SUMMARY
Background
SARS-CoV-2 pandemic affected tuberculosis (TB) management. This Italian nationwide survey assessed COVID-19 impact on TB care and outcomes.
Materials and methods
Twenty-one hospitals or referral centres fulfilled an online survey. Primary objective was to describe clinical features, outcomes and retention in care in subjects with latent TB infection (LTBI) or disease over the first wave of COVID-19 pandemic. Secondary objectives were the assessment of risk factors, co-morbidities, diagnostics, radiological findings, and outcomes of COVID-19 in the study population.
Results
254 patients with LTBI or active TB were included. In co-infected (SARS-CoV-2, LTBI/TB) patients, recovery occurred in 29/32 (90.6%) cases, death in one case. High retention in care was preserved.
Conclusion
in our cohort, outcomes did not seem to be adversely conditioned by incident COVID-19.
Keywords: Tuberculosis, TB, LTBI, SARS-CoV-2, COVID-19, Follow-up, Pandemic
INTRODUCTION
Healthcare systems have been significantly affected by the pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1, 2].
Worldwide, over 527 million cases have been reported and more than 6 million deaths have been attributed to coronavirus disease 2019 (COVID-19) until 1 June 2022 [3, 4].
The high incidence of COVID-19 has changed the natural history and management of other diseases, including tuberculosis (TB) [4, 5]. An overall reduction of TB diagnoses and increased difficulties in its management have been reported [5–7]. The World Health Organization (WHO) predicted that limited access to health services and TB diagnostic tests could have caused a 25% decrease in TB detection, and a 13% increase in mortality [5]. Large epidemiological studies found a significant decrease in new TB diagnoses in outpatient services and in new drug-resistant (DR)-TB, and time series forecasting came out in favor of longer-term effects of COVID-19 for the TB epidemics [6, 8].
Only limited data are available on the impact of COVID-19 in case of pre-existing TB disease or sequelae, as well as on retention-in-care, clinical outcomes, timely TB diagnosis and treatment, especially in developed countries [9, 10]. The analysis of the first cohorts of patients with active TB and COVID-19 by Tadolini et al. and Stochino et al. did not show significant clinical deterioration in TB course in patients with COVID-19 [11, 12]. In addition, complex interactions between HIV/AIDS, TB and COVID-19 have been described in terms of in vitro immune response and clinical outcomes [13–17].
An Italian observational study was performed to assess the impact of SARS-CoV-2 on TB care and outcomes.
PATIENTS AND METHODS
Study design
We conducted an observational retrospective study through an online survey from March 1st 2020 to September 30th 2020. The survey was sent to the Italian TB reference centres (Figure 1) and it was designed according to methodological recommendations for surveys by Pulcini et al. [18].
Figure 1.
Map of participating centres. Blue squares represent teaching (university) hospitals, green squares non-teaching hospitals, red squares Italian outpatient referral centres for TB treatment. (Details of the participating Centres are provided in the Acknowledgments).
Outcomes
The primary objective was to give a descriptive analysis of clinical features, outcomes, and retention in care of an Italian cohort of individuals with TB infection or disease during the first wave of COVID-19 pandemic (February to June 2020), to assess the impact of the pandemic on TB management [2]. Secondary objectives were:
to assess risk factors for poor COVID-19 outcomes and co-morbidities;
to assess the diagnostic method for TB or latent TB infection (LTBI) and imaging findings at the time of diagnosis;
to assess clinical and imaging signs and outcomes of SARS-CoV-2 infection.
Setting and participants
Twenty-one Italian teaching (university) and non-teaching hospitals and referral centres for TB diagnosis and treatment were invited (Figure 1). Only one physician from each centre fulfilled the survey to avoid duplication of data.
Survey design
Demographic and clinical data of patients with TB infection or disease during the first peak of SARS-CoV-2 epidemic were collected to assess the impact on TB care. All patients being newly diagnosed or being treated at the time the survey at the participating centres were included. The questionnaire contained 40 items, divided in 5 sections:
Demographic information (seven questions);
Risk factors for poor COVID-19 outcomes (two questions);
Co-morbidities (nine questions);
LTBI/TB diagnosis (thirteen questions);
COVID-19 diagnosis and outcomes (nine questions).
Ethical approval and informed consent
Ethical approval (n. 224_2020) was issued by the Ethics Committee of the Istituto Nazionale per le Malattie Infettive “Lazzaro Spallanzani” I.R.C.C.S., in agreement with the Declaration of Helsinki (1964) and its later amendments. All data collected remained anonymous and it was not possible to link any patients to the answers. Due to the observational nature of the study, general consent for use of data was used to include data in the statistical analysis. No financial benefits were provided to participants.
Statistical analysis
All data were collected in an ad hoc electronic form. Qualitative variables were described with absolute and relative frequencies, whereas quantitative variables were summarized with means (standard deviations, SD) or medians (interquartile ranges, IQR), depending on their normal or non-normal distribution, respectively. Percentages are calculated on the total number of answers given to each question.
RESULTS
Twenty-one Italian centres responded (response rate 100%). Ten (47.6%) were teaching hospitals, seven (33.3%) non-teaching hospitals, and four (19.0%) were referral centres for TB diagnosis and treatment.
A total of 254 patients (159 male, 92 female, 3 data not available) with TB infection or disease were enrolled in the period March - September 2020 (Table 1). 52% were Caucasian, 19.0% Asiatic, 17.3% African, 11.3% Latin-American. Median (IQR) age was 37 (25–59) years. 169 (66.5%) were ambulatory patients, whereas the others were admitted to the hospital, 80 of whom (31.5%) in non-critical wards. Median (IQR) age was 37 (25–59) years. All patients were Italian residents, with a median (IQR) stay of 5 (2–15) years. 211/249 (84.7%) lived at home, 29/249 (11.7%) in shelters or dormitories, whereas 9/249 (3.6%) were homeless. Most (68.1%) patients shared their living solution with one to five people, 5.5% with six to ten people, 8.7% with more than ten people. Bacillus Calmette-Guérin (BCG) vaccination had been administered to 16/76 (21.1%) patients. Active alcohol abuse was reported in 44/206 (21.4%) patients. 40/212 (18.9%) and 28/212 (13.2%) patients were active and former smokers, respectively (Table 2). The most frequently reported co-morbidities were arterial hypertension (33/155, 21.3%), diabetes (23/155, 14.8%), tumors (16/154, 10.4%), and cardio-vascular disorders (15/146, 10.3%) (Figure 2). Ten patients were underweight (Body Mass Index, BMI ≤17 kg/m2). Seven patients suffered from psychiatric disorders. All patients were receiving medications according to their co-morbidities.
Table 1.
Demographic information of the study population.
| Admission, N (%) | Outpatients | 169/254 (66.5) |
| Non-critical ward | 80/254 (31.5) | |
| Critical ward | 5/254 (2.0) | |
| Gender, N (%) | Male | 159/254 (62.6) |
| Female | 92/254 (36.2) | |
| Not specified | 3/254 (1.2) | |
| Race/ethnic group, N (%) | Caucasian | 127/248 (51.2) |
| Asiatic | 47/248 (19.0) | |
| African | 43/248 (17.3) | |
| Latin-American | 28/248 (11.3) | |
| Arabic | 2/248 (0.8) | |
| Others | 1/248 (0.4) | |
| Age, median (IQR) | 37 (25–59) | |
| Years in Italy, median (IQR) | 5 (2–15) | |
| Household setting, N (%) | Home | 211/249 (84.7) |
| Community/shelters/ dormitory | 29/249 (11.7) | |
| Homeless | 9/249 (3.6) | |
| Household members, N (%) | 0 | 45/254 (17.7) |
| 1–5 | 173/254 (68.1) | |
| 6–10 | 17/254 (5.5) | |
| >10 | 22/254 (8.7) | |
| BCG vaccination, n (%) | 16/76 (21.1) | |
IQR: interquartile range; BCG: Bacillus Calmette-Guérin.
Table 2.
Risk factors and co-morbidities.
| Risk factors | ||
|---|---|---|
| Smoking, N (%) | Non-smoker | 144/212 (67.9) |
| Smoker | 40/212 (18.9) | |
| Former smoker | 28/212 (13.2) | |
| Alcoholism, n (%) | 44/206 (21.4) | |
| Comorbidities | ||
| Arterial hypertension, N (%) | 33/155 (21.3) | |
| Cardio-vascular disorders, N (%) | 15/146 (10.3) | |
| Obesity, N (%) | 8/150 (5.3) | |
| Diabetes, N (%) | 23/155 (14.8) | |
| HIV/AIDS, N (%) | 9/246 (3.7) | |
| COPD, N (%) | 7/155 (4.5) | |
| Asthma, N (%) | 8/147 (5.4) | |
| Tumour, N (%) | 16/154 (10.4) | |
| Kidney disorder, N (%) | 8/149 (5.4) | |
Notes: HIV: human immunodeficiency virus; AIDS: acquired immuno-deficiency syndrome; COPD: chronic obstructive pulmonary disease.
Figure 2.
Prevalence of co-morbidities in the study population that are commonly linked to poor outcomes in SARS-CoV-2 infection.
Notes: HIV: human immunodeficiency virus; AIDS: acquired immunodeficiency syndrome; COPD: chronic obstructive pulmonary disease.
Thirty-seven (14.6%) had LTBI, whereas 217 (85.4%) had TB active disease, with 130/229 (56.8%) pulmonary TB, 51/229 (22.3%) extrapulmonary TB, and 48/229 (21%) with both forms (Table 3). Multidrug-resistant TB (MDR-TB) was diagnosed in 18/194 (9.3%) patients. Treatment was prescribed to 15/37 (40.5%) with LTBI. Only one patient reported a previous LTBI treatment. One hundred and fifty-four patients underwent tuberculin skin testing (TST): of these, 50 (32.5%) were positive and showed a median (IQR) induration diameter of 15 (11–20) mm. Interferon gamma release assay (IGRA) was positive in 140/150 (93.3%) individuals. Nucleic acid amplification test (NAAT) was positive in 173 (92.5%) cases. Culture and smear positivity was found in 136/189 (72.0%) and 129/189 (68.3%) cases, respectively. Chest x-ray was negative in 146/253 (57.7%) patients. Nodules, consolidations, and cavities were described in 9/253 (3.6%), 81/253 (32.0%), and 17/253 (6.7%), respectively. The radiological signs were bilateral in 40/253 (15.8%) patients. CT scan was performed in 134 patients: consolidation was the most frequent finding (73/134, 54.5%), followed by cavity (31/134, 23.1%), and nodule (16/134, 11.9%). CT findings were bilateral in 51/125 (40.8%) patients.
Table 3.
LTBI/TB diagnosis.
| LTBI/TB diagnosis | ||
|---|---|---|
| TB disease and infection, n (%) | TB infection | 37/254 (14.6) |
| TB disease | 217/254 (85.4) | |
| TB form, N (%) | Pulmonary TB | 119/216 (55.0) |
| Extra-pulmonary TB | 97/216 (44.9) | |
| Active TB, N (%) | Drug resistant TB | 18/194 (9.3) |
| Drug susceptible TB | 176/194 (90.7) | |
| TB infection, N (%) | Need to start PTP | 21/37 (56.8) |
| Ongoing PTP | 15/37 (40.5) | |
| Treated TB infection | 1/37 (2.7) | |
| TST positive, N (%) | 50/154 (32.5) | |
| TST (mm), median (IQR) | 15 (11–20) | |
| IGRA positive, N (%) | 140/150 (93.3) | |
| NAAT positive, N (%) | 173/187 (92.5) | |
| Culture positive, N (%) | 136/189 (72.0) | |
| Smear positive, N (%) | 129/189 (68.3) | |
| Chest x-ray, N (%) | Negative | 146/253 (57.7) |
| Nodule | 9/253 (3.6) | |
| Consolidation | 81/253 (32.0) | |
| Cavity | 17/253 (6.7) | |
| Chest x-ray, N (%) | Negative | 146/253 (57.7) |
| Unilateral | 67/253 (26.5) | |
| Bilateral | 40/253 (15.8) | |
| CT findings, N (%) | Negative | 9/134 (6.7) |
| Bilateral interstitial disease | 11/134 (8.2) | |
| Nodule | 16/134 (11.9) | |
| Consolidation | 73/134 (54.5) | |
| Cavity | 31/134 (23.1) | |
| CT, n (%) | Unilateral | 74/125 (59.2) |
| Bilateral | 51/125 (40.8) | |
Notes: COPD: chronic obstructive pulmonary disease; TB: tuberculosis; LTBI: latent tuberculosis infection; PTB: pulmonary tuberculosis; TST: tuberculin skin test; IGRA: interferon gamma release assay; NAAT: nucleic acid amplification test; CT: computed tomography.
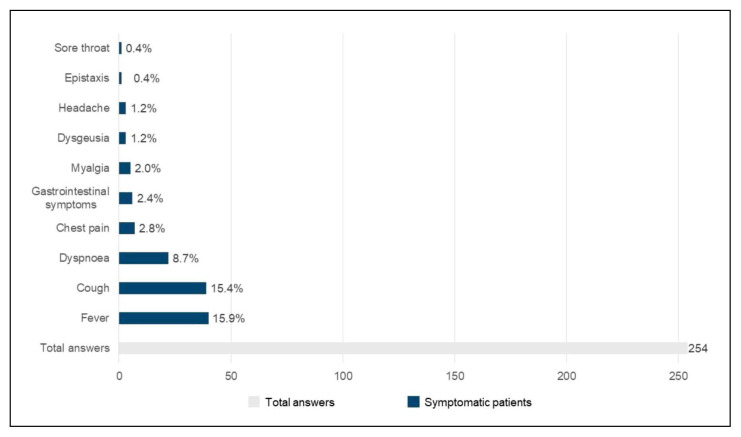
Overall, most patients were asymptomatic. The most frequent symptoms were fever (40, 15.9%), followed by cough (39, 15.4%), dyspnea (22, 8.7%), and chest pain (7, 2.8%) (Figure 3).
Figure 3.
Prevalence of most common symptoms in SARSCoV- 2 infection in the study population.
Results on TB/COVID-19 co-infected subjects
Twenty out of 134 (14.9%) patients reported on a SARS-CoV-2 positive contact (Table 4).
Table 4.
COVID-19 diagnosis and outcomes.
| COVID-19 diagnosis and outcomes | ||
|---|---|---|
| Contact with a positive case, N (%) | 20/134 (14.9) | |
| Dysgeusia, N (%) | 3/254 (1.2) | |
| Headache, N (%) | 3/254 (1.2) | |
| Epistaxis, N (%) | 1/254 (0.4) | |
| Sore throat, N (%) | 1/254 (0.4) | |
| Cough, N (%) | 39/254 (15.4) | |
| Dyspnoea, N (%) | 22/254 (8.7) | |
| Fever, N (%) | 40/254 (15.9) | |
| Myalgia, N (%) | 5/254 (2.0) | |
| Chest pain, N (%) | 7/254 (2.8) | |
| Gastrointestinal symptoms, N (%) | 6/254 (2.4) | |
| Positive nasopharyngeal swab, N (%) | 32/110 (29.1) | |
| Presence of antibodies against SARS-CoV-2, N (%) | 10/32 (31.3) | |
| COVID-19-related radiological findings, N (%) | 17/252 (6.8) | |
| COVID-19-related CT findings, N (%) | 9/14 (64.3) | |
| COVID-19 therapy, N (%) | Nothing | 2/32 (6.3) |
| Chloroquine/ hydroxychloroquine | 24/32 (75.0) | |
| Remdesivir | 2/32 (6.3) | |
| Steroids | 1/32 (3.1) | |
| Tocilizumab | 1/32 (3.1) | |
| Not specified | 2/32 (6.3) | |
| COVID-19 treatment duration (days), median (SD) | 8.8 (5.4) | |
| COVID-19 outcomes, N (%) | Death | 1/32 (3.1) |
| Recovery | 29/32 (90.6) | |
| Unknown | 2/32 (6.3) | |
| Complications related to the co-infection SARS-CoV-2 and M. tuberculosis, N (%) | 3/32 (9.4) | |
Notes: SD: standard deviation; CT: computed tomography.
A positive nasopharyngeal swab was reported in 32 out of 110 tested subjects (29.1%).
COVID-19-related radiological findings were described in 17/252 (6.8%) patients: interstitial lung disease, parenchymal hyperintensities, and ground-glass opacities. Fourteen patients underwent CT, which was positive for 9/14 (64.3%) patients (ground-glass opacities, pleural effusion, interstitial pneumonia). Patients were treated with chloroquine/hydroxychloroquine (24/32, 75.0%), remdesivir (2/32, 6.3%), systemic steroids (1/32, 3.1%), and tocilizumab (1/32, 3.1%). The mean (SD) treatment duration was 8.8 (5.4) days. Clinical recovery was recorded in 29/32 (90.6%) patients, whereas 1/32 (3.1%) died. Retention in care was preserved in 30 out of 32 cases of co-infection (94%). Complications (not specified in the survey) related to the co-infection of SARS-CoV-2 and Mycobacterium tuberculosis were reported in 3/32 (9.4%) patients.
The outcome of patients with TB infection and without COVID-19 is not reported, since the questionnaire contained items only on COVID-19 outcomes.
DISCUSSION
Our study describes a cohort of patients with TB/LTBI during the first COVID-19 pandemic wave in Italy, with epidemiological, clinical and radiological description. In particular, among patients co-infected with M. tuberculosis and SARS-CoV-2, 90.6% recovered and one death occurred, with high retention in care in co-infected patients. Other study groups tried to estimate the impact of SARS-CoV-2 spread on TB management, incidence and mortality. Migliori et al. reported a comparison between the first four months of 2019 and 2020 on TB services on a global scale, which considered 43 specialized centres placed in 19 countries and five continents [6]. Overall, they observed a sizable decrease in TB disease diagnoses in the first part of 2020 compared to 2019, as well as in outpatient new TB disease diagnoses, new TB infections, and drug-resistant TB cases. Few countries, such as Australia, showed opposite trends, possibly due to their negligible number of COVID-19 cases at the start of pandemic thanks to closed borders and strict lockdown, as well as to high TB surveillance [6]. Furthermore, countries with similar TB burden experienced different levels of Health System turmoil according to response approaches to pandemic [7].
A first report including Italy was made by the Global Tuberculosis Network (GTN), with 49 cases of TB and COVID-19 collected from March to April 2020 in eight countries across three continents [11]. A 12.3% case fatality rate was measured, with five of six deaths occurring in people over 60 years old and with at least one co-morbidity. Interestingly, in nine cases TB diagnosis was carried out by reason of admission for COVID-19 suspects. In 14 cases, TB diagnosis followed the COVID-19 one, a fact that stimulated debate on the potential role of SARS-CoV-2 both in unmasking hidden tubercular diseases and in boosting the development of symptomatic TB [19, 20]. A recent large global cohort study realized by the TB/COVID-19 Global Study Group measured a 11% co-infection mortality rate, higher in Europe (14.2%) than outside Europe (9.2%) and associated with older age, male gender, co-morbidities and need for invasive ventilation [10]. This study underlined some indirect evidence against a major contribution of COVID-19 in advancing TB infection to pulmonary disease, in particular the insufficient time between COVID-19 onset and the identification of tuberculous cavities.
Motta et al. focused on patients who died with TB and COVID-19 in a cohort of 69 subjects from eight countries (Italy included) with a 62% prevalence of migrants [20]. They reported a 11.6% case fatality rate, with higher mortality in elderly patients with co-morbidities, thus less frequent among migrants due to younger age. Among the youngest subjects, mortality was related to advanced clinical manifestations and drug-resistant TB.
Our cohort of 254 cases of TB, diagnosed as latent infection (14.6%) or disease (85.4%) over the seven-month period March-September 2020, was characterized by low median age (37 years, IQR 25–59), high prevalence of TB-associated risk factors (i.e., active smoking and alcoholism recorded in 18.9% and 21.4% of 212 and 206 patients, respectively), low rate of severe or complicated TB presentation (249/254 patients managed on clinic or non-critical ward). Within those patients with a median age of 37 years, the more frequent co-morbidities were arterial hypertension (33/155, 21.3%), diabetes (23/155, 14.8%) and tumors (16/154, 10.4%).
Out of 110 patients who underwent nasopharyngeal swab, 32 (29.1%) resulted positive. In most cases, chest x-ray TB lesions did not worsen and only a minority of patients (6.8%) presented new COVID-19 radiological signs of pneumonia. Only 14 subjects with COVID-19 underwent CT scan, 9 of whom presented typical COVID-19 findings. Treatment was known for 30 of 32 diagnoses: 24 cases (80%) were treated with chloroquine or hydroxychloroquine, only few cases (2, 1 and 1) with remdesivir, steroids and tocilizumab, respectively, reflecting the sequence of therapies experimented in the period of observation. After a nine-day median length of treatment, recovery occurred in 29 out of 32 cases, death in one case, retention in care in 30 out of 32 cases (94%). Complications were pointed out in three cases without further details reported in the survey.
In this context, Stochino et al reported the first Italian outbreak of COVID-19 among twenty TB in-patients, characterized by an overall benign clinical course (5% case fatality rate) and described as manageable with meticulous practices of infection control [12].
With the limits related to an observational study, our results suggest a moderate and manageable impact of COVID-19 infection on retention in care, outcomes, and sequelae of people affected by LTBI/TB in Italian TB reference centres [22].
We argue that local low TB burden, together with the chance to manage TB and COVID-19 co-infection inside reference centres for TB, might have played a protective role as compared with the cited experiences in high TB burden countries [23, 24]. Nevertheless, we strongly agree with the recommendation of the Indian Ministry of Health and Family Welfare to perform bi-directional TB-COVID screening for any case of influenza- like illness and severe acute respiratory infection, based upon a 0.37–4.47% prevalence of TB among COVID-19 patients and a reported 26% reduction in local TB notification from January to June 2020 as compared to previous years [23].
Many efforts are still needed to understand the intricacy of TB and COVID-19 co-infection, which even at this moment is conditioning every step of TB control [7, 25]. The effects of treatment interruptions and increased vulnerability to TB are still hidden and will become tangible only in the next years, that is why a relentless commitment both in COVID-19 and TB fight is fundamental.
Limitations of the study
The main limitations of our study are represented by the observational and retrospective design of the research, and by the unavailability of historical data on TB/LTBI incidence and features in the same period of 2019. The survey structure was intentionally simple, to allow a time-sparing and uniform data collection. Nevertheless, the interpretation of results was limited due to some missing details. A selection bias should be considered before generalizing these data, as non-referral centres for TB were not included in the present survey. An addition, a recall bias cannot be excluded as the survey was performed in the period March - September 2020.
CONCLUSIONS
LTBI/TB outcomes were not affected by COVID- 19 in reference centres for TB in Italy during the first pandemic wave, with preserved high retention in care despite the state of emergency. Larger prospective studies with longer follow-up are needed to get a better understanding of the interactions between LTBI/TB and COVID-19.
Acknowledgements
StopTB Italia research group: Luigi Ruffo Codecasa, Riccardo Alagna, Daniela Cirillo, Ilaria Motta, Mariantonietta Purgatorio, Tullio Prestileo, Lorenzo Antonio Surace, Giovanni Cenderello, Graziamaria Vicentini, Danilo Buonsenso, Maurizio Ferrarese, Paola Castellotti, Mirko Compagno, Elisa Vanino, Jessica Mencarini, Alberto Matteelli, Marina Tadolini, Alessandro Sanduzzi, and Giorgio Besozzi.
Participating Centres: Centro di Riferimento per la Tubercolosi, Regione Lombardia, ASST Niguarda, Milano (Coordinator centre); III Divisione Malattie Infettive, ASST Fatebenefratelli Sacco, Milano; Malattie Infettive, I.R.C.C.S. Ospedale S. Raffaele, Milano; U.O. Tisiologia, Ospedale di Sondalo, ASST Valtellina, Sondalo; U.O. Malattie Infettive, Dipartimento Scienze Cliniche e Sperimentali, Università di Brescia; U.O. Malattie Infettive, Ospedale di Trento; I.R.C.C.S. Ospedale Sacro Cuore Don Calabria, Negrar di Valpolicella, Verona; U.O. Malattie Infettive, Ospedale Santa Maria delle Croci di Ravenna, AUSL Romagna, Ravenna; U.O. Malattie Infettive, Policlinico Sant’Orsola Malpighi, Bologna; U.O. Malattie Infettive, ASL1, Ospedale di Sanremo, Sanremo, Imola; SOD Malattie Infettive e Tropicali, A.O.U. Careggi, Firenze; U.O.C. Malattie Infettive, Università degli Studi di Roma Tor Vergata, Roma; U.O.C. di Pediatria, Fondazione Policlinico Universitario “A. Gemelli”, Roma; Istituto Nazionale per le Malattie Infettive Lazzaro Spallanzani I.R.C.C.S.; U.O.C. Malattie Infettive ad indirizzo respiratorio, Ospedale Cotugno, AORN Ospedali dei Colli, Napoli; Cattedra di Pneumologia, Università Federico II, Napoli; U.O.C Malattie Infettive, Ospedale San Carlo, Potenza; O.O. Pneumologia, UTIR, Presidio Ospedaliero Madonna delle Grazie, Matera; Centro di Medicina del Viaggiatore e delle Migrazioni, ASP Catanzaro, Presidio Ospedaliero “Giovanni Paolo II”, Lamezia Terme, Catanzaro; ARNAS Ospedale Civico Benfratelli, UOC Malattie Infettive e Centro per la salute dei Migranti, Palermo; U.O. Malattie Infettive, Università degli Studi di Sassari.
Footnotes
Conflict of interest
The authors declare they have no conflicts of interest.
Ethical disclosure
The authors state that they have obtained ethical approval (n. 224_2020) by the Ethics Committee of the Istituto Nazionale per le Malattie Infettive “Lazzaro Spallanzani” I.R.C.C.S., in agreement with the Declaration of Helsinki (1964) and its later amendments. Informed consent has been obtained from the participants involved.
Author contribution
Conceptualization: G.S., N.R.; methodology: G.S., L.S.; software, N.R., M.G.; validation: All the Authors, StopTB Italia research group; formal analysis: G.S., L.S.; investigation: D.C., R.M.A., N.R.; data curation: N.R., R.M.A., G.S., L.S.; writing- original draft preparation: D.C., R.M.A, N.R.; writing, review and editing: D.C., R.M.A, N.R., L.S., P.R., M.B., M.S., D.G., G.S.; visualization: D.C., R.M.A.; supervision: D.G., N.R., G.S.; project administration: M.G.; funding acquisition: NONE. All authors have read and agreed to the published version of the manuscript.
Financial disclosure
This research received no external funding.
REFERENCES
- 1.Deledda G, Riccardi N, Gori S, et al. The Impact of the SARS-CoV-2 Outbreak on the psychological flexibility and behaviour of cancelling medical appointments of Italian patients with pre-existing medical condition: the “ImpACT-COVID-19 for patients” Multi-Centre Observational Study. Int J Environ Res Public Health. 2021;18:340. doi: 10.3390/ijerph18010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantica G, Riccardi N, Terrone C, Gratarola A. Non-COVID-19 admissions to the emergency department during the pandemic second wave in Italy: What has changed from the first wave? Am J Emerg Med. 2021;45:625–6. doi: 10.1016/j.ajem.2020.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roser M, Ritchie H, Ortiz-Ospina E, Hasell J. Published online at OurWorldInData.org. University of Oxford; 2020. Coronavirus pandemic (COVID-19) [Google Scholar]
- 4.Mantica G, Riccardi N, Terrone C, Gratarola A. Non-COVID-19 visits to emergency departments during the pandemic: the impact of fear. Public Health. 2021;186:17. doi: 10.1016/j.puhe.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Migliori GB, Thong PM, Akkerman O, et al. Worldwide effects of Coronavirus disease pandemic on tuberculosis services, January-April 2020. Emerg Infect Dis. 2020;26:2709–12. doi: 10.3201/eid2611.203163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Migliori GB, Thong PM, Alffenaar J-W, et al. Gauging the impact of the COVID-19 pandemic on tuberculosis services: a global study. Eur Respir J. 2021;58( 5):2101786. doi: 10.1183/13993003.01786-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McQuaid CF, Vassall A, Cohen T, Fiekert K, White RG. The impact of COVID-19 on TB: a review of the data. Int J Tuberc Lung Dis. 2021;25( 6):436–46. doi: 10.5588/ijtld.21.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding W, Li Y, Bai Y, Li Y, Wang L, Wang Y. Estimating the effects of the COVID-19 outbreak on the reductions in tuberculosis cases and the epidemiological trends in China: a causal impact analysis. Infect Drug Resist. 2021;14:4641–55. doi: 10.2147/IDR.S337473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glaziou P. Predicted impact of the COVID-19 pandemic on global tuberculosis deaths in 2020. MedRxiv. Available at: https://www.medrxiv.org/content/10.1101/2020.04.28.20079582v1.
- 10.TB/COVID-19 Global Study Group. Tuberculosis and COVID-19 co-infection: description of the global cohort. Eur Respir J. 2021;11:2102538. [Google Scholar]
- 11.Tadolini M, Codecasa LR, García-García J-M, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. 2020;56:2001398. doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stochino C, Villa S, Zucchi P, Parravicini P, Gori A, Raviglione MC. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur Respir J. 2020;56:2001708. doi: 10.1183/13993003.01708-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulle A, Davies M-A, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020;73:e2005–e2015. doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrone L, Petruccioli E, Vanini V, et al. Coinfection of tuberculosis and COVID-19 limits the ability to in vitro respond to SARS-CoV-2. Int J Infect Dis. 2021;113(Suppl 1):S82–S87. doi: 10.1016/j.ijid.2021.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musso M, Di Gennaro F, Gualano G, et al. Concurrent cavitary pulmonary tuberculosis and COVID-19 pneumonia with in vitro immune cell anergy. Infection. 2021;49( 5):1061–4. doi: 10.1007/s15010-021-01576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riou C, du Bruyn E, Stek C, et al. Relationship of SARS-CoV-2-specific CD4 response to COVID-19 severity and impact of HIV-1 and tuberculosis coinfection. J Clin Invest. 2021;131( 12):e149125. doi: 10.1172/JCI149125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canetti D, Riccardi N, Martini M, et al. HIV and tuberculosis: The paradox of dual illnesses and the challenges of their fighting in the history. Tuberculosis (Edinb) 2020;122:101921. doi: 10.1016/j.tube.2020.101921. [DOI] [PubMed] [Google Scholar]
- 18.Pulcini C, Leibovici L CMI Editorial Office. CMI guidance for authors of surveys. Clin Microbiol Infect. 2016;22:901–2. doi: 10.1016/j.cmi.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Khurana AK, Aggarwal D. The (in)significance of TB and COVID-19 co-infection. Eur Respir J. 2020;56:2002105. doi: 10.1183/13993003.02105-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tadolini M, García-García J-M, Blanc F-X, et al. On tuberculosis and COVID-19 co-infection. Eur Respir J. 2020;56:2002328. doi: 10.1183/13993003.02328-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motta I, Centis R, D’Ambrosio L, et al. Tuberculosis, COVID-19 and migrants: Preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. 2020;26:233–40. doi: 10.1016/j.pulmoe.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Y, Liu M, Chen Y, Shi S, Geng J, Tian J. Association between tuberculosis and COVID-19 severity and mortality: A rapid systematic review and meta-analysis. J Med Virol. 2021;93:194–6. doi: 10.1002/jmv.26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guidance note on Bi-directional TB-COVID screening and screening of TB among ILI/SARI cases. Available at: https://covid19.india.gov.in/document/guidance-note-on-bi-directional-tb-covid-screening/
- 24.European Centre for Disease Prevention and Control, World Health Organization Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2018–2016 data. 2018. Available at: https://apps.who.int/iris/handle/10665/342133.
- 25.Aznar ML, Espinosa-Pereiro J, Saborit N, et al. Impact of the COVID-19 pandemic on tuberculosis management in Spain. Int J Infect Dis. 2021;108:300–5. doi: 10.1016/j.ijid.2021.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]