Summary
As COVID-19 cases exceed hundreds of millions globally, many survivors face cognitive challenges and prolonged symptoms. However, important questions about the cognitive effects of COVID-19 remain unresolved. In this cross-sectional online study, 478 adult volunteers who self-reported a positive test for COVID-19 (mean = 30 days since most recent test) perform significantly worse than pre-pandemic norms on cognitive measures of processing speed, reasoning, verbal, and overall performance, but not short-term memory, suggesting domain-specific deficits. Cognitive differences are even observed in participants who did not require hospitalization. Factor analysis of health- and COVID-related questionnaires reveals two clusters of symptoms—one that varies mostly with physical symptoms and illness severity, and one with mental health. Cognitive performance is positively correlated with the global measure encompassing physical symptoms, but not the one that broadly describes mental health, suggesting that the subjective experience of “long COVID” relates to physical symptoms and cognitive deficits, especially executive dysfunction.
Keywords: COVID-19, long COVID, cognition, mental health, physical health, cross-sectional online study
Graphical abstract
Highlights
-
•
Survivors of COVID-19 exhibit cognitive differences in specific domains
-
•
Speed of processing, verbal, and reasoning are affected, but not memory function
-
•
Performance in affected domains is linked to physical but not mental health
-
•
These effects are observed in mild and hospitalized cases of COVID-19
In 478 survivors of COVID-19 tested approximately 30 days after infection, Wild et al. show that cognition is affected in specific domains. Cognitive performance is positively correlated with physical but not mental health, suggesting that the subjective experience of “long COVID” consists of physical and cognitive, especially executive, symptoms.
Introduction
As the number of people recovering from the effects of coronavirus disease 2019 (COVID-19) infection continues to grow, it is becoming increasingly clear that many experience ongoing cognitive challenges, including problems with memory, attention, reasoning, and problem-solving.2 These issues could be caused by direct viral effects on the brain (e.g., neuroinflammation, stroke, autoimmune responses), as elevated cerebrospinal fluid autoantibodies and significant white matter changes have been reported in patients with neurological symptoms following infection with COVID-19,3, 4, 5, 6 as well as signs of microvascular damage.7 The indirect effects of infection may also be attributed to changes in cognition resulting from inflammation, blood clots, low oxygen levels, sedation, and ventilation. In a recent prospective study of mechanically ventilated critical illness survivors, we reported that all of the patients emerged from the intensive care unit (ICU) with cognitive impairments, regardless of their etiology at admission (sepsis, cardiac arrest, respiratory failure).8, 9, 10
Nevertheless, as the worldwide incidence rates of proven COVID-19 infections exceed 400 million, many questions of importance for post-COVID-19 treatment and recovery remain unanswered. First, are these cognitive deficits, where they occur, generalized or domain specific; that is, do they affect certain cognitive systems more than others, and, if so, which cognitive systems are most susceptible? This issue has gained import in recent months as poorly specified terms such as “brain fog” have entered both common parlance and the scientific literature describing “long COVID” or COVID “long-haulers.”11, 12, 13, 14 Unfortunately, the widespread use of “blunt” screening tools such as the Mini-Mental State Examination (MMSE15) and the Montreal Cognitive Assessment (MoCA16) to evaluate the effects of COVID-19 infection only adds to this confusion, as both were designed to detect the emergence of dementia in the elderly, rather than to provide a comprehensive picture of cognitive performance.17, 18, 19, 20, 21 For example, in one study, 28% of recovered COVID-19 patients scored below the established cutoff of 26 (for dementia) on the MoCA, compared to only 17% of controls, although median MoCA scores in the patients were not statistically different from those of the controls.21 Other studies have suggested a specific domain of cognitive impairment; however, this has varied across studies from primary deficits in attention22 to visuospatial deficits.21 Most studies report multidomain cognitive impairments, although there appears to be a high degree of disagreement about which domains are most affected.2,17, 18, 19, 20,23, 24, 25, 26
Second, how does the emergence of cognitive deficits following COVID-19 infection relate to the severity of the primary infection? Several preliminary studies have suggested that, in patients who required hospitalized treatment, cognitive deficits following COVID-19 infection are dependent on the level of medical assistance received2,23 and the degree of inflammation,22 with severe infections being associated with significant cognitive deficits18,19,24 although cognitive impairments have also been reported in asymptomatic patients,17 and one small study reported no correlation with hospitalization and cognitive impairments.25 Given the current knowledge about longlasting cognitive deficits from post ICU syndrome, we may expect a worse cognitive outcome with more severe COVID-19 illness.8, 9, 10 Alternatively, perhaps the longstanding and untreated hypoxia reported in asymptomatic COVID-19 patients27,28 may lead to long lasting cognitive decline, as has been shown to be the case in other conditions that cause longstanding hypoxia.29,30
Third, how does the pattern of cognitive deficits observed following COVID-19 infection relate to aspects of mental health such as anxiety, depression, and fatigue that may be a consequence of the disease process itself, or a more general effect of living during the time of a global pandemic? Research conducted before the worldwide spread of COVID-19 clearly established that associations exist between impaired cognitive function and both anxiety disorders31 and depression,32 although the relationship between fatigue and specific cognitive deficits seems rather less clear.33,34 Several recent studies have reported an increased risk of psychiatric disturbance in patients recovering from COVID-19,21,35, 36, 37 although others have reported no association between cognitive outcomes and psychiatric symptoms,26 including anxiety,18,25 depression,18,25 and fatigue.25
To fully address these questions, large numbers of patients need to be examined to mitigate the effects of infection severity, stage of recovery, and concomitant mental health issues. To date, most studies have focused on single-case reports or relatively small (<25) cohorts,18, 19, 20,24,25,38 confounding these issues. With many countries in virtual lockdown, opportunities for face-to-face testing are limited, resulting in the widespread use of telephone screening and/or self-reported cognitive status, which have obvious limitations.24,25,38
In this study, we report data from a cohort of 478 patients who self-reported having had a positive COVID-19 test, who were assessed using a comprehensive and widely validated battery of cognitive tests that measures aspects of memory, attention, problem-solving and reasoning. Every patient also completed a questionnaire to fully document his or her COVID-19 experience, infection severity, extent of recovery, and physical, mental, and emotional health status. Here, we report the results of this initial assessment, conducted in the first few months following a positive COVID-19 test.
Results
Participants
Of the 478 volunteers in the COVID+ group (mean age = 42.6 years, 70% female), 66 required treatments in a hospital for their illness, and of those, 34 required supplemental oxygen therapy, 17 were in the ICU, and 11 were on a ventilator (Table 1). Patients who were not hospitalized (N = 412) were asked about their daily functioning, and 283 of them reported being negatively affected by their illness. A COVID severity score, based on the World Health Organization’s 8-point ordinal scale of COVID-19 severity,39 was assigned to each participant based on their responses to these questions; scores ranged from unaffected (score = 0) to hospitalized with severe disease (score = 6; Figure S1; Table S1). Participants also indicated the year and month of their most recent positive test for COVID-19 to approximate the elapsed time since infection (mean = 3 months, SD = 2 months). Also, 159 (33%) and 151 (31.6%) of participants met the criterion for a suspected generalized anxiety disorder (i.e., GAD2 ≥ 3) or major depressive disorder (i.e., PHQ2 ≥3 [PHQ, Patient Health Questionnaire]). A total of 112 participants (23%) indicated having ≥1 preexisting medical conditions before COVID-19: diabetes (N = 23; 5%), obesity (N = 80; 17%), hypertension (N = 47; 10%), stroke (N = 4; <1%), heart attack (N = 1; <1%), or memory problems (N = 2; <1%). A large normative dataset (N = 7,832) collected before the COVID-19 pandemic was used as a comparative baseline group and to adjust for known confounding socio-demographic variables. Details about the COVID+ cohort and the pre-pandemic norms are provided in Table 2.
Table 1.
Responses to questions about COVID-19 illness
| Question | Yes | No | Don’t know | No. of responses |
|---|---|---|---|---|
| Symptoms | 450 (94.1%) | 28 (5.9%) | – | 478 (100%) |
| Selected at least one of “cough,” “fever,” “difficulty breathing,” “pneumonia,” or “loss of smell” | ||||
| Hospital | 66 (13.8%) | 412 (86.2%) | – | 478 (100%) |
| “Did you have to stay in the hospital for COVID-19 symptoms?” | ||||
| Daily routine | 129 (27.0%) | 283 (59.2%) | – | 412 (86.2%) |
| “Were you mostly able to go about your daily routine (e.g., mostly normal sleep, work, activity)?” | ||||
| Supplemental O2 | 34 (7.1%) | 31 (6.5%) | 1 (0.2%) | 66 (13.8%) |
| “Did you require supplemental oxygenation (e.g., with a mask or nasal prongs) while in the hospital? (this does not include being on a ventilator)” | ||||
| Intensive care | 17 (3.6%) | 49 (10.3%) | – | 66 (13.8%) |
| “Were you in the ICU (intensive care unit)?” | ||||
| Ventilator | 11 (2.3%) | 6 (1.3%) | – | 17 (3.6%) |
| “Were you on a ventilator (breathing machine)?” | ||||
Italicized text presents the questionnaire wording, and percentages are relative to the total COVID+ sample (N = 478). Possible responses to each question, except the checklist of symptoms, included “Yes,” “No,” and “I don’t know.”
Table 2.
Descriptive statistics for the COVID+ group and normative data (norms)
| COVID+, N = 478 (%) | Norms, N = 7,832 (%) | |||
|---|---|---|---|---|
| Age, y | mean = 42.6, SD = 14.5 | mean = 43.2, SD = 13.0 | ||
| Female gender | 336 (70%) | 4,857 (62%) | ||
| Completed some post-secondary education | 394 (82%) | 5,917 (76%) | ||
| Grew up “At or above poverty level” | 443 (93%) | 7,312 (93%) | ||
| Languages spoken at home | ||||
| English | 368 (77%) | 6,387 (82%) | ||
| French | 45 (9%) | 485 (6%) | ||
| Spanish | 23 (5%) | 176 (2%) | ||
| Location (top 5 countries) | ||||
| 1 | USA | 202 (42.3%) | Canada | 2,453 (31.3%) |
| 2 | Canada | 143 (29.9%) | UK | 2,420 (30.9%) |
| 3 | UK | 42 (8.8%) | USA | 607 (7.8%) |
| 4 | Spain | 11 (2.3%) | Portugal | 382 (4.9%) |
| 5 | Germany | 7 (1.5%) | Australia | 128 (1.6%) |
| Exercises at least once per week | 335 (70%) | 4,777 (61%) | ||
| ≥1 units of nicotine per day | 40 (8%) | 717 (9%) | ||
| >7 units of alcohol per week | 17 (4%) | 541 (7%) | ||
| ≥1 units of cannabis per day | 51 (11%) | 553 (7%) | ||
| Other stimulantsa | 7 (1%) | 90 (1%) | ||
| Other depressantsa | 4 (1%) | 44 (1%) |
SD, standard deviation; UK, United Kingdom.
Consumed at least once in the past 4 weeks.
Domain specificity of the cognitive profile associated with COVID-19
To examine the effects of COVID-19 infection on cognitive functioning and the domains affected, we compared the COVID+ cohort to normative data on 5 composite scores (i.e., domains) of cognitive performance, instead of testing each of the 12 cognitive tasks separately. Three of these measures were derived from a principal-components analysis of the 12 cognitive test scores. Replicating our previous work,40 these three components could generally be described as representing cognitive performance in three separate domains: visuospatial short-term memory (STM), reasoning, and verbal domains (Figure 1A). Similar factor solutions were produced from the cognitive test scores in the normative and COVID+ samples (see Figure S2 and Table S3). The remaining two composite scores were overall performance across the cognitive task battery and average processing speed (i.e., a reaction-time based measure across all tests). Before the statistical analysis, all of the cognitive scores were corrected for socio-demographic confounding variables (e.g., age, gender, level of education, socioeconomic status [SES], drug use, exercise) using the parameters estimated from the normative data.
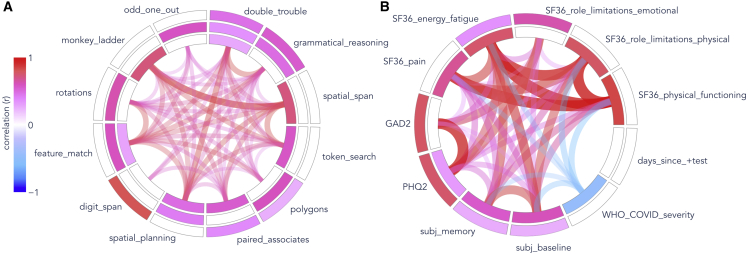
Figure 1.
Factor analyses of cognitive test scores and health-related measures
(A) Twelve cognitive test scores from normative data (N = 7,832) and (B) health-related measures in COVID+ participants (N = 478). Concentric rings represent factors, ordered by decreasing variance explained from inside to outside. Colored cells show the loadings of observed variables on each factor. Curves connecting observed variables indicate pairwise correlations. Pink indicates positive relationships, whereas blues indicate negative correlations. (A) 3 (of 5) composite cognitive scores analyzed in this study were derived from a factor analysis of 12 cognitive tasks: STM (inner ring), reasoning (center), and verbal (outer) domains.
(B) Two factors explained health-related questionnaire variables: overall physical health, including COVID severity (F1; inner ring), and mental health and wellness (F2; outer ring). Note that the GAD2 and PHQ2 scales were reversed to make higher scores correspond to better health, like other measures (except disease severity).
Two-sample tests between the COVID+ and normative groups revealed significant differences in the reasoning and verbal domains, and on the measures of processing speed and overall cognitive performance (all adjusted p < 0.001; Table 3), such that COVID+ participants’ scores were, on average, lower than the norms by 0.20, 0.18, 0.29, and 0.16 SDs, respectively, on these measures. The one exception was STM performance, which did not differ between groups.
Table 3.
Results of comparisons between COVID+ and normative groups on 5 composite cognitive scores
| Score | Difference | t | Df | padj | CI |
|---|---|---|---|---|---|
| STM | 0.06 | 1.38 | 543.80 | 1.000 | (−0.064 to 0.177) |
| Reasoning | −0.20 | −4.26 | 530.10 | <0.001 | (−0.334 to −0.061) |
| Verbal | −0.18 | −4.07 | 540.49 | <0.001 | (−0.318 to −0.051) |
| Processing speed | −0.29 | −6.67 | 527.97 | <0.001 | (−0.423 to −0.164) |
| Overall | −0.16 | −3.96 | 532.52 | 0.001 | (−0.284 to −0.041) |
The cognitive measures were adjusted for nuisance covariates. The mean difference is between groups, COVID+ versus norms, and measured in units of standard deviations (i.e., analogous to Cohen’s d). p values and confidence intervals are Bonferroni corrected for 15 comparisons. The bold entries indicate significant effects (padj < 0.05). t, t statistic; df, degrees of freedom; padj, adjusted p value; CI, confidence interval; STM, short-term memory.
Dissociable health factors associated with COVID-19 infection
Strong correlations were observed between health-related questionnaire variables from the COVID+ group (Figure 1B). Bartlett’s test of sphericity (χ2(45) = 2,168, p < 0.001) and a Kaiser-Meyer-Olkin value of 0.87 confirmed that the multivariate data were factorable. A factor analysis revealed a two-factor structure (i.e., two factors had eigenvalues >1.0), which we interpreted as broadly representing overall physical health, including COVID severity (henceforth referred to as F1; Figure 1B, inner ring), and mental health and wellness (henceforth referred to as F2; Figure 1B, outer ring). Illness severity (WHO_COVID_severity) correlated most strongly (and negatively) with the SF-36 (36-Item Short Form Health Survey) measures of physical functioning, physical-role limitations, energy and fatigue, and pain scales, and the two subjective measures of cognition (Figure 1B). The approximate elapsed time since infection demonstrated negligible correlations with all of the measures. Descriptive statistics (mean and SD) of each health variable, along with their loadings on F1 and F2, can be found in Table S2.
The two health factors F1 and F2 were dissociable with respect to their relationships with demographic variables (Figure S3). Linear regression models (Table S4) showed that the overall F1 was negatively correlated with age (t(473) = −3.20, padj < 0.05, f2 = 0.022), with an average decline of approximately 0.1 SDs per decade, whereas F2 increased with age (t(473) = 2.82, padj < 0.05, f2 = 0.017) by 0.1 SDs per decade. Completion of post-secondary education was also associated with significantly better F2 (t(473) = 3.36, padj < 0.01, d = 0.40) but not F1, and males reported better F1 (t(473) = 5.91, padj < 0.001, d = 0.57), yet there was no difference between males and females in terms of F2. The fact that these measures were clearly dissociable in terms of the demographic variables that they correlated with suggests that they represent two distinct and separable, although not mutually exclusive, effects of COVID-19 infection.
Within-group associations between health and cognition
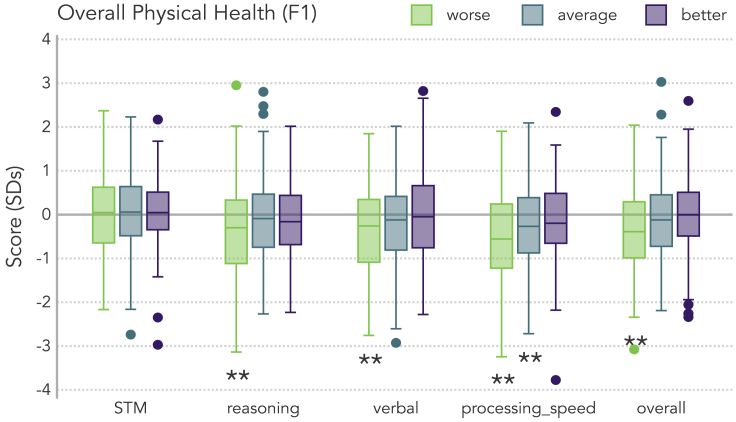
Figure 2 illustrates the cognitive performance of COVID+ participants relative to the normative baseline (Y = 0) as a function of overall physical health. To simplify this visualization and reduce the number of comparisons, participants were grouped into tercile bins (i.e., percentiles that each contained one-third of the sample, corresponding to worse, average, and better physical health). COVID+ participants who had better than average physical health (Figure 2, purple boxes) performed similarly to the norms in all of the cognitive domains. In contrast, those participants with worse than average physical health (Figure 2, green boxes) exhibited significant differences relative to normative data on the four cognitive scores already identified as being significantly lower in the COVID+ cohort: reasoning, verbal, processing speed, and overall performance (all corrected p < 0.05; Table S5). A similar pattern was not apparent when data were re-grouped by the F2 factor scores.
Figure 2.
Within-group associations between physical health and cognition
Participants in the COVID+ sample were grouped into tercile bins based on their F1 scores: Below average (“worse”; left group, green traces), average (center group, blue traces), and above average (“better”; right group, purple traces) physical health (F1). Cognitive scores (corrected for nuisance variables) are relative to the normative sample mean (Y = 0.0). Boxes span from the 1st to 3rd quartiles, horizontal lines within a box indicate the median, whiskers span 1.5 times the interquartile range (limited to minimum/maximum of the sample), and points outside the whiskers (i.e., outliers) are individually plotted. Double asterisks below a box trace indicate a significant difference between that COVID+ subgroup and the norms (p < 0.05 corrected for 15 comparisons).
Linear regression models were used to test the unique contribution of each factor in predicting cognitive performance, within the COVID+ group. F1 (the physical factor) exhibited strong and statistically reliable linear associations with three cognitive scores—verbal, processing speed, and overall performance (all adjusted p < 0.05; Table 4)—when controlling for F2. The directions of these coefficients in Table 4 revealed positive relationships; that is, better physical health (and milder COVID severity) was associated with better verbal and overall performance and faster processing speed. In contrast, F2 did not exhibit a linear relationship with any measure of cognitive performance after accounting for physical health (Table 4). The same pattern of results was observed even when controlling for additional covariates, including the socio-demographic variables (that had already been accounted for using the normative data) and the presence of a preexisting condition (see Table S6).
Table 4.
Linear regression results from models that included both overall physical health (F1) and mental health and wellness (F2) factor scores as simultaneous predictors of cognitive scores
| DV | IV | β | T | df | padj | CI | ΔR2 | f2 |
|---|---|---|---|---|---|---|---|---|
| STM | F1 | 0.03 | 0.72 | 475 | 1.000 | −0.088 to 0.146 | 0.001 | 0.001 |
| F2 | 0.01 | 0.19 | 475 | 1.000 | −0.109 to 0.125 | 0.000 | 0.000 | |
| Reasoning | F1 | 0.09 | 1.90 | 475 | 0.861 | −0.047 to 0.219 | 0.008 | 0.008 |
| F2 | 0.04 | 1.00 | 475 | 1.000 | −0.088 to 0.178 | 0.002 | 0.002 | |
| Verbal | F1 | 0.14 | 3.23 | 475 | 0.020 | 0.012–0.269 | 0.021 | 0.022 |
| F2 | −0.02 | −0.35 | 475 | 1.000 | −0.144 to 0.114 | 0.000 | 0.000 | |
| Processing speed | F1 | 0.15 | 3.48 | 475 | 0.008 | 0.022–0.273 | 0.025 | 0.025 |
| F2 | −0.06 | −1.41 | 475 | 1.000 | −0.185 to 0.065 | 0.004 | 0.004 | |
| Overall | F1 | 0.14 | 3.43 | 475 | 0.010 | 0.019–0.253 | 0.024 | 0.025 |
| F2 | 0.03 | 0.69 | 475 | 1.000 | −0.090 to 0.144 | 0.001 | 0.001 |
p values and confidence intervals are Bonferroni corrected for 15 comparisons. The bold entries indicate significant effects (padj < 0.05). DV, dependent variable; IV, independent variable; β, estimated coefficient; f2, Cohen’s f.
Does hospitalization explain physical/cognitive associations?
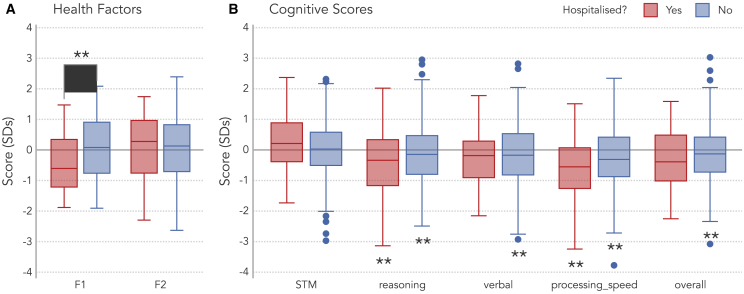
Next, we tested whether the pattern of results found in the COVID+ cohort were driven by more severe cases of COVID-19 that required hospitalization. Comparisons of hospitalized and non-hospitalized subgroups revealed significant differences in terms of F1 (t(88.55) = −3.92, padj < 0.001, d = 0.50) with hospitalized cases reporting worse overall physical health than non-hospitalized cases (Figure 3A). However, these two groups did not significantly differ in terms of their F2 or cognitive performance (Table S7).
Figure 3.
Does hospitalization explain physical/cognitive associations?
(A) Distributions of health factor scores—physical (F1) and mental (F2) health—in the hospitalized (N = 66) and non-hospitalized (N = 412) COVID+ subgroup. The x axis (Y = 0) corresponds to the COVID+ sample mean. The brace and asterisks indicate a significant group difference (p < 0.001).
(B) Cognitive scores (corrected for nuisance variables), for which Y = 0 indicates the normative sample mean. Double asterisks below boxes indicate significant differences between the COVID+ subgroup and the normative sample (p < 0.05 corrected for 10 comparisons). No cognitive differences between hospitalized and non-hospitalized groups were significant at a corrected level. Boxes span from the 1st to 3rd quartiles, horizontal lines within a box indicate the median, whiskers span 1.5 times the interquartile range (limited to minimum/maximum of the sample), and points outside the whiskers (i.e., outliers) are individually plotted.
Comparisons of each of these subgroups to the normative group showed that the non-hospitalized group performed significantly worse in the reasoning and verbal domains and in terms of speed of processing and overall performance, and the hospitalized group performed significantly worse in the reasoning domain and in processing speed (Figure 3B, all corrected p < 0.05; Table S8). In contrast, the STM scores for both groups were not significantly different from normative data.
We included hospitalization status in our regression analyses to see whether it explained away the relationships between F1 and cognitive performance. The pattern of results remained similar: There were positive relationships between F1 and verbal domain performance, speed of processing, and overall cognitive performance (all p < 0.05 corrected for 15 comparisons; Table S9), showing that physical health predicted cognitive performance even when accounting for mental health measures and hospitalization.
Relationships between cognition and other variables
Exploratory analyses examined whether any of the socio-demographic variables (age, gender, post-secondary education, and SES, amount of exercise, or use of various drugs) were related to cognitive performance. Given that the cognitive scores were already corrected for these variables using parameters estimated from the normative sample data, a significant effect of one of these variables would suggest that this effect differed between the normative and COVID+ groups (i.e., a group by demographic-variable interaction). However, no significant associations were found between any of these variables and cognitive scores (Figure S5). This suggests that (1) using the normative dataset to estimate and remove the effects of these potential confounders from cognitive scores was successful, (2) the cognitive differences associated with these variables did not differ between the normative and COVID+ datasets, and hence that (3) the impact of COVID-19 infection on cognition did not differ between males and females or depend on age.
Discussion
In this study, we present data from a cohort of 478 individuals who self-reported having a confirmed positive COVID-19 test and who were assessed using a comprehensive and widely validated battery of cognitive tests that measures aspects of memory, attention, verbal abilities, problem solving, and reasoning. Cognitive scores in multiple broad domains were related to participants’ self-reported COVID-19 physical and mental health experiences, including infection severity, extent of recovery, and measures of anxiety and depression.
The results support the existence of cognitive impairments in the aftermath of COVID-19 infection and include several important additional findings. First, there is striking specificity in the domains that were affected. Speed of processing, verbal, reasoning, and global cognition scores were impaired, whereas a measure of memory performance was unaffected. Second, when all of the physical, cognitive, and mental health factors were considered, two distinct patterns of subjective symptoms emerged. On the one hand, there exists a collection of mostly physical health symptoms, including fatigue, pain, and limitations in performing everyday physical activities, that tend to vary together and are strongly associated with COVID-19 infection severity. Thus, unsurprisingly, more severe disease and older age are associated with poorer physical well-being post-infection. On the other hand, there exists a second set of mental health symptoms that include depression, anxiety, and self-reported limitations in emotional well-being that tend to co-occur and are unrelated to disease severity. Third, the cognitive deficits are strongly and consistently associated with the physical sequelae of the disease, rather than the mental health symptoms. That is, better physical health was correlated with faster processing speed, better verbal ability, and overall cognitive performance, while no associations were found between these measures and the mental health and wellness factor.
It is possible that our normative data do not make a good comparative group because these data were collected before the global pandemic, and some unmeasured confounding factor, such as job security, financial instability, social isolation, an elevated sense of community fear, or anything that undoubtedly affects the well-being of people throughout the world, irrespective of whether they have received a positive COVID-19 diagnosis, could underlie the cognitive differences observed in the COVID+ group. However, two critical observations suggest otherwise. First, we also found cognitive differences within the infected group that were tightly coupled to illness severity and physical symptoms. Second, the subgroup of participants with better than average physical health (relative to the entire COVID+ group) performed similarly to normative data on all cognitive measures. The mutually reinforcing nature of these results, specifically, between-group differences that were replicated within the patient group along a dimension tied to illness severity, strongly suggests that these cognitive differences, and the physical/cognitive associations identified here, are specifically related to COVID-19 infection itself.
It is important to understand that we are not describing two types of people in the post COVID-19 infection population, but two distinct factors that contribute to and characterize the post-COVID-19 syndrome. That these measures were clearly dissociable in terms of the demographic variables that they correlated with suggests that they represent two distinct and separable, although not mutually exclusive, effects of COVID-19 infection. For example, physical health was negatively correlated with age (with an average decline of approximately 0.1 SDs per decade), whereas the other factor, mental health and wellness, increased with age (by 0.1 SDs for every 10 years). It is perhaps to be expected that older COVID-19 survivors would be most affected in terms of their physical and cognitive outcomes (given the greater likelihood of comorbidities in that group), but we also observed the young were more severely affected in terms of their mental health and well-being—a finding that is entirely consistent with research on mental health outcomes during the early stages of the pandemic.41,42 Completion of post-secondary education was also associated with significantly better mental health, but not physical health outcomes, and males reported better physical health than females, yet there was no difference between males and females in terms of mental health and wellness. Again, the differing patterns of correlations between socio-demographic variables and the physical and mental health factors further confirm the existence of two distinct outcomes of COVID-19 infection that are dissociable in multiple ways.
The fact that an aggregate measure of mental health encompassing anxiety, depression, role limitations, and fatigue was not associated with cognitive outcomes in the context of the COVID-19 pandemic is surprising, as numerous studies have shown an association between anxiety, depression, and cognition in the pre-pandemic era.31,32 However, it is important to clarify that those results come from studies that focused on clinical populations—that is, patients who have been diagnosed with a major mental health condition, such as depression or anxiety. In our study, the fact that no association was observed between measures of mental health and cognition may be due to a predominance of detectable, yet subclinical, mental health issues among the COVID-19 survivors. It may be the case that mental health is associated with cognitive performance such as physical health, but to a lesser degree, as is suggested by the observation that our F1 factor included small but non-zero contributions from the GAD2, PHQ2, and variables that may also be thought to have some emotional association (i.e., fatigue and subjective evaluations of cognition). It is also possible that measures of anxiety and depression that are more sensitive to symptom severity, compared to the two-item screeners used in this study, could reveal a relationship between psychopathology and cognitive performance. Importantly, we are not stating that psychopathology is not a consequence of COVID-19 infection, but rather that those particular symptoms are not correlated with objective measures of cognitive function in our sample. Regardless, our study highlights the importance of carefully examining the relationship between physical wellness, mental health, and cognition in other patient populations, to determine what may be driving any observed cognitive impairments.
We also found that lower cognitive performance was not driven by the more severe cases of COVID-19: both hospitalized and non-hospitalized COVID+ subgroups had significantly lower cognitive performance than normative data on some measures, and the correlations between physical health and these cognitive scores persisted even when controlling for hospitalization. This is an important observation because long-lasting cognitive deficits have been reported in non-COVID-19 patients following treatment in the ICU, suggesting that factors such as mechanical ventilation, sedation, drug therapy, and disturbed sleep may contribute to the emergent cognitive profile, independent of infection.8 Indeed, several preliminary studies have suggested that cognitive impairments following COVID-19 infection are dependent on the level of medical assistance received,2,23 although at least one study has reported no correlation between hospitalization and cognitive impairments.25 Our findings support the hypothesis that cognitive impairments are a consequence of COVID-19 infection, rather than a secondary effect (e.g., due to intensive treatments).
Although the present study provides clear evidence for cognitive impairment following COVID-19 infection, the effect was, at least to some extent, domain specific. That is, significant differences were found relative to the norms in speed of processing and in the reasoning and verbal domains, but not in STM performance. These findings shed some light on the nature and extent of the subjective experience of COVID-19 survivors, often called long COVID, the expression now used widely to describe the subjective symptoms that include a sense of cognitive impairment following COVID-19 infection. Specifically, that the cognitive sequelae of COVID-19 in this context includes processing (or “thinking”) speed, reasoning, and verbal abilities, but leaves short-term memory relatively spared. Indeed, the pattern of this functional dissociation is consistent with that observed in a smaller sample of COVID-19 survivors.2
In conclusion, we have shown clear cognitive impairments following COVID-19 infection. These are likely not the result of a “global” impact on cognitive processing, as STM performance was relatively preserved. Crucially, in the domains that were affected, cognitive performance was related to a factor that varied most strongly with variables related to physical health and COVID severity, but not to a factor that broadly described mental health. This has implications from a clinical viewpoint, as survivors who exhibit increased anxiety or depression may or may not have cognitive deficits, whereas cognitive difficulties may be more likely in patients who experience a greater physical toll from the illness. Our findings underscore the fact that the physical, emotional, mental, and cognitive sequelae of COVID-19 are not bound together as a single neurocognitive syndrome and that executive function and verbal abilities are key domains that can be affected in COVID-19 survivors.
Limitations of the study
There are, of course, limitations to this study and the conclusions drawn from our results. Notably, due to online recruitment methods, our sample is likely biased to include individuals who were concerned about their health and well-being post-COVID (i.e., selection bias). This may reduce the generalizability of our findings to the broader population of people who have had COVID, and we cannot speak to the prevalence of the cognitive effects of COVID-19. We also cannot conclusively demonstrate a causal link between COVID-19 infection and cognitive outcomes. As discussed previously, some of our results in the COVID-19 cohort were observed relative to a historical normative dataset, and that may have influenced those apparent differences. However, this seems unlikely given that there were strong associations between cognitive performance and physical symptoms within the COVID+ group, and that the subgroup of participants with good physical health performed “normally” on all cognitive measures. Future research could address this more directly by recruiting true contemporaneous control subjects with confirmed negative COVID-19 tests, or even by comparing individuals’ cognitive performance to their pre-pandemic selves by linking historic datasets to those collected during the pandemic. Another limitation to our approach is that there are many other outcomes of COVID-19 that we did not measure, such as post-traumatic stress disorder (PTSD), that may be interrelated with the sets of symptoms described here. However, regardless of whether cognitive effects are directly caused by COVID-19 or indirectly through another factor, such as PTSD, the fact remains that these impairments are symptoms experienced by survivors of COVID-19 in the weeks to months following infection.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Questionnaire and cognitive test data | This paper | Borealis: https://doi.org/10.5683/SP2/ZQR9QQ |
| Software and algorithms | ||
| Data analysis scripts (Python) | This paper | Zenodo: https://doi.org/10.5281/zenodo.7015795 |
Resource availability
Lead contact
Further Information and requests for resources should be directed to and will be fulfilled by the lead contact, Conor J. Wild (cwild@uwo.ca, conorwild@gmail.com).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Human participants
Data for this study were collected entirely online, from human volunteers, during the period June 23rd 2020 to February 2nd 2021, and 95% of participants signed up before October 14th 2020. During this time, the original SARS-CoV-2 virus was the only (or dominant) strain and PCR testing was the most commonly available form of COVID screening. The study was advertised through online social media channels and mainstream media outlets and reached potential participants around the world. Recruitment was targeted towards individuals who currently had (or previously had) COVID-19, and materials specifically mentioned the potential longer-term effects of COVID-19. Visitors to the study website (www.covidbrainstudy.com) could sign up if interested, and there was no compensation for volunteering. Participants had to be older than 18 years of age, have had a confirmed case of COVID-19 (i.e., self-reported positive test), and be fluent in either French, English, or Spanish – all study materials were available in these languages. The study procedures and materials were approved by Western University’s Health Sciences Research Ethics Board, and participants could withdraw at any point.
Normative cognitive data came from by adult volunteers who had participated in a similar online study from 2017 (well before the onset of the COVID-19 pandemic) that examined the effects of sleep on cognition using the same 12 cognitive tasks and a subset of the same demographic questionnaire items.43 Like in the current study, participants in 2017 were recruited from around the world using a variety of mainstream and social media advertisements. This dataset did not comprise a true control sample for the current COVID + group because the same health-related questionnaires (i.e., SF-36, GAD/PHQ-2, and obviously COVID-19 history) were not collected. Details about the final pre-processed samples for both groups are reported in Table 1.
Method details
After consenting to participate in the study, volunteers completed an online questionnaire about their health followed by 12 cognitive tasks (the order of which was randomized across participants) using a laptop or desktop computer. Cognitive testing was administered via the Cambridge Brain Sciences (CBS) platform, which we have used for other online studies of cognition.43,44 Mobile devices were not supported by CBS when data for the current study were collected (in contrast to the 2017 study) because of compatibility and support issues for more modern browsers, operating systems, and devices. Participants were not blinded to the nature of this study, given that recruitment was targeted towards individuals who had, or had had, COVID-19, and the nature of the questionnaire and cognitive tests being administered.
Health questionnaire
Participants were asked about their COVID-19 history (Table 2), including: presentation of symptoms (according to common screening tools used at that time), the month and year of their most recent positive COVID-19 test, and hospitalisation status (e.g., required treatment in hospital, intensive care unit, ventilator, etc.). A COVID severity score, based on the World Health Organization’s 8-point ordinal scale of COVID-19 severity,45 was assigned to each participant based on these questions; scores ranged from unaffected to hospitalised with severe disease (Figure S1 and Table S1).
Measures of physical and mental health were captured using the RAND Short-Form Health Survey39 (SF-36) – specifically, the physical functioning, role functioning (physical), role functioning (emotional), energy & fatigue, and pain scales – and the GAD-2 & PHQ-2 scales.40,46 The GAD-2 and PHQ-2 were reversed during analysis to make them consistent with the other scales where higher scores indicated better health. Two additional questions were included for subjective evaluations of cognitive functioning: 1) “Do you feel that you are back to your baseline level of cognitive functioning?” and 2) “How would you rate your memory?” on a 5-point scale from “miserable” to “excellent”. Statistics about these measures (means and SDs) are reported in Table S2.
Cognitive test battery
Detailed descriptions of the 12 CBS tasks can be found in the supplementary materials of some of our previous studies,43,44 but briefly they are: 1) Spatial Span (SS; short-term memory); 2) Monkey Ladder (ML; visuospatial working memory), 3) Paired Associates (PA; episodic memory), 4) Token Search (TS; working memory and strategy), 5) Digit Span (DS; verbal working memory), 6) Odd One Out (OOO; deductive reasoning), 7) Rotations (RT; mental rotation), 8) Feature Match (FM; feature-based attention and concentration), 9) Spatial Planning (SP; planning and executive function), 10) Interlocking Polygons (PO, visuospatial processing), 11) Grammatical Reasoning (GR, verbal reasoning), and 12) Double Trouble (DT; a modified Stroop task). For tasks 1–5, the primary outcome measure was the number of items in the hardest problem correctly solved, whereas scores for tasks 6–12 were variations of the sum of correct, minus incorrect, answers within the given time window. Means and SDs for each primary outcome measure, for the normative and COVID + groups, are reported in Tables S3 and S4, respectively.
Each task, except for SP, also provided an aggregate measure of reaction time which was the average duration of correctly answered trials; for tasks 1–5, the individual trial durations were first normalized by the number of items in the problem.
Quantification and statistical analysis
Software
All data preprocessing and analysis was done in Python (3.9.12, https://www.python.org/). Specific packages included: Pandas (1.2.4) for data preprocessing and manipulation, scikit-learn (0.24.1) for estimating and applying data transformations, statsmodels (0.13.2) for building and fitting general linear models and calculating related statistics, pingouin (0.5.1) for performing t-tests, factor analyzer (0.3.2) for performing principal component and factor analyses, and Numpy (1.20.2) for all mathematical operations. All figures were created with custom code written in Python, supported by the Plotly (5.9.0) and Matplotlib (3.4.1) packages.
Data preprocessing
In total, 3,243 people registered to participate in this study. Of those, 1,745 progressed through the questionnaire and 1,456 completed the cognitive tests. One dataset was removed for using an unsupported (i.e., mobile) device and 265 datasets were omitted for indicating an age less than 18 years (no volunteers reported being older than 100 years). As we have done previously,43 test score outliers were filtered in two iterative passes. First, extreme outliers more than 6 SDs from the mean (e.g., technical/database errors) were removed to obtain reasonable estimates of the test means and SDs; four participants had at least one score greater than this threshold. Then, outlier scores were identified as being more than 4 SDs from the re-calculated mean, and 18 participants were excluded for having at least one outlier. 222 cases were excluded because of missing test scores or incomplete (i.e., optionally omitted) questionnaire responses. Finally, only participants who self-reported having received a positive test for COVID-19 were retained for analysis, resulting in a final dataset of 478 COVID-positive cases (i.e., the COVID + group). In this final step, we discarded participants’ data if they did not indicate having received a positive test for COVID-19, even if they had indicated symptoms and/or risks for exposure, due to the uncertainty of their diagnosis. For example, they may have had COVID but not been tested (e.g., no access to testing), or they have been tested because of COVID-like symptoms but received a negative result.
The normative data were preprocessed in a similar way. From 26,256 participants, 7,833 were removed for using a mobile device (because mobile devices were not supported by the present study), 1,831 participants indicated an age less than 18 or greater than 100 years, and 7,594 had missing test scores or questionnaire items. Cases with outliers were removed (N = 289 with a score >6 SDs, followed by N = 877 with a score >4 SDs from the mean), yielding a final normative sample 7,832 participants.
Health factor and composite cognitive scores
Rather than examine every pairwise relationship between 11 individual heath variables and 12 cognitive test scores, we reduced the number of independent and dependent variables using factor analysis. Each multivariate dataset was decomposed into a smaller number of statistically independent underlying, or “latent”, factors that summarized the major modes of covariation. This approach allowed us to simplify interpretations, reduce the total number of model parameters, and avoid multicollinearity between predictors.
The set of health-related measures (i.e., SF-36 scales, GAD-2, PHQ-2, COVID severity, and subjective measures of cognition) from the COVID+ group was summarized using factor analysis with a Varimax rotation. Factors with eigenvalues greater than 1.0 were retained for further analysis, and their scores were calculated for each participant by transforming questionnaire responses using the factor loadings matrix. The resulting factor scores were mean centred (M = 0.0) and had SD = 1.0.
To factorize the 12 cognitive test scores, we performed a PCA with three components and a Varimax rotation as we have done previously. The solution (Figure 1A) was consistent with our previous findings,44 and these components were interpreted as broadly representing short-term memory (STM), reasoning, and verbal ability. Composite scores representing performance in these domains were calculated by transforming participants’ 12 test scores using the PCA loadings.43,44 We also calculated an “overall” score of cognitive performance (the mean of the 12 z-scored primary outcomes) and a measure of “processing speed” (the 1st principal component of the 11 reaction-time based features). Composite scores were positively correlated with higher individual test scores and faster responses, and a supplementary analysis showed similar factor solutions were derived from both groups.
Prior to the PCA analysis and score calculations, cognitive test data for both groups were standardized (M = 0.0, SD = 1.0) using the means and standard deviations from the normative sample. A power transformation47 was applied (again, using parameters estimated from the normative data) to reduce skewness and improve normality of the test score features. The models, parameters, and transformations for these composite score calculations were derived using norms, and therefore: 1) had M = 0.0 and SD = 1.0 in the normative group, and 2) for COVID + participants represented performance relative to norms in units of standard deviations.
Statistical analysis
Data analysis proceeded in two stages. First, we leveraged the power of our larger normative dataset to estimate the relationship between each composite cognitive score and confounding variables common to both datasets. A linear regression model was constructed for each cognitive score that included the predictors: age as a 2nd-order polynomial, and 10 binary predictors for gender (male / female), post-secondary education (none / some), SES (above or below poverty level), exercise (at least weekly or not), and consumption of nicotine (>1 unit per day), alcohol (>7 units per week), cannabis (>1 unit per day), other stimulants (in the past 4 weeks), or other depressants (in the past 4 weeks). The estimated parameters were then used to regress out the expected effects of these variables from the COVID+ and normative cognitive data, and the corrected scores were carried forward to subsequent analyses. This approach was taken to avoid over-fitting the effects of these nuisance variables on the smaller dataset. For example, if COVID-19 infection has a greater impact on cognition for older individuals, then simply controlling for age in an analysis of the COVID + dataset would obscure or reduce this critical finding. Following this, a residual significant relationship between a socio-demographic variable and a corrected score would imply that the effect of that variable differed in the COVID + group (i.e., an interaction).
In the second step, a general linear model was used to estimate the relationship, in the COVID + group, between each (corrected) composite score and the health-related factor scores (i.e., five regression models, each of which predicted a cognitive score from the two health factors). Effect sizes for these continuous predictors included: , the change in variance accounted for by adding the parameter, and Cohen’s , a measure of local effect size.48 Student’s t test was used to test significance of the estimated regression parameters, whereas group comparisons used Welch’s t-test to account for unequal sample sizes.49 All t-tests (for regression parameters and group differences) were two-tailed and reported p values and confidence intervals were Bonferroni corrected across all scores and tests within each analysis set (e.g., 5 cognitive scores × 3 comparisons = 15 statistical tests). Given that the dependent variables were standardized in units of standard deviations (SDs), the parameter estimates for categorical variables, and differences between groups, amounted to standardized mean differences – analogous to Cohen’s d. The parametric statistics used in this study assume the data or model residuals are normally distributed, so where appropriate we used quantile-quantile plots (i.e., “qq” plots) to validate this assumption (e.g., see Figure S4).
Acknowledgments
We would like to thank Sophie Kelly and Mike Battista for their support in advertising the study on social media platforms and helping volunteers with their technical issues. Funding was generously provided by the Canada Excellence Research Chairs, Government of Canada (CERC) program (grant #215063 awarded to A.M.O.), and CIFAR (Manulife Grant CF-0159, awarded to A.M.O. and D.K.M.). R.H.S. receives salary support for research from a Heart & Stroke Clinician-Scientist Phase II Award, the Ontario Brain Institute, and the Department of Medicine at the University of Toronto, Canada.
Author contributions
All of the authors conceived and designed the study. R.H.S. and A.M.O. supervised the project. C.J.W. implemented the online data collection, analyzed the data, and prepared figures, tables, and so forth. C.J.W. and A.M.O. wrote the paper. All of the authors provided valuable insights and suggestions, aided with data interpretation and manuscript revisions, and have approved the final version of the manuscript.
Declaration of interests
The cognitive tests used in this study are marketed by Cambridge Brain Sciences (CBS), of which A.M.O. is the chief scientific officer. Under the terms of the existing licensing agreement, A.M.O. and his collaborators are free to use the platform at no cost for their scientific studies, and that such research projects neither contribute to, nor are influenced by, the activities of the company. C.J.W. provides consulting services to CBS. Consequently, there is no overlap between the present study and the activities of CBS, nor was there any cost to the authors, funding bodies, or participants who were involved in the study. R.H.S. is founder and owner of FollowMD, Inc., a vascular risk-reduction clinic. The authors declare no other competing interests.
Inclusion and diversity
We worked to ensure ethnic or other types of diversity in the recruitment of human subjects. We worked to ensure that the study questionnaires were prepared in an inclusive way. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science.
Published: September 6, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2022.100750.
Supplemental information
Data and code availability
-
•
De-identified data (i.e., cognitive test data and questionnaires) collected for this study are publicly available as a part of this record1 at Borealis: https://doi.org/10.5683/SP2/ZQR9QQ as of the date of publication. They can be used to recreate all statistics, tables, and figures in this manuscript.
-
•
All original code, including a notebook that recreates all analyses, has been deposited in Github: https://github.com/TheOwenLab/2021-Wild-et-al-COVID-Cognition, archived at Zenodo: https://doi.org/10.5281/zenodo.7015795, and is publicly available as of the date of publication.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Wild C.J., Norton L., Swartz R.H., Owen A.M. Cognitive testing and questionnaire data from survivors of COVID-19. [DOI]
- 2.Hampshire A., Trender W., Chamberlain S.R., Jolly A.E., Grant J.E., Patrick F., Mazibuko N., Williams S.C., Barnby J.M., Hellyer P., Mehta M.A. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine. 2021;39:101044. doi: 10.1016/j.eclinm.2021.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franke C., et al. 2020. Brain Behavior and Immunity High Frequency of Cerebrospinal Fluid Autoantibodies in COVID-19 Patients with Neurological Symptoms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varatharaj A., Thomas N., Ellul M.A., Davies N.W.S., Pollak T.A., Tenorio E.L., Sultan M., Easton A., Breen G., Zandi M., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatr. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanin L., Saraceno G., Panciani P.P., Fontanella M.M. 2020. SARS-CoV-2 Can Induce Brain and Spine Demyelinating Lesions; pp. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee M.-H., Perl D.P., Nair G., Li W., Maric D., Murray H., Dodd S.J., Koretsky A.P., Watts J.A., Cheung V., et al. Microvascular injury in the brains of patients with covid-19. N. Engl. J. Med. 2021;384:481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honarmand K., Malik S., Wild C., Gonzalez-Lara L.E., McIntyre C.W., Owen A.M., Slessarev M. Feasibility of a web-based neurocognitive battery for assessing cognitive function in critical illness survivors. PLoS One. 2019;14 doi: 10.1371/journal.pone.0215203. e0215203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandharipande P.P., Girard T.D., Jackson J.C., Morandi A., Thompson J.L., Pun B.T., Brummel N.E., Hughes C.G., Vasilevskis E.E., Shintani A.K., et al. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler J., Borchers F., Endres M., Weiss B., Spies C., Emmrich J.V. Cognitive deficits following intensive care. Dtsch. Arztebl. Int. 2019;116:627–634. doi: 10.3238/arztebl.2019.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yelland G.W. Gluten-induced cognitive impairment (“brain fog”) in coeliac disease. J. Gastroenterol. Hepatol. 2017;32:90–93. doi: 10.1111/jgh.13706. [DOI] [PubMed] [Google Scholar]
- 12.Ocon A.J. Caught in the thickness of brain fog: exploring the cognitive symptoms of Chronic Fatigue Syndrome. Front. Physiol. 2013;4:63–68. doi: 10.3389/fphys.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovalchuk A., Kolb B. Chemo brain: from discerning mechanisms to lifting the brain fog—an aging connection. Cell Cycle. 2017;16:1345–1349. doi: 10.1080/15384101.2017.1334022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wise J. Long covid: doctors call for research and surveillance to capture disease. BMJ. 2020;370:m3586. doi: 10.1136/bmj.m3586. [DOI] [PubMed] [Google Scholar]
- 15.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 17.Amalakanti S., Arepalli K.V.R., Jillella J.P. Cognitive assessment in asymptomatic COVID-19 subjects. VirusDisease. 2021;32:146–149. doi: 10.1007/s13337-021-00663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tay M.R.J., Low Y.H., Lim C.C.T., Umapathi T., Thio J.M.L., Lui W.L., Chan W.L.W., Chua K.S.G. Covert subclinical neurocognitive sequelae during the rehabilitation course of severe coronavirus disease 2019. Am. J. Phys. Med. Rehabil. 2021;100:39–43. doi: 10.1097/PHM.0000000000001633. [DOI] [PubMed] [Google Scholar]
- 19.Beaud V., Crottaz-Herbette S., Dunet V., Vaucher J., Bernard-Valnet R., Du Pasquier R., Bart P.A., Clarke S. Pattern of cognitive deficits in severe COVID-19. J. Neurol. Neurosurg. Psychiatry. 2020;92:567–568. doi: 10.1136/jnnp-2020-325173. 325173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Negrini F., Ferrario I., Mazziotti D., Berchicci M., Bonazzi M., de Sire A., Negrini S., Zapparoli L. Neuropsychological features of severe hospitalized coronavirus disease 2019 patients at clinical stability and clues for postacute rehabilitation. Arch. Phys. Med. Rehabil. 2021;102:155–158. doi: 10.1016/j.apmr.2020.09.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raman B., Cassar M.P., Tunnicliffe E.M., Filippini N., Griffanti L., Alfaro-Almagro F., Okell T., Sheerin F., Xie C., Mahmod M., et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou H., Lu S., Chen J., Wei N., Wang D., Lyu H., Shi C., Hu S. The landscape of cognitive function in recovered COVID-19 patients. J. Psychiatr. Res. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrucci R., Dini M., Groppo E., Rosci C., Reitano M.R., Bai F., Poletti B., Brugnera A., Silani V., D'Arminio Monforte A., Priori A. Long-lasting cognitive abnormalities after COVID-19. Brain Sci. 2021;11:235. doi: 10.3390/brainsci11020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiteside D.M., Oleynick V., Holker E., Waldron E.J., Porter J., Kasprzak M. Neurocognitive deficits in severe COVID-19 infection: case series and proposed model. Clin. Neuropsychol. 2021;35:799–818. doi: 10.1080/13854046.2021.1874056. [DOI] [PubMed] [Google Scholar]
- 25.Woo M.S., Malsy J., Pöttgen J., Seddiq Zai S., Ufer F., Hadjilaou A., Schmiedel S., Addo M.M., Gerloff C., Heesen C., et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020;2 doi: 10.1093/braincomms/fcaa205. fcaa205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaywant A., Vanderlind W.M., Alexopoulos G.S., Fridman C.B., Perlis R.H., Gunning F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. medRxiv. 2020 doi: 10.1101/2020.10.28.20221887. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brouqui P., Amrane S., Million M., Cortaredona S., Parola P., Lagier J.C., Raoult D. Asymptomatic hypoxia in COVID-19 is associated with poor outcome. Int. J. Infect. Dis. 2021;102:233–238. doi: 10.1016/j.ijid.2020.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tobin M.J., Laghi F., Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am. J. Respir. Crit. Care Med. 2020;202:356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Areza-Fegyveres R., Kairalla R.A., Carvalho C.R.R., Nitrini R. Cognition and chronic hypoxia in pulmonary diseases. Dement. Neuropsychol. 2010;4:14–22. doi: 10.1590/S1980-57642010DN40100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilyukov R.G., Nikolov M.S., Pencheva V.P., Petrova D.S., Georgiev O.B., Mondeshki T.L., Milanova V.K. Cognitive impairment and affective disorders in patients with obstructive sleep apnea syndrome. Front. Psychiatry. 2018;9:357–411. doi: 10.3389/fpsyt.2018.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gayete S., Giné A., Miret M., Ayuso-Mateos J.L., Haro J.M., Olaya B. Cognitive function associated with different diagnoses of anxiety disorders over the lifespan: results from a Spanish representative sample. J. Anxiety Disord. 2020;75:102296. doi: 10.1016/j.janxdis.2020.102296. [DOI] [PubMed] [Google Scholar]
- 32.Semkovska M., Quinlivan L., O'Grady T., Johnson R., Collins A., O'Connor J., Knittle H., Ahern E., Gload T. Cognitive function following a major depressive episode: a systematic review and meta-analysis. Lancet Psychiatr. 2019;6:851–861. doi: 10.1016/S2215-0366(19)30291-3. [DOI] [PubMed] [Google Scholar]
- 33.Parsey C.M., Schmitter-Edgecombe M. Using actigraphy to predict the ecological momentary assessment of mood, fatigue, and cognition in older adulthood: mixed-Methods study. JMIR Aging. 2019;2:1–12. doi: 10.2196/11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagogianni C., Thomas S., Lincoln N. Examining the relationship between fatigue and cognition after stroke: a systematic review. Neuropsychol. Rehabil. 2018;28:57–116. doi: 10.1080/09602011.2015.1127820. [DOI] [PubMed] [Google Scholar]
- 35.Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatr. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poyraz B.Ç., Poyraz C.A., Olgun Y., Gürel Ö., Alkan S., Özdemir Y.E., Balkan İ.İ., Karaali R. Psychiatric morbidity and protracted symptoms after COVID-19. Psychiatry Res. 2021;295:113604. doi: 10.1016/j.psychres.2020.113604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soldati A.B., Almeida C., Lima M., Araujo A., Araujo-Leite M.A., Silva M.T.T. Telephone Screening of Cognitive Status (TICS) in severe COVID-19 patients: utility in the era of social isolation. eNeurologicalSci. 2021;22:100322. doi: 10.1016/j.ensci.2021.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hays R.D., Sherbourne C.D., Mazel R.M. The rand 36-item health survey 1.0. Health Econ. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 40.Kroenke K., Spitzer R.L., Williams J.B.W. The patient health questionnaire-2: validity of a two-item depression screener. Med. Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 41.Bruine de Bruin W. Age differences in COVID-19 risk perceptions and mental health: evidence from a national U.S. Survey conducted in march 2020. J. Gerontol.: Ser. Bibliogr. 2021;76:E24–E29. doi: 10.1093/geronb/gbaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwong A.S.F., Pearson R.M., Adams M.J., Northstone K., Tilling K., Smith D., Fawns-Ritchie C., Bould H., Warne N., Zammit S., et al. Mental health before and during the COVID-19 pandemic in two longitudinal UK population cohorts. Br. J. Psychiatry. 2021;218:334–343. doi: 10.1192/bjp.2020.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wild C.J., Nichols E.S., Battista M.E., Stojanoski B., Owen A.M. Dissociable effects of self-reported daily sleep duration on high-level cognitive abilities. Sleep. 2018;41:1–11. doi: 10.1093/sleep/zsy182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hampshire A., Highfield R.R., Parkin B.L., Owen A.M. Fractionating human intelligence. Neuron. 2012;76:1225–1237. doi: 10.1016/j.neuron.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 45.Organization W.H. WHO R&D Blueprint: Novel Coronavirus: Outline of Trial Designs for Experimental Therapeutics. 2020. https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/grc-740953
- 46.Kroenke K., Spitzer R.L., Williams J.B.W., Monahan P.O., Löwe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann. Intern. Med. 2007;146:317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- 47.Yeo I.-K., Johnson R.A. A new family of power transformations to improve normality or symmetry. Biometrika. 2000;87:954–959. http://www.jstor.org/stable/2673623 [Google Scholar]
- 48.Selya A.S., Rose J.S., Dierker L.C., Hedeker D., Mermelstein R.J. A practical guide to calculating Cohen’s f 2, a measure of local effect size, from PROC MIXED. Front. Psychol. 2012;3:1–6. doi: 10.3389/fpsyg.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmerman D.W. A note on preliminary tests of equality of variances. Br. J. Math. Stat. Psychol. 2004;57:173–181. doi: 10.1348/000711004849222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
De-identified data (i.e., cognitive test data and questionnaires) collected for this study are publicly available as a part of this record1 at Borealis: https://doi.org/10.5683/SP2/ZQR9QQ as of the date of publication. They can be used to recreate all statistics, tables, and figures in this manuscript.
-
•
All original code, including a notebook that recreates all analyses, has been deposited in Github: https://github.com/TheOwenLab/2021-Wild-et-al-COVID-Cognition, archived at Zenodo: https://doi.org/10.5281/zenodo.7015795, and is publicly available as of the date of publication.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.