Abstract
Lipid risk factors for cardiovascular disease depend in part on lifestyle, but optimum control of lipids often demands additional measures. Low-density lipoprotein (LDL) doubtless contributes causally to atherosclerosis. Recent human genetic findings have substantiated a number of novel targets for lipid-lowering therapy including apolipoprotein C-III, angiopoietin-like protein 3 and 4, apolipoprotein V, and ATP citrate lyase. These discoveries coupled with advances in biotechnology development afford new avenues for management of LDL and other aspects of lipid risk. Beyond LDL, new treatments targeting triglyceride-rich lipoproteins and lipoprotein(a) have become available and have entered clinical development. Biological and RNA-directed agents have joined traditional small-molecule approaches, which themselves have undergone considerable refinement. Innovative targeting strategies have increased efficacy of some of these novel interventions and markedly improved their tolerability. Gene-editing approaches have appeared on the horizon of lipid management. This article reviews this progress offering insight into novel biological and therapeutic discoveries, and places them into a practical patient care perspective.
Keywords: Lipoproteins, Triglycerides, Lipoprotein(a), Apolipoprotein C-III, Angiopoietin-like proteins, RNA therapeutics
Graphical Abstract
Introduction
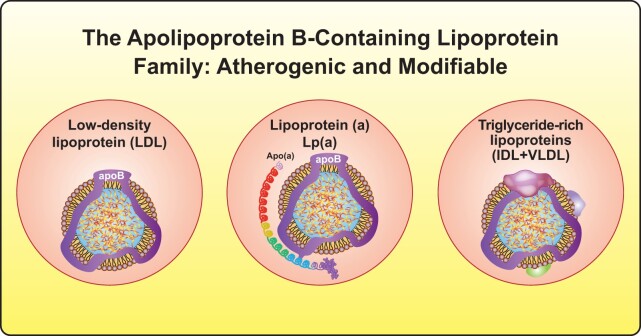
Lipids comprise key modifiable risk factors for atherosclerotic vascular disease (ASVD), a chronic immunoinflammatory process in the arterial wall that causes most cardiovascular (CV) events. The accumulation and retention of apolipoprotein B (apoB)-containing lipoproteins, mainly low-density lipoprotein (LDL) in the arterial intima, accompanies early atherogenesis.1 An inflammatory response ensues that promotes plaque progression and eventually plaque disruption.2 LDL particles constitute 90% of apoB-containing lipoproteins in fasting humans, and have become the prime treatment target in clinical practice. But other apoB-containing lipoproteins also contribute causally to atherosclerosis3 (Figure 1). Triglyceride-rich lipoproteins that are <70 nm in diameter such as chylomicron remnants, very low density lipoprotein (VLDL) remnants, and intermediate-density lipoprotein (IDL) can traverse the endothelium, accumulate, and promote atherogenesis. Recent epidemiologic and genetic studies have established that cholesterol-rich remnant particles that accumulate in individuals with hypertriglyceridaemia are atherogenic and contribute to ASVD.4,5
Figure 1.
New targets for lipid-lowering therapies. Beyond low-density lipoprotein, lipoprotein(a) and triglyceride-rich lipoproteins or remnant lipoproteins have become actionable targets in lipid management. IDL, intermediate-density lipoprotein; VLDL, very low-density lipoprotein.
In general, LDL-cholesterol (LDL-C) correlates tightly with apoB, but in some circumstances like diabetes, obesity, or very low LDL-C, it may underestimate the risk conferred by other apoB-containing lipoproteins. In these conditions, the simple calculation of non-high-density lipoprotein cholesterol (HDL-C) (total cholesterol – HDL) captures all apoB-containing lipoproteins including remnant cholesterol. The measurement of apoB also yields a more accurate estimation of risk than measurement of LDL concentrations in such individuals. Emerging evidence also suggests that non-HDL-C and apoB reflect residual risk better than LDL in statin-treated patients.6 Thus, non-HDL-C has become a secondary target in European and other guidelines. Emerging novel therapies can target these non-LDL lipid fractions and promise to provide practitioners with new tools to confront residual risk.
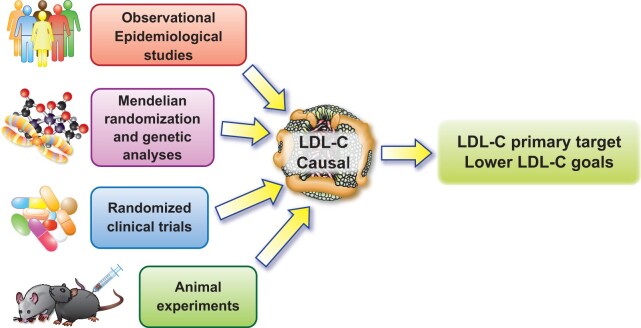
Strong and consistent evidence from monogenic disorders, Mendelian randomization and genome-wide association studies (GWAS), and observational epidemiological, clinical, and interventional investigations have established that LDL satisfies modified Koch’s postulates for causing atherosclerosis7 (Figure 2). Without elevated LDL, atherosclerosis would likely be an orphan disease. LDL is the most extensively studied and targeted lipoprotein and remains justifiably the main lipid focus in clinical practice.
Figure 2.
Multiple lines of evidence showing low-density lipoprotein cholesterol is causal for cardiovascular disease. Data that have accrued from observational data, human genetic analyses, randomized clinical trial results, and animal experimentation in multiple species, all concordantly support a causal contribution of low-density lipoprotein to atherosclerosis.
Multiple lines of evidence show that the magnitude and duration of exposure to LDL determine the risk of ASVD and its complications.8 Thus, more and earlier LDL-C reduction provide greater CV prevention. This observation underscores the urgency of identification and early treatment of high LDL. Based on accumulating clinical trial evidence, guidelines and practice have evolved towards the achievement of more stringent LDL-C goals, especially in higher risk patients.9 Recent studies that lowered LDL-C with combination therapy have not shown a threshold for clinical benefit and have allayed many safety concerns, thus reinforcing the ‘lower is better’ concept.10–12
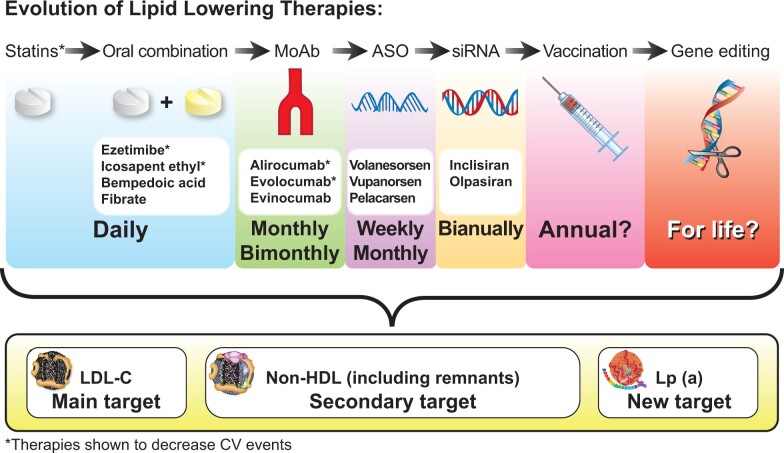
Recent successes of the trials with non-statin lipid-lowering agents in decreasing CV events have shown that LDL-C lowering by a variety of mechanisms including increased LDL receptor expression or reduced cholesterol adsorption yields CV benefit.13 Focus has therefore broadened from ‘high-intensity statin therapy’ to ‘high-intensity lipid-lowering therapy’ for LDL-C management. This recognition, along with the considerable remaining CV risk even in statin-treated individuals, has accelerated the quest for therapies that reduce atherogenic apoB-containing lipoproteins. Targeted delivery of nucleic acid-based therapies has progressed substantially, enabling safe and effective modulation of causal atherogenic particles, thus ushering in a new era in lipid management (Graphical Abstract).
Graphical Abstract.
The future evolution of lipid-lowering therapies. The quest for new lipid-lowering therapies enabling less frequent administration is continuing. Outcome trials to show cardiovascular event reduction will determine their clinical application. ASO, antisense oligonucleotide; CV, cardiovascular; IPE, icosapent ethyl; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); MoAb, monoclonal antibodies; siRNA, small-interfering RNA.
The emergence of new targets for management of dyslipidaemia
In the past decades, advances in genetics, analytical techniques, and increased understanding of signalling molecules have uncovered a diversity of novel targetable mechanisms for lipid-lowering therapy.14,15 For example, such studies identified the genetic defect in autosomal dominant hypercholesterolaemia as gain of function of proprotein convertase subtilisin/kexin Type 9 (PCSK9).16–18
Mendelian randomization approaches can help to differentiate truly causal factors from biomarkers that merely associate with events. The application of Mendelian randomization methodology has validated traditional targets like hydroxymethylglutaryl coenzyme A (HMG-CoA, the enzyme that statins inhibit) and has also identified or buttressed new targets. Examples include NPC1L1, an intestinal transporter inhibited by ezetimibe, apolipoprotein C-III, angiopoietin like-protein (ANGPTL) 3 and 4, apolipoprotein V, and ATP citrate lyase.19–22 The addition of ezetimibe to simvastatin produced incremental LDL lowering and reduction in events in post-acute coronary syndrome patients in IMPROVE-IT.23
Advanced small-molecule, biological, or nucleic acid-based approaches to targeting these newly recognized mediators have undergone rapid development. Monoclonal antibodies and RNA therapeutics have become a reality in the modulation of lipid metabolism. The engineering of antisense RNA and small interfering RNAs (siRNAs) now permits the selective inhibition of the production of specific proteins. These technologies have set the stage for new therapeutic approaches aimed at hepatocytes, key cells in lipid metabolism. Conjugation of RNA therapeutics to a carbohydrate ligand with a terminal N-acetyl galactosamine (GalNAc) residue permits engagement of a hepatocyte-specific receptor that can direct drug delivery to these liver cells. This selective targeting can markedly reduce the dose of inhibitory RNAs and limit unwanted effects that plagued early generations of RNA therapeutics, including sometimes serious injection-site reactions.24 Some of these therapies have already entered testing in Phase 3 trials. Looking forward, the maturation of gene therapies and the recent gene-editing revolution offer exciting new possibilities to treat atherogenic lipoproteins.25 Such developments promise to advance ASVD management substantially.
Targeting low-density lipoprotein-cholesterol with new tools
Highly efficacious therapies can lower LDL-C. Statins, ezetimibe, or PCSK9 inhibition by monoclonal antibodies have all improved CV outcomes in randomized controlled trials (RCTs), and guidelines include their use.9 Yet, implementation of these tools in practice has lagged. A recent study in Europe showed that moderate dose statin monotherapy predominated as a mode of lipid-lowering therapy, with only 18% of very high-risk patients getting to goal.26 In addition to better implementation and adherence strategies, the quest continues for new therapies to lower LDL-C effectively. Most of the emphasis on novel treatments in atherosclerosis have focused on apoB-containing lipoproteins because of their proven causality in ASVD (Figures 1 and 2, and Table 1).
Table 1.
Efficacy, event reduction, and approval status of lipid-lowering therapies
| Class of agent | LDL reduction efficacy (%) | Event reduction | Approval status |
|---|---|---|---|
| Statins | 30–50 | + | + |
| Ezetimibe | 15–20 | + Combined with statin | + |
| PCSK9 inhibitors | 50–60 | + For MoAb combined with statin | + |
| Bempedoic acid | 17–25 | Outcome trial in progress | + |
This table summarizes the efficacy and approval status for different therapies that target LDL.
LDL, low-density lipoprotein.
The finding that PCSK9 regulates LDL homeostasis has provided new opportunities for therapeutic manipulation. PCSK9 acts as a chaperone that delivers the LDL receptor to the lysosome for degradation. PCSK9 reduction promotes recycling of LDL receptors to the cell surface, boosting LDL clearance. The discovery that loss of function mutations in PCSK9 lead to lifelong low LDL-C and decreased CV risk reinforced the development of targeted therapies to inhibit PCSK9.15 The human monoclonal antibodies evolocumab and alirocumab promote removal of PCSK9 from the circulation. This intervention decreases LDL-C levels by 60% even in statin-treated individuals. Furthermore, in two large, rigorous, RCTs (FOURIER and ODYSSEY OUTCOMES) that enrolled very high-risk patients who were already taking statins, treatment with evolocumab and alirocumab significantly reduced CV events.27,28 The cost and need for once or twice monthly injections have, however, hampered the widespread use of PCSK9 monoclonal antibodies, despite their high efficacy and excellent tolerability.
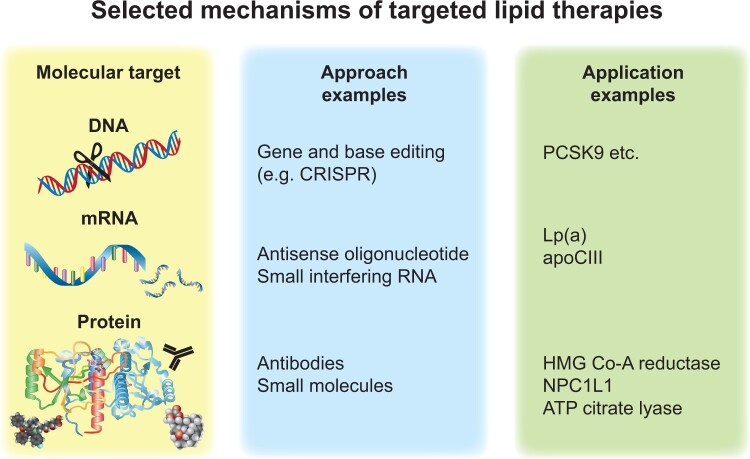
Alternative approaches to inhibit PCSK9 either by blocking function or interfering with expression are under development. Current trials are testing reduction of PCSK9 function with small molecules, mimetic peptides, adnectin, or vaccination as well as interfering with PCSK9 expression using antisense oligonucleotides, siRNA, or genome editing with CRISPR29 (Figure 3). PCSK9-specific gene silencing by siRNA with the agent inclisiran has seen rapid translation to clinical use. Such new approaches, if proven to decrease CV events in outcome trials, and if they demonstrate safety, may confer advantages over antibody therapies including improved durability, more convenient dosage regimens, and possibly cost-effectiveness.
Figure 3.
Selected mechanisms of targeted lipid therapies. Current approaches to interventions that modify lipid metabolism include targeting genomic DNA, messenger RNA, or proteins. The strategies available include various strategies ranging from traditional small-molecule medicinal chemistry approaches through biological agents such as monoclonal antibodies, RNA therapeutics, and, on the horizon, gene editing. ApoC-III, apolipoprotein C-III; Lp(a), lipoprotein(a); HMG-CoA, hydroxymethylglutaryl coenzyme A; PCSK9, proprotein convertase subtilisin/kexin type 9.
The monoclonal antibodies directed against PCSK9 neutralize the target extracellularly, while the approaches that target gene expression act intracellularly. These mechanistic differences could have clinical consequence. Human genetic studies have hinted that germline interference with PCSK9 expression might worsen glucose tolerance, an issue not seen in FOURIER or ODYSSEY OUTCOMES.30 This issue merits monitoring in the large-scale trials of anti-PCSK9 inhibitors that act intracellularly.
Other efforts to develop PCSK9 inhibitors that act via different mechanisms are in very early stages. Studies of permanent gene-editing have begun for two targets: PCSK9 and ANGPTL3. A recent study has shown in primates that a single infusion of CRISPR base editor delivered by nanoparticles reduces PCSK9 production in the liver and lowers LDL-C by 60% for 8 months after a single treatment.31 The use of gene-editing methods in the clinic requires resolution of ethical and safety issues.
Inclisiran
The anti-PCSK9 siRNA agent inclisiran delivered preferentially to hepatocytes via GalNAc targeting can be injected only twice or even once a year. It reduces the hepatic synthesis of PCSK9.32 Inclisiran has a modified nucleic acid backbone that limits its breakdown. One molecule of this siRNA can direct the degradation of multiple mRNA copies serially, producing a durable effect of 3–6 months when administered subcutaneously. When tested in a Phase 2 long-term safety and efficacy trial (ORION-1), inclisiran reduced LDL-C levels up to 53% at 6 months in subjects on the maximum dose of a statin with or without additional lipid-lowering therapy.33 Except for predominantly mild injection-site reactions occurring in 5% of the inclisiran group, the incidence of adverse events did not differ significantly from placebo during the period of observation.
These findings have received support from two Phase 3 trials that enrolled patients with atherosclerotic CV disease (ORION-10) and patients with atherosclerotic CV disease or an equivalent risk (ORION-11), with elevated LDL-C levels despite maximum tolerated dose statin therapy randomized to inclisiran or placebo.34 In these trials, inclisiran, administered subcutaneously every 6 months, reduced LDL-C by ∼ 50% and lowered non-HDL-C, apoB, triglycerides, and lipoprotein(a) [Lp(a)] significantly. The treatment did not alter liver or kidney function, creatine kinase, high-sensitivity C-reactive protein (hsCRP), or platelet count compared with placebo. Current studies with inclisiran cover different clinical settings such as homozygous familial hypercholesterolaemia, hepatic or renal impairment, ASVD, and healthy volunteers. An ongoing randomized, double-blind, placebo-controlled trial (ORION-4, NCT03705234) is examining the effects of inclisiran on CV morbidity and mortality. Recent reports show that antidotes can reverse siRNA-mediated gene silencing. Oligonucleotides that target the RNA-induced silencing complex that mediates siRNA inhibition of gene expression can inactivate this apparatus in vivo, thus providing an ‘antidote’ to the usually prolonged action of the siRNA blockade of PCSK9 expression.35
The siRNA attack on PCSK9 may offer prolonged duration of action, more convenient dosage regimens, and possibly cost-effectiveness if proven safe and effective in decreasing CV events. European and U.S. regulators have approved the use of inclisiran for the treatment of certain adults with hypercholesterolaemia or mixed dyslipidaemia. Because of consistent and durable LDL-C lowering, inclisiran may increase adherence to lipid-lowering therapy and help more patients to attain and ultimately maintain LDL-C goals.
Bempedoic acid
Bempedoic acid is a small molecule with a novel mechanism of action. It blocks ATP-citrate lyase (ACL), a cytosolic enzyme upstream of HMG-CoA in the pathway for de novo cholesterol synthesis.36 Bempedoic acid also activates adenosine monophosphate-activated protein kinase (AMPK), which regulates phosphorylation of substrates that affect inflammatory signalling and lipid metabolism.37 Earlier attempts to block ACL were hampered by low bioavailability and cell permeability but have been overcome with the current, once-daily oral formulation. Bempedoic acid is a prodrug that requires activity of very long-chain acyl-CoA synthetase-1 for conversion to an active modulator.38 The liver, but not most other tissues, contains this enzyme, minimizing the exposure of the active drug to muscle and other extra-hepatic tissues. By virtue of this selective activation in hepatocytes, bempedoic acid should minimize muscle-related side effects, although it acts on the same pathway as statins. Statins can cause muscle symptoms (although much less commonly than generally perceived),12 and very rarely can cause serious muscle issues including rhabdomyolysis, generally when combined with other agents that raise plasma statin concentrations (e.g. gemfibrozil). Observational studies report a 10–15% incidence of statin-associated muscle symptoms unlike the double-blind RCTs or blinded crossover studies that show lower levels of statin intolerance.39 Furthermore, recent trials have demonstrated that some of the perceived muscle symptoms may result from the nocebo effect.40
Clinical trials have shown that bempedoic acid monotherapy or its addition to background lipid-lowering therapy significantly lowers LDL-C, non-HDL-C, apoB, and hsCRP concentrations.41 A pooled analysis of 3623 patients with hypercholesterolaemia showed that bempedoic acid reduced LDL-C between 17% and 25% vs. placebo depending on the background use of statins.42 Studies have evaluated the LDL-lowering efficacy of bempedoic acid in patients with ASVD, heterozygous familial hypercholesterolaemia, and primary prevention using bempedoic acid either as monotherapy or against a background of different doses of statins and ezetimibe.
In an RCT in patients with atherosclerotic CV disease and/or heterozygous familial hypercholesterolaemia on maximally tolerated lipid-lowering therapy, compared with placebo, bempedoic acid treatment reduced LDL-C by 18%, non-HDL-C by 13.3%, apoB by 11.9%, and hsCRP by 21.5% (CLEAR Harmony).43 In patients with a history of statin intolerance requiring additional LDL-C lowering, bempedoic acid 180 mg once daily reduced LDL-C by 23.6% and hsCRP by 25.4% when added to ezetimibe with or without additional lipid-lowering therapy (CLEAR Serenity).44 A recent meta-analysis of 4391 patients and 11 RCTs of bempedoic acid showed that in addition to a reduction in LDL-C and hsCRP, composite CV outcomes and rates of new-onset or worsening diabetes also fell.45 A multicenter, randomized, double-blind, placebo-controlled trial (CLEAR Outcomes) is evaluating whether bempedoic acid (180 mg daily) can reduce the risk of CV morbidity and mortality in 14 014 patients with statin intolerance.46
That bempedoic acid and ezetimibe lower LDL-C by different mechanisms provides a compelling rationale for their combination. In a Phase 3 double-blind trial, a bempedoic acid/ezetimibe fixed-dose combination significantly lowered LDL-C by 38% vs. placebo with a favourable safety profile.47 A recent small, randomized, double-blind, Phase 2 study evaluated LDL-C lowering with the combination of bempedoic acid, ezetimibe, and atorvastatin (10 mg/day). This oral triple therapy lowered LDL-C levels up to 60.5% compared with placebo with good tolerability.48 The addition of bempedoic acid to an anti-PCSK9 antibody yielded an incremental drop in LDL of over 25%.49
Bempedoic acid treatment had rates of myalgia comparable to placebo, as documented in a pooled analysis of four Phase 3 studies.50 Bempedoic acid causes mild increases in blood urea nitrogen, creatinine, and uric acid and gout, effects that reverse after treatment cessation. The observed increases in creatinine and uric acid levels likely result from an effect of bempedoic acid on organic anion transporter 2, a renal transporter involved in excretion of creatinine and uric acid.51 Rare and mild reversible reductions in haemoglobin levels have associated with bempedoic acid. The mechanism is unknown, with no qualitative changes in red blood cells and no evidence that supports plasma dilution as a potential cause.50
Unlike the effects of statins on glycaemia, new-onset diabetes and hyperglycaemia occurred less frequently with bempedoic acid compared with placebo.50 This finding may relate to stimulation of AMPK by bempedoic acid which leads to reduction in gluconeogenesis. AMPK activation also inhibits fatty acid synthesis, an effect not shared by statins.36
The US Food and Drug Administration (FDA) approved bempedoic acid and the bempedoic acid/ezetimibe fixed-dose combination for the treatment of hypercholesterolaemia in adults with established CV disease or familial hypercholesterolaemia requiring additional LDL lowering despite maximally tolerated statin therapy. The European Medicines Agency has authorized its use for the treatment of hypercholesterolaemia and mixed dyslipidaemia. These new agents will complement existing lipid-modifying therapy regimens and will facilitate personalized treatment.
Is high-density lipoprotein modulation to mitigate cardiovascular events a lost cause?
Decades of observational epidemiologic data have documented a highly reproducible inverse relationship between HDL-C concentrations and CV outcomes.52 The heritable components of HDL concentrations depend primarily on small effect variants than on the rarer large effect mutations.53 Extensive pre-clinical literature documents the effects of HDL and its constituents that could mitigate CV risk. The beneficial effects ascribed to HDL include mediating an efflux of cholesterol from foam cells (reverse cholesterol transport), delivering it to hepatocytes via binding to scavenger receptor B1, endothelial protection, anti-oxidant actions, and combatting inflammation.54–57 Cholesterol derived from HDL can undergo conversion to bile acids by the hepatocyte and be eliminated in the faeces. Despite the plausibility of the reverse cholesterol transport hypothesis, and the consistent association of low HDL concentrations with poorer prognosis, accumulating human data suggest that high levels of HDL may actually worsen outcomes. Indeed, the relationship of HDL to mortality appears U-shaped, and higher strata of HDL may associate with increased risk of non-CV diseases including infections.58
At odds with the abundant observational epidemiologic literature and expansive experimental work, attempts to modulate atherosclerotic risk by raising HDL have met with considerable frustration.59 Nicotinic acid raises HDL and produces marked HDL raising and LDL lowering when combined with statins. Yet two well-conducted large-scale clinical trials, HPS2-THRIVE and AIM-HIGH, showed no clinical benefit.60,61 Indeed, in HPS2-THRIVE, the combination of nicotinic acid extended release and a prostaglandin D receptor inhibitor, laropiprant, not only failed to improve CV outcomes, but actually produced a number of unwanted actions.60
The inhibition of cholesterol ester transfer protein (CETP) raised great hopes that the robust elevation in HDL that they produce would improve CV outcomes. Yet several rigorous and well-powered studies revealed no clinical benefit of several CETP inhibitors. Indeed, torcetrapib caused hazard including increased mortality that led to premature termination of its Phase 3 trial.62 An outcome study with dalcetrapib yielded null results.63 A trial with evacetrapib halted prematurely for futility.64 The REVEAL trial with anacetrapib showed a very modest clinical benefit with prolonged follow-up, but this effect likely arose from a reduction in LDL rather an increase in HDL.65 The concept that high concentrations of HDL might deliver rather than remove free cholesterol to macrophages provides one potential contributor to the lack of benefit generally shown by CETP inhibitors.66 Qualitative alterations in HDL particles not captured in HDL-C measurements might also contribute to the disappointing results of trials of CETP inhibitors. Administration of various preparations of apolipoprotein A reconstituted in phospholipid particles likewise have not shown clinical benefit. A trial investigating this approach in individuals within 90 days of an acute coronary syndrome (AEGIS-II) is currently underway.67 In sum, multiple attempts to improve CV outcomes by raising HDL pharmacologically by several mechanisms have so far yielded consistent disappointment.
Some contemporary human genetic studies have cast doubt on the ability of HDL to limit CV risk. Mendelian randomization analyses have not shown significant benefits of increased HDL due to inheritance of various variants that raise its concentration.8 More recent Mendelian randomization analyses, taking pleiotropy into account, have provided evidence for some benefit from lifelong genetically-elevated increases in HDL-C concentration.68
Given the complexity of the HDL particle family and the mutable proteome associated with HDL particles, a simple measurement of HDL-C may obscure the potential benefits of particular classes of HDL or particles endowed with particular protein constituents.69,70 The exploration of therapeutic manipulation to augment the concentrations of particles with beneficial properties remains under investigation. Thus, while HDL has proven to be a frustrating therapeutic target thus far, some experts believe that exploiting the detailed knowledge of the proposed beneficial effects of HDL may yet provide a useful therapeutic avenue.66
Triglyceride-rich lipoproteins ascendant as an anti-atherosclerotic target
Triglyceride measurement in clinical laboratories serves a biomarker for a family of triglyceride-rich lipoproteins that include remnant lipoproteins, among them chylomicron remnants, VLDL, and IDL. The atherogenicity of these particles likely resides in their ability to deliver cholesterol to contribute to foam cell formation rather than on their triglyceride content.71–73 Remnant particles provoke inflammation, as gauged by hsCRP measurements, more potently than LDL itself, another property that may promote atherosclerotic events.74 In an apparently low-risk population enrolled in the PESA study, higher quantiles of triglycerides associated with augmented 18F-fluorodeoxyglucose uptake in several arterial beds, supporting triglycerides’ pro-inflammatory potential.75
Triglyceride concentrations in a population tend to vary inversely with HDL levels. Given the convincing observational epidemiologic evidence supporting a protective role of HDL, traditionally investigations of the contribution of triglycerides to CV risk have adjusted this lipoprotein fraction for HDL. This approach greatly attenuated the relation of triglyceride-rich lipoproteins with CV outcomes and led to relegating triglyceride-rich lipoproteins to a lower echelon as a causal CV risk factor. This situation underwent reassessment as evidence accumulated that raising HDL-C by several pharmacologic maneuvers does not improve CV outcomes, as detailed above.76 Triglyceride concentrations tracked very well with CV events and in long-term follow-up, with CV mortality.75,77
Human genetic studies have also renewed interest in the causal role of triglyceride-rich lipoproteins in atherosclerotic events.78–80 Triglyceride concentrations depend in large part on the activity of the enzyme lipoprotein lipase (LPL) that associates with the surface of microvascular endothelial cells (Figure 4). This enzyme releases free fatty acids from the triglycerides, reducing triglyceride concentrations. Genetic variants that lower LPL activity raise triglyceride-rich lipoprotein particle concentrations. Human genetic studies have shown a consistent increase in CV events in individuals who have inherited variants that boost the action of inhibitors of LPL including apolipoprotein C-III, and ANGPTL3 and 4.78,79,81 On the other hand, individuals with augmented activity of apolipoprotein A V, which increases LPL activity, have reduced CV risk.82 Mutations in LPL itself that impair its function also raise CV risk.80
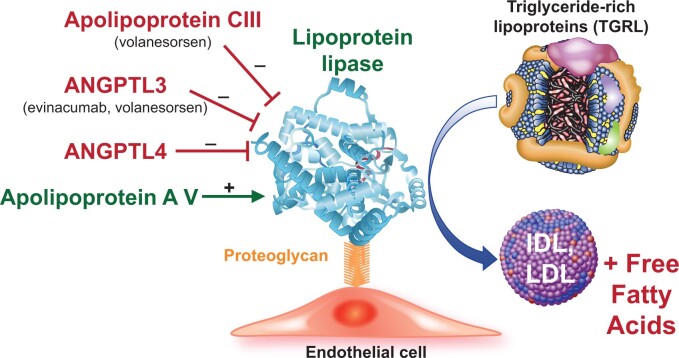
Figure 4.
Lipoprotein lipase modifiers. The enzyme lipoprotein lipase (depicted by the ribbon structure) associates with the surface of endothelial cells by binding to proteoglycans. This enzyme trims triglyceride from triglyceride-rich lipoproteins which include remnants of chylomicrons produced by intestinal cells from dietary lipid and very low-density lipoproteins synthesized endogenously by the liver. Lipoprotein lipase-mediated hydrolysis yields free fatty acids and low-density lipoprotein and intermediate-density lipoproteins. The proteins named in red inhibit lipoprotein lipase, and thus raise blood triglyceride-rich lipoprotein concentrations by limiting triglyceride-rich lipoprotein catabolism. The novel therapeutic agents listed inhibit these inhibitors and thus lower triglyceride-rich lipoprotein levels. Apolipoprotein AV activates lipoprotein lipase (shown in green.) Very strong human genetic evidence support the causality of each of the modulatory proteins depicted in regulating triglyceride-rich lipoproteins. ANGPTL, angiopoietin-like protein.
The reassessment of the causal role of triglyceride-rich lipoproteins in atherothrombosis has spurred the development of interventions that can reduce their concentration. Agents that reduce apolipoprotein C-III and inhibit ANGPTL3 and 4 are currently undergoing clinical evaluation for event reduction (Figure 4). These agents include vupanorsen, an antisense oligonucleotide (targeted to hepatocytes by a GalNAc moiety) that inhibits production of ANGPTL3.83 Evinacumab, a monoclonal antibody, neutralizes ANGPTL3.21 The LDL-lowering effects of ANGPTL3 do not depend on the LDL receptor but rather on VLDL/remnant receptors. In patients with homozygous familial hypercholesterolaemia who lack functioning LDL receptors, and thus respond poorly to agents that depend on raising LDL receptor levels, evinacumab reduced LDL-C by 49% in a Phase 3 trial.84 This result led to the FDA approval of evinacumab as an add-on treatment for adult and paediatric patients aged 12 and above with homozygous familial hypercholesterolaemia. Volanesorsen, an antisense oligonucleotide, targets apolipoprotein C-III, an inhibitor of LPL that elevates triglyceride-rich lipoproteins and may also exert independent proinflammatory effects. In patients with hyperchylomicronaemia, volanesorsen lowered triglycerides by over 70%, but caused injection-site reactions in almost a quarter of patients.85 A new formulation of antisense oligonucleotide with GalNAc-mediated targeting to hepatocytes limits unwanted actions such as injection-site reactions and thrombocytopaenia seen with earlier generations of RNA therapeutics.86,87
Fibric acid derivatives can also raise HDL and lower triglycerides. But fenofibrate has not demonstrated CV benefit in statin-treated patients. Fibric acid derivatives act by stimulating peroxisome proliferator-activated receptor-alpha (PPAR-α). A novel selective PPAR-α modulator, pemafibrate, lowers triglycerides, apolipoprotein C-III, and is currently under investigation in a large-scale clinical outcomes trial, PROMINENT.88,89 This trial, as opposed to most previous studies with PPAR-α stimulators, will target individuals with elevated baseline levels of triglycerides (>200 mg/dL). PROMINENT includes diabetic individuals both with or without established coronary artery disease.90
The increasing recognition of the causality of triglyceride-rich lipoproteins in atherosclerosis supports the recommendation in some guidelines to consider non-HDL-C a secondary target of therapy. In addition to LDL particles, the non-HDL compartment contains remnant cholesterol, now considered atherogenic. ApoB measurements capture all the atherogenic lipoprotein classes and correlate very well with non-HDL-C in a population, but may not be readily available in all practice settings.
Usual clinical practice seldom requires advanced lipid testing such as nuclear magnetic resonance assays, gradient gel electrophoresis, or ultracentrifugation, although their use in selected patients may be appropriate in specialized lipidology clinics.
Lipoprotein(a), a causal risk factor for atherosclerotic events and aortic valve disease: new therapies on the horizon
Lipoprotein(a) denotes a special form of LDL to which an apoprotein known as Apo(a) (unrelated to apolipoprotein A) has bound covalently to the signature protein apoB that encircles the LDL particle (Figure 1). Multiple observational epidemiologic studies showed an association of elevated Lp(a) with increased CV risk. Human genetic studies including GWAS and Mendelian randomization investigations have established the causality of Lp(a) not only in atherosclerotic CV disease but also in calcific aortic valve disease.91–94
The prevalence of elevated Lp(a) does not follow a bell-shaped Gaussian curve, but rather a skewed distribution. Most individuals have normal levels but there is a long tail of individuals with higher concentrations of Lp(a). Heredity strongly influences Lp(a) concentrations and populations of different ethnicities have distinct distributions and concentrations of Lp(a). African Americans have higher mean Lp(a) concentrations and a bell-shaped distribution.95
Measurement and reporting of Lp(a) concentrations has proven daunting. Apo(a) varies in structure depending on inherited genotype. In particular, a looped motif in the secondary structure of Apo(a), known as a kringle (based on its shapes’ resemblance to the eponymous Danish pastry), can vary in number quite widely in individuals due to different repeats of the fourth of these kringle domains. Those with fewer kringle IV repeats have higher Apo(a) blood concentrations. The variable structure from person-to-person in Lp(a) has rendered immunoassays very confusing. As the molecular weight of different versions of Lp(a) varies depending on the length of the Apo(a), reporting the concentrations as mass (mg/dL) can be misleading, prompting the current recommendation to report concentrations of Lp(a) in millimoles, obviating the differences in molecular weight. Yet, many clinical laboratories still report in mg/dL. As LDL or apoB measurements incorporate Lp(a), what we read in laboratory reports as LDL includes Lp(a). Various formulas are available for adjusting the LDL concentrations for the fraction contributed by Lp(a). As a person’s Lp(a) changes little over time, some current guidelines suggest, and we advocate, measurement of Lp(a) one time in all individuals. Certainly, those with unexplained premature atherosclerotic events, coincident calcific aortic stenosis, those with a family history of elevated Lp(a) or premature CV disease, and patients with suboptimal LDL-C-lowering response to statins merit assessment of Lp(a) concentrations.
Unfortunately, Lp(a) has proven a difficult therapeutic target.96 The usual panel of pharmacologic agents that lower LDL have little or no effect on Lp(a), with the exception of anti-PCSK9 antibodies that modestly reduce Lp(a). Recent advances in RNA technology have led to the development of hepatocyte-targeted antisense oligonucleotides or siRNA agents that can lower Lp(a) concentrations strikingly.93,97 The anti-sense oligonucleotide pelacarsen lowers Lp(a) independent of isoform size or genetic variant.98 An siRNA agent, olpasiran, likewise can decrease Lp(a). Current clinical trials with these novel therapeutics provide optimism that we will have in hand effective therapeutics for this causal CV risk factor. Given the ageing of the population and the concomitant increase in calcific aortic valve disease, exploring the ability of Lp(a) lowering to prevent or slow the progression of aortic sclerosis/stenosis in those with Lp(a) elevations likewise merits testing.
Shining a new light on omega-3 fatty acids: recent learnings
Since pioneering observational studies in populations with high fish consumption showing associations with reduced CV risk, interest has focused on the hypothesis that marine fatty acids, notably the omega-3 polyunsaturated fatty acids, might mitigate CV risk. Some intervention studies suggested that high consumption of omega-3 fatty acids, either as fish or supplements, could reduce sudden cardiac death, presumably due to limiting lethal ventricular arrhythmias. The GISSI–Prevenzione study suggested a slight benefit of omega-3 fatty acid supplementation on heart failure outcomes.99 Yet, multiple randomized placebo-controlled clinical trials failed to show a consistent decrease in events in individuals allocated to various omega-3 fatty acid preparations.100 The non-blinded JELIS study in Japanese individuals, which used a higher dose than most other studies [1.5 g/day of purified eicosapentaenoic acid (EPA)], did show a significant reduction in the primary endpoint.101
Recent large-scale trials have yielded mixed results. VITAL, which used 1 g/day of a mixture of EPA and docosahexaenoic acid (DHA), did not meet its primary endpoint.102 The ASCEND study of diabetic people without known CV disease tested a similar EPA + DHA mixture (1 g/day) and also yielded a null result.103 STRENGTH used a higher dose (4 g/day) of a mixture of EPA and DHA as free fatty acids, but halted prematurely for futility.104
REDUCE-IT, however, reported a striking reduction in first and total CV events in a study of some 20 000 individuals randomly allocated to 4 g/day of purified EPA as ethyl esters (icosapent ethyl).105 The treatment was well tolerated save for a small but significant signal for atrial fibrillation. The placebo used in ASCEND was olive oil, STRENGTH used corn oil, while REDUCE-IT used mineral oil. The choice of comparators in these studies has generated considerable controversy. In some but not all studies, mineral oil placebo has led to small increases in LDL-C, triglycerides, and hsCRP but did not increase plaque progression by computed tomography angiography .106
The difference in outcomes in these two superbly designed and conducted studies with disparate results could be due to differences in the omega-3 fatty acid preparation employed or possibly differences in the placebo. EPA and DHA have differential effects on endothelial function, cellular membranes, inflammation, and LDL-C. EPA and DHA differentially modulate membrane elasticity in the presence of cholesterol.107 Potential detrimental effects of DHA could explain the discrepant results, although increases in DHA levels were modest and did not correlate with events in STRENGTH.104,108
Considerable confusion currently reigns regarding the CV effects of omega-3 fatty acids given the disparate results from REDUCE-IT and STRENGTH. Each of these rigorous studies enrolled individuals with similar elevated estimates of CV risk. The US FDA has discounted the mineral oil vs. corn oil comparator issue as a major contributor to the differences between these two studies.109 The plethora of null trials with omega-3 fatty acids have used mixtures that used lower doses or that contain varying amounts of EPA and DHA, and in the case of many non-prescription products, undefined other components that may include saturated fat and oxidized lipids.100 The two trials that have shown positive effects on reducing CV events have used purified EPA in higher dose (REDUCE-IT and JELIS). While the achieved EPA concentrations did not correlate with events in an analysis of STRENGTH,110 they did so in REDUCE-IT. The median achieved levels of EPA in REDUCE-IT (169 µg/mL) were much higher than those in STRENGTH (90 µg/mL) that used the mixture of EPA with DHA. While this controversial area remains unsettled, the preponderance of the data indicates that purified prescription-grade EPA, as ethyl esters, exerts a beneficial effect, while mixtures of omega-3 fatty acids do not. DHA could counter some of the beneficial effects of EPA. That said, DHA is enriched in the retina and central nervous system and may play important protective roles there.
In both REDUCE-IT and STRENGTH, atrial fibrillation increased in the groups treated with omega-3 fatty acids. In REDUCE-IT, much of the atrial fibrillation documented was recurrent rather than new. Yet, in REDUCE-IT, there was no increase in ischaemic strokes. The net benefit on primary and total events in REDUCE-IT suggest an overall protective effect in the study population of high-dose prescription EPA despite the significant increase in atrial fibrillation. While these issues merit further discussion and investigation, icosapent ethyl has received approval in many jurisdictions for reducing CV events in individuals who meet the entry criteria for REDUCE-IT. A further analysis from this trial showed that the benefits of icosapent ethyl increased along with the risk level. The absolute risk reduction was 4% in patients with diabetes without CV disease, and 6% in patients with CV disease without diabetes. The highest benefit with a 10% absolute risk reduction was seen in patients with diabetes and established CV disease.111
Although some of the benefit observed in REDUCE-IT might have accrued due to a reduction in triglyceride-rich lipoproteins, there was no heterogeneity in event reduction in individuals with different tertiles of baseline triglyceride concentrations. While the mechanisms of the benefit observed in REDUCE-IT remain uncertain and are probably multiple, the success of REDUCE-IT provides us with a new tool for CV risk reduction, and should stimulate further exploration of icosapent ethyl as CV therapeutics.112
New tools permit individual tailoring of therapies
We are entering a new era in lipid lowering (Figure 5). Efforts to personalize therapy and target the right patient at the right time include further refinement of risk stratification tools including genetic risk scores and the integration of imaging studies to management decisions.113,114 Lifestyle measures include a healthy diet, weight control, and incorporation of physical activity into daily life to the fullest extent possible. Unfortunately, such behaviours alone often do not suffice to achieve control of lipid risk. Many of our patients have physical or financial limitations that deter them from achieving an ideal cardioprotective lifestyle.
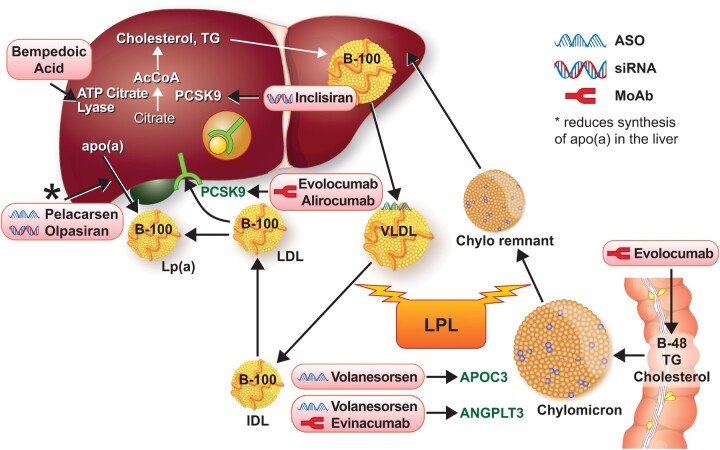
Figure 5.
Newer and emerging lipid-lowering therapies target different aspects of lipid metabolism. The statins target hydroxymethylglutaryl coenzyme A reductase. The newer and emerging agents target other aspects of lipid metabolism as shown here. B48 refers to the shorter form of apolipoprotein B produced by RNA editing in the intestine. B100 refers to the longer form produced in the liver. See the list for explanations of other abbreviations.
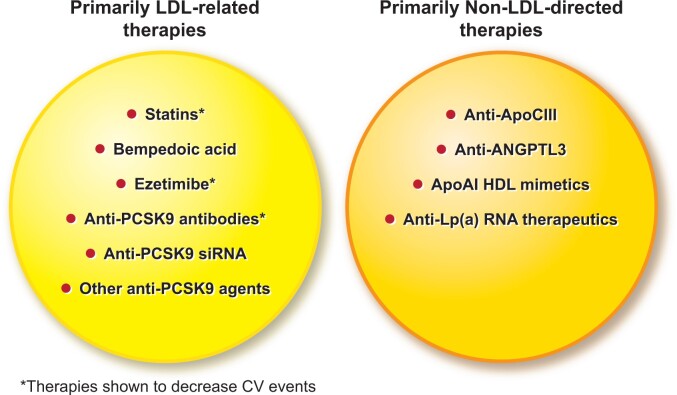
Fortunately, we have in hand or close at hand a flourishing armamentarium of lipid-lowering therapies that target new pathways and causal lipoproteins beyond LDL (Figure 6). While statins remain the first choice for lipid lowering, the availability of complementary therapies allow for individual tailoring according to the needs of the patient if the CV outcome trials with the novel therapies yield favourable results. Implementation of evidence-based lipid management remains inconsistent, requiring education of both physicians and patients as well as consideration of nuanced behavioural interventions. As a community, we face the additional challenge of achieving equitable distribution of and access to the proven and novel therapies to address dyslipidaemias for all segments of society to confront the continued and growing and now global epidemic of atherosclerotic CV disease.
Figure 6.
Current and emerging therapies not only deepen our ability to manage low-density lipoprotein, but to target other aspects of lipid risk factors. See text for explanation.
Funding
P.L. receives funding support from the National Heart, Lung, and Blood Institute (1R01HL134892), the American Heart Association (18CSA34080399), the Fondation Leducq, the RRM Charitable Fund, and the Simard Fund.
Contributor Information
Lale Tokgözoğlu, Department of Cardiology, Hacettepe University Faculty of Medicine, Sıhhiye, Ankara 06100, Turkey.
Peter Libby, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 77 Avenue Louis Pasteur, Boston, MA 02115,USA.
Data Availability
No new data were generated or analysed in support of this research.
References
- 1. Borén J, Chapman MJ, Krauss RMet al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2020;41:2313–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Libby P, Buring JE, Badimon Let al. Atherosclerosis. Nat Rev Dis Primers 2019;5:56. [DOI] [PubMed] [Google Scholar]
- 3. Balling M, Nordestgaard BG, Langsted A, Varbo A, Kamstrup PR, Afzal S. Small dense low-density lipoprotein cholesterol predicts atherosclerotic cardiovascular disease in the Copenhagen General Population Study. J Am Coll Cardiol 2020;75:2873–2875. [DOI] [PubMed] [Google Scholar]
- 4. Laufs U, Parhofer KG, Ginsberg HN, Hegele RA. Clinical review on triglycerides. Eur Heart J 2020;41:99c–109c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ginsberg HN, Packard CJ, Chapman MJet al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies—a consensus statement from the European Atherosclerosis Society. Eur Heart J; doi:10.1093/eurheartj/ehab551. Published online ahead of print 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johannesen CDL, Mortensen MB, Langsted A, Nordestgaard BG. Apolipoprotein B and non-HDL cholesterol better reflect residual risk than LDL cholesterol in statin-treated patients. J Am Coll Cardiol 2021;77:1439–1450. [DOI] [PubMed] [Google Scholar]
- 7. Ference BA, Ginsberg HN, Graham Iet al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emerging Risk Factors C, Di Angelantonio E, Gao P, et al. Lipid-related markers and cardiovascular disease prediction. JAMA 2012;307:2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mach F, Baigent C, Catapano ALet al. ESC Scientific Document Group . 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 10. Giugliano RP, Mach F, Zavitz Ket al. Cognitive function in a randomized trial of evolocumab. N Engl J Med 2017;377:633–643. [DOI] [PubMed] [Google Scholar]
- 11. Ray KK, Ginsberg HN, Davidson MHet al. Reductions in atherogenic lipids and major cardiovascular events. Circulation 2016;134:1931–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collins R, Reith C, Emberson Jet al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–2561. [DOI] [PubMed] [Google Scholar]
- 13. Lobo LM, Molinero G, Masson Wet al. Non-statin lipid-lowering therapy in coronary atherosclerosis regression: a meta-analysis and meta-regression. Eur Heart J 2020;41:2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kathiresan S; Myocardial Infarction Genetics Consortium . A PCSK9 missense variant associated with a reduced risk of early-onset myocardial infarction. N Engl J Med 2008;358:2299–2300. [DOI] [PubMed] [Google Scholar]
- 15. Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–1272. [DOI] [PubMed] [Google Scholar]
- 16. Abifadel M, Varret M, Rabes JPet al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003;34:154–156. [DOI] [PubMed] [Google Scholar]
- 17. Benjannet S, Hamelin J, Chrétien M, Seidah NG. Loss- and gain-of-function PCSK9 variants: cleavage specificity, dominant negative effects, and low density lipoprotein receptor (LDLR) degradation. J Biol Chem 2012;287:33745–33755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tan YD, Xiao P, Guda C. In-depth Mendelian randomization analysis of causal factors for coronary artery disease. Sci Rep 2020;10:9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ference BA, Majeed F, Penumetcha R, Flack JM, Brook RD. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 x 2 factorial Mendelian randomization study. J Am Coll Cardiol 2015;65:1552–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hegele RA, Tsimikas S. Lipid-lowering agents. Circ Res 2019;124:386–404. [DOI] [PubMed] [Google Scholar]
- 21. Dewey FE, Gusarova V, Dunbar RLet al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med 2017;377:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ference BA. Mendelian randomization studies: using naturally randomized genetic data to fill evidence gaps. Curr Opin Lipidol 2015;26:566–571. [DOI] [PubMed] [Google Scholar]
- 23. Cannon CP, Blazing MA, Giugliano RPet al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 24. Dowdy SF. Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol 2017;35:222–229. [DOI] [PubMed] [Google Scholar]
- 25. Landmesser U, Poller W, Tsimikas S, Most P, Paneni F, Lüscher TF. From traditional pharmacological towards nucleic acid-based therapies for cardiovascular diseases. Eur Heart J 2020;41:3884–3899. [DOI] [PubMed] [Google Scholar]
- 26. Ray K, Molemans B, Schoonen Wet al. ; DA VINCI Study . EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI Study. Eur J Prev Cardiol 2021;28:1279–1289. [DOI] [PubMed] [Google Scholar]
- 27. Sabatine MS, Giugliano RP, Keech ACet al. ; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 28. Schwartz GG, Steg PG, Szarek Met al. ; ODYSSEY OUTCOMES Committees and Investigators . Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 29. Nishikido T, Ray KK. Non-antibody approaches to proprotein convertase subtilisin kexin 9 inhibition: siRNA, antisense oligonucleotides, adnectins, vaccination, and new attempts at small-molecule inhibitors based on new discoveries. Front Cardiovasc Med 2018;5:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ference BA, Robinson JG, Brook RDet al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 2016;375:2144–2153. [DOI] [PubMed] [Google Scholar]
- 31. Musunuru K, Chadwick AC, Mizoguchi Tet al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 2021;593:429–434. [DOI] [PubMed] [Google Scholar]
- 32. Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya Set al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet 2014;383:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ray KK, Stoekenbroek RM, Kallend Det al. Effect of an siRNA therapeutic targeting PCSK9 on atherogenic lipoproteins. Circulation 2018;138:1304–1316. [DOI] [PubMed] [Google Scholar]
- 34. Ray KK, Wright RS, Kallend Det al. ; ORION-10 and ORION-11 Investigators . Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020;382:1507–1519. [DOI] [PubMed] [Google Scholar]
- 35. Zlatev I, Castoreno A, Brown CRet al. Reversal of siRNA-mediated gene silencing in vivo. Nat Biotechnol 2018;36:509–511. [DOI] [PubMed] [Google Scholar]
- 36. Pinkosky SL, Filippov S, Srivastava RAet al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res 2013;54:134–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verberk SGS, Kuiper KL, Lauterbach MA, Latz E, Van den Bossche J. The multifaceted therapeutic value of targeting ATP-citrate lyase in atherosclerosis. Trends Mol Med 2021;27:1095–1105. [DOI] [PubMed] [Google Scholar]
- 38. Pinkosky SL, Newton RS, Day EAet al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat Commun 2016;7:13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO Study. Cardiovasc Drugs Ther 2005;19:403–414. [DOI] [PubMed] [Google Scholar]
- 40. Wood FA, Howard JP, Finegold JAet al. N-of-1 trial of a statin, placebo, or no treatment to assess side effects. N Engl J Med 2020;383:2182–2184. [DOI] [PubMed] [Google Scholar]
- 41. Ballantyne CM, Davidson MH, Macdougall DEet al. Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in patients with hypercholesterolemia: results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. J Am Coll Cardiol 2013;62:1154–1162. [DOI] [PubMed] [Google Scholar]
- 42. Banach M, Duell PB, Gotto AM Jret al. Association of bempedoic acid administration with atherogenic lipid levels in phase 3 randomized clinical trials of patients with hypercholesterolemia. JAMA Cardiol 2020;5:1124–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ray KK, Bays HE, Catapano ALet al. ; CLEAR Harmony Trial . Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019;380:1022–1032. [DOI] [PubMed] [Google Scholar]
- 44. Laufs U, Banach M, Mancini GBJet al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc 2019;8:e011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang X, Zhang Y, Tan Het al. Efficacy and safety of bempedoic acid for prevention of cardiovascular events and diabetes: a systematic review and meta-analysis. Cardiovas Diabetol 2020;19:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nicholls SJ, Lincoff AM, Bays HEet al. Rationale and design of the CLEAR-Outcomes trial: evaluating the effect of bempedoic acid on cardiovascular events in patients with statin intolerance. Am Heart J 2021;235:104–112. [DOI] [PubMed] [Google Scholar]
- 47. Ballantyne CM, Laufs U, Ray KKet al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol 2020;27:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rubino J, MacDougall DE, Sterling LR, Hanselman JC, Nicholls SJ. Combination of bempedoic acid, ezetimibe, and atorvastatin in patients with hypercholesterolemia: a randomized clinical trial. Atherosclerosis 2021;320:122–128. [DOI] [PubMed] [Google Scholar]
- 49. Rubino J, Macdougall DE, Sterling LR, Kelly SE, Mckenney JM, Lalwani ND. Lipid lowering with bempedoic acid added to a proprotein convertase subtilisin/kexin type 9 inhibitor therapy: a randomized, controlled trial. J Clin Lipidol 2021;15:593–601. doi: 10.1016/j.jacl.2021.05.002 [DOI] [PubMed] [Google Scholar]
- 50. Bays HE, Banach M, Catapano ALet al. Bempedoic acid safety analysis: pooled data from four phase 3 clinical trials. J Clin Lipidol 2020;14:649.e6–659.e6. [DOI] [PubMed] [Google Scholar]
- 51. Lepist EI, Zhang X, Hao Jet al. Contribution of the organic anion transporter OAT2 to the renal active tubular secretion of creatinine and mechanism for serum creatinine elevations caused by cobicistat. Kidney Int 2014;86:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rosenson RS, Brewer HB, Barter PJet al. HDL and atherosclerotic cardiovascular disease: genetic insights into complex biology. Nature Rev Cardiol 2018;15:9–19. [DOI] [PubMed] [Google Scholar]
- 53. Dron JS, Wang J, Low-Kam Cet al. Polygenic determinants in extremes of high-density lipoprotein cholesterol. J Lipid Res 2017;58:2162–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rye KA, Bursill CA, Lambert G, Tabet F, Barter PJ. The metabolism and anti-atherogenic properties of HDL. J Lipid Res 2009;50 Suppl:S195–S200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol 2011;8:222–232. [DOI] [PubMed] [Google Scholar]
- 56. Robert J, Osto E, Von Eckardstein A. The endothelium is both a target and a barrier of HDL’s protective functions. Cells 2021;10:1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rohatgi A, Khera A, Berry JDet al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 2014;371:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Madsen CM, Varbo A, Nordestgaard BG. Novel insights from human studies on the role of high-density lipoprotein in mortality and noncardiovascular disease. Arterioscler Thromb Vasc Biol 2021;41:128–140. [DOI] [PubMed] [Google Scholar]
- 59. Siddiqi HK, Kiss D, Rader D. HDL-cholesterol and cardiovascular disease: rethinking our approach. Curr Opin Cardiol 2015;30:536–542. [DOI] [PubMed] [Google Scholar]
- 60. Landray MJ, Haynes R, Hopewell JCet al. ; Hps2-Thrive Collaborative Group Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–212. [DOI] [PubMed] [Google Scholar]
- 61. Boden WE, Probstfield JL, Anderson Tet al. ; AIM-HIGH Investigators . Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 62. Barter PJ, Caulfield M, Eriksson Met al. ; ILLUMINATE Investigators . Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–2122. [DOI] [PubMed] [Google Scholar]
- 63. Schwartz GG, Olsson AG, Abt Met al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367:2089–2099. [DOI] [PubMed] [Google Scholar]
- 64. Lincoff AM, Nicholls SJ, Riesmeyer JSet al. ; ACCELERATE Investigators . Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med 2017;376:1933–1942. [DOI] [PubMed] [Google Scholar]
- 65. Bowman L, Hopewell JC, Chen Fet al. ; HPS3/TIMI55–REVEAL Collaborative Group . Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 2017;377:1217–1227. [DOI] [PubMed] [Google Scholar]
- 66. Pownall HJ, Rosales C, Gillard BK, Gotto AM Jr. High-density lipoproteins, reverse cholesterol transport and atherogenesis. Nat Rev Cardiol 2021;18:712–723. [DOI] [PubMed] [Google Scholar]
- 67. Gibson CM, Kastelein JJP, Phillips ATet al. Rationale and design of ApoA-I Event Reducing in Ischemic Syndromes II (AEGIS-II): a phase 3, multicenter, double-blind, randomized, placebo-controlled, parallel-group study to investigate the efficacy and safety of CSL112 in subjects after acute myocardial infarction. Am Heart J 2021;231:121–127. [DOI] [PubMed] [Google Scholar]
- 68. Thomas DG, Wei Y, Tall AR. Lipid and metabolic syndrome traits in coronary artery disease: a Mendelian randomization study. J Lipid Res 2021;62:100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mendivil CO, Furtado J, Morton AM, Wang L, Sacks FM. Novel pathways of apolipoprotein A-I metabolism in high-density lipoprotein of different sizes in humans. Arterioscler Thromb Vasc Biol 2016;36:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sacks FM, Jensen MK. From high-density lipoprotein cholesterol to measurements of function. Arterioscler Thromb Vasc Biol 2018;38:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014;384:626–635. [DOI] [PubMed] [Google Scholar]
- 72. Duran EK, Pradhan AD. Triglyceride-rich lipoprotein remnants and cardiovascular disease. Clin Chem 2021;67:183–196. [DOI] [PubMed] [Google Scholar]
- 73. Packard CJ, Boren J, Taskinen M-R. Causes and consequences of hypertriglyceridemia. Front Endocrinol (Lausanne) 2020;11:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hansen SEJ, Madsen CM, Varbo A, Nordestgaard BG. Low-grade inflammation in the association between mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis: a study of more than 115000 individuals from the general population. Clin Chem 2019;65:321–332. [DOI] [PubMed] [Google Scholar]
- 75. Raposeiras-Roubin S, Rosselló X, Oliva Bet al. Triglycerides and residual atherosclerotic risk. J Am Coll Cardiol 2021;77:3031–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Libby P. Triglycerides on the rise: should we swap seats on the seesaw? Eur Heart J 2015;36:774–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Klempfner R, Erez A, Sagit B-Zet al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease. Circ Cardiovasc Qual Outcomes 2016;9:100–108. [DOI] [PubMed] [Google Scholar]
- 78. Rosenson RS, Davidson MH, Hirsh BJ, Kathiresan S, Gaudet D. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J Am Coll Cardiol 2014;64:2525–2540. [DOI] [PubMed] [Google Scholar]
- 79. Musunuru K, Kathiresan S. Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circ Res 2016;118:579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Khera AV, Won HH, Peloso GMet al. ; Myocardial Infarction Genetics Consortium, DiscoverEHR Study Group, CARDIoGRAM Exome Consortium, and Global Lipids Genetics Consortium . Association of rare and common variation in the lipoprotein lipase gene with coronary artery disease. JAMA 2017;317:937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Stitziel NO, Khera AV, Wang Xet al. ; PROMIS and Myocardial Infarction Genetics Consortium Investigators . ANGPTL3 deficiency and protection against coronary artery disease. J Am Coll Cardiol 2017;69:2054–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Grosskopf I, Baroukh N, Lee SJet al. Apolipoprotein A-V deficiency results in marked hypertriglyceridemia attributable to decreased lipolysis of triglyceride-rich lipoproteins and removal of their remnants. Arterioscler Thromb Vasc Biol 2005;25:2573–2579. [DOI] [PubMed] [Google Scholar]
- 83. Gaudet D, Karwatowska-Prokopczuk E, Baum SJet al. ; Vupanorsen Study Investigators . Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur Heart J 2020;41:3936–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rosenson RS, Burgess LJ, Ebenbichler CFet al. Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med 2020;383:2307–2319. [DOI] [PubMed] [Google Scholar]
- 85. Gouni-Berthold I, Alexander VJ, Yang Qet al. ; COMPASS Study Group . Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2021;9:264–275. [DOI] [PubMed] [Google Scholar]
- 86. Qamar A, Libby P, Bhatt DL. Targeting RNA to lower triglycerides: long strides from short molecules. Eur Heart J 2019;40:2797–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Alexander VJ, Xia S, Hurh Eet al. N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels. Eur Heart J 2019;40:2785–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fruchart JC, Santos RD, Aguilar-Salinas Cet al. The selective peroxisome proliferator-activated receptor alpha modulator (SPPARMα) paradigm: conceptual framework and therapeutic potential. Cardiovasc Diabetol 2019;18:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pradhan AD, Paynter NP, Everett BMet al. Rationale and design of the pemafibrate to reduce cardiovascular outcomes by reducing triglycerides in patients with diabetes (PROMINENT) study. Am Heart J 2018;206:80–93. [DOI] [PubMed] [Google Scholar]
- 90. Fruchart JC, Santos RD, Yamashita S, Libby P;International Atherosclerosis Society/R3i Foundation Consensus Panel . Residual vascular risk in diabetes—will the SPPARM alpha concept hold the key? Diabetes Metab Syndr 2019;13:2723–2725. [DOI] [PubMed] [Google Scholar]
- 91. Tsimikas S, Fazio S, Ferdinand KCet al. ; NHLBI Working Group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol 2018;71:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gudbjartsson DF, Thorgeirsson G, Sulem Pet al. Lipoprotein(a) concentration and risks of cardiovascular disease and diabetes. J Am Coll Cardiol 2019;74:2982–2994. [DOI] [PubMed] [Google Scholar]
- 93. Tsimikas S. Potential causality and emerging medical therapies for lipoprotein(a) and its associated oxidized phospholipids in calcific aortic valve stenosis. Circ Res 2019;124:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Thanassoulis G, Campbell CY, Owens DSet al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med 2013;368:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Enkhmaa B, Anuurad E, Berglund L. Lipoprotein (a): impact by ethnicity and environmental and medical conditions. J Lipid Res 2016;57:1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Libby P. Lipoprotein (a)—a frustrating final frontier in lipid management? JACC Basic Transl Sci 2016;1:428–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stiekema LCA, Prange KHM, Hoogeveen RMet al. Potent lipoprotein(a) lowering following apolipoprotein(a) antisense treatment reduces the pro-inflammatory activation of circulating monocytes in patients with elevated lipoprotein(a). Eur Heart J 2020;41:2262–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Karwatowska-Prokopczuk E, Clouet-Foraison N, Xia Set al. Prevalence and influence of LPA gene variants and isoform size on the Lp(a)-lowering effect of pelacarsen. Atherosclerosis 2021;324:102–108. [DOI] [PubMed] [Google Scholar]
- 99. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 1999;354:447–455. [PubMed] [Google Scholar]
- 100. Aung T, Halsey J, Kromhout Det al. ; Omega-3 Treatment Trialists’ Collaboration . Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol 2018;3:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yokoyama M, Origasa H, Matsuzaki Met al. ; Japan EPA Lipid Intervention Study (JELIS) Investigators . Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–1098. [DOI] [PubMed] [Google Scholar]
- 102. Manson JE, Cook NR, Lee IMet al. ; VITAL Research Group . Marine n−3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bowman L, Mafham M, Wallendszus Ket al. ; ASCEND Study Collaborative Group . Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018;379:1540–1550. [DOI] [PubMed] [Google Scholar]
- 104. Nicholls SJ, Lincoff AM, Garcia Met al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk. JAMA 2020;324:2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bhatt DL, Steg PG, Miller Met al. REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 106. Lakshmanan S, Shekar C, Kinninger Aet al. Comparison of mineral oil and non-mineral oil placebo on coronary plaque progression by coronary computed tomography angiography. Cardiovasc Res 2020;116:479–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jacobs ML, Faizi HA, Peruzzi JA, Vlahovska PM, Kamat NP. EPA and DHA differentially modulate membrane elasticity in the presence of cholesterol. Biophys J 2021;120:2317–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. So J, Wu D, Lichtenstein AHet al. EPA and DHA differentially modulate monocyte inflammatory response in subjects with chronic inflammation in part via plasma specialized pro-resolving lipid mediators: a randomized, double-blind, crossover study. Atherosclerosis 2021;316:90–98. [DOI] [PubMed] [Google Scholar]
- 109. US Food & Drug Administration . Endocrinologic and Metabolic Drugs Advisory Committee. https://www.fda.gov/advisory-committees/human-drug-advisory-committees/endocrinologic-and-metabolic-drugs-advisory-committee (10 December 2021).
- 110. Nissen SE, Lincoff AM, Wolski Ket al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk: a secondary analysis of the STRENGTH trial. JAMA Cardiol 2021;6:910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bhatt DL, Brinton EA, Miller Met al. 4-LB: substantial cardiovascular benefit from icosapent ethyl in patients with diabetes: REDUCE-IT DIABETES. Diabetes 2020;69:4-LB. [Google Scholar]
- 112. Mason RP, Libby P, Bhatt Deepak L. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol 2020;40:1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mega JL, Stitziel NO, Smith JGet al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet 2015;385:2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Orringer CE, Blaha MJ, Blankstein Ret al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol 2021;15:33–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.