Keywords: collagen, fibro/adipogenic progenitors, mechanical overload, satellite cells, skeletal muscle
Abstract
Multinuclear muscle fibers are the most voluminous cells in skeletal muscle and the primary drivers of growth in response to loading. Outside the muscle fiber, however, is a diversity of mononuclear cell types that reside in the extracellular matrix (ECM). These muscle-resident cells are exercise-responsive and produce the scaffolding for successful myofibrillar growth. Without proper remodeling and maintenance of this ECM scaffolding, the ability to mount an appropriate response to resistance training in adult muscles is severely hindered. Complex cellular choreography takes place in muscles following a loading stimulus. These interactions have been recently revealed by single-cell explorations into muscle adaptation with loading. The intricate ballet of ECM remodeling involves collagen production from fibrogenic cells and ECM modifying signals initiated by satellite cells, immune cells, and the muscle fibers themselves. The acellular collagen-rich ECM is also a mechanical signal-transducer and rich repository of growth factors that may directly influence muscle fiber hypertrophy once liberated. Collectively, high levels of collagen expression, deposition, and turnover characterize a well-trained muscle phenotype. The purpose of this review is to highlight the most recent evidence for how the ECM and its cellular components affect loading-induced muscle hypertrophy. We also address how the muscle fiber may directly take part in ECM remodeling, and whether ECM dynamics are rate limiting for muscle fiber growth.
INTRODUCTION
Skeletal muscle has a striking capacity to accommodate mechanical load, with the ability to double in size within a matter of weeks concomitant with improved functionality (1). Significant molecular and structural adaptations within muscle fibers define mechanical load-induced muscle hypertrophy (2), yet critical to this process is coordinated turnover and signaling from the acellular extracellular matrix (ECM). The ECM provides structure, stiffness, and extra-to-intracellular signal transduction that affect many functions central to muscle hypertrophy; these functions include force transmission, mechanotransduction, migration, growth, and differentiation (3).
The skeletal muscle ECM comprises a variety of macromolecules (4), of which collagens and proteoglycans are the most abundant. Hierarchical organization of the ECM can be defined into three layers; the epimysium, a fibrous and elastic sheet-like structure supporting the entire muscle; the perimysium, which subdivides the muscle into fascicles; and the endomysium, which adheres to and encloses each individual muscle fiber (5) (Fig. 1). The ECM is produced, assembled, and modified by several cell types within the skeletal muscle environment, including the muscle fiber itself. Recent reviews highlight the communication between the ECM and cardiomyocytes to maintain striated muscle homeostasis in the myocardium (6), as well as the coordination of skeletal muscle repair in response to injury through the ECM microenvironment (7). The bidirectional communication between muscle and connective tissue to support embryonic myogenesis has also been described (8). The goal of this review is to provide information on the remodeling of the ECM that occurs during loading-induced adult muscle hypertrophy. We specifically focus on how the ECM integrates signaling between various cell types within muscle to support adult tissue growth.
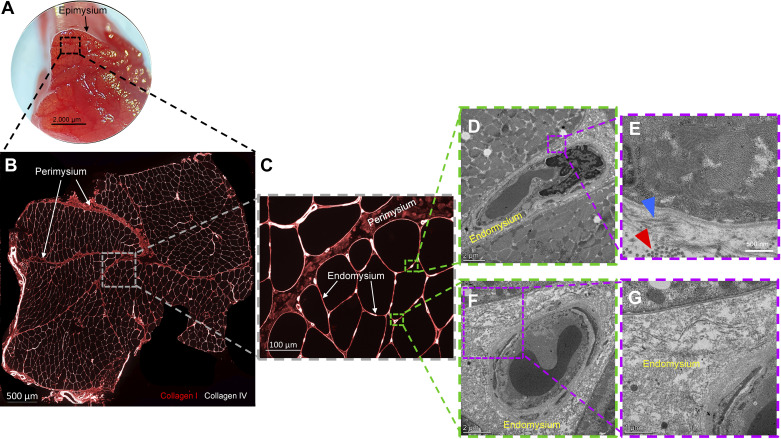
Figure 1.
Hierarchical organization of the extracellular matrix in human skeletal muscle. A: muscle biopsy from the vastus lateralis showing gross organization of fascicles and epimysium surrounding the muscle. B: cross section showing (×20 magnification) several fascicles and perimysium present. Immunohistochemical identification of collagen type I (red) and type IV (white) denote fibrillar and basement membrane collagens, respectively, in skeletal muscle. C: representative higher magnification immunohistochemical image denoting perimysium and endomysium. D–G: 1 mm3 portion of a human vastus lateralis biopsy was fixed in a paraformaldehyde/glutaraldehyde solution prior to observation on a transmission electron microscope. The endomysium is shown in greater detail with transverse (red arrowhead) and longitudinal (blue arrowhead) collagen fibers denoted.
CELLS THAT CONTRIBUTE TO ECM REMODELING DURING HYPERTROPHY
Advances in single cell and single nuclear-RNA sequencing have revealed significant heterogeneity of various cell types that contribute to ECM remodeling within skeletal muscle (9). ECM adaptation is facilitated through the biosynthesis of collagens, proteoglycans, and glycoproteins within the matrix as well as turnover of these macromolecules via enzymes such as matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs). Fibro/adipogenic progenitors (FAPs), fibroblasts, immune cells, satellite cells, and muscle fibers themselves (Fig. 2A) interact through a choreographed network to regulate ECM homeostasis and turnover to support hypertrophy in response to loading (Fig. 2B).
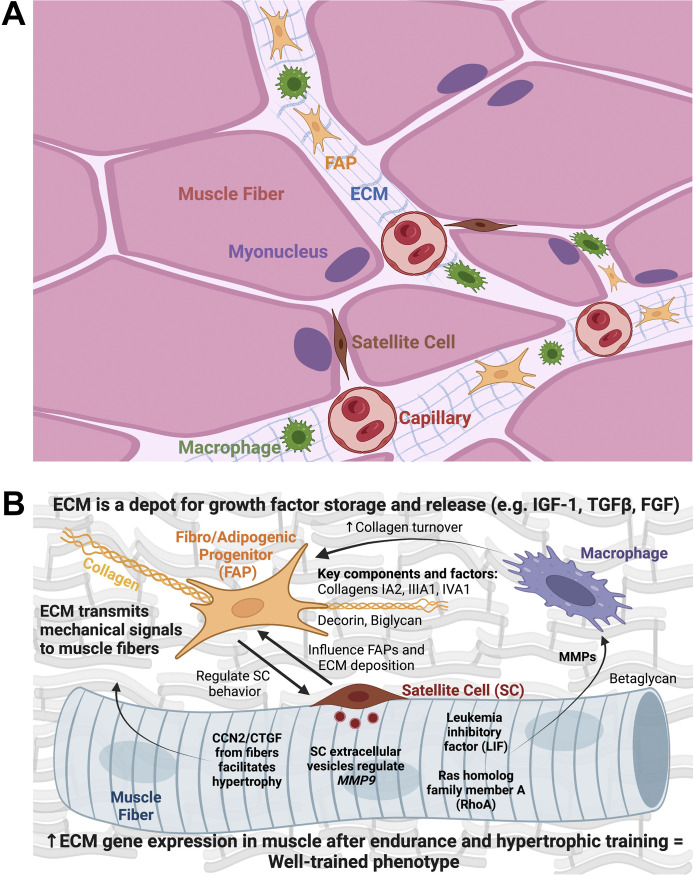
Figure 2.
Overview of how the extracellular matrix (ECM) and its cellular components influence muscle fiber adaptation to exercise and contribute to muscle growth. A: muscle fibers are surrounded by the ECM that contains several mononuclear cell types that influence the microenvironment. The ECM also relays mechanical signals that can trigger growth. B: in response to mechanical loading, the ECM and its cellular constituents produce and release growth factors that may affect intracellular muscle fiber hypertrophic processes. Fibro/adipogenic progenitors (FAPs) deposit collagen in the ECM and signal to satellite cells, thereby influencing their propensity to fuse and contribute myonuclei to the muscle fiber (10). Satellite cells also proliferate and communicate signals to fibrogenic cells (1, 11) and muscle fibers (12) via extracellular vesicles to control collagen deposition and remodeling during loading. The muscle fiber itself can release factors during loading that act on macrophages, specifically affecting the production of matrix metalloproteinases (MMPs) (13, 14). MMPs influence collagen turnover to facilitate a proper adaptive response. Globally, an upregulation in collagens and ECM turnover factors in skeletal muscle tissue is characteristic of a well-conditioned phenotype following various types of chronic exercise, including resistance training (15, 16). (Created with BioRender.com).
FAPs are interstitial mesenchymal progenitors that are believed to represent the primary cell of origin for the majority of ECM proteins (17). FAPs are generally characterized by PDGFRα+ expression and exist in dynamic cell states, facilitating their ability to adopt multiple lineages including fibrogenic, adipogenic, and osteogenic (18). A recent investigation identified six Pdgfrα+ nonmyogenic cell subtypes that can contribute to remodeling of the skeletal muscle microenvironment (19). Reciprocal interaction between FAPs and satellite cells facilitates adaptation to mechanical loading (Fig. 2B) (20). Satellite cell proliferation and myonuclear accrual during mechanical loading were shown to be dependent on FAP-derived thrombospondin-1 (20). In addition, genetic depletion of Pdgfrα+ FAPs attenuated plantaris hypertrophy following overload (20). Collagen and matricellular biosynthesis by FAPs is responsive to mechanical signals and inherent stiffness of the surrounding microenvironment via YAP/TAZ signaling (21). When cultured on substrates approximating fibrotic muscle stiffness, FAPs show increased myofibroblast activation with corresponding YAP nuclear translocation (22). Surprisingly, culturing FAPs on collagen substrates with greater crosslinking and larger fibril size inhibited myofibroblast activation, independent of YAP localization (22). These data would suggest an uncoupling between mechanical stiffness and collagen architecture in the regulation of FAP activation. Further evidence is gleaned from cardiac fibroblasts, where exposure to lower substrate modulus initiates downregulation of a myofibroblast phenotype and restoration of fibroblast quiescence (23). These findings underscore the mechanosensitivity of FAPs to their native environment as well as their critical role during muscle adaptation.
Within human skeletal muscle, single-cell transcriptome analysis has identified multiple FAP cell clusters that express the canonical PDGFRα marker as well as collagen types I, III, and VI (24, 25). Single-cell clustering in human and mouse skeletal muscle often does not reveal mature fibroblasts (25, 26), which suggests that mature fibroblasts and FAPs have similar gene expression, or the presence of mature fibroblasts within the clusters is limited. Immunohistochemical analysis also reveals congruency between canonical fibroblast markers [e.g., transcription factor 7-like 2 (TCF7L2)] and PDGFRα in human and murine muscle, further pointing to an overlap in fibrogenic cell identity in muscle (27, 28).
Skeletal muscle resident and infiltrating macrophages are a significant source of transforming growth factor-β (TGF-β), which is a major regulator of ECM deposition and remodeling. Macrophage interactions with FAPs can initiate, sustain, or resolve a profibrotic muscle environment (29). Both pro- and anti-inflammatory macrophage subpopulations increase in skeletal muscle during resistance exercise training (30), and macrophage recruitment is necessary to support hypertrophy during loading (31). Recent work has identified a novel mechanism whereby macrophages directly participate in ECM remodeling in response to a resistance loading stimulus (13). Single-cell analysis of mechanically overloaded murine muscle showed a striking elevation of Mmp14 in macrophages that may facilitate ECM remodeling through collagen type I degradation (13). The signal for Mmp14 regulation may come from muscle fibers via the release of leukemia inhibitory factor (LIF) upon muscle fiber contraction. Greater abundance of proinflammatory macrophages as well as less-dense collagen organization is correlated with poorer hypertrophic adaptation in older adults (32). These recent studies highlight new immune cell-mediated and acellular mechanisms underlying response heterogeneity during resistance training.
Satellite cells, the bona fide muscle stem cells, reside between the basal lamina and sarcolemma and interact closely with their anatomical niche to participate in muscle formation, maintenance, and repair (7). Work over the past several years has yielded insight into novel roles for satellite cells in modulating their surrounding ECM. The genetic depletion of satellite cells results in greater homeostatic ECM deposition across the lifespan in resting muscle (33). The absence of satellite cells during mechanical loading affects ECM adaptation via excessive collagen expression in fibrogenic cells/FAPs (1, 12, 34) and aberrant expression of ECM-remodeling genes (e.g., Timp4 and Mmp9) in endothelial cells (12) as well as muscle fibers (12). In addition, excessive collagen deposition occurs following mechanical overload and high-intensity interval training in muscle containing fusion-incompetent satellite cells (35, 36). Satellite cell-derived contributions to ECM remodeling occur quickly during the hypertrophic process (1, 36). The presence of satellite cells for the initial 96 h of mechanical overload is sufficient to support proper ECM remodeling and hypertrophy, underpinning the powerful secretory function of these cells in supporting adult muscle adaptation to loading (12). Satellite cells may also influence the fate of FAPs (fibrogenic vs. mineralizing) via secretory signaling during muscle loading (12).
In addition to interstitial cells, recent evidence supports an active role for muscle fibers in the remodeling of the ECM during hypertrophy. Satellite cell-derived extracellular vesicles repress expression of ECM-related genes, in particular Mmp9, in muscle cells (12). MMP9 is pleiotropic in muscle, likely exerting direct effects on the ECM via proteolytic cleavage of collagen IV and laminin. MMP9 may also influence angiogenesis (37) to affect ECM turnover and muscle hypertrophy. Mmp9 overexpression results in hypertrophic fibers (38) while also attenuating satellite cell-mediated fiber repair (39). Satellite cell-derived communication with muscle fibers may therefore fine-tune MMP9 to maintain ECM integrity and facilitate mechanical overload-induced hypertrophy (12). RhoA within muscle fibers also directly influences Mmp expression, macrophage recruitment, and ECM remodeling during loading (14). Recent work leveraging gain/loss of function of cellular communication network factor 2 (Ccn2)/connective tissue growth factor (Ctgf) specifically within muscle fibers further highlights fiber-intrinsic regulation of the ECM during overload. Muscle fiber-specific overexpression of Ccn2 resulted in greater collagen deposition but preserved fiber hypertrophy following muscle overload (40). Conversely, muscle fiber-specific depletion of Ccn2 attenuated hypertrophic capacity while supporting expansion of the ECM similar to wild-type mice (40). Depletion of Ccn2 disrupted mechanotransduction and protein synthetic capacity through focal adhesion kinase to blunt cellular growth during overload (40). Taken together, these recent studies support the notion that muscle fiber-derived growth factors and enzymes are essential to support healthy ECM remodeling and propagate hypertrophic adaptation to mechanical loading (Fig. 2B).
GROWTH FACTORS IN THE ECM THAT FACILITATE MUSCLE HYPERTROPHY
Autocrine and paracrine skeletal muscle insulin-like growth factor (IGF), TGF-β, and fibroblast growth factor (FGF) signaling orchestrate key cellular processes such as muscle protein synthesis, protein degradation, and satellite cell activity (41–43). Resident and infiltrating macrophages are a critical source of these growth factors to support muscle adaptation and ECM remodeling (29). Cross talk between muscle fibers, macrophages, and the ECM modulates these and other growth factor signaling cascades to regulate loading-induced muscle fiber hypertrophy. Although the above list of growth factors is not exhaustive, the available literature regarding mechanistic action of the acellular ECM on these signaling cascades provides a conceptual framework for ECM contributions to hypertrophy during adaptation to exercise.
Insulin-like growth factor-1 is a positive regulator of muscle hypertrophy during growth, regeneration, and exercise adaptation (44). IGF-1 signaling stimulates protein synthesis, inhibits protein degradation, and increases satellite cell proliferation and differentiation (45). In the ECM, IGF-1 can be sequestered by binding to components such as heparin and decorin (46). These ECM molecules neutralize specific IGF-1 isoforms, providing a latent IGF-1 pool that allows for ECM regulation of the IGF-1 signaling cascade. Decorin also modulates myoblast differentiation downstream of IGF-1 receptor signaling, suggesting it plays an important role in IGF-1 signal transduction in addition to passive sequestration (47). Decorin can then be degraded by exercise-responsive MMPs (e.g., MMP2) (48) to facilitate release and bioavailability of these growth factors.
In contrast to IGF1, the TGF-β family of cytokines is a negative regulator of hypertrophy, but is perhaps equally important for muscle development, regeneration, and adaptation to exercise (49). TGF-β signaling inhibits muscle protein synthesis and satellite cell proliferation and differentiation, and stimulates protein degradation (50). Similar to IGF1, TGF-β can be sequestered in the ECM by binding to proteoglycans such as decorin, biglycan, and betaglycan (51). Competent ECM regulation of TGF-β signaling leads to healthy muscle growth and development (50), whereas dysregulation of ECM competency and TGF-β signaling is best exemplified in muscular dystrophy and other muscle wasting diseases (52).
Although TGF-β and IGF-1 are well-defined effectors of muscle atrophy and hypertrophy signaling cascades within the muscle fiber, FGF signaling stimulates satellite cell proliferation and inhibits differentiation (53). The ECM regulates the local concentration of FGF within muscle. The proteoglycan glypican-1 is required for FGF2 signal transduction to stimulate satellite cell proliferation but also acts temporally to sequester FGF2, permitting differentiation (54). The syndecan family of transmembrane proteoglycans is proposed to play a similar role in FGF signal transduction (55). However, research in avian muscle suggests that syndecan-4 may act to promote proliferation and inhibit differentiation in an FGF2-independent manner (56), providing evidence for direct action of ECM components on hypertrophic processes.
ECM ADAPTATION AS A SIGN OF A WELL-TRAINED MUSCLE PHENOTYPE
Skeletal muscle plasticity with exercise is purportedly mediated via the accumulated effects of acute gene expression “pulses” that cause altered protein abundance and a subsequently well-adapted phenotype (57). Differences in resting gene expression caused by chronic exercise also contribute to a trained phenotype (57). Generally, consistently high levels of ECM-related gene expression (specifically collagens) are viewed as deleterious since it may contribute to fibrosis and decreased contractile function. However, in the context of exercise, pronounced ECM-related gene expression strongly characterizes highly conditioned muscle. It is intuitive that ECM-related gene expression is induced following acute resistance (58) as well as endurance exercise (59, 60) in the untrained state. Exercise can be injurious in the early phases of unaccustomed training and cause disruption to the ECM (61). As a consequence, acute resistance and endurance exercise bouts have broad but strikingly similar gene expression signatures that comprise a generalized stress response that includes ECM reorganization (62). Specifically, collagens IA2, IIIA1, and IVA1 are among the top five most upregulated genes in a meta-analysis profile of the skeletal muscle transcriptome in response to endurance and resistance exercise (62).
Over time, the ECM-related gene expression response to acute endurance (63) and resistance exercise (64) is altered by training status, but ECM organization is still a core node of regulation. Indeed, transcriptome alterations with acute exercise in chronic resistance trained skeletal muscle of humans remain enriched for ECM remodeling genes (64). Collagen expression, namely, COL IIIA1 and COL IVA1, represent two of the largest differentially expressed genes under chronic resistance training conditions, underscoring habitual ECM turnover that supports muscle adaptation (64). Once well-trained, levels of collagen and ECM remodeling genes (e.g., MMPs) are positively associated with the magnitude of adaptation in muscle (65). Similarly, expression and secretion of the matrix proteoglycan decorin increase dramatically following acute resistance exercise, and decorin expression positively correlates with hypertrophy and strength improvements following chronic resistance training (66). Circulating levels of N-terminal peptide of procollagen type III are also significantly correlated with improvements in lean body mass following resistance training in older adults (67). The extracellular matrix protein tenascin-C supports angiogenesis, and mutations in tenascin-C limit capillary remodeling during exercise adaptation (68). Collagen and turnover-related gene expression at rest are also augmented by chronic interval (69), resistance (15, 16), and hypertrophic concurrent training (59, 70) in humans. Since ECM remodeling is reportedly regulated at the mRNA level with exercise (71), increases in ECM-related gene expression with training are reflected at the protein level in muscle; this includes numerous collagens and proteoglycans (71, 72). Specifically regarding hypertrophy and strength, collagen accrual and reorganization are characteristic of (73) and essential for successful adaptation to extreme loading in rodents (74). Collectively, abundant collagen production and turnover accompany all modes of exercise adaptation, presumably to provide a robust scaffold for more functional muscle fibers (75).
IS ECM DEPOSITION RATE LIMITING OR PERMISSIVE FOR GROWTH?
To facilitate hypertrophic and functional adaptation in skeletal muscle in response to mechanical overload, ECM remodeling and fibrogenic cell proliferation occur rapidly and simultaneously with muscle fiber protein anabolism and growth (Fig. 3). Fibrillar collagens (types I and III) show the greatest upregulation in response to mechanical overload (1, 76) (Fig. 3), supporting the enhanced load-bearing capacity of the hypertrophying muscle. Likewise, Col XIIa1, localized at tissue junctions, shows marked upregulation to support and stabilize the musculotendinous junction during mechanical loading (78). Whether ECM remodeling and deposition are rate limiting or permissive for muscle fiber hypertrophy is less clear. There exist clear early temporal requirements for satellite cells to facilitate healthy ECM remodeling and muscle fiber hypertrophy (1, 12, 36). In the absence of satellite cell-secretory function, excess ECM accumulates and results in impaired hypertrophy in response to 8 wk of mechanical overload in rodents (1, 11, 12, 34). Likewise, when satellite cell fusion is inhibited, fibrotic ECM deposition occurs that is associated with blunted hypertrophy (35, 36). Conversely, muscle growth is attenuated following concurrent exercise training in the absence of satellite cells without a concomitant increase in ECM content (79). It is difficult to experimentally separate fusogenic and secretory functions of satellite cells to directly ascertain whether restoration of the myonuclear domain or proper ECM remodeling is a greater determinant of hypertrophic adaptation to mechanical load (12). Initial remodeling of the ECM in response to mechanical overload occurs independent from myonuclear accretion (11, 12), supportive of ECM turnover as a rate-limiting step during load-induced hypertrophy.
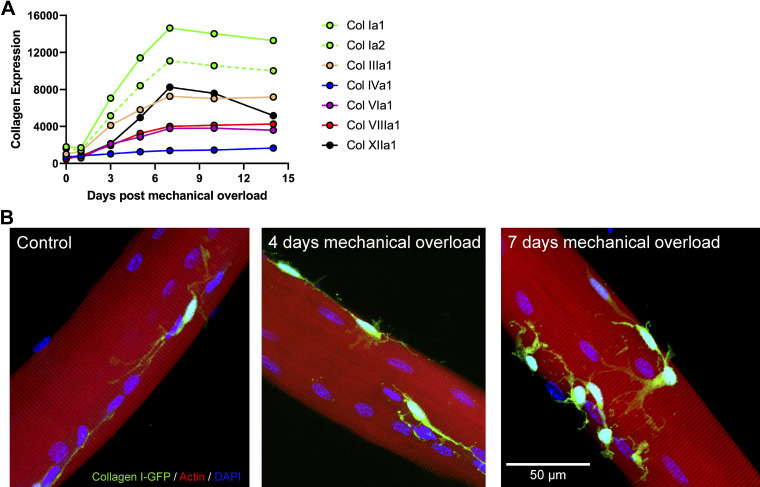
Figure 3.
Rapid increase in collagen expression and collagen I-expressing cell abundance during mechanical overload. A: quantification of time course microarray analysis of various collagen isoform transcripts following mechanical overload (GEO: GSE47098) (1, 76). Note the peak for the most abundant collagen transcripts (Col Ia1, Col Ia2, Col XIIa1, and Col IIIa1) at 7 days post-mechanical overload in a murine model. B: cells expressing collagen I, the most abundant collagen isoform in skeletal muscle, were identified using a mouse line expressing green fluorescent protein (GFP) under the control of the collagen-α1(I) promoter (77). Four-month-old mice were subjected to sham surgery (Control), or 4 or 7 days of mechanical overload via synergist ablation surgery. A portion of the plantaris was fixed prior to staining with phalloidin. Fibers and adherent collagen I-GFP+ cells were visualized at ×40 magnification using a Nikon A1R Inverted Confocal Microscope.
Recent work further underscores muscle-ECM cross talk as a critical effector of muscle growth. Perturbations in TGF-β receptor signaling and ECM remodeling are sufficient to induce muscle fiber hypertrophy in the absence of mechanical load (80). In muscle fiber-specific Tgfbr1 and Acvr1b knockout mice, elevated ECM content and related gene expression are associated with enhanced protein synthesis and depressed protein degradation to induce substantial hypertrophy (80). Importantly, this effect does not occur with single receptor knockout, indicating coordinated signaling through TGF-β, myostatin, and activin A to affect muscle size. Following cardiotoxin injury, muscle fiber-specific Tgfbr1 and Acvr1b knockout mice show augmented regeneration and ECM gene transcription, purported to be due to enhanced TGF-β signaling in fibrogenic cells following injury (80). Conversely, muscle fiber-specific Ccn2/Ctgf knockout mice demonstrated comparable muscle fiber hypertrophy following mechanical overload with disproportionately greater collagen deposition compared with wild-type controls (40). These results challenge the concept that greater ECM accumulation is a rate-limiting step during mechanical load-induced muscle growth. Limited inference on ECM quality can be gleaned from the semiquantitative histological assessment of collagen/ECM content in these studies (40, 80). It is likely that ECM quality affects muscle function and force transmission (81), which would in turn impact adaptation to mechanical load. Smaller collagen fibrils and a lower degree of collagen cross linking support greater satellite cell proliferation and differentiation, whereas collagen content alone does not have measurable effects on satellite cell dynamics (82). Future studies should ascertain the effects of collagen architecture and ECM quality on mechanical load adaptation.
Conclusions
Recent research has deepened our knowledge about cell-to-cell interactions that govern ECM remodeling to facilitate muscle hypertrophy. The ECM provides critical enzymatic, biochemical, and mechanical support to permit mechanical overload-induced hypertrophy. Our understanding of the cell-to-cell interactions that support ECM remodeling during hypertrophy continues to evolve, bolstered by advances in skeletal muscle single-cell analyses. Future additional studies exploring ECM quality and targeted manipulation of ECM components in vivo are necessary to refine loading-induced ECM adaptation to optimize muscle growth during hypertrophy.
GRANTS
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award No. R01 AR072061 (to C. S. Fry) and R00 AG063994 (to K. A. Murach).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
This article is part of the special collection “Musculoskeletal Biology and Bioengineering.” Frank Zaucke, PhD, Thomas Hawke, PhD, and Liliana Schaefer, MD, served as Guest Editors of this collection. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
K.A.M. and C.S.F. prepared figures; C.R.B., C.M.L., N.T.T., A.R.K., K.A.M., and C.S.F. drafted manuscript; C.R.B., C.M.L., N.T.T., A.R.K., K.A.M., and C.S.F. edited and revised manuscript; C.R.B., C.M.L., N.T.T., A.R.K., K.A.M., and C.S.F. approved final version of manuscript.
REFERENCES
- 1. Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA. Myogenic progenitor cells control extracellular matrix production by fibroblasts during skeletal muscle hypertrophy. Cell Stem Cell 20: 56–69, 2017. doi: 10.1016/j.stem.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jorgenson KW, Phillips SM, Hornberger TA. Identifying the structural adaptations that drive the mechanical load-induced growth of skeletal muscle: a scoping review. Cells 9: 1658, 2020. doi: 10.3390/cells9071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15: 802–812, 2014. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Csapo R, Gumpenberger M, Wessner B. Skeletal muscle extracellular matrix - what do we know about its composition, regulation, and physiological roles? A narrative review. Front Physiol 11: 253, 2020. doi: 10.3389/fphys.2020.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kjaer M, Magnusson P, Krogsgaard M, Moller JB, Olesen J, Heinemeier K, Hansen M, Haraldsson B, Koskinen S, Esmarck B, Langberg H. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat 208: 445–450, 2006. doi: 10.1111/j.1469-7580.2006.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bowers SLK, Meng Q, Molkentin JD. Fibroblasts orchestrate cellular crosstalk in the heart through the ECM. Nat Cardiovasc Res 1: 312–321, 2022. doi: 10.1038/s44161-022-00043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loreti M, Sacco A. The jam session between muscle stem cells and the extracellular matrix in the tissue microenvironment. NPJ Regen Med 7: 16, 2022. doi: 10.1038/s41536-022-00204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nassari S, Duprez D, Fournier-Thibault C. Non-myogenic contribution to muscle development and homeostasis: the role of connective tissues. Front Cell Dev Biol 5: 22, 2017. doi: 10.3389/fcell.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKellar DW, Walter LD, Song LT, Mantri M, Wang MFZ, De Vlaminck I, Cosgrove BD. Large-scale integration of single-cell transcriptomic data captures transitional progenitor states in mouse skeletal muscle regeneration. Commun Biol 4: 1280, 2021. doi: 10.1038/s42003-021-02810-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaneshige A, Kaji T, Zhang L, Saito H, Nakamura A, Kurosawa T, Ikemoto-Uezumi M, Tsujikawa K, Seno S, Hori M. Relayed signaling between mesenchymal progenitors and muscle stem cells ensures adaptive stem cell response to increased mechanical load. Cell Stem Cell 29: 265–280.e6, 2022. doi: 10.1016/j.stem.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 11. Murach KA, Peck BD, Policastro RA, Vechetti IJ, Van Pelt DW, Dungan CM, Denes LT, Fu X, Brightwell CR, Zentner GE, Dupont-Versteegden EE, Richards CI, Smith JJ, Fry CS, McCarthy JJ, Peterson CA. Early satellite cell communication creates a permissive environment for long-term muscle growth. Iscience 24: 102372, 2021. doi: 10.1016/j.isci.2021.102372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murach KA, Vechetti IJ Jr, Van Pelt DW, Crow SE, Dungan CM, Figueiredo VC, Kosmac K, Fu X, Richards CI, Fry CS, McCarthy JJ, Peterson CA. Fusion-independent satellite cell communication to muscle fibers during load-induced hypertrophy. Function (Oxf) 1: zqaa009, 2020. doi: 10.1093/function/zqaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peck BD, Murach KA, Walton RG, Simmons AJ, Long DE, Kosmac K, Dungan CM, Kern PA, Bamman MM, Peterson CA. A muscle cell-macrophage axis involving matrix metalloproteinase 14 facilitates extracellular matrix remodeling with mechanical loading. FASEB J 36: e22155, 2022. doi: 10.1096/fj.202100182RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noviello C, Kobon K, Delivry L, Guilbert T, Britto F, Julienne F, Maire P, Randrianarison-Huetz V, Sotiropoulos A. RhoA within myofibers controls satellite cell microenvironment to allow hypertrophic growth. iScience 25: 103616, 2022. doi: 10.1016/j.isci.2021.103616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guzzoni V, Ribeiro MB, Lopes GN, de Cássia Marqueti R, de Andrade RV, Selistre-de-Araujo HS, Durigan JL. Effect of resistance training on extracellular matrix adaptations in skeletal muscle of older rats. Front Physiol 9: 374, 2018. doi: 10.3389/fphys.2018.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gordon PM, Liu D, Sartor MA, IglayReger HB, Pistilli EE, Gutmann L, Nader GA, Hoffman EP. Resistance exercise training influences skeletal muscle immune activation: a microarray analysis. J Appl Physiol (1985) 112: 443–453, 2012. doi: 10.1152/japplphysiol.00860.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schüler SC, Kirkpatrick JM, Schmidt M, Santinha D, Koch P, Di Sanzo S, Cirri E, Hemberg M, Ori A, von Maltzahn J. Extensive remodeling of the extracellular matrix during aging contributes to age-dependent impairments of muscle stem cell functionality. Cell Rep 35: 109223, 2021. doi: 10.1016/j.celrep.2021.109223. [DOI] [PubMed] [Google Scholar]
- 18. Malecova B, Gatto S, Etxaniz U, Passafaro M, Cortez A, Nicoletti C, Giordani L, Torcinaro A, De Bardi M, Bicciato S, De Santa F, Madaro L, Puri PL. Dynamics of cellular states of fibro-adipogenic progenitors during myogenesis and muscular dystrophy. Nat Commun 9: 3670, 2018. doi: 10.1038/s41467-018-06068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leinroth AP, Mirando AJ, Rouse D, Kobayahsi Y, Tata PR, Rueckert HE, Liao Y, Long JT, Chakkalakal JV, Hilton MJ. Identification of distinct non-myogenic skeletal-muscle-resident mesenchymal cell populations. Cell Rep 39: 110785, 2022. doi: 10.1016/j.celrep.2022.110785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaneshige A, Kaji T, Zhang L, Saito H, Nakamura A, Kurosawa T, Ikemoto-Uezumi M, Tsujikawa K, Seno S, Hori M, Saito Y, Matozaki T, Maehara K, Ohkawa Y, Potente M, Watanabe S, Braun T, Uezumi A, Fukada S-I. Relayed signaling between mesenchymal progenitors and muscle stem cells ensures adaptive stem cell response to increased mechanical load. Cell Stem Cell 29: 265–280.e6, 2022. doi: 10.1016/j.stem.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 21. Stearns-Reider KM, D'Amore A, Beezhold K, Rothrauff B, Cavalli L, Wagner WR, Vorp DA, Tsamis A, Shinde S, Zhang C, Barchowsky A, Rando TA, Tuan RS, Ambrosio F. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 16: 518–528, 2017. doi: 10.1111/acel.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loomis T, Hu L-Y, Wohlgemuth RP, Chellakudam RR, Muralidharan PD, Smith LR. Matrix stiffness and architecture drive fibro-adipogenic progenitors’ activation into myofibroblasts. Sci Rep 12: 13582, 2022. doi: 10.1038/s41598-022-17852-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang H, Haeger SM, Kloxin AM, Leinwand LA, Anseth KS. Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PLoS One 7: e39969, 2012. doi: 10.1371/journal.pone.0039969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farup J, Just J, de Paoli F, Lin L, Jensen JB, Billeskov T, Roman IS, Cömert C, Møller AB, Madaro L, Groppa E, Fred RG, Kampmann U, Gormsen LC, Pedersen SB, Bross P, Stevnsner T, Eldrup N, Pers TH, Rossi FMV, Puri PL, Jessen N. Human skeletal muscle CD90 + fibro-adipogenic progenitors are associated with muscle degeneration in type 2 diabetic patients. Cell Metab 33: 2201–2214.e1, 2021. doi: 10.1016/j.cmet.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubenstein AB, Smith GR, Raue U, Begue G, Minchev K, Ruf-Zamojski F, Nair VD, Wang X, Zhou L, Zaslavsky E, Trappe TA, Trappe S, Sealfon SC. Single-cell transcriptional profiles in human skeletal muscle. Sci Rep 10: 229, 2020. doi: 10.1038/s41598-019-57110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muhl L, Genové G, Leptidis S, Liu J, He L, Mocci G, Sun Y, Gustafsson S, Buyandelger B, Chivukula IV, Segerstolpe Å, Raschperger E, Hansson EM, Björkegren JLM, Peng XR, Vanlandewijck M, Lendahl U, Betsholtz C. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat Commun 11: 3953, 2020. [Erratum in Nat Commun 11: 4493, 2020]. doi: 10.1038/s41467-020-17740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fry CS, Johnson DL, Ireland ML, Noehren B. ACL injury reduces satellite cell abundance and promotes fibrogenic cell expansion within skeletal muscle. J Orthop Res 35: 1876–1885, 2017. doi: 10.1002/jor.23502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138: 3625–3637, 2011. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21: 786–794, 2015. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 30. Walton RG, Dungan CM, Long DE, Tuggle SC, Kosmac K, Peck BD, Bush HM, Villasante Tezanos AG, McGwin G, Windham ST, Ovalle F, Bamman MM, Kern PA, Peterson CA. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: a randomized, double-blind, placebo-controlled, multicenter trial: the MASTERS trial. Aging Cell 18: e13039, 2019. doi: 10.1111/acel.13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DiPasquale DM, Cheng M, Billich W, Huang SA, van Rooijen N, Hornberger TA, Koh TJ. Urokinase-type plasminogen activator and macrophages are required for skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol 293: C1278–C1285, 2007. doi: 10.1152/ajpcell.00201.2007. [DOI] [PubMed] [Google Scholar]
- 32. Long DE, Peck BD, Lavin KM, Dungan CM, Kosmac K, Tuggle SC, Bamman MM, Kern PA, Peterson CA. Skeletal muscle properties show collagen organization and immune cell content are associated with resistance exercise response heterogeneity in older persons. J Appl Physiol (1985) 132: 1432–1447, 2022. doi: 10.1152/japplphysiol.00025.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med 21: 76–80, 2015. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu HL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28: 1654–1665, 2014. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goh Q, Millay DP. Requirement of myomaker-mediated stem cell fusion for skeletal muscle hypertrophy. eLife 6: e20007, 2017. doi: 10.7554/eLife.20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goh Q, Song T, Petrany MJ, Cramer AA, Sun C, Sadayappan S, Lee SJ, Millay DP. Myonuclear accretion is a determinant of exercise-induced remodeling in skeletal muscle. eLife 8: e44876, 2019. doi: 10.7554/eLife.44876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deryugina EI, Quigley JP. Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: contrasting, overlapping and compensatory functions. Biochim Biophys Acta 1803: 103–120, 2010. doi: 10.1016/j.bbamcr.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dahiya S, Bhatnagar S, Hindi SM, Jiang C, Paul PK, Kuang S, Kumar A. Elevated levels of active matrix metalloproteinase-9 cause hypertrophy in skeletal muscle of normal and dystrophin-deficient mdx mice. Hum Mol Genet 20: 4345–4359, 2011. doi: 10.1093/hmg/ddr362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chenette DM, Cadwallader AB, Antwine TL, Larkin LC, Wang J, Olwin BB, Schneider RJ. Targeted mRNA decay by RNA binding protein AUF1 regulates adult muscle stem cell fate, promoting skeletal muscle integrity. Cell Rep 16: 1379–1390, 2016. doi: 10.1016/j.celrep.2016.06.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Petrosino JM, Longenecker JZ, Angell CD, Hinger SA, Martens CR, Accornero F. CCN2 participates in overload-induced skeletal muscle hypertrophy. Matrix Biol 106: 1–11, 2022. doi: 10.1016/j.matbio.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-β1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol 164: 1007–1019, 2004. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Forcina L, Miano C, Scicchitano BM, Musarò A. Signals from the niche: insights into the role of IGF-1 and IL-6 in modulating skeletal muscle fibrosis. Cells 8: 232, 2019. doi: 10.3390/cells8030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Robertson TA, Maley MA, Grounds MD, Papadimitriou JM. The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res 207: 321–331, 1993. doi: 10.1006/excr.1993.1199. [DOI] [PubMed] [Google Scholar]
- 44. Rotwein P. Insulin-like growth factor action and skeletal muscle growth, an in vivo perspective. Growth Horm IGF Res 13: 303–305, 2003. doi: 10.1016/j.ghir.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 45. Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-β, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol 138: 311–315, 1989. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- 46. Iozzo RV, Buraschi S, Genua M, Xu SQ, Solomides CC, Peiper SC, Gomella LG, Owens RC, Morrione A. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J Biol Chem 286: 34712–34721, 2011. doi: 10.1074/jbc.M111.262766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suzuki K, Kishioka Y, Wakamatsu J, Nishimura T. Decorin activates Akt downstream of IGF-IR and promotes myoblast differentiation. Anim Sci J 84: 669–674, 2013. doi: 10.1111/asj.12055. [DOI] [PubMed] [Google Scholar]
- 48. Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y. Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-β1 release. Biochem J 322: 809–814, 1997. doi: 10.1042/bj3220809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gumucio JP, Sugg KB, Mendias CL. TGF-β superfamily signaling in muscle and tendon adaptation to resistance exercise. Exerc Sport Sci Rev 43: 93–99, 2015. doi: 10.1249/JES.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Massagué J, Cheifetz S, Endo T, Nadal-Ginard B. Type β transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci USA 83: 8206–8210, 1986. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Droguett R, Cabello-Verrugio C, Riquelme C, Brandan E. Extracellular proteoglycans modify TGF-β bio-availability attenuating its signaling during skeletal muscle differentiation. Matrix Biol 25: 332–341, 2006. doi: 10.1016/j.matbio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 52. Demonbreun AR, Fallon KS, Oosterbaan CC, Vaught LA, Reiser NL, Bogdanovic E, Velez MP, Salamone IM, Page PGT, Hadhazy M, Quattrocelli M, Barefield DY, Wood LD, Gonzalez JP, Morris C, McNally EM. Anti-latent TGFβ binding protein 4 antibody improves muscle function and reduces muscle fibrosis in muscular dystrophy. Sci Transl Med 13: eabf0376, 2021. doi: 10.1126/scitranslmed.abf0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pawlikowski B, Vogler TO, Gadek K, Olwin BB. Regulation of skeletal muscle stem cells by fibroblast growth factors. Dev Dyn 246: 359–367, 2017. doi: 10.1002/dvdy.24495. [DOI] [PubMed] [Google Scholar]
- 54. Velleman SG, Liu C, Coy CS, McFarland DC. Effects of glypican-1 on turkey skeletal muscle cell proliferation, differentiation and fibroblast growth factor 2 responsiveness. Dev Growth Differ 48: 271–276, 2006. doi: 10.1111/j.1440-169X.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 55. Pisconti A, Bernet JD, Olwin BB. Syndecans in skeletal muscle development, regeneration and homeostasis. Muscles Ligaments Tendons J 2: 1–9, 2012. [PMC free article] [PubMed] [Google Scholar]
- 56. Velleman SG, Coy CS, McFarland DC. Effect of syndecan-1, syndecan-4, and glypican-1 on turkey muscle satellite cell proliferation, differentiation, and responsiveness to fibroblast growth factor 2. Poult Sci 86: 1406–1413, 2007. doi: 10.1093/ps/86.7.1406. [DOI] [PubMed] [Google Scholar]
- 57. Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 58. Wessner B, Liebensteiner M, Nachbauer W, Csapo R. Age-specific response of skeletal muscle extracellular matrix to acute resistance exercise: a pilot study. Eur J Sport Sci 19: 354–364, 2019. doi: 10.1080/17461391.2018.1526974. [DOI] [PubMed] [Google Scholar]
- 59. Hjorth M, Norheim F, Meen AJ, Pourteymour S, Lee S, Holen T, Jensen J, Birkeland KI, Martinov VN, Langleite TM, Eckardt K, Drevon CA, Kolset SO. The effect of acute and long-term physical activity on extracellular matrix and serglycin in human skeletal muscle. Physiol Rep 3: e12473, 2015. doi: 10.14814/phy2.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Neubauer O, Sabapathy S, Ashton KJ, Desbrow B, Peake JM, Lazarus R, Wessner B, Cameron-Smith D, Wagner K-H, Haseler LJ, Bulmer AC. Time course-dependent changes in the transcriptome of human skeletal muscle during recovery from endurance exercise: from inflammation to adaptive remodeling. J Appl Physiol (1985) 116: 274–287, 2014. doi: 10.1152/japplphysiol.00909.2013. [DOI] [PubMed] [Google Scholar]
- 61. Damas F, Libardi CA, Ugrinowitsch C, Vechin FC, Lixandrão ME, Snijders T, Nederveen JP, Bacurau AV, Brum P, Tricoli V, Roschel H, Parise G, Phillips SM. Early-and later-phases satellite cell responses and myonuclear content with resistance training in young men. PLoS One 13: e0191039, 2018. doi: 10.1371/journal.pone.0191039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pillon NJ, Gabriel BM, Dollet L, Smith JAB, Sardón Puig L, Botella J, Bishop DJ, Krook A, Zierath JR. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat Commun 11: 470, 2020. doi: 10.1038/s41467-019-13869-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schmutz S, Däpp C, Wittwer M, Vogt M, Hoppeler H, Flück M. Endurance training modulates the muscular transcriptome response to acute exercise. Pflugers Arch 451: 678–687, 2006. doi: 10.1007/s00424-005-1497-0. [DOI] [PubMed] [Google Scholar]
- 64. Damas F, Ugrinowitsch C, Libardi CA, Jannig PR, Hector AJ, McGlory C, Lixandrão ME, Vechin FC, Montenegro H, Tricoli V, Roschel H, Phillips SM. Resistance training in young men induces muscle transcriptome-wide changes associated with muscle structure and metabolism refining the response to exercise-induced stress. Eur J Appl Physiol 118: 2607–2616, 2018. doi: 10.1007/s00421-018-3984-y. [DOI] [PubMed] [Google Scholar]
- 65. Timmons JA, Jansson E, Fischer H, Gustafsson T, Greenhaff PL, Ridden J, Rachman J, Sundberg CJ. Modulation of extracellular matrix genes reflects the magnitude of physiological adaptation to aerobic exercise training in humans. BMC Biol 3: 1–10, 2005. doi: 10.1186/1741-7007-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kanzleiter T, Rath M, Görgens SW, Jensen J, Tangen DS, Kolnes AJ, Kolnes KJ, Lee S, Eckel J, Schürmann A, Eckardt K. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun 450: 1089–1094, 2014. doi: 10.1016/j.bbrc.2014.06.123. [DOI] [PubMed] [Google Scholar]
- 67. Fragala MS, Jajtner AR, Beyer KS, Townsend JR, Emerson NS, Scanlon TC, Oliveira LP, Hoffman JR, Stout JR. Biomarkers of muscle quality: N-terminal propeptide of type III procollagen and C-terminal agrin fragment responses to resistance exercise training in older adults. J Cachexia Sarcopenia Muscle 5: 139–148, 2014. doi: 10.1007/s13539-013-0120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Valdivieso P, Toigo M, Hoppeler H, Flück M. T/T homozygosity of the tenascin-C gene polymorphism rs2104772 negatively influences exercise-induced angiogenesis. PLoS One 12: e0174864, 2017. doi: 10.1371/journal.pone.0174864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Miyamoto-Mikami E, Tsuji K, Horii N, Hasegawa N, Fujie S, Homma T, Uchida M, Hamaoka T, Kanehisa H, Tabata I, Iemitsu M. Gene expression profile of muscle adaptation to high-intensity intermittent exercise training in young men. Sci Rep 8: 16811, 2018. doi: 10.1038/s41598-018-35115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wen Y, Dungan CM, Mobley CB, Valentino T, von Walden F, Murach KA. Nucleus type-specific DNA methylomics reveals epigenetic “memory” of prior adaptation in skeletal muscle. Function (Oxf) 2: zqab038, 2021. doi: 10.1093/function/zqab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Makhnovskii PA, Zgoda VG, Bokov RO, Shagimardanova EI, Gazizova GR, Gusev OA, Lysenko EA, Kolpakov FA, Vinogradova OL, Popov DV. Regulation of proteins in human skeletal muscle: the role of transcription. Sci Rep 10: 3514, 2020. doi: 10.1038/s41598-020-60578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Deshmukh AS, Steenberg DE, Hostrup M, Birk JB, Larsen JK, Santos A, Kjøbsted R, Hingst JR, Schéele CC, Murgia M, Kiens B, Richter EA, Mann M, Wojtaszewski JFP. Deep muscle-proteomic analysis of freeze-dried human muscle biopsies reveals fiber type-specific adaptations to exercise training. Nat Commun 12: 600, 2021. doi: 10.1038/s41467-021-22015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mendias CL, Schwartz AJ, Grekin JA, Gumucio JP, Sugg KB. Changes in muscle fiber contractility and extracellular matrix production during skeletal muscle hypertrophy. J Appl Physiol (1985) 122: 571–579, 2017. doi: 10.1152/japplphysiol.00719.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stantzou A, Relizani K, Morales-Gonzalez S, Gallen C, Grassin A, Ferry A, Schuelke M, Amthor H. Extracellular matrix remodelling is associated with muscle force increase in overloaded mouse plantaris muscle. Neuropathol Appl Neurobiol 47: 218–235, 2021. doi: 10.1111/nan.12655. [DOI] [PubMed] [Google Scholar]
- 75. Kritikaki E, Asterling R, Ward L, Padget K, Barreiro E, Simoes DCM. Exercise training-induced extracellular matrix protein adaptation in locomotor muscles: a systematic review. Cells 10: 1022, 2021. doi: 10.3390/cells10051022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chaillou T, Lee JD, England JH, Esser KA, McCarthy JJ. Time course of gene expression during mouse skeletal muscle hypertrophy. J Appl Physiol (1985) 115: 1065–1074, 2013. doi: 10.1152/japplphysiol.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yata Y, Scanga A, Gillan A, Yang L, Reif S, Breindl M, Brenner DA, Rippe RA. DNase I-hypersensitive sites enhance α1(I) collagen gene expression in hepatic stellate cells. Hepatology 37: 267–276, 2003. doi: 10.1053/jhep.2003.50067. [DOI] [PubMed] [Google Scholar]
- 78. Koch M, Schulze J, Hansen U, Ashwodt T, Keene DR, Brunken WJ, Burgeson RE, Bruckner P, Bruckner-Tuderman L. A novel marker of tissue junctions, collagen XXII. J Biol Chem 279: 22514–22521, 2004. doi: 10.1074/jbc.M400536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Englund DA, Figueiredo VC, Dungan CM, Murach KA, Peck BD, Petrosino JM, Brightwell CR, Dupont AM, Neal AC, Fry CS, Accornero F, McCarthy JJ, Peterson CA. Satellite cell depletion disrupts transcriptional coordination and muscle adaptation to exercise. Function (Oxf) 2: zqaa033, 2021. doi: 10.1093/function/zqaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hillege MMG, Shi A, Galli RA, Wu G, Bertolino P, Hoogaars WMH, Jaspers RT. Lack of Tgfbr1 and Acvr1b synergistically stimulates myofibre hypertrophy and accelerates muscle regeneration. eLife 11: e77610, 2022. doi: 10.7554/eLife.77610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Brashear SE, Wohlgemuth RP, Gonzalez G, Smith LR. Passive stiffness of fibrotic skeletal muscle in mdx mice relates to collagen architecture. J Physiol 599: 943–962, 2021. doi: 10.1113/JP280656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hu LY, Mileti CJ, Loomis T, Brashear SE, Ahmad S, Chellakudam RR, Wohlgemuth RP, Gionet-Gonzales MA, Leach JK, Smith LR. Skeletal muscle progenitors are sensitive to collagen architectural features of fibril size and cross linking. Am J Physiol Cell Physiol 321: C330–C342, 2021. doi: 10.1152/ajpcell.00065.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]