Abstract
Background:
We have previously reported activation in reward, salience and executive control regions during functional MRI (fMRI) using an approach–avoidance conflict (AAC) decision-making task with healthy adults. Further investigations into how anxiety and depressive disorders relate to differences in neural responses during AAC can inform their understanding and treatment. We tested the hypothesis that people with anxiety or depression have altered neural activation during AAC.
Methods:
We compared 118 treatment-seeking adults with anxiety or depression and 58 healthy adults using linear mixed-effects models to examine group-level differences in neural activation (fMRI) during AAC decision-making. Correlational analyses examined relationships between behavioural and neural measures.
Results:
Adults with anxiety or depression had greater striatal engagement when reacting to affective stimuli (p = 0.008, d = 0.31) regardless of valence, and weaker striatal engagement during reward feedback (p = 0.046, d = −0.27) regardless of the presence of monetary reward. They also had blunted amygdala activity during decision-making (p = 0.023, d = −0.32) regardless of the presence of conflict. Across groups, approach behaviour during conflict decision-making was inversely correlated with striatal activation during affective stimuli (p < 0.001, r = −0.28) and positively related to striatal activation during reward feedback (p < 0.001, r = 0.27).
Limitations:
Our transdiagnostic approach did not allow for comparisons between specific anxiety disorders, and our cross-sectional approach did not allow for causal inference.
Conclusion:
Anxiety and depression were associated with altered neural responses to AAC. Findings were consistent with the role of the striatum in action selection and reward responsivity, and they point toward striatal reactivity as a future treatment target. Blunting of amygdala activity in anxiety or depression may indicate a compensatory response to inhibit affective salience and maintain approach.
Introduction
Approach–avoidance conflict (AAC) occurs when a person makes a behavioural choice that might have rewarding or threatening outcomes.1–3 Neural and behavioural mechanisms during AAC decision-making are relevant across several psychiatric disorders, particularly anxiety4 but also depression.5 Neuroimaging paradigms targeting the neural processing of threat, reward or decision-making have separately identified disruptions in people with anxiety or depression,6–8 characterized by increased reactivity to threat in the amygdala and insula, decreased reactivity to reward in the striatum and dys-regulated engagement of the prefrontal-cingulate region during decision-making.9 The robustness of these findings should be qualified by mixed findings from previous metaanalyses,10,11 and could be further supported by replication studies with increased statistical power.12 However, AAC decision-making paradigms can also build on this previous work by allowing us to examine these constructs and their neural underpinnings simultaneously in a dynamic context.13
Tasks for fMRI with ecological validity that can effectively simulate the complex experience of AAC decision-making in the context of anxiety or depression might improve mechanistic understandings of these disorders14 at the group level and at the level of individual differences.15 AAC decisionmaking paradigms could also help guide future treatment research in these disorders, given that current therapies often try to reduce avoidance and increase approach,16 but the optimal balance of these efforts for specific disorders and patients does not yet have an empirical foundation.
The AAC paradigms were derived from animal models of anxiety17,18 and have been validated in humans.19–24 However, they have been tested primarily in nonclinical populations, contributing to normative understandings of the behavioural and neural patterns that underlie AAC decision-making, but not yet contributing to an understanding of the disruptions relevant to mental health disorders.25,26 Further, determining the functional relationships between task behaviours and neural activations and how they might differ for those with anxiety or depression could facilitate a more nuanced understanding of AAC decision-making as a potential contributor to anxiety or depression,27,28 similarly or differentially across the disorders and for people living with these disorders.
Behavioural AAC studies that compare healthy and clinical populations are rare and results have been mixed, suggesting either greater avoidance in adults with anxiety disorders29 or similar avoidance with greater response inconsistency in an adult transdiagnostic anxiety or depression sample.30 Differences between AAC task paradigms might partially explain these mixed behavioural findings. Neurally, 1 AAC fMRI study demonstrated decreased activation in the bilateral prefrontal cortex and striatum in adults with major depressive disorder.5 Adults with anxiety disorders might well be expected to show similar prefrontal and striatal disruptions during AAC tasks,4 but this concept has not yet been examined. One fMRI study of AAC in adolescents with generalized anxiety disorder found reduced responsivity to rewards and punishment in the striatum (i.e., caudate and putamen) compared to typically developing adolescents, but no neural differences during decision-making.31 Given the high comorbidity of anxiety and depression,32–35 studies of AAC decision-making could be useful for delineating transdiagnostic and disorder-specific disruptions.
Our AAC task paradigm19,20 has good test–retest reliability for behavioural responses36 and fMRI-measured neural activation,37 supporting its utility for studying individual differences. This task allows for the separate manipulation of decision-making (varying conflict level), affective outcomes (using negative or positive stimuli) and reward feedback (varying reward values). In 2 previous fMRI studies with samples of 15 and 30 healthy adults,20,37 participants showed distinct regional activations across the following dimensions: for decision-making, greater conflict trials elicited less activity in the amygdala and more in the dorsal anterior cingulate cortex (dACC), dorsal and ventral striatum, and right dorsolateral prefrontal cortex (dlPFC); for affective outcomes, the amygdala, dACC, dorsal and ventral striatum, and anterior insula were more activated by negatively valenced stimuli than by positively valenced stimuli; and for reward feedback, the dorsal and ventral striatum was significantly activated relative to baseline, but its activity did not differ by reward value. However, the neural activations elicited during this task have been examined only in healthy adult samples.20,37
In the present study, we compared a large sample of treatment-seeking adults with anxiety or depression to a group of healthy adults; we used our AAC decision-making task during fMRI to examine behavioural and neural differences.19,20 We also examined the functional relationships between behavioural and neural measures and assessed whether these relationships differed across diagnostic groups.
Our primary analyses focused on activation during each task phase (decision-making, affective outcomes, reward feedback) in the same a priori regions of interest (ROIs) used in our previous test–retest reliability study.37 We hypothesized that adults with anxiety or depression would demonstrate reduced approach behaviour during AAC and lower activation of the dlPFC and dorsal and ventral striatum during decisionmaking; greater activation of the amygdala and anterior insula during affective outcomes; and less activation of the dorsal and ventral striatum during reward. We also hypothesized that increased activity in the dlPFC and striatum during decision-making would predict greater approach behaviour, that activity in the amygdala and anterior insula during affective outcomes would predict less approach, and that activity in the striatum during reward feedback would predict greater approach. Exploratory analyses examined behavioural and neural differences between diagnostic subgroups.
Methods
Participant selection
Overall, 139 adults with anxiety or depression and 78 healthy adult participants completed the AAC task during fMRI. We compiled the data set across several recently completed or ongoing studies,16,37,38 all of which took place at the same site and are fully described in Appendix 1, available at www.jpn.ca/lookup/doi/10.1503/jpn.220083/tab-related-content. However, only 30 fMRI scans included in the present study (all from healthy comparisons) have been previously described,37 and that previous study focused on the reliability of behaviour and neural activation during the AAC task over time.
We assessed participants for psychiatric diagnoses using the Mini-International Neuropsychiatric Interview (MINI),39 version 6.0.0 for DSM-IV-TR40 or version 7.0.2 for DSM-5,41 and participants completed the Patient-Reported Outcomes Measurement Information System42 Anxiety and Depression Scales (data missing for n = 8). Diagnostic interviewers received comprehensive assessment training from a senior-level licensed clinician and were required to achieve a sufficient interrater reliability rating (Cohen κ > 0.8) for the MINI before they conducted interviews independently.
For the anxiety or depression group, inclusion criteria were a primary diagnosis of generalized anxiety disorder or major depressive disorder. Comorbid diagnoses of generalized anxiety disorder or major depressive disorder and additional anxiety disorder and related diagnoses (i.e., social anxiety disorder, panic disorder, posttraumatic stress disorder) were permitted. Exclusion criteria included the following: current severe suicidal ideation; diagnosis of psychotic disorder, bipolar disorder, obsessive–compulsive disorder, substance use disorder or eating disorder; medical diagnoses that affected brain function (e.g., neurologic disease); a history of moderate or severe traumatic brain injury; metallic MRI contraindications; noncorrectable vision or hearing problems; and psychotropic medications that acutely affected brain function (e.g., anxiolytics, antipsychotics or mood stabilizers). Participants with anxiety or depression who reported current use of antidepressants (e.g., selective serotonin reuptake inhibitors) were included if their dosage had been stable for 6 weeks before the study. Healthy participants had no psychiatric diagnoses, and they were not taking any psychotropic medications.
Of the 217 participants who completed fMRI, 41 (21 with anxiety or depression, 20 healthy participants) were excluded because of poor fMRI data quality (e.g., excess motion, scanner acquisition errors, software malfunctions). Excess motion was defined as 20% or more MRI repetition times with an average Euclidean norm value of 0.30 or greater. A consort diagram with detailed exclusion information is provided in Appendix 1, Figure S1. The final sample of 176 participants included 118 people with anxiety or depression and 58 healthy participants. To examine for potential bias resulting from data exclusions, we compared the included and excluded samples based on demographic characteristics,43,44 and we found no significant differences between the samples in terms of age, sex, or race or ethnicity (Appendix 1, Table S1).
Participants provided informed consent and received monetary compensation for study procedures following the guidelines of the Western Institutional Review Board, which approved the study protocol. Research was conducted in accordance with the World Medical Association Declaration of Helsinki.
Experimental paradigm and stimuli
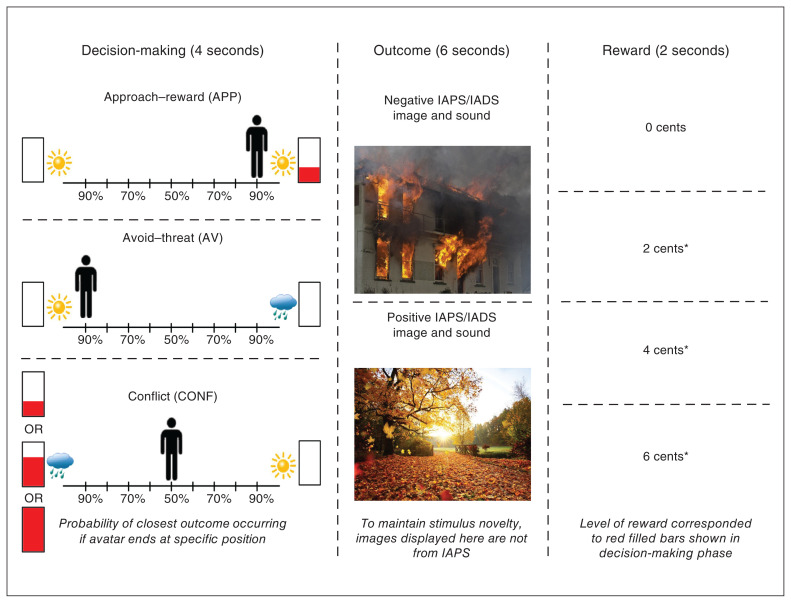
The AAC task was conducted as previously described20 and is pictured in Figure 1. Briefly, the task consisted of 3 phases: decision-making, affective outcome and reward feedback. During decision-making, participants were shown a runway with images on each side that represented 2 possible outcomes; each outcome was an affective stimulus paired with a monetary reward (in US cents). An image of a sun or cloud indicated a stimulus outcome with a positive or negative valence, respectively. The filling (in red) of an adjacent rectangular meter indicated the level of reward.
Figure 1.
Approach–avoidance conflict task. The 3 phases of the approach–avoidance conflict task are displayed in order from left to right. Left: During the decision-making phase, participants had 4 seconds to move the avatar (by moving a joystick) to a position that accurately reflected their preference of 2 potential outcomes. The position to which they moved the avatar determined the relative probability that each of the outcomes would occur (e.g., 90%:10% or 50%:50%). For approach–reward (APP) trials, participants were presented with a choice of 2 positive outcomes; 1 of the outcomes was paired with a 2-cent reward, as indicated by the filling of the red bar. For avoid–threat (AV) trials, participants were presented with a choice of a positive or negative outcome, and neither was paired with a reward. For conflict (CONF) trials, participants were presented with a choice of a positive outcome not paired with a reward and a negative outcome paired with a reward. The reward level was indicated by the level of filling of the red bar (2, 4 or 6 cents). Middle: During the affective outcome phase, participants were presented with a positive or negative affective stimulus picture and sound pairing. The pictures and sounds were drawn from the IAPS,45 IADS46 and other public-domain audio files. (The pictures displayed in this figure are from the public domain and not from IAPS to maintain stimulus novelty). Right: During the reward feedback phase, participants were presented with text indicating their level of reward for the trial (i.e., 0, 2, 4 or 6 cents), their total accumulated award and a trumpet sound when they received a reward (indicated by an asterisk). IADS = International Affective Digitized Sounds; IAPS = International Affective Picture System.
Approach–reward (APP) trials involved no threat (i.e., positive affective outcomes on both sides) and 2 cents were offered for 1 side. Avoid–threat (AV) trials had no possibility of reward: 0 cents were offered for both positive and negative affective outcomes. In conflict (CONF) trials, 2, 4 or 6 cents were offered for negative affective outcomes (CONF2, CONF4 or CONF6), and 0 cents were offered for positive affective outcomes. Participants used a joystick to indicate their preference for the probability of each of the 2 affective outcomes (each runway position equated to 10%–90% probability on each side). The avatar’s starting position and the side on which the offered outcome or reward appeared were counterbalanced across trials. During affective outcomes, participants were presented with positively or negatively valenced pictures or sounds drawn from the International Affective Picture System,45 International Affective Digitized Sounds46 and other public-domain audio files. During reward feedback, participants were given 0, 2, 4 or 6 cents, and different tones were played depending on whether or not a reward was given.
The task was programmed in PsychoPy (version 1.84.2) and used an event-related design with 90 trials in total (18 of each trial type: APP, AV, CONF2, CONF4 and CONF6) over 3 fMRI scans (30 trials, 480 s per scan). The decisionmaking phase lasted 4 s, the affective outcome phase lasted 6 s, the reward feedback phase lasted 2 s and the intertrial interval lasted 1–7 s (mean 4 s). We measured task performance using approach behaviour and reaction time. We measured approach behaviour by determining the avatar’s end position on the runway relative to the negative outcome or reward; this ranged from −4 (full avoidance) to +4 (full approach). We measured reaction time as the time it took for participants to move the joystick during the decision-making phase. We calculated approach behaviour and reaction time for each participant and averaged them by trial type (reaction time data were missing for 9 participants).
fMRI data acquisition and imaging parameters
We acquired functional and structural images using a Discovery MR750 whole-body 3.0 T MRI scanner (GE Healthcare). We used a receive-only 8-element phased array coil optimized for parallel imaging for MRI signal reception. Three fMRI scans collected blood-oxygenation-level-dependent signals using single-shot, gradient-recalled echo-planar imaging sequences with sensitivity encoding (matrix 96 × 96, field of view 240 mm, in-plane resolution 1.875 × 1.875 mm2, 39 axial slices, slice thickness 2.9 mm, repetition time 2.0 s, echo time 27 ms, flip angle 40°, sampling bandwidth 250 kHz, 256 volumes and acceleration factor R = 2 in the phase-encoding direction).
We used a single T1-weighted magnetization-prepared rapid gradient echo imaging sequence with sensitivity encoding for anatomic reference and alignment (matrix size 256 × 256, field of view 240 mm, in-plane resolution 0.938 × 0.938 mm2, slice thickness 1.0 mm, repetition time 5.94 ms, echo time 1.96 ms, flip angle 8°, sampling bandwidth 31.2 kHz, 186 axial slices per volume and acceleration factor R = 2).
Data preprocessing and participant-level analyses
We processed and analyzed all structural and functional imaging data using Analysis of Functional NeuroImages (AFNI) software.47 We discarded the first 3 volumes and performed slice timing correction for each remaining volume. The anatomic image was aligned to an echo-planar image and warped to the MNI152_T1_2009c T1-weighted anatomic template. Echo-planar images were then realigned to the first volume, template-normalized and resampled to a 2 mm3 voxel size. We resampled anatomic data to a 1 mm3 voxel size.
We analyzed time series data using AFNI’s 3dDeconvolve program (using a γ variate hemodynamic response function; i.e., AFNI’s BLOCK function) with 9 regressors of interest: the APP, AV, CONF2, CONF4 and CONF6 decisionmaking blocks; the negative and positive affective stimuli outcome blocks; and the reward and no-reward blocks. Regressors of noninterest included motion parameters (x, y or z translations, and roll, pitch or yaw rotations); baseline, linear and quadratic trends; and the average time series from a mask of each participant’s ventricles (constructed using FreeSurfer).48 We divided regression coefficients by the baseline regressor to calculate percent signal change. Finally, we applied a Gaussian filter with 4 mm full width at half maximum.
ROI selection
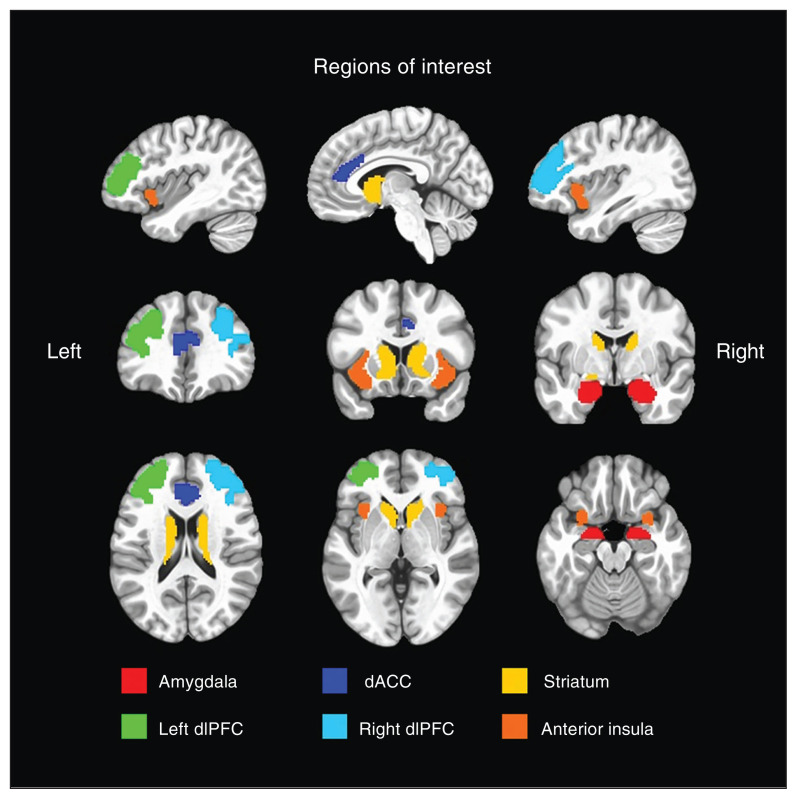
We constructed a priori composite ROIs using subregions of the Brainnetome atlas (atlas.brainnetome.org),49 identical to our previous test–retest reliability study of the AAC task.37 Decision-making included the amygdala, dACC, striatum (combined dorsal caudate, ventral caudate and nucleus accumbens), and the left and right dlPFC (separated into left and right to account for laterality effects). Affective outcomes included the amygdala (separated into left and right to account for valence effects), dACC, striatum and anterior insula. Reward feedback included only the striatum. See Figure 2 for a visualization of the 6 composite ROIs. We found previously that these composite ROIs were robustly active and reliable during AAC task performance.37
Figure 2.
Brainnetome composite regions of interest. We used 6 composite regions of interest for primary analyses, constructed using the Brainnetome atlas.49 We overlaid these regions of interest on the MNI152_T1_2009c T1-weighted anatomic template brain (https://afni.nimh.nih.gov/MNI_Atlas) in neurologic orientation (i.e., left is left). dACC = dorsal anterior cingulate cortex; dlPFC = dorsolateral prefrontal cortex.
Statistical analyses
We conducted statistical analyses using the R statistical package;50 the primary analyses were linear mixed-effects models. We examined the data for normality and identified individual outliers using a standard deviation cut-off of ± 3 standard deviations and Cook’s distance of 0.023 (R packages psych,51 Routliers52 and influence. ME53). Final sample sizes and additional details for each analysis are reported in corresponding tables in Appendix 1. For each behavioural and ROI measure, we used linear mixed-effects models to examine the fixed effects of AAC task trial type (i.e., APP, AV, CONF2, CONF4, CONF6; negative or positive; reward or no reward) and group status (i.e., anxiety or depression or healthy comparisons), including participant as a random effect. All effects that were significant at p < 0.05 are reported. For the decision-making and affective outcome task phases, findings that exceeded a Bonferroni-corrected α threshold of p < 0.01 based on 5 ROIs are denoted in the tables and figures.
Because the full anxiety or depression sample differed significantly from healthy participants in terms of age and sex, we included age and sex as covariates in the linear mixed-effects models. We conducted a sensitivity analysis with an anxiety or depression subsample (n = 58) that was propensity-matched for age and sex to healthy comparisons (n = 58) as an additional check to ensure that the findings in the unmatched samples were not driven by differences in these parameters (Appendix 1).
For ROI data analyses, we included approach behaviour as a covariate to model its relationship with neural activity and ensure that the effects of the other variables of interest (e.g., trial type and group) were not confounded by task behaviour. For decision-making, the approach behaviour covariate was separated by individual trial type (i.e., APP, AV, CONF2, CONF4, CONF6). For affective outcomes and reward feedback, we averaged the approach behaviour covariate across conflict trial types (i.e., CONF2, CONF4, CONF6). Adding approach behaviour as a covariate removed the need for follow-up Pearson r correlations and allowed us to examine brain–behaviour relationships while controlling for age or sex confounds. For each statistical model, we estimated the effect sizes for significant findings using Cohen d, Pearson r or η2.
We also conducted follow-up exploratory analyses to examine disorder-specific differences by dividing the anxiety or depression group into 3 diagnostic subgroups: anxiety only (n = 32), depression only (n = 25), and comorbid anxiety and depression (n = 61). We used linear mixed-effects models as before, recoding the group effect into these 3 subgroups. We expected these models to have an unbalanced design based on differences in the sex characteristics of these diagnostic groups as reported in epidemiological studies.54 As before, we included age and sex as covariates, and we added psychotropic medication usage as another covariate. Raw data and analysis scripts for all statistical analyses, along with whole-brain data, are publicly available in a data repository at https://osf.io/cdxqj/ (Open Science Framework).
Results
Demographic and clinical characteristics for the participant samples are reported in Table 1. Demographic and clinical characteristics of the anxiety or depression subgroups are reported in Table 2.
Table 1.
Demographic and clinical characteristics, full sample (n = 176)
| Characteristic | Adults with anxiety or depression* n = 118 |
Healthy participants* n = 58 |
p value† |
|---|---|---|---|
| Age, yr | 34.08 ± 10.79 | 24.21 ± 8.59 | < 0.001 |
| Female | 77.97% | 62.07% | 0.031 |
| Race or ethnicity other than white | 35.59% | 37.93% | 0.87 |
| Education, yr | 14.92 ± 2.24 | 13.60 ± 2.20 | < 0.001 |
| Anxiety only‡ | 27.12% | 0.00% | — |
| Depression only | 22.03% | 0.00% | — |
| Comorbid anxiety and depression | 50.85% | 0.00% | — |
| PROMIS score | |||
| Anxiety | 63.54 ± 5.94 | 46.76 ± 6.74 | < 0.001 |
| Depression | 59.34 ± 6.83 | 43.89 ± 6.23 | < 0.001 |
| Psychotropic medications§ | |||
| None | 69.50% | 0.00% | — |
| 1 | 25.42% | 0.00% | — |
| 2–3 | 5.08% | 0.00% | — |
| Substance use (past 30 days) | |||
| Cannabis | 15.25% | 11.54% | 0.48 |
| Alcohol | 60.17% | 62.07% | 0.87 |
| Tobacco | 9.26% | 5.17% | 0.55 |
PROMIS = Patient-Reported Outcomes Measurement Information System.
Values are mean ± standard deviation or %.
We used independent-samples t tests for comparisons of continuous variables between groups, and Fisher’s exact tests for frequency comparisons of categorical variables between groups.
Anxiety disorder and related diagnoses included generalized anxiety disorder (n = 89), social anxiety disorder (n = 30), panic disorder (n = 10), posttraumatic stress disorder (n = 6) and obsessive–compulsive disorder (n = 1).
Psychotropic medications included escitalopram (n = 10), bupropion (n = 9), sertraline (n = 8), citalopram (n = 3), fluoxetine (n = 2), paroxetine (n = 2), desvenlafaxine (n = 1), mirtazapine (n = 1), trazodone (n = 1) and lamotrigine (n = 1).
Table 2.
Demographic and clinical characteristics, anxiety and depression subgroups (n = 118)
| Characteristic | Anxiety only* n = 32 |
Depression only* n = 25 |
Comorbid anxiety and depression* n = 61 |
p value | Significant pair-wise differences (p value) |
|---|---|---|---|---|---|
| Age, yr | 34.72 ± 11.32 | 34.36 ± 11.18 | 33.62 ± 10.50 | 0.89 | — |
| Female | 90.63% | 52.00% | 85.61% | — | Depression < anxiety = anxiety and depression (p < 0.001) |
| Race or ethnicity other than white | 28.13% | 32.00% | 40.98% | — | Depression = anxiety = anxiety and depression (p > 0.22) |
| Education, yr | 15.69 ± 2.24 | 14.00 ± 1.76 | 14.89 ± 2.30 | 0.017 | Depression < anxiety (p = 0.003) |
| PROMIS score | |||||
| Anxiety | 63.37 ± 4.95 | 57.16 ± 7.48 | 65.87 ± 3.89 | < 0.001 | Depression < anxiety = anxiety and depression (p < 0.001) |
| Depression | 54.04 ± 5.81 | 61.32 ± 7.13 | 61.45 ± 5.68 | < 0.001 | Anxiety < depression = anxiety and depression (p < 0.001) |
PROMIS = Patient-Reported Outcomes Measurement Information System.
Values are mean ± standard deviation or %.
Behavioural data
Approach behaviour
Full results for this outcome appear in Appendix 1, Table S2. We found no main effect of group on approach behaviour. However, we did find a significant effect of trial type on approach behaviour (p < 0.001, η2 = 0.54): as expected, participants across groups exhibited the most approach behaviours during APP trials, the fewest approach behaviours during AV trials and moderate approach behaviours during CONF2, CONF4 and CONF6 trials, showing slight increases with higher rewards. A significant group × trial type interaction (p = 0.020, η2 = 0.003) revealed a reduced tendency toward avoidance or approach among participants with anxiety or depression (i.e., lower response extremity). Follow-up analysis used the absolute value of approach behaviour as an indicator of response extremity and revealed a significant effect of group (p = 0.002, d = −0.31): we found a lower absolute value of approach behaviour in participants with anxiety or depression relative to healthy participants across trial types (Appendix 1, Table S2). Age and sex covariates were not significant.
Reaction time
Full results for this outcome appear in Appendix 1, Table S2. Reaction time analyses revealed significant effects of trial type (p < 0.001, η2 = 0.009): participants responded more quickly during APP trials than in AV, CONF2 and CONF4 trials (p < 0.038). We found no differences between other trial types. We found no main effect of group on reaction time, and no group × trial type interaction. Age and sex covariates were not significant.
Subgroup comparisons
We found no significant differences between diagnostic subgroups (anxiety only, depression only, and comorbid anxiety and depression) on approach behaviour, absolute value of approach behaviour or reaction time (Appendix 1, Table S3).
ROI data
Decision-making phase
Significant group effects are depicted in Figure 3, with trial-type effects in Appendix 1, Figure S3 (full results in Appendix 1, Table S4).
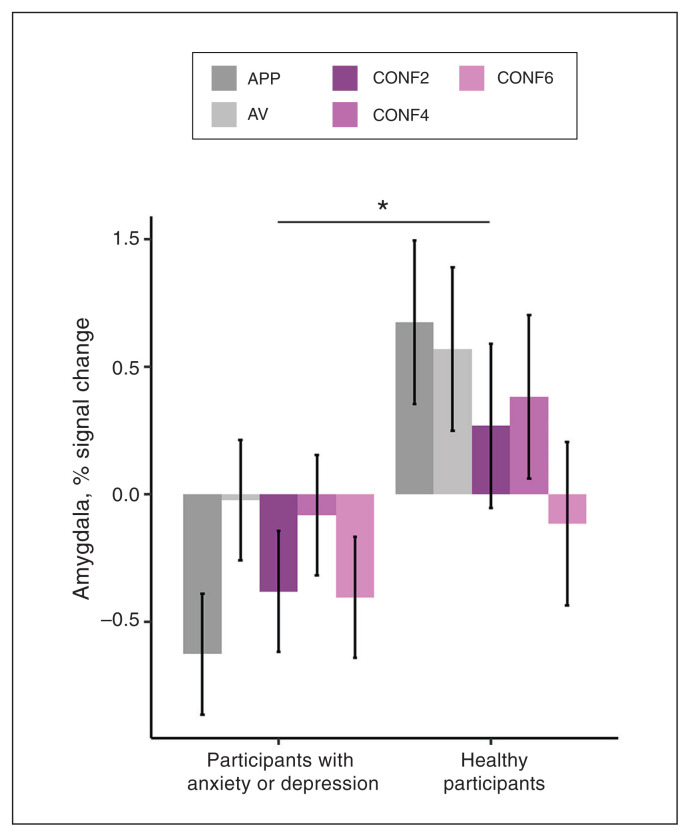
Figure 3.
Decision-making group effect for the bilateral amygdala region of interest. Bar graphs depict percent signal change estimated marginal means (error bars depict ± 1 standard error) for a significant group effect for the bilateral amygdala in the decisionmaking phase (p = 0.023; d = −0.32); *p < 0.05. Compared to healthy participants, participants with anxiety or depression showed blunted activation of the amygdala during decision-making, regardless of the presence of conflict. We observed no significant group × trial type interaction. This amygdala blunting effect in participants with anxiety or depression was consistent across each decision-making trial type. APP = approach–reward trial; AV = avoid–threat trial; CONF2 = conflict trial with a 2-cent reward; CONF4 = conflict trial with a 4-cent reward; CONF6 = conflict trial with a 6-cent reward.
We found a main effect of group in the bilateral amygdala (p = 0.023, d = −0.32), indicating blunted activation in participants with anxiety or depression compared to healthy participants. We found significant trial-type effects in the dACC, striatum, left dlPFC and right dlPFC (p < 0.042, η2 > 0.02), but no group × trial type interactions. Associations between approach behaviour and neural activity during decisionmaking were not significant for any of the 5 ROIs identified a priori (p > 0.09). Given the group differences we identified in the absolute value of approach behaviour, we reran linear mixed-effects models for each ROI using absolute value of approach behaviour as a covariate to examine for relationships between neural activity and response extremity, but none of the ROIs showed significant associations (p > 0.09). Age and sex covariates were not significant.
Affective outcome and reward feedback phases
Significant group effects for ROI measures are depicted in Figure 4, with trial-type effects in Appendix 1, Figure S4 (full results in Appendix 1, Table S5).
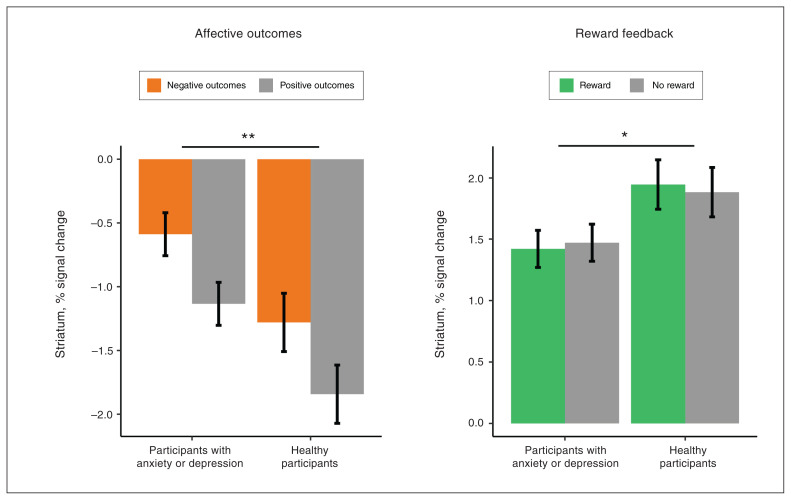
Figure 4.
Affective outcomes and reward feedback group effects for the striatum region of interest. Bar graphs depict percent signal change estimated marginal means (error bars depict ± 1 standard error) for significant group effects for the striatum during affective outcomes and reward feedback; *p < 0.05, **p < 0.01. Compared to healthy participants, participants with anxiety or depression showed increased activation of the striatum during affective outcomes, regardless of valence (p = 0.008, d = 0.31), and decreased activation of the striatum during reward feedback, regardless of reward presence (p = 0.046, d = −0.27).
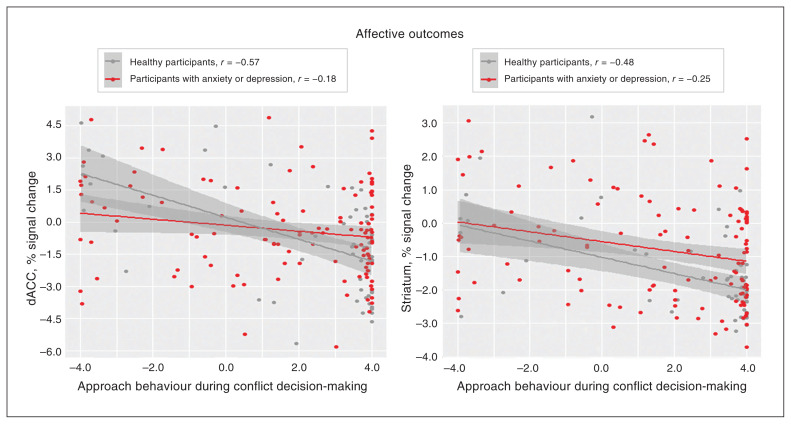
During affective outcomes, the group effect was significant in the striatum (p = 0.008, d = 0.31), reflecting increased striatal activation for participants with anxiety or depression compared to healthy participants. We found significant trial-type effects (p < 0.001, d > 0.47) in all 5 ROIs identified a priori: negative outcomes showed increased activation compared to positive outcomes. We found no group × trial type interactions. Approach behaviour during conflict showed a significant negative association (p < 0.007) with neural activity in the right amygdala (r = −0.18), dACC (r = −0.28), striatum (r = −0.28) and anterior insula (r = −0.21). In the dACC and striatum, the magnitude of this negative association was significantly stronger for healthy participants than for participants with anxiety or depression (p < 0.05). Scatterplots depicting these differential associations (dACC, striatum) are provided in Figure 5, and scatterplots for the remaining associations that were similar across groups (right amygdala, anterior insula) are depicted in Appendix 1, Figure S5.
Figure 5.
Group differences for negative associations between activation and approach behaviour. Scatterplots depict the linear relationships between approach behaviour during conflict decision-making trials (i.e., CONF2, CONF4 and CONF6) and percent signal change in the dACC or striatum; the line of best fit for each group is plotted with ± 1 standard error. Approach behaviour during conflict decision-making trials was inversely associated with neural activation in the dACC and striatum during affective outcomes. The magnitude of the association was significantly stronger for healthy participants than for participants with anxiety or depression in the dACC (p = 0.018) and the striatum (p = 0.049). CONF2 = conflict trial with a 2-cent reward; CONF4 = conflict trial with a 4-cent reward; CONF6 = conflict trial with a 6-cent reward; dACC = dorsal anterior cingulate cortex.
Follow-up linear mixed-effects models using the absolute value of approach behaviour as a covariate showed a significant negative association with activity in the striatum (p = 0.017, r = −0.16) but not for any other ROIs (p > 0.41). The magnitude of this association did not differ between participants with anxiety or depression and healthy participants (p = 0.31); the scatterplot is shown in Appendix 1, Figure S3. Age was a significant covariate for the right amygdala (p = 0.01, r = −0.17): younger participants showed more neural activation for affective outcomes. The age covariate was not significant for the remaining ROIs, and the sex covariate was not significant for any ROI.
During the reward feedback phase, the group effect was significant in the striatum (p = 0.046; d = −0.27), this time reflecting decreased activation for patients with anxiety or depression. We found no significant trial-type effect or group × trial type interaction. Approach behaviour during conflict showed a significant positive association with activity in the striatum during the reward phase (p < 0.001, r = 0.27). The magnitude of this positive association did not differ significantly between participants with anxiety or depression and healthy participants (p = 0.51). The scatterplot depicting this association is shown in Appendix 1, Figure S3. A follow-up linear mixed-effects model using the absolute value of approach behaviour as a covariate showed no significant association with activity in the striatum during reward feedback (p = 0.06). Age and sex covariates were not significant.
Subgroup comparisons
The diagnostic subgroups (anxiety only, depression only and comorbid anxiety and depression) showed no differences in activation during decision-making, affective outcomes or reward feedback (Appendix 1, Tables S6 and S7).
Discussion
The current study examined the hypothesis that people with anxiety or depression show altered behavioural performance and neural activation during an AAC decision-making task19,20 completed during fMRI. We had 3 main results. First, behavioural analyses found a group difference for response extremity: participants with anxiety or depression exhibited lower absolute values of approach behaviour than healthy participants. Second, analyses of fMRI data revealed that participants with anxiety or depression showed differences in striatal activation during affective outcomes (increased for anxiety or depression) and reward feedback (decreased for anxiety or depression). Participants with anxiety or depression also showed blunted activation of the amygdala during decision-making. Third, participants with greater striatal reactivity during affective outcomes showed less approach behaviour during conflict. In contrast, those with greater striatal reactivity during reward feedback exhibited more approach behaviour during conflict. Taken together, these findings support the hypothesis that people with anxiety or depression show altered neural activation during AAC, specifically in salience regions such as the striatum and the amygdala. Further, striatal responses that occur during emotion and reward inform decision-making during conflict.
Perhaps the most impactful and novel results were those found in the striatum, which related to anxiety or depression and approach behaviour during conflict decision-making. These striatal group differences suggest that AAC decisionmaking in participants with anxiety or depression compared with healthy participants might be affected more by stimuli with immediate, inherent affective salience (e.g., negative or positive stimuli) than by abstract reward goals (e.g., winning money). Furthermore, brain–behaviour associations suggested that difficulty disengaging the striatum during affective outcomes was linked to more avoidance behaviour (stronger association for healthy participants than for participants with anxiety or depression) and less response extremity; heightened engagement during reward feedback was linked to more approach behaviour.
These results were in some ways consistent with many previous studies using fMRI and event-related potentials that reported blunted striatal reactivity to reward in depression.55–58 They were also consistent with a more limited number of studies that reported similar findings for people with anxiety disorders.9,59,60 However, given the complementary role of the striatum in updating behavioural strategies by incorporating new information,61,62 a weaker link between the brain and behaviour during affective outcomes for participants with anxiety or depression (compared to healthy participants) could indicate that these people were less effective in updating their behavioural strategy based on emotionally salient information. Overall, these novel results suggest that the striatum plays a crucial role in AAC decision-making.
Examinations of neural activations during the decisionmaking task phase demonstrated that participants with anxiety or depression showed blunting effect in the amygdala. This effect was unexpected given previous work using animal models, which has shown threat-related increases in amygdala activity during conflict tasks.17 One possible interpretation of the present findings is that they indicate a compensatory inhibition of affective salience during AAC decision-making, which could explain why we observed similar approach behaviour across groups. However, activity in the amygdala was not directly associated with approach behaviour, so our interpretation remains unclear. The influence of anxiety and depression on the activation of the amygdala during decision-making in humans is likely complex and process-specific, warranting further investigation. As well, we have not previously identified increased activation of the anterior hippocampus during conflict in this task,20,37 although other AAC tasks have.13,21,63 Therefore, we did not include the hippocampus as an ROI in the current analysis. Further work is needed to better understand the task contexts and parameters that are most sensitive to hippocampal involvement.
Regarding behavioural group differences, participants with anxiety or depression were characterized by lower absolute values of approach behaviour (i.e., less extreme approach–avoidance behaviour). This finding suggests that people with anxiety or depression might be more likely to respond to conflict with uncertainty, and it might relate to results from previous work that used an active inference modelling approach in a large transdiagnostic sample who completed the same AAC task behaviourally without neuroimaging.30 That study reported that participants with anxiety or depression and participants with substance use disorder had higher values on a “decision uncertainty” parameter, and that those findings were consistent across time.36 The current study was focused on a priori hypotheses with predefined ROIs and task conditions; future exploratory work using a computational approach with our AAC task could have utility in refining spatiotemporal brain–behaviour relationships during AAC decision-making.
We found no differences between participants with anxiety and depression, consistent with previous task-based fMRI studies64,65 but not with other resting-state fMRI studies, which have indicated neural differences between anxiety and depression.66,67 These findings support a transdiagnostic approach for studying AAC in anxiety and depression to further examine the independent and shared effects of anxiety and depression on neural activity.
Limitations
The current study had several limitations to consider. First, although a transdiagnostic sample might be a strength in terms of clinical utility for biomarker discovery,68,69 it also limits the ability to detect subtle differences between specific anxiety disorders. In addition, our cross-sectional, observational approach did not allow for causal inference.70,71 Finally, only 36% of the participants in the current study were from a racial or ethnic group other than white; future studies should recruit samples with greater representation across diverse racial or ethnic groups.72
Conclusion
The current study demonstrates blunted activity in the amygdala during decision-making and aberrant striatal reactivity in participants with anxiety and depression during the emotion and reward phases of our AAC task. Furthermore, striatal reactivity during emotion and reward tracked with approach behaviour during conflict. These results highlight the striatum as a potential treatment target to directly influence approach behaviour, with implications for future work examining neural mechanisms of change in response to established mental health treatments or to inform novel treatment strategies.
Supplementary Material
Footnotes
Competing interests: M. Paulus is an advisor to Spring Care, Inc., a behavioural health startup; has received royalties for an article about methamphetamine in UpToDate; and serves on the board of directors of the Anxiety and Depression Association of America. R. Aupperle has received payment for invited lectures at Harvard Medical School and the University of Michigan, and has participated in a data safety monitoring board at New York University Langone Health. No other competing interests declared.
Contributors: E. Akeman, J. Santiago, K. Cosgrove, A. Clausen, N. Kirlic, R. Smith and R. Aupperle designed the study. J. Touthang and M. Cannon acquired the data, which T. McDermott, H. Berg, M. Craske, J. Abelson and M. Paulus analyzed. T. McDermott wrote the article, which H. Berg, J. Touthang, E. Akeman, M. Cannon, J. Santiago, K. Cosgrove, A. Clausen, N. Kirlic, R. Smith, M. Craske, J. Abelson, M. Paulus and R. Aupperle reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors made substantial contributions to the paper.
Funding: Funding for this study was provided by the National Institute of Mental Health under award number K23MH108707 (principal investigator: R. Aupperle) and by the William K. Warren Foundation. T. McDermott received support from the National Institute of Mental Health (NIMH) under award number F31MH122090. K. Cosgrove received support from the National Institute of Child Health and Human Development under award number F31HD103340. N. Kirlic and R. Smith received support from the National Institute of General Medical Sciences (NIGMS) under award number P20GM121312. M. Craske received support from NIMH under award numbers R33MH115138, R61MH119270 and R61MH113772. J. Abelson received support from NIMH under award number R01MH113574. M. Paulus received support from NIGMS under award number P20GM121312 and from the National Institute on Drug Abuse (NIDA) under award numbers U01DA041089 and U01DA050989. Finally, R. Aupperle also received support from NIMH under award number R01MH123691 and from NIDA under award number U01DA050989.
References
- 1.Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol Psychiatry 1998;44:1248–63. [DOI] [PubMed] [Google Scholar]
- 2.Gray JA. A critique of Eysenck’s theory of personality. In: Eysenck HJ, editor. A model for personality. Heidelberg: Springer; 1981: 246–76. [Google Scholar]
- 3.Gray JA, McNaughton N. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. Oxford: Oxford University Press; 2000. [Google Scholar]
- 4.Aupperle RL, Paulus MP. Neural systems underlying approach and avoidance in anxiety disorders. Dialogues Clin Neurosci 2010; 12:517–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ironside M, Amemori KI, McGrath CL, et al. Approach-avoidance conflict in major depressive disorder: congruent neural findings in humans and nonhuman primates. Biol Psychiatry 2020;87:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britton JC, Lissek S, Grillon C, et al. Development of anxiety: the role of threat appraisal and fear learning. Depress Anxiety 2011; 28:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hägele C, Schlagenhauf F, Rapp M, et al. Dimensional psychiatry: reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology (Berl) 2015;232:331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu P, Gu R, Broster LS, et al. Neural basis of emotional decision making in trait anxiety. J Neurosci 2013;33:18641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillon DG, Rosso IM, Pechtel P, et al. Peril and pleasure: an rdoc-inspired examination of threat responses and reward processing in anxiety and depression. Depress Anxiety 2014;31:233–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng TH, Alloy LB, Smith DV. Meta-analysis of reward processing in major depressive disorder reveals distinct abnormalities within the reward circuit. Transl Psychiatry 2019;9:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picó-Pérez M, Radua J, Steward T, et al. Emotion regulation in mood and anxiety disorders: a meta-analysis of fMRI cognitive reappraisal studies. Prog Neuropsychopharmacol Biol Psychiatry 2017; 79:96–104. [DOI] [PubMed] [Google Scholar]
- 12.Poldrack RA, Baker CI, Durnez J, et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 2017;18:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Neil EB, Newsome RN, Li IH, et al. Examining the role of the human hippocampus in approach-avoidance decision making using a novel conflict paradigm and multivariate functional magnetic resonance imaging. J Neurosci 2015;35:15039–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonkusare S, Breakspear M, Guo C. Naturalistic stimuli in neuroscience: critically acclaimed. Trends Cogn Sci 2019;23:699–714. [DOI] [PubMed] [Google Scholar]
- 15.McDermott TJ, Kirlic N, Aupperle RL. Roadmap for optimizing the clinical utility of emotional stress paradigms in human neuroimaging research. Neurobiol Stress 2018;8:134–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santiago J, Akeman E, Kirlic N, et al. Protocol for a randomized controlled trial examining multilevel prediction of response to behavioral activation and exposure-based therapy for generalized anxiety disorder. Trials 2020;21:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirlic N, Young J, Aupperle RL. Animal to human translational paradigms relevant for approach avoidance conflict decision making. Behav Res Ther 2017;96:14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.La-Vu M, Tobias BC, Schuette PJ, et al. To approach or avoid: an introductory overview of the study of anxiety using rodent assays. Front Behav Neurosci 2020;14:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aupperle RL, Sullivan S, Melrose AJ, et al. A reverse translational approach to quantify approach-avoidance conflict in humans. Behav Brain Res 2011;225:455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aupperle RL, Melrose AJ, Francisco A, et al. Neural substrates of approach-avoidance conflict decision-making. Hum Brain Mapp 2015; 36:449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bach DR, Guitart-Masip M, Packard PA, et al. Human hippocampus arbitrates approach-avoidance conflict. Curr Biol 2014;24:1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zorowitz S, Rockhill AP, Ellard KK, et al. The neural basis of approach-avoidance conflict: a model based analysis. eNeuro 2019;6:ENEURO.0115–19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bublatzky F, Alpers GW, Pittig A. From avoidance to approach: the influence of threat-of-shock on reward-based decision making. Behav Res Ther 2017;96:47–56. [DOI] [PubMed] [Google Scholar]
- 24.Fung BJ, Qi S, Hassabis D, et al. Slow escape decisions are swayed by trait anxiety. Nat Hum Behav 2019;3:702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castanheira L, Silva C, Cheniaux E, et al. Neuroimaging correlates of depression — implications to clinical practice. Front Psychiatry 2019;10:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goossen B, van der Starre J, van der Heiden C. A review of neuroimaging studies in generalized anxiety disorder: “So where do we stand?” J Neural Transm (Vienna) 2019;126:1203–16. [DOI] [PubMed] [Google Scholar]
- 27.Duval ER, Javanbakht A, Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Ther Clin Risk Manag 2015;11:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 2007;10:1116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittig A, Boschet JM, Gluck VM, et al. Elevated costly avoidance in anxiety disorders: patients show little downregulation of acquired avoidance in face of competing rewards for approach. Depress Anxiety 2021;38:361–71. [DOI] [PubMed] [Google Scholar]
- 30.Smith R, Kirlic N, Stewart JL, et al. Greater decision uncertainty characterizes a transdiagnostic patient sample during approach-avoidance conflict: a computational modelling approach. J Psychiatry Neurosci 2021;46:E74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bashford-Largo J, Aloi J, Zhang R, et al. Reduced neural differentiation of rewards and punishment during passive avoidance learning in adolescents with generalized anxiety disorder. Depress Anxiety 2021;38:794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulus MP, Yu AJ. Emotion and decision-making: affect-driven belief systems in anxiety and depression. Trends Cogn Sci 2012;16:476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi KW, Kim YK, Jeon HJ. Comorbid anxiety and depression: clinical and conceptual consideration and transdiagnostic treatment. Adv Exp Med Biol 2020;1191:219–35. [DOI] [PubMed] [Google Scholar]
- 34.Gorman JM. Comorbid depression and anxiety spectrum disorders. Depress Anxiety 1996–1997;4:160–8. [DOI] [PubMed] [Google Scholar]
- 35.Brown GW, Harris TO, Eales MJ. Social factors and comorbidity of depressive and anxiety disorders. Br J Psychiatry Suppl 1996;30:50–7. [PubMed] [Google Scholar]
- 36.Smith R, Kirlic N, Stewart JL, et al. Long-term stability of computational parameters during approach-avoidance conflict in a transdiagnostic psychiatric patient sample. Sci Rep 2021;11:11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDermott TJ, Kirlic N, Akeman E, et al. Test-retest reliability of approach-avoidance conflict decision-making during functional magnetic resonance imaging in healthy adults. Hum Brain Mapp 2021;42:2347–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akeman E, Kirlic N, Clausen AN, et al. A pragmatic clinical trial examining the impact of a resilience program on college student mental health. Depress Anxiety 2020;37:202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(Suppl 20):22–33, quiz 34–57. [PubMed] [Google Scholar]
- 40.Diagnostic and statistical manual of mental disorders. Fourth edition. Text revision. Washington (DC): American Psychiatric Association; 2000. [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fifth edition. Arlington (VA): American Psychiatric Association Publishing; 2013. [Google Scholar]
- 42.Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment 2011;18:263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cosgrove KT, McDermott TJ, White EJ, et al. Limits to the generalizability of resting-state functional magnetic resonance imaging studies of youth: an examination of ABCD Study® baseline data. Brain Imaging Behav 2022;16:1919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker TC, Ricard JA. Structural racism in neuroimaging: perspectives and solutions. Lancet Psychiatry 2022;9:e22. [DOI] [PubMed] [Google Scholar]
- 45.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical report A-8. Gainesville (FL): University of Florida; 2008. [Google Scholar]
- 46.Lang PJ, Bradley MM, Cuthbert BN. The International Affective Digitized Sounds (2nd edition; IADS-2): affective ratings of sounds and instruction manual. Technical report B-3. Gainesville (FL): University of Florida; 2007. [Google Scholar]
- 47.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–73. [DOI] [PubMed] [Google Scholar]
- 48.Fischl B. FreeSurfer. Neuroimage 2012;62:774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan L, Li H, Zhuo J, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex 2016;26:3508–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Team RCR. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. [Google Scholar]
- 51.psych. version 2.1.9. Evanston (IL): Northwestern University; 2021. Available: www.rdocumentation.org/packages/psych/versions/2.1.9/topics/00.psych (accessed 2022 July 22). [Google Scholar]
- 52.Leys C, Delacre M, Mora YL, et al. How to classify, detect, and manage univariate and multivariate outliers, with emphasis on pre-registration. Rev Int Psychol Soc 2019;32:1–10. [Google Scholar]
- 53.Nieuwenhuis R, te Grotenhuis M, Pelzer B. influence. ME: tools for detecting influential data in mixed effects models. R J 2012;4:38–47. [Google Scholar]
- 54.Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol 2014;35:320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burkhouse KL, Gorka SM, Afshar K, et al. Neural reactivity to reward and internalizing symptom dimensions. J Affect Disord 2017;217:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lieberman L, Gorka SM, Funkhouser CJ, et al. Impact of posttraumatic stress symptom dimensions on psychophysiological reactivity to threat and reward. J Psychiatr Res 2017;92:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White EJ, Nacke M, Akeman E, et al. P300 amplitude during a monetary incentive delay task predicts future therapy completion in individuals with major depressive disorder. J Affect Disord 2021;295:873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keren H, O’Callaghan G, Vidal-Ribas P, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG Studies. Am J Psychiatry 2018;175:1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sambuco N, Bradley M, Herring D, et al. Transdiagnostic trauma severity in anxiety and mood disorders: functional brain activity during emotional scene processing. Psychophysiology 2020;57:e13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sambuco N, Bradley MM, Lang PJ. Trauma-related dysfunction in the fronto-striatal reward circuit. J Affect Disord 2021;287:359–66. [DOI] [PubMed] [Google Scholar]
- 61.Kim H, Sul JH, Huh N, et al. Role of striatum in updating values of chosen actions. J Neurosci 2009;29:14701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ballard I, Miller EM, Piantadosi ST, et al. Beyond reward prediction errors: human striatum updates rule values during learning. Cereb Cortex 2018;28:3965–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loh E, Kurth-Nelson Z, Berron D, et al. Parsing the role of the hippocampus in approach-avoidance conflict. Cereb Cortex 2017; 27:201–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janiri D, Moser DA, Doucet GE, et al. Shared neural phenotypes for mood and anxiety disorders: a meta-analysis of 226 task-related functional imaging studies. JAMA Psychiatry 2020;77:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacNamara A, Klumpp H, Kennedy AE, et al. Transdiagnostic neural correlates of affective face processing in anxiety and depression. Depress Anxiety 2017;34:621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oathes DJ, Patenaude B, Schatzberg AF, et al. Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging. Biol Psychiatry 2015;77:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bijsterbosch JD, Ansari TL, Smith S, et al. Stratification of MDD and GAD patients by resting state brain connectivity predicts cognitive bias. Neuroimage Clin 2018;19:425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McQuaid RJ. Transdiagnostic biomarker approaches to mental health disorders: consideration of symptom complexity, comorbidity and context. Brain Behav Immun Health 2021;16:100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petersen CS, Miskowiak KW. Toward a transdiagnostic neurocircuitry-based biomarker model for pro-cognitive effects: challenges, opportunities, and next steps. CNS Spectr 2021;26:333–7. [DOI] [PubMed] [Google Scholar]
- 70.Weichwald S, Peters J. Causality in cognitive neuroscience: concepts, challenges, and distributional robustness. J Cogn Neurosci 2021;33:226–47. [DOI] [PubMed] [Google Scholar]
- 71.Dijkstra N, de Bruin L. Cognitive neuroscience and causal inference: implications for psychiatry. Front Psychiatry 2016;7:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dotson VM, Duarte A. The importance of diversity in cognitive neuroscience. Ann N Y Acad Sci 2020;1464:181–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.