Abstract
Introduction:
As breast cancer treatment options have multiplied and biologic diversity within breast cancer has been recognized, the use of the same treatment strategies for patients with early-stage and favorable disease, and for those with biologically aggressive disease, has been questioned. In addition, as patient-reported outcome measures have called attention to the morbidity of many common treatments, and as the cost of breast cancer care has continued to increase, reduction in the overtreatment of breast cancer has assumed increasing importance.
Areas covered:
Here we review selected aspects of surgery, radiation oncology, and medical oncology for which scientific evidence supports de-escalation for invasive carcinoma and ductal carcinoma in situ, and assess strategies to address overtreatment.
Expert opinion:
The problems of breast cancer overtreatment we face today are based on improved understanding of the biology of breast cancer and abandonment of the “one-size-fits-all” approach. As breast cancer care becomes increasingly complex, and as our knowledge base continues to increase exponentially, these problems will only be magnified in the future. To continue progress, the move must be made from advocating the maximum-tolerated treatment to advocating the minimum-effective one.
Keywords: breast cancer, overtreatment, radiotherapy, chemotherapy, ductal carcinoma in situ, breast-conserving therapy, axilla
1. Introduction
In recent years, the overdiagnosis and overtreatment of breast cancer have assumed increasing importance as treatment options have multiplied, as patient-reported outcome measures have called attention to the morbidity of many common treatments, and as the cost of breast cancer care has continued to increase.
In the setting of breast cancer, overdiagnosis refers to the identification on screening mammography of biologically indolent cancers, which, if left untreated, would not cause a problem during a patient’s lifetime [1], while overtreatment describes the use of therapies with minimal benefit to patients. Benefit has classically been defined as prolongation of survival; other endpoints are often important to patients, however, and patient-centered decision making often results in the use of treatments which do not improve survival, but which do improve local control or provide peace of mind. Although incorporation of patient preferences may make defining overtreatment challenging, many of the treatment paradigms for breast cancer were developed based upon the results of trials conducted 20-30 years ago. Until relatively recently, the treatment recommended for patients with favorable early-stage breast cancer was often the same as that recommended for those with locally advanced cancers. Improved understanding of breast cancer biology, the detection of smaller cancers due to the uptake of screening mammography, and recognition that systemic therapy decreases both local and distant recurrence, led to a new generation of trials focusing on treatment de-escalation, which have demonstrated equivalent oncologic outcomes for less surgery, more limited radiotherapy, and more tailored use of chemotherapy (Figure 1). In spite of the availability of high-quality evidence, many aspects of therapeutic de-escalation have been adopted very slowly. In this article, we will review selected aspects of surgery, radiation oncology, and medical oncology where scientific evidence supports de-escalation, and assess progress in minimizing overtreatment.
Figure 1.
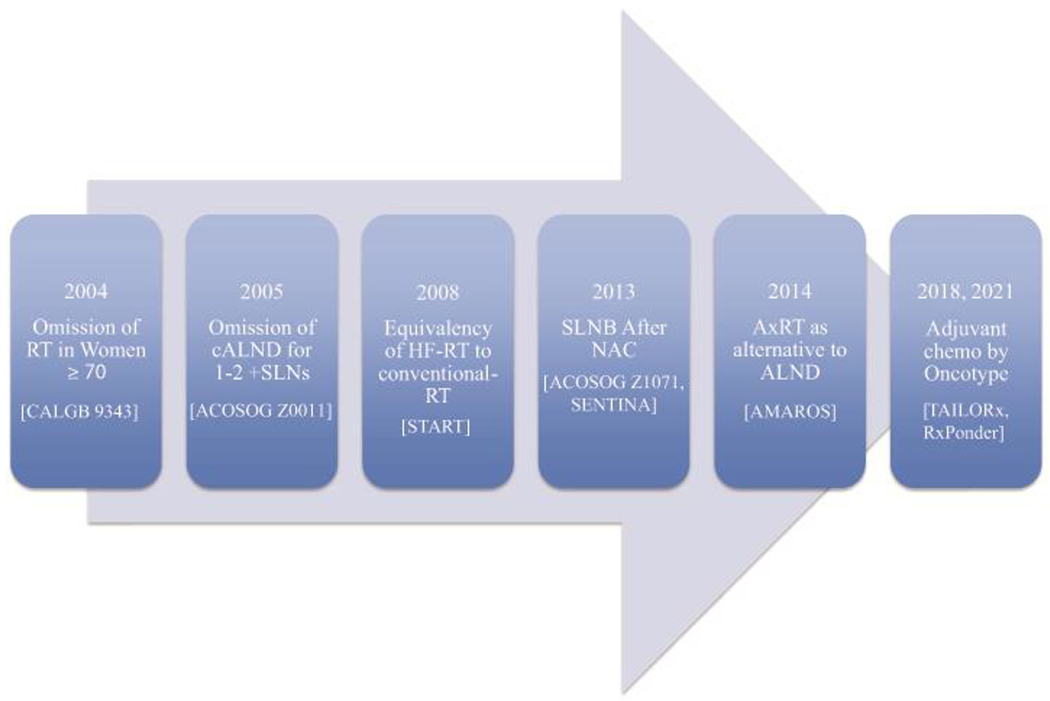
Practice-changing trials of de-escalation.
Abbreviations: RT, radiation therapy; cALND, completion axillary lymph node dissection; SLN, sentinel lymph node; HF-RT, hypofractionated radiation therapy; SLNB, sentinel lymph node biopsy; NAC, neoadjuvant chemotherapy; AxRT, axillary radiation therapy; ALND, axillary lymph node dissection; chemo, chemotherapy
2. Therapy of Invasive Cancer
2.1. Surgical management of the breast in invasive carcinoma
In the 1980s initial results were published from trials demonstrating the equivalency of breast-conserving therapy (BCT) to mastectomy [2,3]. Long-term results from 6 randomized trials in a meta-analysis from the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) confirmed the lack of a survival benefit for mastectomy [4], and BCT rates in the United States rose steadily from 1998-2005, but these then began to decline, accompanied by an increase in bilateral mastectomy (BM) for the treatment of unilateral cancer [5]. This trend, paradoxically, occurred at a time when improvements in breast imaging and the widespread use of adjuvant systemic therapy for early-stage breast cancers resulted in significantly lower rates of local recurrence after BCT than were seen in the initial randomized trials and which did not differ from rates of local recurrence after mastectomy for the majority of patients [6].
The increased use of mastectomy was initially attributed to surgeon preference, but studies subsequently showed that this was a patient-driven trend. Katz et al. surveyed a population-based sample of women with stage 0-2 breast cancer and found that among White women, if the patient reported that she was the primary surgical decision maker, the mastectomy rate was 27%, compared to 17% if the decision was shared, and 5% when the patient identified the surgeon as the primary decision maker (p<0.001)[7]. This association was not consistent across racial and ethnic groups, with Black women being more likely to undergo mastectomy when they identified the surgeon as the primary decision maker. Concern about recurrence was cited by patients as the most important determinant of treatment choice [7]. High rates of needing a second surgery, with large studies documenting re-excision in approximately 23% of patients when BCT was attempted, were also thought to contribute to patient preference for mastectomy. Approximately half of the re-excisions were performed for a negative margin of no ink on tumor, apparently in the belief that a more widely clear margin would reduce the chance of local recurrence [8], although the only microscopically defined margin width in the initial studies establishing the safety of BCT was no ink on tumor (National Surgical Adjuvant Breast and Bowel Project B-06 [3]). In a survey of a population-based sample of surgeons treating breast cancer, only 11% accepted this definition, while 19% endorsed a margin width of greater than 1 cm [9]. Preference for more widely clear margins was significantly associated with treatment of a lower volume of breast cancer cases, suggesting a need for provider education.
In response to high rates of re-excision and the declining use of BCT, in 2013 the Society of Surgical Oncology (SSO) and the American Society of Radiation Oncology (ASTRO) developed an evidence-based consensus statement on margins in invasive breast cancer treated with whole-breast irradiation (WBI), and endorsed no ink on tumor as the standard acceptable margin width for BCT [8]. This guideline was subsequently endorsed by the American Society of Clinical Oncology and the 2015 St. Gallen Consensus Conference, and incorporated into the National Comprehensive Cancer Network guidelines in 2016. A meta-analysis of 559,016 patients in 30 studies comparing rates of re-excision before and after publication of the margins consensus statement found a statistically significant reduction in the odds ratio (OR) for re-operation in single-institution studies (OR 0.62, 95% confidence interval [CI] 0.52-0.74) and in population-based studies (OR 0.76; 95% CI 0.72-0.80) in the period following the consensus [10]. In a population-based study, Morrow et al. demonstrated that the decreased rate of re-operation translated to a statistically significant increase in overall rates of BCT with a decline in both unilateral and bilateral mastectomy [11], indicating that in the absence of level 1 evidence, guidelines addressing areas of clinical uncertainty can reduce overtreatment.
Additional efforts to increase the use of BCT have involved a re-examination of contraindications to BCT. In 2002 the joint guidelines of the American College of Surgeons and American College of Radiology [12] included multicentricity, inability to achieve negative margins, and a history of prior radiotherapy to the breast region as absolute contraindications to BCT, while a large tumor-to-breast ratio was considered a relative contraindication. The NSABP B-18 and B-27 trials demonstrated no difference in long-term disease-free and overall survival for patients who received chemotherapy in the preoperative versus postoperative setting [13]. These trials and other subsequent studies demonstrated the benefit of neoadjuvant therapy in downstaging breast tumor size and increasing eligibility for BCT [13]. With the introduction of modern chemotherapy regimens and targeted therapies, up to 75% of patients ineligible for BCT due to large tumor size relative to breast size became BCT-eligible following neoadjuvant chemotherapy (NAC) [13]. The availability of NAC, coupled with oncoplastic surgical approaches which maintain cosmetic appearance even after larger breast resections, means that a large tumor-to-breast size is rarely a contraindication to BCT today, although a substantial minority of BCT-eligible patients still opt to undergo mastectomy, particularly in the United States [14].
Another traditional contraindication to BCT which has been recently challenged is multicentric disease. In a pooled analysis of 6134 patients from 3 NAC trials, patients with multicentric disease who underwent BCT had similar local relapse-free, disease-free, and overall survival (92%, 82%, and 89%, respectively) as patients with unifocal disease (94%, 86%, and 92%, respectively) [15]. In the surgery-first setting, the initial results from the ACOSOG [American College of Surgeons Oncology Group] Z11102 trial of BCT in multicentric cancer reported successful BCT with a single operation in 67.6% of the 198 patients enrolled, and that only 7.1% of patients required conversion to mastectomy [16]. Long-term follow-up will provide additional data on the oncologic safety of this technique in the upfront surgery setting. The St. Gallen expert consensus panel has strongly endorsed the use of BCT in multicentric disease, provided negative margins are achieved and WBI is given [17].
The presence of a BRCA1/BRCA2 mutation as a contraindication to BCT has also been questioned. Mastectomy was thought to be the preferred treatment based on retrospective studies reporting increased local recurrence rates in BRCA1/BRCA2 mutation carriers treated with BCT compared to mastectomy [18]. A meta-analysis of 526 BRCA mutation carriers and 2328 patients not known to have mutations found no difference in the incidence of local recurrence between groups for studies with follow-up durations of less than 7 years, while in studies with longer follow-up periods, 24% of BRCA carriers experienced local recurrence compared to 16% of those with sporadic cancers, suggesting an increase in second primary cancers rather than true local recurrence [18]. Rates of new primary cancer vary with mutation type and age at diagnosis of initial cancer, and no benefit in breast-specific or overall survival has been demonstrated for mastectomy over BCT in BRCA1/BRCA2 mutation carriers. Management guidelines issued by ASCO, ASTRO, and the SSO in 2020 recommend that BRCA status should not preclude BCT in otherwise eligible patients [19].
In women who have previously undergone breast conservation with radiotherapy and develop a new primary cancer, mastectomy has been the standard of care due to concerns about the complications of repeat radiation, the high rate of local failure with repeat lumpectomy only, and the potential for a poor cosmetic outcome. The RTOG 1014 trial demonstrated acceptable toxicity and safety of partial breast irradiation (PBI) after a second lumpectomy for patients who had undergone prior BCT with WBI [20]. At median follow-up of 5.5 years, the distant metastasis-free and overall survival was 95% [20]. These data confirm similar findings from retrospective studies supporting the safety of repeat BCT as an alternative to mastectomy for ipsilateral breast tumor recurrence (IBTR). Of note, this approach has largely been studied in postmenopausal women with unicentric estrogen receptor (ER) positive second breast cancers.
In contrast to the progress made in decreasing medical contraindications to BCT, efforts to decrease the use of BM in women with unilateral breast cancer have met with limited success. In spite of a steady decrease in the risk of contralateral cancer development and lack of a survival benefit for BM, use of the procedure has continued to increase [21]. Traditional educational approaches and decision aids improve patient understanding about the risks and benefits of the procedure, but have failed to decrease its use [22,23]. Interestingly, in a population-based study by Jagsi et al., only 30% of breast cancer patients reported that their surgeon actively recommended against BM, perhaps out of respect for patient autonomy or concern of losing the patient to another surgeon. The BM rate in those who received a recommendation against the procedure was 1.9% compared to 19% in those who received no recommendation [24]. Approaches to addressing the overuse of BM have been reviewed in detail recently [21], but there is little evidence at this point in time that patient enthusiasm for the procedure is waning.
2.2. Management of the axilla
For many years, axillary lymph node dissection (ALND) was thought to be an essential part of curative breast cancer treatment. ALND effectively maintains local control in the axilla and provides prognostic information to direct the use of adjuvant systemic therapy. In the late 1970s the landmark NSABP B-04 trial demonstrated that ALND did not improve breast cancer survival [25], but the study did not lead to abandonment of ALND because at that time, nodal status was the determinant of the need for systemic therapy. The advent of screening mammography and the diagnosis of smaller tumors with a lower likelihood of axillary nodal metastases meant that large numbers of women were subjected to the morbidity of ALND to demonstrate that their nodes were normal, an unsatisfactory situation. The demonstration that sentinel lymph node biopsy (SLNB) accurately staged the axilla as node positive or negative led to the rapid demise of ALND as a staging procedure. The recognition that systemic therapy contributes to local control subsequently led to studies examining the safety of SLNB alone in patients with a limited axillary tumor burden. The ACOSOG Z0011 trial demonstrating no benefit for ALND in patients with metastases in 1 or 2 sentinel lymph nodes (SLNs) undergoing BCT with WBI and systemic therapy, although initially quite controversial, resulted in a rapid decline in the use of ALND, with rates of ALND in the United States for patients with 1-2 involved nodes declining from 62% to 39% in the year after its presentation [26], and falling from 75% to 52% in patients undergoing BCT in The Netherlands [27]. The subsequent demonstration in the AMAROS trial that SLN biopsy with nodal irradiation was oncologically equivalent to ALND with less morbidity resulted in rates of ALND after BCT in The Netherlands falling to 17% by 2015 [27]. Implementation of the Z0011 criteria in practice has been shown to spare ALND and its associated morbidities in 84% of clinically node-negative patients found to have SLN metastases [28]. The replacement of ALND with SLNB in patients undergoing initial surgery represents one of the greatest success stories in surgical de-escalation and avoidance of overtreatment, and efforts are ongoing to decrease the use of ALND in other populations.
The safety of avoiding ALND in mastectomy patients with limited SLN involvement is less clear. Two of the randomized trials comparing axillary radiation and ALND in patients with 1-2 positive SLNs included a small number of mastectomy patients (248 AMAROS, 84 OTOASOR) [29], but outcomes were not analyzed separately from the BCT patients comprising the majority of the study population. These trials demonstrated no difference in rates of axillary recurrence after ALND or SLNB and nodal radiotherapy, but less lymphedema was seen after nodal radiotherapy, supporting omission of ALND in patients with involvement of 1-2 SLNs who have indications for postmastectomy radiotherapy (PMRT). While the need for PMRT is not always clear intraoperatively in clinically node-negative patients, in those considered to be likely candidates for PMRT based on information available preoperatively who are found to have 1 or 2 positive SLNs intraoperatively, the decision to proceed with ALND can be deferred until final surgical pathology is available. In a single-institution prospective study where this approach was implemented, 13 of 79 patients (16.5%) with 1-2 positive SLNs required return to the operating room for ALND; however, ALND was spared in 84% of patients and reduced the combined use of ALND and PMRT by 32% [30]. Radiation after ALND is associated with lymphedema in up to 30% of patients [31], and treatment with both modalities should be avoided when possible. Whether ALND is necessary for positive SLNs in mastectomy patients who will not receive PMRT is unclear at present, and data from the POSNOC [32] and SENOMAC trials [33] will directly address this question (Table 1) [32–37]. ALND remains standard in patients with involvement of ≥3 SLNs with tumor regardless of the type of breast surgery performed since the safety of radiotherapy in patients with a heavier tumor burden has not been established. The TAXIS trial will address whether SLNB and removal of palpable axillary nodes (tailored axillary surgery) followed by nodal radiotherapy is non-inferior to ALND and nodal radiotherapy in patients presenting with clinically node-positive breast cancer [35].
Table 1.
Clinical trials investigating de-escalation of axillary treatment.
| Trial | Study Size | Inclusion Criteria | Randomization Arms | Primary Endpoint |
|---|---|---|---|---|
| POSNOC [32] | 1900 | cT1-2 undergoing BCT or mastectomy, with 1-2 SLN macrometastases | Adjuvant therapy and axillary treatment (ALND or axillary radiation) vs adjuvant therapy only | Axillary recurrence |
| SENOMAC [33] | 3500 | cT1-3 undergoing BCT or mastectomy, with 1-2 SLN macrometastases | Completion ALND vs no additional axillary surgery | Breast cancer-specific survival |
| Alliance A011202 | 1660 | Biopsy-proven cN1 patients who converted to cN0 after neoadjuvant chemotherapy, with positive SLN intraoperatively | ALND and nodal radiation vs axillary and nodal radiation | Invasive breast cancer recurrence-free interval |
| TAXIS [35] | 1500 | Biopsy-proven axillary metastases (including those with residual axillary disease after neoadjuvant chemotherapy), clipped axillary lymph node | Tailored axillary surgery (TAS) (removal of SLNs and palpable suspicious nodes) completion ALND, nodal radiation vs TAS and nodal radiation | Disease-free survival |
| SOUND [34] | 1560 | cT1N0 undergoing BCT, negative preoperative axillary ultrasound | SLNB vs no axillary surgery | Distant disease-free survival |
| INSEMA [36] | 6000 | cT1-2N0 undergoing BCT, negative preoperative axillary ultrasound | SLNB vs no axillary surgery | Invasive disease-free survival |
| BOOG 2013-08 [37] | 1644 | cT1-2N0 undergoing BCT, negative preoperative axillary ultrasound | SLNB vs no axillary surgery | Regional recurrence rate |
Abbreviations: BCT, breast-conserving therapy; SLN, sentinel lymph node; ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy
More recently, the use of SLNB to de-escalate axillary treatment in patients presenting with node-positive disease who convert to clinically node negative after NAC has been studied. Several trials [38] demonstrated the feasibility and accuracy of SLNB in this setting. Although overall false-negative rates of 12-14% were reported, the use of dual-tracer mapping, removal of at least 3 SLNs, clipping of positive nodes at biopsy to allow localization and removal, and the use of immunohistochemistry have all been shown to reduce the false-negative rate to <10%. Axillary recurrence after SLNB alone after NAC has been reported in several retrospective studies with rates of axillary recurrence of ≤3% and follow-up periods of 3-10 years [39]. The avoidance of ALND with NAC requires a nodal pathologic complete response (pCR) and thus is highly dependent upon ER, progesterone receptor (PR), and HER2 status. In a consecutive series of 630 patients with biopsy-proven nodal disease receiving NAC, rates of nodal pCR ranged from 20% in ER+/HER2− patients to 78% in ER−/HER2+ patients, and ALND was avoided in 41% of the group [40]. These data provide a compelling rationale for the treatment of all node-positive HER2+ and triple negative (TN) patients with NAC. While ALND remains the standard of care for patients with any residual nodal disease after NAC, the ongoing Alliance A011202 trial is examining whether further de-escalation can occur when these patients are treated with axillary radiation as an alternative to ALND.
In certain patient groups, even SLNB may not be warranted. In the CALGB 9343 trial of women age ≥70 years with cT1N0 ER+ cancers treated with lumpectomy and tamoxifen, with or without radiation, there were only 6 axillary recurrences in the 392 patients who did not undergo axillary surgery [41]. As older patients have competing risks of mortality, and as axillary staging is less likely to affect adjuvant treatment decisions, the utility of SLNB is limited in this population. Despite the 2016 Choosing Wisely campaign recommending against routine use of SLNB in patients age >70 years with ER+ breast cancers, uptake has been variable[42], with implementation barriers coming from both physicians and patients. In a survey of members of the American Society of Breast Surgeons, 83% favored performing SLNB in a 75-year-old patient with a cT1N0 ER+ breast cancer [43]. Practice in a non-academic setting, absence of fellowship training, and increased time in practice were associated with decreased likelihood of guideline adherence, indicating that while these recommendations are available and widely endorsed, their dissemination can still be improved. Similarly, in interviews with 18 surgeons who treat breast cancer, multiple surgeons reported being unfamiliar with this Choosing Wisely recommendation and the supporting evidence [44]. Many surveyed surgeons believed that the staging information provided by SLNB was important in informing subsequent treatments, and nearly all would perform a SLNB if a medical or radiation oncologist indicated this would guide subsequent treatment. Given the multidisciplinary nature of breast cancer care, each individual discipline must integrate these de-escalation guidelines into their group practice to ensure their successful implementation. This was exemplified in the rapid decrease in re-excisions following the issuance of the joint SSO/ASTRO margin guidelines in 2014.
Patients themselves are also hesitant to de-escalate therapy. In a survey study of 30 women age ≥70 without breast cancer, participants cited a wide range of reservations toward the Choosing Wisely recommendation, including association of treatment de-escalation with poor prognosis, and wariness about applying population-based data to their personal situations [45]. Patients also have different approaches to their care, with some consistently preferring a more-aggressive approach and more interventions regardless of medical necessity. Clinician understanding of the individual patient’s rationale for her or his treatment preference is critical in developing a tailored approach to successful de-escalation.
Future opportunities for SLNB omission in a larger group of patients exist, as treatment recommendations are increasingly determined by tumor biology rather than nodal status. For postmenopausal women with ER+ breast cancers, the TAILORx and RxPonder trials demonstrated that the benefit of chemotherapy is dependent upon the Oncotype DX (Exact Sciences, Redwood City, CA) score both for node-negative patients as well as those with 1-3 positive nodes [46], suggesting that SLNB might be eliminated in this large group of patients. The major barrier to implementation of elimination of SLNB in ER+ postmenopausal women is that decision making regarding the use of regional node irradiation and postmastectomy radiotherapy is largely based upon the extent of nodal involvement. For other patient groups, knowledge of nodal status remains essential for systemic therapy decision making (Table 2), and elimination of SLN biopsy is inappropriate.
Table 2.
Impact of nodal status on systemic therapy recommendations.
| HER2+ | ||
|---|---|---|
| T1aN0 | T1bc N0 | Stage II/III |
| Consider TH | TH chemotherapy | ACT or TC plus trastuzumab, ± pertuzumab |
| Triple Negative | ||
| T1a N0 | T1bc N0 | Stage II/III |
| Consider chemotherapy | TC or ACT | ACT |
| HR+, HER2− | |
|---|---|
| Premenopausal* Node Negative |
Postmenopausal Node Negative, 1-3+ nodes |
| Oncotype score >25 Chemotherapy + endocrine therapy |
Oncotype score >25 Chemotherapy + endocrine therapy |
| Oncotype score ≤15 Endocrine therapy |
Oncotype score ≤25 Endocrine therapy |
| Oncotype score 16-25 Consider chemotherapy |
|
| Node positive | |
| Chemotherapy | |
Nodal status primary determinant of need for chemotherapy
Abbreviations: HER2, human epidermal growth factor receptor 2; TH, taxane and trastuzumab; ACT, anthracycline, cyclophosphamide, Taxane; TC, carboplatin taxane; HR, hormone receptor;
Another strategy to decrease the use of SLNB is to identify patients at low risk for nodal metastases; 3 ongoing clinical trials are investigating the omission of SLNB in patients with early breast cancer, regardless of tumor subtype (Table 1). In the SOUND and INSEMA trials, patients with cT1N0 (SOUND) or cT1/2N0 (INSEMA) disease undergoing BCT with a negative preoperative axillary ultrasound are randomized to SLNB or no axillary surgery, with the primary endpoint of distant disease-free survival [34] or invasive disease-free survival, respectively [36]. Preliminary results from these studies indicate that the in the largely postmenopausal ER+ participants, ≥3 involved lymph nodes were found in only 0.5% and 1.3% of those randomized to ALND, while 8.6% and 14%, respectively, had any nodal macrometastases. No results have been reported from the Dutch BOOG 2013-08 study which includes T1 and T2 patients of any receptor status having primary surgery or surgery after NAC [37]. These studies demonstrate that a heavy nodal tumor burden is uncommon in patients meeting study eligibility criteria, making a difference in survival outcomes unlikely. Elimination of SLNB raises the possibility that it may be replaced by more morbid and costly radiotherapy due to uncertainty about nodal status. Ongoing trials attempting to refine selection criteria for radiotherapy may address this issue, but until results are available, knowledge of nodal status for radiotherapy decision making is a compelling reason to continue to perform SLNB in the majority of invasive breast cancer patients. Trials examining de-escalation of axillary surgery are summarized in Table 1.
2.3. Radiation
Radiation is a key component for local control after breast-conserving surgery (BCS). The initial trials demonstrating this utilized conventional fractionated WBI of 50 Gray (Gy) administered in 25 fractions over 5 weeks [4]. After the EBCTCG meta-analysis of studies conducted in the 1980s and 1990s demonstrated that the 15.7% reduction in any recurrence with WBI translated to a 3.8% absolute reduction in the risk of death at 15 years, with an 8.5% absolute reduction in death among node-positive patients, WBI became a standard part of BCT [47]. However, when node-negative patients were risk-stratified based upon age, tumor grade, ER status, use of tamoxifen, and surgery type, the reduction in the risk of death for the low-risk group was only 0.1%. Hypofractionated regimens have since been developed which deliver higher doses per fraction, allowing a similar cumulative radiation dose to be given over a shorter period of time, a treatment scheme which is more convenient for patients and reduces cost. Randomized controlled trials of hypofractionated radiation have shown no difference in local recurrence and a reduced incidence of adverse effects to normal tissue. The extensive body of evidence supporting hypofractionated WBI is summarized in Table 3 [48,49]. Current ASTRO and National Institute for Heath and Care Excellence (NICE) guidelines recommend hypofractionated radiation as standard practice. While the use of hypofractionation has increased since the publication of these trials and guidelines, it has had variable uptake internationally. In the United States, analysis of claims data covering 7.4% of U.S. adult women demonstrated an increase in the use of hypofractionated radiation from 10.6% in 2008 to 34.5% in 2013 [50]. Data from the National Cancer Database reflected a similar pattern, with the use of hypofractionated WBI increasing exponentially from 0.7% in 2004 to 38.1% in 2016, but still not reaching widespread adoption [51]. In comparison, in Ontario, Canada, 71% of patients undergoing WBI received a hypofractionated regimen, and among institutions of the European Organisation for Research and Treatment of Cancer (EORTC), 72% of institutions used a hypofractionated schedule [52]. This variability in adoption is likely due to a combination of patient, facility, and provider factors, including patient age, treatment facility type and volume, and reimbursement structure.
Table 3.
Randomized controlled trials of hypofractionated radiation.
| Trial (Enrollment Period) | No. of Patients | Inclusion Criteria | Radiation Regimen | Locoregional Recurrence | Overall Survival |
|---|---|---|---|---|---|
| Ontario Multicenter Trial (1993-1996) | 1234 | Lumpectomy only, pT1-3, N0 |
Treatment: 42.5 Gy in 16 fractions over 22 days Control: 50 Gy in 25 fractions over 35 days No tumor bed boost |
No difference at 10 years: 6.2% (treatment) vs 6.7% (control) | No difference in 10-year OS: 84.6% (treatment) vs 84.4% (control) |
| START-A (1999-2002) | 2236 | pT1-3a, pN0-1 |
Treatment: 41.6 Gy in 13 fractions or 39 Gy in 13 fractions over 5 weeks Control: 50 Gy in 25 fractions over 5 weeks Tumor bed boost permitted |
No difference at 10 years: 6.3% (41.6 Gy), 8.8% (39 Gy) vs 7.4% (control) | No difference in 10-year OS: 81.6%, 79.7% (treatment) vs 80.2% (control) |
| START-B (1999-2002) | 2215 | pT1-3a, pN0-1 |
Treatment: 40 Gy in 15 fractions over 3 weeks Control: 50 Gy in 25 fractions over 5 weeks Tumor bed boost permitted |
No difference at 10 years: 4.3% (treatment) vs 5.5% (control) | Improved 10-year OS in treatment arm: 84.1% vs 80.8% |
| DBCG HYPO (2009-2014) | 1854 | >40 years, Lumpectomy only, pTis-2, pN0 |
Treatment: 40 Gy in 15 fractions Control: 50 Gy in 25 fractions Tumor bed boost permitted |
No difference at 9 years: 3.0% (treatment) vs 3.3% (control) | No difference in 9-year OS: 93.4% (treatment) vs 93.4% (control) |
| Chinese Multicenter Trial [49] (2010-2015) |
734 | Lumpectomy only, pT1-2, pN0-3 |
Treatment: 43.5 Gy in 15 fractions over 3 weeks Control: 50 Gy in 25 fractions over 5 weeks Mandatory tumor bed boost |
No difference at 5 years: 1.2% (treatment) vs 2.0% (control) | No difference in 5-year OS: 97.5% (treatment) vs 98.0% (control) |
| FAST-Forward [48] (2011-2014) |
4110 | pT1-3, pN0-1 |
Treatment: 27 Gy in 5 fractions over 1 week, 26 Gy in 5 fractions over 1 week Control: 40 Gy in 15 fractions over 3 weeks Tumor bed boost permitted |
No difference at 5 years: 1.7% (27 Gy), 1.4% (26 Gy) vs 2.1% (control) | No difference in 5-year OS: 93.1% (27 Gy), 93.3% (26 Gy), vs 94.6% (control) |
Abbreviations: OS, overall survival
The FAST-Forward trial examined a further reduction in treatment time and reported no difference in the 5-year local recurrence rate, with similar normal tissue effects with the administration of 26-27 Gy in 5 fractions over 1 week compared to 40 Gy in 15 fractions over 3 weeks [48]. Additional follow-up data are needed to confirm the long-term safety and efficacy of these condensed hypofractionated regimens and to determine their acceptance.
Partial-breast irradiation (PBI), another effort to tailor treatment by radiating the area of the breast at highest risk for local recurrence, limits the delivery of radiation to the portion of the breast around the tumor bed and is also administered in an accelerated regimen (APBI). PBI/APBI has been proposed for patients at low risk for local recurrence, and randomized controlled trials comparing PBI/APBI to WBI in patients with low recurrence risk characteristics have demonstrated similar rates of local control and adverse effects to normal tissue [53]. The NSABP B-39/RTOG 0413 trial did not demonstrate statistical equivalence between APBI and WBI [53], but the absolute difference in 10-year local recurrence was less than 1%. Limited information is available regarding the uptake of PBI in clinical practice, but a 2020 survey of 74 Italian radiotherapy centers (38% response rate) indicated that while 95% routinely used hypofractionated WBI, only 40% employed PBI [54].
At present, the only group of patients with invasive cancer for which there is some consensus that radiotherapy can be omitted completely is women age ≥70 years with T1, clinically node-negative, ER+/HER2− breast cancers. The Cancer and Leukemia Group B (CALGB) 9343 trial demonstrated no survival difference when radiotherapy was added to lumpectomy and tamoxifen, and the rate of locoregional recurrence at 10 years was only 8.1% in the no-radiotherapy group, with no difference in the rate of breast preservation between groups [41]. This study was initially published in 2004, but an analysis of 120,308 women age ≥70 years with stage 1 ER+ breast cancers undergoing lumpectomy demonstrated that radiotherapy usage decreased by only 4.1% between 2005 and 2012 compared to before 2004, with almost one-third of women age ≥85 years continuing to receive radiotherapy [55]. Many of the variables significantly associated with a higher likelihood of omission of radiotherapy (low income level, Medicaid, rural residence, African-American race, and lack of receipt of chemotherapy or endocrine therapy) are suggestive of disparities in care rather than an evidence-based desire to de-escalate care. A 2015-2016 survey of a United States-wide sample of surgeons and radiation oncologists treating breast cancer found that omission of radiotherapy for patients meeting CALGB 9343 eligibility criteria was felt to be unreasonable by 40% of surgeons and 20% of radiation oncologists because they erroneously believed radiotherapy was associated with survival benefit or they greatly overestimated the risk of local recurrence associated with radiotherapy omission [56]. More recently, a number of trials attempting to identify patients with biologically favorable cancers suitable for omission of radiotherapy have been launched. The majority of these are single-arm studies (reviewed by Montagna et al. [57]) seeking to establish that postmenopausal ER+ patients with low risk scores on commercially available genomic assays have a low risk of local recurrence after treatment with excision and endocrine therapy. The EXPERT study is a multicenter, non-inferiority randomized trial in which women age ≥50 years with unifocal, grade I-II, pT1N0 cancers and a Prosigna (Veracyte, South San Francisco, CA) genomic score ≤60 are randomized to BCS and endocrine therapy alone or with radiotherapy [58]. Results of these trials have the potential to substantially change practice in a large number of breast cancer patients.
3. Chemotherapy
3.1. Patient selection for chemotherapy benefit
Historically, patient and tumor features such as age, tumor size, and tumor grade, were used to determine prognosis and the benefit of adjuvant chemotherapy. The knowledge that metastatic breast cancer is incurable, coupled with improvements in systemic therapy, has led to increasingly large numbers of women being recommended to receive chemotherapy, and current recommendations include treatment of node-negative cancers between 0.5 cm and 1 cm for high-risk subtypes such as TN and HER2 overexpressing breast cancer [59]. The determination of what constitutes overtreatment with chemotherapy is complicated by the fact that patients have variable benefit thresholds for which they are willing to accept chemotherapy. Among patients receiving contemporary anthracycline-based chemotherapy, 57% would consider 6 months of chemotherapy worthwhile for a 2-month survival benefit; this increased to 88% for a 9-month survival benefit [60]. In a survey of 100 patients with newly diagnosed solid tumors, 53% were willing to accept intensive treatments with considerable side effects for even a 1% increase in cure rate [61]. The introduction of multi-gene assays has aided in further tailoring the use of adjuvant chemotherapy by identifying a large subset of ER+ breast cancer patients with an excellent prognosis after treatment with endocrine therapy alone.
In the TAILORx trial of 10,253 women with hormone receptor-positive/HER2− node-negative breast cancers, those with an Oncotype DX recurrence score of 0-10 had a distant recurrence risk of 0.7% after treatment with endocrine therapy alone, while those with Oncotype DX recurrence scores of 11-25 had similar rates of recurrence-free and overall survival when treated with endocrine therapy alone or with chemotherapy [62]. A suggestion of chemotherapy benefit was seen in women age <50 years with scores 16-25, and clinical risk factors can be coupled with the Oncotype DX recurrence score to more accurately identify this patient subgroup [63]. Prospective and pooled analyses have demonstrated Oncotype DX recurrence score use to alter chemotherapy treatment recommendations in 30-33% of patients, with some series reporting up to a 69% reduction in chemotherapy use within certain patient subgroups [64,65].
The results of the RxPonder trial demonstrated that the addition of chemotherapy to endocrine therapy did not improve survival outcomes in postmenopausal patients with ER+/HER2− node-positive breast cancer and Oncotype DX recurrence scores <26. In contrast, a significant benefit for chemotherapy was seen in premenopausal women regardless of Oncotype DX recurrence score [46]. In a Surveillance, Epidemiology, and End Results Program (SEER) study of patients diagnosed from 2013-2015, use of chemotherapy was found to decline by 34.5%, and increasing use of the Oncotype DX recurrence score in node-positive patients accounted for one-third of the decline, even in the absence of randomized data indicating the safety of this approach. With the publication of RxPonder, a further decrease in chemotherapy use is likely.
For patients with HER2+ cancers, clinical trials have addressed the use of shorter, less-toxic chemotherapy regimens. The APT trial demonstrated a 7-year disease-free and overall survival of 93% and 95%, respectively, for patients with HER2+ tumors ≤ 3 cm in size treated with adjuvant paclitaxel weekly for 12 weeks and trastuzumab only, illustrating the efficacy of an anthracycline-sparing approach in patients with small tumors [66]. Chemotherapy has also been increasingly administered in the neoadjuvant setting, particularly for HER2+ and TN tumors. A National Cancer Database study reported the proportion of breast cancer patients who received NAC increased from 12.2% in 2003 to 24.0% in 2022 [67]. One of the benefits of this treatment sequence is for adjuvant therapy to be tailored based on extent of residual disease. The KATHERINE trial and the CREATE-X study demonstrated the benefit of additional chemotherapy in patients who failed to achieve pCR with NAC [68,69]. The potential sparing of additional chemotherapy in patients who have no residual disease after NAC is being addressed in the ongoing CompassHER2-pCR trial in patients with stage II/IIIA HER2+ disease who are treated with neoadjuvant taxane-based HER2 targeted therapy, with only those with residual disease receiving additional adjuvant chemotherapy.
4. DCIS
The incidence of ductal carcinoma in situ (DCIS) has increased significantly with the widespread use of screening mammography, and DCIS represents approximately 20% of all newly diagnosed breast cancers. Historically, DCIS was considered an obligate precursor to invasive cancer leading to the current standard-of-care approaches of excision, radiation, and anti-estrogen therapy. However, the relative incidence of invasive breast cancer has remained unchanged despite a marked increase in the detection and treatment of DCIS, and breast cancer-specific survival after treatment of DCIS exceeds 95% regardless of treatment approach [70]. These observations have raised concerns that DCIS is not only being overtreated, but also overdiagnosed. Strategies to de-escalate the treatment of DCIS or eliminate treatment altogether are predicated upon the ability to identify subsets of DCIS patients with a low risk of progression to invasive carcinoma.
4.1. Treatment de-escalation
One area in which there is a clear opportunity to decrease overtreatment of DCIS is in axillary management. DCIS, by definition, does not have the potential to spread to regional lymph nodes. While SLN metastases have been reported in pure DCIS, in the NSABP B-24 trial only 6 of 1799 patients treated without axillary surgery experienced axillary recurrence at a median follow-up of 11.6 years, and 1 of these patients was found to have undiagnosed microinvasion [71]. SLNB has been suggested for patients undergoing mastectomy for DCIS given the 20% upgrade rate to invasive carcinoma in patients diagnosed by core biopsy and the questionable accuracy of SLNB after mastectomy [72]. Reported rates of axillary involvement in patients initially diagnosed with DCIS are variable, ranging from 1-3%, with an increased likelihood of nodal positivity in patients with larger-size DCIS, DCIS with necrosis, or high-grade DCIS [72], but predictive models have been largely unsuccessful at identifying patients with a >50% risk of invasive cancer and, by extension, a higher likelihood of nodal metastases. Despite this, axillary surgery continues to be performed for DCIS in patients undergoing lumpectomy as well as mastectomy. Analysis of nationwide trends using the SEER database demonstrated that 18% of patients with DCIS undergoing lumpectomy underwent an SLNB in 2010 compared to only 1.4% in 1990 (p<0.001 for trend), and that 67% of patients undergoing mastectomy had concurrent SLNB [73].
The issue of treatment de-escalation in the breast is more complex. Although cause-specific survival exceeds 95% whether DCIS is treated with mastectomy, BCS with radiotherapy, or BCS alone, rates of local recurrence at 10 years are 2.5%, 13.6%, and 25.5% [74], respectively, and half of the local recurrences are invasive carcinoma. Four randomized controlled trials have demonstrated that adjuvant radiation reduces the risk of local recurrence by 50% [75], although it does not improve survival. Even in patients with low-volume, non-high-grade DCIS, radiation confers a significant risk reduction [76,77], and a subset of women with DCIS not benefitting from radiotherapy has not been identified. Studies have shown that women with DCIS estimate their risk of breast cancer metastases and mortality to be equivalent to those with invasive cancers [78], so it is not surprising that many choose mastectomy or radiotherapy to mitigate this risk. Although the risk of local recurrence of DCIS has declined over time [79], a SEER study demonstrated an increase in the use of lumpectomy with radiotherapy and BM for treatment of DCIS between 1990 and 2010, with corresponding decreases in lumpectomy alone and unilateral mastectomy [73]. Given that concern about recurrence is said by many patients to be their primary concern in local therapy decision making, and that local recurrence is traumatic for patients even when not associated with an increased mortality risk, it is difficult to classify patient preference for radiotherapy or even mastectomy as overtreatment. Nonetheless, there is potential for improvement. An older study showed that while surgeon preference for mastectomy as treatment for DCIS varied with DCIS size and grade, preference for radiotherapy did not [80]. The more recent studies of radiotherapy in lower-risk DCIS discussed earlier [76,77] provide greater clarity on the benefit of radiotherapy in subsets of women with DCIS, and assist in informed decision making.
Recurrence-risk models have been proposed to provide individualized estimates of recurrence and may assist in identifying patients unlikely to benefit from radiotherapy. The Memorial Sloan Kettering Cancer Center (MSKCC) DCIS nomogram incorporates 10 clinical, pathologic, and treatment variables to provide a risk estimate of IBTR at 5 and 10 years after excision [81]. The Oncotype DX DCIS score (Exact Sciences, Redwood City, CA) utilizes a 12-gene genomic assay to estimate the 10-year risk of local recurrence and has been validated both as an independent risk-estimation tool and when used in conjunction with clinicopathologic factors [82]. Although not predictive of radiotherapy benefit, use of the Oncotype DX DCIS score has been shown to alter adjuvant radiation recommendations in approximately one-third of patients undergoing planned BCS [83].
Another area of concern regarding overtreatment in DCIS is the use of endocrine therapy. Two randomized controlled trials have demonstrated the benefit of tamoxifen in reducing both ipsilateral and contralateral breast events following excision of DCIS. After a median follow-up of 14.6 years, the NSABP B-24 trial demonstrated a significant reduction in the rate of invasive breast cancer (no tamoxifen 19%, tamoxifen 12%) but not in the rate of DCIS (12% versus 9%, p=0.12) [84]. The UK/ANZ DCIS trial demonstrated a reduction in 10-year risk of recurrent ipsilateral DCIS (no tamoxifen 12.1%, tamoxifen 8.6%) and contralateral tumors (4.2% versus 1.9%), but not of ipsilateral invasive disease (6.9% versus 6.8%) [85]. No difference in survival was seen in either trial. Tamoxifen and other anti-estrogen therapies have side effects, including vasomotor symptoms, thromboembolic events, and endometrial cancer. For many women, these side effects outweigh the small absolute reduction in new invasive events. Uptake of endocrine therapy for DCIS is relatively low, ranging from 21-47% [86]. Endocrine therapy should be discussed as an option for those who wish to minimize the risk of future breast cancer events and have a favorable risk-benefit ratio for treatment (premenopausal, 2 breasts at risk), but should not be considered a mandatory part of DCIS management.
4.2. Active surveillance trials
The marked increase in DCIS diagnosis in recent years, coupled with the lack of a corresponding decrease in breast cancer mortality, as well as the identification of DCIS in asymptomatic women at autopsy, has led to the question of whether some subsets of women with DCIS can be observed with no surgical treatment, with or without endocrine therapy, with surgery reserved for those who progress to invasive cancer. The 3 ongoing randomized controlled trials and the single-arm prospective trial currently investigating the safety of active surveillance in patients with low-risk DCIS are summarized in Table 4 [87].
Table 4.
Current trials of active surveillance for DCIS.
| Trial | Target Enrollment | Inclusion Criteria | Surveillance Regimen | Primary Endpoint |
|---|---|---|---|---|
| LORD [87] | 1240 | Age ≥45 years, low-grade DCIS | Annual mammogram. Anti-estrogen therapy not permitted. |
10-year ipsilateral invasive breast cancer-free rate |
| LORIS [87] | 932 | Age ≥46 years , low- or intermediate-grade DCIS | Annual mammogram. Anti-estrogen therapy not permitted. |
5-year ipsilateral invasive breast cancer-free survival |
| COMET [87] | 1200 | Age ≥40 years, low- or intermediate-grade DCIS | Clinical breast exam every 6 months, mammogram of affected breast every 6 months, mammogram of unaffected breast every year. Anti-estrogen therapy encouraged. |
2-, 5-, 7-year rate of ipsilateral invasive breast cancer |
| LORETTA [87] | 340 | Age ≥40 years, ≤2.5 cm of low- or intermediate-grade DCIS | Mammogram and ultrasound every 6 months for 1 year, then every year. Mandatory anti-estrogen therapy. |
5-year cumulative incidence of ipsilateral invasive breast cancer |
Abbreviations: DCIS, ductal carcinoma in situ
Several concerns persist regarding active surveillance for DCIS. While multiple biopsies and large-volume vacuum-assisted biopsies are required in all trials to reduce the risk of missing invasive cancer, the upstaging rate of patients with low-risk DCIS as defined by these trials is up to 20%[88]. Additionally, the frequency of follow-up imaging is increased when surgery is omitted, which often leads to additional biopsies. Lastly, the lack of surgical intervention may heighten the anxiety that patients already have in association with their DCIS diagnosis [89]. Active surveillance is applicable to a relatively small proportion of DCIS patients who are often candidates for lumpectomy alone [90], and it is unclear that the use of endocrine therapy for 5 years or the risk of invasive carcinoma without intervention will be preferable to a small lumpectomy to patients. Traditional oncologic outcomes as well as patient-reported outcomes will help inform the safety and practicality of this approach.
5. Strategies to Address Overtreatment
It is apparent from the previous discussion that the causes of overtreatment are multifactorial, and that both physicians and patients contribute to overtreatment. It is puzzling that some de-escalation strategies have been rapidly embraced, while others with equally strong supporting evidence have been slowly adopted. While no single strategy will reduce overtreatment, there is evidence that some measures are effective. A review of 160 interventions designed to change physician practice found that 89% of those that targeted specific areas previously identified as problems successfully achieved change compared to 42% of more-general interventions [91], a concept illustrated by the prompt decrease in use of re-excision for negative margins after publication of the SSO-ASTRO margin guidelines [10]. The rapid pace of change in breast cancer management makes it challenging for physicians, particularly those with less-specialized practices, to be aware of which de-escalation strategies are ready for adoption in practice and which require further evaluation. A population-based study in The Netherlands demonstrated that development of an integrated oncological care pathway increased compliance with 7 of 8 medical indicators, including 4 of 4 measures based on national guidelines [92]. Addressing overtreatment is also dependent upon a well-functioning multidisciplinary breast team, as little is achieved if surgical de-escalation is replaced with increased extent of radiotherapy and greater use of systemic therapy. In a study of a random sample of breast care teams in the United Kingdom, Haward et al. found that the proportion of breast care nurses on the team and the caseload per team member were significantly associated with improved clinical performance. Dispersed leadership among team members was also associated with concern for quality, increased participation, and self-rated effectiveness [93].
Addressing patient desire for more radical treatment than is necessary is equally challenging. The concept of anticipated regret—fear of not doing enough now, having a bad outcome later, and feeling regret—is a powerful driver of patient choice [7]. A detailed explanation of the lack of benefit of more radical approaches, and of the increased risk of complications associated with a clear medical recommendation against the more radical procedure, is helpful. The use of framing strategies derived from negotiating techniques to present information has been shown to increase the use of active surveillance rather than radical prostatectomy in low-risk prostate cancer [94], but whether this approach would be effective in breast cancer is unknown.
6. Expert Opinion
Current problems with overtreatment in breast cancer are the result of a perfect storm of events—rapidly changing care standards which are ill-defined, increased patient participation in the decision-making process (with opinions often formulated based upon medically incorrect internet information), a reimbursement system which rewards doing more, not less, and care which is multidisciplinary in name but not in practice. The desire to produce evidence-based, high-quality guidelines often involves a multi-year development process, resulting in a product that provides little useful guidance for practicing physicians because the quality of evidence is deemed insufficient to support definitive recommendations. In the absence of clear guidance, physicians are left to adopt new approaches based on their own comfort levels as early or late adopters.
Some clarity in this regard could be achieved by developing clear, relatively simple statements on acceptable, unacceptable, and evolving approaches to areas of controversy, rapidly update-able as the evidence base matures. Talks at conferences should also clearly state current practice and what remains to be proven to help avoid both overtreatment and undertreatment.
Breast cancer is often considered a model for multidisciplinary cancer management, but what often occurs is serial management by various disciplines without an integrated treatment plan. Illustratively, only 9% of patients receiving chemotherapy between 1998-2021 received NAC despite multiple randomized trials and meta-analyses demonstrating equal survival compared to adjuvant chemotherapy, but greater eligibility for BCT and fewer axillary nodal metastases with the NAC approach [95]. The demonstration that additional chemotherapy use in HER2+ and TN patients post-NAC who do not have a pCR improves survival increases the importance of appropriate referral. Additionally, ongoing trials are exploring NAC as a means to identify patients requiring less-intensive chemotherapy regimens. The proposal that all patients be seen by a multidisciplinary team at initial presentation to address failure of surgeons to refer patients for a discussion of NAC is not practical in high-volume practice settings. As care becomes increasingly complex, proactive, multidisciplinary agreement on how institutions or practices will manage evolving and controversial situations, with the goal of avoiding both overtreatment and undertreatment, is useful.
Physicians should understand that anticipatory regret influences physician as well as patient decision making. While financial gain associated with overtreatment may be a motivator for some physicians, most are anxious to minimize the likelihood of recurrence and death from breast cancer. When faced with the anxious patient asking a physician to do “everything,” it is easy to succumb to the belief that doing more may be beneficial, while forgetting that doing more may simply mean a greater burden of treatment with no benefit. The problems of overtreatment we face today are based on improved understanding of breast cancer biology and abandonment of the “one-size-fits-all” approach. As our knowledge base increases, these will only be magnified in the future. To continue progress, we must, as advocated by Dr. Umberto Veronesi, move from advocating the maximum-tolerated treatment to the minimum-effective one [96].
Article Highlights.
Recognition of the biologic diversity of breast cancer offers the opportunity to reduce overtreatment.
Neoadjuvant chemotherapy increases eligibility for breast conservation, decreases the need for axillary dissection, and, in the future, may allow identification of patients suitable for shorter, less-toxic chemotherapy regimens.
The duration and extent of radiotherapy, and the use of chemotherapy have been reduced in patients with biologically favorable tumors, and genomic assays offer the promise of improved tailoring, or even elimination of radiotherapy, in some patient subsets.
Treatments that do not prolong survival may be sought by patients because they value other outcomes such as improved local control, making de-escalation of treatment challenging.
A functioning multidisciplinary breast team that develops integrated treatment plans across disciplines is essential to reducing overtreatment as care becomes more complex.
Funding
The preparation of this manuscript was supported in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Footnotes
Declaration of interest
M Morrow has received speaking honoraria from Exact Sciences and Roche. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Welch HG, Prorok PC, O’Malley AJ, et al. Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness. N Engl J Med. 2016. Oct 13;375(15):1438–1447. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981. Jul 2;305(1):6–11. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985. Mar 14;312(11):665–73. [DOI] [PubMed] [Google Scholar]
- 4.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005. Dec 17;366(9503):2087–106. [DOI] [PubMed] [Google Scholar]
- 5.**.Kummerow KL, Du L, Penson DF, et al. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015. Jan;150(1):9–16. [DOI] [PubMed] [Google Scholar]; This study of considerable interest demonstrates the increase in unilateral and bilateral mastectomies performed in the United States in BCS-eligible patients.
- 6.Bouganim N, Tsvetkova E, Clemons M, et al. Evolution of sites of recurrence after early breast cancer over the last 20 years: implications for patient care and future research. Breast Cancer Res Treat. 2013. Jun;139(2):603–6. [DOI] [PubMed] [Google Scholar]
- 7.*.Katz SJ, Lantz PM, Janz NK, et al. Patient involvement in surgery treatment decisions for breast cancer. J Clin Oncol. 2005. Aug 20;23(24):5526–33. [DOI] [PubMed] [Google Scholar]; This study of interest shows the effect of patient-reported participation in the treatment decision process on the rate of mastectomy.
- 8.Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014. May 10;32(14):1507–15. [DOI] [PubMed] [Google Scholar]
- 9.Azu M, Abrahamse P, Katz SJ, et al. What is an adequate margin for breast-conserving surgery? Surgeon attitudes and correlates. Ann Surg Oncol. 2010. Feb;17(2):558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.*.Marinovich ML, Noguchi N, Morrow M, et al. Changes in Reoperation After Publication of Consensus Guidelines on Margins for Breast-Conserving Surgery: A Systematic Review and Meta-analysis. JAMA Surg. 2020. Oct 1;155(10):e203025. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of interest illustrates the impact of consensus guidelines on clinical practice, with variation in effect between the institution and population level.
- 11.Morrow M, Abrahamse P, Hofer TP, et al. Trends in Reoperation After Initial Lumpectomy for Breast Cancer: Addressing Overtreatment in Surgical Management. JAMA Oncol. 2017. Oct 1;3(10):1352–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrow M, Strom EA, Bassett LW, et al. Standard for breast conservation therapy in the management of invasive breast carcinoma. CA Cancer J Clin. 2002. Sep-Oct;52(5):277–300. [DOI] [PubMed] [Google Scholar]
- 13.Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J Clin Oncol. 2021. May 1;39(13):1485–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.*.Golshan M, Loibl S, Wong SM, et al. Breast Conservation After Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer: Surgical Results From the BrighTNess Randomized Clinical Trial. JAMA Surg. 2020. Mar 1;155(3):e195410. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of interest shows worldwide variations in patients’ choice of surgical intervention (BCT versus mastectomy) and extent of surgery (unilateral versus bilateral), among BCT-eligible women.
- 15.Ataseven B, Lederer B, Blohmer JU, et al. Impact of multifocal or multicentric disease on surgery and locoregional, distant and overall survival of 6,134 breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2015. Apr;22(4):1118–27. [DOI] [PubMed] [Google Scholar]
- 16.Rosenkranz KM, Ballman K, McCall L, et al. The Feasibility of Breast-Conserving Surgery for Multiple Ipsilateral Breast Cancer: An Initial Report from ACOSOG Z11102 (Alliance) Trial. Ann Surg Oncol. 2018. Oct;25(10):2858–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017. Aug 1;28(8):1700–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davey MG, Davey CM, Ryan ÉJ, et al. Combined breast conservation therapy versus mastectomy for BRCA mutation carriers - A systematic review and meta-analysis. Breast. 2021. Apr;56:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tung NM, Boughey JC, Pierce LJ, et al. Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J Clin Oncol. 2020. Jun 20;38(18):2080–2106. [DOI] [PubMed] [Google Scholar]
- 20.Arthur DW, Winter KA, Kuerer HM, et al. Effectiveness of Breast-Conserving Surgery and 3-Dimensional Conformal Partial Breast Reirradiation for Recurrence of Breast Cancer in the Ipsilateral Breast: The NRG Oncology/RTOG 1014 Phase 2 Clinical Trial. JAMA Oncol. 2020. Jan 1;6(1):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montagna G, Morrow M. Contralateral prophylactic mastectomy in breast cancer: what to discuss with patients. Expert Rev Anticancer Ther. 2020. Mar;20(3):159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawley ST, Li Y, An LC, et al. Improving Breast Cancer Surgical Treatment Decision Making: The iCanDecide Randomized Clinical Trial. J Clin Oncol. 2018. Mar 1;36(7):659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manne SL, Smith BL, Frederick S, et al. B-Sure: a randomized pilot trial of an interactive web-based decision support aid versus usual care in average-risk breast cancer patients considering contralateral prophylactic mastectomy. Transl Behav Med. 2020. May 20;10(2):355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.*.Jagsi R, Hawley ST, Griffith KA, et al. Contralateral Prophylactic Mastectomy Decisions in a Population-Based Sample of Patients With Early-Stage Breast Cancer. JAMA Surg. 2017. Mar 1;152(3):274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of interest shows the effect of surgeon recommendation on patients’ decisions regarding CPM.
- 25.Fisher B, Montague E, Redmond C, et al. Comparison of radical mastectomy with alternative treatments for primary breast cancer. A first report of results from a prospective randomized clinical trial. Cancer. 1977. Jun;39(6 Suppl):2827–39. [DOI] [PubMed] [Google Scholar]
- 26.**.Howard DH, Soulos PR, Chagpar AB, et al. Contrary To Conventional Wisdom, Physicians Abandoned A Breast Cancer Treatment After A Trial Concluded It Was Ineffective. Health Aff (Millwood). 2016. Jul 1;35(7):1309–15. [DOI] [PubMed] [Google Scholar]; This study of significant interest demonstrates the uptake and practice changes in the United States following publication of the ACOSOG Z0011 trial results.
- 27.**.Poodt IGM, Spronk PER, Vugts G, et al. Trends on Axillary Surgery in Nondistant Metastatic Breast Cancer Patients Treated Between 2011 and 2015: A Dutch Population-based Study in the ACOSOG-Z0011 and AMAROS Era. Ann Surg. 2018. Dec;268(6):1084–1090. [DOI] [PubMed] [Google Scholar]; This study of significant interest illustrates the success in altering clinical practice following publication of the ACOSOG Z0011 and AMAROS trial results.
- 28.Morrow M, Van Zee KJ, Patil S, et al. Axillary Dissection and Nodal Irradiation Can Be Avoided for Most Node-positive Z0011-eligible Breast Cancers: A Prospective Validation Study of 793 Patients. Ann Surg. 2017. Sep;266(3):457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castelo M, Hu SY, Dossa F, et al. Comparing Observation, Axillary Radiotherapy, and Completion Axillary Lymph Node Dissection for Management of Axilla in Breast Cancer in Patients with Positive Sentinel Nodes: A Systematic Review. Ann Surg Oncol. 2020. Aug;27(8):2664–2676. [DOI] [PubMed] [Google Scholar]
- 30.*.Kantor O, Means J, Grossmith S, et al. Optimizing Axillary Management in Clinical T1-2N0 Mastectomy Patients with Positive Sentinel Lymph Nodes. Ann Surg Oncol. 2021. Sep 1. [DOI] [PubMed] [Google Scholar]; This study of interest shows the real-world results of deferring completion ALND at the time of initial mastectomy in AMAROS-eligible patients.
- 31.Warren LE, Miller CL, Horick N, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: a prospective cohort study. Int J Radiat Oncol Biol Phys. 2014. Mar 1;88(3):565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goyal A, Dodwell D. POSNOC: A Randomised Trial Looking at Axillary Treatment in Women with One or Two Sentinel Nodes with Macrometastases. Clin Oncol (R Coll Radiol). 2015. Dec;27(12):692–5. [DOI] [PubMed] [Google Scholar]
- 33.de Boniface J, Frisell J, Andersson Y, et al. Survival and axillary recurrence following sentinel node-positive breast cancer without completion axillary lymph node dissection: the randomized controlled SENOMAC trial. BMC Cancer. 2017. May 26;17(1):379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation after axillary UltraSouND). Breast. 2012. Oct;21(5):678–81. [DOI] [PubMed] [Google Scholar]
- 35.Henke G, Knauer M, Ribi K, et al. Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically node-positive breast cancer (TAXIS): study protocol for a multicenter, randomized phase-III trial. Trials. 2018. Dec 4;19(1):667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reimer T, Stachs A, Nekljudova V, et al. Restricted Axillary Staging in Clinically and Sonographically Node-Negative Early Invasive Breast Cancer (c/iT1-2) in the Context of Breast Conserving Therapy: First Results Following Commencement of the Intergroup-Sentinel-Mamma (INSEMA) Trial. Geburtshilfe Frauenheilkd. 2017. Feb;77(2):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Roozendaal LM, Vane MLG, van Dalen T, et al. Clinically node negative breast cancer patients undergoing breast conserving therapy, sentinel lymph node procedure versus follow-up: a Dutch randomized controlled multicentre trial (BOOG 2013-08). BMC Cancer. 2017. Jul 1;17(1):459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simons JM, van Nijnatten TJA, van der Pol CC, et al. Diagnostic Accuracy of Different Surgical Procedures for Axillary Staging After Neoadjuvant Systemic Therapy in Node-positive Breast Cancer: A Systematic Review and Meta-analysis. Ann Surg. 2019. Mar;269(3):432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrio AV, Montagna G, Mamtani A, et al. Nodal Recurrence in Patients With Node-Positive Breast Cancer Treated With Sentinel Node Biopsy Alone After Neoadjuvant Chemotherapy-A Rare Event. JAMA Oncol. 2021. Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montagna G, Mamtani A, Knezevic A, et al. Selecting Node-Positive Patients for Axillary Downstaging with Neoadjuvant Chemotherapy. Ann Surg Oncol. 2020. Oct;27(11):4515–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013. Jul 1;31(19):2382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKevitt E, Cheifetz R, DeVries K, et al. Sentinel Node Biopsy Should Not be Routine in Older Patients with ER-Positive HER2-Negative Breast Cancer Who Are Willing and Able to Take Hormone Therapy. Ann Surg Oncol. 2021. Oct;28(11):5950–5957. [DOI] [PubMed] [Google Scholar]
- 43.**.Armani A, Douglas S, Kulkarni S, et al. Controversial Areas in Axillary Staging: Are We Following the Guidelines? Ann Surg Oncol. 2021. Oct;28(10):5580–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of significant interest highlights physician-associated variables that present as barriers in adopting new treatment guidelines.
- 44.**.Smith ME, Vitous CA, Hughes TM, et al. Barriers and Facilitators to De-Implementation of the Choosing Wisely(®) Guidelines for Low-Value Breast Cancer Surgery. Ann Surg Oncol. 2020. Aug;27(8):2653–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of significant interest highlights the approach and attitude of surgeons toward integrating de-escalation of care guidelines into their practice.
- 45.*.Wang T, Mott N, Miller J, et al. Patient Perspectives on Treatment Options for Older Women With Hormone Receptor-Positive Breast Cancer: A Qualitative Study. JAMA Netw Open. 2020. Sep 1;3(9):e2017129. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of interest reports on patients’ perspectives regarding Choosing Wisely recommendations.
- 46.Kalinsky K, Barlow WE, Gralow JR, et al. 21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. N Engl J Med. 2021;385(25):2336–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011. Nov 12;378(9804):1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray Brunt A, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020. May 23;395(10237):1613–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang SL, Fang H, Hu C, et al. Hypofractionated Versus Conventional Fractionated Radiotherapy After Breast-Conserving Surgery in the Modern Treatment Era: A Multicenter, Randomized Controlled Trial From China. J Clin Oncol. 2020. Nov 1;38(31):3604–3614. [DOI] [PubMed] [Google Scholar]
- 50.*.Bekelman JE, Sylwestrzak G, Barron J, et al. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008-2013. Jama. 2014. Dec 17;312(23):2542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of interest highlights the limited adoption of hypofractioned WBI in the United States.
- 51.Kang MM, Hasan Y, Waller J, et al. Has Hypofractionated Whole-Breast Radiation Therapy Become the Standard of Care in the United States? An Updated Report from National Cancer Database. Clin Breast Cancer. 2021. Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashworth A, Kong W, Whelan T, et al. A population-based study of the fractionation of postlumpectomy breast radiation therapy. Int J Radiat Oncol Biol Phys. 2013. May 1;86(1):51–7. [DOI] [PubMed] [Google Scholar]
- 53.Hickey BE, Lehman M. Partial breast irradiation versus whole breast radiotherapy for early breast cancer. Cochrane Database Syst Rev. 2021. Aug 30;8(8):Cd007077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gregucci F, Fozza A, Falivene S, et al. Present clinical practice of breast cancer radiotherapy in Italy: a nationwide survey by the Italian Society of Radiotherapy and Clinical Oncology (AIRO) Breast Group. Radiol Med. 2020. Jul;125(7):674–682. [DOI] [PubMed] [Google Scholar]
- 55.*.Chu QD, Zhou M, Medeiros KL, et al. Impact of CALGB 9343 Trial and Sociodemographic Variation on Patterns of Adjuvant Radiation Therapy Practice for Elderly Women (≥70 Years) with Stage I, Estrogen Receptor-positive Breast Cancer: Analysis of the National Cancer Data Base. Anticancer Res. 2017. Oct;37(10):5585–5594. [DOI] [PubMed] [Google Scholar]; This study of interest illustrates how the publication of the CALGB 9343 results has had minimal impact on reducing radiotherapy utilization in elderly women.
- 56.*.Shumway DA, Griffith KA, Sabel MS, et al. Surgeon and Radiation Oncologist Views on Omission of Adjuvant Radiotherapy for Older Women with Early-Stage Breast Cancer. Ann Surg Oncol. 2017. Nov;24(12):3518–3526. [DOI] [PubMed] [Google Scholar]; This study of interest reports misconceptions that physicians have regarding the benefit of radiotherapy in patients meeting CALGB 9343 eligibility criteria.
- 57.Montagna G, Morrow M. Breast-conserving Surgery Without Radiation Therapy for Invasive Cancer. Clin Breast Cancer. 2021. Apr;21(2):112–119. [DOI] [PubMed] [Google Scholar]
- 58.Breast International Group, International Breast Cancer Study Group. EXamining PErsonalised Radiation Therapy for Low-risk Early Breast Cancer (EXPERT) 2021 [Accessed January 3, 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT02889874
- 59.National Comprehensive Cancer Network (NCCN). NCCN Guidelines for Treatment of Cancer by Type, Version 5 2020 [Accessed January 3, 2022]. Available from: https://www.nccn.org/guidelines/recently-published-guidelines
- 60.**.Vaz-Luis I, O’Neill A, Sepucha K, et al. Survival benefit needed to undergo chemotherapy: Patient and physician preferences. Cancer. 2017. Aug 1;123(15):2821–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of significant interest demonstrates the differing thresholds for which patients would be willing to undergo chemotherapy for incremental improvements in survival.
- 61.Slevin ML, Stubbs L, Plant HJ, et al. Attitudes to chemotherapy: comparing views of patients with cancer with those of doctors, nurses, and general public. Bmj. 1990. Jun 2;300(6737):1458–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2018. Jul 12;379(2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and Genomic Risk to Guide the Use of Adjuvant Therapy for Breast Cancer. N Engl J Med. 2019. Jun 20;380(25):2395–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.*.Carlson JJ, Roth JA. The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013. Aug;141(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of interest demonstrates the impact of Oncotype DX in altering chemotherapy recommendations.
- 65.Dieci MV, Guarneri V, Zustovich F, et al. Impact of 21-Gene Breast Cancer Assay on Treatment Decision for Patients with T1-T3, N0-N1, Estrogen Receptor-Positive/Human Epidermal Growth Receptor 2-Negative Breast Cancer: Final Results of the Prospective Multicenter ROXANE Study. Oncologist. 2019. Nov;24(11):1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tolaney SM, Guo H, Pernas S, et al. Seven-Year Follow-Up Analysis of Adjuvant Paclitaxel and Trastuzumab Trial for Node-Negative, Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer. J Clin Oncol. 2019. Aug 1;37(22):1868–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mougalian SS, Soulos PR, Killelea BK, et al. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer. 2015. Aug 1;121(15):2544–52. [DOI] [PubMed] [Google Scholar]
- 68.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017. Jun 1;376(22):2147–2159. [DOI] [PubMed] [Google Scholar]
- 69.von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019. Feb 14;380(7):617–628. [DOI] [PubMed] [Google Scholar]
- 70.Narod SA, Iqbal J, Giannakeas V, et al. Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ. JAMA Oncol. 2015. Oct;1(7):888–96. [DOI] [PubMed] [Google Scholar]
- 71.Julian TB, Land SR, Fourchotte V, et al. Is sentinel node biopsy necessary in conservatively treated DCIS? Ann Surg Oncol. 2007. Aug;14(8):2202–8. [DOI] [PubMed] [Google Scholar]
- 72.El Hage Chehade H, Headon H, Wazir U, et al. Is sentinel lymph node biopsy indicated in patients with a diagnosis of ductal carcinoma in situ? A systematic literature review and meta-analysis. Am J Surg. 2017. Jan;213(1):171–180. [DOI] [PubMed] [Google Scholar]
- 73.Worni M, Akushevich I, Greenup R, et al. Trends in Treatment Patterns and Outcomes for Ductal Carcinoma In Situ. J Natl Cancer Inst. 2015. Dec;107(12):djv263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stuart KE, Houssami N, Taylor R, et al. Long-term outcomes of ductal carcinoma in situ of the breast: a systematic review, meta-analysis and meta-regression analysis. BMC Cancer. 2015. Nov 10;15:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Correa C, McGale P, Taylor C, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010(41):162–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Solin LJ, Gray R, Hughes LL, et al. Surgical Excision Without Radiation for Ductal Carcinoma in Situ of the Breast: 12-Year Results From the ECOG-ACRIN E5194 Study. J Clin Oncol. 2015. Nov 20;33(33):3938–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCormick B, Winter K, Hudis C, et al. RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol. 2015. Mar 1;33(7):709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.**.Hawley ST, Janz NK, Griffith KA, et al. Recurrence risk perception and quality of life following treatment of breast cancer. Breast Cancer Res Treat. 2017. Feb;161(3):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of significant interest illustrates how a significant proportion of women with DCIS overestimate their risk of systemic recurrence.
- 79.Subhedar P, Olcese C, Patil S, et al. Decreasing Recurrence Rates for Ductal Carcinoma In Situ: Analysis of 2996 Women Treated with Breast-Conserving Surgery Over 30 Years. Ann Surg Oncol. 2015. Oct;22(10):3273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katz SJ, Lantz PM, Janz NK, et al. Patterns and correlates of local therapy for women with ductal carcinoma-in-situ. J Clin Oncol. 2005. May 1;23(13):3001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rudloff U, Jacks LM, Goldberg JI, et al. Nomogram for predicting the risk of local recurrence after breast-conserving surgery for ductal carcinoma in situ. J Clin Oncol. 2010. Aug 10;28(23):3762–9. [DOI] [PubMed] [Google Scholar]
- 82.Solin LJ, Gray R, Baehner FL, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013. May 15;105(10):701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rakovitch E, Parpia S, Koch A, et al. DUCHESS: an evaluation of the ductal carcinoma in situ score for decisions on radiotherapy in patients with low/intermediate-risk DCIS. Breast Cancer Res Treat. 2021. Jul;188(1):133–139. [DOI] [PubMed] [Google Scholar]
- 84.Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 2012. Apr 20;30(12):1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011. Jan;12(1):21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zujewski JA, Harlan LC, Morrell DM, et al. Ductal carcinoma in situ: trends in treatment over time in the US. Breast Cancer Res Treat. 2011. May;127(1):251–7. [DOI] [PubMed] [Google Scholar]
- 87.Hwang ES, Solin L. De-Escalation of Locoregional Therapy in Low-Risk Disease for DCIS and Early-Stage Invasive Cancer. J Clin Oncol. 2020. Jul 10;38(20):2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pilewskie M, Stempel M, Rosenfeld H, et al. Do LORIS Trial Eligibility Criteria Identify a Ductal Carcinoma In Situ Patient Population at Low Risk of Upgrade to Invasive Carcinoma? Ann Surg Oncol. 2016. Oct;23(11):3487–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Partridge A, Adloff K, Blood E, et al. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. J Natl Cancer Inst. 2008. Feb 20;100(4):243–51. [DOI] [PubMed] [Google Scholar]
- 90.Pilewskie M, Olcese C, Patil S, et al. Women with Low-Risk DCIS Eligible for the LORIS Trial After Complete Surgical Excision: How Low Is Their Risk After Standard Therapy? Ann Surg Oncol. 2016. Dec;23(13):4253–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.**.Davis DA, Thomson MA, Oxman AD, et al. Changing physician performance. A systematic review of the effect of continuing medical education strategies. Jama. 1995. Sep 6;274(9):700–5. [DOI] [PubMed] [Google Scholar]; The study of significant interest demonstrates the improved success rate in affecting change in clinical practice with targeted interventions.
- 92.van Hoeve J, de Munck L, Otter R, et al. Quality improvement by implementing an integrated oncological care pathway for breast cancer patients. Breast. 2014. Aug;23(4):364–70. [DOI] [PubMed] [Google Scholar]
- 93.Haward R, Amir Z, Borrill C, et al. Breast cancer teams: the impact of constitution, new cancer workload, and methods of operation on their effectiveness. Br J Cancer. 2003. Jul 7;89(1):15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ehdaie B, Assel M, Benfante N, et al. A Systematic Approach to Discussing Active Surveillance with Patients with Low-risk Prostate Cancer. Eur Urol. 2017. Jun;71(6):866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. 2015. Jun;12(6):335–43. [DOI] [PubMed] [Google Scholar]
- 96.Veronesi U, Stafyla V, Luini A, et al. Breast cancer: from “maximum tolerable” to “minimum effective” treatment. Front Oncol. 2012;2:125. [DOI] [PMC free article] [PubMed] [Google Scholar]


