Abstract
Purpose
Mesenchymal stem cells (MSCs) and their derivant are among the promising treatments for intrauterine adhesion (IUA); they have been reported to repair the endometrial injury by proliferating endometrial cells. However, the signal pathways involved are not clear. This study investigated the role of human umbilical cord mesenchymal stem cell-derived conditioned medium (hUCMSC-CM) in relieving IUA to find out whether Wnt/β-catenin signaling was involved, and if so, to determine the possible ligands.
Methods
After endometrial epithelial cells (EECs) were treated with hUCMSC-CM, their proliferation and migration were measured by the CCK8 assay and the scratch assay. The activation of Wnt/β-catenin signaling was measured by Western blots, fluorescent staining, and T-cell factor/lymphoid enhancer factor (TCF/LEF) luciferase. A Wnt inhibitor (XAV393) was used to inhibit the proliferation effect of hUCMSC-CM in EECs. Wnt5a expression in hUCMSC was measured by Western blots and fluorescent staining, and Wnt5a in hUCMSC-CM was detected by enzyme-linked immunosorbent assay (ELISA), to further clarify the mechanism.
Results
As shown by the CCK8 assay, hUCMSC-CM promoted proliferation and migration of EECs. The expression of β-catenin, c-myc, and cyclin D1 increased in EECs after being treated with hUCMSC-CM. Moreover, hUCMSC-CM was found to promote β-catenin delivery into nuclei by Western blot and fluorescent staining; meanwhile, the inhibitor (XAV393) could restrain this process and inhibit the effect of hUCMSC-CM on EEC proliferation. Wnt5a was detected in hUCMSCs and hUCMSC-CM, which might be a potential therapeutic target.
Conclusion
This study demonstrated that hUCMSC-CM promoted human endometrial cell proliferation through Wnt/β-catenin signaling, and Wnt5a might be a potential activator. This would be one of the activating signal pathways in the MSC-related treatment of IUA.
1. Introduction
Intrauterine adhesion (IUA), also known as Asherman's syndrome (AS), is a complex gynecological pathology mainly caused by the intrauterine operation, infection, and congenital anomaly of the uterus. It can be characterized by fibrous, connective tissue bands in the uterine cavity, with or without glandular tissue [1], leading to hypomenorrhea or amenorrhea, repeated pregnancy loss, and infertility [2]. Hysteroscopy is the first-line diagnostic investigation, and hysteroscopic adhesiolysis combined with hormone therapy had been used. However, with a high rate of recurrence after adhesiolysis [2], a new treatment was still being investigated. Human umbilical cord mesenchymal stem cell (hUCMSCs) transplantation was reported to repair the endometrial injury by proliferating endometrial cells [3]. Stem cell transplantation might be a potential treatment for IUA, but the tumorigenicity remained a problem since stem cell had the self-renewal capacity and multiple differentiation potentials. Recently, the conditioned medium (CM) of mesenchymal stem cells (MSCs) was shown to have a therapeutic potential similar to that of MSCs, which were repairing injured endometrium and restoring fertility, indicating that MSC-based therapy could be achieved through paracrine secretion [1]. Many cytokines were detected in MSC-CM [4], which is cell-free and rich in secreted bioactive factors, including a variety of protein, peptide, RNA, and lipid mediators [4]. It was still unclear which one played key role in IUA repairing.
Wnt5a/β-catenin signaling is critical in estrogen-mediated uterine development [5]. It can be induced by estradiol [6], participating in the development and functional maintenance of the endometrium [7, 8] and modulating the self-renewal activity of endometrial mesenchymal stem cells (eMSCs) [9–11], indicating that it might be a potential target in IUA treatment. Wnt ligands were found in hUCMSC-derived exosomes [12], promoting β-catenin nuclear translocation and enhancing the proliferation of skin cells. Wnt5a could also be secreted and transported in extracellular vesicles [13].
In this study, we investigated the role of hUCMSC-CM in promoting the proliferation and migration of endometrial epithelial cells (EECs) to find out whether Wnt5a derived from hUCMSC-CM could activate β-catenin signaling in endometrial cells.
2. Methods
2.1. Ethics Statement
For this study, hUCMSCs were isolated from umbilical cords acquired from the Department of Obstetrics of Beijing Hospital, according to the protocol approved by the Ethics Committee of Beijing Hospital (no. [2019]04). The donors also provided informed consent, in accordance with the Declaration of Helsinki. Human EECs were purchased from Procell Life Science & Technology Co., Ltd., Wuhan, China. The authors had no conflict of interest.
2.2. Isolation and Characterization of Human Umbilical Cord Mesenchymal Stem Cells (hUCMSCs)
The umbilical cords were washed with phosphate buffer saline (PBS, 0.1 M, pH 7.4) containing 5% penicillin/streptomycin (P/S). Wharton's jelly was cut into small pieces and exposed to collagenase type IV solution (1 : 1 in PBS) for 1 h. They were cultured in Dulbecco's modified Eagle's medium (DMEM/F12, Gibco, Hyclone), supplemented with 10% fetal bovine serum (Gibico) and 1% P/S, and incubated in a humidified atmosphere containing 5% CO2 at 37°C. The medium was changed every day. Next, the large particle pieces were removed, and the adherent cells were subcultured using 0.25% trypsin. The hUCMSCs from the third to the fifth passages were used for further experiments [14].
The hUCMSCs were verified by a flow cytometer (Beckman Coulter, Brea, CA, USA) after staining with the specific antibodies, including CD 34, 44, 45, 73, 90, and 11b conjugated with FITC and APC, respectively.
2.3. Preparation of hUCMSC-CM
The hUCMSCs (third passage) were washed with PBS three times until they were 70–80% confluent, then they were preincubated with serum-free fresh DMEM/F12 for 48 h. The CM was collected, centrifuged at 1,000 rpm for 20 min, and filtered through a 0.22 um filter.
2.4. ELISA
Wnt5a in the CMs was measured by the enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (MLBio, Shanghai, China).
2.5. Proliferation Assay
EECs (P3) were seeded in 96-well plates (1 × 104 cells/well) after being cultured with hUCMSC-CM or serum-free DMEM/F12 for 48 h. The CCK-8 reagent (10 μl; Beyotime, Jiangsu, China) was then added to the plates, which were incubated in a humidified atmosphere containing 5% CO2 at 37°C for 3 hours, and each sample was measured at 450 nm.
2.6. mRNA Isolation, cDNA Synthesis, and Real-Time RT-PCR
EECs were pretreated with hUCMSC-CM or serum-free MDEM/F12 in 6 cm plates for 48 h. The cells were collected, and the total RNA was extracted by an RNA isolation kit (Vazyme, Nanjing, Jiangsu Province, China), followed by a reverse transcription using a cDNA synthesis kit (TransGen Biotech, Beijing, China). The cDNA was followed by a real-time quantitative polymerase chain reaction (PCR) using the KAPA SYBR® FAST Master Mix (KAPA BIO, Boston, MA, USA) with a primer. Each gene expression level was normalized, with GAPDH used as a housekeeping control. The primer sequences were as follows: Wnt5a, forward 5′-CTCGCCATGAAGAAGTCCA-3′ and reverse 5′- TACCTAGCGACCACCAAGAA -3′; cyclin D1, forward 5′- GCTGCGAAGTGGAAACCATC -3′ and reverse 5′- CCTCCTTCTGCACACATTTGAA -3′; c-myc, forward 5′- GTCAAGAGGCGAACACACAAC -3′ and reverse 5′- TTGGACGGACAGGATGTATGC -3′; CTNNB1, 5′- GGTGGGCTGCAGAAAATGGTT -3′ and reverse 5′- GATGGCAGGCTCAGTGATGTCTTC -3′.
2.7. Western Blotting
Cells were lysed with RIPA lysis buffer (Solarbio, Beijing, China) containing 1 × PMSF (Solarbio, Beijing, China) to extract total protein.
Nuclear and cytoplasmic proteins were extracted separately with the Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, Jiangsu, China). Protein lysates (20 μg) were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (0.45 μm; Millipore, Burlington, MA, USA). The membranes were incubated with antibodies against β–catenin (1 : 4000 in antibody dilution buffer (Beyotime, Jiangsu, China), the following antibodies used the same dilution buffer), C-myc (1 : 1000), Cyclin D1(1 : 1000), GAPDH (1 : 5000), Wnt5a (1 : 1000), β-tubulin (1 : 2000) (Abcam, Cambridge, UK), and Histone (1 : 2000) (Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight and then incubated with corresponding horseradish peroxidase- (HRP-) conjugated second antibodies (1 : 1000) (Cell Signaling Technology, Danvers, MA, USA) at 37°C for 1 h. The bands were visualized with enhanced chemiluminescence (Millipore, Burlington, MA, USA). The intensity of each band was calculated using Image J software (National Institutes of Health, MD, USA).
2.8. Immunofluorescence Staining
After treatment, the cells were fixed in 4% paraformaldehyde, permeabilized with 0.3% Triton X-100 in PBS, and blocked in 1% bovine serum albumin for 1 h. Then, the cells were incubated with primary β-catenin antibodies (1 : 200) (Abcam, Cambridge, UK) at 4°C overnight in a humidified atmosphere. They were then incubated with Alexa Fluor488-conjugated secondary antibodies (1 : 500) (Proteintech, Chicago, IL, USA) for 1 h, and the nucleus was stained with DAPI (Beyotime, Jiangsu, China) for 5 min. Immunofluorescence staining was imaged using fluorescence microscopy (Cannon, Tokyo, Japan).
2.9. TOP/FOP Luciferase Reporter Assay
The cells were seeded into a 24-well plate at a density of 50,000 cells per well. The cells were cotransfected with 0.5 μg of either TOPflash or FOPflash vector (Beyotime, Jiangsu, China) and 0.1 μg of pRL-TK (Renilla-TK-luciferase vector) as a control using Lipofectamine 3000 (Invitrogen). Some cells were subsequently treated with hUCMSC-CM, while the control group was treated with the serum-free DMEM/F12 for 48 h. The cells were then lysed, and the luciferase activities were measured using the Dual-Luciferase Reporter Gene Assay Kit (Beyotime, Jiangsu, China), according to the manufacturer's instructions. Firefly luciferase activity was normalized against the Renilla luciferase activity, and the TOP/FOP ratio was used as a measure to evaluate the activation of the T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription.
2.10. Scratch Assay
The cells were seeded in 6-well plates at 5 × 105 cells per well. After cell adherence, scratches were made with a sterilized P200 pipette tip (500-μm width), and the cells were cultured in hUCMSC-CM or serum-free DMEM/F12 after washing with PBS. After 0 h, 48 h, and 96 h, the scratches were photographed by microscopy (Canon, Tokyo, Japan) and measured manually with Image J software. The data were reported as the ratio of migration relative to the control.
2.11. Statistics
The results are expressed as mean ± SEM. All experiments were repeated three times with independent cultures. Statistical significance was assessed using Student's t-test, and a P value < 0.05 indicated significant differences.
3. Results
3.1. Characterization of hUCMSCs
Flow cytometry was used to detect the positive markers (CD44, CD73, and CD90) and the negative markers (CD45, CD34, and CD11b) of the MSCs to identify the hUCMSCs. The results demonstrated that the positive markers were highly expressed, but the negative markers were not expressed (Figure 1).
Figure 1.
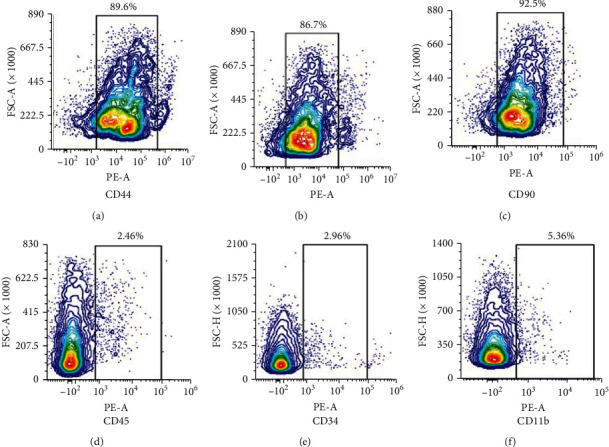
Characterization of MSCs. Flow cytometry analysis of common MSC markers such as (a) CD44, (b) CD73, and (c) CD90. MSCs are negative for (d) CD45, (e) CD34, and (f) CD11b.
3.2. hUCMSC-CM Promoted the Proliferation and Migration of Endometrial Cells
The CCK8 assay was performed to investigate the effect of hUCMSC-CM on endometrial cells proliferation. The results showed that hUCMSC-CM promoted the proliferation of EECs (Figure 2(a)). The scratch assay showed that hUCMSC-CM enhanced the EECs' migration property compared with the control group (Figure 2(b)).
Figure 2.
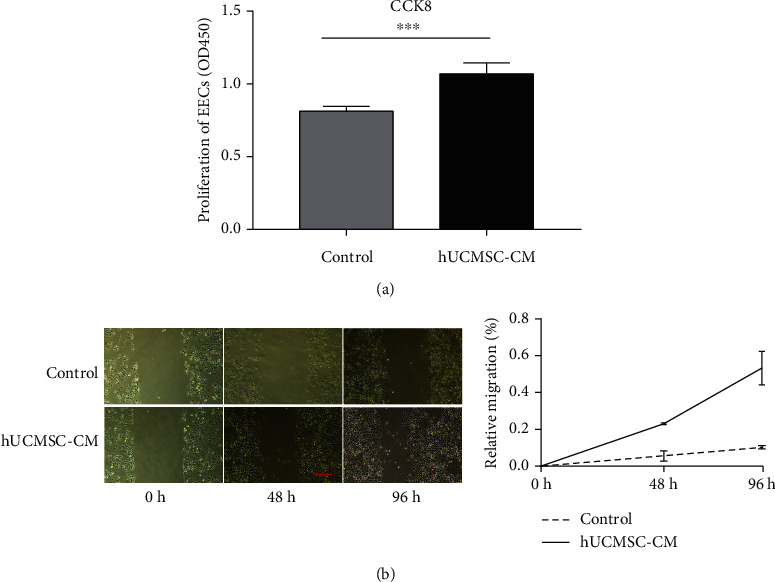
Cell proliferation and migration activity of EECs treated with hUCMSC-CM. (a) Cell proliferation of EECs with serum-free DMEM/F12 (control group) or hUCMSC-CM (experimental group). (b) The image of cell migration of EECs cultured with serum-free DMEM/F12 (control group) or hUCMSC-CM. Scale bar: 20 μm. ∗∗∗P < 0.001.
3.3. hUCMSC-CM Activated Wnt/β-Catenin Pathway of Endometrial Cells In Vitro
After being treated with hUCMSC-CM for 48 h, the mRNA expression of CTNNB1 in EECs was detected. The results of the CTNNB1 gene expression were significantly higher in the hUCMSC-CM group than in the control group (treated with serum-free DMEM/F12) (Figure 3(a)), and the results were confirmed (by Western blotting) in the protein levels (Figure 3(b)).
Figure 3.
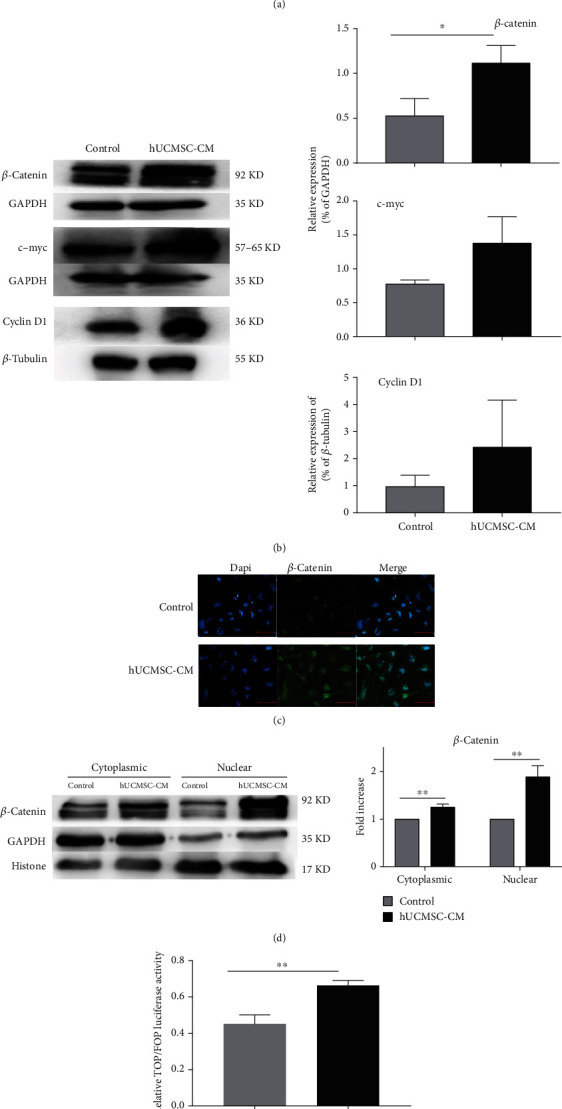
hUCMSC-CM activated Wnt/catenin signaling pathway. (a) CTNNB1, CMYC, and CYCLIND1 gene expressions were measured by RT-PCR in EECs treated with serum-free DMEM/F12 (control group) or hUCMSC-CM. (b) Western blotting images and relative quantitative analysis of total β-catenin, c-myc, and cyclin D1 protein in EECs. EECs were treated with serum-free DMEM/F12 (control group) or hUCMSC-CM. (c) Immunofluorescence staining of β-catenin protein in EECs after serum-free DMEM/F12 (control group) or hUCMSC-CM treatments. Scale bar: 20 μm. (d) Western blotting determined the nuclear and cytoplasmic β-catenin levels in EEC cells after serum-free DMEM/F12 (control group) or hUCMSC-CM treatments. (e) The TCF/LEF luciferase signals in EECs after serum-free DMEM/F12 (control group) or hUCMSC-CM treatments. ∗P < 0.05; ∗∗P < 0.01.
After being treated with hUCMSC-CM for 48 h, the mRNA expression of downstream gene CMYC and CYCLIND1 in EECs was detected. The CYCLIND1 gene expression was significantly higher in the hUCMSC-CM group than that in the control group (treated with serum-free DMEM/F12), while C-MYC showed no statistical difference (Figure 3(a)). Both had no statistical difference in protein levels (Figure 3(b)).
Furthermore, the β-catenin protein levels in nucleus and cytoplasm were detected, respectively. In the hUCMSC-CM groups, the β-catenin protein levels were higher in nucleus than those of the control groups (Figure 3(d)), and they showed nuclei located in immunofluorescence staining (Figure 3(c)). TCF/LEF luciferase activities were measured in these two groups. The results showed that hUCMSC-CM increased the TCF/LEF luciferase activities of endometrial cells (Figure 3(e)), indicating that hUCMSC-CM can promote nuclear localization of β-catenin and transcriptional activation of the Wnt/β-catenin pathway in EECs in vitro.
3.4. Wnt5a Expression in hUCMSCs and hUCMSC-CM
To explore which protein might activate the Wnt/β-catenin in endometrial cells, the expression of Wnt5a in hUCMSCs was confirmed by Western blotting. The protein expression levels of Wnt5a were significantly higher in hUCMSCs than that in EECs (Figure 4(a)). By immunofluorescence staining, we also detected the Wnt5a protein located in the plasma of hUCMSCs (Figure 4(b)). By ELISA, we detected the concentration of Wnt5a in the culture supernatant (Figure 4(c)). The results showed that the level of secreted Wnt5a was higher than that in the control group (serum-free DMEM/F12). This data showed that Wnt5a was produced by hUCMSCs and secreted into hUCMSC-CM.
Figure 4.
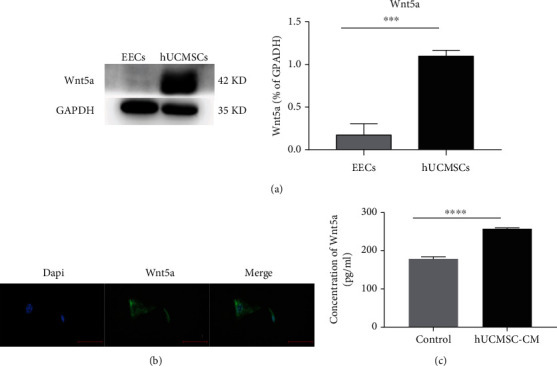
Wnt5a expression in human MSCs. (a) Western blots of Wnt5a and corresponding semiquantitative analysis using EECs as control. (b) Immunofluorescence staining of Wnt5a in hUCMSCs. Scale bar: 10 μm. (c) Wnt5a was quantified in the hUCMSC-CM by ELISA, with serum-free DMEM/F12 as control. ∗∗∗P < 0.001; ∗∗∗∗P < 0.0001.
3.5. XAV939 Inhibited the Proliferation Effect of hUCMSC-CM in Endometrial Cells
Loss-of-function approaches were used to assess the role of Wnt/β-catenin signaling in the proliferation of endometrial cells. XAV939 is a potent tankyrase inhibitor that targets Wnt/β-catenin signaling, stabilizing axin, thereby stimulating β-catenin degradation. The addition of XAV939 (at a concentration of 50 μmol/ml) to hUCMSC-CM inhibited the proliferation of endometrial cells in the control group compared with the hUCMSC-CM group (Figure 5(a)). Being degraded, β-catenin protein delivered into nuclei would also be inhibited (Figure 5(b)).
Figure 5.
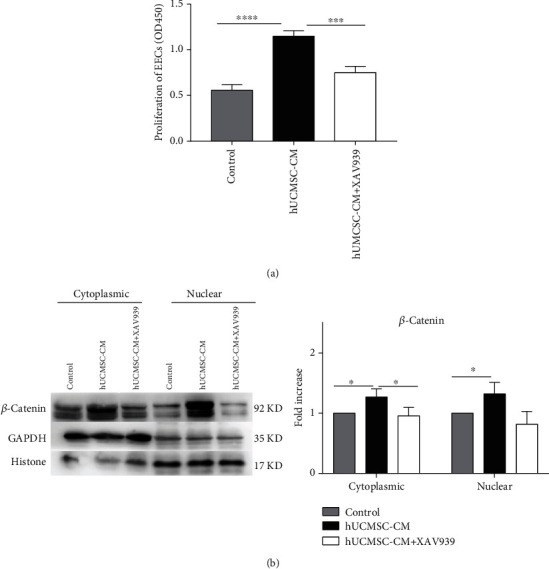
The proliferation of endometrial cells treated with hUCMSC-CM is inhibited by XAV939. (a) The proliferation of EECs treated with serum-free DMEM/F12 + DMSO (control group), hUCMSC − CM + DMSO, and hUCMSC − CM + XAV939 (50 μmol/ml). (b) Western blotting images and quantitative analysis of β-catenin protein levels in nucleus and cytoplasm ∗P < 0.05; ∗∗∗P < 0.001; ∗∗∗∗P < 0.0001.
4. Discussion
In this study, we explored the effect of hUCMSC-CM on promoting human endometrial cell proliferation and found that Wnt/β-catenin signaling was activated in this process. Our results showed that hUCMSC-CM could activate Wnt/β-catenin signaling in promoting EECs proliferation; meanwhile, by inhibiting the signaling, the proliferation of EECs was restrained. We also found that the signaling was probably activated by Wnt5a secreted by hUCMSCs, which was detected in the cytoplasm, and also in the hUCMSC-CM.
Recently, there have been several studies on stem cells therapy in IUA in animals and human beings. Bone marrow stem cell intrauterine transplantation can improve the fertility outcome in IUA mice [15]. The therapeutic effect of MSCs had been verified in IUA patients [16, 17]. As we know, the endometrium of the uterus has a regenerative capacity. During each menstrual cycle, it undergoes a process of degeneration, proliferation, differentiation, and regeneration. Stem cells were believed to reside in the basal layer of the endometrium [18, 19]. When the uterine injury occurred, stem cells were recruited and differentiated to repair the impairment, likely due to immunomodulation, recruitment of growth factors, and paracrine effects [2]. In our previous study, MSCs secreted a multitude of cytokines and signaling molecules that promote the endometrial proliferation and glandular reformation after trauma, including epidermal growth factor (EGF), insulin-like growth factor-binding protein (IGFBP), insulin-like growth factor-1 (IGF-1), and fibroblast growth factor (FGF) [20]. MSC-CM contained a variety of protein, peptide, RNA, and lipid mediators [4], also shown to have a therapeutic effect on IUA as in the case of stem cells. However, the mechanisms and signaling pathways involved remained unclear.
The Wnt signaling pathway is an essential cellular signaling pathway in organisms, which often involves in embryonic development and cell proliferation [21]. This pathway can be divided into two forms: the canonical and the noncanonical types. The noncanonical pathway includes the noncanonical planar cell polarity (PCP) pathway and the Wnt/calcium pathway.
In the canonical Wnt signaling pathway, also known as the Wnt/β-catenin pathway, Wnt ligands bind to the frizzled (Fzd) receptor and coreceptor and low-density lipoprotein receptor-related protein 5/6 (LRP 5/6), initiating β-catenin nuclear translocation. β-Catenin is an unstable protein and is strictly regulated in the cytoplasm. In the absence of a Wnt ligand, intracellular β-catenin is targeted by a degradation complex. The complex consists of tumor suppressor protein (APC), scaffold protein Axin, and two kinases: tyrosine kinase 1α (CK1α) and glycogen synthase kinase 3β (GSK-3β). CK1α and GSK-3β phosphorylated β-catenin N-terminal serine and threonine, phosphorylated β-catenin recognized by transducers, which are part of the ubiquitin ligase complex, then β-catenin was degraded after ubiquitination. When the Wnt ligand binds to Fzd and LRP5/6, it induces phosphorylation of disheveled protein (DVL/DSH), recruits Axin protein, and then disintegrates the degradation complex. β-Catenin accumulates in the cytoplasm and translocates into the nucleus, where it binds to the TCF/LEF to regulate the target gene of the Wnt pathway [21].
The canonical Wnt signaling pathway plays a role in endometrial cell proliferation. Nei and his colleagues [22] collected the normal endometrial tissue, endometrial hyperplasia tissue, and endometrial cancer. They found that β-catenin was expressed in endometrial tissue in the middle and the late proliferation phases and increased in the nucleus of proliferative endometrial cells stimulated with high-dose estrogen without progesterone. In the secretion phase, when progesterone increases and estrogen decreases, the nucleus β-catenin decreases. Hou et al. [23] detected β-catenin in the nucleus of the endometrial epithelium after giving exogenous estrogen to mice. Furthermore, they delivered adenoviral expressed SFRP2, the Wnt signaling inhibitor, in mice and observed that estrogen-induced promotion of EECs proliferation was inhibited. Gunin et al. [24] inhibited GSK3β by giving LiCl, activator of the Wnt/β-catenin pathway, and successfully simulated the proliferation of endometrium in mice.
Wnt signaling molecules can also activate eMSCs and participate in endometrial periodic regeneration. Cao et al. [9] found that coculture of myometrial cells enhanced the colony forming and self-renewal ability of eMSCs. It is believed that Wnt/β-catenin signaling was involved. By using the Wnt pathway activator (Wnt3a CM) and the Wnt inhibitor (XAV939 and IWP-2), the participation of β-catenin signaling in the self-renewal of eMSCs was confirmed. The high expression of Wnt5a was detected in uterine myocytes. The introduction of recombinant Wnt5a can stimulate eMSCs colony formation, while the Wnt5a antibody inhibited the colony formation. It is inferred that uterine myocytes are the activated microenvironment components of eMSCs, and the Wnt5a/β-catenin signaling pathway is an important signaling pathway to activate eMSCs. Bukowska et al. [25] found that the inhibitor (XAV939) and the activator (BIO) of the canonical Wnt signaling pathway could upregulate and downregulate the stem cell markers (CD73 and CD105), respectively, indicating that the activation of the canonical Wnt signaling pathway led to the differentiation of eMSCs.
C-myc and cyclin D1 are the downstream genes of Wnt/β-catenin signaling [26, 27]; they are also estrogen-regulated genes implicated in cell proliferation. C-myc is a proto-oncogene, which had been reported to encode a transcriptional regulatory protein required for cell growth [28] and modulate the expression of cyclin D1, a cell cycle control gene [29]. It can be upregulated by estrogen and found increased expression in endometriosis [30]. In this study, they were upregulated by hUCMSC-CM along with β-catenin, indicating that the component in the hUCMSC-CM might activate Wnt/β-catenin signaling, upregulate the expression of c-myc, and regulate the cell cycle of EECs, promoting the cells' proliferation.
Many proteins are responsible for the development and regeneration of the endometrium, of which Wnt, c-kit (CD117), Oct-4, CD34/KLF4, and Musashi-1 are the best classified [2]. In this study, Wnt/β-catenin signaling was activated by hUCMSC-CM, participating in EEC proliferation. Wnt genes were found expressed in hUCMSCs; among them, a relatively high mRNA expression of the Wnt5a was detected [31]. Consistent with our results, the Wnt5a protein was found expressed in hUCMSCs and enriched in hUCMSC-CM, indicating that Wnt5a/β-catenin signaling might be involved.
5. Limitations
Regarding the limitations of our work, first, the complex signal network and cross-talk are not discussed in this article. Second, other Wnt-ligand proteins, such as Wnt7a and Wnt4, were reported to play an essential role in endometrial development and regeneration of reproductive tracts; however, whether or not they relate to IUA treatment has not been addressed in this paper. Third, the Wnt/β-catenin pathway has been examined in vitro; in vivo studies need to be conducted in future work.
6. Conclusion
Our study demonstrated that hUCMSC-CM promoted human endometrial cell proliferation through Wnt/β-catenin signaling, and Wnt5a might be an activator. This would be one of the activating signal pathways in the MSC-related therapy of IUA.
Acknowledgments
This work was supported by the Beijing Dongcheng Department of Science, Technology, and Information (BJ-2019-103 to Shaowei Wang).
Contributor Information
Jianping Cai, Email: caijp61@vip.sina.com.
Shaowei Wang, Email: w_sw999@163.com.
Data Availability
The data used to support the findings of this study are currently under embargo while the research findings are commercialized. Requests for data, 12 months after publication of this article, will be considered by the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Liu F., Hu S., Yang H., et al. Hyaluronic acid hydrogel integrated with mesenchymal stem cell-secretome to treat endometrial injury in a rat model of Asherman's syndrome. Advanced Healthcare Materials . 2019;8(14, article e1900411) doi: 10.1002/adhm.201900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benor A., Gay S., DeCherney A. An update on stem cell therapy for Asherman syndrome. Journal of Assisted Reproduction and Genetics . 2020;37(7):1511–1529. doi: 10.1007/s10815-020-01801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L., Li Y., Guan C. Y., et al. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell Research & Therapy . 2018;9(1):p. 36. doi: 10.1186/s13287-018-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogatcheva N. V., Coleman M. E. Conditioned medium of mesenchymal stromal cells: a new class of therapeutics. Biochemistry Biokhimiia . 2019;84(11):1375–1389. doi: 10.1134/s0006297919110129. [DOI] [PubMed] [Google Scholar]
- 5.St-Jean G., Boyer A., Zamberlam G., Godin P., Paquet M., Boerboom D. Targeted ablation ofWnt4andWnt5ain müllerian duct mesenchyme impedes endometrial gland development and causes partial müllerian agenesis†. Biology of Reproduction . 2019;100(1):49–60. doi: 10.1093/biolre/ioy160. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y., van der Zee M., Fodde R., Blok L. J. Wnt/β-catenin and sex hormone signaling in endometrial homeostasis and cancer. Oncotarget . 2010;1(7):674–684. doi: 10.18632/oncotarget.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farah O., Biechele S., Rossant J., Dufort D. Porcupine-dependent Wnt signaling controls stromal proliferation and endometrial gland maintenance through the action of distinct WNTs. Developmental Biology . 2017;422(1):58–69. doi: 10.1016/j.ydbio.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Mericskay M., Kitajewski J., Sassoon D. Wnt5ais required for proper epithelial-mesenchymal interactions in the uterus. Development (Cambridge, England) . 2004;131(9):2061–2072. doi: 10.1242/dev.01090. [DOI] [PubMed] [Google Scholar]
- 9.Cao M., Chan R. W. S., Cheng F. H. C., et al. Myometrial cells stimulate self-renewal of endometrial mesenchymal stem-like cells through wnt5a/β-catenin signaling. Stem cells (Dayton, Ohio) . 2019;37(11):1455–1466. doi: 10.1002/stem.3070. [DOI] [PubMed] [Google Scholar]
- 10.Cha J., Bartos A., Park C., et al. Appropriate crypt formation in the uterus for embryo homing and implantation requires Wnt5a-ROR signaling. Cell Reports . 2014;8(2):382–392. doi: 10.1016/j.celrep.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi K., Yoshioka S., Reardon S. N., et al. Wnts in the neonatal mouse uterus: potential regulation of endometrial gland development. Biology of Reproduction . 2011;84(2):308–319. doi: 10.1095/biolreprod.110.088161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B., Wang M., Gong A., et al. Hucmsc-exosome mediated-wnt4 signaling is required for cutaneous wound healing. Stem cells (Dayton, Ohio) . 2015;33(7):2158–2168. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- 13.Działo E., Rudnik M., Koning R. I., et al. Wnt3a and wnt5a transported by exosomes activate wnt signaling pathways in human cardiac fibroblasts. International Journal of Molecular Sciences . 2019;20(6) doi: 10.3390/ijms20061436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravan A. P., Goudarzi F., Rafieemehr H., et al. Human umbilical cord-mesenchymal stem cells conditioned medium attenuates CCl4induced chronic liver fibrosis. Toxin Reviews . 2021;40(2):238–249. doi: 10.1080/15569543.2019.1590849. [DOI] [Google Scholar]
- 15.Alawadhi F., Du H., Cakmak H., Taylor H. S. Bone marrow-derived stem cell (bmdsc) transplantation improves fertility in a murine model of Asherman's syndrome. PLoS One . 2014;9(5, article e96662) doi: 10.1371/journal.pone.0096662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh N., Mohanty S., Seth T., Shankar M., Dharmendra S., Bhaskaran S. Autologous stem cell transplantation in refractory Asherman′s syndrome: a novel cell based therapy. Journal of human reproductive sciences . 2014;7(2):93–98. doi: 10.4103/0974-1208.138864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santamaria X., Cabanillas S., Cervelló I., et al. Autologous cell therapy with cd133+ bone marrow-derived stem cells for refractory Asherman's syndrome and endometrial atrophy: a pilot cohort study. Human Reproduction (Oxford, England) . 2016;31(5):1087–1096. doi: 10.1093/humrep/dew042. [DOI] [PubMed] [Google Scholar]
- 18.Santamaria X., Mas A., Cervelló I., Taylor H., Simon C. Uterine stem cells: from basic research to advanced cell therapies. Human Reproduction Update . 2018;24(6):673–693. doi: 10.1093/humupd/dmy028. [DOI] [PubMed] [Google Scholar]
- 19.Taylor H. S. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA . 2004;292(1):81–85. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S., Zhu D., Mei X., et al. Advances in biomaterials and regenerative medicine for primary ovarian insufficiency therapy. Bioactive materials . 2021;6(7):1957–1972. doi: 10.1016/j.bioactmat.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duchartre Y., Kim Y. M., Kahn M. The wnt signaling pathway in cancer. Critical reviews in oncology/hematology . 2016;99:141–149. doi: 10.1016/j.critrevonc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nei H., Saito T., Yamasaki H., Mizumoto H., Ito E., Kudo R. Nuclear localization of?-catenin in normal and carcinogenic endometrium. Molecular Carcinogenesis . 1999;25(3):207–218. doi: 10.1002/(SICI)1098-2744(199907)25:3<207::AID-MC7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Hou X., Tan Y., Li M., Dey S. K., das S. K. Canonical wnt signaling is critical to estrogen-mediated uterine growth. Molecular endocrinology (Baltimore, Md) . 2004;18(12):3035–3049. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunin A. G., Emelianov V. U., Mironkin I. U., Morozov M. P., Tolmachev A. S. Lithium treatment enhances estradiol-induced proliferation and hyperplasia formation in the uterus of mice. European Journal of Obstetrics, Gynecology, and Reproductive Biology . 2004;114(1):83–91. doi: 10.1016/j.ejogrb.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Bukowska J., Ziecik A. J., Laguna J., Gawronska-Kozak B., Bodek G. The importance of the canonical wnt signaling pathway in the porcine endometrial stromal stem/progenitor cells: implications for regeneration. Stem Cells and Development . 2015;24(24):2873–2885. doi: 10.1089/scd.2015.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohammed M. K., Shao C., Wang J., et al. Wnt/β-catenin signaling plays an ever-expanding role in stem cell self- renewal, tumorigenesis and cancer chemoresistance. Genes & diseases . 2016;3(1):11–40. doi: 10.1016/j.gendis.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris S. L., Huang S. Crosstalk of the Wnt/β-catenin pathway with other pathways in cancer cells. Genes & diseases . 2016;3(1):41–47. doi: 10.1016/j.gendis.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhanasekaran R., Deutzmann A., Mahauad-Fernandez W. D., Hansen A. S., Gouw A. M., Felsher D. W. The MYC oncogene -- the grand orchestrator of cancer growth and immune evasion. Nature reviews Clinical oncology . 2022;19(1):23–36. doi: 10.1038/s41571-021-00549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connor M. J., Thakar T., Nicolae C. M., Moldovan G. L. PARP14 regulates cyclin D1 expression to promote cell-cycle progression. Oncogene . 2021;40(30):4872–4883. doi: 10.1038/s41388-021-01881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellegrini C., Gori I., Achtari C., et al. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertility and Sterility . 2012;98(5):1200–1208. doi: 10.1016/j.fertnstert.2012.06.056. [DOI] [PubMed] [Google Scholar]
- 31.An integrated encyclopedia of DNA elements in the human genome. Nature . 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are currently under embargo while the research findings are commercialized. Requests for data, 12 months after publication of this article, will be considered by the corresponding author.


