Keywords: neuronal, signalling, zinc, sensor
Abstract
Zinc is an essential trace element that stabilizes protein structures and allosterically modulates a plethora of enzymes, ion channels and neurotransmitter receptors. Labile zinc (Zn2+) acts as an intracellular and intercellular signalling molecule in response to various stimuli, which is especially important in the central nervous system. Zincergic neurons, characterized by Zn2+ deposits in synaptic vesicles and presynaptic Zn2+ release, are found in the cortex, hippocampus, amygdala, olfactory bulb and spinal cord. To provide an overview of synaptic Zn2+ and intracellular Zn2+ signalling in neurons, the present paper summarizes the fluorescent sensors used to detect Zn2+ signals, the cellular mechanisms regulating the generation and buffering of Zn2+ signals, as well as the current perspectives on their pleiotropic effects on phosphorylation signalling, synapse formation, synaptic plasticity, as well as sensory and cognitive function.
1. Introduction
As the second most abundant trace element after iron, zinc has the highest concentration in the brain (6–95 µg g−1) [1–3], and is essential for brain function. Prenatal zinc deficiency during the critical period of rapid brain growth in fetuses causes irreversible damage to neural development [4–10], impairs learning and memory [11], and leads to development of autism-like behaviour [12]. Such nutritional zinc deficiency impedes neurogenesis, neuronal migration and synaptogenesis during brain development [13]. Zinc deficiency in adults has been clinically linked to psychological disorders such as depression [14,15], attention-deficit/hyperactivity disorder (ADHD) [16,17], and autism [18], as well as neurodegenerative diseases such as Parkinson's disease [19–21].
Zinc's importance in the brain is due to its high protein binding capacity [22], which serves critical roles in the structural stability, catalytic activity and regulatory function of thousands of proteins. In the past few decades, the focus of biological study on zinc has expanded from its structural and catalytical roles to regulatory function and its signalling roles have been extensively investigated, especially in the immune system and central nervous system [23–25]. This review focuses particularly on neurons, the fundamental units that generate electrical and chemical signals in the brain.
Neuronal signalling is partially mediated by free or labile zinc (referred to as Zn2+ ) via intracellular and intercellular signalling pathways. Inside neurons, the majority of zinc is present in a protein-bound state, leaving only a subnanomolar concentration of labile Zn2+ in the cytosol [26–29]. Intracellular Zn2+ homeostasis is efficiently maintained at stable levels with buffering and muffling mechanisms involving Zn2+ transporters, metallothioneins and metal-responsive transcription factor-1 [26,27,30,31]. A high concentration of Zn2+ is stored in the synaptic vesicles of certain neurons [28,29], which can be released into the synaptic cleft and act as intercellular signalling molecules when neurons are activated by physiological stimuli [31,32]. In addition, cytosolic Zn2+, via liberation from intracellular stores, can mediate a series of intracellular signalling events. In this review, we will provide an overview of neuronal Zn2+ signalling, both intracellularly and intercellularly. We will first summarize the fluorescent sensors that have been used to illuminate Zn2+ signals within subcellular compartments of neurons and synapses. Next, we will discuss the current understanding about synaptic Zn2+ signalling in the brain. We will then move onto discussing different players that modulate intracellular Zn2+ signals, followed by a discussion of the targets and function of Zn2+ in intracellular signalling pathways.
2. Detection of neuronal Zn2+ signals
The prerequisite of a signalling molecule is that its concentration can fluctuate alongside physiological events. Fluorescent sensors, along with time-lapse fluorescence microscopy, have been enormously instrumental in revealing spatio-temporal dynamic changes of Zn2+ signals in neurons and brain tissues. Fluorescent Zn2+ sensors involve a Zn2+-binding unit with one or two fluorescent components so that the sensors can display changes in spectral properties in response to the binding of Zn2+ ions. These tools include synthetic small molecule sensors that are constructed by organic chemistry and genetically encoded sensors that are constructed by molecular protein engineering. Cell impermeable small molecule sensors can be used to detect extracellular Zn2+ signals in brain slices, while detection of intracellular Zn2+ can be achieved by the addition of an ester moiety, which allows sensors to cross the plasma membrane into cells, followed by cleavage via intracellular esterases [33,34]. However, incomplete hydrolysis of these esters can cause nonspecific localization of small molecule sensors to subcellular compartments [35]. Small molecule sensors generally display a very large dynamic range and are easy to use in comparison to genetically encoded sensors. Genetically encoded sensors, on the other hand, are superior in spatio-temporal detection because they can be precisely targeted to specific cell types and subcellular compartments [36–38]. Genetically encoded sensors are ideal for long-term imaging due to their ability to be retained in cells for days to weeks [36,39]. Please see these review papers [38,40,41] for a comprehensive overview of different types of Zn2+ sensors. Here we will focus on the sensors that have been successfully used to detect dynamic changes in Zn2+ signals in neuron cultures and brain slices (table 1).
Table 1.
List of some sensors used to measure and detect neuronal Zn2+ signals.
| localization | name | dissociation constant (Kd) | application | references |
|---|---|---|---|---|
| cytosol | ZP1 | 0.7 nM | hippocampal slices | [35,42–44] |
| primary neurons | ||||
| ZP3 | 0.7 nM | hippocampal neurons and slices | [30,45] | |
| ZnAF-2DA | 2.7 nM | hippocampal slices | [46] | |
| FluoZin-3 AM | 15 nM | primary neurons | [33,47–51] | |
| hippocampal slices | ||||
| GZnP3 | 1.3 nM | primary neurons | [52] | |
| mitochondria | RhodZin-3 | 65 nM | primary neurons | [39,53–57] |
| PC12 cells | ||||
| Mito-ZapCY1 | 1.6 pM | C. elegans PVD neurons | [58,59] | |
| lumen of synaptic vesicles and acid compartments | TSQ | 155 nM–48 µM | hippocampal slices | [35,60–66] |
| Zinquin | 620 nM | primary neurons | [60,64–67] | |
| SpiroZin-2 | 3.6 nM | hippocampal slices | [51,68] | |
| extracellular regions (synaptically released Zn2+) | FluoZin-3 | 15 nM | hippocampal slices | [33,69,70] |
| ZP4 | 0.65 nM | hippocampal slices | [71–73] | |
| primary neurons | ||||
| ZnAF-2 | 2.7 nM | hippocampal slices | [34,42,46,74–77] | |
| NewPort Green DCF | 1 μM | hippocampal slices | [78–81] | |
| LZ9 | 0.57 nM | coronal brain slices containing dorsal cochlear nucleus | [82–84] |
Steady-state Zn2+ concentration in the cytoplasm of mammalian cells is maintained at a subnanomolar range (100 pM–1 nM) [33,85–87]. To detect cytosolic Zn2+ signals changing from baseline concentrations to high nanomolar concentrations, we need sensors with nanomolar sensitivity which is determined by both binding affinity and dynamic range. A number of cell permeable small molecule sensors with nanomolar affinity have been reported including ZnAF-2DA (Kd: 2.7 nM) [88], ZP1 (Zinpyr-1, Kd: 0.7 nM) [89], ZP3 (Kd: 0.7 nM) [45], ZP4 (Kd: 0.65 nM) [71] and FluoZin-3 acetoxymethyl (AM) ester (Kd: 15 nM) [47]. Among these sensors, FluoZin-3 AM is one of the most popular sensors used to detect cytosolic Zn2+ signals because it displays large response to Zn2+ [47]. The fluorescence of FluoZin-3 is not affected by other cations such as Ca2+ and Mg2+, which allows simultaneous recording of Zn2+ and Ca2+ by using FluoZin-3 alongside the Ca2+ dye Fura Red [48], Fura-2FF [49,50] or Fura-6F [50] in primary cultured neurons and hippocampal slices. FluoZin-3 also has low sensitivity to changes in pH, with its signal remaining unchanged from pH 6 to pH 9 [48,90]. FluoZin-3 has successfully been used with pH sensor pHrodo Red to record dynamic changes in Zn2+ and pH simultaneously in cultured hippocampal neurons [48]. One limitation of using small molecule sensors to detect cytosolic Zn2+ in neurons is that their nonspecific subcellular localization generates variable fluorescence between the cytosol and bright punctate compartments, making it difficult to interpret the subcellular locations of Zn2+ signals. Genetically encoded sensors with more specific subcellular localization allow distinguishing Zn2+ signals coming from various subcellular compartments. For example, GZnP3 (Kd: 1.3 nM) has been used to show that endolysosomal vesicles can release Zn2+ into the cytosol in hippocampal neurons [52].
Measurement of Zn2+ in the mitochondrial matrix by different sensors has determined that mitochondrial Zn2+ concentration is several orders of magnitude lower than the cytosol, approximately 0.2–300 pM [58,85,91–94]. RhodZin-3 (Kd: 65 nM) is the first reported mitochondrial Zn2+ sensor, which has a 75-fold fluorescence change from quenched N,N,N′,N′-tetrakis(2-pyridinylmethyl)-1,2-ethanediamine (TPEN) to saturated Zn2+ levels in vitro [53,54]. The positive charges carried by RhodZin-3 allow it to follow the electrical gradient and accumulate in the mitochondria [54]. RhodZin-3 has been used to record increases in mitochondrial Zn2+ following ischemia [53], N-ethylmaleimide treatment [55] or Aβ42 incubation [39,56] in primary cortical and hippocampal neurons. Mitochondrial Zn2+ was also detected in the neuron-related PC12 cells using RhodZin-3 [57]. However, due to its positive charge, RhodZin-3 signals are reduced in mitochondria when the mitochondrial membrane is depolarized, which limits its proper localization [39,56]. Other small molecule Zn2+ sensors targeted to mitochondria include DA-ZP1-TPP [95] and ZP1BG which requires co-transfection with mitochondrial-targeted alkylguaninetransferase [96], but these probes have not been widely used in live cells. Three genetically encoded mitochondrial Zn2+ sensors based on fluorescence resonance energy transfer (FRET) have been created with various binding affinities: mito-ZapCY1 (Kd: 1.6 pM, [58]), mito-eCALWY-4 (Kd: 60 pM, [92]), and mito-eZinCh-2 (Kd: 5–10 pM, [91]). These ratiometric probes are useful in quantification of mitochondrial Zn2+ concentrations. For example, mito-ZapCY1 has been used to measure mitochondrial Zn2+ in C. elegans posterior ventral dorsal (PVD) neurons [59]. Mitochondrial intermembrane space (IMS) is separated from mitochondrial matrix by the inner mitochondrial membrane, where oxidative phosphorylation occurs. A single fluorescent protein-based Zn2+ sensor, GZnP2, has been targeted to the mitochondrial matrix and the IMS [85], demonstrating that the concentration of labile Zn2+ in the IMS is 100 pM, revealing differences in Zn2+ concentration across inner mitochondrial membrane by three orders of magnitude [85].
Synaptic vesicles are unique secretory organelles found in neurons, which are categorized by the different neurotransmitters they contain. High concentrations of Zn2+ are concentrated in the synaptic vesicles of certain glutamatergic neurons [29,31,97–100], but also in some glycinergic and GABAergic neurons in neocortex [101,102], hippocampus [100,103–111], amygdala [31,107], auditory brainstem [112,113] and spinal cord [114,115]. The vesicular Zn2+ concentrations are estimated to be in the high nanomolar to low millimolar range [11,29,98,109,116–121]. This pool of Zn2+ inside the synaptic vesicles was visualized by small molecule sensors such as TSQ (Kd: 155 nM to 48 µM), which is a quinoline-based membrane-permeable sensor [60,61]. TSQ is lipophilic making it ideal for measuring vesicular Zn2+ in brain tissue [35,62,63]. TSQ's derivative, Zinquin (Kd: 620 nM) was developed to increase cellular retention [64]. However, both TSQ and Zinquin were found to coordinate non-labile Zn2+, that is pre-bound with proteins [60,65] and low molecular weight ligands (glutamic acid, glutathione, histidine and ATP) [66]. Recently, a pH insensitive sensor SpiroZin2 (Kd: 3.6 nM) was used to detect vesicular Zn2+ in hippocampal mossy fibres [68] and lysosomal Zn2+ in lactating mouse mammary epithelial cells [51].
Synapse activity causes Zn2+ to release along with neurotransmitters, subsequently increasing extracellular Zn2+ concentration in the synaptic cleft. Direct quantitative measurement of such brief (within a few milliseconds) [122] and localized Zn2+ signals within the synaptic cleft remains challenging, but a large body of evidence has detected synaptically released Zn2+ and estimated that extracellular Zn2+ can increase from 1–20 nM baseline concentration to high micromolar concentration [34,78,98,123–126]. The cell membrane impermeable version of Zn2+ sensors such as FluoZin-3 (Kd: 15 nM), ZP4 (Kd: 0.65 nM) and ZnAF-2 (Kd: 2.7 nM) have been used to examine Zn2+ release at hippocampal mossy fibre synapses [34,42,46,71–77,101,126,127]. By utilizing different affinities of sensors ZnAF-2 and ZnAF-3 (Kd: 790 nM), it has been demonstrated that membrane depolarization induces differential amounts of Zn2+ release in the hippocampus, with the highest in the dentate gyrus compared to CA1 and CA3 [34]. Cell impermeable Newport Green DCF has low affinity for Zn2+ (Kd: 1 µM), making it ideal for detecting high concentrations of Zn2+, and it has been used to visualize vesicular Zn2+ release in the hippocampal hilus [78,79]. The ratiometric Zn2+ sensor LZ9 (Kd = 0.57 nM) was developed for more precise quantification of extracellular Zn2+ to correct for the varieties in tissue thickness, sensor concentrations and imaging acquisitions [82]. LZ9 was designed by linking a green Zn2+ sensor with lissamine rhodamine B (LRB), which is a Zn2+-insensitive red fluorophore [82,83]. With LZ9, the extracellular Zn2+ concentration in the mice dorsal cochlear nucleus (DCN) was measured and detected under electrical stimulations [82].
3. Synaptic Zn2+ signalling in the brain
The presence of abundant Zn2+ in the synaptic vesicles of zincergic neurons has been confirmed by histochemical staining [29,31,97–100,128], electron microscopy [111], and microscopy imaging using the fluorescent sensors discussed in the previous section. This pool of Zn2+ is concentrated into synaptic vesicles through vesicular transporter ZnT3 [129,130] (figure 1), and co-released with neurotransmitters, such as glutamate, to the synaptic cleft during physiological neuronal excitation [31,101,131]. Synaptically released Zn2+ has been suggested to act via phasic mode, which refers to the situation that free Zn2+ immediately increases in the synaptic cleft, diffuses away from released sites and acts on target cells [118,132–135]. There is evidence that such synaptic Zn2+ can diffuse into extrasynaptic regions during repetitive synaptic stimulations [82]. Synaptically released Zn2+ acts as an intercellular signalling molecule to regulate activity of presynaptic or postsynaptic neurons, astrocytes and microglia cells [118,133–135] by targeting a variety of ionotropic and metabotropic receptors located on cells. The neurobiological roles of synaptic Zn2+ have been investigated extensively by three strategies: (a) eliminating Zn2+ inside synaptic vesicles using ZnT3 knockout mice created by Dr Richard Palmiter's team [130], (b) chelating extracellular Zn2+ with membrane-impermeable chelators such as CaEDTA, Tricine and ZX1[84,136–140], or (c) mutating the Zn2+ binding sites on the receptor proteins such as glycine receptor alpha1 subunit [140] and glutamate receptor GluN2A subunit [98]. In addition to the transient high concentrations of synaptic Zn2+, low concentrations of ambient extracellular Zn2+ (less than 10 nM) might also act as a signalling molecule via tonic mode. Such ambient Zn2+ was suggested to derive from accumulation of synaptically released Zn2+ that is still coordinated with membrane proteins [141], or from efflux of cytoplasmic Zn2+ in postsynaptic cells by Zn2+ transporter ZnT1 [142] (figure 1). Different results were reported regarding whether tonic Zn2+ affects cell excitability, which might be due to the differences in the synapses chosen to study, Mg2+ concentration in the experimental buffers, or chelators used [82,98].
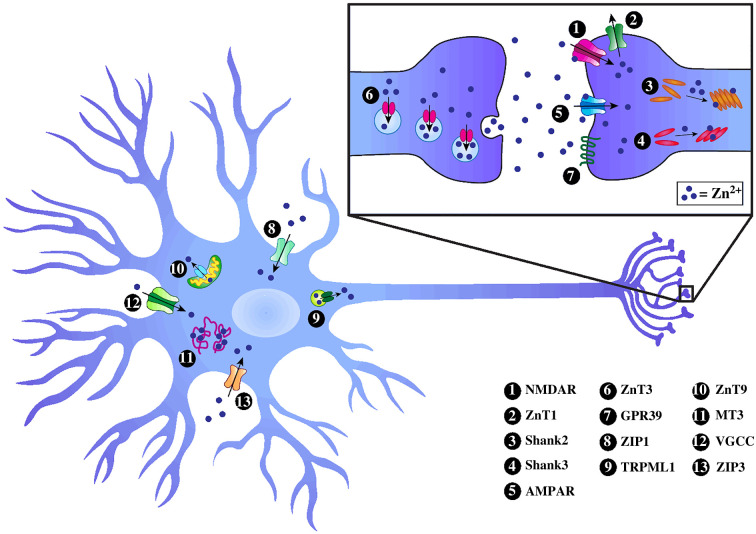
Figure 1.
Modulation and targets of intracellular and synaptic Zn2+ signals in glutamatergic neurons. Intracellular Zn2+ signals can be generated via influx from extracellular environments mediated by ZIP1, ZIP3, and opening of ion channels (NMDAR, GluA2-lacking AMPAR, VGCC), liberation from metallothioneins (MT3), or release through the TRPML1 channel from lysosomes and late endosomes. Cytosolic Zn2+ is transported out of neurons by ZnT1 and sequestered into synaptic vesicles by ZnT3. Low concentrations of mitochondrial Zn2+ are maintained by ZnT9. Synaptically released Zn2+ can inhibit AMPAR containing GluA2 subunits and NMDAR to regulate synaptic activity. Zn2+ can also induce GPR39-mediated signalling. Postsynaptic Zn2+ signals translocate Shank2 and Shank3 to postsynaptic regions, thereby enhancing recruitments of AMPAR's GluA2 subunits and promoting removal of GluA1 subunits.
A number of ionotropic receptors can be regulated by Zn2+ including N-methyl-D-aspartate receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) without GluA2 subunit [143], gamma-aminobutyric acid receptors (GABARs), glycine receptors, L-type and N-type voltage-gated calcium channels (VGCCs), voltage-gated sodium channels, voltage-gated potassium channels, P2X purinergic receptors and transient receptor potential ankyrin 1 (TRPA1) channels [144–149] (figure 1). Zn2+ displays differential modulation (inhibition, excitation or activation) of these targets with varying potency depending on target isoform types, Zn2+ concentration and synaptic activity [138]. For example, NMDAR subtypes containing GluN2A subunits possess a nanomolar-affinity Zn2+ binding site, while GluN2B subunits have a micromolar-affinity site for Zn2+ [150–152]. For AMPAR, hundreds of micromolar Zn2+ demonstrates inhibition towards the AMPAR containing GluA2 subunits [153]. By tuning the gating of these ion channels, synaptically released Zn2+ modulates excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs respectively), and hence synaptic activity [120,121,126,140,154]. The effects of synaptic Zn2+ on EPSCs have been studied in brain regions that are rich in glutamatergic Zn2+, including hippocampal mossy fibre-CA3, hippocampal Schaffer collateral-CA1 synapses and DCN parallel fibre synapses, where it was discovered that presynaptic stimulation discharges Zn2+ that inhibits NMDAR EPSCs [11,82,98,109] and AMPAR ESPCs [84]. In addition, synaptically released Zn2+ inhibits GABAAR IPSCs in principal neurons in the lateral amygdala [120], but enhances GABAAR IPSCs in somatostatin interneurons in the auditory cortex [155] as well as glycinergic IPSCs in hypoglossal motoneurons of the brainstem [140,156,157].
Emerging studies also revealed the roles of synaptic Zn2+ in regulating metabotropic receptors. Hershfinkel's group demonstrated that synaptically released Zn2+ from mossy fibre stimulation interacts with a ‘Zn2+-sensing receptor’ (ZnR/GPR39) on postsynaptic cells in CA3 of hippocampal slices (figure 1), which triggers the G protein-coupled receptor pathway and subsequently induces the release of Ca2+ from thapsigargin-sensitive intracellular pools [118,158]. Such Zn2+-evoked Ca2+ signals induce a series of MAPK-dependent cascades, promoting interaction between SNAP23 and K+/Cl− cotransporter 2 (KCC2), enhancing the surface expression and activity of KCC2 in hippocampal neurons [158,159]. The increased KCC2-mediated Cl− outward current maintains the hyperpolarizing GABAAR reversal potentials, reducing glutamate excitotoxicity in hippocampal neurons. This has further been supported by the finding that GPR39 knockout mice are more susceptible to kainate-induced seizures compared to wild-type groups [160]. The ZnR was also reported to be present in the DCN, where activation of ZnR by synaptic Zn2+ promotes synthesis of endocannabinoids, resulting in the reduction of presynaptic glutamate release in a retrograde manner [113]. However, ZnR/GPR39 has very low binding affinity for Zn2+ (Kd: 150 µM), raising the question of whether it acts as a physiological receptor of Zn2+. Early studies have suggested that Zn2+ exerts effects via tropomyosin receptor kinase B (TrkB), through either activating metalloprotease to increase mature brain-derived neurotropic factor (BDNF) [161], or transactivating TrkB directly [154]. However, this hypothesis was challenged by inconsistent results in ZnT3 knockout mice. Although several studies showed that there is an increased level of BDNF in ZnT3 knockout mice [162–165], the TrkB protein levels in ZnT3 knockout mice have been reported to be increased [165], downregulated [162,163], or unchanged [164,166].
Through modulating the activity of the vast array of receptors, the signals of synaptic Zn2+ are transduced to impact brain function. For example, synaptically released Zn2+ facilitates long-term potentiation (LTP) at the cortico-amygdala synapses via depressing feedforward GABAergic inhibition of principal neurons [120]. Partially due to the roles of synaptic Zn2+ in amygdala synaptic plasticity, ZnT3 knockout mice showed deficiency in subtle and complex learning of fear [167]. Synaptic Zn2+ in the hippocampus was suggested to enhance LTP in the CA1 region [168] and modulate the mossy fibre LTP in the CA3 regions [11,109]. As hippocampal LTP is the basis of normal cognitive function, synaptic Zn2+ might be involved in learning and memory [77,169]. ZnT3 knockout mice showed mild deficits in long-term memory and spatial memory [170,171]. In addition, synaptically released Zn2+ in the auditory cortex and somatosensory cortex aids in sensory processes as ZnT3 knockout mice have been shown to have deficits in distinguishing different sound frequencies [136,172] and detecting fine textural differences [173].
4. Modulation and function of intracellular Zn2+ signals in neurons
Dynamic changes in intracellular Zn2+ concentration are tightly regulated in neurons by a multitude of membrane transporters, ion channels and buffering proteins, which control Zn2+ signals both spatially and temporally. The Zn2+ transporters include 10 efflux transporters (ZnTs, SLC30A) and 14 influx transporters (ZIPs, SLC39A). ZnTs extrude Zn2+ into the extracellular space or intracellular compartments [31,129,130,174,175], while ZIPs allow Zn2+ inflow by or coupled with H+ or HCO−3 gradients [176–179]. The expression of Zn2+ transporters has distinct localization and roles in various brain areas and subcellular compartments. ZnT1 (SLC30A1), which is primarily localized on the plasma membrane [180–182], was shown to be closely tied with NMDA receptors at postsynaptic densities [183] (figure 1). ZnT3 (SLC30A3) is localized on the membrane of synaptic vesicles and highly co-localized with synaptic Zn2+ [31,129,130,174,175] (figure 1). ZnT9 (SLC30A9) is a mitochondrial Zn2+ transporter which maintains low Zn2+ inside mitochondria [184] (figure 1). TMEM163 is another Zn2+ transporter expressed in the synaptic vesicles and has been categorized as ZnT11 [185]. Both ZIP1 and ZIP3 are ubiquitous plasma membrane transporters, but they display distinct expressions in the hippocampus [178,186] (figure 1). ZIP1 is enriched in the stratum pyramidale of CA3, while ZIP3 is highly expressed in dentate gyrus granule cells [187]. ZIP4 has high expression in the soma and postsynaptic regions of Purkinje neurons in the cerebellum [188].
Metallothioneins (MTs) are a family of cysteine-rich (30%) proteins, including four human isoforms (MT1, MT2, MT3, MT4), which play critical roles in regulating gene expression, controlling cellular metal metabolism and adjusting cellular adaptation to stress [189]. The MT3 protein consists of two subdomains (α and β domain), and has the capacity of binding up to seven Zn2+ ions [190] (figure 1). MT3 is the brain-specific MT, responsible for buffering cellular Zn2+ in neurons and astrocytes [191–194]. The high amounts of cysteine residues in MTs are accessible to modification by reactive oxygen or nitrogen species, thus liberating Zn2+ to the cytosol [195–197]. Compared to MT1 and MT2, MT3 demonstrated higher reactivity to release more Zn2+ when treated with S-nitrosothiols due to the enhanced nitrosylation of multiple cysteines adjacent to basic and acid residues in MT3 [198]. Accordingly, exogenous nitric oxide increases intracellular Zn2+ in neurons [199]. In addition, cytosolic acidification following Ca2+ influx has been suggested to induce intracellular Zn2+ release in neurons [48,49].
Labile Zn2+ is maintained at nanomolar concentration (approx. 20 nM) in cerebrospinal fluid (CSF) in the normal brain [200], while the concentration of Zn2+ can reach up to 15 000 fold (approx. 300 µM) in the synaptic cleft under both spontaneous and electrically stimulated conditions [109,179,200,201]. It is still unclear whether the synaptically released Zn2+ can flux into neurons, but immediate increases in cellular Zn2+ can be mediated through opening of ion channels on the plasma membrane or lysosomal membrane. Both fluorescence-based imaging techniques and electrophysiology have provided strong evidence that activated voltage-gated Ca2+ channels are permeable to Zn2+ in mammalian neurons [147,149,202–204]. Recent electrophysiology and Zn2+ fluorescent imaging studies show that some TRP subfamilies are Zn2+ permeable [205]. TRPA1 channels, which are mainly located in dorsal root ganglia neurons, are permeable to Zn2+ [146]. Another TRP channel, transient receptor potential canonical type 6 (TRPC6), demonstrates Zn2+ permeability in mouse cortical neurons and contributes to nuclear Zn2+ accumulation [206]. In addition, the lysosomal channel transient receptor potential mucolipin 1 (TRPML1) mediates Zn2+ release from late endosomes in primary rat neuron culture [52] (figure 1).
Increases in cellular Zn2+ signals might act similarly to Ca2+ signals, mediating a cascade of phosphorylation signalling events that involve protein kinases and proteases. The enzymes that can be regulated by intraneuronal Zn2+ should have a half-maximal inhibitory concentration (IC50) within physiological range of Zn2+ concentrations (high picomolar to low nanomolar). Several enzymes demonstrate this high Zn2+ binding affinity in vitro including tyrosine phosphatase beta activity (IC50 = 21 pM) [207], caspase 3 (IC50 < 10 nM) [208], and Ca2+-ATPase (IC50 = 80 pM) [209]. Tyrosine phosphatases are expressed in neurons and play a role in modifying synaptic formation as well as neuronal development [210]. Intraneuronal Zn2+ release, mediated by nitric oxide, was found to activate p38 MAPK, resulting in apoptosis in cortical neurons [199]. Zn2+ reuptake into the hippocampal mossy fibre terminal was suggested to inhibit MAPK tyrosine phosphatase, thereby inducing Erk activation [211]. However, a recent study in HeLa cells and mouse hippocampal neuronal cell line (HT-22) suggests that nanomolar Zn2+ activates Erk and Akt signalling pathways via the upstream molecule Ras, while activation of Erk phosphatase requires a Zn2+ concentration higher than is reached by physiological fluctuations [212]. In addition, members of the MAPK pathway proteins (e.g. MAPK1, MAPK4, Fibroblast growth factor receptor 3, Fibroblast Growth Factor Receptor Substrate 2, Rap guanine nucleotide exchange factor 2, c-Jun) were also upregulated at the transcriptional level by increases in intraneuronal Zn2+ [213].
The Shank proteins are another target of intraneuronal Zn2+ signals and the interaction between Zn2+ and Shank proteins regulates synapse formation and function. The Shank proteins, a family of scaffolding proteins located in the excitatory synapses of neurons, consist of three members (Shank1, Shank2 and Shank3) [214,215] (figure 1). Shank protein is a ‘master’ scaffolding protein, interacting and coordinating with intermediate scaffolding proteins at the postsynaptic regions [214]. Shank proteins indirectly interact with NMDA receptors by linking PSD95 to guanylate kinase (GK)-associated proteins [216,217]. There is a direct interaction between AMPAR's GluA1 subunit and Shank3's PDZ domain [218]. In addition, Shank assists synaptic growth and maturation through affecting the internalization of the transmembrane Wnt receptor Frizzled-2 (Fz2) [219]. It has been well studied that internalization and cleavage of Fz2 play an important role in modulating synapse development [220,221]. Among the three members, Shank2 and Shank3 contain a sterile-alpha-motif domain at their C-terminal that can bind Zn2+, while Shank1 is insensitive to Zn2+ [222,223]. Binding of Zn2+ is required for oligomerization and assembly of both Shank2 and Shank3 [223,224], essential for their proper postsynaptic localization during synaptogenesis and synapse maturation [225]. The Zn2+ sensitive Shank proteins are expressed before Shank1 in neurons, resulting in a difference in Zn2+ sensitivity between young neurons and mature neurons [225]. Neuron depolarization-induced Zn2+ signals interact with Shank2 and Shank3, recruiting AMPAR GluA2 subunits and removing GluA1 subunits at the glutamatergic synapses, hence enhancing AMPAR synaptic efficacy in young neurons [224,226] (figure 1). In mouse models of Zn2+ deficiency, Shank protein levels were significantly reduced in the striatum, hippocampus, cortex and cerebellum and the mice displayed developmental and behavioural issues mimicking autism spectrum disorders [227].
5. Conclusion
The surge in development of a full suite of new fluorescent Zn2+ sensors, chelators and genetic mice models allows us to gain a greater understanding about the neuronal signalling of Zn2+ in live primary neuron culture, brain slices and live animals. The dynamic changes in neuronal Zn2+ signals have been evidenced by the detection of extracellular Zn2+ signals during synaptic activity and intracellular Zn2+ signals during influx, neuron excitation and oxidative stress. Simultaneous measurement of intracellular Zn2+ concentrations and signalling molecules in live cells has confirmed that physiologically relevant Zn2+ dynamics regulate Erk signalling events. Behavioural studies in live mice with the application of fast and selective Zn2+ chelators along with genetic knockdown of ZnT3 further elucidated the involvement of synaptic Zn2+ signals in learning, memory, emotion, sensory function and social interaction.
However, it is still not completely unveiled when, where and how physiological changes in intracellular and intercellular Zn2+ signals can regulate their targets. We still lack tools that are sensitive and bright enough to track localized Zn2+ signals within synapses and neurons in whole organisms, preventing us from tackling many of these unanswered questions. Another challenge is that occurrence of Zn2+ signals is accompanied with the changes in other cellular signals such as Ca2+, pH and redox potential, which adds to the complexity of investigating the Zn2+ signalling roles. New sensors are needed to resolve these challenges. In addition, given that there are multiple zincergic neurons and Zn2+ targets, the function of synaptic Zn2+ signals in a specific brain region is hard to clarify from whole-body ZnT3 knockout mice. Conditional knockout of ZnT3 in specific brain regions and neuron types will further elucidate the roles of synaptic Zn2+ in the brain.
Data accessibility
This article has no additional data.
Authors' contributions
C.Z.: writing—original draft, writing—review and editing; A.D.: writing—original draft, writing—review and editing; K.G.: writing—original draft, writing—review and editing; Y.Q.: conceptualization, funding acquisition, project administration, resources, supervision, validation, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was funded by the NIH R01NS110590.
References
- 1.Yanagisawa H. 2008. Zinc deficiency and clinical practice—validity of zinc preparations—. Yakugaku Zasshi. 128, 333-339. ( 10.1248/yakushi.128.333) [DOI] [PubMed] [Google Scholar]
- 2.Mocchegiani E, Bertoni-Freddari C, Marcellini F, Malavolta M. 2005. Brain, aging and neurodegeneration: role of zinc ion availability. Prog. Neurobiol. 75, 367-390. ( 10.1016/j.pneurobio.2005.04.005) [DOI] [PubMed] [Google Scholar]
- 3.Grochowski C, Blicharska E, Krukow P, Jonak K, Maciejewski M, Szczepanek D, Jonak K, Flieger J, Maciejewski R. 2019. Analysis of trace elements in human brain: its aim, methods, and concentration levels. Front. Chem. 7, 115. ( 10.3389/fchem.2019.00115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi BY, Kim IY, Kim JH, Lee BE, Lee SH, Kho AR, Sohn M, Suh SW. 2016. Zinc plus cyclo-(His-Pro) promotes hippocampal neurogenesis in rats. Neuroscience 339, 634-643. ( 10.1016/j.neuroscience.2016.10.035) [DOI] [PubMed] [Google Scholar]
- 5.Choi BY, Kim IY, Kim JH, Lee BE, Lee SH, Kho AR, Sohn M, Suh S. 2017. Administration of zinc plus cyclo-(His-Pro) increases hippocampal neurogenesis in rats during the early phase of streptozotocin-induced diabetes. Int. J. Mol. Sci. 18, 73. ( 10.3390/ijms18010073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi BY, Kim JH, Kim HJ, Lee BE, Kim IY, Sohn M, Suh SW. 2014. Zinc chelation reduces traumatic brain injury-induced neurogenesis in the subgranular zone of the hippocampal dentate gyrus. J. Trace Elem. Med. Biol. 28, 474-481. ( 10.1016/j.jtemb.2014.07.007) [DOI] [PubMed] [Google Scholar]
- 7.Gao HL, Zheng W, Xin N, Chi ZH, Wang ZY, Chen J, Wang Z-Y. 2009. Zinc deficiency reduces neurogenesis accompanied by neuronal apoptosis through caspase-dependent and-independent signaling pathways. Neurotox. Res. 16, 416-425. ( 10.1007/s12640-009-9072-7) [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Jang BG, Choi BY, Kwon LM, Sohn M, Song HK, Suh SW. 2012. Zinc chelation reduces hippocampal neurogenesis after pilocarpine-induced seizure. PLoS ONE 7, e48543. ( 10.1371/journal.pone.0048543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levenson CW, Morris D. 2011. Zinc and neurogenesis: making new neurons from development to adulthood. Adv. Nutr. 2, 96-100. ( 10.3945/an.110.000174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suh SW, Won SJ, Hamby AM, Yoo BH, Fan Y, Sheline CT, Tamano H, Takeda A, Liu J. 2009. Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J. Cereb. Blood Flow Metab. 29, 1579-1588. ( 10.1038/jcbfm.2009.80) [DOI] [PubMed] [Google Scholar]
- 11.Pan E, Zhang XA, Huang Z, Krezel A, Zhao M, Tinberg CE, Lippard SJ, Mcnamara JO. et al. 2011. Vesicular zinc promotes presynaptic and inhibits postsynaptic long-term potentiation of mossy fiber-CA3 synapse. Neuron 71, 1116-1126. ( 10.1016/j.neuron.2011.07.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grabrucker S, Boeckers TM, Grabrucker AM. 2016. Gender dependent evaluation of autism like behavior in mice exposed to prenatal zinc deficiency. Front. Behav. Neurosci. 10, 37. ( 10.3389/fnbeh.2016.00037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dvergsten CL, Johnson LA, Sandstead HH. 1984. Alterations in the postnatal development of the cerebellar cortex due to zinc deficiency. III. Impaired dendritic differentiation of basket and stellate cells. Dev. Brain Res. 16, 21-26. ( 10.1016/0165-3806(84)90058-0) [DOI] [PubMed] [Google Scholar]
- 14.Swardfager W, Herrmann N, Mazereeuw G, Goldberger K, Harimoto T, Lanctôt KL. 2013. Zinc in depression: a meta-analysis. Biol. Psychiatry 74, 872-878. ( 10.1016/j.biopsych.2013.05.008) [DOI] [PubMed] [Google Scholar]
- 15.Siwek M, et al. 2013. Zinc as a marker of affective disorders. Pharmacol. Rep. 65, 1512-1518. ( 10.1016/S1734-1140(13)71512-3) [DOI] [PubMed] [Google Scholar]
- 16.Viktorinova A, Ursinyova M, Trebaticka J, Uhnakova I, Durackova Z, Masanova V. 2016. Changed plasma levels of zinc and copper to zinc ratio and their possible associations with parent-and teacher-rated symptoms in children with attention-deficit hyperactivity disorder. Biol. Trace Elem. Res. 169, 1-7. ( 10.1007/s12011-015-0395-3) [DOI] [PubMed] [Google Scholar]
- 17.Zhou F, Wu F, Zou S, Chen Y, Feng C, Fan G. 2016. Dietary, nutrient patterns and blood essential elements in Chinese children with ADHD. Nutrients 8, 352. ( 10.3390/nu8060352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjørklund G. 2013. The role of zinc and copper in autism spectrum disorders. Acta Neurobiol. Exp. (Wars) 73, 225-236. [DOI] [PubMed] [Google Scholar]
- 19.Brewer GJ, Kanzer SH, Zimmerman EA, Molho ES, Celmins DF, Heckman SM, Dick R. 2010. Subclinical zinc deficiency in Alzheimer's disease and Parkinson's disease. Am. J. Alzheimer's Dis. Other Dement. 25, 572-575. ( 10.1177/1533317510382283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsleff L, Schauss AG, Bier ID, Stuart S. 1999. Evidence of functional zinc deficiency in Parkinson's disease. J. Altern. Complement. Med. 5, 57-64. ( 10.1089/acm.1999.5.57) [DOI] [PubMed] [Google Scholar]
- 21.Du K, Liu MY, Zhong X, Wei MJ. 2017. Decreased circulating Zinc levels in Parkinson's disease: A meta-analysis study. Sci. Rep. 7, 1-8. ( 10.1038/s41598-016-0028-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreini C, Banci L, Bertini I, Rosato A. 2006. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 5, 196-201. ( 10.1021/pr050361j) [DOI] [PubMed] [Google Scholar]
- 23.Fukada T, Kambe T. 2018. Welcome to the world of zinc signaling. Int. J. Mol. Sci. 19, 785. ( 10.3390/ijms19030785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T. 2011. Zinc homeostasis and signaling in health and diseases. J. Biol. Inorg. Chem. 16, 1123-1134. ( 10.1007/s00775-011-0797-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maret W. 2017. Zinc in cellular regulation: the nature and significance of ‘zinc signals’. Int. J. Mol. Sci. 18, 2285. ( 10.3390/ijms18112285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda A. 2001. Zinc homeostasis and functions of zinc in the brain. Biometals 14, 343-351. ( 10.1023/A:1012982123386) [DOI] [PubMed] [Google Scholar]
- 27.Colvin RA, Fontaine CP, Laskowski M, Thomas D. 2003. Zn2+ transporters and Zn2+ homeostasis in neurons. Eur. J. Pharmacol. 479, 171-185. ( 10.1016/j.ejphar.2003.08.067) [DOI] [PubMed] [Google Scholar]
- 28.Frederickson CJ. 1989. Neurobiology of zinc and zinc-containing neurons. Int. Rev. Neurobiol. 31, 145-238. ( 10.1016/S0074-7742(08)60279-2) [DOI] [PubMed] [Google Scholar]
- 29.Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB. 2000. Importance of zinc in the central nervous system: the zinc-containing neuron. J. Nutr. 130(5S Suppl), 1471S-1483S. ( 10.1093/jn/130.5.1471S) [DOI] [PubMed] [Google Scholar]
- 30.Colvin RA, Holmes WR, Fontaine CP, Maret W. 2010. Cytosolic zinc buffering and muffling: their role in intracellularzinc homeostasis. Metallomics 2, 306-317. ( 10.1039/b926662c) [DOI] [PubMed] [Google Scholar]
- 31.Sensi SL, Paoletti P, Bush AI, Sekler I. 2009. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 10, 780-791. ( 10.1038/nrn2734) [DOI] [PubMed] [Google Scholar]
- 32.Liang X, Dempski RE, Burdette SC. 2016. Zn2+ at a cellular crossroads. Curr. Opin. Chem. Biol. 31, 120-125. ( 10.1016/j.cbpa.2016.02.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin Y, Miranda JG, Stoddard CI, Dean KM, Galati DF, Palmer AE. 2013. Direct comparison of a genetically encoded sensor and small molecule indicator: implications for quantification of cytosolic Zn2+. ACS Chem. Biol. 8, 2366-2371. ( 10.1021/cb4003859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komatsu K, Kikuchi K, Kojima H, Urano Y, Nagano T. 2005. Selective zinc sensor molecules with various affinities for Zn2+, revealing dynamics and regional distribution of synaptically released Zn2+ in hippocampal slices. J. Am. Chem. Soc. 127, 10 197-10 204. ( 10.1021/ja050301e) [DOI] [PubMed] [Google Scholar]
- 35.Woodroofe CC, Masalha R, Barnes KR, Frederickson CJ, Lippard SJ. 2004. Membrane-permeable and-impermeable sensors of the Zinpyr family and their application to imaging of hippocampal zinc in vivo. Chem. Biol. 11, 1659-1666. ( 10.1016/j.chembiol.2004.09.013) [DOI] [PubMed] [Google Scholar]
- 36.Palmer AE, Qin Y, Park JG, McCombs JE. 2011. Design and application of genetically encoded biosensors. Trends Biotechnol. 29, 144-152. ( 10.1016/j.tibtech.2010.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadoine M, Ishikawa Y, Kleist TJ, Wudick MM, Nakamura M, Grossmann G, Frommer WB, Ho C-H. 2021. Designs, applications, and limitations of genetically encoded fluorescent sensors to explore plant biology. Plant Physiol. 187, 485-503. ( 10.1093/plphys/kiab353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carpenter MC, Lo MN, Palmer AE. 2016. Techniques for measuring cellular zinc. Arch. Biochem. Biophys. 611, 20-29. ( 10.1016/j.abb.2016.08.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dittmer PJ, Miranda JG, Gorski JA, Palmer AE. 2009. Genetically encoded sensors to elucidate spatial distribution of cellular zinc. J. Biol. Chem. 284, 16 289-16 297. ( 10.1074/jbc.M900501200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pratt EPS, Damon LJ, Anson KJ, Palmer AE. 2021. Tools and techniques for illuminating the cell biology of zinc. Biochim. Biophys. Acta (BBA) - Mol. Cell Res. 1868, 118865. ( 10.1016/j.bbamcr.2020.118865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Z, Lippard SJ. 2012. Illuminating mobile zinc with fluorescence from cuvettes to live cells and tissues. Methods Enzymol. 505, 445-468. ( 10.1016/B978-0-12-388448-0.00031-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyman LM, Franz KJ. 2012. Probing oxidative stress: Small molecule fluorescent sensors of metal ions, reactive oxygen species, and thiols. Coord. Chem. Rev. 256, 2333-2356. ( 10.1016/j.ccr.2012.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stork CJ, Li YV. 2010. Zinc release from thapsigargin/IP3-sensitive stores in cultured cortical neurons. J. Mol. Signal. 5, 5. ( 10.1186/1750-2187-5-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu Q, Haragopal H, Slepchenko KG, Stork C, Li YV. 2016. Intracellular zinc distribution in mitochondria, ER and the Golgi apparatus. Int. J. Physiol. Pathophysiol. Pharmacol. 8, 35. [PMC free article] [PubMed] [Google Scholar]
- 45.Chang CJ, Nolan EM, Jaworski J, Burdette SC, Sheng M, Lippard SJ. 2004. Bright fluorescent chemosensor platforms for imaging endogenous pools of neuronal zinc. Chem. Biol. 11, 203-210. ( 10.1016/j.chembiol.2004.01.017) [DOI] [PubMed] [Google Scholar]
- 46.Takeda A. 2012. Zinc signaling in the hippocampus and its relation to pathogenesis of depression. J. Trace Elem. Med. Biol. 26, 80-84. ( 10.1016/j.jtemb.2012.03.016) [DOI] [PubMed] [Google Scholar]
- 47.Gee KR, Zhou ZL, Ton-That D, Sensi SL, Weiss JH. 2002. Measuring zinc in living cells: a new generation of sensitive and selective fluorescent probes. Cell Calcium 31, 245-251. ( 10.1016/S0143-4160(02)00053-2) [DOI] [PubMed] [Google Scholar]
- 48.Zhang C, Maslar D, Minckley TF, LeJeune KD, Qin Y. 2021. Spontaneous, synchronous zinc spikes oscillate with neural excitability and calcium spikes in primary hippocampal neuron culture. J. Neurochem. 157, 1838-1849. ( 10.1111/jnc.15334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiedrowski L. 2012. Cytosolic acidification and intracellular zinc release in hippocampal neurons. J. Neurochem. 121, 438-450. ( 10.1111/j.1471-4159.2012.07695.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medvedeva YV, Lin B, Shuttleworth CW, Weiss JH. 2009. Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia. J. Neurosci. 29, 1105-1114. ( 10.1523/JNEUROSCI.4604-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han Y, Goldberg JM, Lippard SJ, Palmer AE. 2018. Superiority of SpiroZin2 versus FluoZin-3 for monitoring vesicular Zn2+ allows tracking of lysosomal Zn2+ pools. Sci. Rep. 8, 1-15. ( 10.1038/s41598-018-33102-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minckley TF, Zhang C, Fudge DH, Dischler AM, LeJeune KD, Xu H, Qin Y. 2019. Sub-nanomolar sensitive GZnP3 reveals TRPML1-mediated neuronal Zn2+ signals. Nat. Commun. 10, 4806. ( 10.1038/s41467-019-12761-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonanni L, et al. 2006. Zinc-dependent multi-conductance channel activity in mitochondria isolated from ischemic brain. J. Neurosci. 26, 6851. ( 10.1523/JNEUROSCI.5444-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sensi S, Ton-That D, Sullivan P, Jonas E, Gee K, Kaczmarek L, Weiss JH. 2003. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc. Natl Acad. Sci. USA 100, 6157-6162. ( 10.1073/pnas.1031598100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gibon J, Tu P, Frazzini V, Sensi SL, Bouron A. 2010. The thiol-modifying agent N-ethylmaleimide elevates the cytosolic concentration of free Zn2+ but not of Ca2+ in murine cortical neurons. Cell Calcium 48, 37-43. ( 10.1016/j.ceca.2010.06.004) [DOI] [PubMed] [Google Scholar]
- 56.Li X, Jiang L. 2018. Multiple molecular mechanisms form a positive feedback loop driving amyloid β42 peptide-induced neurotoxicity via activation of the TRPM2 channel in hippocampal neurons. Cell Death Dis. 9, 1-6. ( 10.1038/s41419-017-0012-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang DM, Huang CC, Chang YF. 2020. Combinatorial roles of mitochondria and cGMP/PKG pathway in the generation of neuronal free Zn2+ under the presence of nitric oxide. J. Chin. Med. Assoc. 83, 357-366. ( 10.1097/jcma.0000000000000280) [DOI] [PubMed] [Google Scholar]
- 58.Park JG, Qin Y, Galati DF, Palmer AE. 2012. New sensors for quantitative measurement of mitochondrial Zn2+. ACS Chem. Biol. 7, 1636-1640. ( 10.1021/cb300171p) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deng H, Qiao X, Xie T, Fu W, Li H, Zhao Y, Miao L. 2021. SLC-30A9 is required for Zn2+ homeostasis, Zn2+ mobilization, and mitochondrial health. Proc. Natl Acad. Sci. USA 118, e2023909118. ( 10.1073/pnas.2023909118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meeusen JW, Tomasiewicz H, Nowakowski A, Petering DH. 2011. TSQ (6-methoxy-8-p-toluenesulfonamido-quinoline), a common fluorescent sensor for cellular zinc, images zinc proteins. Inorg. Chem. 50, 7563-7573. ( 10.1021/ic200478q) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asahina K. 2022. Induction of Cell Death in Pancreatic Tumors by Zinc and Its Fluorescence Chelator TSQ. Biol. Trace Elem. Res. 200, 1667-1676. ( 10.1007/s12011-021-02770-7) [DOI] [PubMed] [Google Scholar]
- 62.Tønder N, Johansen FF, Frederickson CJ, Zimmer J, Diemer NH. 1990. Possible role of zinc in the selective degeneration of dentate hilar neurons after cerebral ischemia in the adult rat. Neurosci. Lett. 109, 247-252. ( 10.1016/0304-3940(90)90002-Q) [DOI] [PubMed] [Google Scholar]
- 63.Lee JY, Cho E, Seo JW, Hwang JJ, Koh JY. 2012. Alteration of the cerebral zinc pool in a mouse model of Alzheimer disease. J. Neuropathol. Exp. Neurol. 71, 211-222. ( 10.1097/NEN.0b013e3182417387) [DOI] [PubMed] [Google Scholar]
- 64.Nowakowski AB, Petering DH. 2011. Reactions of the fluorescent sensor, Zinquin, with the zinc-proteome: adduct formation and ligand substitution. Inorg. Chem. 50, 10 124-10 133. ( 10.1021/ic201076w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sauer GR, Smith DM, Cahalane M, Wu LNY, Wuthier RE. 2003. Intracellular zinc fluxes associated with apoptosis in growth plate chondrocytes. J. Cell. Biochem. 88, 954-969. ( 10.1002/jcb.10446) [DOI] [PubMed] [Google Scholar]
- 66.Marszałek I, Goch W, Bal W. 2019. Ternary Zn(II) complexes of fluorescent zinc probes Zinpyr-1 and Zinbo-5 with the low molecular weight component of exchangeable cellular zinc pool. Inorg. Chem. 58, 14 741-14 751. ( 10.1021/acs.inorgchem.9b02419) [DOI] [PubMed] [Google Scholar]
- 67.Colvin RA, Laskowski M, Fontaine CP. 2006. Zinquin identifies subcellular compartmentalization of zinc in cortical neurons. Relation to the trafficking of zinc and the mitochondrial compartment. Brain Res. 1085, 1-10. ( 10.1016/j.brainres.2006.02.043) [DOI] [PubMed] [Google Scholar]
- 68.Rivera-Fuentes P, Wrobel AT, Zastrow ML, Khan M, Georgiou J, Luyben TT, Roder JC, Okamoto K, Lippard SJ. 2015. A far-red emitting probe for unambiguous detection of mobile zinc in acidic vesicles and deep tissue. Chem. Sci. 6, 1944-1948. ( 10.1039/C4SC03388D) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao J, Bertoglio BA, Gee KR, Kay AR. 2008. The zinc indicator FluoZin-3 is not perturbed significantly by physiological levels of calcium or magnesium. Cell Calcium 44, 422-426. ( 10.1016/j.ceca.2008.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krenn BM, Gaudernak E, Holzer B, Lanke K, Van Kuppeveld FJM, Seipelt J. 2009. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J. Virol. 83, 58-64. ( 10.1128/JVI.01543-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burdette SC, Frederickson CJ, Bu W, Lippard SJ. 2003. ZP4, an improved neuronal Zn2+ sensor of the Zinpyr family. J. Am. Chem. Soc. 125, 1778-1787. ( 10.1021/ja0287377) [DOI] [PubMed] [Google Scholar]
- 72.Zhu L, Wang HD, Yu XG, Jin W, Qiao L, Lu TJ, Hu Z, Zhou J. 2009. Erythropoietin prevents zinc accumulation and neuronal death after traumatic brain injury in rat hippocampus: In vitro and in vivo studies. Brain Res. 1289, 96-105. ( 10.1016/j.brainres.2009.07.015) [DOI] [PubMed] [Google Scholar]
- 73.Frederickson CJ, et al. 2004. Method for identifying neuronal cells suffering zinc toxicity by use of a novel fluorescent sensor. J. Neurosci. Methods 139, 79-89. ( 10.1016/j.jneumeth.2004.04.033) [DOI] [PubMed] [Google Scholar]
- 74.Hirano T, Kikuchi K, Urano Y, Higuchi T, Nagano T. 2000. Highly zinc-selective fluorescent sensor molecules suitable for biological applications. J. Am. Chem. Soc. 122, 12 399-12 400. ( 10.1021/ja002467f) [DOI] [Google Scholar]
- 75.Nydegger I, Rumschik SM, Kay AR. 2010. Zinc Is externalized rather than released during synaptic transmission. ACS Chem. Neurosci. 1, 728-736. ( 10.1021/cn100065s) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takeda A, Takada S, Ando M, Itagaki K, Tamano H, Suzuki M, Iwaki H, Oku N. 2010. Impairment of recognition memory and hippocampal long-term potentiation after acute exposure to clioquinol. Neuroscience 171, 443-450. ( 10.1016/j.neuroscience.2010.09.017) [DOI] [PubMed] [Google Scholar]
- 77.Ueno S, Tsukamoto M, Hirano T, Kikuchi K, Yamada MK, Nishiyama N, Nagano T, Matsuki N, Ikegaya Y. 2002. Mossy fiber Zn2+ spillover modulates heterosynaptic N-methyl-D-aspartate receptor activity in hippocampal CA3 circuits. J. Cell Biol. 158, 215-220. ( 10.1083/jcb.200204066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Y, Hough CJ, Suh SW, Sarvey JM, Frederickson CJ. 2001. Rapid translocation of Zn2+ from presynaptic terminals into postsynaptic hippocampal neurons after physiological stimulation. J. Neurophysiol. 86, 2597-2604. ( 10.1152/jn.2001.86.5.2597) [DOI] [PubMed] [Google Scholar]
- 79.Suh SW. 2009. Detection of zinc translocation into apical dendrite of CA1 pyramidal neuron after electrical stimulation. J. Neurosci. Methods. 177, 1-13. ( 10.1016/j.jneumeth.2008.09.016) [DOI] [PubMed] [Google Scholar]
- 80.Thompson RB, Peterson D, Mahoney W, Cramer M, Maliwal BP, Suh SW, Frederickson C, Fierke C, Herman P. 2002. Fluorescent zinc indicators for neurobiology. J. Neurosci. Methods 118, 63-75. ( 10.1016/S0165-0270(02)00144-9) [DOI] [PubMed] [Google Scholar]
- 81.Cadosch D, Meagher J, Gautschi OP, Filgueira L. 2009. Uptake and intracellular distribution of various metal ions in human monocyte-derived dendritic cells detected by Newport Green™ DCF diacetate ester. J. Neurosci. Methods 178, 182-187. ( 10.1016/j.jneumeth.2008.12.008) [DOI] [PubMed] [Google Scholar]
- 82.Anderson CT, Radford RJ, Zastrow ML, Zhang DY, Apfel UP, Lippard SJ, Tzounopoulos T. et al. 2015. Modulation of extrasynaptic NMDA receptors by synaptic and tonic zinc. Proc. Natl Acad. Sci. USA 112, E2705-E2714. ( 10.1073/pnas.1421567112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vogler NW, Betti VM, Goldberg JM, Tzounopoulos T. 2020. Mechanisms underlying long-term synaptic zinc plasticity at mouse dorsal cochlear nucleus glutamatergic synapses. J. Neurosci. Methods 40, 4981. ( 10.1523/JNEUROSCI.0175-20.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalappa BI, Anderson CT, Goldberg JM, Lippard SJ, Tzounopoulos T. 2015. AMPA receptor inhibition by synaptically released zinc. Proc. Natl Acad. Sci. USA 112, 15 749-15 754. ( 10.1073/pnas.1512296112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fudge DH, Black R, Son L, LeJeune K, Qin Y. 2018. Optical recording of Zn(2+) dynamics in the mitochondrial matrix and intermembrane space with the GZnP2 sensor. ACS Chem. Biol. 13, 1897-1905. ( 10.1021/acschembio.8b00319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vinkenborg JL, Nicolson TJ, Bellomo EA, Koay MS, Rutter GA, Merkx M. 2009. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat. Methods 6, 737-740. ( 10.1038/nmeth.1368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bozym RA, Thompson RB, Stoddard AK, Fierke CA. 2006. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chem. Biol. 1, 103-111. ( 10.1021/cb500043a) [DOI] [PubMed] [Google Scholar]
- 88.Hirano T, Kikuchi K, Urano Y, Nagano T. 2002. Improvement and biological applications of fluorescent probes for zinc, ZnAFs. J. Am. Chem. Soc. 124, 6555-6562. ( 10.1021/ja025567p) [DOI] [PubMed] [Google Scholar]
- 89.Walkup GK, Burdette SC, Lippard SJ, Tsien RY. 2000. A new cell-permeable fluorescent probe for Zn2+. J. Am. Chem. Soc. 122, 5644-5645. ( 10.1021/ja000868p) [DOI] [Google Scholar]
- 90.Devinney MJ, Reynolds IJ, Dineley KE. 2005. Simultaneous detection of intracellular free calcium and zinc using fura-2FF and FluoZin-3. Cell Calcium 37, 225-232. ( 10.1016/j.ceca.2004.10.003) [DOI] [PubMed] [Google Scholar]
- 91.Hessels AM, Chabosseau P, Bakker MH, Engelen W, Rutter GA, Taylor KM, Merkx M. 2015. eZinCh-2: a versatile, genetically encoded FRET sensor for cytosolic and intraorganelle Zn2+ imaging. ACS Chem. Biol. 10, 2126-2134. ( 10.1021/acschembio.5b00211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chabosseau P, et al. 2014. Mitochondrial and ER-targeted eCALWY probes reveal high levels of free Zn2+. ACS Chem. Biol. 9, 2111-2120. ( 10.1021/cb5004064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xue L, Li G, Yu C, Jiang H. 2012. A ratiometric and targetable fluorescent sensor for quantification of mitochondrial zinc ions. Chemistry. 18, 1050-1054. ( 10.1002/chem.201103007) [DOI] [PubMed] [Google Scholar]
- 94.McCranor BJ, Bozym RA, Vitolo MI, Fierke CA, Bambrick L, Polster BM, Fiskum G, Thompson RB. 2012. Quantitative imaging of mitochondrial and cytosolic free zinc levels in an in vitro model of ischemia/reperfusion. J. Bioenerg. Biomembr. 44, 253-263. ( 10.1007/s10863-012-9427-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chyan W, Zhang DY, Lippard SJ, Radford RJ. 2014. Reaction-based fluorescent sensor for investigating mobile Zn2+ in mitochondria of healthy versus cancerous prostate cells. Proc. Natl Acad. Sci. USA 111, 143-148. ( 10.1073/pnas.1310583110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tomat E, Nolan EM, Jaworski J, Lippard SJ. 2008. Organelle-specific zinc detection using zinpyr-labeled fusion proteins in live cells. J. Am. Chem. Soc. 130, 15 776-15 777. ( 10.1021/ja806634e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takeda A. 2011. Insight into glutamate excitotoxicity from synaptic zinc homeostasis. Int. J. Alzheimer's Dis. 2011, 1-8. ( 10.4061/2011/491597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vergnano A, Rebola N, Savtchenko L, Pinheiro P, Casado M, Kieffer B, Rusakov DA, Mulle C, Paoletti P. 2014. Zinc dynamics and action at excitatory synapses. Neuron 82, 1101-1114. ( 10.1016/j.neuron.2014.04.034) [DOI] [PubMed] [Google Scholar]
- 99.Paoletti P, Vergnano AM, Barbour B, Casado M. 2009. Zinc at glutamatergic synapses. Neuroscience 158, 126-136. ( 10.1016/j.neuroscience.2008.01.061) [DOI] [PubMed] [Google Scholar]
- 100.Sindreu C, Storm DR. 2011. Modulation of neuronal signal transduction and memory formation by synaptic zinc. Front. Behav. Neurosci. 5, 68. ( 10.3389/fnbeh.2011.00068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Frederickson CJ, Bush AI. 2001. Synaptically released zinc: physiological functions and pathological effects. Biometals 14, 353-366. ( 10.1023/A:1012934207456) [DOI] [PubMed] [Google Scholar]
- 102.Bush AI. 2003. The metallobiology of Alzheimer's disease. Trends Neurosci. 26, 207-214. ( 10.1016/S0166-2236(03)00067-5) [DOI] [PubMed] [Google Scholar]
- 103.Ruiz A, Walker MC, Fabian-Fine R, Kullmann DM. 2004. Endogenous zinc inhibits GABAA receptors in a hippocampal pathway. J. Neurophysiol. 91, 1091-1096. ( 10.1152/jn.00755.2003) [DOI] [PubMed] [Google Scholar]
- 104.Sindreu CB, Varoqui H, Erickson JD, Pérez-Clausell J. 2003. Boutons containing vesicular zinc define a subpopulation of synapses with low AMPAR content in rat hippocampus. Cerebral Cortex 13, 823-829. ( 10.1093/cercor/13.8.823) [DOI] [PubMed] [Google Scholar]
- 105.Haug F-MŠ. 1967. Electron microscopical localization of the zinc in hippocampal mossy fibre synapses by a modified sulfide silver procedure. Histochemie 8, 355-368. ( 10.1007/BF00401978) [DOI] [PubMed] [Google Scholar]
- 106.Hassler O, Söremark R. 1968. Accumulation of zinc in mouse brain: An autoradiographic study with 65Zn. Archives Neurol. 19, 117-120. ( 10.1001/archneur.1968.00480010135011) [DOI] [PubMed] [Google Scholar]
- 107.Sikora J, Ouagazzal A-M. 2021. Synaptic zinc: an emerging player in Parkinson's disease. Int. J. Mol. Sci. 22, 4724. ( 10.3390/ijms22094724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu H. 1993. Chelation of zinc by diethyldithiocarbamate facilitates bursting induced by mixed antidromic plus orthodromic activation of mossy fibers in hippocampal slices. Brain Res. 624, 162-170. ( 10.1016/0006-8993(93)90074-W) [DOI] [PubMed] [Google Scholar]
- 109.Vogt K, Mellor J, Tong G, Nicoll R. 2000. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 26, 187-196. ( 10.1016/S0896-6273(00)81149-6) [DOI] [PubMed] [Google Scholar]
- 110.Li Y, Hough CJ, Frederickson CJ, Sarvey JM. 2001. Induction of mossy fiber→CA3 long-term potentiation requires translocation of synaptically released Zn2+. J. Neurosci. 21, 8015-8025. ( 10.1523/JNEUROSCI.21-20-08015.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pérez-Clausell J, Danscher G. 1985. Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res. 337, 91-98. ( 10.1016/0006-8993(85)91612-9) [DOI] [PubMed] [Google Scholar]
- 112.Frederickson CJ, Howell GA, Haigh MD, Danscher G. 1988. Zinc-containing fiber systems in the cochlear nuclei of the rat and mouse. Hearing Res. 36, 203-211. ( 10.1016/0378-5955(88)90062-7) [DOI] [PubMed] [Google Scholar]
- 113.Perez-Rosello T, et al. 2013. Synaptic Zn2+ inhibits neurotransmitter release by promoting endocannabinoid synthesis. J. Neurosci. 33, 9259-9272. ( 10.1523/JNEUROSCI.0237-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Birinyi A, Parker D, Antal M, Shupliakov O. 2001. Zinc co-localizes with GABA and glycine in synapses in the lamprey spinal cord. J. Comp. Neurol. 433, 208-221. ( 10.1002/cne.1136) [DOI] [PubMed] [Google Scholar]
- 115.Wang Z, Li JY, Dahlström A, Danscher G. 2001. Zinc-enriched GABAergic terminals in mouse spinal cord. Brain Res. 921, 165-172. ( 10.1016/S0006-8993(01)03114-6) [DOI] [PubMed] [Google Scholar]
- 116.Zhang Y, Keramidas A, Lynch JW. 2016. The free zinc concentration in the synaptic cleft of artificial glycinergic synapses rises to at least 1 μM. Front. Mol. Neurosci. 9, 88. ( 10.3389/fnmol.2016.00088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wolf C, Weth A, Walcher S, Lax C, Baumgartner W. 2018. Modeling of zinc dynamics in the synaptic cleft: Implications for CADHERIN mediated adhesion and synaptic plasticity. Front. Mol. Neurosci. 11, 306. ( 10.3389/fnmol.2018.00306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Besser L, Chorin E, Sekler I, Silverman WF, Atkin S, Russell JT, Hershfinkel M. 2009. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J. Neurosci. Methods 29, 2890-2901. ( 10.1523/JNEUROSCI.5093-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frederickson CJ, et al. 2006. Synaptic release of zinc from brain slices: factors governing release, imaging, and accurate calculation of concentration. J. Neurosci. Methods 154, 19-29. ( 10.1016/j.jneumeth.2005.11.014) [DOI] [PubMed] [Google Scholar]
- 120.Kodirov SA, Takizawa S, Joseph J, Kandel ER, Shumyatsky GP, Bolshakov VY. 2006. Synaptically released zinc gates long-term potentiation in fear conditioning pathways. Proc. Natl Acad. Sci. USA 103, 15 218-15 223. ( 10.1073/pnas.0607131103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qian J, Noebels JL. 2006. Exocytosis of vesicular zinc reveals persistent depression of neurotransmitter release during metabotropic glutamate receptor long-term depression at the hippocampal CA3–CA1 synapse. J. Neurosci. Methods 26, 6089-6095. ( 10.1523/JNEUROSCI.0475-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kay AR, Tóth K. 2006. Influence of location of a fluorescent zinc probe in brain slices on its response to synaptic activation. J. Neurophysiol. 95, 1949-1956. ( 10.1152/jn.00959.2005) [DOI] [PubMed] [Google Scholar]
- 123.Assaf SY, Chung SH. 1984. Release of endogenous Zn2+ from brain tissue during activity. Nature 308, 734-736. ( 10.1038/308734a0) [DOI] [PubMed] [Google Scholar]
- 124.Aniksztejn L, Charton G, Ben-Ari Y. 1987. Selective release of endogenous zinc from the hippocampal mossy fibers in situ. Brain Res. 404, 58-64. ( 10.1016/0006-8993(87)91355-2) [DOI] [PubMed] [Google Scholar]
- 125.Howell GA, Welch MG, Frederickson CJ. 1984. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature 308, 736-738. ( 10.1038/308736a0) [DOI] [PubMed] [Google Scholar]
- 126.Qian J, Noebels JL. 2005. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J. Physiol. 566, 747-758. ( 10.1113/jphysiol.2005.089276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ketterman JK, Li YV. 2008. Presynaptic evidence for zinc release at the mossy fiber synapse of rat hippocampus. J. Neurosci. Res. 86, 422-434. ( 10.1002/jnr.21488) [DOI] [PubMed] [Google Scholar]
- 128.Frederickson CJ, Kasarskis EJ, Ringo D, Frederickson RE. 1987. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J. Neurosci. Methods 20, 91-103. ( 10.1016/0165-0270(87)90042-2) [DOI] [PubMed] [Google Scholar]
- 129.Palmiter RD, Cole TB, Quaife CJ, Findley SD. 1996. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl Acad. Sci. USA 93, 14 934-14 939. ( 10.1073/pnas.93.25.14934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. 1999. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl Acad. Sci. USA 96, 1716-1721. ( 10.1073/pnas.96.4.1716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Frederickson CJ, Koh JY, Bush AI. 2005. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 6, 449-462. ( 10.1038/nrn1671) [DOI] [PubMed] [Google Scholar]
- 132.Kay AR, Tóth K. 2008. Is zinc a neuromodulator? Sci. Signal. 1, re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smart TG, Hosie AM, Miller PS. 2004. Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist 10, 432-442. ( 10.1177/1073858404263463) [DOI] [PubMed] [Google Scholar]
- 134.Kauppinen TM, Higashi Y, Suh SW, Escartin C, Nagasawa K, Swanson RA. 2008. Zinc triggers microglial activation. J. Neurosci. 28, 5827-5835. ( 10.1523/JNEUROSCI.1236-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huiliang Z, et al. 2021. Zinc induces reactive astrogliosis through ERK-dependent activation of Stat3 and promotes synaptic degeneration. J. Neurochem. 159, 1016-1027. ( 10.1111/jnc.15531) [DOI] [PubMed] [Google Scholar]
- 136.Anderson CT, Kumar M, Xiong S, Tzounopoulos T. 2017. Cell-specific gain modulation by synaptically released zinc in cortical circuits of audition. Elife 6, e29893. ( 10.7554/elife.29893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li Y, et al. 2017. Mobile zinc increases rapidly in the retina after optic nerve injury and regulates ganglion cell survival and optic nerve regeneration. Proc. Natl Acad. Sci. USA 114, E209-E218. ( 10.1073/pnas.1616811114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Krall RF, Tzounopoulos T, Aizenman E. 2021. The function and regulation of zinc in the brain. Neuroscience 457, 235-258. ( 10.1016/j.neuroscience.2021.01.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Radford RJ, Lippard SJ. 2013. Chelators for investigating zinc metalloneurochemistry. Curr. Opin. Chem. Biol. 17, 129-136. ( 10.1016/j.cbpa.2013.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hirzel K, Müller U, Latal AT, Hülsmann S, Grudzinska J, Seeliger MW, Betz H, Laube B. 2006. Hyperekplexia phenotype of glycine receptor alpha1 subunit mutant mice identifies Zn2+ as an essential endogenous modulator of glycinergic neurotransmission. Neuron 52, 679-690. ( 10.1016/j.neuron.2006.09.035) [DOI] [PubMed] [Google Scholar]
- 141.Nydegger I, Rumschik SM, Zhao J, Kay AR. 2012. Evidence for an extracellular zinc-veneer in rodent brains from experiments with Zn-ionophores and ZnT3 knockouts. ACS Chem. Neurosci. 3, 761-766. ( 10.1021/cn300061z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Krall RF, Moutal A, Phillips MB, Asraf H, Johnson JW, Khanna R, Hershfinkel M, Aizenman E, Tzounopoulos T. 2020. Synaptic zinc inhibition of NMDA receptors depends on the association of GluN2A with the zinc transporter ZnT1. Sci. Adv. 6, eabb1515. ( 10.1126/sciadv.abb1515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weiss JH, Sensi SL. 2000. Ca2+–Zn2+ permeable AMPA or kainate receptors: possible key factors in selective neurodegeneration. Trends Neurosci. 23, 365-371. ( 10.1016/S0166-2236(00)01610-6) [DOI] [PubMed] [Google Scholar]
- 144.Blakemore LJ, Trombley PQ. 2017. Zinc as a neuromodulator in the central nervous system with a focus on the olfactory bulb. Front. Cell. Neurosci. 11, 297. ( 10.3389/fncel.2017.00297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schwiebert EM, Liang L, Cheng NL, Williams CR, Olteanu D, Welty EA, Zsembery A. 2005. Extracellular zinc and ATP-gated P2X receptor calcium entry channels: new zinc receptors as physiological sensors and therapeutic targets. Purinergic Signal. 1, 299-310. ( 10.1007/s11302-005-0777-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu H, Bandell M, Petrus MJ, Zhu MX, Patapoutian A. 2009. Zinc activates damage-sensing TRPA1 ion channels. Nat. Chem. Biol. 5, 183-190. ( 10.1038/nchembio.146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Atar D, Backx PH, Appel MM, Gao WD, Marban E. 1995. Excitation-transcription coupling mediated by zinc influx through voltage-dependent calcium channels (∗). J. Biol. Chem. 270, 2473-2477. ( 10.1074/jbc.270.6.2473) [DOI] [PubMed] [Google Scholar]
- 148.Gyulkhandanyan AV, Lee SC, Bikopoulos G, Dai F, Wheeler MB. 2006. The Zn2+-transporting pathways in pancreatic β-cells: a role for the L-type voltage-gated Ca2+ channel. J. Biol. Chem. 281, 9361-9372. ( 10.1074/jbc.M508542200) [DOI] [PubMed] [Google Scholar]
- 149.Kerchner GA, Canzoniero LMT, Yu SP, Ling C, Choi DW. 2000. Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J. Physiol. 528, 39-52. ( 10.1111/j.1469-7793.2000.00039.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen N, Moshaver A, Raymond LA. 1997. Differential sensitivity of recombinant N-methyl-D-aspartate receptor subtypes to zinc inhibition. Mol. Pharmacol. 51, 1015-1023. ( 10.1124/mol.51.6.1015) [DOI] [PubMed] [Google Scholar]
- 151.Fayyazuddin A, Villarroel A, Le Goff A, Lerma J, Neyton J. 2000. Four residues of the extracellular N-terminal domain of the NR2A subunit control high-affinity Zn2+ binding to NMDA receptors. Neuron 25, 683-694. ( 10.1016/S0896-6273(00)81070-3) [DOI] [PubMed] [Google Scholar]
- 152.Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. 2005. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J. Neurosci. 25, 308-317. ( 10.1523/JNEUROSCI.3967-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Carrillo E, Bhatia NK, Akimzhanov AM, Jayaraman V. 2020. Activity dependent inhibition of AMPA receptors by Zn2+. J. Neurosci. 40, 8629-8636. ( 10.1523/JNEUROSCI.1481-20.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang YZ, Pan E, Xiong ZQ, McNamara JO. 2008. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron 57, 546-558. ( 10.1016/j.neuron.2007.11.026) [DOI] [PubMed] [Google Scholar]
- 155.Kouvaros S, Kumar M, Tzounopoulos T. 2020. Synaptic zinc enhances inhibition mediated by somatostatin, but not parvalbumin, cells in mouse auditory cortex. Cerebral Cortex 30, 3895-3909. ( 10.1093/cercor/bhaa005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Suwa H, Saint-Amant L, Triller A, Drapeau P, Legendre P. 2001. High-affinity zinc potentiation of inhibitory postsynaptic glycinergic currents in the zebrafish hindbrain. J. Neurophysiol. 85, 912-925. ( 10.1152/jn.2001.85.2.912) [DOI] [PubMed] [Google Scholar]
- 157.Laube B. 2002. Potentiation of inhibitory glycinergic neurotransmission by Zn2+: a synergistic interplay between presynaptic P2X2 and postsynaptic glycine receptors. European J. Neurosci. 16, 1025-1036. ( 10.1046/j.1460-9568.2002.02170.x) [DOI] [PubMed] [Google Scholar]
- 158.Chorin E, Vinograd O, Fleidervish I, Gilad D, Herrmann S, Sekler I, Aizenman E, Hershfinkel M. 2011. Upregulation of KCC2 activity by zinc-mediated neurotransmission via the mZnR/GPR39 receptor. J. Neurosci. Methods 31, 12 916-12 926. ( 10.1523/JNEUROSCI.2205-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Asraf H, Bogdanovic M, Gottesman N, Sekler I, Aizenman E, Hershfinkel M. 2022. SNAP23 regulates KCC2 membrane insertion and activity following mZnR/GPR39 activation in hippocampalneurons. iScience 25, 103751. ( 10.1016/j.isci.2022.103751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gilad D, Shorer S, Ketzef M, Friedman A, Sekler I, Aizenman E, Hershfinkel M. 2015. Homeostatic regulation of KCC2 activity by the zinc receptor mZnR/GPR39 during seizures. Neurobiol. Dis. 81, 4-13. ( 10.1016/j.nbd.2014.12.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hwang JJ, Park MH, Choi SY, Koh JY. 2005. Activation of the Trk signaling pathway by extracellular zinc. Role of metalloproteinases. J. Biol. Chem. 280, 11 995-12 001. ( 10.1074/jbc.M403172200) [DOI] [PubMed] [Google Scholar]
- 162.Adlard PA, Parncutt JM, Finkelstein DI, Bush AI. 2010. Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer's disease? J. Neurosci. Methods 30, 1631-1636. ( 10.1523/JNEUROSCI.5255-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nakashima AS, Butt RH, Dyck RH. 2011. Alterations in protein and gene expression within the barrel cortices of ZnT3 knockout mice: experience-independent and dependent changes. Neurochem. Int. 59, 860-870. ( 10.1016/j.neuint.2011.08.007) [DOI] [PubMed] [Google Scholar]
- 164.Helgager J, Huang YZ, McNamara JO. 2014. Brain-derived neurotrophic factor but not vesicular zinc promotes TrkB activation within mossy fibers of mouse hippocampus in vivo. J. Comp. Neurol. 522, 3885-3899. ( 10.1002/cne.23647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yoo MH, Kim TY, Yoon YH, Koh JY. 2016. Autism phenotypes in ZnT3 null mice: Involvement of zinc dyshomeostasis, MMP-9 activation and BDNF upregulation. Sci. Rep. 6, 1-15. ( 10.1038/s41598-016-0001-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McAllister BB, Bihelek N, Mychasiuk R, Dyck RH. 2020. Brain-derived neurotrophic factor and TrkB levels in mice that lack vesicular zinc: effects of age and sex. Neuroscience 425, 90-100. ( 10.1016/j.neuroscience.2019.11.009) [DOI] [PubMed] [Google Scholar]
- 167.Martel G, Hevi C, Friebely O, Baybutt T, Shumyatsky GP. 2010. Zinc transporter 3 is involved in learned fear and extinction, but not in innate fear. Learning Memory 17, 582-590. ( 10.1101/lm.1962010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Takeda A, Fuke S, Ando M, Oku N. 2009. Positive modulation of long-term potentiation at hippocampal CA1 synapses by low micromolar concentrations of zinc. Neuroscience 158, 585-591. ( 10.1016/j.neuroscience.2008.10.009) [DOI] [PubMed] [Google Scholar]
- 169.Takeda A, Tamano H. 2017. The impact of synaptic Zn2+ dynamics on cognition and its decline. Int. J. Mol. Sci. 18, 2411. ( 10.3390/ijms18112411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Martel G, Hevi C, Kane-Goldsmith N, Shumyatsky GP. 2011. Zinc transporter ZnT3 is involved in memory dependent on the hippocampus and perirhinal cortex. Behav. Brain Res. 223, 233-238. ( 10.1016/j.bbr.2011.04.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cole TB, Martyanova A, Palmiter RD. 2001. Removing zinc from synaptic vesicles does not impair spatial learning, memory, or sensorimotor functions in the mouse. Brain Res. 891, 253-265. ( 10.1016/S0006-8993(00)03220-0) [DOI] [PubMed] [Google Scholar]
- 172.Kumar M, Xiong S, Tzounopoulos T, Anderson CT. 2019. Fine control of sound frequency tuning and frequency discrimination acuity by synaptic zinc signaling in mouse auditory cortex. J. Neurosci. Methods 39, 854-865. ( 10.1523/JNEUROSCI.1339-18.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wu HP, Dyck RH. 2018. Signaling by synaptic zinc is required for whisker-mediated, fine texture discrimination. Neuroscience 369, 242-247. ( 10.1016/j.neuroscience.2017.11.020) [DOI] [PubMed] [Google Scholar]
- 174.Bafaro E, Liu Y, Xu Y, Dempski RE. 2017. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduction Targeted Therapy 2, 1-12. ( 10.1038/sigtrans.2017.29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Colvin RA. 1998. Characterization of a plasma membrane zinc transporter in rat brain. Neurosci. Lett. 247, 147-150. ( 10.1016/S0304-3940(98)00302-4) [DOI] [PubMed] [Google Scholar]
- 176.Gaither LA, Eide DJ. 2000. Functional expression of the human hZIP2 zinc transporter. J. Biol. Chem. 275, 5560-5564. ( 10.1074/jbc.275.8.5560) [DOI] [PubMed] [Google Scholar]
- 177.Dufner-Beattie J, Langmade SJ, Wang F, Eide D, Andrews GK. 2003. Structure, function, and regulation of a subfamily of mouse zinc transporter genes. J. Biol. Chem. 278, 50 142-50 150. ( 10.1074/jbc.M304163200) [DOI] [PubMed] [Google Scholar]
- 178.Belloni-Olivi L, Marshall C, Laal B, Andrews GK, Bressler J. 2009. Localization of zip1 and zip4 mRNA in the adult rat brain. J. Neurosci. Res. 87, 3221-3230. ( 10.1002/jnr.22144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Emmetsberger J, Mirrione MM, Zhou C, Fernandez-Monreal M, Siddiq MM, Ji K, Tsirka SE. 2010. Tissue plasminogen activator alters intracellular sequestration of zinc through interaction with the transporter ZIP4. J. Neurosci. Methods 30, 6538-6547. ( 10.1523/JNEUROSCI.6250-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim AH, Sheline CT, Tian M, Higashi T, McMahon RJ, Cousins RJ, Choi DW. 2000. L-type Ca2+ channel-mediated Zn2+ toxicity and modulation by ZnT-1 in.PC12 cells. Brain Res. 886, 99-107. ( 10.1016/S0006-8993(00)02944-9) [DOI] [PubMed] [Google Scholar]
- 181.Lichten LA, Cousins RJ. 2009. Mammalian zinc transporters: nutritional and physiologic regulation. Ann. Rev. Nutr. 29, 153-176. ( 10.1146/annurev-nutr-033009-083312) [DOI] [PubMed] [Google Scholar]
- 182.Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. 2004. Overview of mammalian zinc transporters. Cell. Mol. Life Sci. 61, 49-68. ( 10.1007/s00018-003-3148-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mellone M, Pelucchi S, Alberti L, Genazzani AA, Di Luca M, Gardoni F. 2015. Zinc transporter-1: a novel NMDA receptor-binding protein at the postsynaptic density. J. Neurochem. 132, 159-168. ( 10.1111/jnc.12968) [DOI] [PubMed] [Google Scholar]
- 184.Kowalczyk A, et al. 2021. Evolutionary rate covariation identifies SLC30A9 (ZnT9) as a mitochondrial zinc transporter. Biochem. J. 478, 3205-3220. ( 10.1042/BCJ20210342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sanchez VB, Ali S, Escobar A, Cuajungco MP. 2019. Transmembrane 163 (TMEM163) protein effluxes zinc. Arch. Biochem. Biophys. 677, 108166. ( 10.1016/j.abb.2019.108166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Qian J, Xu K, Yoo J, Chen TT, Andrews G, Noebels JL. 2011. Knockout of Zn transporters Zip-1 and Zip-3 attenuates seizure-induced CA1 neurodegeneration. J. Neurosci. 31, 97-104. ( 10.1523/JNEUROSCI.5162-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bogdanovic M, Asraf H, Gottesman N, Sekler I, Aizenman E, Hershfinkel M. 2022. The ZIP3 zinc transporter is localized to mossy fiber terminals and is required for kainate-induced degeneration of CA3 neurons. J. Neurosci. 42, 2824-2834. ( 10.1523/JNEUROSCI.0908-21.2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.De Benedictis CA, Haffke C, Hagmeyer S, Sauer AK, Grabrucker AM. 2021. Expression analysis of zinc transporters in nervous tissue cells reveals neuronal and synaptic localization of ZIP4. Int. J. Mol. Sci. 22, 4511. ( 10.3390/ijms22094511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aschner M. 1996. The functional significance of brain metallothioneins. FASEB J. 10, 1129-1136. ( 10.1096/fasebj.10.10.8751715) [DOI] [PubMed] [Google Scholar]
- 190.Koh JY, Lee SJ. 2020. Metallothionein-3 as a multifunctional player in the control of cellular processes and diseases. Molecular Brain 13, 1-12. ( 10.1186/s13041-019-0541-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nakajima K, Suzuki K. 1995. Immunochemical detection of metallothionein in brain. Neurochem. Int. 27, 73-87. ( 10.1016/0197-0186(94)00169-U) [DOI] [PubMed] [Google Scholar]
- 192.Lee SJ, Park MH, Kim HJ, Koh JY. 2010. Metallothionein-3 regulates lysosomal function in cultured astrocytes under both normal and oxidative conditions. Glia 58, 1186-1196. ( 10.1002/glia.20998) [DOI] [PubMed] [Google Scholar]
- 193.Masters BA, Quaife CJ, Erickson JC, Kelly EJ, Froelick GJ, Zambrowicz BP, Brinster RL, Palmiter RD. 1994. Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J. Neurosci. 14, 5844-5857. ( 10.1523/JNEUROSCI.14-10-05844.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yamada M, Hayashi S, Hozumi I, Inuzuka T, Tsuji S, Takahashi H. 1996. Subcellular localization of growth inhibitory factor in rat brain: light and electron microscopic immunohistochemical studies. Brain Res. 735, 257-264. ( 10.1016/0006-8993(96)00586-0) [DOI] [PubMed] [Google Scholar]
- 195.Frederickson CJ, Maret W, Cuajungco MP. 2004. Zinc and excitotoxic brain injury: a new model. Neuroscientist 10, 18-25. ( 10.1177/1073858403255840) [DOI] [PubMed] [Google Scholar]
- 196.Shuttleworth CW, Weiss JH. 2011. Zinc: new clues to diverse roles in brain ischemia. Trends Pharmacol. Sci. 32, 480-486. ( 10.1016/j.tips.2011.04.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Maret W. 2000. The function of zinc metallothionein: a link between cellular zinc and redox state. J. Nutr. 130, 1455S-1458S. ( 10.1093/jn/130.5.1455S) [DOI] [PubMed] [Google Scholar]
- 198.Chen Y, Irie Y, Keung WM, Maret W. 2002. S-nitrosothiols react preferentially with zinc thiolate clusters of metallothionein III through transnitrosation. Biochemistry 41, 8360-8367. ( 10.1021/bi020030+) [DOI] [PubMed] [Google Scholar]
- 199.Bossy-Wetzel E, et al. 2004. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron 41, 351-365. ( 10.1016/S0896-6273(04)00015-7) [DOI] [PubMed] [Google Scholar]
- 200.Frederickson CJ, et al. 2006. Concentrations of extracellular free zinc (pZn) e in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp. Neurol. 198, 285-293. ( 10.1016/j.expneurol.2005.08.030) [DOI] [PubMed] [Google Scholar]
- 201.Klitenick MA, Frederickson CJ, Manton WI. 1983. Acid-vapor decomposition for determination of zinc in brain tissue by isotope dilution mass spectrometry. Anal. Chem. 55, 921-923. ( 10.1021/ac00257a023) [DOI] [PubMed] [Google Scholar]
- 202.Sensi SL, Canzoniero LM, Yu SP, Ying HS, Koh JY, Kerchner GA, Choi DW. 1997. Measurement of intracellular free zinc in living cortical neurons: routes of entry. J. Neurosci. 17, 9554-9564. ( 10.1523/JNEUROSCI.17-24-09554.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Weiss JH, Hartley DM, Koh JY, Choi DW. 1993. AMPA receptor activation potentiates zinc neurotoxicity. Neuron 10, 43-49. ( 10.1016/0896-6273(93)90240-R) [DOI] [PubMed] [Google Scholar]
- 204.Sanford L, Palmer AE. 2020. Dissociated hippocampal neurons exhibit distinct Zn2+ dynamics in a stimulation-method-dependent manner. ACS Chem. Neurosci. 11, 508-514. ( 10.1021/acschemneuro.0c00006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Inoue K, O'Bryant Z, Xiong ZG. 2015. Zinc-permeable ion channels: effects on intracellular zinc dynamics and potential physiological/pathophysiological significance. Curr. Med. Chem. 22, 1248-1257. ( 10.2174/0929867322666150209153750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gibon J, Tu P, Bohic S, Richaud P, Arnaud J, Zhu M, Boulay G, Bouron A. 2011. The over-expression of TRPC6 channels in HEK-293 cells favours the intracellular accumulation of zinc. Biochim. Biophys. Acta (BBA)-Biomembr. 1808, 2807-2818. ( 10.1016/j.bbamem.2011.08.013) [DOI] [PubMed] [Google Scholar]
- 207.Wilson M, Hogstrand C, Maret W. 2012. Picomolar concentrations of free zinc(II) ions regulate receptor protein-tyrosine phosphatase β activity. J. Biol. Chem. 287, 9322-9326. ( 10.1074/jbc.C111.320796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Maret W, Jacob C, Vallee BL, Fischer EH. 1999. Inhibitory sites in enzymes: zinc removal and reactivation by thionein. Proc. Natl Acad. Sci. USA 96, 1936-1940. ( 10.1073/pnas.96.5.1936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hogstrand C, Verbost PM, Wendelaar Bonga SE. 1999. Inhibition of human erythrocyte Ca2+-ATPase by Zn2+. Toxicology 133, 139-145. ( 10.1016/S0300-483X(99)00020-7) [DOI] [PubMed] [Google Scholar]
- 210.Lee JR. 2015. Protein tyrosine phosphatase PTPRT as a regulator of synaptic formation and neuronal development. BMB Rep. 48, 249. ( 10.5483/BMBRep.2015.48.5.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sindreu C, Palmiter RD, Storm DR. 2011. Zinc transporter ZnT-3 regulates presynaptic Erk1/2 signaling and hippocampus-dependent memory. Proc. Natl Acad. Sci. USA 108, 3366-3370. ( 10.1073/pnas.1019166108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Anson KJ, Corbet GA, Palmer AE. 2021. Zn2+ influx activates ERK and Akt signaling pathways. Proc. Natl Acad. Sci. USA 118, e2015786118. ( 10.1073/pnas.2015786118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sanford L, Carpenter MC, Palmer AE. 2019. Intracellular Zn2+ transients modulate global gene expression in dissociated rat hippocampal neurons. Sci. Rep. 9, 1-14. ( 10.1038/s41598-018-37186-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Monteiro P, Feng G. 2017. SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat. Rev. Neurosci. 18, 147-157. ( 10.1038/nrn.2016.183) [DOI] [PubMed] [Google Scholar]
- 215.Sheng M, Kim E. 2000. The Shank family of scaffold proteins. J. Cell Sci. 113(Pt 11), 1851-1856. ( 10.1242/jcs.113.11.1851) [DOI] [PubMed] [Google Scholar]
- 216.Tu JC, et al. 1999. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23, 583-592. ( 10.1016/S0896-6273(00)80810-7) [DOI] [PubMed] [Google Scholar]
- 217.Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M. 1999. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 23, 569-582. ( 10.1016/S0896-6273(00)80809-0) [DOI] [PubMed] [Google Scholar]
- 218.Uchino S, et al. 2006. Direct interaction of post-synaptic density-95/Dlg/ZO-1 domain-containing synaptic molecule Shank3 with GluR1 α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor. J. Neurochem. 97, 1203-1214. ( 10.1111/j.1471-4159.2006.03831.x) [DOI] [PubMed] [Google Scholar]
- 219.Harris KP, Akbergenova Y, Cho RW, Baas-Thomas MS, Littleton JT. 2016. Shank modulates postsynaptic Wnt signaling to regulate synaptic development. J. Neurosci. 36, 5820-5832. ( 10.1523/JNEUROSCI.4279-15.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Budnik V, Salinas PC. 2011. Wnt signaling during synaptic development and plasticity. Curr. Opin. Neurobiol. 21, 151-159. ( 10.1016/j.conb.2010.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Restrepo LJ, DePew AT, Moese ER, Tymanskyj SR, Parisi MJ, Aimino MA, Duhart JC, Fei H, Mosca TJ. 2022. γ-secretase promotes Drosophila postsynaptic development through the cleavage of a Wnt receptor. Dev. Cell 57, 1643-1660.37. (doi:1016/j.devcel.2022.05.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gundelfinger ED, Boeckers TM, Baron MK, Bowie JU. 2006. A role for zinc in postsynaptic density asSAMbly and plasticity? Trends Biochem. Sci. 31, 366-373. ( 10.1016/j.tibs.2006.05.007) [DOI] [PubMed] [Google Scholar]
- 223.Baron MK, Boeckers TM, Vaida B, Faham S, Gingery M, Sawaya MR, Salyer D, Gundelfinger ED, Bowie JU. 2006. An architectural framework that may lie at the core of the postsynaptic density. Science 311, 531-535. ( 10.1126/science.1118995) [DOI] [PubMed] [Google Scholar]
- 224.Ha HT, et al. 2018. Shank and zinc mediate an AMPA receptor subunit switch in developing neurons. Front. Mol. Neurosci. 11, 405. ( 10.3389/fnmol.2018.00405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Grabrucker AM, et al. 2011. Concerted action of zinc and ProSAP/Shank in synaptogenesis and synapse maturation. EMBO J. 30, 569-581. ( 10.1038/emboj.2010.336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Arons MH, Lee K, Thynne CJ, Kim SA, Schob C, Kindler S, Montgomery JM, Garner CC. 2016. Shank3 is part of a zinc-sensitive signaling system that regulates excitatory synaptic strength. J. Neurosci. 36, 9124-9134. ( 10.1523/JNEUROSCI.0116-16.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Grabrucker S, et al. 2014. Zinc deficiency dysregulates the synaptic ProSAP/Shank scaffold and might contribute to autism spectrum disorders. Brain 137, 137-152. ( 10.1093/brain/awt303) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.