Abstract
The expression of any given bacterial protein is predicted to depend on (i) the transcriptional regulation of the promoter and the translational regulation of its mRNA and (ii) the synthesis and translation of total (bulk) mRNA. This is because total mRNA acts as a competitor to the specific mRNA for the binding of initiation-ready free ribosomes. To characterize the effects of mRNA competition on gene expression, the specific activity of β-galactosidase expressed from three different promoter-lacZ fusions (Pspc-lacZ, PRNAI-lacZ, and PRNAII-lacZ) was measured (i) in a relA+ background during exponential growth at different rates and (ii) in relA+ and ΔrelA derivatives of Escherichia coli B/r after induction of a mild stringent or a relaxed response to raise or lower, respectively, the level of ppGpp. Expression from all three promoters was stimulated during slow exponential growth or at elevated levels of ppGpp and was reduced during fast exponential growth or at lower levels of ppGpp. From these observations and from other considerations, we propose (i) that the concentration of free, initiation-ready ribosomes is approximately constant and independent of the growth rate and (ii) that bulk mRNA made during slow growth and at elevated levels of ppGpp is less efficiently translated than bulk mRNA made during fast growth and at reduced levels of ppGpp. These features lead to an indirect enhancement in the expression of LacZ (or of any other protein) during growth in media of poor nutritional quality and at increased levels of ppGpp.
The expression of a bacterial gene can be studied by measuring the relative abundance of either its mRNA or its protein product. Intuitively these two methods might seem to be equivalent, but in fact they are not. Using artificially constructed promoter-lacZ fusions integrated into the Escherichia coli chromosome, we have previously determined the activities of a number of constitutive mRNA promoters expressed as lacZ transcripts per minute per promoter and as units of β-galactosidase activity (19). The transcriptional activities of these promoters increased with increasing growth rate, whereas the specific activity of β-galactosidase decreased. The rate of translation initiation of lacZ mRNA was found to be rather constant, with no indication of growth rate-dependent translational control (17). Therefore, the discrepancy was not caused by any control on the translation of the lacZ reporter mRNA.
What causes gene expression at the transcriptional and translational levels to respond in opposite directions to changes in the growth rate? The answer to this question is rather simple: the abundance in the cytoplasm of any given protein, or the specific activity of an enzyme (activity per amount of protein), reflects the distribution of translating ribosomes between the encoding mRNA and bulk mRNA. This distribution depends on two factors: (i) the relative amounts of the encoding mRNA and bulk mRNA and (ii) the translation frequencies of the encoding mRNA and bulk mRNA (see, e.g., reference 26).
In this report, we have considered the effects of bulk mRNA and free ribosomes on the synthesis of β-galactosidase expressed from three artificial promoter-lacZ fusions carrying the promoters for the spc ribosomal protein operon (Pspc), the pBR322 plasmid replication inhibitor RNAI (PRNAI), and the pBR322 replication primer RNAII (PRNAII). In previous studies involving measurements of transcripts by hybridization, Pspc and PRNAI were found to be constitutive, without specific control; PRNAII was positively regulated by ppGpp but was constitutive in the absence of ppGpp (19). The experiments presented here suggest to us that many poorly translated mRNAs (e.g., those with weak ribosome binding sites) accumulate during slow growth in poor media and, conversely, many frequently translated mRNAs (e.g., those with strong ribosome binding sites) accumulate during fast growth in rich media. This keeps the concentration of free ribosomes approximately constant as the growth rate increases, in spite of an increasing concentration of total ribosomes. Moreover, it produces an apparent stimulation in the expression of any given protein under conditions of slow growth or at increased intracellular levels of ppGpp. These results have implications for the expression of any bacterial gene, including the control of the synthesis of ribosomal RNA and ribosomal proteins, and for the interpretation of data obtained with reporter systems.
MATERIALS AND METHODS
Bacterial strains used.
The Escherichia coli strains used in this work and their origins or constructions are described in Table 1. Fusions of lacZ with Pspc and the plasmid pBR322 promoters PRNAI and PRNAII were constructed as previously described (17, 19). The promoters were originally cloned in the plasmid vector pSL03 and then recombined into the mal locus of the chromosome of lac deletion derivatives of E. coli B/r (see Table 1 for details). pSL03 was derived from the W205 trp-lac operon fusion (24) from which the trp transcription terminator upstream of lacZ (25) has been deleted (17). The operon fusions carried the following promoter fragments: Pspc, from nucleotide (nt) −51 relative to the transcription start through rplN (the first gene of the spc operon, encoding ribosomal protein L14), ending at nt +453; PRNAI, from nt −77 to +32; and PRNAII, from nt −63 to +63. All promoter fragments carried EcoRI and BamHI sites at their 5′ or 3′ ends, respectively, for insertion into the multiple cloning site of pSL03. For the experiment for which results are shown in Fig. 2, a spc-lac operon fusion was used in which Pspc was directly linked to lacZ (from nt −51 to +59) without rplN. Previous studies have shown that strains in which lacZ is directly linked to Pspc may show an anomalous growth rate-dependent reduction in the accumulation of lacZ mRNA (17). The reason for this effect is not known; possibly, sequences close to the 5′ end of the spc operon transcript interact with sequences in the trp-lac mRNA leader to produce a fortuitous signal which either causes transcription termination or shortens the mRNA lifetime. Inclusion of rplN in the construct ensures that such effects are absent (17). Inclusion of additional sequences upstream of Pspc (up to 105 bp upstream of position −51) had no measurable effect on β-galactosidase expression; this suggests that the region upstream of Pspc is devoid of regulatory elements.
TABLE 1.
Bacterial strains
| E. coli strain | Genotype | Reference or construction |
|---|---|---|
| HB181 | B/r A malK+ Δ(argF-lacIOZYA) phe(Am) thr(Am) hsdR/M (K-12) | 35 |
| VH2732 | MC4100 ΔrelA251::kan argA::Tn10 malB::malE′-rrnB UAR-P1-φX174E′-′lacZ-T1T2-kan-T1T2-′malKa | 12 |
| XZ241 | HB181 ΔrelA251::kan argA::Tn10 spoT+ malB::malE′-rrnB UAR-P1-box BAC-λ′-lacZ-T1T2-kan-T1T2-′malK | Zhang and Bremer, unpublished data; the ΔrelA251::kan argA::Tn10 alleles of VH2732 were transduced with phage P1 into XZ213 (reference 35), selecting for Tcr and screening for the Rel− phenotype |
| SL104 | HB181 malB::malE′-Pspc-lacZ-T1T2-kan-T1T2-′malK | 17 |
| SL106 | HB181 malB::malE′-Pspc-rplN-lacZ-T1T2-kan-T1T2-′malK | 17 |
| SL111 | SL104 ΔrelA251::kan | The ΔrelA251::kan argA::Tn10 alleles from XZ241 were transduced with phage P1 into SL104, selecting for Tcr and screening for the RelA phenotype; the strain was cured of Tn10 and checked for Arg+ |
| YX101 | HB181 malB::malE′-PRNAI-lacZ-T1T2-kan-T1T2-′malK | Construction as described for SL104, except that a PRNAI promoter fragment was cloned |
| YX102 | HB181 malB::malE′-PRNAII-lacZ-T1T2-kan-T1T2-′malK | Construction as described for SL104, except that a PRNAII promoter fragment was cloned |
| YX103 | YX101 ΔrelA251::kan | The ΔrelA251::kan argA::Tn10 alleles from XZ241 were transduced with phage P1 into YX101, selecting for Tcr and screening for the RelA phenotype; the strain was then cured of Tn10 and checked for the Arg+ phenotype |
| YX104 | YX102 ΔrelA251::kan | Construction as described for YX103, except that strain XZ102 was used as a recipient |
T1T2, transcription terminators of the rrnB operon.
FIG. 2.
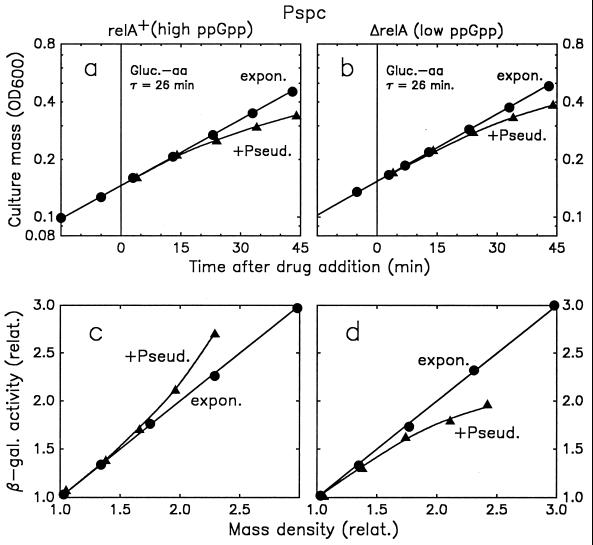
β-Galactosidase expression from Pspc during the stringent and the relaxed response. The stringent (relA+) strain SL104 (a and c) and the relaxed (ΔrelA) strain SL111 (b and d) were grown exponentially (●) in glucose-amino acids medium. Mild amino acid deprivation was induced in part of the culture (▴) by treatment with 3 μg of pseudomonic acid/ml. (a and b) culture mass density (OD600) as a function of time; (c and d) β-galactosidase activity plotted against cell mass density. Values are normalized to the β-galactosidase activity and culture mass (OD600) observed at the time of drug addition. τ, doubling time.
Conditions of growth.
Cultures were grown at 37°C in medium C (11) supplemented with either 0.2% (vol/vol) glycerol or 0.2% (wt/vol) glucose, with or without 0.8% Difco Casamino Acids plus 50 μg of tryptophan/ml, or in Luria-Bertani (LB) medium (23) with 0.2% glucose. Minimal media were supplemented with phenylalanine and threonine as required, at 50 μg/ml. Experimental cultures were inoculated from overnight cultures in glycerol minimal medium by diluting at least 250-fold into minimal media or 2,000-fold into amino acid-supplemented media.
Growth was measured as the increase in turbidity at 600 nm with a 1-cm light path (optical density at 600 nm [OD600]). Since the turbidity is not exactly proportional to the culture density, the observed values, after subtraction of the medium blank, were corrected for nonlinearity (2). The corrected OD values deviated by less than 1% from the average exponential curve, so that the inaccuracy of the average OD used for determination of the specific enzyme activity was about 1%.
β-Galactosidase assays.
Assays for β-galactosidase activity were performed with four to five 10-μl samples of culture taken at different times during exponential growth as described previously (18). The specific activity of β-galactosidase was expressed as the increase in A420 per hour of incubation of the assay at 30°C per OD600 unit of culture in the assay. The specific activity values obtained from different samples of a given culture generally deviated from the average by less than 2%. Greater deviations, up to 10%, were sometimes observed in repeat experiments carried out with cultures grown on different days. The reproducibility of the assays can also be seen from Fig. 2c and d: the fact that, for growth without pseudomonic acid, all measured points lie on a straight line with a slope of 1.0 implies that the specific enzyme activity was exactly the same for all assays during threefold exponential growth of the culture.
RESULTS
Enzyme expression during exponential growth at different rates.
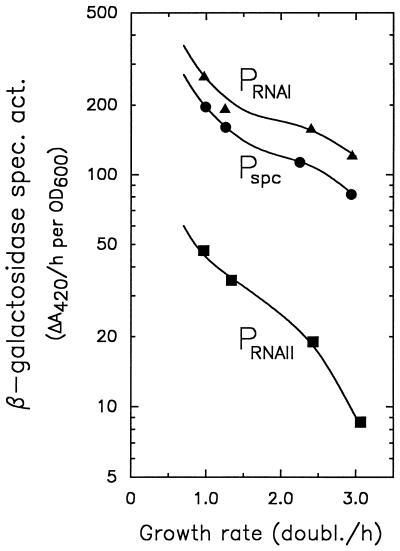
The specific activities of β-galactosidase expressed from Pspc, PRNAI, and PRNAII in the ppGpp-proficient E. coli B/r strains SL106, YX101, and YX102 (Table 1; the promoter-lacZ fusions in these strains are integrated into the mal locus of the bacterial chromosome [see Materials and Methods]), respectively, were measured during exponential growth in different media (Fig. 1). For all three promoters, the specific activity of β-galactosidase decreased with increasing growth rate in the range between 1.0 and 3.0 doublings/h. The specific activities for Pspc and PRNAI decreased in parallel, about 2.5-fold for the threefold increase in growth rate, whereas the specific activity for PRNAII decreased more than fivefold over this range of growth rates (Fig. 1). Previous transcription assays with E. coli K-12 strains showed that the activities of Pspc and PRNAI are not significantly affected by the presence of ppGpp at the low (“basal”) levels accumulating during slow exponential growth in ppGpp-proficient (relA+ spoT+) strains. In contrast, PRNAII was stimulated by the low level of ppGpp under those conditions (19). Since cytoplasmic levels of ppGpp also decrease with increasing growth rate (31), the steeper decrease in the enzyme activity curve for PRNAII in comparison to the curves for the other two promoters is consistent with positive control of PRNAII by ppGpp. Results similar to those for Pspc in Fig. 1 have been reported previously (17); the data are shown here for the purposes of comparison and further analysis.
FIG. 1.
Growth rate dependence of β-galactosidase expression from Pspc, PRNAI, and PRNAII. Strains SL106, YX101, and YX102, respectively, were used, and the growth media (given in order from the lowest to the highest growth rate) were glycerol minimal medium, glucose minimal medium, glucose-amino acids, and LB-glucose (see Materials and Methods).
Enzyme expression during the stringent and relaxed response.
The stimulation or reduction of the production of a protein under slow- or fast-growth conditions can be mimicked by artificially altering the intracellular level of ppGpp. Pseudomonic acid is a competitive inhibitor of isoleucyl tRNA synthetase and, at low concentrations, causes mild amino acid deprivation (14). A relA+ and a ΔrelA strain carrying Pspc-lacZ operon fusions (strains SL104 and SL111, respectively [Table 1]) were treated with pseudomonic acid. Culture mass accumulation was always reduced by the addition of pseudomonic acid (Fig. 2a and b). Under these conditions, expression of lacZ from Pspc was stimulated by a high ppGpp concentration (the stringent response [Fig. 2c]) and reduced by a low ppGpp concentration (the relaxed response [Fig. 2d]). As during exponential growth, the expression of lacZ from this promoter correlates with the ppGpp concentration.
The relaxed response can also be induced in relA+ strains by treatment with chloramphenicol (5, 16). When a culture of the relA+ strain SL104 was treated with chloramphenicol at a low concentration (1 μg/ml), the accumulation of β-galactosidase activity relative to the accumulation of total culture mass decreased in the same manner as that observed during mild amino acid starvation of a ΔrelA strain (data not shown).
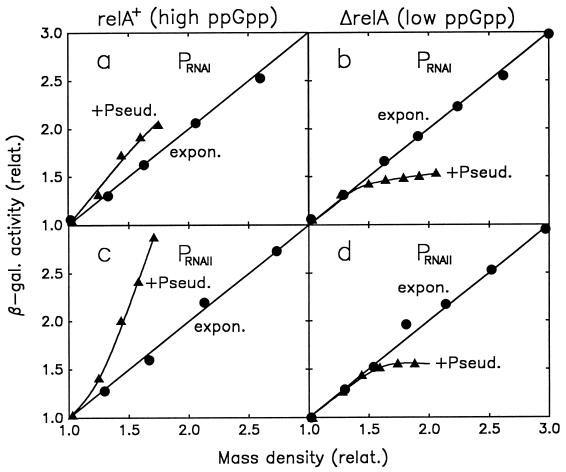
Amino acid deprivation experiments as shown in Fig. 2 for Pspc were also performed with relA+ and ΔrelA strains carrying a PRNAI-lacZ or a PRNAII-lacZ fusion (strains YX101 through YX104). Again, enzyme expression was stimulated when the level of ppGpp was raised and was inhibited when the ppGpp level was lowered (Fig. 3). However, the stimulation during the stringent response and the inhibition during the relaxed response were exaggerated with the PRNAII-lacZ strains in comparison to those for the PRNAI strains. Apparently, changes in the level of ppGpp during a mild stringent or a relaxed response affect enzyme expression from PRNAI and Pspc (Fig. 2 and 3a and b) only indirectly, whereas enzyme expression from PRNAII (Fig. 3c and d) is, in addition, stimulated by a direct effect of ppGpp on transcription. Together, the direct and indirect effects produce the stronger response of β-galactosidase expression from PRNAII in comparison to those from the other two promoters, whose transcriptional activity is not affected by ppGpp during exponential growth (19).
FIG. 3.
β-Galactosidase expression from PRNAI and PRNAII during the stringent and the relaxed response. Stringent and relaxed strains carrying PRNAI-lacZ and PRNAII-lacZ operon fusions were grown exponentially in glucose minimal medium and treated with pseudomonic acid (3 μg/ml), as described in the legend for Fig. 2. (a and b) PRNAI-lacZ fusion strains YX101 (relA+) and YX103 (ΔrelA); (c and d) PRNAII-lacZ fusion strains YX102 (relA+) and YX104 (ΔrelA). The culture mass (OD600) values and β-galactosidase activities were normalized to the values observed at time zero (addition of pseudomonic acid); only the differential plots, of β-galactosidase activity versus culture mass, are shown.
Experiments similar to those for which results are shown in Fig. 2 and 3 were carried out with lacZ fusions carrying the phage λ promoter (PL) and the β-lactamase promoter (Pbla), whose transcription is also not significantly affected by ppGpp during exponential growth (19). During the stringent and the relaxed response, the expression of lacZ from the promoters showed the same apparent positive correlation to the ppGpp concentration as that observed for Pspc and PRNAI (data not shown). Since Pspc, PRNAI, PL, and Pbla are not known to have any common control elements, it is unlikely that the effect of ppGpp on enzyme expression is specific for these particular promoters. Rather, the effect is more likely to be related to general physiological aspects of bacterial growth that are affected by ppGpp (see Discussion).
DISCUSSION
Translational competition between bulk mRNA and specific mRNA.
In the following, we consider the effect of total (bulk) mRNA and its translation on enzyme expression during exponential growth in different media. For this purpose, the expression of β-galactosidase from Pspc (Fig. 1) was chosen as an example. The same logic applies to the expression of any stable protein, whether it is measured by enzyme assays, radioactive labeling, protein staining, Western blotting, or some other means. This discussion requires additional information about spc promoter activity, bulk mRNA synthesis, total RNA synthesis, and protein synthesis. This information was obtained from previous measurements in the same E. coli B/r background and is summarized (with references) in Table 2.
TABLE 2.
Parameters related to lacZ expression from Pspc in strain SL106a
| Parameter | Symbol | Unitb | Interpolated value at the indicated μ (τ)c
|
|||||
|---|---|---|---|---|---|---|---|---|
| 0.6 (100) | 1.0 (60) | 1.5 (40) | 2.0 (30) | 2.5 (24) | 3.0 (20) | |||
| Total proteind | Pt/OD | 1016 aa/OD460 | 58 | 55 | 51 | 48 | 45 | 40 |
| Total RNAd | Rt/OD | 1016 nt/OD460 | 3.3 | 3.8 | 4.4 | 5.3 | 6.3 | 6.7 |
| β-Gal sp acte | β-Gal/OD | (ΔA420/h)/OD600 | 280 | 195 | 140 | 120 | 105 | 80 |
| β-Gal sp actf | β-Gal/Pt | (ΔA420/h)/1016 aa | 3.0 | 2.2 | 1.7 | 1.6 | 1.5 | 1.3 |
| lacZ mRNA/total RNAg | Rlac/Rt | Relative units | 1.20 | 1.18 | 1.15 | 1.10 | 1.05 | 1.00 |
| lacZ mRNA translation rateh | (dβ-Gal/dt)/Rlac | Relative units | 0.31 | 0.32 | 0.30 | 0.30 | 0.29 | 0.26 |
| Stable RNA synthesis ratei | rs/rt | Fraction | 0.41 | 0.52 | 0.68 | 0.78 | 0.85 | 0.90 |
| Stable RNA synthesis ratej | rs/OD | 1014 nt/min/OD460 | 2.7 | 5.1 | 8.8 | 14.4 | 21.2 | 27.3 |
| mRNA synthesis ratek | rm/rt | Fraction | 0.59 | 0.48 | 0.32 | 0.22 | 0.15 | 0.10 |
| mRNA synthesis ratel | rm/OD | 1014 nt/min/OD460 | 3.9 | 4.7 | 4.2 | 4.1 | 3.7 | 3.0 |
| mRNA avg lifem | τm | min | 1.9 | 2.0 | 2.1 | 2.2 | 2.3 | 2.4 |
| mRNA/ODn | Rm/OD | 1014 nt/OD460 | 7.4 | 9.4 | 8.8 | 9.0 | 8.6 | 7.3 |
| mRNA/total RNAo | Rm/Rt | Fraction | 0.022 | 0.025 | 0.020 | 0.017 | 0.014 | 0.0011 |
| lacZ mRNA/total mRNAp | Rlac/Rm | Relative units | 54 | 47 | 57 | 65 | 76 | 92 |
| Protein synthesis rate/total RNAq | (dP/dt)/Rt | aa/min/nt | 0.12 | 0.17 | 0.20 | 0.21 | 0.21 | 0.21 |
| Peptide chain elongation rater | cp | aa polymerized/active ribosome | 13 | 18 | 22 | 22 | 22 | 22 |
| Distribution of ribosomes on mRNAs | dr | nt/ribosome | 143 | 160 | 129 | 108 | 88 | 70 |
| Avg protein synthesis rate/mRNAt | (dP/dt)/Rm | aa/min/nt | 5.5 | 6.8 | 10.0 | 12.4 | 15.1 | 19.0 |
| mRNA translation rateu | (di/dt)/Rm | translations/min/mRNA | 16 | 20 | 30 | 37 | 45 | 57 |
Values are interpolated from observed data to match growth rate values in reference 4, Tables 2 and 3.
aa, amino acids.
μ, growth rate, expressed as doublings per hour; τ, doubling time in minutes.
Per OD460 unit of culture mass (2).
Per OD600 unit (Fig. 1) (17). The value at 0.6 doubling/h has been obtained by extrapolation and is consistent with similar data from E. coli K-12 strains, which grow more slowly in glycerol minimal medium than B/r strains (19).
Per amount of protein, calculated as (β-Gal/OD600)/(1.6 Pt/OD460); the factor 1.6 converts OD460 units into OD600 units (2).
Amount of lacZ mRNA per amount of total RNA in relative units, normalized to the hybridization value observed in LB medium at 3.0 doublings/h, which was set at 1.0 (17).
Calculated as (ln2/τ) · (β-Gal/P) · (Pt/Rt)/(Rlac/Rt).
Rate of stable RNA (rRNA plus tRNA) synthesis as a fraction of total RNA synthesis rate (30; Table 3 of reference 4). The value at 3.0 doublings/h was obtained by extrapolation.
Per OD460 unit of culture mass; calculated as (ln2/τ) · 0.98 · 1.2 · Rt. The factors 0.98 and 1.2 reflect the facts that 98% of total RNA is stable RNA (about 2% is mRNA; see values for Rm/Rt below) and 20% of stable RNA precursors are rapidly degraded spacers.
As a fraction of total RNA synthesis rate, calculated as 1 − rs/rt.
Per OD460 unit of culture mass; calculated as rs/OD · (rm/rt)/(rs/rt).
The average functional life of mRNA is assumed to be equal to the average functional life of lacZ mRNA (18) (see the text). For E. coli B/r in glucose minimal medium, the average life of total mRNA has previously been estimated from pulse-labeling data to be about 1 min (1).
Amount of total mRNA/OD460 unit of culture mass; calculated as (rm/OD) · τm.
Calculated as (Rm/OD)/(Rt/OD).
Calculated as (Rlac/Rt)/(Rm/Rt).
Calculated as (ln2/τ) · (Pt/Rt).
Calculated as (dP/dt)/Rt · 4,566/(0.84 · 0.85 · 60). The factor 4,566 is the number of rRNA nucleotides per ribosome; 0.84 is the fraction of total RNA that is rRNA (14% is tRNA, and 2% is mRNA); 0.85 is the fraction of total ribosomes that is active at any given time; and 60 is the number of seconds per minute (see Table 3 in reference 4 for details).
Average distance in mRNA nucleotides between translating ribosomes for average (bulk) mRNA; calculated as 60 · cp/[(dP/dt)/Rm].
Calculated as [(dP/dt)/Rt]/(Rm/Rt).
Average rate of initiation (i) of translation (initiations per minute) per mRNA molecule; calculated as 3 · (dP/dt)/Rm = 60 · 3 · cp/dr, where the factor 3 represents the coding ratio (3 nt per amino acid) and the factor 60 is the number of seconds in a minute.
During exponential growth, the relative amounts of stable components reflect their relative synthesis rates; e.g., the specific activity of β-galactosidase in the cytoplasm, expressed as the amount of enzyme as a proportion of total protein (β-Gal/Pt), equals the quotient of the rates of synthesis of β-galactosidase and total protein [(dβ-Gal/dt)/(dPt/dt)]. If lacZ mRNA and average bulk mRNA were translated with equal efficiencies (equal translation initiations per minute per mRNA molecule), then the ratio of the synthesis rate of β-galactosidase to that of total protein (i.e., the specific activity) would reflect the proportion of lacZ mRNA in total (bulk) mRNA (Rlac/Rm). However, since lacZ mRNA and bulk mRNA may be translated differently, one has to consider the translation rate of lacZ mRNA [(dβ-Gal/dt)/Rlac] relative to the average translation rate of total mRNA [(dPt/dt)/Rm]. Using these parameters and notations, the specific activity of β-galactosidase can be related to the transcription and translation of lacZ and bulk mRNA as follows:
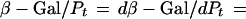 |
1 |
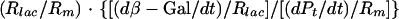 |
The first factor of the product on the right side of the equation (Rlac/Rm) reflects the relative abundance of lacZ mRNA, and the second factor, {[(dβ-Gal/dt)/Rlac]/[(dPt/dt)/Rm]}, reflects the translation efficiency of lacZ mRNA relative to that of bulk mRNA.
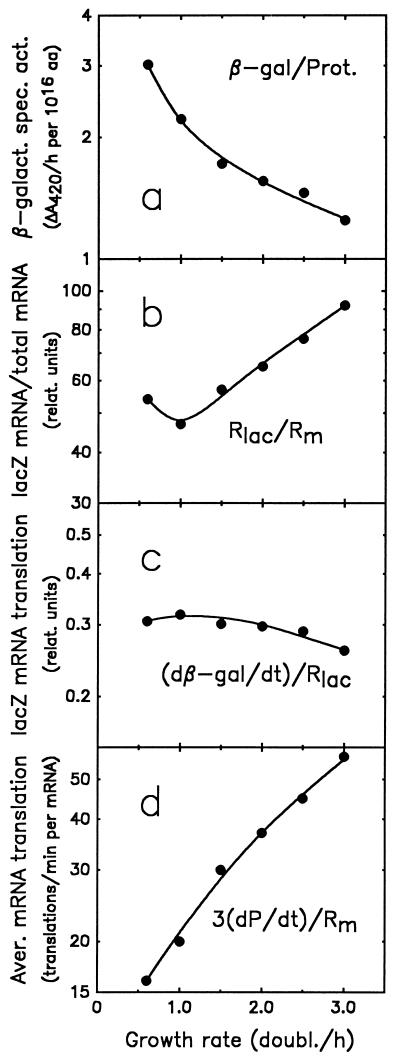
The amount of lacZ mRNA (Rlac, in relative units) expressed from Pspc in E. coli B/r has been determined previously with hybridization assays applied to given amounts of total RNA (Rt) (17). As a consequence, Rlac/Rt, rather than Rlac/Rm, is the parameter actually observed. To obtain Rlac/Rm, the observed Rlac/Rt, is divided by the fraction of total RNA that is mRNA (Rm/Rt). Since no hybridization probe is available for total mRNA, Rm/Rt was found indirectly. By using a hybridization probe for rRNA with pulse-labeled total RNA and correcting for the synthesis of tRNA, the rate of stable RNA synthesis (sum of rRNA plus tRNA) has previously been determined as a fraction of the rate of total RNA synthesis (rs/rt) (31). From rs/rt, the rate of mRNA synthesis was found, also as a fraction of the rate of total RNA synthesis, as the difference rm/rt = (1 − rs/rt). By combining rm/rt with the average mRNA lifetime, τm, the ratio of the amounts, Rm/Rt, can be obtained. Using published data for Rlac/Rt, rs/rt, and τm, and additional data on the macromolecular composition of E. coli B/r (i.e., total RNA and protein), all parameters occurring in equation 1 above have been calculated (Table 2; for details and references, see table footnotes) and plotted as functions of the growth rate (Fig. 4). It can be seen that the specific activity of β-galactosidase decreases (Fig. 4a) even though the amount of lacZ mRNA as a proportion of total mRNA (Rlac/Rm) increases (Fig. 4b), and the rate of translation initiation per lacZ mRNA [(dβ-Gal/dt)/Rlac] is approximately constant (Fig. 4c). This apparent discrepancy is explained by the higher rate of translation of bulk mRNA at high growth rates (Fig. 4d). In other words, the specific activity of β-galactosidase decreases with increasing growth rate (Fig. 4a), despite an increasing abundance of its mRNA (Fig. 4b), mainly because of an increasing translation of bulk mRNA (Fig. 4d). The reason for this increased translation of bulk mRNA is discussed below.
FIG. 4.
Growth rate dependence of the β-galactosidase specific activity expressed from Pspc in strain SL106 and of transcription and translation parameters that determine this specific activity (see the text and equation 1). (a) β-Galactosidase specific activity; (b) relative abundance of lacZ mRNA as a proportion of total mRNA; (c) rate of translation initiation of lacZ mRNA in relative units; (d) average rate of translation initiation of total (bulk) mRNA in translations per minute per average mRNA molecule. This rate can be found either from the total rate of protein synthesis per amount of mRNA [(dPt/dt)/Rm] or from the peptide chain elongation rate (cp) and the average distance of ribosomes on the mRNA (dr [see Table 2, footnote u]). Data represent interpolated values taken from Table 2 (see Table 2, footnotes f, p, h, and u, for the data in panels a, b, c, and d, respectively).
The rate of translation of bulk mRNA [(dPt/dt)/Rm] was calculated both as the number of amino acid residues polymerized into polypeptides per minute per mRNA nucleotide (Table 2) and as the number of translation initiations per minute per average mRNA molecule [(di/dt)/Rm] (Table 2; Fig. 4d). The latter was obtained from the former by multiplication by the coding ratio, 3 nt per amino acid residue. For example, according to Fig. 4d, an average mRNA molecule is translated almost once every second at a growth rate of 3 doublings/h.
Decay of bulk mRNA.
In Table 2, the average life of total (bulk) mRNA (τm) has not been observed. The decay of total mRNA is expected to be complex, with higher-order kinetics, reflecting at first the fast decay of the least-stable mRNAs and later the slower decay of the more-stable mRNAs. Since no data about the growth rate dependence of the decay of total mRNA are available, we have used instead the average functional lifetimes of lacZ mRNA, which are 1.9 and 2.4 min in E. coli B/r growing at 1 and 3 doublings/h, respectively (18). These lifetimes were assumed to be representative for bulk mRNA. If the lifetimes of different mRNA species differ by constant factors from the lacZ mRNA lifetimes (i.e., a different factor for each mRNA species, but the same mRNA species-dependent factor for all growth rates), then the curves for Rlac/Rm (Fig. 4b) and (dPt/dt)/Rm (Fig. 4d) would shift in parallel either up or down, depending on whether the average τm values for bulk mRNA are greater or smaller than those of lacZ mRNA. However, if the τm of bulk mRNA increases with the growth rate more than the τm of lacZ mRNA, then the curves in Fig. 4b and d would increase less steeply. The lifetime of ribosomal protein mRNAs does indeed appear to increase with increasing growth rate more than the lifetime of lacZ mRNA (18); therefore, the relative abundance of lacZ mRNA (Fig. 4b) and the translation of bulk mRNA (Fig. 4d) might increase somewhat less steeply with the growth rate than is shown.
Translation frequency of lacZ mRNA at different growth rates.
The approximately constant rate of translation of lacZ mRNA (Fig. 4c) could have several explanations: either (i) the lacZ ribosome binding site is saturated with ribosomes and initiation factors at all growth rates, (ii) the translation of lacZ mRNA is subject to a specific control that keeps it constant at all growth rates, or (iii) the concentrations of “initiation-ready” free ribosomes are nearly constant at all growth rates. Since the rate of translation of lacZ mRNA increases severalfold in the presence of the antibiotic rifampin when the amount of total mRNA decreases due to the inhibition of transcription (18), we conclude that, during normal exponential growth, the lacZ ribosome binding site is not saturated. Moreover, despite extensive study, there is no evidence to suggest that translation of lacZ mRNA is subject to any specific control. This leads us to suggest that the concentration of initiation-ready ribosomes (i.e., mature 30S ribosomal subunits with IF3, ready to bind to an mRNA ribosome binding site in the presence of saturating or constant concentrations of IF1, IF2, and initiator tRNA) is relatively constant and subsaturating at different growth rates. This causes the observed constant frequency of lacZ mRNA translation (Fig. 4c).
Translation frequency of bulk mRNA at different growth rates.
Several features could contribute to the increasing average rate of translation initiation of bulk mRNA at increasing growth rates (Fig. 4d) despite a constant concentration of free, initiation-ready ribosomes and/or factors (see above). First, the average mRNA present during growth in rich media may have more-efficient ribosome binding sites than mRNA made during growth in poor media. Alternatively or in addition, the rate of translation of some mRNAs may be controlled by special regulatory sites such that translation is favored at high growth rates. The second possibility, i.e., control that favors translation at high growth rates, has been found to occur for ribosomal protein mRNAs (reviewed in reference 15), but it is not known to be the rule for most other mRNAs. Therefore, both more-efficient ribosome binding sites on constitutive mRNAs made during growth in rich media compared to more-repressible mRNAs made during growth in poor media and translational control of certain mRNAs are inferred to contribute to the increased translation of bulk mRNA at high growth rates. Whatever the exact cause, the increased translation makes bulk mRNA an increasingly better competitor for ribosome binding to lacZ mRNA as the population of mRNAs changes with increasing growth rate.
Effect of ppGpp on protein and enzyme expression.
During a mild stringent or a mild relaxed response, the β-galactosidase specific activities expressed from both Pspc-lacZ and PRNAI-lacZ operon fusions were increased and reduced, respectively (Fig. 2 and 3). Based on the analysis of the growth rate dependence of enzyme expression above, we suggest that ppGpp affects the accumulation and quality of bulk mRNAs and thereby causes the apparent positive control of enzyme expression by ppGpp. Indirect stimulation of lacZ expression by ppGpp (Fig. 2 and 3) could be produced in a number of ways. For example, ppGpp might directly or indirectly stimulate the synthesis of mRNAs with weak ribosome binding sites, or it might inhibit the synthesis of mRNAs with strong ribosome binding sites. Indirect effects of ppGpp on transcription are expected for promoters controlled by DNA binding factors like Fis, H-NS, or Lrp (see, e.g., references 9, 29, 34 and 36), whose syntheses depend on ppGpp. LacZ expression may also be increased if ppGpp stimulates the decay of mRNAs with strong ribosome binding sites (e.g., ribosomal protein mRNAs [18]). Any one or all of these effects might contribute to the apparent stimulation of enzyme expression by ppGpp.
Combined effects of growth rate and ppGpp on bacterial gene expression.
The effects of ppGpp and growth rate on bulk mRNA synthesis and translation are superimposed on the direct transcriptional control by ppGpp. Three cases are to be distinguished, as follows.
(i) If a promoter is under positive transcriptional control by ppGpp, as is PRNAII, then the direct and indirect effects of ppGpp are additive, so that the specific activity of β-galactosidase decreases with increasing growth rate or at reduced levels of ppGpp more than the specific activity expressed from most other mRNA promoters that are not affected by ppGpp (e.g., Pspc, PRNAI, λ PL, and Pbla [19]), as observed (Fig. 1). Earlier reports suggest that the promoters of the histidine biosynthetic operon and the lac operon are also under positive control by ppGpp (27, 28, 32).
(ii) If a promoter is under negative transcriptional control by ppGpp, as are the P1 promoters of rrn operons (19), then both transcriptional activity and the expression of a reporter enzyme from P1 increase with increasing growth rate (10, 13, 19, 35). But in this instance, the transcriptional activity of rrnB P1 increases about 40-fold in the range of growth rates studied (between 0.7 and 3.0 doublings/h), whereas the specific activity of β-galactosidase expressed from rrnB P1 increases only about 15-fold (19, 35). Thus, the indirect effects of bulk mRNA subtract from the direct transcriptional effect of ppGpp on P1.
(iii) An intermediate situation is represented by the P2 promoters of rrn operons, whose transcriptional activity increases with growth rate less than transcription from rrn P1 but more than transcription from mRNA promoters (19). β-Galactosidase expression from rrnB P2 is approximately constant and independent of the bacterial growth rate (10, 19, 35). Thus, for rrnB P2, the effects of an increasing rate of transcription from the promoter and increasing competition of bulk mRNA for translation compensate for one another. The constancy of the specific activity of β-galactosidase expressed from rrnB P2 has previously been interpreted as an indication of a lack of “growth rate-dependent control” (see, e.g., reference 10). We suggest that it is more likely to be a coincidence of two opposing effects on gene expression.
Control of rRNA synthesis: role of free RNA polymerase concentration and ppGpp.
Using a lacZ reporter system and lacZ mRNA hybridization assays, the absolute activities of the rrnB P1 and P2 promoters, expressed as number of transcripts per minute per promoter, were previously found to increase with increasing growth rate (19). Since no specific control factors or factor binding sites have ever been associated with rrn P2 promoters, we have assumed that rrn P2 promoters are constitutive, so that their activity is affected only by the concentration of free RNA polymerase and the promoter-specific parameters Vmax and Km, representing the maximum promoter activity at saturation with free RNA polymerase and the free RNA polymerase concentration at half-maximal activity. In the absence of ppGpp, the rrn P1 promoters are also constitutive; accordingly, their activity also depends only on the free RNA polymerase concentration and the associated Vmax and Km values. The Km value for P1 promoters is affected by the DNA binding factors Fis and H-NS. In addition, in normal ppGpp-proficient strains, P1 activity was found to be inhibited by ppGpp. As a result, the P1 activity was lower than the P2 activity during slow bacterial growth, when basal (exponential-growth) levels of ppGpp are highest, and higher than the P2 activity during fast bacterial growth, when basal levels of ppGpp are lowest. Thus, in our model (19), the growth rate regulation of ribosome synthesis depends both on the concentration of free RNA polymerase, which determines the frequency of transcription initiation at both P1 and P2, and on the concentration of ppGpp, which selectively reduces expression from P1 under slow-growth conditions.
Role of ppGpp in the control of ribosomal protein synthesis.
It has been reported that the rate of synthesis of spc mRNA from the normal spc operon relative to the rate of total mRNA synthesis increases with increasing growth rate (8, 20). This is similar to the growth rate-dependent increase in the mRNA amounts, Rlac/Rm, determined above for the Pspc-rplN-lacZ fusion (Fig. 4b). Since the rate of ribosomal protein synthesis as a proportion of total protein synthesis (denoted by αr [3]) increases with increasing growth rate similarly to the rate of spc mRNA synthesis per total mRNA synthesis, it has been suggested that the control of ribosomal protein synthesis occurs mainly at the transcriptional level (4, 8, 20). This interpretation was based on the two implicit assumptions that (i) spc mRNA and bulk mRNA are translated approximately equally and (ii) spc mRNA and bulk mRNA have approximately equal lifetimes. Based on the considerations above (Fig. 4) and previous measurements of spc mRNA lifetimes (18), both assumptions appear to be unjustified. The increasing rate of synthesis of ribosomal proteins from the spc operon with increasing growth rate is mediated through control of the decay of spc mRNA; similar mechanisms presumably control the decay of other ribosomal protein mRNAs (18, 22). This control of the mRNA lifetime adjusts the synthesis of ribosomal proteins to the ppGpp-dependent synthesis of rRNA, overriding any transcriptional regulation of ribosomal protein operons or superimposed effects of bulk mRNA translation.
The synthesis rates of spc ribosomal proteins and of spc operon mRNA have been determined previously during mild amino acid starvation or chloramphenicol treatment of stringent and relaxed strains (3, 5, 6, 7, 21). Those studies suggested that the activity of Pspc is stringently controlled, i.e., reduced when ppGpp levels increase and enhanced when ppGpp levels decrease. Those observations contradict the apparent positive control by ppGpp of β-galactosidase synthesis expressed from Pspc observed here (Fig. 2). This discrepancy can be explained as follows. The wild-type spc operon mRNA carries a control site at which the regulatory ribosomal protein S8 binds under conditions of reduced rRNA synthesis (e.g., during the stringent response). The binding of S8 to its own mRNA initiates a regulatory pathway that accelerates the decay of spc operon mRNA, thereby adjusting ribosomal protein synthesis to the accumulation of rRNA (reviewed in reference 15). Therefore, we suggest that the changes in the synthesis of spc ribosomal proteins that have been observed previously during the stringent and the relaxed response or during chloramphenicol treatment are the result of a regulation of spc mRNA decay in response to ppGpp-dependent changes in rRNA synthesis (18). This regulation requires specific control sites on the normal spc mRNA which are not present in the spc-lac fusion mRNA used here.
ACKNOWLEDGMENTS
This work has been supported by grants from the NIH and MRC.
We thank Mans Ehrenberg for valuable comments regarding the biochemistry of translation initiation.
REFERENCES
- 1.Baracchini E, Bremer H. Determination of synthesis rate and lifetime of bacterial mRNAs. Anal Biochem. 1987;167:245–260. doi: 10.1016/0003-2697(87)90160-6. [DOI] [PubMed] [Google Scholar]
- 2.Bipatnath M, Dennis P P, Bremer H. Initiation and velocity of chromosome replication in Escherichia coli B/r and K-12. J Bacteriol. 1998;180:265–273. doi: 10.1128/jb.180.2.265-273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenthal R M, Lemaux P G, Neidhardt F C, Dennis P P. The effects of the relA gene on the synthesis of aminoacyl-tRNA synthetases and other transcription and translation proteins in Escherichia coli. B Mol Gen Genet. 1976;149:291–296. doi: 10.1007/BF00268530. [DOI] [PubMed] [Google Scholar]
- 4.Bremer H, Dennis P P. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1553–1569. [Google Scholar]
- 5.Dennis P P. Effects of chloramphenicol on the transcriptional activities of ribosomal RNA and ribosomal protein genes in Escherichia coli. J Mol Biol. 1976;108:535–546. doi: 10.1016/s0022-2836(76)80135-0. [DOI] [PubMed] [Google Scholar]
- 6.Dennis P P, Nomura M. Stringent control of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci USA. 1974;71:3819–3823. doi: 10.1073/pnas.71.10.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis P P, Nomura M. Stringent control of the transcriptional activities of ribosomal protein genes in Escherichia coli. Nature (London) 1975;255:460–465. doi: 10.1038/255460a0. [DOI] [PubMed] [Google Scholar]
- 8.Gausing K. Regulation of ribosome production in Escherichia coli: synthesis and stability of ribosomal RNA and ribosomal protein mRNA at different growth rates. J Mol Biol. 1977;115:335–354. doi: 10.1016/0022-2836(77)90158-9. [DOI] [PubMed] [Google Scholar]
- 9.Gosink K K, Ross W, Leirmo S, Osuna R, Finkel S E, Johnson R C, Gourse R. DNA binding and bending are necessary but not sufficient for Fis-dependent activation of rrnB P1. J Bacteriol. 1993;175:1580–1589. doi: 10.1128/jb.175.6.1580-1589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gourse R L, De Boer H A, Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate-dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986;44:197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- 11.Helmstetter C. Rate of DNA synthesis during the division cycle of Escherichia coli B/r. J Mol Biol. 1967;24:417–427. [Google Scholar]
- 12.Hernandez V J, Bremer H. Escherichia coli ppGpp synthetase II activity requires spoT. J Biol Chem. 1991;266:5991–5999. [PubMed] [Google Scholar]
- 13.Hernandez V J, Bremer H. Characterization of RNA and DNA synthesis in Escherichia coli strains devoid of ppGpp. J Biol Chem. 1993;268:10851–10862. [PubMed] [Google Scholar]
- 14.Hughes J, Mellows G. Inhibition of isoleucyl-transfer ribonucleic acid synthetase in Escherichia coli by pseudomonic acid. Biochem J. 1978;176:305–318. doi: 10.1042/bj1760305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keener J, Nomura M. Regulation of ribosome synthesis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1417–1431. [Google Scholar]
- 16.Lagosky P, Chang F. Correlation between RNA synthesis and basal level guanosine 5′-diphosphate 3′-diphosphate in relaxed mutants of Escherichia coli. J Biol Chem. 1981;256:11651–11656. [PubMed] [Google Scholar]
- 17.Liang S, Dennis P P, Bremer H. Expression of lacZ from the promoter of the Escherichia coli spc operon cloned into vectors carrying the W205 trp-lac fusion. J Bacteriol. 1998;180:6090–6100. doi: 10.1128/jb.180.23.6090-6100.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang S, Ehrenberg M, Dennis P P, Bremer H. Decay of rplN and lacZ mRNA in Escherichia coli. J Mol Biol. 1999;288:521–538. doi: 10.1006/jmbi.1999.2710. [DOI] [PubMed] [Google Scholar]
- 19.Liang S, Bipatnath M, Xu Y-C, Chen S-L, Dennis P P, Ehrenberg M, Bremer H. Activities of constitutive promoters in Escherichia coli. J Mol Biol. 1999;292:19–37. doi: 10.1006/jmbi.1999.3056. [DOI] [PubMed] [Google Scholar]
- 20.Little R, Bremer H. Transcription of ribosomal component genes and lac in a relA+/relA− pair of Escherichia coli strains. J Bacteriol. 1984;159:863–869. doi: 10.1128/jb.159.3.863-869.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maher D, Dennis P. In vivo transcription of E. coli genes coding for rRNA, ribosomal proteins and subunits of RNA polymerase: influence of the stringent control system. Mol Gen Genet. 1977;155:203–211. doi: 10.1007/BF00393161. [DOI] [PubMed] [Google Scholar]
- 22.Mattheakis L, Vu L, Sor F, Nomura M. Retroregulation of the synthesis of ribosomal proteins L14 and L24 by feedback repressor S8 in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:448–452. doi: 10.1073/pnas.86.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller J. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 24.Mitchell D H, Reznikoff W S, Beckwith J R. Genetic fusions defining trp and lac operon regulatory elements. J Mol Biol. 1975;93:331–350. doi: 10.1016/0022-2836(75)90281-8. [DOI] [PubMed] [Google Scholar]
- 25.Mott J E, Galloway J L, Platt T. Maturation of Escherichia coli tryptophan operon mRNA: evidence for 3′ exonucleolytic processing after rho-dependent termination. EMBO J. 1985;4:1887–1891. doi: 10.1002/j.1460-2075.1985.tb03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pease A J, Wolf R E. Determination of the growth rate-regulated steps in expression of the Escherichia coli K-12 gnd gene. J Bacteriol. 1994;176:115–122. doi: 10.1128/jb.176.1.115-122.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Primakoff P, Artz S W. Positive control of lac operon expression in vitro by guanosine 5′-diphosphate 3′-diphosphate. Proc Natl Acad Sci USA. 1979;76:1726–1730. doi: 10.1073/pnas.76.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riggs D C, Mueller R D, Kwan H S, Artz S W. Promoter domain mediates guanosine tetraphosphate activation of the histidine operon. Proc Natl Acad Sci USA. 1986;83:9333–9337. doi: 10.1073/pnas.83.24.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross W, Thompson J F, Newlands J T, Gourse R L. E. coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 1990;9:3733–3742. doi: 10.1002/j.1460-2075.1990.tb07586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryals J, Bremer H. RelA-dependent RNA polymerase activity in Escherichia coli. J Bacteriol. 1982;150:168–179. doi: 10.1128/jb.150.1.168-179.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryals J, Little R, Bremer H. Control of rRNA and tRNA syntheses in Escherichia coli by guanosine tetraphosphate. J Bacteriol. 1982;151:1261–1268. doi: 10.1128/jb.151.3.1261-1268.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shand R F, Blum P H, Mueller R D, Riggs D C, Artz S W. Correlation between histidine operon expression and guanosine 5′-diphosphate 3′-diphosphate levels during amino acid downshift in stringent and relaxed strains of Salmonella typhimurium. J Bacteriol. 1989;171:737–743. doi: 10.1128/jb.171.2.737-743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schleif R. Control of the production of ribosomal protein. J Mol Biol. 1967;27:41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- 34.Wagner R. The regulation of ribosomal RNA synthesis and bacterial cell growth. Arch Microbiol. 1994;161:100–109. doi: 10.1007/BF00276469. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X-Y, Bremer H. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J Biol Chem. 1995;270:11181–11195. doi: 10.1074/jbc.270.19.11181. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X-Y, Bremer H. Effects of Fis on ribosome synthesis and activity and on promoter activities in E. coli. J Mol Biol. 1996;259:27–40. doi: 10.1006/jmbi.1996.0299. [DOI] [PubMed] [Google Scholar]