Abstract
Background: Nonalcoholic fatty liver disease (NAFLD) is one of the most common liver disorders with a relatively high mortality rate. Berberine has recently been found to have some antidiabetic and antihyperlipidemic effects, although the evidence of its effectiveness in NAFLD is limited. To assess the efficacy of berberine among patients with NAFLD.
Methods: The patients with NAFLD were randomly assigned to treatment with (n = 25) or without (n = 25) berberine. The patients in the intervention group took berberine 6.25 g per day and the control group had no berberine. All patients in the 2 groups had been recommended to have lifestyle training, including a low-fat diet and physical activity before randomization. Independent student t tests or Mann-Whitney U tests along paired t tests or Wilcoxon signed-rank tests were used. Analysis of covariance was also used to estimate the difference of the variables between the 2 groups adjusting for baseline characteristics.
Results: The results indicated that berberine, compared with the control group, had no significant impact on lipid levels, including triglyceride (P = 0.350), total cholesterol (P = 0.120), high-density lipoproteins (P = 0.401), and low-density lipoproteins (P = 0.100). Similarly, no significant difference was observed between the treatment arms in the level of fasting blood glucose (P = 0.055) and liver enzymes, such as alkaline phosphatase (P = 0.109), serum glutamic-oxaloacetic transaminase (P = 0.366), and serum glutamic pyruvic transaminase (P = 0.436). The effect of berberine on body weight was also nonsignificant (P = 0.494) and even smaller than that of liver enzymes, with a mean difference of 1.8 kg (P = 0.304) in body weight.
Conclusion: Berberine was not associated with a significant decrease in lipid profile, fasting blood glucose, or liver enzymes among patients with NAFLD.
Keywords: Nonalcoholic Fatty Liver Disease, Berberine, Liver Enzymes, FBS, Lipid Profiles
Introduction
↑What is “already known” in this topic:
Berberine is known as an antidiabetic, antiobesity, hypotensive, and hypolipidemic agent and is recommended as an effective intervention in non-alcoholic fatty liver and metabolic syndrome.
→What this article adds:
Despite its widespread use in the treatment of nonalcoholic fatty liver disease, berberine does not outperform lifestyle changes such as a low-fat, low-calorie diet, and exercise therapy.
Nonalcoholic fatty liver disease (NAFLD), one of the most common liver dysfunctions, affects about one-fourth of the adult population and its mortality rate is between 6.3% and 33%.(1) Distribution of the disease is geographically heterogeneous, with a prevale nce of 31.8% in the Middle East (2), 20% to 30% in Western countries (3), and 13.5% in Africa (2).
NAFLD can be discovered incidentally with elevated liver enzymes or fatty deposits on the liver.
NAFLD can result in serious illnesses and is associated with a group of metabolic disorders, including type 2 diabetes mellitus, hypercholesterolemia, obesity, and hypertension, which can be considered as potential risk factors for progressive liver pathologies and cardiovascular diseases (2). It has already been established that the association between NAFLD and components of metabolic syndrome (MetS) is bidirectional, with morbidity being 58% and 74% in overweight and diabetic patients, respectively (1). The disease is currently one of the top etiologies for hepatocellular carcinoma (HCC), a major indication for liver transplantation. Additionally, there is a body of evidence indicating that around 30% of NAFLD patients could develop nonalcoholic steatohepatitis (NASH), which significantly increases the risk of HCC and liver failure (4). Although metabolic comorbidities, lifestyle habits, and patient characteristics, including age and gender, are found to be associated with NAFLD (5), the pathogenesis of the disease is yet to be determined completely.
Management of NAFLD must address both liver dysfunction and potential comorbid components of MetS. Therefore, lifestyle modifications consisting of diet, weight loss, and physical activity, are recommended; weight loss is key to improvement in the histopathological features of NASH. Given the very close association between NAFLD and insulin resistance, metformin and thiazolidinediones are the main pharmaceuticals prescribed for the treatment of the disease along with concurrent statins to control blood fat (1,6). It should be noted, however, that a meta-analysis did not find metformin to be effective in improving liver histology among patients with NAFLD and NASH (7). In addition, controversial findings on potential treatment-related adverse events have led to restrictions in the prescription of thiazolidinediones (7). In this context, research and development should focus on effective NAFLD treatments to fill a significant gap in the market. Berberine, also known as berberine hydrochloride, the main active alkaloid with a benzyl tetra hydroxy quinoline chemical structure and also a molecular formula of C20H18NO4, is derived from Berberis Vulgaris fruits. This fruit is also known as Iranian Zereshk (barberries) and is cultivated across the world, specially in the Khorasan province of Iran.
Berberis vulgaris and its main constituent berberine have been used in traditional medicine for a long time. It has recently been announced to have some biological effects in several clinical indications and laboratory settings as well (1,8-15).
A large body of literature claims that berberine has some pharmacological properties, such as antidiabetic, antiobesity, hypotensive and hypolipidemic, anti-inflammatory, antioxidant, and antibacterial effects, that have been supported in both in vitro and in vivo experiments (8-12,15,16). Berberine modulates glucose and lipid metabolism through a multipath way mechanism that includes AMP-activated protein kinase-(AMPK-) p38 MAPK-GLUT4, JNK pathway, and PPARα pathway (12). In regard to many reports on the protective effects of berberine, numerous studies have reviewed the literature and suggest that it is necessary to have more investigations, especially reliable clinical trials to evaluate its effectiveness (1). Reviewing the articles discovers the low evidence on its effectiveness and underlying mechanisms in the treatment of NAFLD and associated comorbid components of MetS among these patients. Though the beneficial action of berberine has been obvious, further studies are still in need to optimize its clinical application in NAFLD. This study aimed to evaluate the therapeutic effect of berberine on the liver function and metabolic profiles of the patients with NAFLD.
Methods
Participants
This study was a randomized clinical trial conducted among the staff with NAFLD in Takestan Social Security hospital, Qazvin, Iran. A screening program for fatty liver disease was conducted in Takestan Social Security Hospital using ultrasonography and blood tests. The patients aged between 18 and 65 years (inclusive) with a fatty liver confirmed by abdominal ultrasonography (Grades 1-3 based on the intensity of liver/kidney contrast) were invited to participate in the study if they met all inclusion criteria. Patients with serious comorbid conditions, blood pressure more than 140/90 mmHg, body mass index (BMI) lower than 25, liver and renal failure, hepatitis B or C, hepatic cirrhosis, cancer, serious dyslipidemia, or other endocrine illnesses were excluded from the trial. Patients also were ineligible if they had excessive alcohol intake per week (>140 g for men and >70 g for women within 6 months before enrollment to the study), organ dysfunction or other types of fatty liver disease caused by alcohol, viral or autoimmune hepatitis, and the Wilson disease. Patients could not have taken any medication for liver problems 6 weeks before enrolling in the study. They also had to cease taking lipid-regulating drugs 6 weeks before starting the treatment. Breastfeeding and pregnant women were excluded from the study.
Study Design
A 7-week (45-day), open-label, double-blinded randomized controlled trial was conducted in a medical center affiliated to the Ministry of Welfare and Social Security in Iran to study the impact of berberine on the liver function and metabolic profiles of patients with NAFLD. The hospital employees who met the enrollment criteria were selected via a human resource database related to employee routine annual examinations. Using a computer-generated random-allocation sequence, the patients were equally assigned (1:1) to 2 groups: 1 group taking berberine 6.25 g per day (arm A) and the other group taking no berberine (Arm B). The patients were prescribed to receive berberine orally (5L water including 100 g dried berberine boiled at 167°F until 4L). The secondary investigator generated 3 computerized allocation sequences. The secretory of the Nutrition department randomly selected one of the sequences for the study. The secondary investigator enrolled the participants and performed the interventions in the 2 groups. Providing berberine and prescribing exercise and diet was performed by the secondary investigator. The primary investigator who was not informed about the group allocation and interventions assessed the outcomes and also a statistic analyzer out of the hospital who was blinded to group allocations analyzed the data. To control the potential effect of confounders on the outcome and to keep a balance between the daily dietary and physical activity among the 2 groups, all participants were trained by a skilled dietitian with more than 20 years’ work experience and an expert sports medicine specialist on modifying lifestyle and behavior improvement. Both groups were trained with the aim of subtracting 500 kcal from their mean daily calorie intake and achieving approximately 150 minutes of moderate-intensity aerobic exercise (40%-60% of heart rate reserve) per week. The study was based on the CONSORT (Consolidated Standards of Reporting Trials (www.consort-statement.org)), approved by a regulatory board and ethics review committee at the study center, and was conducted in accordance with the guidelines of the Declaration of Helsinki. All patients provided written informed consent before study entry.
Outcome Measures
Primary outcomes measures were change in alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) levels before and after the intervention, and the difference between the study groups. We also measured the impact of the intervention on lipid profile of the patients (triglyceride [TG], total cholesterol [TC], high-density lipoprotein [HDL], and low-density lipoprotein [LDL]), and fasting blood sugar (FBS) levels. Prior to study enrollment, participants underwent an interview on diet and physical activity by a trained investigator using 2 structured forms: the Food Frequency Questionnaire (FFQ) and the Baecke Questionnaire. The FFQ, a common dietary assessment tool, is a reliable semi-quantitative questionnaire that obtains frequency and, in some cases, portion size information about food and beverage consumption over time (147 items) with a standard serving size; it has already been validated in previous studies in Iran (17). The Baecke questionnaire measures usual physical activities during the last 12 months. This questionnaire includes 16 questions within 3 main domains of individual physical activities (occupational, sport, and recreational), and has already been validated in previous studies in Iran (18). Blood examinations of glucose, liver enzymes, and lipid profile (laboratory kits), both at the beginning and at the end of the trial (day 45) were performed. Compliance to the intervention and any possible adverse events were assessed in the both groups by weekly follow-up visits.
Statistical Analysis
Differences between the study groups were compared using independent Student t tests or Mann-Whitney U tests for continuous variables, along with Pearson chi-square or Fisher exact tests for categorical variables. Within-group differences between baseline values and those after 6 weeks were also analyzed using paired t tests or Wilcoxon signed-rank tests. The Analysis of Covariance (ANCOVA) was also used in estimating the difference of the variables between the 2 groups adjusting for baseline characteristics. The mean values and standard deviations were used unless otherwise stated. For variables with a skewed distribution, medians and interquartile ranges were applied (marked by "+" in Table 1). The online calculator “sealed envelope” was used for calculating the sample size (www.sealedenvelop.com). Depending on the questionnaire and the corresponding instructions, missing data were addressed. Statistical analyses were performed using IBM SPSS Software (Version 24) (IBM Corp).
Table 1. Baseline Characteristics .
| Variable | Intervention Group (n = 24), mean (SD) |
Control Group (n = 24), mean (SD) |
Between-Group Differences, P Value |
| Age, y | 40.6 (8.8) | 42.2 (3.8) | 0.2251 |
| Gender (male %) | 82.6 | 69.5 | 0.3003 |
| Academic education, % | 60.8 | 73.9 | 0.345 |
| Weight, kg | 89.7 (12.4) | 88.3 (10.8) | 0.3491 |
| BMI, kg/m2 | 29.9 (3.8) | 30.1 (4.1) | 0.4401 |
| FBS, mg/dL | 107.2 (38.04) | 93.01 (8.9) | 0.0661 |
| Physical activity, min | 7.02 (1.4) | 7.02 (1.02) | 0.4821 |
| Energy intake (kcal)+ | 3178.01 (721.5) | 3006.3 (359.8) | 0.0102 |
| ALP U/L | 186.9 (81.5) | 199.8 (47.4) | 0.2891 |
| SGOT U/L | 30.5 (13.5) | 28.9 (6.4) | 0.3001 |
| SGPT U/L+ | 42.0 (25.0) | 46.0 (28.0) | 0.4462 |
| TG (mg/dl) | 182.3 (111.6) | 247.6 (144.01) | 0.0461 |
| TC, (mg/dl) | 184.6 (47.7) | 197.9 (36.05) | 0.1461 |
| LDL, mg/dL | 105.1 (33.8) | 113.02 (28.9) | 0.2291 |
| HDL, (mg/dL | 33.1 (6.6) | 33.1 (4.2) | 0.4961 |
| Fatty liver grading (number) Grade 1 Grade 2 Grade 3 |
18 5 1 |
14 7 3 |
0.0811 0.1972 0.1563 |
Abbreviations: ALP, alkaline phosphatase; BMI, body mass index; FBS, fasting blood sugar; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SGOT, serum glutamic-oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; TC, total cholesterol; TG, triglyceride.
+ median (interquartile range)
1Independent Student t tests, 2Mann-Whitney U tests, 3Pearson chi-square
The sample size was calculated based on previous studies (1). The 50 patients were required to have a 95% chance of detecting, with a significant level of 5%, a lipid profile, and a blood glucose difference of 10% between groups, considering a standard deviation of 10%, and a dropout rate of 5%. To take into account possible missing data or dropouts, we included a minimum of 25 patients in each group.
Results
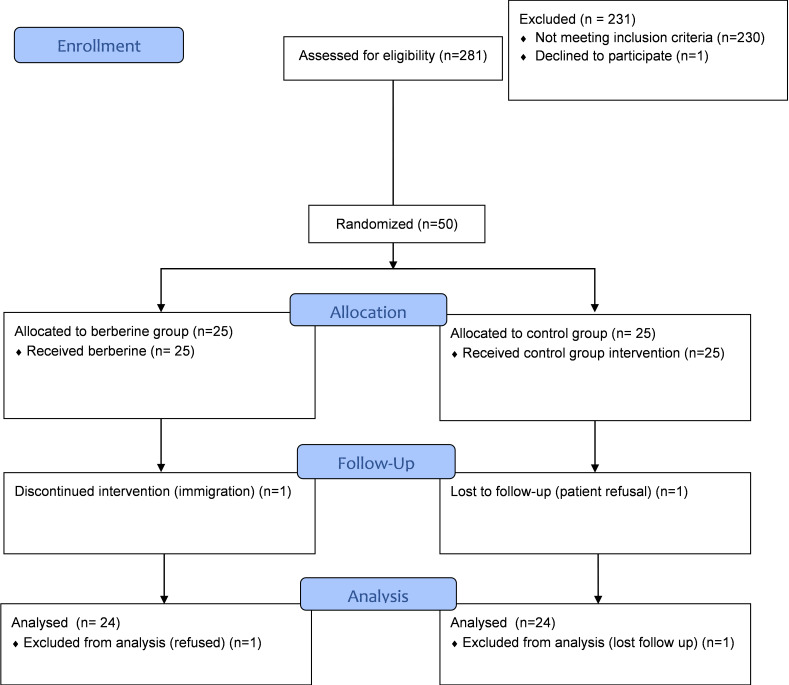
A total of 281 patients diagnosed with NAFLD were initially enrolled into the trial. After screening for comorbid conditions (per the protocol) and other exclusion criteria, 50 patients were eligible and randomized to receive berberine (n = 25) or assigned to the control group (n = 25) (Fig. 1).
Fig. 1.
Study enrollment and participation flowchart (CONSORT 2010 flow diagram)
Baseline characteristics were well-balanced across the study groups. Overall, 48 patients completed the trial; 1 patient in the berberine group refused to continue the intervention, and 1 patient in the control group was lost to follow-up. Compliance with the lifestyle intervention was over 90% in both groups based on the secondary investigator observation.
Study samples were predominately men (82.6% in the berberine group and 69.5% in the controls). Patient demographic and clinical characteristics, including baseline values for liver enzymes and lipid profile, are presented in Table 1. The mean weight at baseline was slightly higher in the intervention arm (89.7 ± 12.4 kg) compared with the control group (88.3 ± 10.8 kg) (P =.349). Patients receiving berberine had greater baseline fasting glucose and physical activity compared with the control group (107.2 ± 38 mg/dL vs 93 ± 8.9 mg/dL and 3322 ± 571.7 min vs 3004 ± 265.7 min, respectively). However, none of the differences was statistically significant. Baseline parameters except TG and energy intake were also similar between the study groups. Thereafter, the ANCOVA was performed to evaluate whether the baseline TG as a covariate influences the effect of berberine as an independent variable on the TG at the sixth week as a dependent variable. According to the results of this analysis, there was no significant difference in TG between the 2 groups (P = 0.464).
Comparing the results between a 6-week treatment and before the intervention showed that TG reduction in the control group was more than the intervention group (247.6 reduced to 193.2 in the control group vs 182.3 reduced to 179.8 in the intervention group) and TC reduction in the both groups was not prominent. However, the values showed no statistically significant difference between the groups in terms of TG or TC (P = 0.464 and P = 0.326, respectively; Table 2). Moreover, TG and TC changes within groups was not statistically significant (P = 0.397 and P = 0.176, respectively. Findings showed that the levels of fasting glucose, ALP, SGOT, and SGPT did not drop in the patients who received berberine, and also between-group differences were not statistically significant. The effect of berberine on body weight was even smaller than that of liver enzymes, with a mean difference of 1.8 kg in patient weight (P = 0.335); similar BMIs were observed between the study arms (P = 0.344).
Table 2. Postintervention Outcomes at Week 6 .
| Variable | Intervention Group (n = 24), mean (SD) | Control Group (n = 24), mean (SD) |
Between Group Differences, P Value |
| Weight, kg | 89.9 (11.8) | 88.1 (11.2) | 0.304 |
| BMI, kg/m2 | 30.04 (3.7) | 30.02 (3.1) | 0.494 |
| FBS, mg/dL | 104.9 (37.2) | 91.9 (8.9) | 0.055 |
| ALP, IU/L | 181.4 (55.3) | 175.7 (43.0) | 0.109 |
| SGOT, U/L | 22.6 (6.2) | 21.9 (6.8) | 0.366 |
| SGPT, U/L+ | 30.5(33.5) | 31.0 (21.5) | 0.436 |
| TG, mg/dL | 179.8 (100.5) | ) 193.2 (128.0) | 0.350 |
| TC, mg/dL | 181.3 (35.1) | 193.2(31.3) | 0.120 |
| HDL, mg/dL | 37.1 (6.9) | 37.8 (6.9) | 0.401 |
| LDL, mg/dL | 105.8 (28.7) | 120.1 (31.1) | 0.100 |
Abbreviations: ALP, alkaline phosphatase; BMI, body mass index; FBS, fasting blood sugar; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SGOT, serum glutamic-oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; TC, total cholesterol; TG,triglyceride.
* Statistically significant difference (P < 0.05)
ANCOVA was used to compare the groups.
+ median (interquartile range).
The comparison of outcome measures in the berberine group from the baseline to post completion of the treatment at week 6 showed that berberine was significantly effective in decreasing SGOT from 30.4 to 22.6 unit/liter (P = 0.005) and SGPT from 46.3/L to 36.9/L (P = 0.001). Berberine also significantly increased patient’s HDL level from 33.4 at the baseline to 37.1 at week 6 (P = 0.006). The changes in FBS level, ALP, TC, and LDL after 6-week berberine treatment, however, was not statistically significant (Fig. 2).
Fig. 2.
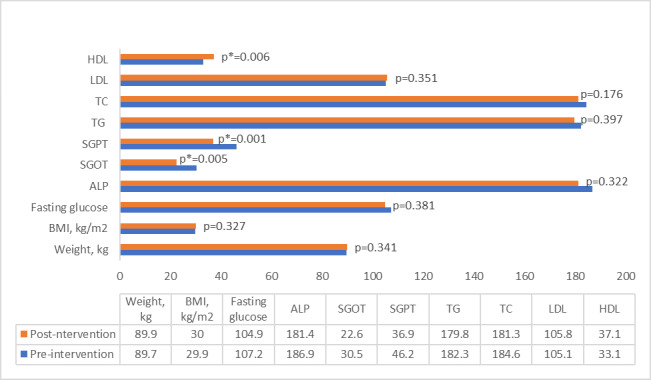
Postintervention vs baseline measures in berberine group.
Abbreviations: ALP, alkaline phosphatase; BMI, body mass index;, fasting blood sugar; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SGOT, serum glutamic-oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; TC, total cholesterol; TG, triglyceride.
* Statistically significant difference (P < 0.05).
** Number of patients n = 24
Discussion
Even though the effect of berberine on liver function has been increasingly under investigation over the past few years, its therapeutic effect and associated mechanisms are still unclear. The current study evaluated the effect of 6-week berberine treatment in patients with NAFLD. The comparison between the intervention and control arms showed no statistically significant effect on lipid profiles, level of fasting glucose, and liver enzymes.
Berberine was found to have no significant effect on patient’s BMI and weight, which is consistent with the findings of a study conducted by Mamaghani et al (2009), where berberine was determined to have no significant impact on the BMI (19). Unlike this study participants were not given any instructions on lifestyle modification. In this trial, patients in both arms were required to participate in the preintervention training to maintain their low-calorie diet over the course of treatment, which was monitored during the follow-up visits. There is a strong body of evidence indicating the effectiveness of low-calorie diets on weight loss and BMI control (20,21). Daily intake of calories is proven to be superior to the type of diet and the ratio of fat to protein, in particular when it is accompanied by routine exercise. Our findings were consistent with the results of another trial in which low-fat and standard diets had a similar effect on
weight loss (22). However, it should be noted that patients are less likely to lose weight with a low-fat diet in such a short period of time.
Similarly, we did not find any significant differences in FBS, TG, and TC among the patients who received or did not receive berberine over 6 weeks. Similar findings were demonstrated in another trial that investigated the effectiveness of an 8-week treatment (5g berberine per day) on metabolic syndrome. In contrast to our study, berberine was shown to be effective in changing both LDL and HDL concentrationamong patients who took berberine compared with the controls (19). In another setting, 3 months of treatment with a higher dose of 8.3 g berberine per day was effective not only in decreasing liver enzymes, ALT, AST, and FBG but also in reducing TG and TC compared with placebo (23). Reviewing the papers demonstrates that 5 gr berberine could not change the metabolic parameters while 1.5 g or 3 g berberine a day in other studies had a positive effect on blood glucose and lipid profiles (13,15). The same positive effect of berberine in different doses (1 g a day or 8.3 g a day) on metabolic parameters and also different effect of berberine on liver enzymes (6.25 gr vs 15 gr a day) show the necessity for considering the berberine dose in different variables. As a result, the period, type of berberis from which the berberine is derived, and outcome variable or study design could all be important.
Two systematic literature reviews assessed treatment options for MetS and liver disease. In their review, Wei et.al. (2016) assessed the efficacy of berberine in the treatment of fatty liver disease and conducted a meta-analysis on six included trails (1). The results showed that the reductions in TC, LDL, ALT, and FBS in the berberine group were significantly higher than those in the control group. Post-intervention TG levels also dropped among NAFLD patients. However, berberine (0.5 g, three times per day) was combined with metformin in two of the included trials, and treatment duration was heterogenous across the studies, ranging from 12 to 16 weeks. It is also notable that the trials had no similar comparison groups, and none of them compared berberine to placebo. According to the other systematic literature review, B. vulgaris may be a promising treatment option for controlling the different components of MetS including diabetes, dyslipidemia, hypertension and obesity, and consequently cardiovascular disease (24).
With respect to the mechanism of action, the efficacy of berberine in protecting liver function could be associated with antioxidant and anti-inflammatory characteristics of its phenolic ingredients (19), as several animal studies have demonstrated similar associations between phenol ingredients of berberine and lipid profile improvement, as well as reductions in liver enzymes among rats and other animal populations (23,25,26). Meanwhile, phenol ingredients are found to be associated with diabetes, hypertension, and dyslipidemia (27).
Although the current study found no significant differences in liver enzymes among NAFLD patients in the berberine arm compared with the control group, significant changes from the baseline to the 6th week were observed in both arms. Our findings indicated that over 6 weeks, there was a significant increase in HDL concentration, along with reductions in SGOT and SGPT, in each group regardless of the berberine administration. This was probably due to the lifestyle modification training of both groups that were given prior to treatment, which included maintaining a low-calorie diet during the 6-week study period.
Despite our study, the findings of a similar study (28) illustrated that reduction of ALT and AST was more significant in the lifestyle interventions plus BBR group compared with life style interventions group at the 16th week. Considering the similar life style interventions between both studies, the cause of this difference can be the duration (6w vs 16w) or the daily intake of berberine (6.25 g vs 15 g).
None of the study groups showed significant changes in LDL, FBS, and TC levels from the baseline to the 6th week. However, TG and ALP both dropped among patients in the control group. It appears that the low-fat diet caused the TG and ALP levels to drop at the end of the trial among these patients, as the association of the diet with lipid profile and liver function improvements has been fully demonstrated in previously published trials (29-32). For example, Ghaemi et al (2013) established the efficacy of a low-fat diet in reducing TG, TC, and LDL levels among patients with NAFLD (29). Likewise, the diet was found to be effective in improving liver function among NAFLD patients due to changes in liver enzymes (30). Unlike our study, participants in these trials were given a low-fat diet accompanied by routine physical activity to control calorie intake and lose weight, both of which were well-achieved at the end of the intervention. In our trial, no changes were observed in the BMI or weight over 6 weeks. The efficacy of a low-fat diet on improving liver function in previous trials may be due to weight loss over time, as the link between weight change and liver enzymes has already been established, with improvements in fatty-acid metabolism and liver cell apoptosis being the main contributing factors (29,33,34).
There are several limitations to the findings of this study. We recruited a small sample of patients with NAFLD from a human resource database of a particular organization who met the inclusion criteria and were motivated to participate in our trial. Therefore, the result cannot be generalized to all patients with fatty liver disease. Both study groups were trained to achieve and maintain their low-fat diet, and although they were monitored through the study period, we lacked interventions targeting physical exercise and calorie intake. Other limitations of the current study included not providing placebo to patients in the control group and the unblinded design of the trial, both of which could be potential sources of bias.
Published studies evaluating the efficacy of berberine in the treatment of NAFLD are limited in scope and number. Therefore, the current study is one of the few that provides evidence on how berberine affects lipid profiles and liver function in obese patients with fatty liver disease. Future research might involve evaluating the therapeutic efficacy of berberine by increasing the sample size and the length of the treatment to better determine the level of adherence over time.
Conclusion
A 6-week berberine treatment was not associated with improved lipid profile, FBS, or liver enzymes among the patients with NAFLD. Postintervention measures indicated significant increases in HDL, as well as reductions in SGOT and SGPT from the baseline to the 6th week in both groups.
Acknowledgment
The authors would like to thank the head and staff of Takestan hospital for their support.
Conflict of Interests
The authors declare that they have no competing interests.
Cite this article as: Nejati L, Movahedi A, Salari GR, Moeineddin R, Nejati P. The Effect of Berberine on Lipid Profile, Liver Enzymes, and Fasting Blood Glucose in Patients with Non-alcoholic Fatty Liver Disease (NAFLD): A Randomized Controlled Trial. Med J Islam Repub Iran. 2022 (20 Apr);36:39. https://doi.org/10.47176/mjiri.36.39
References
- 1. Wei X, Wang C, Hao S, Song H, Yang L. The therapeutic effect of berberine in the treatment of nonalcoholic fatty liver disease: a meta-analysis. Evid Based Complementary Alter Med 2016;2016. [DOI] [PMC free article] [PubMed]
- 2.Younossi ZM, Marchesini G, Pinto-Cortez H, Petta S. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation. 2019;103(1):22–7. doi: 10.1097/TP.0000000000002484. [DOI] [PubMed] [Google Scholar]
- 3.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of Non-Alcoholic Fatty Liver Disease. Digest Dis. 2010;28(1):155–61. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 4.Tziomalos K, Athyros VG, Paschos P, Karagiannis A. Nonalcoholic fatty liver disease and statins. Metabolism. 2015;64(10):1215–23. doi: 10.1016/j.metabol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Araújo AR, Rosso N, Bedogni G, Tiribelli C, Bellentani S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018;38(S1):47–51. doi: 10.1111/liv.13643. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Zhu C, Ying Y, Luo L, Huang D, Luo Z. Metformin and berberine, two versatile drugs in treatment of common metabolic diseases. Oncotarget. 2017;9(11):10135–46. doi: 10.18632/oncotarget.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 8.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–7. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 10.Kong WJ, Zhang H, Song DQ, Xue R, Zhao W, Wei J, et al. Berberine reduces insulin resistance through protein kinase C-dependent up-regulation of insulin receptor expression. Metab Clin Exp. 2009;58:109–19. doi: 10.1016/j.metabol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Dong H, Zhao Y, Zhao L, Lu F. The effects of berberine on blood lipids: a systemic review and meta-analysis of randomized controlled trials. Planta Med. 2013 Apr;79(6):437–46. doi: 10.1055/s-0032-1328321. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Xiao X, Feng K, Wang T, Li W, Yuan T, et al. Berberine Moderates Glucose and Lipid Metabolism through Multipathway Mechanism. Evid Based Complementry Alternat Med. 2011;2011:924851. doi: 10.1155/2011/924851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lao-ong T, Chatuphonprasert W, Nemoto N, Jarukamjorn K. Alteration of hepatic glutathione peroxidase and superoxide dismutase expression in streptozotocin-induced diabetic mice by berberine. Pharm Biol. 2012 Aug;50(8):1007–12. doi: 10.3109/13880209.2012.655377. [DOI] [PubMed] [Google Scholar]
- 14.Hwang JM, Wang CJ, Chou FP, Tseng TH, Hsieh YS, Lin WL, et al. Inhibitory effect of berberine on tert-butyl hydroperoxide-induced oxidative damage in rat liver. Arch Toxicol. 2002 Nov;76(11):664–70. doi: 10.1007/s00204-002-0351-9. [DOI] [PubMed] [Google Scholar]
- 15.Aryaeian N, Sedehi SK, Khorshidi M, Zarezadeh M, Hosseini A, Shahram F. Effects of hydroalcoholic extract of Berberis Integerrima on the anthropometric indices and metabolic profile in active rheumatoid arthritis patients. Complement Ther Med. 2020;50:102331. doi: 10.1016/j.ctim.2020.102331. [DOI] [PubMed] [Google Scholar]
- 16.Aryaeian N, Sedehi SK, Arablou T. Polyphenols and their effects on diabetes management: A review. Med J Islam Repub Iran. 2017 Dec 26;31:134. doi: 10.14196/mjiri.31.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezazadeh A, Omidvar N, Tucker KL. Food frequency questionnaires developed and validated in Iran: a systematic review. Epidemiol Health. 2020;42:e2020015. doi: 10.4178/epih.e2020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadeghisani M, Manshadi FD, Azimi H, Montazeri A. Validity and reliability of the Persian version of Baecke habitual physical activity questionnaire in healthy subjects. Asian J Sports Med. 2016;7(3) doi: 10.5812/asjsm.31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebrahimi-Mamaghani M, Arefhosseini S, Golzarand M, Aliasgarzadeh A, Vahed-Jabbary M. Long-term effects of processed Berberis vulgaris on some metabolic syndrome components. Iran J Endocrinol Metab. 2009;11(1) [Google Scholar]
- 20. Mahan LK, Escott-Stump S. Krause's food, nutrition, & diet therapy: Saunders Philadelphia; 2004.
- 21.Barak F, Falahi E, Hassanzadeh A. Adherence to the Dietary Approaches to Stop Hypertension (DASH) diet in relation to obesity among Iranian female nurses. Public Health Nutr. 2015;18(4):705–12. doi: 10.1017/S1368980014000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noakes M, Keogh JB, Foster PR, Clifton PM. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am J Clin Nutr. 2005;81(6):1298–306. doi: 10.1093/ajcn/81.6.1298. [DOI] [PubMed] [Google Scholar]
- 23.Iloon RK, Najafi SS, Sharif F, Hamedi A, Hoseini MA, Najafi MK, et al. The effect of berberis vulgaris extract on transaminase activities in non-alcoholic Fatty liver disease. Hepat Mon. 2015;15(2):e25067–e. doi: 10.5812/hepatmon.25067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabeshpour J, Imenshahidi M, Hosseinzadeh H. A review of the effects of Berberis vulgaris and its major component, berberine, in metabolic syndrome. Iran J Basic Med Sci. 2017;20(5):557–68. doi: 10.22038/IJBMS.2017.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shidfar F, Seyyed Ebrahimia S, Hosseini S, Heydari I, Shidfard S, Hajhassanid G. The Effects of Berberis vulgaris Fruit Extract on Serum Lipoproteins, apoB, apoA-I, Homocysteine, Glycemic Control and Total Antioxidant Capacity in Type 2 Diabetic Patients. Iran J Pharm Res. 2012;11(2):643–52. [PMC free article] [PubMed] [Google Scholar]
- 26.Taheri S, Zarei A, Changizi Ashtiyani S, Rezaei A, Zaheiri S. Evaluation of the effects of hydroalcoholic extract of Berberis vulgaris root onthe activity of liver enzymes in male hypercholesterolemic rats. Avicenna J Phytomed. 2012;2(3) [PMC free article] [PubMed] [Google Scholar]
- 27.Federico A, Trappoliere M, Loguercio C. Treatment of patients with non-alcoholic fatty liver disease: current views and perspectives. Digest Liv Dis. 2006;38(11):789–801. doi: 10.1016/j.dld.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Yan HM, Xia MF, Wang Y, Chang XX, Yao XZ, Rao SX, et al. Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease. PLoS One. 2015;10(8):e0134172. doi: 10.1371/journal.pone.0134172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghaemi A, Taleban F, Hekmatdoost A, Rafiei A, Hosseini V, Amiri Z, et al. Effect of weight reduction diet on non-alcoholic fatty liver disease. Iran J Nutr Sci Food Technol. 2013;8(2):123–34. [Google Scholar]
- 30.Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, et al. One-year intense nutritional counseling results in histological improvement in patients with nonalcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100(5):1072. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 31.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42(1):44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 32.Rebollo A, Roglans N, Alegret M, Laguna JC. Way back for fructose and liver metabolism: bench side to molecular insights. World J Gastroenterol. 2012;18(45):6552. doi: 10.3748/wjg.v18.i45.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49(1):306–17. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamimi TIA-R, Elgouhari HM, Alkhouri N, Yerian LM, Berk MP, Lopez R, et al. An apoptosis panel for nonalcoholic steatohepatitis diagnosis. J Hepatol. 2011;54(6):1224–9. doi: 10.1016/j.jhep.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]