Abstract
Background: Bell's palsy is a rare adverse event reported in COVID-19 vaccines. Given the importance of neurological manifestations, the necessity to highlight and scrutinize the incidence of them following COVID-19 vaccination is needed. This study aimed to systematically review the reported cases of Bell's palsy following vaccination against COVID-19.
Methods: This systematic review is conducted based on the Cochrane Collaboration Handbook and PRISMA Statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyzes) and using the Joanna Briggs Institute (JBI) methodology for systematic reviews. The inclusion criteria for the included published studies were patient age ≥18 years, history of Bell's palsy after COVID-19 vaccination and established diagnosis in the patients with COVID-19 vaccination. The exclusion criteria were repeated cases and missing clinical information. The search strategy aimed to find both published and unpublished studies in August 2021 and updated by hand searching in May 2022 using the identified keywords and index terms in Cochrane Library, MEDLINE (PubMed), Web of Science, Scopus, ProQuest, and Google scholar. Finally, the reference lists of all identified reports and articles were searched for additional studies. The JBI critical appraisal tools for case reports or case series were used to assess the risk of bias in the included studies.
Results: During the electronic search, hand search, and reference check, we identified 1281 citations, and in hand searching, we detected additional 15 studies. After omitting duplicated citations and assessing the title, abstract, and full text 15 case-report and two case-series studies were included for the critical appraisal process and were included in this study. Pfizer and Moderna vaccines were the most common vaccines among articles that reported the cases of Bell’s palsy. Left-sided paralysis was more common than right-sided paralysis. The interval between receiving the vaccine and the onset of facial weakness was between 1 and 48 days.
Conclusion: Further studies with larger sample sizes are necessary to assess the association between Bell's palsy and the dose-response of the COVID-19 vaccine.
Keywords: Bell's Palsy, COVID-19 Vaccination, Systematic Review
Introduction
↑What is “already known” in this topic:
Bell’s palsy is a rare complication reported after COVID-19 vaccination. Given the importance of neurological manifestations, the necessity to highlight and scrutinize the incidence of them following vaccination is needed.
→What this article adds:
Pfizer and Moderna vaccines were the most common vaccines that reported to cause Bell’s palsy. Left-sided paralysis was reported to be more common. The interval between receiving the vaccine and the onset of facial weakness was between 1 and 48 days. Further studies with a larger sample size are necessary to assess the association between Bell’s palsy and the dose-response of the COVID-19 vaccine.
The coronavirus disease 19 (COVID-19) pandemic, which is the result of infection with severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), is a major threat to world health (1). Most patients show flu-like symptoms such as fever, cough, fatigue, and in severe cases, shortness of breath (2). The disease has neurological manifestations such as headache, decreased sense of smell, hypogeusia, and rarely central and peripheral nervous system involvement, including Bell’s palsy (3).
Vaccines prevent the spread of viral infection and reduce its mortality (4). In December 2020, the US Food and Drug Administration (FDA) introduced two mRNA vaccines produced by Pfizer and Moderna as the first COVID-19 vaccines, followed by Johnson & Johnson’s adenovirus vaccine. Janssen Biotech has been added to the list of two vaccines, and since then, a number of vaccines have been developed and marketed with different mechanisms to combat COVID-19. Ten vaccines are overall approved by the WHO, and about 48% of people are fully vaccinated around the world. Although in many cases, mild side effects such as injection site pain, mild fever, fatigue, headache, arthralgia, and myalgia are reported, several cases with neurological manifestations after COVID-19 vaccination have been observed (5). One of the neurological manifestations of COVID-19 vaccines is Bell’s palsy (6).
Scottish anatomist Charles Bell first named Bell’s palsy. Bell’s palsy is defined as partial or complete, unilateral, acute paralysis of the facial nerve that results in a complete or incomplete inability to move the muscles of one side of the face voluntarily. This defect is due to edema and inflammation of nerve VII in its passage through the temporal bone, especially the labyrinth part, which can lead to pressure on the nerve and disruption of blood flow, resulting in temporary or permanent nerve damage. The most common age of onset is 15 to 45 years, and the incidence of this disease is 11.5 to 53.3 per 100,000 people per year (7). Although the exact cause of Bell’s palsy is not known, viral infections including herpes simplex virus, herpes zoster virus, CMV, EBV, adenovirus, and influenza B virus, are the most probable causes of this defect. Despite the self-limiting nature of the disease (onset of recovery is 2 to 3 weeks after the onset of symptoms and complete recovery is 3 to 4 months after the onset of the disease), 30% of patients do not entirely recover (7,8).
Following the COVID-19 vaccination, several cases of Bell’s palsy have been reported (9,10). Regarding the importance of immunization against COVID-19, this study aimed to systematically review the reported cases of Bell’s palsy following vaccination against COVID-19.
Methods
This systematic review is conducted based on the Cochrane Collaboration Handbook and PRISMA Statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyzes) and using the Joanna Briggs Institute (JBI) methodology for systematic reviews.
Eligibility criteria
Types of participants
The inclusion criteria for the included published studies were patient age ≥18 years, history of Bell’s palsy after COVID-19 vaccination, and established diagnosis in the patients with COVID-19 vaccination. The exclusion criteria were repeated cases and missing clinical information.
Types of exposure(s)
The exposure (s) were assumed to different types of COVID-19 vaccination.
Types of outcomes
This review included the studies that contained Bell’s Palsy diagnosis.
Types of studies
The quantitative component of the review was observational studies, including case reports, or case-series studies.
Search strategy
To find both published and unpublished studies, a three-step search strategy was utilized. An initial limited search of MEDLINE was undertaken, followed by analyzing the text words, including the title and abstract, and the index terms used to describe the article. The second search using all identified keywords and index terms was done across all included databases on August 2021 and was updated by hand searching in May 2022 through various databases. Finally, the reference lists of all identified reports and articles were searched for additional studies. Studies published in any language and on any date were considered to be included in this review.
Using the identified keywords and index terms, we searched Cochrane Library, MEDLINE (PubMed), Web of Science, Scopus, ProQuest, and Google scholar.
The search for unpublished studies from seminars and congresses was included, too.
Our search strategy in PubMed was as below:
(“COVID-19”[Mesh] OR “SARS-CoV-2”[Mesh]) OR (COVID 19[Text Word]) OR (COVID-19[Text Word]) OR (2019-nCoV[Text Word]) OR (2019 nCoV[Text Word]) OR (Coronavirus Disease-19[Text Word]) OR (2019 Novel Coronavirus[Text Word]) OR (SARS-CoV-2[Text Word]) OR (SARS CoV 2[Text Word]) OR (SARS Coronavirus 2[Text Word]) OR (Wuhan Coronavirus[Text Word]) AND (“Vaccines”[Mesh]) OR “Vaccination”[Mesh]) OR (vaccin*[Text Word]) OR (Active Immunization*[Text Word]) AND (“Bell Palsy”[Mesh]) OR “Facial Paralysis”[Mesh]) OR (bell* pals*[Text Word]) OR (Facial Paralys*[Text Word]) OR (Facial pals*[Text Word]) OR (Facial Neuropath*[Text Word]).
Study selection
All identified citations from the search were collated and uploaded into EndNote, and repeated studies were eliminated. Two independent reviewers were in charge of screening the titles and abstracts to assess the studies’ eligibility based on inclusion and exclusion criteria. Full texts of potentially relevant studies were retrieved, and details of citations were imported into the JBI System for the Unified Management, Assessment, and Review of Information (JBI SUMARI) (Joanna Briggs Institute, Adelaide, Australia). Two reviewers assessed the full texts of selected citations in detail. The reviewers resolved disagreements that arose at each level of the study selection process, through discussion or with a third reviewer. The search results and details of the screening process are present in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram (11).
Assessment of methodological quality
The Joanna Briggs Institute’s (JBI) critical appraisal tools for case reports or case series were used to assess the risk of bias in the included studies. The probable disagreements that arose between the reviewers were resolved through discussion or with a third reviewer.
Data collection
Two independent reviewers extracted the data from included articles using the modified standardized JBI data extraction tool. Specific details were selected from included studies, including the first author, publication year, study design, country, type of vaccine, age, received shots, method of diagnosis, the interval between receiving vaccine and onset of facial weakness, and outcome. Any disagreement among all reviewers was resolved by discussion.
Data synthesis
Descriptive statistical analysis was performed using the SPSS/ver 24.
Study inclusion
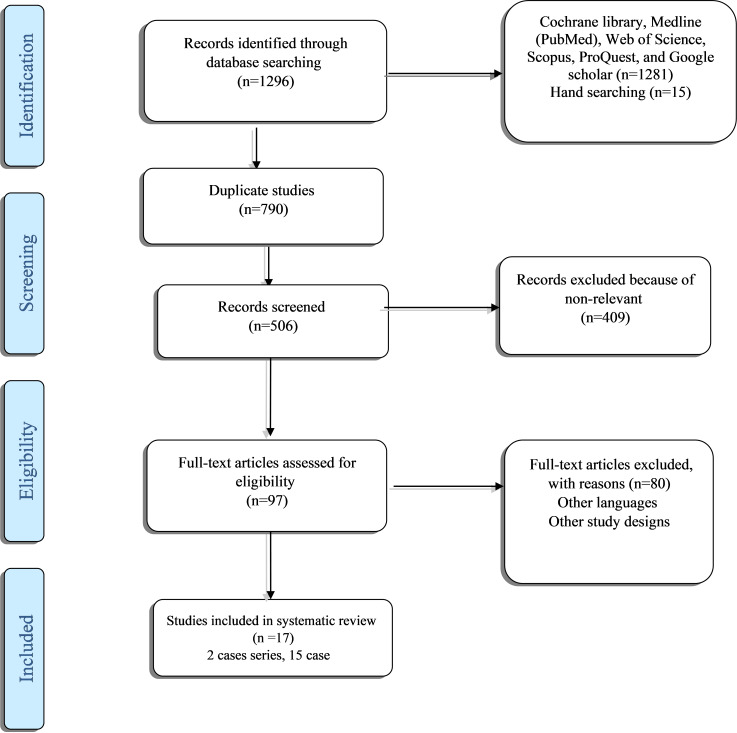
During the electronic search, hand search, and reference check, we identified 1296 citations. After omitting duplicated citations, 1206 studies remained for the screening process. Ninety-seven studies were selected based on titles and abstracts. In the full-text selection step, 80 studies were excluded; finally, 17 case-report or case-series studies were included for the critical appraisal process and were included in this study. Additional information on the article selection process is presented in the PRISMA flow chart (Fig. 1).
Fig. 1.
PRISMA 2020 flow diagram (Covid-BellPlasy)
Results
Characteristics of included studies and findings
Our results showed that among 33 cases with the diagnosis of Bells’ palsy, 22 received Pfizer, seven cases were injected Moderna, and four cases received other vaccines (Janssen, COVAXIN, and Sputnik V). Most individuals were female (51.7% vs. 48.3%). In the analysis based on the vaccine type, in receivers of Pfizer, most cases were male (71.4% vs. 53.3%), while in Moderna receivers, females were predominant cases of Bells’ palsy (33.3% vs. 14.3%). Left-side paralysis happened more frequently than on the rightside (48.5% vs. 27.3%). The mean interval between receiving the vaccine and onset of facial weakness (Days) was 10.25 days with a minimum of 1 and a maximum of 48 days. In males, this range varied between 1-21 days, while in females, it happened in the range of 1-32 days (Table 1).
Table 1. Characteristics of cases with Bell’s palsy .
| Vaccine Type | N (%) | Male | Female | |
| Pfizer-BioNTech | 22 (66.7) | 10 (71.4) | 8 (53.3) | |
| Moderna | 7 (21.2) | 2 (14.3) | 5 (33.3) | |
| Janssen(Ad26.COV2.S) | 1 (3.0) | - | 1 (6.7) | |
| COVAXIN | 1 (3.0) | 1 (6.7) | - | |
| Sputnik V (recombinant vector-based: Ad26. Ad5) | 2 (6.1) | 1 (7.1) | 1 (7.1) | |
| Paralysis | ||||
| Right-side | 9 (27.3) | 5 (35.7) | 4 (26.7) | |
| Left-side | 16 (48.5) | 8 (57.1) | 8 (53.3) | |
| Two-side | 1 (3.0) | 1 (7.1) | - | |
| Not-mentioned | 7 (21.2) | - | 3 (20.0) | |
| The interval between receiving vaccine and onset of facial weakness (Days) | Minimum | 1 | 1 | 1 |
| Maximum | 48 | 21 | 32 | |
| Mean | 10.25 | 6.50 | 10.03 | |
The age range of patients in the studied groups was between 17 to 86 years old. Fourteen cases were male, and 15 were female. Other studies did not report the patient’s sex.
Most reported cases were from Israel, USA, Spain, Turkey and less common in other countries. Fifteen patients (45.5%) had received two vaccine shots, and 18 cases received one shot. In most cases left side of the face was paralyzed (n=16; 48.5%), while 9 (27.3%) were on the right side.
House-Brackmann grade or seriousness of disease were as follows: In 6 studies, the cases had no severe complications; in one case, it was severe, and in one case, it was moderate. In other cases, it was not mentioned, and the remained cases were Grade II, III, or IV. In the included studies, nine patients had a history of underlying hypertension, asthma (n=1), dyslipidemia (n=3), stroke (n=1), prostate cancer (n=1), previous Bell’s Palsy episode (n=2), Meniere’s disease (n=1), obstructive sleep apnea (n=1), cardiac pacemaker (n=1), Type 2 diabetes mellitus (n=3), and one HIV positive patient from 20 years ago, with stage 3 CKD, and prediabetes. While in some studies, the patients had no past medical history.
Appraising the articles by JBI critical appraisal checklists showed that all case reports met the conditions by a proportion of at least 60% compatibility. This proportion was not high enough for the two included case series; however, they were not excluded due to meeting the necessary conditions required in our study. Moreover, a limited quantity of appropriate articles constrained us to include the case series (Table 2).
Table 2. Critical appraisal using JBI checklists (https://jbi.global/critical-appraisal-tools) .
| Case Reports | ||||||||||
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 |
| Haris Iftikhar (28) | Yes | No | Yes | Yes | Yes | Yes | UC | Yes | ||
| Sohil Pothiawala (29) | Yes | Yes | Yes | Yes | Yes | No | UC | Yes | ||
| Michael Repajic (30) | Yes | Yes | Yes | Yes | No | No | UC | Yes | ||
| C. Martin-Villares (31) | Yes | Yes | Yes | Yes | Yes | Yes | UC | Yes | ||
| Giuseppe Colella (32) | Yes | Yes | Yes | Yes | Yes | Yes | UC | Yes | ||
| Mark Obermann (33) | Yes | No | Yes | Yes | Yes | Yes | UC | Yes | ||
| Gómez de Terreros Caro G (34) | Yes | Yes | Yes | Yes | Yes | Yes | UC | Yes | ||
| Y. Nishizawa (35) | Yes | Yes | Yes | Yes | No | No | UC | Yes | ||
| Ish, Somya (36) | Yes | Yes | Yes | Yes | Yes | Yes | UC | Yes | ||
| Abigail Burrows (37) | Yes | Yes | Yes | Yes | Yes | Yes | UC | Yes | ||
| Ender Salbaş (38) | Yes | No | Yes | Yes | Yes | Yes | UC | Yes | ||
| Caroline C. Mussatto (39) | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | ||
| Sujan Poudel (40) | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | ||
| Omid Mirmosayyeb (41) | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | ||
| Rocco Galimi (42) | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | ||
| Case series | ||||||||||
| Thomas Soeiro (20) | UC | UC | UC | Yes | UC | Yes | No | NA | No | NA |
| Asaf Shemer (43) | UC | UC | UC | Yes | UC | Yes | Yes | NA | No | NA |
Discussion
Our results showed that among 17 included studies, Pfizer-BioNTech, and Moderna were the most commonly reported vaccine in case-report studies of Bell’s palsy. Left-sided paralysis was more common than right-sided paralysis. The interval between receiving the vaccine and the onset of facial weakness was between 1 and 48 days.
Although, in this systematic review, we do not confirm or deny the existence of a causal relationship between COVID-19 vaccination and the incidence of Bell’s palsy, and the discovery of such a link requires extensive case-control studies; however, given the importance of neurological manifestations, the necessity to highlight and scrutinize the incidence of them following COVID-19 vaccination is needed.
A case-control study on 37 patients admitted for facial nerve palsy examined the Pfizer-BioNTech COVID-19 vaccine association with an increased risk of acute-onset peripheral facial nerve palsy. In 21 (56.7%) cases with a mean and standard deviation (SD) time from vaccination to the occurrence of palsy was 9.3 (4.2) days from the first dose and 14.0 (12.6) days from the second dose, palsy happened. In 74 matched controls, 59.5% were vaccinated recently, too. The authors did not find any association between recent vaccination with the Pfizer-BioNTech vaccine and the risk of facial nerve palsy (The adjusted odds ratio for exposure was 0.84 (95%CI, 0.37-1.90; P = 0.67) (12).
Michos et al., in a prospective cohort study, evaluated the association between antibody levels after immunization with the BNT162b2 mRNA COVID-19 vaccine and epidemiological and clinical parameters on 268 Healthcare workers. Most serious adverse events included a 45- year-old man who experienced Bell’s palsy 20 days after the 2nd dose (13).
Wan (14) evaluated the risk of Bell’s palsy within 42 days following vaccination with BNT162b2 (Fosun–BioNTech [equivalent to Pfizer–BioNTech]) or CoronaVac (from Sinovac Biotech, Hong Kong) in a case series study on individuals who were immunized by the first dose of CoronaVac and individuals who were vaccinated by the first dose of BNT162b2. They used the data from voluntary surveillance reporting with the Hospital Authority, the COVID-19 Vaccine Adverse Event Online Reporting system for all healthcare professionals, and the Hospital Authority’s territory-wide electronic health records from the Clinical Data Analysis and Reporting System. Among the individuals, 54 clinically confirmed Bell’s palsy cases were reported (28 following CoronaVac with age-standardized incidence of 66·9 cases per 100 000 person-years (95% CI 37·2 to 96·6), and 16 following BNT162b2 with age-standardized incidence of 42·8 per 100 000 person-years (19·4 to 66·1)). They showed an additional 4·8 cases per 100 000 people vaccinated for CoronaVac and 2·0 cases per 100 000 people vaccinated for BNT162b2. In terms of a nested case-control study, after matching 298 Bell’s Palsy cases with 1181 controls, the adjusted ORs were 2·385 (95% CI 1·415 to 4·022) for CoronaVac and 1·755 (0·886 to 3·477) for BNT162b2.
Avcı et al. (15), in their cross-sectional study, reported only 1 (0.1%) patient with facial paralysis among 1710 (of a total 3383) health care workers vaccinated against COVID 19 who (51%) participants agreed to answer the study questions.
El-Shitany, in a retrospective cross-sectional study, reported COVID-19 vaccination side effects among both participants who were previously infected with coronavirus and uninfected individuals. Their results showed that Bell’s palsy was observed in 3 (1.3%) individuals after the first vaccine dose and among 455 respondents to an online survey. Among previously infected participants, this rate was 5.3%, and for uninfected participants, it was 0.7% (16).
Another prospective monitor for potential adverse events associated with mRNA SARS-CoV-2 vaccination study among U.S. nursing home residents showed that unadjusted 15-day rates of adverse effects per 100,000 residents after the first dose of vaccine (n= 8553 nursing home residents) were 12 (95% CI: 2, 66) for Bell’s Palsy; and unlike the vaccinated, in unvaccinated individuals (n=11,072), there were no occurrences of Bell’s Palsy (17).
Sato (18), in a disproportionality analysis on the USA Vaccine Adverse Event Reporting System with age and sex adjusting from age of 18 years or over individuals who were vaccinated between January 2010 and April 2021, showed an increased risk of facial nerve palsy following COVID-19 mRNA vaccines both for BNT162b2 (reporting odds ratio [ROR] 1.84; 95% confidence interval [CI] 1.65–2.06) and mRNA-1273 (ROR 1.54; 95% CI 1.39–1.70), which were similar to influenza vaccination (ROR 2.04; 95% CI 1.76–2.36). However, due to the nature of self-reporting, the results of Sato’s study (18) had limitations, such as over or under-reporting side effects. The lack of information about concomitant medications or medical history was another limitation of this study. Therefore, at present, the results of this study can not be considered as evidence for facial nerve palsy as a post-vaccination complication.
However, the study’s main strength was that it is based on a database containing global and real-world data from many patients, which is appropriate to provide a preliminary hypothesis to guide future epidemiological studies. Further observational studies are needed to conclude that COVID-19 mRNA vaccines actually raise the incidence of facial nerve palsy.
The level of facial nerve palsy after COVID-19 mRNA vaccination was equal to influenza vaccination before the COVID-19 epidemic. Despite the different underlying mechanisms, observations of facial nerve palsy with this frequency may be instructive for neuroscientists and physicians in the global promotion of COVID-19 vaccines. By tracking the procedure and the process of this inflammatory effect, a more precise evaluation of vaccines’ adverse effects would be possible. Furthermore, regarding the medical and pharmacological history of the patients, these observations may lead to a practical guide for selecting a suitable kind of vaccine for each case in order to minimize the incidence of adverse effects (19,20).
The mechanism of Bell’s palsy in patients after vaccination is unclear. One hypothesis links Bell’s palsy to an autoimmune phenomenon, which is thought to occur through the mimicry of host molecules by vaccine antigen or the activation of reactive dormant T cells (21). Another potential mechanism is the reactivation of latent herpes simplex type 1 infections (22). Bell’s palsy can also be secondary to immune-mediated segmental demyelination, similar to Guillain-Barré syndrome (23). Inactivated vaccines have been widely used to combat other viral infections, like the flu. The inactivated virus has been shown to contain various viral antigens that may make changes to the immune response in a wider group of patients (24).
In contrast, the BNT162b2 vaccine may activate innate immunity and result in interferon protein producing by combining mRNA and lipids (10). Interferon treatment has been reported to cause facial nerve palsy (25). Although numerous possible pathways, including viral, autoimmune, or innate immune activation, have been implicated in causing Bell’s palsy after COVID-19 vaccination, these mechanisms may be multifactorial and not applicable for all cases. Further research is needed to confirm the mechanism of Bell’s palsy after COVID-19 immunization (26).
In terms of BNT162b2 receptors, there were no gender differences (27), which did not match the WHO Drug Monitoring Database that reported 67.8% of patients developing Bell’s palsy after COVID-19 mRNA vaccination were female. The inconsistency may be due to reporting bias. However, observations revealed that a larger percentage of patients with Bell’s palsy following the CoronaVac vaccination were male, and they did not rule out the possibility of a gender difference in the risk of Bell’s palsy after the CoronaVac vaccination. In addition, the sample size was not large enough to detect any significant results in the analysis of female patients. Post hoc analysis identified potential signals in CoronaVac and BNT162b2. In addition, noteworthy the subsequent analysis to test the dose-response relationship between the COVID-19 vaccine and Bell’s palsy was weak. Hence, they should just be a heuristic analysis in their study.
Our results showed that there was a weak association between different types of COVID-19 vaccination and the risk of Bell’s palsy in most included studies.
In our study, only 17 case reports or case-series studies met our inclusion criteria which led to caution in interpreting the results. Further epidemiological studies with a sufficient sample size to perform a meta-analysis are essential to evaluate the association between Bell’s palsy and the COVID-19 vaccine.
Conclusion
Pfizer-BioNTech and Moderna were the most commonly reported vaccines in case reports of Bell’s palsy. The interval between receiving the vaccine and the onset of facial weakness was between 1 and 48 days. Some epidemiological studies showed an association. Further studies with larger sample sizes are necessary to assess the association between Bell’s palsy and the dose-response of the COVID-19 vaccine.
Ethics Approval
The regional Ethics Committee approved the protocol of this study (IR.TBZMED.REC.1400.720).
Acknowledgment
We would like to thank Vice Chancellor for Research and Technology for approving the protocol.
Conflict of Interests
The authors declare that they have no competing interests.
Cite this article as: Shahsavarinia K, Mahmoodpoor A, Sadeghi-Ghyassi F, Nedayi A, Razzaghi A, Zehi Saadat M, Salehi-Pourmehr H. Bell's Palsy and COVID-19 Vaccination: A Systematic Review. Med J Islam Repub Iran. 2022 (30 Jul);36:85. https://doi.org/10.47176/mjiri.36.85
References
- 1.Shi Y, Wang G, Cai XP, Deng JW, Zheng L, Zhu HH, et al. An overview of COVID-19. J Zhejiang Univ Sci B. 2020;21(5):343–60. doi: 10.1631/jzus.B2000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baloch S, Baloch MA, Zheng T, Pei X. The Coronavirus Disease 2019 (COVID-19) Pandemic. Tohoku J Exp Med. 2020;250(4):271–8. doi: 10.1620/tjem.250.271. [DOI] [PubMed] [Google Scholar]
- 3.Nepal G, Rehrig JH, Shrestha GS, Shing YK, Yadav JK, Ojha R, et al. Neurological manifestations of COVID-19: a systematic review. Crit Care (London, England) 2020;24(1):421. doi: 10.1186/s13054-020-03121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maciorowski D, Ogaugwu C, Durvasula SR, Durvasula R, Kunamneni A. Therapeutic and Vaccine Options for COVID-19: Status after Six Months of the Disease Outbreak. SLAS Discov. 2021;26(3):311–29. doi: 10.1177/2472555220979579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sriwastava S, Sharma K, Khalid SH, Bhansali S, Shrestha AK, Elkhooly M, et al. COVID-19 Vaccination and Neurological Manifestations: A Review of Case Reports and Case Series. Brain Sci. 2022;12(3) doi: 10.3390/brainsci12030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burrows A, Bartholomew T, Rudd J. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep. 2021;14(7) doi: 10.1136/bcr-2021-243829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baugh RF, Basura GJ, Ishii LE, Schwartz SR, Drumheller CM, Burkholder R, et al. Clinical practice guideline: Bell’s palsy. Otolaryngol Head Neck Surg. 2013;149(3 Suppl):S1–27. doi: 10.1177/0194599813505967. [DOI] [PubMed] [Google Scholar]
- 8.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med. 2004;350(9):896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 9.Couch RB. Nasal vaccination, Escherichia coli enterotoxin, and Bell’s palsy. N Engl J Med. 2004;350(9):860–1. doi: 10.1056/NEJMp048006. [DOI] [PubMed] [Google Scholar]
- 10.Ozonoff A, Nanishi E, Levy O. Bell’s palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021;21(4):450–2. doi: 10.1016/S1473-3099(21)00076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Shemer A, Pras E, Einan-Lifshitz A, Dubinsky-Pertzov B, Hecht I. Association of COVID-19 Vaccination and Facial Nerve Palsy: A Case-Control Study. JAMA Otolaryngol Head Neck Surg. 2021;147(8):739–43. doi: 10.1001/jamaoto.2021.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michos A, Tatsi EB, Filippatos F, Dellis C, Koukou D, Efthymiou V, et al. Association of total and neutralizing SARS-CoV-2 spike -receptor binding domain antibodies with epidemiological and clinical characteristics after immunization with the 1(st) and 2(nd) doses of the BNT162b2 vaccine. Vaccine. 2021;39(40):5963–7. doi: 10.1016/j.vaccine.2021.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wan EYF, Chui CSL, Lai FTT, Chan EWY, Li X, Yan VKC, et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2021. [DOI] [PMC free article] [PubMed]
- 15. Avcı H, Karabulut B, Eken HD, Faraşoğlu A, Çakil T, Çoruk S, et al. Otolaryngology-Specific Symptoms May Be Highly Observed in Patients With a History of Covid-19 Infection After Inactivated Coronavirus Vaccination. Ear Nose Throat J. 2021:1455613211028493. [DOI] [PubMed]
- 16.El-Shitany NA, Harakeh S. Minor to Moderate Side Effects of Pfizer-BioNTech COVID-19 Vaccine Among Saudi Residents: A Retrospective Cross-Sectional Study. Int J Gen Med. 2021;14:1389–401. doi: 10.2147/IJGM.S310497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bardenheier BH, Gravenstein S, Blackman C, Gutman R, Sarkar IN, Feifer RA, et al. Adverse events following mRNA SARS-CoV-2 vaccination among US nursing home residents. Vaccine. 2021;39(29):3844–51. doi: 10.1016/j.vaccine.2021.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato K, Mano T, Niimi Y, Toda T, Iwata A, Iwatsubo T. Facial nerve palsy following the administration of COVID-19 mRNA vaccines: analysis of a self-reporting database. Int J Infect Dis. 2021;111:310–2. doi: 10.1016/j.ijid.2021.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–49. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soeiro T, Salvo F, Pariente A, Grandvuillemin A, Jonville-Béra AP, Micallef J. Type I interferons as the potential mechanism linking mRNA COVID-19 vaccines to Bell’s palsy. Therapie. 2021;76(4):365–7. doi: 10.1016/j.therap.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Principi N, Esposito S. Do Vaccines Have a Role as a Cause of Autoimmune Neurological Syndromes? Front Public Health. 2020;8:361. doi: 10.3389/fpubh.2020.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami S, Mizobuchi M, Nakashiro Y, Doi T, Hato N, Yanagihara N. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med. 1996;124(1 Pt 1):27–30. doi: 10.7326/0003-4819-124-1_part_1-199601010-00005. [DOI] [PubMed] [Google Scholar]
- 23.Koski CL. Humoral mechanisms in immune neuropathies. Neurol Clin. 1992;10(3):629–49. [PubMed] [Google Scholar]
- 24.Dong Y, Dai T, Wei Y, Zhang L. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020;5(1):237. doi: 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang I, Calvit TB, Cash BD, Holtzmuller KC. Bell’s palsy: a rare complication of interferon therapy for hepatitis C. Dig Dis Sci. 2004;49(4):619–20. doi: 10.1023/b:ddas.0000026389.56819.0c. [DOI] [PubMed] [Google Scholar]
- 26.Renoud L, Khouri C, Revol B, Lepelley M, Perez J, Roustit M, et al. Association of Facial Paralysis With mRNA COVID-19 Vaccines: A Disproportionality Analysis Using the World Health Organization Pharmacovigilance Database. JAMA Intern Med. 2021;181(9):1243–5. doi: 10.1001/jamainternmed.2021.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan EYF, Chui CSL, Lai FTT, Chan EWY, Li X, Yan VKC, et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22(1):64–72. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iftikhar H, Noor SMU, Masood M, Bashir K. Bell’s Palsy After 24 Hours of mRNA-1273 SARS-CoV-2 Vaccine. Cureus. 2021;13(6):e15935. doi: 10.7759/cureus.15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pothiawala S. Bell’s Palsy After Second Dose of Moderna COVID-19 Vaccine: Coincidence or Causation? Acta Med Litu. 2021;28(2):298–301. doi: 10.15388/Amed.2021.28.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Repajic M, Lai XL, Xu P, Liu A. Bell’s Palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell’s palsy. Brain Behav Immun Health. 2021;13:100217. doi: 10.1016/j.bbih.2021.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin-Villares C, Vazquez-Feito A, Gonzalez-Gimeno MJ, de la Nogal-Fernandez B. Bell’s palsy following a single dose of mRNA SARS-CoV-2 vaccine: a case report. J Neurol. 2022;269(1):47–8. doi: 10.1007/s00415-021-10617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colella G, Orlandi M, Cirillo N. Bell’s palsy following COVID-19 vaccination. J Neurol. 2021;268(10):3589–91. doi: 10.1007/s00415-021-10462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obermann M, Krasniqi M, Ewers N, Fayad J, Haeberle U. Bell’s palsy following COVID-19 vaccination with high CSF antibody response. Neurol Sci. 2021;42(11):4397–9. doi: 10.1007/s10072-021-05496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gómez de Terreros Caro G, Gil Díaz S, Pérez Alé M, Martínez Gimeno ML. Bell’s palsy following COVID-19 vaccination: a case report. Neurologia (Engl Ed) 2021;36(7):567–8. doi: 10.1016/j.nrleng.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishizawa Y, Hoshina Y, Baker V. Bell’s palsy following the Ad26COV2S COVID-19 vaccination. QJM. 2021;114(9):657–8. doi: 10.1093/qjmed/hcab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ish S, Ish P. Facial nerve palsy after COVID-19 vaccination - A rare association or a coincidence. Indian J Ophthalmol. 2021;69(9):2550–2. doi: 10.4103/ijo.IJO_1658_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burrows A, Bartholomew T, Rudd J, Walker D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep. 2021;14(7) doi: 10.1136/bcr-2021-243829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salbaş E, Özge Ö, Levent Ö, Karahan AY. Facial paralysis following messenger RNA COVID-19 Vaccines: The report of two Cases. Ege Tıp Bilimleri Dergisi. 2021;4(2):73–7. [Google Scholar]
- 39.Mussatto CC, Sokol J, Alapati N. Bell’s palsy following COVID-19 vaccine administration in HIV+ patient. Am J Ophthalmol Case Rep. 2022;25:101259. doi: 10.1016/j.ajoc.2022.101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poudel S, Nepali P, Baniya S, Shah S, Bogati S, Nepal G, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond) 2022;78:103897. doi: 10.1016/j.amsu.2022.103897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirmosayyeb O, Barzegar M, Rezaei M, Baharlouie N, Shaygannejad V. Bell’s palsy after Sputnik V COVID-19 (Gam-COVID-Vac) vaccination. Clin Case Rep. 2022;10(2):e05468. doi: 10.1002/ccr3.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galimi R, Galimi M. Adverse Event Reporting after mRNA COVID-19 Vaccination: A Bell’s Palsy Case the Day after. Neurol Case Rep. 2021;4(2):1026. [Google Scholar]
- 43.Shemer A, Pras E, Hecht I. Peripheral Facial Nerve Palsy Following BNT162b2 (COVID-19) Vaccination. Isr Med Assoc J. 2021;23(3):143–4. [PubMed] [Google Scholar]