Abstract
Much research has shown Bee venom to be an effective neuroprotective agent. However, the usual transdermal injection of bee venom poses many pharmacokinetic disadvantages. Here, we compared the administration of bee venom via subcutaneous injection (SC) and via Microneedle patch (MN). Both administrated routes produce significant recovery effects, however: the MN significantly prolongs the bio-significant-and-yet-lower concentration of bee venom in mice bodies. In contrast, SC could produce only a short period of much higher bee venom levels in the blood and brain. We also see that due to the concentration-response-curve of bee venom (represented by melittin): mice bodies do not require much higher bee venom concentration (seen in the SC group) to produce a much more significant neuroprotective effect (than seen in those treated with the MN method). Therefore, a MN could maintain bee venom levels in mice bodies at lower-yet-more-efficient concentrations. This is important, as bee venom can cause more adverse effects and pain sensations, at higher concentrations. For the first time, we confirmed that the pharmacokinetic advantages of MN delivered bee venom also guarantee a holistic neuroprotection effect (which was shown by SC delivered bee venom in previous research). This was proven via the results of the water maze experiments for long-term learning memory assessment and protein analysis of key neuronal regulatory proteins: BDNF, p-CREB, iNOS, and mArhR 1. In conclusion, for situations where we ought to administrate drugs at a more downward amount, such as bee venom, MN can keep the therapeutic concentrations at a lower, yet interestingly, more-efficient level.
Keywords: Neurodegeneration, bee venom, melittin, microneedle patch, pharmacokinetic
1. Introduction
For centuries, bee venom has been a therapeutic source for curing a diverse array of human disorders (Pucca et al., 2019; Kasozi et al., 2020); recently, the focus on bee venom as a therapy has gained steady interest from researchers and practitioners given bee venom’s anti-neurodegeneration ability, with recent studies have depicted bee venom’s significant anti-neurodegenerative properties via a wide-range of mechanisms (Moon et al., 2006, 2007; Doo et al., 2012; Chung et al., 2015; Gu et al., 2015; Hwang et al., 2015; Im et al., 2016; Ye et al., 2016; Cai et al., 2017; Ham et al., 2019a, 2019b; Nguyen & Lee, 2021; Duc Nguyen et al., 2022).
In most in vivo and clinical bee venom biological efficacy studies (Jang & Kim, 2020), including neuro research above, transdermal injections were used to administrate the bee venom to treating subjects. Drugs administered trans dermally exhibit high bioavailability as they avoid gastrointestinal processing and the first-pass metabolic process (Nagarkar et al., 2020). However, raw drugs injections under the skin usually result in a sharp rise in blood plasma concentrations within the early stage of administration, and dramatically decreases after a max concentration is reached, thereby effectively pushing the drug concentration beyond its useful therapeutic window (Kagan, 2014; Wang et al., 2019). In the case of bee venom solution injections, as concentration levels increase sharply, the injection method can induce sharp and painful sensations and other adverse effects (Chen et al., 2016; Burzyńska & Piasecka-kwiatkowska, 2021). Since effective bee venom concentrations can only be maintained at an effective level for a short period of time, the outcome bioactivity can fall well short of its needed therapeutic levels.
Recently, in other disease research using bee venom products, such as rheumatoid arthritis, ideas emerged that we could administrate bee venom via a microneedles patch (MN) assisted delivering method, however, non in-dept work to uncover possible improvements in pharmacokinetics pattern has been made (Zhao et al., 2016; Du et al., 2021). Bee venom delivering dosages must be conducted repeatedly to enhance the disordered condition over a planned period. Therefore, it may help to develop means to assist patients to take the medication without the hassle of performing self-injections (when the presence of a medical personal is not available) (Schiff et al., 2017; Jung & Jin, 2021), also produce an economical administrative solution (B. Y. Lee et al., 2015; Chen et al., 2020) that causes minimal pain. MN delivery system is an emerging area for both the systemic and local delivery of macromolecules and helps generalize the application of medicine (Bariya et al., 2011; Tran et al., 2021; Zhang et al., 2021). The ability to control the release of the drug via MN and maintain the body’s drug levels in a more-stable pharmacokinetic pattern can act as a good solution for the administration of bee venom (Avcil & Çelik, 2021).
Upon that ground, we proposed this in-depth study of pharmacokinetics improvement of using MN to deliver bee venom trans dermally, especially applied to the case study its’ neuroprotection effect. Clear understandings of MN delivered bee venom advantages are helpful in further promoting bee venom as a neuroprotective medication.
2. Materials and methods
2.1. Animal and drug administration
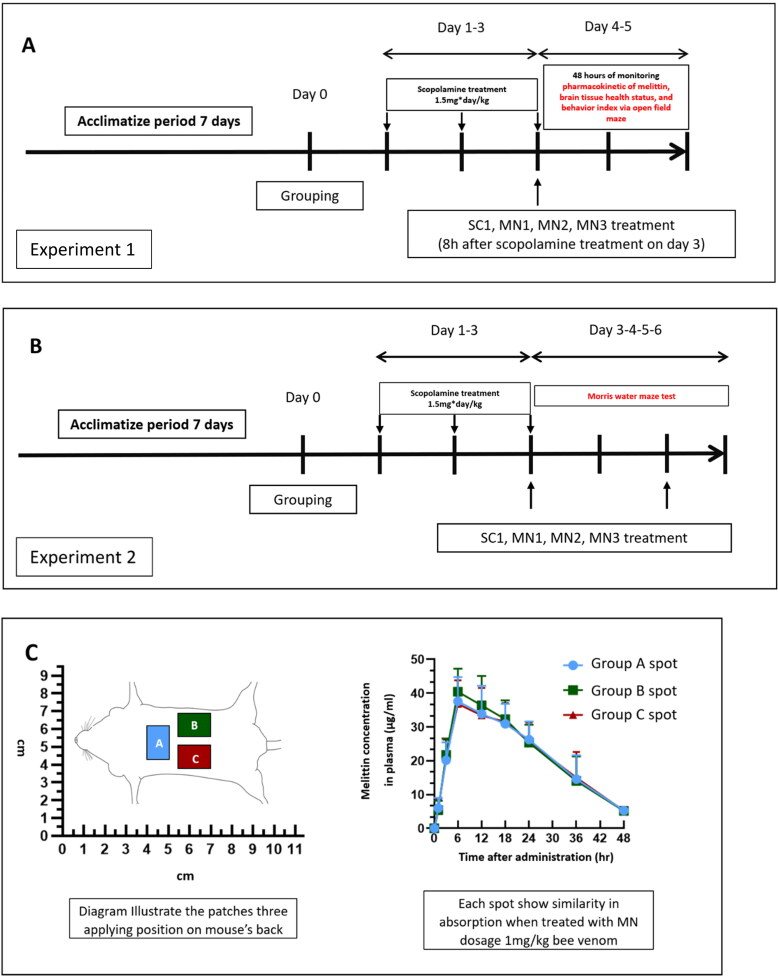
Two experiments 1 and 2 were conducted (Figure 2). Twenty-week-old BALB/c male (38–42 g/mouse) mice were purchased from Samtaco (Gyeonggi-do, Republic of Korea). A 12 h light-dark cycle was maintained at a constant temperature and at a constant level of humidity (room temperature 25 ± 2 °C). Feed and water were supplied to the mice sufficiently, and which they were allowed to ingest freely. After acclimatizing for six days, the mice were randomly divided into groups. Two consecutive experiments were carried out for the purposes of this study: (1) To study the pharmacokinetic effect of a single administration of bee venom via SC and MN (Figure 2A), at each time point for blood plasma and brain sample analysis, four mice were randomly chosen from each group to be sacrificed; and (2) To determine the effect of treatment on long-term memory and learning abilities (Figure 2B). Group SC1 received 1 mg/kg SC of 0.05 ml bee venom solution via SC, at a non-acupoint: Hypochondrium—located 10 mm above the iliac crest (Duc Nguyen et al., 2022). For MN administration, we used the ‘RAPBV21’ product, which is a MN version loaded with bee venom manufactured by RAPHAS (Seoul, Korea), our research partner. Each MN contained 0.04 mg of honeybee venom (Chungjin Bio tech, Gyeonggi-do, Korea) each −40–50% of which was the dried weight of the venom’s melittin, as per the producer’s information.
Figure 2.
Two consecutive in vivo experiments—experiment 1 and experiment 2—were carried out: (A) To explore the pharmacokinetic of Melittin—the chosen marker compound—we administered bee venom via an SC injection and via MN, observing the level of melittin in blood plasma and in the brain, along with the effect of the treatment on brain tissue and locomotor behavior indexes. (B) After observing the effect of MNs regarding bee venom, an experiment was carried out to verify the effect of the SC and MN treatments on long term memory and learning abilities. (C) From one to three patches were needed to be applied in animals among the MN1 to MN3 groups. Therefore, we examined the absorption equivalence of these attached sites: There was three A, B and C spot’s groups, animals in each group were administrated with a single MN (1 mg/kg) at either A, B, or C spots on their backs. Four mice from each group were sacrificed at each time point to provide the data. The result suggested that all three spots A, B, and C facilitate similar bee venom absorption with MN delivery method. SC1, bee venom subcutaneous 1 mg/kg; MN3, applied with 3 × bee venom patches (total 3 mg/kg); MN3, applied with 2 × bee venom patches (total 2 mg/kg); and MN1, applied with 1 × bee venom patch (total 1 mg/kg).
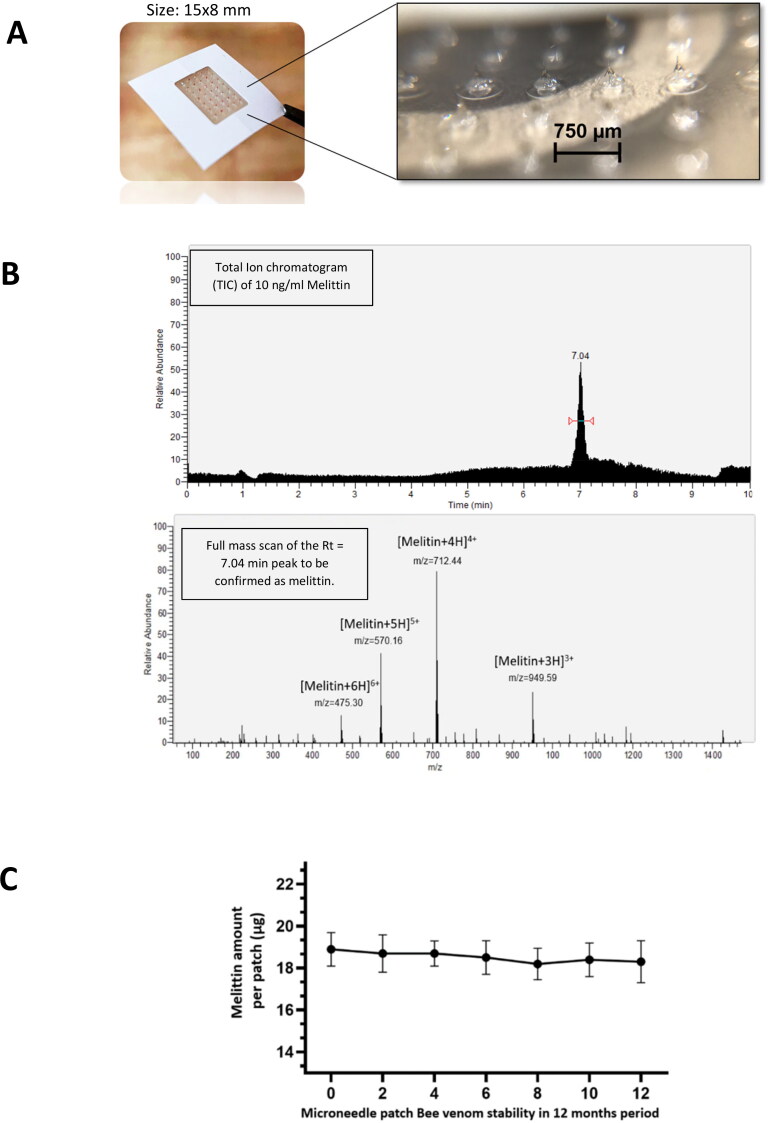
Figure 1.
Bee venom-loaded microneedle patches utilized in the experiment. (A) Needle array on the MN surface. (B) We chose melittin as the marker compound for analysis of bee venom pharmacokinetics. Total ion Chromatogram display of melittin standard compound. We analyze the peak Rt = 7.04 min and showed the representative un-fragmented mass spectrometry profile to confirm as the Melittin peaks: [M + 3H]3+ m/z = 948.59, [M + 4H]4+ m/z = 712.9, Melittin [M + 5H]5+ m/z = 570.16, and Melittin [M + 6H]6+ m/z = 475.30 (Hematyar et al., 2018; Huang et al., 2020). These are standard data for the qualitative and quantitative evaluations of bee venom in all plasma and brain extracts via MN patch samples. (C) Stability of melittin, the main component of the bee venom in the MN.
Applying the patches to the mice was carried out in a similar manner to prior research done by the RAPHAS company (Park et al., 2020). Follow this protocol the patches (15 × 8 mm) were applied to the back of mice, this is also the similar site of many other studies using mouse model to study MN drug delivery (Bhatnagar et al., 2019; Xu et al., 2020; Wang et al., 2021). Since there are mice which needs to be applied up to three patches onto their backs. We needed to examine the absorption equivalence of these attached sites: There was three A, B and C spot’s groups, animals in each group were administrated with a single MN (1 mg/kg) at either A, B, or C spots. Four mice from each group were sacrificed at each time point to provide the data. The result suggested that all three spots facilitate similar bee venom absorption with MN delivery method (Figure 2C), which is a good basement for applying multiple MN on a single mouse. MN3, applied with 3 × bee venom patches (total dosage 3 mg/kg); MN2, applied with 2 × bee venom patches (total dosage 2 mg/kg); and MN1, applied with 1 × bee venom patch (dosage 1 mg/kg). There is only one SC1 group is because 1 mg/kg bee venom delivered via SC has been successfully shown to possess significant biological effect in previous research (Duc Nguyen et al., 2022). Therefore, SC1 is set as the standard group, whereas we selected a range of dosages in MN delivered bee venom to compare the characteristics of MN groups to the SC1 standard group. Animal per group: Experiment 1: From each group, 4 animals were sacrificed at each time point to collect the data, 8 animals were used in the open field maze experiment, totally 66 mice/group. Experiment 2: 8 mice/group.
2.2. Collection of brain and blood
The mice were anesthetized, blood was collected from their hearts and their brains were collected after opening the scalp and immediately rinsed with physiological saline. These samples were subsequently subjected to further analysis in the same day.
2.3. Extraction of melittin from brain tissue and blood plasma
After collection, brain tissue was immediately homogenized with −20 °C methanol (0.5 mL/50 mg tissue); −20 °C chloroform (0.5 mL/50 mg tissue) was then added, mixed, and incubated at 2 °C for 15 min. Subsequently, ice-cold water (0.5 mL/50 mg tissue) was added before the mixing and incubation processes were repeated. Phase separation was conducted via centrifuging the sample at 13,000 rpm for 5 min at 4 °C, all in the supernatant aqueous phase—which contains water solute components, including melittin—was collected, freeze dried and dissolved in 50 μL distilled water for Mass spectrometer analysis.
Blood was let to clot for 30 min and centrifuged at 2000 g for 5 min to obtain serum. The 50 µL serum was added to 50 µL of 5% DMSO with 0.1% acetic acid, then vortexed for 5 min. The mixture was then centrifuged at 3000 × g for 3 min. The supernatant was collected for mass spectroscopy analysis.
2.4. Mass spectrometry analysis
The melittin extracted from the brains was analyzed with LC − MS/MS. Baseline, peak spiking, and calculation establishments closely followed a previous publish research (Nguyen et al., 2022). Separation was conducted via an Ultimate 3000 Ultra-High-Performance Liquid Chromatography (UHPLC) system, and mass detection was achieved using an LTQ-Orbitrap Velos (Thermo Fisher Scientific, San Jose, CA). The LC separation module included an analytical column Accucore™ Vanquish™ C18+ UHPLC Columns of 1.5 μm particle size and a diameter of 2.1 × 100 mm. The flow rate was maintained at 0.3 mL/min. The sample injection volume was set at 2.0 μL. The two eluent solvents of water (A) and acetonitrile (B) were used. A time-dependent gradient was determined as follows—0–3 min: hold B at 5%; 3–9 min: increase B to 100% from 5%; 9–9.5 min: hold B at 100%; and 9–10 min: Reduce B from 100% to 0%. The interface was selected at 4.6 kV and 270 °C, and the detection voltage was set at 1.97 kV. Under a positive ionization mode, the mass survey scans were conducted in the FT cell in the range of 100 to 1,400 m/z (within the range of the instrument). The automatic gain control was 1 × 106 ions. A test with commercial standard melittin (M4171, Sigma-Aldrich, MO, USA) was made to determine the analysis condition and melittin peak retention time. The peak observed via TIC was analyzed to show the representative un-fragmented mass spectrometry profile of melittin. This profile was then confirmed with the results of other studies (Hematyar et al., 2018; Huang et al., 2020); subsequently, this data is used to specifically scan for the detection of the melittin signal at the respective retention times to perform the qualitative and quantitative evaluations of bee venom in all plasma and brain extracts via MN patch samples. The standard melittin concentration was prepared from 0.01 to 500 ng/mL for the setup calibration curve and to calculate the melittin amount in the samples corrodingly.
2.5. Brain ROS, MDA and ATP levels biochemical assay
For reactive oxygen species (ROS), lipid peroxidation (MDA), and adenosine triphosphate (ATP) levels, mice brains were homogenized with phosphate-buffered saline (PBS) at 3 °C, then centrifuged for 10 min at 10,000 × g at 4 °C with 10% homogenates; the supernatant was collected and stored at −80 °C for biochemical assessment.
The total protein content was determined using a bicinchoninic acid (BCA) Protein Assay Kit (ab102536, Abcam, Cambridge, UK). Examinations on ROS were based on previously established methods (Hayashi et al., 2007; J. S. Lee et al., 2015), and lipid peroxidation was conducted using an MDA Assay Kit (ab102536, Abcam, Cambridge, UK), and an ATP kit (ab83355, Abcam, Cambridge, UK).
2.6. Open field behavior test
A chamber of 50 cm length × 50 cm width × 38 cm height was made from white non-porous plastic. In the middle of the maze, a 17 × 17 cm virtual square was placed, as determined by the monitoring software. The chamber was cleaned with ethanol 80% to remove any scent clues from the previously tested mouse. For each test, a mouse was allowed to move around the maze for 10 min, with their behavior recorded via Any-Maze software (Stoelting Co., Wood Dale, USA). In total, eight mice were tested per group/time.
2.7. Morris water maze test
The behavioral experiment commenced 30 min after the scopolamine injection. The Morris water maze was constructed by filling, using tap water in a black circular water tank (diameter = 120 cm; height = 50 cm) surrounded by various visual signals on a pole in a fixed position throughout the entire experiment. Water temperature was maintained at 22 ± 2 °C. The water tank was divided into four quadrants: southeast, northeast, southwest, and northwest. A white platform (diameter = 10 cm; height = 25 cm) was in the middle of the northwest quadrant. The swimming activity of the mice was recorded with an overhead video camera and analyzed using the Any-Maze software (Stoelting Co., Wood Dale, USA).
On day three, adaptation training was performed. The animals were allowed to swim freely for 100 s in the tank with the platform visible for 1 cm above the water. This was conducted three times a day for each mouse; if a mouse was unable to find the platform, it was gently guided to the platform’s position manually.
Between days four to six, the platform was submerged 1 cm below the water’s surface. The mouse was placed at the center of the northeast quadrant in the first test of the day and at the center of the southwest quadrant in the second test. The mouse was then allowed to find the platform within 100 s, and if the platform could not be found, the mouse was guided to the platform and kept in place for 10 s. Then, this mouse was then gently placed in a warm water bag and transferred back to their cage.
After the final hidden platform test on day six, the platform was removed from the water tank, and each mouse was placed in the center of the southeast quadrant and allowed to find the disappeared platform for 120 s. Swimming distributions of all groups were recorded, allowing us to generate heatmaps to represent each group’s recall memory of the platform’s position.
2.8. Western blot analysis
Brain samples were homogenized in a 50 mM tris (hydroxymethyl) aminomethane (Tris-HCl, pH 7.4) solution containing phosphate and protease inhibitors at approximately 0 °C. After centrifugation (12,000 rpm) at 4 °C for 10 min, the total protein content was determined using a BCA protein analysis kit (ab102536, Abcam, Cambridge, UK). The protein sample was mixed with a 1/4 volume loading buffer and then heated at 100 °C for 5 min. 20 μg of protein from each sample was electrophoresed using 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride membrane (PVDF). The membrane was then blocked with 5% nonfat milk at room temperature for 1 h and incubated with anti-BDNF (MABN79, MercK KGaA, Darmstadt, Germany), anti-p-CREB (06-519, Sigma-Aldrich, MO, USA), anti-iNOS (ab178945, Abcam, Cambridge, UK), anti-mAchR (m1M9808, Merck KgaA, Darmstadt, Germany). To quantify protein expression by western blotting, β-actin was used as a control (Zhang et al., 2012; Gilda & Gomes, 2015) therefore membranes were also incubated with anti-β-actin antibody (ab8226, Abcam, Cambridge, UK).
The membrane was subsequently rinsed with a phosphate-buffered saline (PBST) solution with Tween 20 and incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit (G-21234, Thermofisher, Massachusetts, USA) secondary antibody at room temperature for 2 h. After thorough washing with PBST, the protein bands were visualized using an ECL prime kit (GERPN2236, Sigma-Aldrich, MO, USA). Stained images were obtained using the Amersham™ ImageQuant™ system (Boston, USA), and postmortem data processing was performed using ImageJ (http://imagej.nih.gov/).
2.9. Formulation of concentration-response curve
The inhibition rate at a selected time point was calculated as: [OS (Scopolamine only group) − OS (Treatment group)]/[OS ((Scopolamine only group)-OS (Normal group)] × 100(%). OS: oxidative stress ROS or MDA value. This inhibition rate is then match with the respective melittin level at that same time point to form up the concentration-response curve of melittin (represents for bee venom). The data of ROS and MDA level are only used from within the first sixth hour. The reason for this is: we realized that at these early hours, the reduction of ROS and MDA could be mostly thanks to bee venom direct effect, which is actively triggering the neurons inner cellular antioxidant barrier and suppressing oxidative stress such as ROS and MDA (Kurek-Górecka et al., 2020; Duc Nguyen et al., 2022); As the time goes on beyond 6 hours after bee venom administration: the recovery of cell internal living functions had improved (seen via ATP levels enhancement Figure 3(G) (Masasuke Yoshida, 2001)) and these functions can significantly reduce ROS and MDA level themselves (Jo et al., 2020).
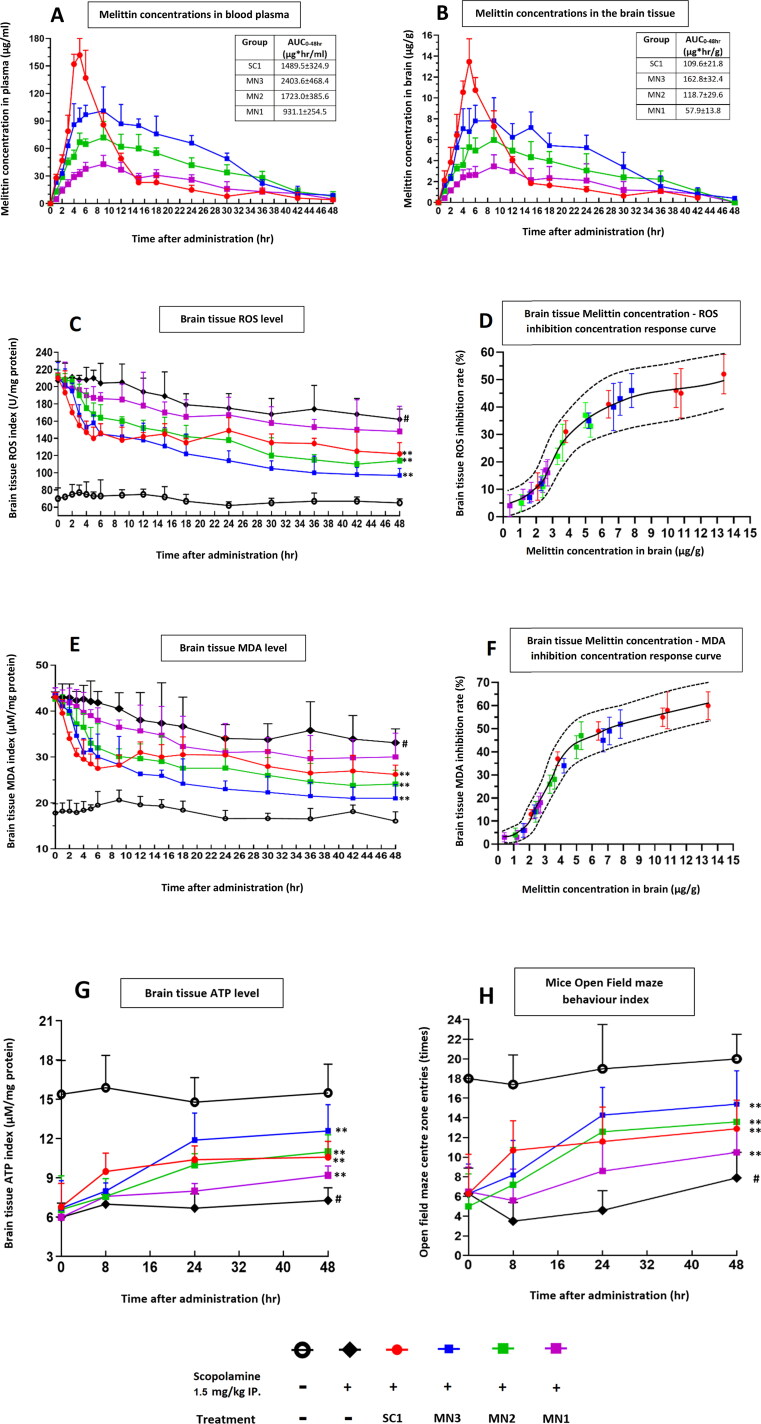
Figure 3.
In experiment 1, the effect of scopolamine and bee venom treatments on brain oxidative and ATP indexes were surveyed at 0, 1, 2, 3, 4, 5, 6, 9, 12, 15, 18, 24, 30, 36, 42, 48 hours after treatment. For each time point, 4 mice from each group were sacrificed and their brains were collected and processed for mass spectroscopy analysis and biochemical assays. (A) Melittin concentrations in blood plasma. (B) Melittin concentrations in brain tissue. (C) Brain tissue ROS index. (D) Correlation between melittin concentration in the brain tissue and ROS level inhibition rate. (E) Brain tissue MDA index. (F) Correlation between melittin concentration in the brain tissue and MDA level inhibition rate. (G) ATP level in the brain tissue. (H) Behavior index of mice tested in an open field maze, the times that the mice enter the center zone were recorded as the index to represent locomotor function, and which is directly related with CNS status. SC1, bee venom subcutaneous 1 mg/kg; MN3, applied with 3 × bee venom patches (total 3 mg/kg); MN2, applied with 2 × bee venom patches (total 2 mg/kg); MN1, applied with 1 × bee venom patch (total 1 mg/kg). #p < .01 compared with Naive group; *p < .05 compared with the Scopolamine group; **p < .01 compared with the Scopolamine group.
2.10. Statistical analysis
Statistical analysis was performed using SPSS version 18.0, and data are presented as the mean ± SD. Escape delay time and path length data presented in the Morris water maze test were analyzed using a two-way repeated measures analysis of variance (ANOVA). Other behavioral data and biomarker changes were analyzed using a one-way ANOVA for multiple comparisons. After ANOVA analysis, a Dunnett’s test was performed as a post hoc test.
3. Results
3.1. Pharmacokinetis of melittin as the marker compound of bee venom in blood and brain samples of tested mouse models
After administration, the SC1 group experienced a rapid spike in their plasma melittin concentrations, from 0 μg/ml at 0 h up to nearly 150 μg/ml after 5 hours. However, the concentration of melittin sharply decreased down to under 30 μg at the 15th hour and was maintained at this low level for the rest of the experiment period. In the brain, similarly, a melittin concentration peak was detected at 12 μg/g by the fifth hour, with the level also exhibiting the same sharp decline trails as seen in plasma; after the 24th hour very low level of melittin (under 2 μg/g) was found in the brain extracts (Figure 3A and 3B).
For the MN3 and MN2 groups, the pharmacokinetic result contrasts to those of the SC1 treatment. Between the onset and the fourth hour, we saw a rapid increase in melittin levels in both plasma (from 0 to 60–90 μg/ml) and brain tissue (from 0 to 4–7 μg/g). The high plateau concentrations of MN melittin was maintained between the fourth and ninth hour. After this period, both groups exhibited much slighter decreases over time until the 30th hour, when compared to the SC1 group; in this period, both MN3 and MN2 treatment could maintain melittin levels much more significant than those seen in SC1 administration. For the MN1 group, the pharmacokinetic pattern is similar to that of MN2 and MN3, albeit at a much lower scale.
The AUC (area under curve) value expresses that at a same dosage of 1 mg/kg bee venom per mouse, the MN method only could deliver into the blood stream about haft of that compared to the SC administration (MN1 931.1 ± 254.5 μg hr/ml vs. SC1 1489.5 ± 324.9 μg hr/ml). MN2 with 2 mg/kg, and SC1 with 1 mg/kg bee venom both could delivered relatively similar bee venom amount the blood and brain (MN2 1723.0 ± 385.6 μg hr/ml vs. SC1 1489.5 ± 324.9 μg hr/ml). Whereas MN3 with a triple bee venom payload only can deliver about 1.6 time more than that of SC1 (MN3 2403.6 ± 468.4 μg hr/ml vs. SC1 1489.5 ± 324.9 μg hr/ml).
3.2. Brain tissue expression of ROS, MDA in correlation with melittin concentration, which showcased MN effectiveness; ATP levels and effects on open maze behavioral activity
Actively promote the intercellular HO-1 antioxidant enzyme to suppress oxidative stress such as ROS and MDA is an ability of bee venom and melittin proven in various studies, and not only to neurological research. Oxidative stress is the sensitive and reactive indexes, and their changes are in the forefront period to any neurodegeneration inducer or neuroprotection promoter phenomenal, therefore ROS and MDA indexes are used to monitor bee venom bioactivity level in mice brain (Hanafi et al., 2018; Hozzein et al., 2018; Abd El-Haleim, 2020; Kurek-Górecka et al., 2020; Kim et al., 2021; Nguyen & Lee, 2021; Duc Nguyen et al., 2022).
The results of these parameter improvements differ between the MN and SC treatments in 2 periods from the 0 to 10th hours and from the 10th to 48th hours:
From the 0th to 10th hour post-treatment, the SC1 group showed a better suppression of ROS (Figure 3C) and MDA levels (Figure 3E), and enhanced ATP levels and open field maze activity index than that produced via MN treatment (Figure 3G and 3H).
From the 10th to 48th hours, the SC1 group’s brain ROS and MDA level, ATP levels and open field maze activity index exhibited few significant changes. In this later period, their melittin concentrations in plasma and the brain dropped significantly, whereas the MN3 and MN2 groups’ melittin concentrations were still maintained much more significant. The mentioned biological scores in the MN3 and MN2 groups were enhanced continuously. In this period, these improvements of the MN3 and MN2 surpassing those of the SC1 group (Figure 3A–C and 3E).
For the MN1 group, all improvements are expressed only slightly improvements when compared to the scopolamine-only treated group throughout the duration of the trial.
The concentration-response-curve of ROS and MDA to melittin (representative compound to bee venom) level: This expressed the ability of melittin (and bee venom) in inhibiting these two oxidative stress levels. Generally, the effect of treatment rises rapidly (from 0 to around 30–50% ROS or MDA inhibition) when melittin level in the brain increases from 0 to 5 μg/g. However, when melittin levels continue to increase above 5 μg/g, the correlation inhibition level of the treatment slow down, for instance when melittin reaches around 14 μg/g, the inhibition ability is only about 1.3–1.5 times more than that when melittin was at 5 μg/g (Figure 3D and 3F).
This meant the SC1 treatment produces an unnecessary high spike of melittin level in mice brain and body (up to around 14 μg/g at the 4–6th hours) and the effect of this high concentration is not much more effective to those of MN3 and MN2 (around 4 to 8 μg/g at the 4–30th hours) (Figure 3A, 3B, 3D, and 3F).
3.3. The improvement in long-term memory and learning ability
After the pharmacokinetic study, we experimented to determine whether the improvements of MN pharmacokinetics could also guarantee a neuroprotective effect on long-term learning and memory ability.
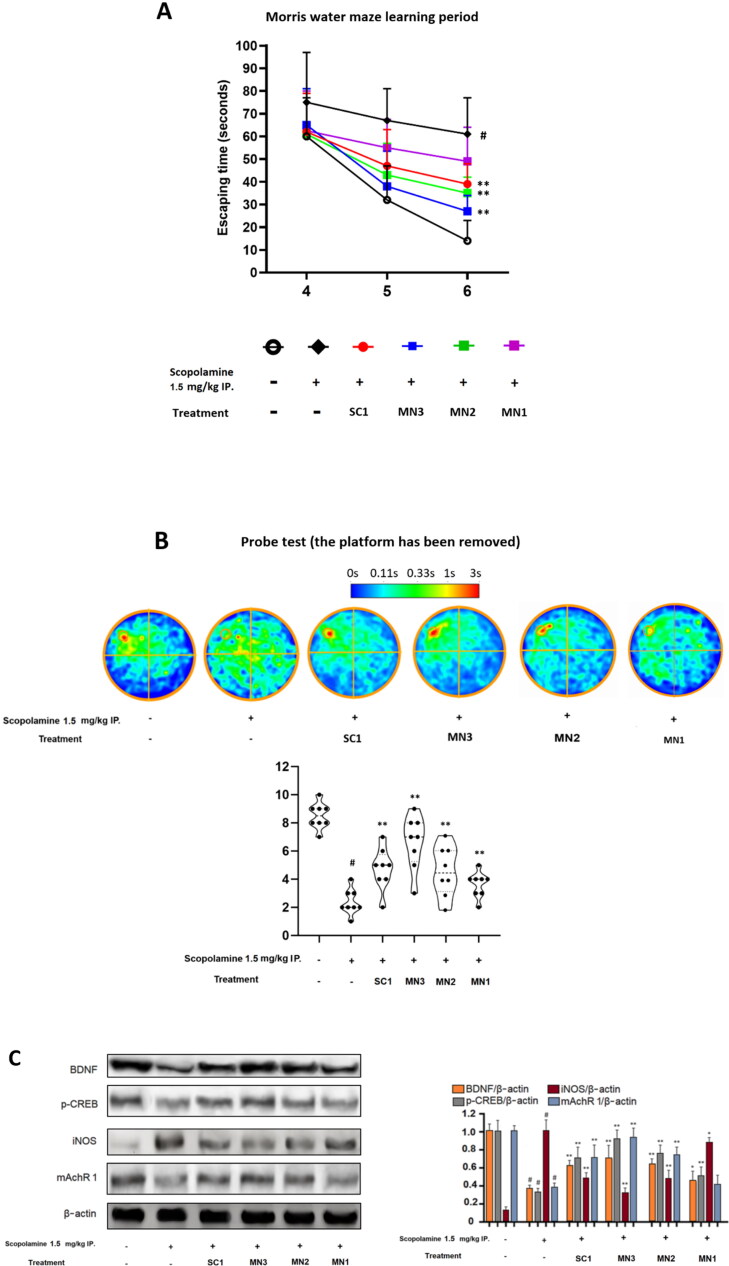
The learning score implied that mice from the MN3 group exhibited a good overall learning ability for the escape time on day six—which is very close to those in the naïve, normal non-treatment group. The SC1 and MN2 exhibited similar recovery level group also showed significant improvements compared to the scopolamine-only treated group—however, the differences are not as stark as that of the MN3 group. The MN1 group only expresses very minor enhancements. In line with these results, the probe test result at the end of day six also confirmed that MN3 was the most effective in protecting mice against scopolamine-induced amnesia. This is followed by the SC1 and MN2 group’s enhancements, whereas the MN1 group exhibited improvement results with a only slight statistically significant when compared to the scopolamine-only treated group (p = .046) (Figure 4A and 4B).
Figure 4.
In experiment 2: The effect the effect of the SC and MN treatments on long term memory and learning ability tested with Morris water maze. (A) Learning performance in the training period from day 4 to 6. (B) Within Morris water maze experiment, on day 6 at the end of the schedule a probe test was carried out, in which the platform was removed, and mice were let to swim freely to assess their remembrance of the platform position. The summarized data were made in the form of heat map for each group. (C) Western blot experiment was carried out to confirm the effect of treatment methods on brain tissue intercellular regulation. SC1, bee venom subcutaneous 1 mg/kg; MN3, applied with 3 × bee venom patches (total 3 mg/kg); MN2, applied with 2 × bee venom patches (total 2 mg/kg); MN1, applied with 1 × bee venom patch (total 1 mg/kg). #p < .01 compared with Naive group; *p < .05 compared with Scopolamine group; **p < .01 compared with Scopolamine group.
3.4. Effect of MN and SC treatment on brain intercellular treatment
Internal protein signaling regulation takes time to consolidate and to express different signals, it is a reason that second experiment was two times longer than the first experiment (four days vs. 2 days). We have validated the data of the general all-around set of proteins that are closely related to neuro functions: neurotrophic BDNF and p-CREB, inflammatory protein iNOS, and cholinergic signaling system protein mArhR 1 (Duc Nguyen et al., 2022). The result shows that this protein was negatively altered by scopolamine administration, however, they are all enhanced by bee venom treatment: The most improvement comes from MN3 groups. SC1 and MN2 groups protein levels are similarly expressed, and these followed by SC1 group. For the MN1 group only exhibits slightly statistically significant results compared to scopolamine only treated group (Figure 4C). For the first time, MN delivered bee venom can be proven to show neurocognitive protection effect, this significantly promotes the usage of bee venom as a practical medication in this field.
4. Discussion
Bee venom, thanks to research conducted across recent years, has been proven to have comprehensive anti-neurodegenerative effects (Moon et al., 2006, 2007; Doo et al., 2012; Chung et al., 2015; Gu et al., 2015; Hwang et al., 2015; Im et al., 2016; Ye et al., 2016; Cai et al., 2017; Ham et al., 2019a, 2019b; Nguyen & Lee, 2021; Duc Nguyen et al., 2022). For instance, in terms of both in vitro and in vivo models, bee venom diminished LPS-induced proinflammatory cytokines GFAP, IBA-1, COX-2, and iNOS, having also resulted in a decrease in amyloid genesis and neuroinflammation. This observation was confirmed via the expression of GFAP, IBA-1, COX-2, and iNOS. Other research mentioned that bee venom promotes inhibition of NF-κB activation through expression of IκB, phospho-IκB, p50, and p65 in animal model. In this study, melittin—a major component of bee venom—directly binds to the C-terminus of p50 where NLS is located and, as such, interrupts translocation of NF-κB (Gu et al., 2015; Im et al., 2016). Bee venom and melittin were also found to enhance neuro stress-induced conditions triggered by Amyloid beta in both in vitro and in vivo studies; this finding was significant since it exhibits the neuroprotective ability of the drug candidate in enhancing the intrinsic antioxidant defense barrier by promoting Nrf2 nuclear translocation and up regulating the antioxidative HO-1 enzyme production; as such, brain tissue redox balance was significantly fostered and subsequent brain-derived neurotrophic factors, inflammation regulations, and learning memory ability were all significantly restored (Nguyen & Lee, 2021; Duc Nguyen et al., 2022). Furthermore, in a report of vascular dementia is caused by ischemia and/or vascular brain lesions, bee venom had significantly recovered and restored the cognitive indexes and brain cells’ metabolisms (Cai et al., 2017).
The neuroprotective effects of bee venom were revealed via much research. With this contextual understanding, bee venom—and especially its dominant component, melittin—is our focus. As such, melittin was chosen to be the marker compound to study bee venom’s pharmacokinetic and bio activity. Actively promote the intercellular HO-1 antioxidant enzyme to suppress oxidative stress such as ROS and MDA is an ability of bee venom and melittin proven in various studies, and not only to neurological research. Oxidative stress are the sensitive and reactive indexes, and their changes are in the forefront period to any neurodegeneration inducer or neuroprotection promoter phenomenal, therefore ROS and MDA indexes are used to monitor bee venom bioactivity level in mice brain (Hanafi et al., 2018; Hozzein et al., 2018; Abd El-Haleim, 2020; Kurek-Górecka et al., 2020; Kim et al., 2021; Nguyen & Lee, 2021; Duc Nguyen et al., 2022).
About the in vivo model used in the research: The scopolamine-induced neuro stress model is a common model for studying neurodegenerative disorders in mice. The result of scopolamine stress includes a rise in ROS and MDA levels, decrease in the levels of APT in the brain, and influent overall cognitive and locomotor function which can be scored via open field maze, and long term memory ability which can be evaluated by water maze (J. S. Lee et al., 2015; Karthivashan et al., 2018; Baghel & Thakur, 2019; Model et al., 2019; Muhammad et al., 2019; Baek et al., 2020; Al-Amin et al., 2022). For the current study, we analyzed the melittin as a marker chemical compound present in the brain tissue of the mice. We believe that scopolamine-induced mice (achieved via the intraperitoneal route at the abdominal area) avoids possible brain damages, better than to utilize models with methods that create an Alzheimer-like state by administrating amyloid beta or lipopolysaccharides delivered by intracerebroventricular injections directly into the brains. Such an invasive intracerebroventricular injection might affect the brain blood barrier—which controls the passing of large molecule such as melittin—and influences the overall reliability of the analysis.
Even though bee venom was discovered a potential medication in various diseases (Pucca et al., 2019; Kasozi et al., 2020), the administration of bee venom in previous studies was carried out via transdermal injections, which is inconvenient as it causes pain and adverse effect for the patient (Chen et al., 2016; Burzyńska & Piasecka-kwiatkowska, 2021). Sensations of pain can affect treatment, a particularly salient concern given that bee venom injections must be repeated so as to achieve a medically significant effect (Hwang et al., 2015; Cai et al., 2017; Nguyen & Lee, 2021; Duc Nguyen et al., 2022). Therefore in therapy, it is favorable if we can the lower the bee venom concentration that the body is exposed to.
Drugs generally possess a concentration-response-curve to behave in therapies (Satoh et al., 2013; Fernando & Soysa, 2015; Light et al., 2019). In this case, the bee venom treatment ability to inhibit ROS and MDA level does not increase linear as concentration of melittin increases in the brain and blood plasma. This means a lower concentration (than the peak levels achieved via the SC method) of melittin can be maintained but maximize the effect-dosage correlation. This provides the benefit of not exposing the experimental subject to a high and un-effective bee venom concentration (represented by melittin in this study) in blood and brain.
As presented in this research, SC injections produce a sharp increase, followed by a rapid decrease, of melittin concentrations in both blood plasma and brain tissue. In the early stages (0–10 hours from treatment), when bee venom levels are high throughout the brain, SC1 treatment can enhance ROS, MDA, ATP levels and cognitive-locomotor functions well and faster than administrations of MN. However, the melittin concentration’s rapid decrease led to the protection of MN3 and MN2 to surpass that of SC1. Therefore, the superior results obtained via the SC1 injection only lasted for a short period of time; by the end of the study period, MN3 and MN2 treatments produced generally equal or greater effective results across all categories.
It should be noted that, as SC1 involves a very high maximum concentration of melittin, from the resulting levels were recorded at 1.5–2 times the max melittin concentration produced by MN2 and MN3. The short-lasted and high max concentration of melittin of the SC1 administration is disadvantageous because melittin and bee venom can cause pain and adverse effects (Chen et al., 2016; Burzyńska & Piasecka-kwiatkowska, 2021). The reduction of the maximum peak concentration, while simultaneously prolonging the drug’s effective concentration, is a must for enhancing the safety of this medication.
MN is an emerging technology, made with special reagents for use with a suitable needle shape—that is, the 0.2 to 1.5 mm long needles, which are hard enough to penetrate through the outmost epidermis layer of the skin and reach the tissue beneath. Once the needle reaches a moist environment, they start to dissolve into liquid. The drug that was mixed within the needle is released simultaneously, and the speed at which the needles dissolve can be designed by altering the reagent content (Bariya et al., 2011; Avcil & Çelik, 2021; Yang et al., 2022). The prolonged drug releasing effect can also be extended to days instead of just hours via the SC method (Tran et al., 2021).
The ‘RAPBV21’ MN product from RAPHAS company (Park et al., 2020) was utilized and showed a good ability to controllably release bee venom into the blood stream, in a different manner to that of the SC method. This is an advantage, as we can see that the peak melittin concentration is not as high as that of the SC1 method and aims to produce less venom-related pain sensations and adverse effects for the subject (Chen et al., 2016; Burzyńska & Piasecka-kwiatkowska, 2021). As the MN slowly releases the drug, it keeps maintaining demonstrably steadier concentration levels.
The MN1 group, using a similar amount of bee venom to the SC1 group, can only release to the body as much as haft compared to SC1 treatment (validated via the AUC0–48 hr values). This low efficiency we believe is a nature of most MN method nowadays, as MN needles are short and cannot penetrate as deep as in SC administration to reach blood circulating areas, and much of the bee venom is believed to remain on the skin up-most and dry layers (Alkilani et al., 2015; Tsakovska et al., 2017). Despite that, this together with the slowly dissolving ability of the MN creates a slow-release effect that avoid high spikes of bee venom concentration seen in SC administration; And facilitated long lasting bee venom content in the body much longer than SC method. Therefore, the MN method is interestingly suitable to deliver drugs which need to reduce max concentration and prolonged tailing release such as bee venom (Bariya et al., 2011; Avcil & Çelik, 2021; Chenyuan Wang, 2022).
In details, at the 24th hour, in brain tissue, the MN3 group’s melittin concentration was still observed to be approximately 70% that of its peak level. This number for the MN2 group is about 66%, whereas the SC1 groups exhibited almost no detection value at the same point in its progression. Thanks to the mentioned concentration response curve details, melittin concentration (represent bee venom existence) does not necessarily have to be as high as SC1 peaks to produce significant effect. Therefore, in the 10th to 48th hours, the protective level of MN3 and MN2 continuously increase and generally surpass that of SC1 administration at the end of the treatment.
If the SC method aims to achieve the same pharmacokinetic advantages outcomes as that displayed via the use of MN, it would need to be conducted multiple times back-to-back with lower doses to keep its peak melittin concentration stable and steady at a continuous rate (within the therapeutic range). Alternately, one can also use a single dose via the SC method with a much higher dosage to facilitate a considerable tailing concentration after the peak had been reached—however, this is seemingly not practical due to the pain-inducing sensation of the treatment and the adverse effects of bee venom (Yassin & Haffejee, 2007; Brocks & Mehvar, 2010). In addition, MN patch are considerable much more convenient if patients need to take the drugs themselves, if they are administrated routinely (Avcil & Çelik, 2021).
When the pharmacokinetic experiment was carried out, we observed that the MN method produces greater advantages in terms of the 48-hour measured indexes. However, only SC injections of bee venom or melittin had been proved for improving wide neurodegenerative effects across the previously conducted research (Hwang et al., 2015; Cai et al., 2017; Nguyen & Lee, 2021; Duc Nguyen et al., 2022), but not has been seen via MN delivered bee venom. To further confirm that the pharmacokinetic advantages of MN also produce real and holistic therapy effects for neuro protection, we conducted additional experiments on the long-term learning and memory ability, as well as the brain’s inner cellular signaling regulations, to confirm these claims. As a result, brain cognitive function, in water maze experiment and the enhancement of key neuro degeneration protein: BDNF, p-CREB, iNOS, and mAchR 1 were all enhanced and MN3 showed a higher improvement then SC1, which is in a similar order in the first experiment. This is in correlation that reducing ROS, MDA oxidative stress (as seem in experiment 1) is a known neuro protective strategy to produce more lasting effect (as seem in experiment 2) of bee venom and melittin (Nguyen & Lee, 2021; Duc Nguyen et al., 2022).
5. Conclusion
The study confirms the improvements in pharmacokinetics aspects of the bee venom MN method provided by RAPHAS company. An clearly advantage of MN treatment is it can prolong these efficient concentrations for a much longer period than the SC method. Also, for drugs that should be used at lower dosage such as bee venom, it is a strong point of MN to keep the peak concentrations under check. Even though the peak concentrations delivered by MN are lower than those seen in the SC treatment, due the mentioned concentration-response-curve, these are lower-yet-more-efficient concentrations than the higher concentrations produced by the SC method. Also, for the first time, MN delivered bee venom was proven to show neurocognitive protection effect, this significantly promotes the usage of bee venom as a practical medication in combating aging-related neurological disorders.
Glossary
Abbreviations
- SC
Subcutaneous injection
- MN
Microneedle patch
- MDA
Lipid Peroxidation
- iNOS
inducible nitric oxide synthase
- MWM
Morris water maze
- Nrf2
nuclear factor erythroid 2-like 2
- HO-1
heme oxygenase-1
- M1
muscarinic acetylcholine receptors
- ROS
reactive oxygen species
- pCREB
phosphorylated cAMP Response Element-Binding
- mAchR 1
Muscarinic Acetylcholine Receptor M1
- SC
Subcutaneous Injection
- BDNF
brain-derived neurotrophic factor
Correction statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (Grant No.: HI19C0553) and by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT)—Nos. NRF 2022R1I1A3068255 and NRF 2021R1A2C2007041.
Data availability statement
The data presented in this study are available in the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Institutional review board statement
This study was conducted in accordance with the Guide for Care and Use of Laboratory Animals of the National Research Council (NRC, 1996) and was approved by the Committee of Animal Care and Experiment of Dongshin University, Korea (DSU2021-01-07).
References
- Abd El-Haleim EA. (2020). “Molecular study on the potential protective effects of bee venom against fructose-induced nonalcoholic steatohepatitis in rats. Pharmacology 105:692–704. Available at: 10.1159/000508511. [DOI] [PubMed] [Google Scholar]
- Al-Amin MY, Lahiry A, Ferdous R, et al. (2022). Stephania japonica ameliorates scopolamine-induced memory impairment in mice through inhibition of acetylcholinesterase and oxidative stress. Adv Pharmacol Pharm Sci 2022:8305271. Available at: 10.1155/2022/8305271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkilani AZ, McCrudden MTC, Donnelly RF. (2015). Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 7:438–70. Available at: 10.3390/pharmaceutics7040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avcil M, Çelik A. (2021). Microneedles in drug delivery: progress and challenges. Micromachines. 12:1321. Available at 10.3390/mi12111321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek SY, Li FY, Kim DH, et al. (2020). Enteromorpha prolifera extract improves memory in scopolamine-treated mice via downregulating amyloid-β expression and upregulating BDNF/TrkB pathway. Antioxidants 9:620. Available at: 10.3390/antiox9070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghel MS, Thakur MK. (2019). Vdac1 downregulation causes mitochondrial disintegration leading to hippocampal neurodegeneration in scopolamine-induced amnesic mice. Mol Neurobiol 56:1707–18. Available at: 10.1007/s12035-018-1164-z. [DOI] [PubMed] [Google Scholar]
- Bariya SH, Gohel MC, Mehta TA, et al. (2011). Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol 64:11–29. Available at: 10.1111/j.2042-7158.2011.01369.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Bankar NG, Kulkarni MV, et al. (2019). Dissolvable microneedle patch containing doxorubicin and docetaxel is effective in 4T1 xenografted breast cancer mouse model. Int J Pharm 556:263–75. Available at: 10.1016/j.ijpharm.2018.12.022. [DOI] [PubMed] [Google Scholar]
- Brocks DR, Mehvar R. (2010). Rate and extent of drug accumulation after multiple dosing revisited. Clin Pharmacokinet 49:421–438. Available at: 10.2165/11531190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Burzyńska M, Piasecka-kwiatkowska D. (2021). A review of honeybee venom allergens and allergenicity. IJMS 22:8371. Available at: 10.3390/ijms22168371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Lee JH, Yang EJ. (2017). Bee venom ameliorates cognitive dysfunction caused by neuroinflammation in an animal model of vascular dementia. Mol Neurobiol 54:5952–60. Available at: 10.1007/s12035-016-0130-x. [DOI] [PubMed] [Google Scholar]
- Chen J, Guan S-M, Sun W, et al. (2016). Melittin, the major pain-producing substance of bee venom. Neurosci Bull 32:265–72. Available at: 10.1007/s12264-016-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Xian Y, Carrier AJ, et al. (2020). A simple and cost-effective approach to fabricate tunable length polymeric microneedle patches for controllable transdermal drug delivery. RSC Adv 10:15541–6. Available at: 10.1039/d0ra01382j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung ES, Lee G, Lee C, et al. (2015). Bee venom phospholipase A2, a novel Foxp3 + regulatory T cell inducer, protects dopaminergic neurons by modulating neuroinflammatory responses in a mouse model of Parkinson’s disease. J Immunol 195:4853–60. Available at: 10.4049/jimmunol.1500386. [DOI] [PubMed] [Google Scholar]
- Doo A-R, Kim S-N, Kim S-T, et al. (2012). Bee venom protects SH-SY5Y human neuroblastoma cells from 1-methyl-4-phenylpyridinium-induced apoptotic cell death. Brain Res 1429:106–15. Available at: 10.1016/j.brainres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Du G, He P, Zhao J, et al. (2021). Polymeric microneedle-mediated transdermal delivery of melittin for rheumatoid arthritis treatment. J Control Release 336:537–48. Available at: 10.1016/j.jconrel.2021.07.005. [DOI] [PubMed] [Google Scholar]
- Duc Nguyen C, et al. (2022) Bee venom activates the Nrf2/OH-1 and TrkB/CREB/BDNF pathways in neuronal cell responses against oxidative stress induced by Aβ 1–42. [DOI] [PMC free article] [PubMed]
- Fernando CD, Soysa P. (2015). Optimized enzymatic colorimetric assay for determination of hydrogen peroxide (H2O2) scavenging activity of plant extracts. MethodsX 2:283–91. Available at: 10.1016/j.mex.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilda JE, Gomes AV. (2015). Western blotting using in-gel protein labeling as a normalization control: stain-free technology. Methods Mol Biol 1295:381–91. [DOI] [PubMed] [Google Scholar]
- Gu SM, Park MH, Hwang CJ, et al. (2015). Bee venom ameliorates lipopolysaccharide-induced memory loss by preventing NF-kappaB pathway. J Neuroinflammation 12:1–15. Available at: 10.1186/s12974-015-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham HJ, Han S-B, Yun J, et al. (2019a). Bee venom phospholipase A2 ameliorates amyloidogenesis and neuroinflammation through inhibition of signal transducer and activator of transcription-3 pathway in Tg2576 mice. Transl Neurodegener 8:1–16. Available at: 10.1186/s40035-019-0167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham HJ, Han JH, Lee YS, et al. (2019b). Bee Venom soluble phospholipase A2 exerts neuroprotective effects in a lipopolysaccharide-induced mouse model of Alzheimer’s disease via inhibition of nuclear factor-kappa B. Front Aging Neurosci 11:287. Available at: 10.3389/fnagi.2019.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafi MY, Zaher ELM, El-Adely SEM, et al. (2018). The therapeutic effects of bee venom on some metabolic and antioxidant parameters associated with HFD-induced non-alcoholic fatty liver in rats. Exp Ther Med 15:5091–9. Available at: 10.3892/etm.2018.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I, Morishita Y, Imai K, et al. (2007). High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutation Research - Genetic Toxicology and Environmental Mutagenesis 631:55–61. Available at: 10.1016/j.mrgentox.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Hematyar M, Soleimani M, Es-Haghi A, et al. (2018). Synergistic co-delivery of doxorubicin and melittin using functionalized magnetic nanoparticles for cancer treatment: loading and in vitro release study by LC–MS/MS. Artif Cells Nanomed Biotechnol 46: S1226–S1235. Available at: 10.1080/21691401.2018.1536063. [DOI] [PubMed] [Google Scholar]
- Hozzein WN, Badr G, Badr BM, et al. (2018). Bee venom improves diabetic wound healing by protecting functional macrophages from apoptosis and enhancing Nrf2, Ang-1 and Tie-2 signaling. Mol Immunol 103:322–35. Available at: 10.1016/j.molimm.2018.10.016. [DOI] [PubMed] [Google Scholar]
- Huang S, Wang J, Guo Z, et al. (2020). Quantitative measurement of melittin in Asian honeybee venom using a new method including UPLC-QqTOF-MS. Toxins 12:437. Available at: 10.3390/toxins12070437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DS, Kim SK, Bae H. (2015). Therapeutic effects of bee venom on immunological and neurological diseases. Toxins (Basel) 7:2413–21. Available at: 10.3390/toxins7072413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im EJ, Kim SJ, Hong SB, et al. (2016). Anti-inflammatory activity of bee venom in BV2 microglial cells: mediation of MyD88-dependent NF-B signaling pathway. Evid Based Complement Alternat Med 2016:3704764. Available at: 10.1155/2016/3704764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Kim KH. (2020). Clinical effectiveness and adverse events of bee venom therapy: a systematic review of randomized controlled trials. Toxins 12:558. Available at: 10.3390/toxins12090558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S-M, Zhang KAI, Wurm FR, et al. (2020). Mimic of the cellular antioxidant defense system for a sustainable regeneration of nicotinamide adenine dinucleotide (NAD). ACS Appl Mater Interfaces 12:25625–32. Available at: 10.1021/acsami.0c05588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Jin SG. (2021). Microneedle for transdermal drug delivery: current trends and fabrication. J Pharm Investig 51:503–17. Available at: 10.1007/s40005-021-00512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan L. (2014). Special section on DMPK of therapeutic proteins - Minireview: pharmacokinetic modeling of the subcutaneous absorption of therapeutic proteins. Drug Metab Dispos 42:1890–905. Available at: 10.1124/dmd.114.059121. [DOI] [PubMed] [Google Scholar]
- Karthivashan G, Park S-Y, Kweon M-H, et al. (2018). Ameliorative potential of desalted Salicornia europaea L. extract in multifaceted Alzheimer’s-like scopolamine-induced amnesic mice model. Sci Rep 8:1–16. Available at: 10.1038/s41598-018-25381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasozi KI, Niedbała G, Alqarni M, et al. (2020). Bee venom—A potential complementary medicine candidate for SARS-CoV-2 infections. Front Public Health 8. Available at: 10.3389/fpubh.2020.594458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Baek SY, Sok D-E, et al. (2020). Neuroprotective activity of polyphenol-rich ribes diacanthum pall against oxidative stress in glutamate-stimulated ht-22 cells and a scopolamine-induced amnesia animal model. Antioxidants 9:895–17. Available at: 10.3390/antiox9090895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Leem J, Hong HL. (2021). Melittin ameliorates endotoxin-induced acute kidney injury by inhibiting inflammation, oxidative stress, and cell death in mice. Oxid Med Cell Longev 2021:8843051. Available at: 10.1155/2021/8843051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek-Górecka A, Komosinska-Vassev K, Rzepecka-Stojko A, et al. (2020). Bee venom in wound healing. Molecules 26:148. Available at: 10.3390/molecules26010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BY, Bartsch SM, Mvundura M, et al. (2015). An economic model assessing the value of microneedle patch delivery of the seasonal influenza vaccine. Vaccine 33:4727–36. Available at: 10.1016/j.vaccine.2015.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-S, Kim H-G, Lee H-W, et al. (2015). Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci Rep 5:9651. Available at: 10.1038/srep09651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light JG, Haidari W, Feldman SR. (2019). Assessing efficacy and the speed of response in psoriasis treatment. J Dermatolog Treat 30:523–4. Available at: 10.1080/09546634.2019.1643588. [DOI] [PubMed] [Google Scholar]
- Masasuke Yoshida EM, TH. (2001). ATP synthase—A marvellousrotary engine of the cell. Nat Rev Mol Cell Biol 2:669–77. Available at: 10.1038/35089509. [DOI] [PubMed] [Google Scholar]
- Model A, et al. (2019). Ethanolic extract of orthosiphon stamineus improves memory in scopolamine-induced. Front Pharmacol 10:1–11. Available at: 10.3389/fphar.2019.01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon D-O, Park S-Y, Heo M-S, et al. (2006). Key regulators in bee venom-induced apoptosis are Bcl-2 and caspase-3 in human leukemic U937 cells through downregulation of ERK and Akt. Int Immunopharmacol 6:1796–807. Available at: 10.1016/j.intimp.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Moon D-O, Park S-Y, Lee K-J, et al. (2007). Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int Immunopharmacol 7:1092–101. Available at: 10.1016/j.intimp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Muhammad T, Ali T, Ikram M, et al. (2019). Melatonin rescue oxidative stress-mediated neuroinflammation/neurodegeneration and memory impairment in scopolamine-induced amnesia mice model. J Neuroimmune Pharmacol 14:278–94. Available at: 10.1007/s11481-018-9824-3. [DOI] [PubMed] [Google Scholar]
- Nagarkar R, Singh M, Nguyen HX, et al. (2020). A review of recent advances in microneedle technology for transdermal drug delivery. J Drug Delivery Sci Technol 59:101923. Available at: 10.1016/j.jddst.2020.101923. [DOI] [Google Scholar]
- Nguyen CD, Lee G. (2021). Neuroprotective activity of melittin—the main component of bee venom—Against oxidative stress induced by Aβ25–35 in in vitro and in vivo models. Antioxidants 10:1654. Available at: 10.3390/antiox10111654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T-T-L, Kim JW, Choi H-I, et al. (2022). Development of an LC-MS/MS method for ARV-110, a PROTAC molecule, and applications to pharmacokinetic studies. Molecules 27:1977. Available at: 10.3390/molecules27061977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, et al. (2020). Efficacy of transdermal immunotherapy with biodegradable microneedle patches in a murine asthma model. Clin Exp Allergy 50:1084–92. Available at: 10.1111/cea.13688. [DOI] [PubMed] [Google Scholar]
- Pucca MB, Cerni FA, Oliveira IS, et al. (2019). Bee updated: current knowledge on bee venom and bee envenoming therapy. Front Immunol 10:2090. Available at: 10.3389/fimmu.2019.02090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, McKercher SR, Lipton SA. (2013). Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol Med 65:645–57. Available at: 10.1016/j.freeradbiomed.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff M, Saunderson S, Mountian I, et al. (2017). Chronic disease and self-injection: ethnographic investigations into the patient experience during treatment. Rheumatol Ther 4:445–63. Available at: 10.1007/s40744-017-0080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KTM, Gavitt TD, Farrell NJ, et al. (2021). Transdermal microneedles for the programmable burst release of multiple vaccine payloads. Nat Biomed Eng 5:998–1007. Available at: 10.1038/s41551-020-00650-4. [DOI] [PubMed] [Google Scholar]
- Tsakovska I, Pajeva I, Al Sharif M, et al. (2017). Quantitative structure-skin permeability relationships. Toxicology 387:27–42. Available at: 10.1016/j.tox.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Wang C, Jiang X, Zeng Y, et al. (2022). Rapidly separable microneedle patches for controlled release of therapeutics for long-acting therapies. Med Drug Discov 13:100118. Available at: 10.1016/j.medidd.2021.100118. [DOI] [Google Scholar]
- Wang EQ, Plotka A, Salageanu J, et al. (2019). Comparative pharmacokinetics and pharmacodynamics of bococizumab following a single subcutaneous injection using drug substance manufactured at two sites or administration via two different devices. Clin Pharmacol Drug Dev 8:40–8. Available at: 10.1002/cpdd.454. [DOI] [PubMed] [Google Scholar]
- Wang Z, Luan J, Seth A, et al. (2021). Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat Biomed Eng 5:64–76. Available at: 10.1038/s41551-020-00672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Li X, Zhang P, et al. (2020). Rapidly dissolving microneedle patch for synergistic gene and photothermal therapy of subcutaneous tumor. J Mater Chem B 8:4331–9. Available at: 10.1039/d0tb00105h. [DOI] [PubMed] [Google Scholar]
- Yang L, Yang Y, Chen H, et al. (2022). Polymeric microneedle-mediated sustained release systems: design strategies and promising applications for drug delivery. Asian J Pharm Sci. 17:70–86. Available at: 10.1016/j.ajps.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin AA, Haffejee M. (2007). Testosterone depot injection in male hypogonadism: a critical appraisal. Clin Interv Aging 2(4):577–90. [PMC free article] [PubMed] [Google Scholar]
- Ye M, Chung H-S, Lee C, et al. (2016). Neuroprotective effects of bee venom phospholipase A2 in the 3xTg AD mouse model of Alzheimer’s disease. J Neuroinflammation 13:10–2. Available at: 10.1186/s12974-016-0476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Zhou X, Liu L, et al. (2021). Dissolving polymer microneedles for transdermal delivery of insulin. Front Pharmacol 12:719905. Available at: 10.3389/fphar.2021.719905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Yang D, Zhou C, et al. (2012). β-Actin as a loading control for plasma-based Western blot analysis of major depressive disorder patients. Anal Biochem 427:116–20. Available at: 10.1016/j.ab.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Zhao M, Bai J, Lu Y, et al. (2016). Anti-arthritic effects of microneedling with bee venom gel. J Tradit Chin Med Sci 3:256–62. Available at: 10.1016/j.jtcms.2016.09.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the article.