molecular Devices, San Jose, CA). Total RNA (0.5 µg) was reverse-transcribed for cDNA synthesis using the QuantiTect Reverse Transcription kit (Cat. No. 205313, Qiagen, Germantown, MD). Quantitative real-time polymerase reaction (qRT-PCR) was then performed on a Bio-Rad CFX96 Real-Time System (Bio-Rad Labs, Hercules, CA) using Sybr green mix (Bio-Rad Labs, Hercules, CA) and forward and reverse primers (custom-designed using NCBI Primer-BLAST online program and ordered from Integrated DNA Technologies, Coralville, IA) (Table 1). The mRNA expression relative to the housekeeping gene ribosomal protein large P0 (Rplp0) was calculated using the 2-△△CT method as described (27).
Table 1.
Quantitative real time-polymerase chain reaction primers
| Afp (mouse) | AAACCTCCAGGCAACAACCA; TTCCTTGGCAACACTCCTCG |
| Anxa8 (mouse) | ATGTGCAGAGGCAGCAGATT; GTATGGCGGGTACATGAGGG |
| Ceacam2 (mouse) | CTGAAGCTGTCTGAGGGCAA; AGGCTGAATGGGTCACTTCG |
| ChgA (mouse) | AAGGTGATGAAGTGCGTCCTGGAA; AGCAGATTCTGGTGTCGCAGGATA |
| Chil1 (mouse) | CCAGATAGCCCAACACCTGG; CTTCTGGCCTTGGAAGAGGG |
| Clca4a (mouse) | GGAGTTTACGCCACCCATCA; TCCTCCAAGACTGGCTGAGT |
| Emp1 (mouse) | CTCCCTGTCCTACGGCAATG; GAGCTGGAACACGAAGACCA |
| Gabra1 (mouse) | TGGAAAGAAGTCTGTGGCCC; TCACGGTCAACCTCATGGTG |
| Hoxb7 (mouse) | CTTCCGGATCTACCCCTGGA; CCGAGTCAGGTAGCGATTGT |
| Il-1β (pig) | CCTCCTCCCAGGCCTTCTGT; GGGCCAGCCAGCACTAGAGA |
| Il6 (mouse/rat) | CCAATTTCCAATGCTCTCCT; ACCACAGTGAGGAATGTCCA |
| Ki67 (mouse) | CCAAGGCCCAAGTTTGATGC; GACTTGGCCCCGAGATGTAG |
| Lyz1 (mouse) | AAGCTGGCTGACTGGGTGTGTTTA; CACTGCAATTGATCCCACAGGCAT |
| Muc2 (pig) | CCTCAACATCGAGAGTGCCA; GCACGTGTCGTATTTGCACC |
| Oxct1 (mouse) | AAAGACGAAGCTGATGCGGA; GTCCCCCTCTAATCATGGCG |
| Ptprz1 (mouse) | GCAAAAGTCCCCAGCAAGTG; ATGCATGCCTCCCTTTTGGA |
| Rasal1 (mouse) | ACACTCAGCCATCACACTGG; ATCCTCCAGCAGTTTCAGCC |
| Serpina3n (mouse) | AGGCTGACCTGTCTGCAATC; CTGCTTCTGTGCCTGTCTCA |
| Sis (mouse) | GCCCATATTCATGGTGGAAC; TCCAATGACAGGAGTCACCA |
| Timp1 (mouse) | CCAGAACCGCAGTGAAGAGT; GTACGCCAGGGAACCAAGAA |
| Tnf-α (mouse) | TTCCGAATTCACTGGAGCCTCGAA; TGCACCTCAGGGAAGAATCTGGAA |
| Tnf-α (pig) | GCCCTTCCACCAACGTTTTC; TCTGGCAAGGGCTCTTGATG |
| TNF-α (human) | GGCGTGGAGCTGAGAGATAAC; GGTGTGGGTGAGGAGCACAT |
| Tlr4 (mouse) | TTTATTCAGAGCCGTTGGTG; CAGAGGATTGTCCTCCCATT |
| Vnn1 (mouse) | CTCAGCGGCACTTTTGGAAC; TTGGCTTCAGGCTAACCAGG |
| Rplp0 (mouse/pig/ human) | GGCGACCTGGAAGTCCAACT; CCATCAGCACCACAGCCTTC |
| Vil1 (mouse) | AAATTGCAGCCTCGGCGTATCAAG; GGCACAGGCTCCAAGTTGTTCTTT |
RNA-Seq Analysis of the Intestinal Epithelium
Total RNA was purified and DNAse I treated using RNeasy mini kit (Qiagen). The total RNA quantity and purity were analyzed using the Bioanalyzer 2100 and RNA 6000 Nano LabChip Kit (Agilent, CA, 5067-1511), and high-quality RNA samples with RIN number >7.0 were used to construct sequencing libraries. After total RNA was extracted, mRNA was purified from total RNA (5 µg) using Dynabeads Oligo (dT) (Thermo Fisher, CA) with two rounds of purification. After purification was completed, the mRNA was fragmented into short fragments using divalent cations under elevated temperature [Magnesium RNA Fragmentation Module (NEB, Cat. No. E6150) at 94°C for 5–7 min]. The cleaved RNA fragments were then reverse-transcribed to create cDNA using SuperScript II Reverse Transcriptase (Invitrogen, Cat. No. 1896649), which were next used to synthesize U-labeled second-stranded DNAs with Escherichia coli DNA polymerase I (NEB, Cat. No. m0209), RNase H (NEB, Cat. No. M0297), and dUTP Solution (Thermo Fisher, Cat. No. R0133). An A-base was then added to the blunt ends of each strand, preparing them for ligation to the indexed adapters. Each adapter contained a T-base overhang for ligating the adapter to the A-tailed fragmented DNA. Dual-index adapters were ligated to the fragments, and size selection was performed with AMPureXP beads. After heat-labile UDG enzyme (NEB, Cat. No. M0280) treatment of the U-labeled second-stranded DNAs, the ligated products were amplified with PCR in the following conditions: initial denaturation at 95°C for 3 min; eight cycles of denaturation at 98°C for 15 s, annealing at 60°C for 15 s, and extension at 72°C for 30 s; and then final extension at 72°C for 5 min. The average insert size for the final cDNA libraries was 300 ± 50 bp. We performed 2 × 150-bp paired-end sequencing (PE150) on an Illumina Novaseq 6000 (LC-Bio Technology, Co., Ltd., Hangzhou, China) following the vendor’s recommended protocol.
Transcript Quantification
After sequencing was completed, read trimming and filtering were performed using Cutadapt (https://cutadapt.readthedocs.io/en/stable/,version:cutadapt-1.9). Quality control was performed using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Read alignment and transcript quantification were performed using Salmon 1.3.0. A mouse reference genome was constructed using the mm10 mouse genome for Salmon analysis. Fragment-level GC biases were corrected by using the “–gcBias” option in Salmon. Next, the transcript quantification files were imported into DESeq2. Gene expression values were adjusted for sequencing depth and RNA composition using DESeq2’s median of ratios method. Differential expression analysis was performed in DESeq2. A log2-fold change of 1 and adjusted P value of 0.05 were used as thresholds to determine significantly differentially expressed genes. P values were adjusted using the Benjamini–Hochberg method. Gene set enrichment analysis was performed using Rpackage cluster Profiler with gene ontologies including biological process, chemical component, and molecular function (1). Plots were generated with R packages ggplot (2) and ComplexHeatmap (3). Data are deposited into GEO accession GSE193177 at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE193177.
Statistical Analysis
All data were analyzed using Graph Pad Prism 9 (GraphPad Software). Data were analyzed for statistical significance by ordinary one-way ANOVA followed by Tukey’s multiple comparison test or unpaired t test. A power analysis was performed to calculate sample size according to our recent publication (31), using an effect size of twofold for gene expression and NEC severity and a difference of 20% for changes in protein expression by immunofluorescence between groups. The standard deviation for each of these parameters was based on our previously published studies (20–22, 27, 32), and we accepted a significance (type 1 error) of 5% (P = 0.05), the probability of finding an effect of 80%, and an attrition rate based on prior studies of 10%. The corrected sample size (including attrition) was then determined according to Charan et al. (33). The mouse pups and piglets of both sexes were randomized to the treatment groups, and blinded analyses. A P value of <0.05 (95% confidence level) was considered statistically significant, and data are presented as means ± SE. All experiments were performed with at least three biological replicates and at least 2–3 mice per group.
RESULTS
ST266 Administration Prevents the Development of NEC in Neonatal Mice
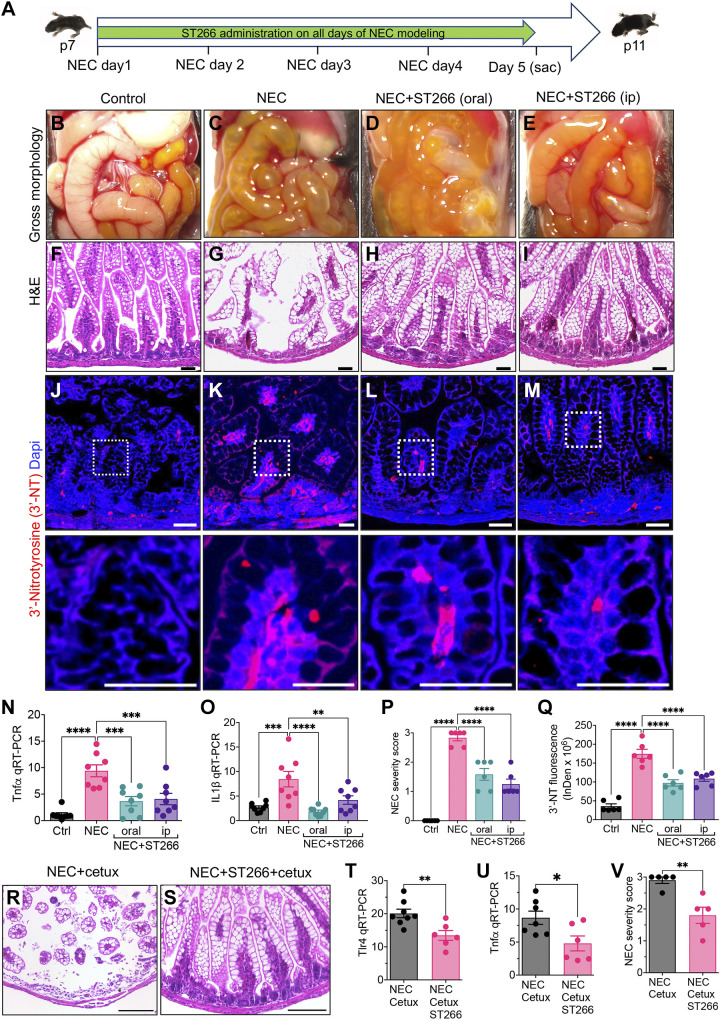
We first sought to assess whether the administration of ST266 could prevent the development of NEC in newborn mice (Fig. 1A). The mouse NEC model involves the administration of infant formula and stool from an infant with severe NEC every 3 h for 4 days, as well as twice daily exposure to hypoxia, to mice at p7 (27). In all cases, control mice remain with their mothers and are nursed. As shown in Fig. 1, B–E, mice subjected to the NEC model exhibit the gross appearance of NEC, including patchy intestinal edema, bowel dilation, and air within the wall of the bowel, a finding that is also known as pneumatosis intestinalis, and is pathognomonic of NEC (34). The intestine of control mice appears pink and healthy (Fig. 1B). The presence of NEC was confirmed histologically via H&E staining of the intestine, revealing disruption of the ileal mucosa and loss of the crypt-villus architecture in mice with NEC (Fig. 1G) as compared with the control mice, in which the integrity of the villi is maintained (Fig. 1F). Having induced NEC in mice, we next assessed the effect of ST266 administration on disease severity. As shown in Fig. 1, the administration of ST266 by either the oral (Fig. 1, D and H) or intraperitoneal (Fig. 1, E and I) routes significantly reduced the severity of NEC in mice, as revealed by the finding of pink and healthy gross appearance of the small intestine (Fig. 1, D and E) and intact histological appearance of the mucosal architecture, as compared with NEC mice that did not receive ST266 (Fig. 1, C and G). The prevention of NEC in response to ST266 was further revealed by examining the expression in the intestinal mucosa of the proinflammatory cytokine Tnfα and IL1β, which were found to be significantly increased in mice with NEC as compared with control mice, and were both significantly reduced in NEC mice after ST266 administration, by either the oral or parenteral route (Fig. 1, N and O). Further evidence that ST266 supplementation can prevent NEC was found in the NEC severity score that quantifies the degree of NEC severity based on the extent of histological injury (32) and revealed that ST266 administration reduced NEC severity after administration via either the oral or parenteral routes (Fig. 1P). As an additional measure of the protective effect of ST266 on mucosal injury in NEC, ST266 supplementation reduced the expression of the proinflammatory marker 3-nitrotyrosine (3′-NT) in the intestinal epithelium in mice, which was increased in mice NEC and reduced after administration of ST266 by either the oral or parenteral routes (Fig. 1, J–M, quantified in Q).
Figure 1.
ST266 administration prevents the development of NEC in neonatal mice. A: schematic illustration of the experimental protocol for the administration of ST266 in the NEC model in mice. Gross images of the small bowel of control (B), NEC (C), NEC mice given ST266 via oral route (D), and NEC mice given ST266 via intraperitoneal route (E). Representative H&E-stained histological images showing the intestinal mucosa of the terminal ileum in control (F), NEC (G), NEC mice given ST266 via oral route (H), and NEC mice given ST266 via intraperitoneal route (I). Representative confocal images of immunofluorescence staining of 3′-nitrotyrosine (3′-NT, red; Dapi-nuclei, blue) in the ileum of control (J), NEC (K), NEC mice given oral ST266 (L), and NEC mice given intraperitoneal ST266 (M). N and O: qRT-PCR expression of proinflammatory cytokines Tnfα and IL1β in the intestinal mucosa (n = 8 mice/group). P: representative NEC severity score (n = 6 mice/group). Q: quantification of fluorescent intensity of 3′-NT staining using the ImageJ software (n = 6 mice/group). Representative H&E-stained histological images showing the intestinal mucosa of the terminal ileum in NEC+Cetuximab (R) and NEC mice given ST266 via oral route+Cetuximab intraperitoneal route (S). qRT-PCR expression of Tlr4 (T) and proinflammatory cytokines Tnfα (U) in the intestinal mucosa (n = 6 mice/group). V: representative NEC severity score (n = 6 mice/group). Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons tests (N–Q) or unpaired t test (T–V) using GraphPad Prism 9 software. **P < 0.05, **P < 0.01, *** or ****P < 0.001. Each dot on the graph represents a different mouse. Scale bars, 100 µm (F–I, R, and S), 25 µm (J–M). H&E, hematoxylin and eosin; NEC, necrotizing enterocolitis.
Given that native amniotic fluid is enriched in epidermal growth factor (EGF) whose activity has been shown to mediate the protective effects of amniotic fluid against NEC (25, 35), we assessed whether the protective effects of ST266 against NEC required EGF signaling. As shown in Fig. 1, R–V, treatment of mice with the EGFR inhibitor cetuximab did not prevent the protective ability of ST266 for NEC, as ST266 restored histology (Fig. 1, R and S), reduced the expression of Tlr4 and Tnfα in the intestinal mucosa, (Fig. 1 T and U), and reduced NEC severity (Fig. 1V), despite pretreatment with cetuximab. Taken together, these findings illustrate that the addition of ST266 can prevent NEC in mice. We next sought to determine whether ST266 administration could treat established NEC in mice.
Administration of ST266 Reduces the Severity of Established NEC in Mice
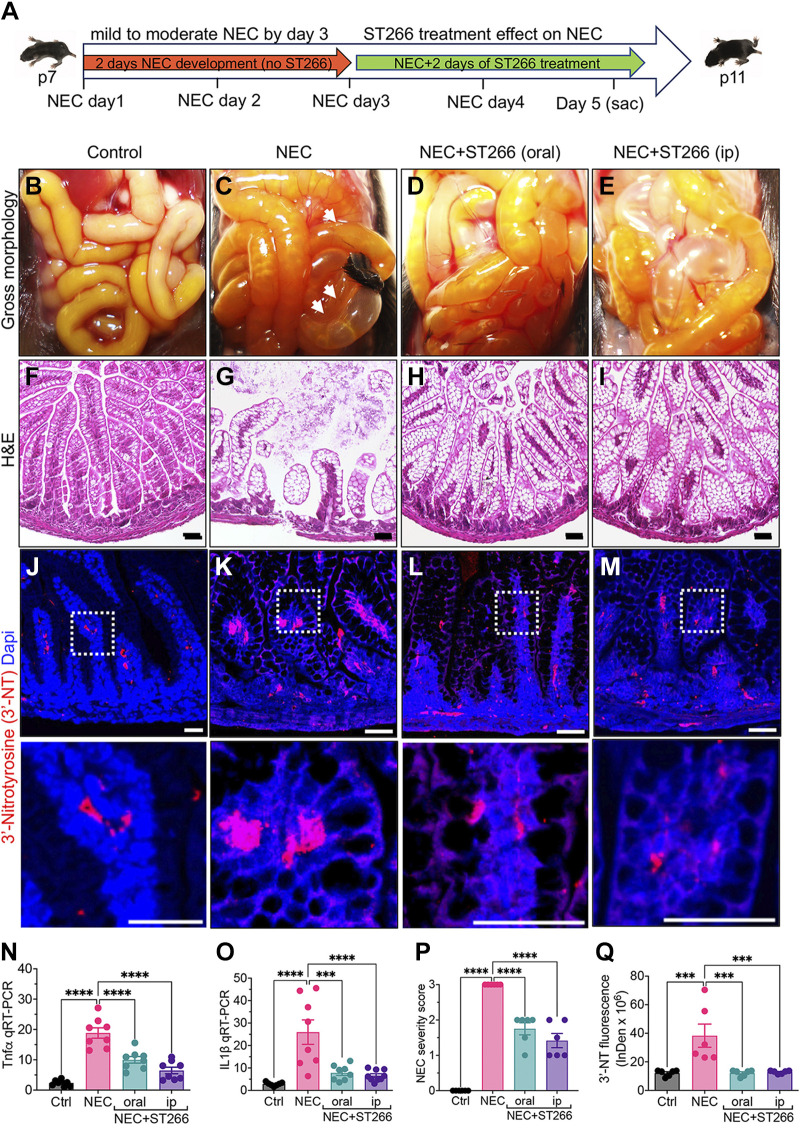
We next sought to determine the ability of ST266 to treat established NEC in mice. To do so, we induced p7 NEC in mice by subjecting mice to 3 days of the model, after which we have shown that moderately severe NEC is established (36) (Fig. 2A). Following this time point, we administered ST266 via either the oral or intraperitoneal route for two additional days. As shown in Fig. 2, D–E and H–I ST266 treatment via either the oral or intraperitoneal routes significantly reduced the severity of NEC when compared with mice who had untreated NEC (Fig. 2, C and G), as revealed by improved gross appearance (Fig. 2, D and E), improved histological appearance of the terminal ileum (Fig. 2, H and I), and reduced expression of Tnfα and IL1β in the terminal ileum (Fig. 2, N and O). ST266 treatment also reduced overall NEC severity as based on the blinded assessment of the histology (Fig. 2P). As an additional measure of the treatment effects of ST266 in NEC, ST266 treatment reduced the expression of the proinflammatory marker 3′-NT in the intestinal epithelium in mice with NEC (Fig. 2, J–M, quantified in Q). These findings reveal that ST266 can both prevent and treat NEC in mice. Based upon these findings, we next sought to assess whether ST266 could prevent NEC in a model performed in an animal that is closer in size to the human premature infant, and so investigated whether ST266 could prevent NEC in premature piglets.
Figure 2.
The administration of ST266 reduces the severity of established NEC in mice. A: schematic illustration of the experimental protocol for the administration of ST266 in the NEC model in mice. Gross images of the small bowel of control (B), NEC (B), NEC mice given ST266 via oral route (D), and NEC mice given ST266 via intraperitoneal route (E). Representative H&E-stained histological images showing the intestinal mucosa of the terminal ileum in control (F), NEC (G), NEC mice given ST266 via oral route (H), and NEC mice given ST266 via intraperitoneal route (I). Representative confocal images of immunofluorescence staining of 3′-nitrotyrosine (3′-NT, red; Dapi-nuclei, blue) in the ileum of control (J), NEC (K), NEC mice given oral ST266 (I), and NEC mice given intraperitoneal ST266 (M). N and O: qRT-PCR expression of proinflammatory cytokines Tnfα and IL1β in the intestinal mucosa (n = 8 mice/group). P: representative NEC severity score (n = 6 mice/group). Q: quantification of fluorescent intensity of 3′-NT staining using the ImageJ software (n = 6 mice/group) Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons tests using GraphPad Prism 9 software. *** or ****P < 0.001. Each dot on the graph represents a different mouse. Scale bars, 100 µm (F–I), 25 µm (J–M). H&E, hematoxylin and eosin; NEC, necrotizing enterocolitis.
Administration of ST266 Prevents Development of NEC in Premature Piglets
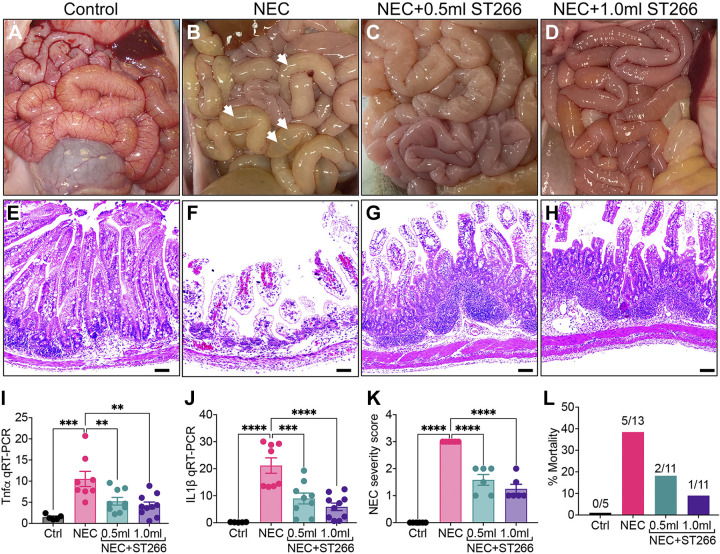
We next assessed the potential role of ST266 in preventing NEC in premature piglets. The premature piglet’s small intestine shares several architectural and physiological similarities with the human infant (37), and with a typical weight of ∼1,000 g, is of a similar size to the typical premature infant who develops NEC (9). NEC was induced in premature piglets that were delivered by cesarean section at 95% gestational age according to a well-validated model (36). Compared with control piglets (Fig. 3, A and E), the small intestine of piglets that were induced to develop NEC exhibit marked edema and inflammation of the small intestine, as well as persistent bloody diarrhea (Fig. 3, B and F). Importantly, administration of ST266 via the intraperitoneal route at two different doses (0.5 mL or 1.0 mL/piglet/day) significantly reduced the severity of NEC in a dose-dependent manner as manifested by an architecturally more intact appearing small intestine (Fig. 3, C and D) and improved histological appearance of the terminal ileal mucosa (Fig. 3, G and H) as compared with piglets with NEC and no ST266 (Fig. 3F). There was an expansion of the area below the crypts in piglets who received ST266 that was associated with improved epithelial architecture (Fig. 3, G and H). As shown in Fig. 3, I and J, ST266 administration to NEC piglets at 0.5 mL or 1.0 mL/piglet/day reduced the induction of the proinflammatory cytokines Tnfα (Fig. 3I) and Il-1β (Fig. 3J). ST266 administration also reduced the NEC severity score at both doses similarly (Fig. 3K). Importantly, ST266 significantly reduced mortality in piglets with NEC (Fig. 3L).
Figure 3.
Administration of ST266 prevents development of NEC in premature piglets. Gross images of the small bowel of control (A), NEC (B), NEC piglets given 0.5-mL ST266 (C), and NEC piglets given 1.0-mL ST266 (D) via intraperitoneal route once daily at 10 AM on each day of the NEC model. Representative H&E-stained histological images showing the intestinal mucosa of the terminal ileum in control (E), NEC (F), NEC piglets given 0.5-mL ST266 (G), and NEC piglets given 1.0-mL ST266 (H). qRT-PCR expression of proinflammatory cytokines Tnfα (I) and IL1β (J) in the intestinal mucosa. NEC severity score (K) and percentage mortality rate (L) (n = 6 piglets/group). Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons tests using GraphPad Prism 9 software. **P < 0.01, *** or ****P < 0.001. Each dot on the graph represents a different piglet. qPCR data were obtained from Ctrl (n = 5), NEC (n = 8), NEC + 0.5 mL ST266 (n = 9), and NEC + 1.0 mL ST266 (n = 10) piglets. Scale bars, 200 µm. H&E, hematoxylin and eosin; NEC, necrotizing enterocolitis.
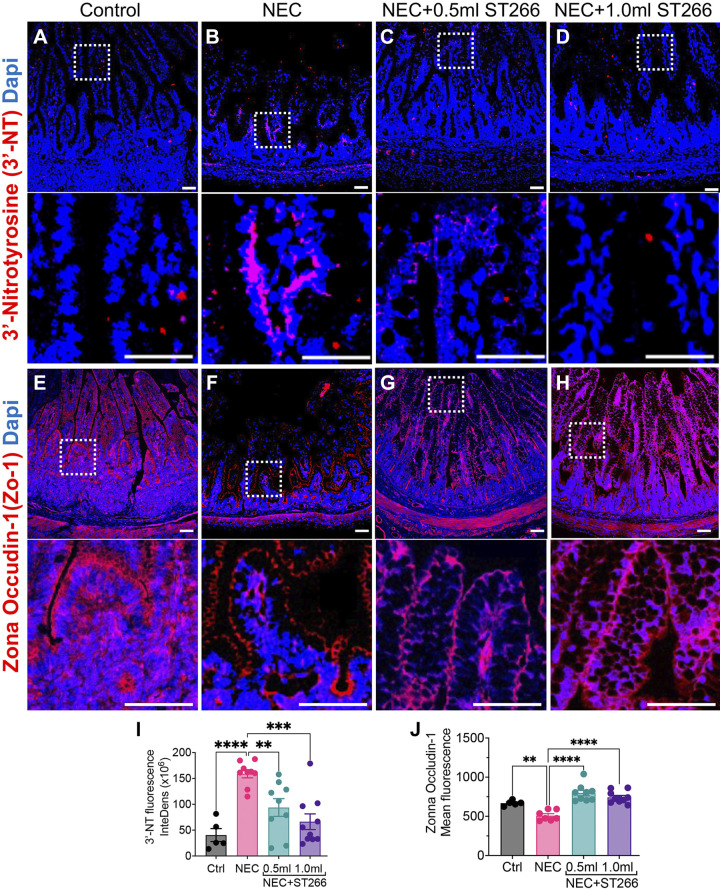
To further quantify the protective effects of ST266 in piglets with NEC, we next assessed the degree of expression of 3′-nitrotyrosine (3′-NT) in the intestinal epithelium, a marker of oxidative-mucosal injury. As shown in Fig. 4, A–D, the expression of 3′-NT was increased in the intestinal epithelium in piglets with NEC as compared with control piglets, yet was reduced to levels similar to control piglets after the administration of ST266 at both the lower and higher doses (Fig. 4, C and D and I). Moreover, the administration of ST266 at both doses restored the integrity of the intestinal barrier in piglets with NEC, as revealed by a loss of expression of the tight junction protein ZO-1 in the intestinal epithelium that was reversed in the presence of ST266 (Fig. 4, E–H, and J) at both low and high doses.
Figure 4.
Administration of ST266 prevents small intestinal mucosal injury and loss of tight junctions in premature piglets induced to develop NEC. Immunofluorescence images of 3′-nitrotyrosine (3′-NT, red fluorescence) as an indicator of oxidative injury in small bowel of control (A), NEC (B), NEC piglets given 0.5-mL ST266 (C), and NEC piglets given 1.0-mL ST266 (D). Immunofluorescence images of Zona occludin-1 (Zo-1, red fluorescence) as an indicator of barrier integrity in small bowel of control (E), NEC (F), NEC piglets given 0.5-mL ST266 (G), and NEC piglets given 1.0-mL ST266 (H) via intraperitoneal route once daily at 10 AM on each day of the NEC model. Quantification of fluorescent intensity of 3′-NT staining (I) and Zo-1 (J) using the ImageJ software. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons tests using GraphPad Prism 9 software. **P < 0.01, *** or ****P < 0.001. Each dot on the graph represents a different piglet. Data were obtained from Ctrl (n = 5), NEC (n = 8), NEC + 0.5 mL ST266 (n = 9), and NEC + 1.0 mL ST266 (n = 10) piglets. Scale bars, 50 µm. NEC, necrotizing enterocolitis.
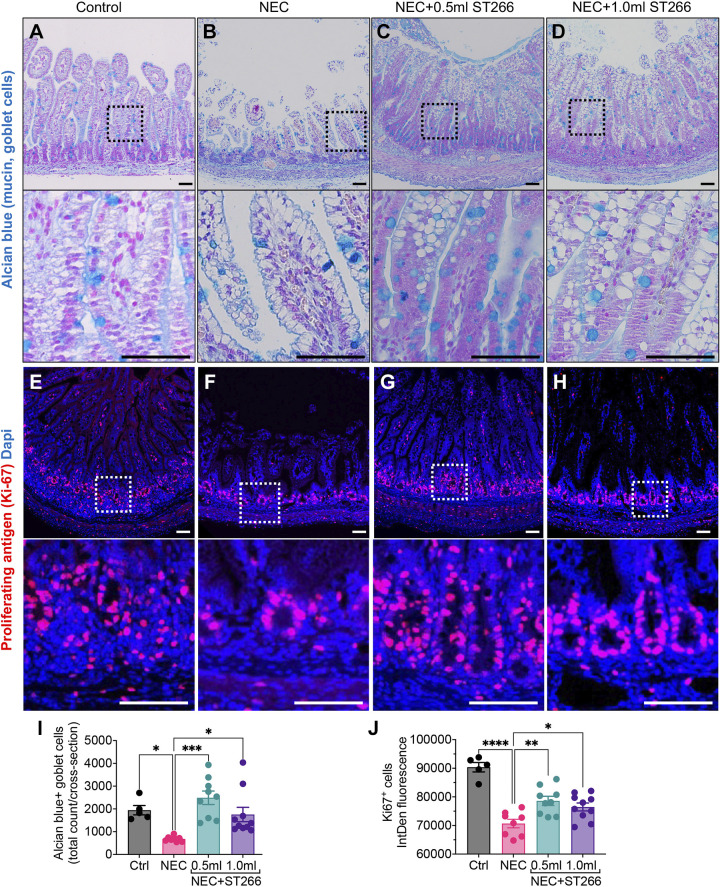
We next evaluated the ability of ST266 to restore two important consequences of NEC, namely, a loss of goblet cells, which serve to secrete mucus and protect the intestinal mucosa from injury (13), and a reduction in intestinal stem cell proliferation (38, 39), which contributes to the blunted intestinal villi and mucosal loss seen in NEC. As shown in Fig. 5, A–D, the induction of NEC in piglets led to a reduction in the number of goblet cells in the intestinal epithelium, as revealed by a loss of staining with the goblet cell dye Alcian blue (Fig. 5, A and B). Importantly, ST266 supplementation restored the number of goblet cells at both doses examined (Fig. 5, C and D, quantified in I). In parallel, NEC induction led to a significant reduction in crypt progenitor cell proliferation, as revealed by reduced immunostaining for Ki67 in NEC piglets as compared with control piglets (Fig. 5, E, F, and J). The administration of ST266 restored proliferation back to control levels, at both doses examined (Fig. 5, G, H, and J). Taken in aggregate, these findings illustrate that ST266 prevents NEC in piglets and mice. We therefore next sought to determine the mechanisms involved and focused first on the potential anti-inflammatory effects of ST266 as an inhibitor of TLR4.
Figure 5.
Administration of ST266 prevents loss of Alcian blue-stained goblet cells and Ki-67-positive proliferative progenitor cells in premature piglets induced to develop NEC. Representative confocal images of Alcian blue-stained goblets cells in small bowel of control (A), NEC (B), NEC piglets given 0.5-mL ST266 (C), and NEC piglets given 1.0-mL ST266 (D). Immunofluorescence images of Ki-67-stained cells (red fluorescence) in small bowel of control (E), NEC (F), NEC piglets given 0.5-mL ST266 (G), and NEC piglets given 1.0-mL ST266 (H) via intraperitoneal route once daily at 10 AM on each day of the NEC model. Quantification of Alcian blue-stained goblet cells (I) and Ki-6-stained proliferative cells (J) using the ImageJ software. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons tests using GraphPad Prism 9 software. *P < 0.05, **P < 0.01, *** or ****P < 0.001. Each dot on the graph represents a different piglet. Data were obtained from Ctrl (n = 5), NEC (n = 8), NEC + 0.5 mL ST266 (n = 9), and NEC + 1.0 mL ST266 (n = 10) piglets. Scale bars, 50 µm. NEC, necrotizing enterocolitis.
ST266 Inhibits TLR4 Signaling in IEC
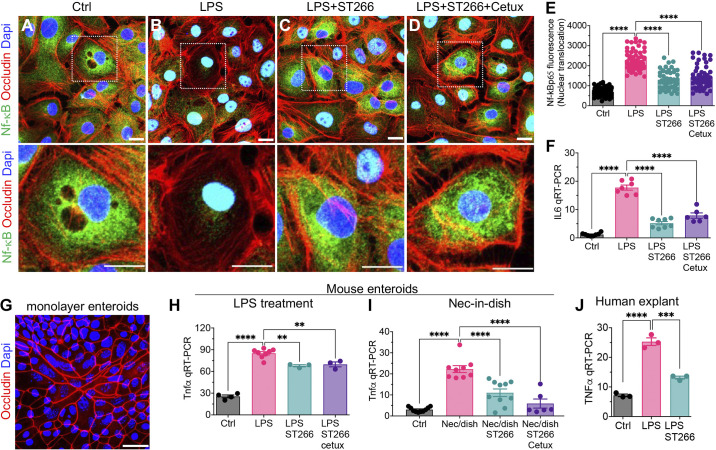
We have shown that the induction of NEC requires the activation of the lipopolysaccharide receptor Toll-like receptor 4 (TLR4) in the intestinal epithelial cells (13). Upon TLR4 activation, there is a rapid translocation of the transcription factor NF-κB from the cytoplasm to the nucleus, resulting in the induction of proinflammatory cytokines including IL6 and Tnfα (20). As shown in Fig. 6, A–D, treatment of IEC-6 enterocytes with LPS resulted in the translocation of NF-κB from the cytoplasm to the nucleus as revealed by confocal microscopy (Fig. 6, A, B, and E) and the induction of IL6 as measured by qRT-PCR (Fig. 6F). Importantly, pretreatment of IEC-6 cells with ST266 significantly reduced the translocation of NF-κB (Fig. 6, C and E) and reduced the expression of IL6 (Fig. 6F). It is noteworthy that the inhibitory effects of ST266 on TLR4 signaling did not require EGF [known to be an abundant molecule in amniotic fluid (40)], as cotreatment of cells with ST266 and the EGF receptor inhibitor cetuximab did not reduce the protective effects of ST266 on either NF-κB translocation (Fig. 6, D and E) or the expression of IL6 (Fig. 6F). The effects of ST266 in inhibiting TLR4 signaling were also observed in primary enteroids obtained from mice (Fig. 6G), in which ST266 prevented the LPS-induced expression of Tnfα (Fig. 6H). We note that cotreatment of mouse enteroids with the EGF receptor inhibitor cetuximab did not prevent the ST266-mediated inhibition of LPS-induced Tnfα expression in mouse enteroids (Fig. 6, G and H).
Figure 6.
ST266 inhibits TLR4 signaling in intestinal epithelial cells (IECs) independent of EGFR signaling. A–E: IEC6 cells. Representative confocal images of IEC6 cells immunostained for NF-κB p65 after treatment with media (Control, Ctrl) (A), LPS (B), LPS+ST266 (C), and LPS + ST266 + Cetuximab (Cetux) (D). E: NF-κB p65 translocation from cytoplasm to nucleus assessed by quantifying NF-κB p65 fluorescence intensity in the nucleus using ImageJ software (each dot in the graph represents an individual cell, and cells were counted from n = 3 independent experiments). F: qRT-PCR expression of proinflammatory cytokine interleukin-6 (IL6) (n = 6–8, as indicated). G and H: primary intestinal enteroids. G: confocal image of enteroids grown to monolayer on fibronectin-coated wells stained for Occludin (red fluorescence) showing viability and integrity of the cultured primary epithelial cells. H: qRT-PCR expression of proinflammatory cytokine Tnfα (n = 3–8 as indicated). I: qRT-PCR expression of proinflammatory cytokine Tnfα in enteroids subjected to NEC-in-dish treatment model (n = 6–9). J: human ileal tissue; qRT-PCR expression of proinflammatory cytokine TNFα in deidentified resected human nonnecrotic (control) small bowel tissue subjected to ex vivo cotreatment with LPS and ST266 for 6 h (n = 3). Each dot in dot graphs of cell culture experiments represents data from an independent cell-culture well/experiment or an individual tissue specimen from mouse or human. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons tests using GraphPad Prism 9 software. 0.05, **P < 0.01, *** or ****P < 0.001. Scale bars, 10 µm. EGFR, epidermal growth factor receptor; LPS, lipopolysaccharide; TLR4, Toll-like receptor 4.
To gain greater insights into the anti-inflammatory potential of ST266 in the intestinal epithelium, we next turned to our recently described “NEC-in-a-dish” model, in which we treated primary enteroids obtained from newborn mice with bacteria harvested from an infant with severe NEC, in the presence or absence of ST266 (21). As shown in Fig. 6I, treatment of enteroids with NEC bacteria resulted in the increased expression of Tnfα that was reduced by ST266 treatment, whereas cetuximab did not prevent the ST266-mediated inhibition of bacteria-induced Tnfα expression in mouse enteroids as shown in Fig. 6I.
Finally, to assess if ST266 could inhibit TLR4 signaling in human tissues, we used IRB-approved deidentified, resected human samples to ex vivo treat with LPS in presence or absence of LPS for 6 h. As data shown in Fig. 6J, ST266 reduced LPS-induced proinflammatory cytokine TNFα expression in the human explants.
In conjunction with the data shown in Fig. 1, these findings implicate ST266 in reducing the response of TLR4 signaling in enterocytes. Given that ST266 could influence several pathways within the intestinal epithelium, we next turned to RNA seq analysis to more fully interrogate the effects of ST266 on the transcriptome of the intestinal epithelium in newborn mice.
Effect of ST266 on the Transcriptomic Profile of the Intestine of Mice with NEC
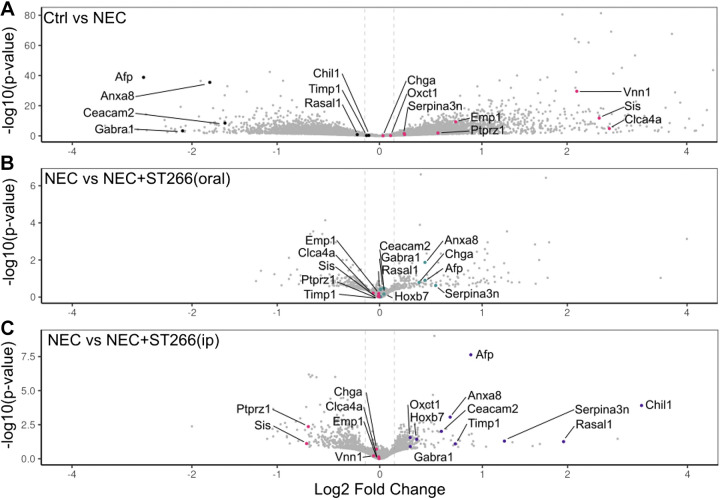
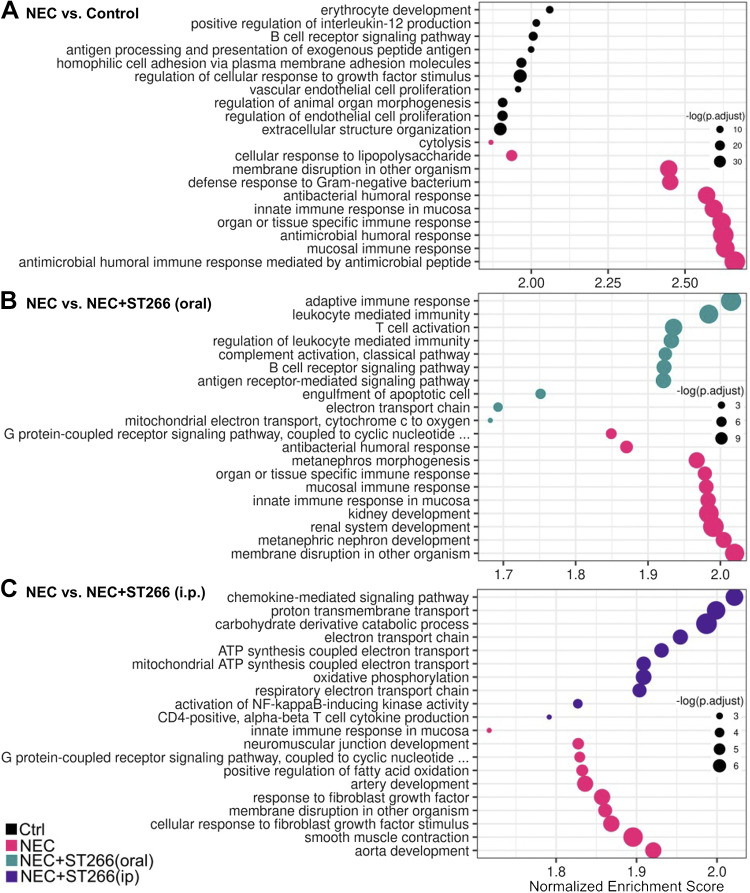
We next performed a transcriptomic analysis of the intestine of mice who were subjected to experimental NEC in the presence or absence of ST266. As shown in Fig. 7, NEC induction significantly altered the transcriptomic profile of the intestinal epithelium in mice (Fig. 7A), which was further altered by treatment with ST266 either orally (Fig. 7B) or intraperitoneally (Fig. 7C) as revealed by volcano plots showing the differentially expressed genes in the indicated groups. A more detailed analysis of the genes whose pattern of expression changes in each treatment group is shown in Fig. 8. Specifically, NEC in mice is characterized by the induction of a series of proinflammatory, metabolic and cell death genes, consistent with prior publications (Fig. 8A) (41). Importantly, the administration of oral ST266 altered this gene expression pattern, which then became characterized by an induction of cell cycle genes including Rac2, as well as adaptive immune genes including Cd28, Cd22, and Lef1. Interestingly, treatment of mice with ST266 via the intraperitoneal route yielded a different transcriptional profile than mice administered ST266 via the oral route, as characterized by induction of antinecrosis genes including Serpina3n and fetal development genes including Afp (Fig. 8, B and C).
Figure 7.
ST266 alters the transcriptomic profile of the intestine in mice with NEC. Volcano plots showing the differentially expressed genes in the intestinal epithelium in NEC mice and breastfed controls (A), NEC mice and NEC mice administered ST266 orally (B), and NEC mice and NEC mice administered ST266 via intraperitoneal route (C). Vertical lines are drawn at a log2-fold change of 0.25. Expression values were z-score scaled. Adjusted P < 0.05, log2FC > 1. NEC, necrotizing enterocolitis.
Figure 8.
ST266 treatment alters the expression pathway of key genes in the intestine of mice. Pathway analyses shown as bubble plots that represent the most upregulated pathways in each sample comparison. Comparisons include those between NEC mice and breastfed controls (A), NEC mice and NEC mice administered ST266 via oral route (B), and NEC mice and NEC mice administered ST266 via intraperitoneal route (C). Size of circle represents significance (P value). NEC, necrotizing enterocolitis.
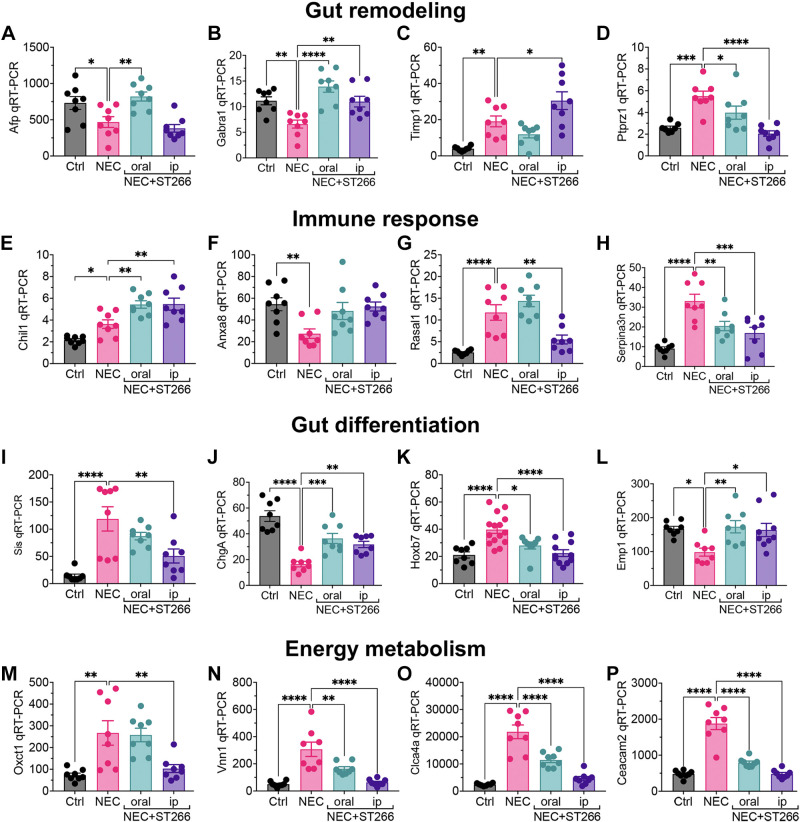
To validate the results of the RNA seq experiments, we performed quantitative RT-PCR on intestinal samples from mice with and without NEC that were treated in the presence or absence of ST266 administered via either the oral or intraperitoneal routes. Gene sets were found to regulate pathways that clustered into four major groups, namely, gut remodeling (Fig. 9, A–D), immune responses (Fig. 9, E–H), gut differentiation (Fig. 9, I–L), and energy metabolism (Fig. 9, M–P). Taken together, these findings illustrate that ST266 alters the transcriptional profile of the intestinal mucosa and that the oral and intraperitoneal routes differ in their effects.
Figure 9.
Validation of key pathway genes of RNA seq transcriptome data by qRT-PCR. qRT-PCR of expression in the intestinal epithelium of gut remodeling genes – α-fetoprotein (Afp) (A), γ-aminobutyric acid A receptor, subunit α 1(Gabra1) (B), tissue inhibitor of metalloproteinase 1 (Timp1) (C), and protein tyrosine phosphatase, receptor type Z, polypeptide 1(Ptprz1) (D). qRT-PCR of expression of immune response genes– chitinase-like 1 (Chil1) (E), Annexin A8 (Anxa8) (F), RAS protein activator-like 1 (GAP1 like) (Rasal1) (G), and serine (or cysteine) peptidase inhibitor, clade A, member 3 N (Serpina3n) (H). qRT-PCR of expression of gut differentiation genes – enterocyte marker, sucrase isomaltase (Sis) (I), enteroendocrine marker, chromogranin A (ChgA) (J), cell proliferation gene, Homoebox 7 (Hoxb7) (K), epithelial cells membrane protein (Emp1) (L). qRT-PCR of expression of energy metabolism and other genes – oxoacid CoA transferase 1 (Oxct1) (M), vanin1 (Vnn1) (N), chloride channel accessory 4 (Clca4a) (O), and carcinoembryonic antigen-related cell adhesion molecule (Ceacam2) (P) in neonatal control and NEC mice without or with ST266 administration. Each dot on the graph represents a different mouse. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons tests using GraphPad Prism 9 software. *P < 0.05, **P < 0.01, *** or ****P < 0.001. NEC, necrotizing enterocolitis.
DISCUSSION
The current study provides evidence that the administration of a secretome from amniotic cells can prevent and/or treat NEC in preclinical models. The protective effects of ST266 were observed in two experimental models (mice and piglets). There was no observable toxicity from the ST266 administration, as evidenced by an absence of depilation, weight loss, reduced activity, or abnormal behavior of any sort with no ill effects noted. The mechanisms of action were related to the inhibition in LPS signaling, as revealed using intestinal epithelial cell cultures, intestinal organoids, human ileal explants, and mouse models of endotoxemia. In view of the critical role that LPS activation of TLR4 plays in the pathogenesis of NEC (13), the current data suggest that the anti-inflammatory effects of ST266 are mediated in part by TLR4 inhibition. TLR4 activation on the intestinal epithelium induces epithelial death via apoptosis and necroptosis (38, 42), prevents mucosal healing through impaired enterocyte proliferation (39) and migration (15), and impairs intestinal perfusion by inducing vasoconstriction in the intestinal mesentery by critically regulating local nitric oxide production (11), which together lead to the induction of NEC (43), and which may be inhibited by ST266. Furthermore, an analysis of the transcriptomic pathways that are altered by ST266 in the intestinal mucosa of mice further revealed that ST266 altered the expression of genes that were involved in gut remodeling, immune responses, gut differentiation, and energy metabolism. Given that ST266 administration was found to be safe and well tolerated, the current findings suggest that the administration of a formulation containing ST266 could represent a novel approach for the prevention or treatment of NEC in infants.
The potential anti-inflammatory benefits of ST266 in the management of NEC are consistent with the benefit shown by ST266 in other model systems of inflammation. For instance, Hasturk et al. (44) have shown that topical ST266 can safely reduce inflammation in the oral cavity in adults after a 6 wk treatment. ST266 can improve optic nerve demyelination in experimental autoimmune encephalomyelitis, a model that resembles multiple sclerosis (45) and that similar protection could be achieved through administration of the amnion-derived multipotent progenitor (AMP) cells themselves (46). Topical treatment with ST266 was also found to reduce ultraviolet-induced inflammation of the skin in humans and to decrease damaged DNA (26). It is noteworthy that in the current study, the transcriptomic profiling of mice treated with ST266 revealed differences in responses when ST266 was administered via the oral as compared with the parenteral route (Fig. 9). In explaining this finding, we posit that the differential gene expression may reflect differences that are induced by ST266 on the intestinal mucosa when administered orally, as compared with on immune cells that then enter the intestinal mucosa, when ST266 is administered parenterally. By evaluating the differential transcriptomic profile that is induced by ST266 in response to two different routes of administration, we can potentially tailor the route of administration to the stage of disease (early may benefit from oral administration, whereas late disease may benefit from parenteral administration), while also understanding off-target effects. These studies in aggregate demonstrate that secreted growth factors and cytokines derived from amniotic progenitor cells can exert anti-inflammatory properties in mice and provide a rationale for the investigation of ST266 as an approach to prevent or treat NEC.
Several studies have described the protective effects of amniotic fluid against NEC, either through their protein components, their cellular components, or microvesicles present within the fluid itself. For instance, we have shown that the administration of amniotic fluid to mice or piglets prevents NEC through the inhibition of the bacterial receptor Toll-like receptor 4 through its component epidermal growth factor (EGF), which is enriched in amniotic fluid, and which was responsible for the inhibition of TLR4 (25). The anti-inflammatory effects of amniotic fluid were validated in a subsequent study in neonatal piglets, in which amniotic fluid was thought to exert its protective effects on dendritic cells (47). Amniotic fluid is also enriched in progenitor cells, which have been shown to integrate within the bowel wall in models in which they protect against NEC development, in part through increasing enterocyte proliferation and reducing apoptosis (48). The importance of Wnt signaling in the efficacy of amniotic fluid stem cells in NEC prevention was seen in a recent study in mice (49), and although the precise mechanisms by which these cells regulate the Wnt pathway is incompletely understood, the amniotic fluid stem cell-derived vesicles appear sufficient to mediate the effect (49). Amniotic fluid stem cells were also found to reduce ascites in a rat model of NEC (48) and to protect against NEC via its component hepatocyte growth factor (50). In a recent comparison of various stem cell populations (amniotic fluid-derived mesenchymal SC, amniotic fluid-derived neural SC, bone marrow-derived mesenchymal SC, and neonatal enteric neural SC) that could be considered for the treatment or prevention of NEC, amniotic fluid-derived stem cells were thought to be the most effective and practical (51). More recently, exosomes derived from amniotic fluid-derived stem cells were sufficient to protect the intestine in experimental NEC in rats (52), a finding that was also seen in mice with NEC in a variation of the rat model (53). In a study supportive of the current work, Pierro and colleagues (54) showed that the effluent from cultures of amniotic fluid stem cells regulates cell development, perhaps explaining their effects in preventing NEC in mice. These findings collectively support a closer examination of the role of amniotic fluid and its components in the management of NEC.
The fact that ST266 represents a secretome with multiple components likely explains the results of the transcriptomic analysis that reveals its effects on pathways as seemingly diverse as gut development, cell metabolism, and inflammation. Although these results are consistent with the overarching effects of amniotic fluid on infant growth and development, they could raise questions regarding the potential utility of such a reagent in the management of premature infants, who by definition are under a state of constant growth and development. It is reassuring that the anti-NEC effects of ST266 occurred even in the presence of inhibition of the EGF pathway using the EGFR cetuximab, indicating that EGF signaling is not required for the anti-inflammatory effects of ST266. This observation avoids concerns related to growth factor receptor activation in the neonatal population. We do note that a synthetic amniotic fluid has been administered to preterm infants without apparent toxicity in two separate studies (55, 56). That said, the therapeutic potential of amniotic fluid administration has been questioned by other authors, who have raised concerns about its harmful effects, in particular its ability to irritate exposed neural tissue (57). It is noteworthy that ST266 itself is produced by a subset of amnion epithelial cells and so does not contain any components of amniotic fluid that could potentially be irritating. These properties suggest that a substance like ST266 that is endowed with the beneficial anti-inflammatory properties of amniotic fluid, without its component irritating factors, may represent a safe approach for administration to infants to prevent or treat NEC.
There are several limitations of the current study that may reduce its immediate applicability to clinical translation. First, although we took care to use two different NEC models (mice and piglets), neither model has all the features that are seen in clinical NEC. For instance, NEC in humans does not develop immediately after birth, but rather at 3–4 wk of age. By contrast, the current animal models are induced to develop either immediately after birth (piglet) or at a week of life (mouse). It is reassuring that the newborn mouse represents the 28-wk human infant at a time point in which NEC occurs (58). Moreover, NEC occurs in infants who are exposed to the unique environmental stressors of the neonatal intensive care unit, which can only be loosely replicated in animal models. Finally, the clinical onset of NEC, which can occur as either an indolent condition or a fulminant process, is difficult to replicate fully in the mouse or the piglet. Despite these limitations, the experimental systems used are excellent approximations of the size (piglet) and the pathogenic underpinnings (mouse) seen in clinical NEC and are highly suited for the detection of potential toxic effects of the compound. Moreover, the mouse and piglet models have been used by our group for the identification of novel compounds that can prevent NEC that have been validated in human NEC tissue ex vivo (19, 32, 36), suggesting the validity of these models in establishing the potential benefit of ST266.
In summary, we have shown that a secretome derived from amniotic cells can prevent or treat NEC in mouse and piglet models. The mechanisms of action include a potent anti-inflammatory effect, as well as pleiotropic effects on gut development, metabolism, and immunity. Given the lack of alternative, specific, effective anti-NEC therapies, these findings raise the possibility that ST266 could be useful in the prevention or treatment of clinical NEC.
GRANTS
D.J.H. is supported by Office of Extramural Research, National Institutes of Health Grant R35GM141956, and this research was funded in part by a Sponsored Research Grant from Noveome Biotherapeutics.
DISCLOSURES
The ST266 therapy studied here is proprietary property of Noveome Biotherapeutics, Inc. D.L.S., H.W., and L.R.B. are all employees of Noveome. All in vivo studies on necrotizing enterocolitis, which represent the majority of experiments in this manuscript, were conducted without influence from any of the Noveome employees to reduce any potential conflicts of interest. These competing interests do not alter our adherence to this journal’s policies on sharing data and materials. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
C.P.S., H.W., L.R.B., P.L., and D.J.H. conceived and designed research; C.P.S., R.A., H.J., W.B.F., C.L., A.J.G.S., A.I., M.S., S.S., S.W., T.P.Jr., M.W., L.R.B., and P.L. performed experiments; C.P.S., R.A., W.F., C.L., A.J.G.S., A.I., S.W., T.P.Jr., M.W., D.L.S., H.W., Z.K., L.R.B., and P.L. analyzed data; C.P.S., R.A., W.F., D.L.S., H.W., Z.K., L.R.B., and D.J.H. interpreted results of experiments; C.P.S. and D.J.H. prepared figures; D.J.H. drafted manuscript; C.P.S., D.L.S., H.W., Z.K., and D.J.H. edited and revised manuscript; C.P.S., R.A., H.J., W.F., D.L.S., H.W., L.R.B., and D.J.H. approved final version of manuscript.
REFERENCES
- 1. Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, Sanchez PJ, Shankaran S, Van Meurs KP, Ball MB, Hale EC, Newman NS, Das A, Higgins RD, Stoll BJ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med 372: 331–340, 2015. doi: 10.1056/nejmoa1403489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alsaied A, Islam N, Thalib L. Global incidence of necrotizing enterocolitis: a systematic review and meta-analysis. BMC Pediatr 20: 344, 2020. doi: 10.1186/s12887-020-02231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu J, Murphy S, Kochanek K, Arias E. Deaths: Final Data for 2019. National Vital Statistics Reports (NVSS) 2021, 70(8): 1–87. https://stacks.cdc.gov/view/cdc/106058/cdc_106058_DS1.pdf. [PubMed] [Google Scholar]
- 4. Samuels N, Van De Graaf RA, De Jonge RCJ, Reiss IKM, Vermeulen MJ. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr 17: 105, 2017. doi: 10.1186/s12887-017-0847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hackam DJ. Small animal models for the study of necrotizing enterocolitis and NEC-in-a-dish. In: Necrotizing Enterocolitis: Pathogenesis, Diagnosis and Treatment. Boca Raton, FL: CRC Press, 2021, p. 232–237. [Google Scholar]
- 6. Hackam DJ. Surgery for Necrotizing Enterocolitis. Boca Raton, FL: CRC Press, 2021, p. 49–57. [Google Scholar]
- 7. Han SM, Knell J, Henry O, Riley H, Hong CR, Staffa SJ, Modi BP, Jaksic T. Long-term outcomes of severe surgical necrotizing enterocolitis. J Pediatr Surg 55: 848–851, 2020. doi: 10.1016/j.jpedsurg.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 8. Sampah MES, Hackam DJ. Prenatal immunity and influences on necrotizing enterocolitis and associated neonatal disorders. Front Immunol 12: 650709, 2021. doi: 10.3389/fimmu.2021.650709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kovler ML, Sodhi CP, Hackam DJ. Precision-based modeling approaches for necrotizing enterocolitis. Dis Model Mech 13: dmm044388, 2020. doi: 10.1242/dmm.044388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hackam DJ, Sodhi CP, Good M. New insights into necrotizing enterocolitis: from laboratory observation to personalized prevention and treatment. J Pediatr Surg 54: 398–404, 2019. doi: 10.1016/j.jpedsurg.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A, Neal MD, Jia H, Lin J, Ma C, Branca MF, Prindle T, Richardson WM, Ozolek J, Billiar TR, Binion DG, Gladwin MT, Hackam DJ. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci USA 110: 9451–9456, 2013. doi: 10.1073/pnas.1219997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu P, Sodhi CP, Hackam DJ. Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology 21: 81–93, 2014. doi: 10.1016/j.pathophys.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, Prindle T Jr, Russo AM, Afrazi A, Good M, Brower-Sinning R, Firek B, Morowitz MJ, Ozolek JA, Gittes GK, Billiar TR, Hackam DJ. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143: 708–718.e5, 2012. doi: 10.1053/j.gastro.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, Thomson RB, Soliman A, Arditi M, Caplan MS. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 177: 3273–3282, 2006. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leaphart CL, Cavallo JC, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 179: 4808–4820, 2007. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 16. Yu W, Haque I, Venkatraman A, Menden HL, Mabry SM, Roy BC, Xia S, Prokop JW, Umar S, Geurts AM, Sampath V. SIGIRR mutation in human necrotizing enterocolitis (NEC) disrupts STAT3-dependent microRNA expression in neonatal gut. Cell Mol Gastroenterol Hepatol 13: 425–440, 2021. doi: 10.1016/j.jcmgh.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, Jakub A, Shi X-H, Shah S, Ozolek JA, Hackam DJ. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol 182: 636–646, 2009. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, Lu P, Ma C, Branca MF, Weyandt S, Fulton WB, Nino DF, Prindle T Jr, Ozolek JA, Hackam DJ. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest 126: 495–508, 2016. doi: 10.1172/JCI83356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neal MD, Jia H, Eyer B, Good M, Guerriero CJ, Sodhi CP, Afrazi A, Prindle T, Ma C, Branca M, Ozolek J, Brodsky JL, Wipf P, Hackam DJ. Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors. PLoS One 8: e65779, 2013. doi: 10.1371/journal.pone.0065779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sodhi CP, Wipf P, Yamaguchi Y, Fulton WB, Kovler M, Nino DF, Zhou Q, Banfield E, Werts AD, Ladd MR, Buck RH, Goehring KC, Prindle T Jr, Wang S, Jia H, Lu P, Hackam DJ. The human milk oligosaccharides 2'-fucosyllactose and 6'-sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll-like receptor 4 signaling. Pediatr Res 89: 91–101, 2021. doi: 10.1038/s41390-020-0852-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Werts AD, Fulton WB, Ladd MR, Saad-Eldin A, Chen YX, Kovler ML, Jia H, Banfield EC, Buck RH, Goehring K, Prindle T, Wang S, Zhou Q, Lu P, Yamaguchi Y, Sodhi CP, Hackam DJ. A novel role for necroptosis in the pathogenesis of necrotizing enterocolitis. Cell Mol Gastroenterol Hepatol 9: 403–423, 2020. doi: 10.1016/j.jcmgh.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jia H, Sodhi CP, Yamaguchi Y, Lu P, Ladd MR, Werts A, Fulton WB, Wang S, Prindle T Jr, Hackam DJ. Toll like receptor 4 mediated lymphocyte imbalance induces Nec-induced lung injury. Shock 52: 215–223, 2019. doi: 10.1097/SHK.0000000000001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hackam DJ, Sodhi CP. Toll-like receptor-mediated intestinal inflammatory imbalance in the pathogenesis of necrotizing enterocolitis. Cell Mol Gastroenterol Hepatol 6: 229–238.e1, 2018. doi: 10.1016/j.jcmgh.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Good M, Sodhi CP, Egan CE, Afrazi A, Jia H, Yamaguchi Y, Lu P, Branca MF, Ma C, Prindle T, Mielo S, Pompa A, Hodzic Z, Ozolek JA, Hackam DJ. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol 8: 1166–1179, 2015. doi: 10.1038/mi.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Good M, Siggers RH, Sodhi CP, Afrazi A, Alkhudari F, Egan CE, Neal MD, Yazji I, Jia H, Lin J, Branca MF, Ma C, Prindle T, Grant Z, Shah S, Slagle D 2nd, Paredes J, Ozolek J, Gittes GK, Hackam DJ. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci USA 109: 11330–11335, 2012. doi: 10.1073/pnas.1200856109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guan L, Suggs A, Galan E, Lam M, Baron ED. Topical application of ST266 reduces UV-induced skin damage. Clin Cosmet Investig Dermatol 10: 459–471, 2017. doi: 10.2147/CCID.S147112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou Q, Nino DF, Yamaguchi Y, Wang S, Fulton WB, Jia H, Lu P, Prindle T Jr, Pamies D, Morris M, Chen LL, Sodhi CP, Hackam DJ. Necrotizing enterocolitis induces T lymphocyte-mediated injury in the developing mammalian brain. Sci Transl Med 13: eaay6621, 2021. doi: 10.1126/scitranslmed.aay6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steed DL, Trumpower C, Duffy D, Smith C, Marshall V, Rupp R, Robson M. Amnion-derived cellular cytokine solution: a physiological combination of cytokines for wound healing. Eplasty 8: e18, 2008. [PMC free article] [PubMed] [Google Scholar]
- 29. Banas RA, Trumpower C, Bentlejewski C, Marshall V, Sing G, Zeevi A. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum Immunol 69: 321–328, 2008. doi: 10.1016/j.humimm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 30. Sodhi CP, Fulton WB, Good M, Vurma M, Das T, Lai CS, Jia H, Yamaguchi Y, Lu P, Prindle T, Ozolek JA, Hackam DJ. Fat composition in infant formula contributes to the severity of necrotising enterocolitis. Br J Nutr 120: 665–680, 2018. doi: 10.1017/S0007114518001836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sodhi CP, Gonzalez Salazar AJ, Kovler ML, Fulton WB, Yamaguchi Y, Ishiyama A, Wang S, Prindle T, Vurma M, Das T, Jia H, Lu P, Hackam DJ. The administration of a pre-digested fat-enriched formula prevents necrotising enterocolitis-induced lung injury in mice. Br J Nutr. In Press. 2021. doi: 10.1017/S0007114521004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu P, Yamaguchi Y, Fulton WB, Wang S, Zhou Q, Jia H, Kovler ML, Salazar AG, Sampah M, Prindle T Jr, Wipf P, Sodhi CP, Hackam DJ. Maternal aryl hydrocarbon receptor activation protects newborns against necrotizing enterocolitis. Nat Commun 12: 1042, 2021. doi: 10.1038/s41467-021-21356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Donovan C, Liu G, Shen S, Marshall JE, Kim RY, Alemao CA, Budden KF, Choi JP, Kohonen‐Corish M, El‐Omar EM, Yang IA, Hansbro PM. The role of the microbiome and the NLRP3 inflammasome in the gut and lung. J Leukoc Biol 108: 925–935, 2020. doi: 10.1002/JLB.3MR0720-472RR. [DOI] [PubMed] [Google Scholar]
- 34. Hackam DJ. Necrotizing enterocolitis, the stories of its tiny patients, and the journey ahead. In: Necrotizing enterocolitis: Pathogenesis, Diagnosis and Treatment. Boca Raton, FL: CRC Press, 2021. p. 1–4. [Google Scholar]
- 35. Dasgupta S, Jain SK. Protective effects of amniotic fluid in the setting of necrotizing enterocolitis. Pediatr Res 82: 584–595, 2017. doi: 10.1038/pr.2017.144. [DOI] [PubMed] [Google Scholar]
- 36. Kovler ML, Gonzalez Salazar AJ, Fulton WB, Lu P, Yamaguchi Y, Zhou Q, Sampah M, Ishiyama A, Prindle T Jr, Wang S, Jia H, Wipf P, Sodhi CP, Hackam DJ. Toll-like receptor 4-mediated enteric glia loss is critical for the development of necrotizing enterocolitis. Sci Transl Med 13: eabg3459, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sangild PT, Siggers RH, Schmidt M, Elnif J, Bjornvad CR, Thymann T, Grondahl ML, Hansen AK, Jensen SK, Boye M, Moelbak L, Buddington RK, Westrom BR, Holst JJ, Burrin DG. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 130: 1776–1792, 2006. doi: 10.1053/j.gastro.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 38. Neal MD, Sodhi CP, Jia H, Dyer M, Egan CE, Yazji I, Good M, Afrazi A, Marino R, Slagle D, Ma C, Branca MF, Prindle T Jr, Grant Z, Ozolek J, Hackam DJ. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J Biol Chem 287: 37296–37308, 2012. doi: 10.1074/jbc.M112.375881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle TJ Jr, Branca M, Russo A, Gribar SC, Ma C, Hackam DJ. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology 138: 185–196, 2010. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hofmann GE, Abramowicz JS. Epidermal growth factor (EGF) concentrations in amniotic fluid and maternal urine during pregnancy. Acta Obstet Gynecol Scand 69: 217–221, 1990. doi: 10.3109/00016349009028683. [DOI] [PubMed] [Google Scholar]
- 41. Cho SX, Rudloff I, Lao JC, Pang MA, Goldberg R, Bui CB, McLean CA, Stock M, Klassert TE, Slevogt H, Mangan NE, Cheng W, Fischer D, Gfroerer S, Sandhu MK, Ngo D, Bujotzek A, Lariviere L, Schumacher F, Tiefenthaler G, Beker F, Collins C, Kamlin COF, König K, Malhotra A, Tan K, Theda C, Veldman A, Ellisdon AM, Whisstock JC, Berger PJ, Nold-Petry CA, Nold MF. Characterization of the pathoimmunology of necrotizing enterocolitis reveals novel therapeutic opportunities. Nat Commun 11: 1–19, 2020. doi: 10.1038/s41467-020-19400-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neal MD, Sodhi CP, Dyer M, Craig BT, Good M, Jia H, Yazji I, Afrazi A, Richardson WM, Beer-Stolz D, Ma C, Prindle T, Grant Z, Branca MF, Ozolek J, Hackam DJ. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J Immunol 190: 3541–3551, 2013. doi: 10.4049/jimmunol.1202264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hackam DJ, Sodhi CP. Bench to bedside - new insights into the pathogenesis of necrotizing enterocolitis. Nat Rev Gastroenterol Hepatol 19: 468–479, 2022. doi: 10.1038/s41575-022-00594-x. [DOI] [PubMed] [Google Scholar]
- 44. Hasturk H, Steed D, Tosun E, Martins M, Floros C, Nguyen D, Stephens D, Cugini M, Starr J, Van Dyke TE. Use of amnion‐derived cellular cytokine solution for the treatment of gingivitis: a 2‐week safety, dose‐ranging, proof‐of‐principle randomized trial. J Periodontol 92: 1317–1328, 2021. doi: 10.1002/jper.20-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Willett K, Khan RS, Dine K, Wessel H, Kirshner ZZ, Sauer JL, Ellis A, Brown LR, Shindler KS. Neuroprotection mediated by ST266 requires full complement of proteins secreted by amnion-derived multipotent progenitor cells. PLoS One 16: e0243862, 2021. doi: 10.1371/journal.pone.0243862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khan RS, Ross AG, Willett K, Dine K, Banas R, Brown LR, Shindler KS. Amnion-derived multipotent progenitor cells suppress experimental optic neuritis and myelitis. Neurotherapeutics 18: 448–459, 2021. doi: 10.1007/s13311-020-00949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Siggers J, Østergaard MV, Siggers RH, Skovgaard K, Mølbak L, Thymann T, Schmidt M, Møller HK, Purup S, Fink LN, Frøkiær H, Boye M, Sangild PT, Bering SB. Postnatal amniotic fluid intake reduces gut inflammatory responses and necrotizing enterocolitis in preterm neonates. In: Am J Physiol Gastrointest Liver Physiol 304: G864–G875, 2013. doi: 10.1152/ajpgi.00278.2012. [DOI] [PubMed] [Google Scholar]
- 48. Zani A, Cananzi M, Lauriti G, Fascetti-Leon F, Wells J, Siow B, Lythgoe MF, Pierro A, Eaton S, De Coppi P. Amniotic fluid stem cells prevent development of ascites in a neonatal rat model of necrotizing enterocolitis. Eur J Pediatr Surg 24: 57–60, 2013. doi: 10.1055/s-0033-1350059. [DOI] [PubMed] [Google Scholar]
- 49. Li B, Lee C, O'Connell JS, Antounians L, Ganji N, Alganabi M, Cadete M, Nascimben F, Koike Y, Hock A, Botts SR, Wu RY, Miyake H, Minich A, Maalouf MF, Zani-Ruttenstock E, Chen Y, Johnson-Henry KC, De Coppi P, Eaton S, Maattanen P, Delgado Olguin P, Zani A, Sherman PM, Pierro A. Activation of Wnt signaling by amniotic fluid stem cell-derived extracellular vesicles attenuates intestinal injury in experimental necrotizing enterocolitis. Cell Death Dis 11: 750, 2020. doi: 10.1038/s41419-020-02964-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jain SK, Baggerman EW, Mohankumar K, Namachivayam K, Jagadeeswaran R, Reyes VE, Maheshwari A. Amniotic fluid-borne hepatocyte growth factor protects rat pups against experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 306: G361–G369, 2014. doi: 10.1152/ajpgi.00272.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McCulloh CJ, Olson JK, Wang Y, Vu J, Gartner S, Besner GE. Evaluating the efficacy of different types of stem cells in preserving gut barrier function in necrotizing enterocolitis. J Surg Res 214: 278–285, 2017. doi: 10.1016/j.jss.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McCulloh CJ, Olson JK, Wang Y, Zhou Y, Tengberg NH, Deshpande S, Besner GE. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J Pediatr Surg 53: 1215–1220, 2018. doi: 10.1016/j.jpedsurg.2018.02.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. O'Connell JS, Lee C, Farhat N, Antounians L, Zani A, Li B, Pierro A. Administration of extracellular vesicles derived from human amniotic fluid stem cells: a new treatment for necrotizing enterocolitis. Pediatr Surg Int 37: 301–309, 2021. [DOI] [PubMed] [Google Scholar]
- 54. Li B, Lee C, Cadete M, O’Connell JS, Alganabi M, Lee D, Ganji N, Miyake H, Botts SR, Johnson-Henry KC, Maattanen P, Sherman PM, Pierro A. Amniotic fluid stem cell administration can prevent epithelial injury from necrotizing enterocolitis. Pediatr Res 91: 101–106, 2022. doi: 10.1038/s41390-021-01657-6. [DOI] [PubMed] [Google Scholar]
- 55. Sullivan SE, Calhoun DA, Maheshwari A, Ashmeade TL, Auerbach DA, Hudak ML, Beltz SE, Christensen RD. Tolerance of simulated amniotic fluid in premature neonates. Ann Pharmacother 36: 1518–1524, 2002. doi: 10.1345/aph.1A439. [DOI] [PubMed] [Google Scholar]
- 56. Hosseini M, Azampour H, Raeisi S, Behtari M, Valizadeh H, Saboohi R. The effects of enteral artificial amniotic fluid-containing erythropoietin on short term outcomes of preterm infants. Turk J Pediatr 61: 392–398, 2019. doi: 10.24953/turkjped.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 57. Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol 25: 341–348, 2005. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- 58. Stanford AH, Gong H, Noonan M, Lewis AN, Gong Q, Lanik WE, Hsieh JJ, Lueschow SR, Frey MR, Good M, McElroy SJ. A direct comparison of mouse and human intestinal development using epithelial gene expression patterns. Pediatr Res 88: 66–76, 2020. doi: 10.1038/s41390-019-0472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]