Abstract
Background
Data regarding outcome of Coronavirus disease 2019 (COVID-19) in vaccinated patients with autoimmune hepatitis (AIH) are lacking. We evaluated the outcome of COVID-19 in AIH patients who received at least one dose of Pfizer- BioNTech (BNT162b2), Moderna (mRNA-1273) or AstraZeneca (ChAdOx1-S) vaccine.
Patients and methods
We performed a retrospective study on AIH patients with COVID-19. The outcomes of AIH patients who had acute respiratory syndrome coronavirus 2 (SARS-CoV-2) breakthrough infection after at least one dose of COVID-19 vaccine were compared to unvaccinated patients with AIH. COVID-19 outcome was classified according to clinical state during the disease course as: (i) no hospitalization, (ii) hospitalization without oxygen supplementation, (iii) hospitalization with oxygen supplementation by nasal cannula or mask, (iv) intensive care unit (ICU) admission with non-invasive mechanical ventilation, (v) ICU admission with invasive mechanical ventilation or (vi) death, and data was analyzed using ordinal logistic regression.
Results
We included 413 (258 unvaccinated and 155 vaccinated) patients (81%, female) with a median age of 52 (range: 17–85) years at COVID-19 diagnosis. The rates of hospitalization were (36.4% vs. 14.2%), need for any supplemental oxygen (29.5% vs. 9%) and mortality (7% vs. 0.6%) in unvaccinated and vaccinated AIH patients with COVID-19. Having received at least one dose of SARS-CoV-2 vaccine was associated with a significantly lower risk of worse COVID-19 severity, after adjusting for age, sex, comorbidities and presence of cirrhosis (adjusted odds ratio [aOR] 0.18, 95% confidence interval [CI], 0.10–0.31). Overall, vaccination against SARS-CoV-2 was associated with a significantly lower risk of mortality from COVID-19 (aOR 0.20, 95% CI 0.11–0.35).
Conclusions
SARS-CoV-2 vaccination significantly reduced the risk of COVID-19 severity and mortality in patients with AIH.
Keywords: Liver failure, Breakthrough infection, Immunosuppression, Vaccine, Autoimmunity
1. Introduction
Coronavirus Disease 2019 (COVID-19) caused by the acute severe respiratory syndrome coronavirus-2 (SARS-CoV-2) infection has rapidly spread worldwide and caused an ongoing pandemic since December 2019 [1]. Most cases with COVID-19 have mild symptoms but COVID-19 can develop into respiratory failure and death [2,3]. Several comorbidities such as cardiovascular diseases, chronic lung diseases, kidney disease, and chronic liver diseases (CLD) have been implicated as risk factors for adverse COVID-19 outcomes [[4], [5], [6], [7]].
The rates of COVID-19-related hospitalization and mortality have significantly decreased in the general population following effective vaccination program [8]. Importantly, patients who were on immunosuppressive therapy for conditions such as autoimmune hepatitis (AIH), inflammatory bowel diseases or after liver transplantation (LT) were not included in the phase 3 trials of mRNA-based vaccines [9,10]. Only 196 (0.6%) patients with liver disease were included in the Moderna mRNA-1273 trial [10].
The current hepatology guidelines strongly recommend vaccination against SARS-CoV-2 for patients with CLD and LT recipients, but little data is available about vaccine response and post-vaccination clinical outcome of COVID-19 in these patient groups [[11], [12], [13]].
In this study, we describe the clinical outcome of COVID-19 among AIH patients who had been vaccinated against SARS-CoV-2 infection. The rates of hospitalization, need for respiratory support, and mortality were compared in vaccinated and unvaccinated AIH patients contracting SARS-CoV-2 infection.
2. Materials and methods
2.1. Study design
AIH-COVID-19 study group (supplementary table 1) independently collected data of AIH patients who were diagnosed with COVID-19. All participants identified patients and collected data from electronic records and patient charts using the same case report form. Only AIH patients (>16 years old) who were diagnosed with COVID-19 (hospital-based PCR test) between March 11, 2020 and April 01, 2022 were included in the study. We also identified those AIH patients who had SARS-CoV-2 infection despite having received at least one dose of mRNA based (BNT162b2mRNA or mRNA-1273) or ChAdOx1-S vaccine. For patients with multiple episodes of COVID-19, data were recorded only for the first COVID-19 episode. The Harran University Hospital of Şanlıurfa was the coordinating center and local ethics review boards of centers providing patient data approved the study.
2.2. Data collection
Collected patient data included demographic characteristics, comorbidities, types and doses of immunosuppression and presence of cirrhosis. Data on the entire COVID-19 disease course was collected until recovery or death. Details about collected variables were described previously by the study group [7,14,15]. Dates, doses and types of administered SARS-CoV-2 vaccines and date of breakthrough infection were also recorded. Data on virus genotypes and spike antibody titers were not available for most patients.
2.3. Outcomes
The severity of COVID-19 was classified using a six-level ordinal scale based on the worst clinical state during the disease course until death or recovery: (i) no hospitalization, (ii) hospitalization without oxygen supplementation, (iii) hospitalization requiring oxygen supplementation by nasal cannula or mask, (iv) intensive care unit (ICU) admission requiring non-invasive mechanical ventilation, (v) ICU admission requiring invasive mechanical ventilation and, (vi) death. This ordinal COVID-19 outcome spectrum was obtained from the WHO clinical progression scale [16].
2.4. Statistical analysis
We used descriptive statistics to summarize patient characteristics and outcome categories overall and by vaccination status. The association between vaccination status and COVID outcome was analyzed using an ordinal logistic regression model, in which the six-level ordinal COVID-19 outcome was the dependent variable and vaccination status was the independent variable of interest. We fitted models without adjustment, with adjustment for age and sex, and with additional adjustment for the number of comorbidities and presence of cirrhosis to estimate crude and adjusted odds ratios (OR) with 95% confidence intervals. We also fitted binary logistic regression models to separately analyze the relative risk of mortality. In an additional analysis we used an interaction term between vaccination status and the presence of cirrhosis to explore a possible heterogeneity in the association between vaccination status and COVID mortality in AIH patients with cirrhosis. All analyses were conducted using the open-source R software v. 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) running under the IDE RStudio v. 1.3.1103 (RStudio, Boston MA) using the MASS package.
3. Results
3.1. General characteristics of the study population
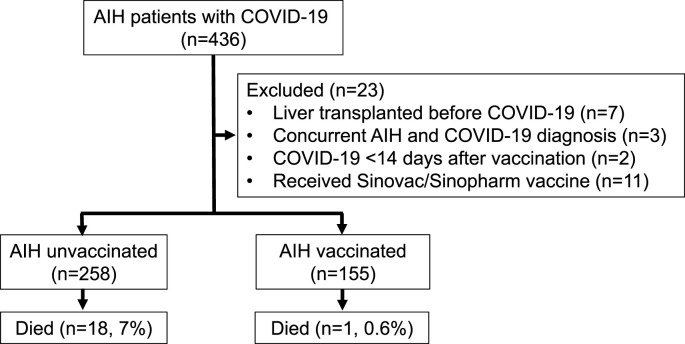
Of the 436 AIH patients with COVID-19, we excluded patients who had undergone liver transplantation (n = 7) prior to COVID-19, those who were concomitantly diagnosed with AIH and COVID-19 (n = 3), patients who developed SARS-CoV-2 infection <14 days after the first vaccine dose (n = 2) and those who received other types of vaccines (Sinovac/Sinopharm) (n = 11). The final study population included 413 AIH patients with COVID-19 (Fig. 1 ). The data of 254 patients were also included in previous studies [7,14]. The general characteristics of the study population are presented in Table 1 .
Fig. 1.
Study flow chart for patient inclusion.
Table 1.
Demographics and clinical features of study population.
| Study population (n = 413) | AIH unvaccinated (n = 258) | AIH vaccinated (n = 155) | |
|---|---|---|---|
| Median age, years (range) | 52 (17–85) | 50 (17–85) | 53 (18–83) |
| Female, n (%) | 333 (80.6) | 205 (79.5) | 128 (82.6) |
| Variant syndromes (PBC/PSC), n (%) | 39 (9.4) | 26 (10.1) | 13 (8.4) |
| Cirrhosis, n (%) | 117 (28.3) | 71 (27.5) | 46 (29.7) |
| Smoking (current), n (%) | 27 (6.5) | 15 (5.8) | 12 (7.7) |
| Alcohol, n (%) | 5 (1.2) | 4 (1.6) | 1 (0.6) |
| Co-morbidity, (%) | 180 (43.6) | 109 (42.2) | 71 (45.8) |
| Arterial Hypertension | 106 (25.7) | 63 (24.4) | 43 (27.7) |
| Diabetes mellitus | 89 (21.5) | 55 (21.3) | 34 (21.9) |
| Cardiac disease | 24 (5.9) | 13 (5) | 11 (7.1) |
| Respiratory disease | 17 (4.1) | 7 (2.8) | 10 (6.5) |
| Kidney insufficiency | 16 (3.9) | 8 (3.1) | 8 (5.2) |
| Active cancer | 7 (1.7) | 3 (1.2) | 4 (2.6) |
| AIH medications, n (%) | 388 (93.9) | 238 (92.2) | 150 (96.8) |
| AZA alone (median dose, mg) | 103 (24.9) 87.5 (25–200) |
65 (25.2) 75 (25–200) |
38 (24.5) 100 (25–200) |
| Prednisolone (equivalent) alone (median dose, mg) | 67 (16.2) 5 (2.5–60) |
49 (19.0) 5 (2.5–60) |
18 (11.6) 5 (2.5–40) |
| MMF alone (median dose, mg) | 14 (3.4) 1500 (500–2000) |
10 (3.9) 1000 (500–2000) |
4 (2.5) 2000 (500–2000) |
| Tacrolimus alone (median dose, mg) | 4 (0.9) 3 (1–7) |
3 (1.2) 3 (1.5–7) |
1 (0.6) 4 (1–6) |
| 6-MP alone | 1 (0.2) | 1 (0.4) | – |
| Methotrexate alone | 1 (0.2) | – | 1 (0.6) |
| Any combination | 198 (49.1) | 110 (42.6) | 88 (56.8) |
| Symptoms at presentation, n (%) | 384 (93) | 237 (91.9) | 147 (94.8) |
| Medical therapies for COVID-19, n (%) | 139 (33.7) | 118 (45.7) | 21 (13.5) |
| Antibiotics, n (%) | 111 (26.9) | 88 (34.1) | 23 (14.8) |
AZA, azathioprine; MMF, mycophenolate mofetil; 6-MP, mercaptopurine; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis.
3.2. Characteristics of AIH patients with breakthrough SARS-CoV-2 infection
A total of 155 patients (82.6%, female) with a median age of 53 (range: 18–83) years had breakthrough infection. The majority (96.8%) were on immunosuppressive therapy at the time of COVID-19 diagnosis. Forty-six (29.7%) patients showed features of cirrhosis and 71 (45.8%) patients had comorbidities (Table 1). Most patients (n = 147, 94.8%) were symptomatic at the time of COVID-19 diagnosis.
The Pfizer-BioNTech vaccine was received by 131 (84.5%) patients, Moderna by 11 (7.1%) and Oxford-AstraZeneca by 13 (8.4%) patients. Three of these patients received heterologous vaccination. Twenty-two patients (16 in the single dose group) had previously received other vaccines, Sinovac/Sinopharm (n = 21) and Sputnik (n = 1), prior to Pfizer-BioNTech (BNT162b2), Moderna (mRNA-1273) or AstraZeneca (ChAdOx1-S) vaccination. Among the identified AIH patients with breakthrough infections, 28 (18%) had received one dose of vaccine, 96 (62%) two doses and 31 (20%) three doses.
Outcome of COVID-19 in vaccinated and unvaccinated patients with AIH.
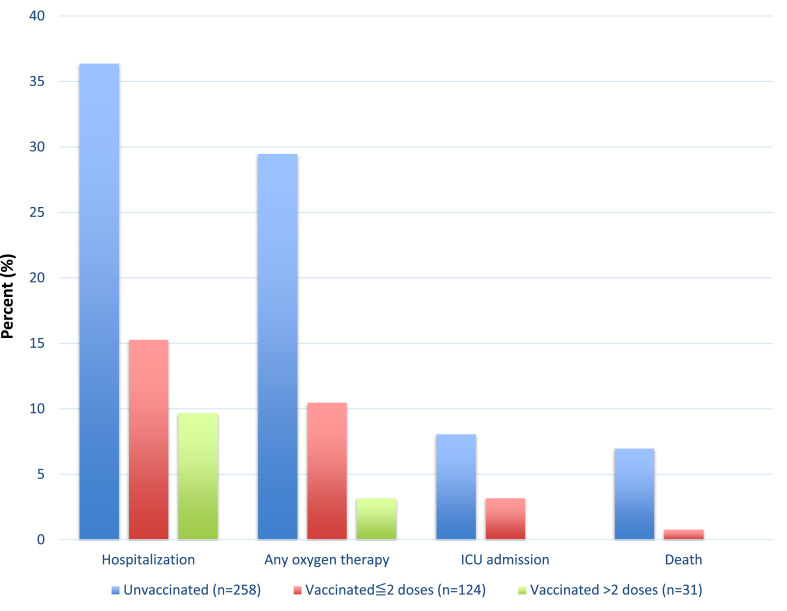
The outcomes of AIH patients with COVID-19 according to vaccination status are presented in Fig. 2 and supplementary table 2. The rates of hospitalization were 36.4% vs. 14.2%, need for supplemental oxygen were 29.5% vs. 9% and mortality were 7% vs. 0.6% among unvaccinated and vaccinated AIH patients with COVID-19, respectively.
Fig. 2.
Outcome of COVID-19 in patients with autoimmune hepatitis.
After adjusting for age, gender, co-existence of comorbid condition and presence of cirrhosis, vaccination (any number of doses) against SARS-CoV-2 was associated with a significantly decreased risk of worse COVID-19 severity (aOR 0.18, 95% CI 0.10–0.31) in AIH patients with breakthrough infection (Table 2 ). Crude, age-sex adjusted, and fully adjusted odds ratios with CIs are presented in supplementary table 3. Vaccination (dose >2) further reduced the risk of COVID-19 severity (aOR 0.11, 95% CI 0.02–0.34). Overall, at least one dose of vaccination was associated with a significantly decreased risk of COVID-19 related mortality (aOR 0.20, 95% CI 0.11–0.35). Adding an interaction term between vaccination status and cirrhosis did not suggest a significant heterogeneity in the association between vaccination status and mortality in patients with or without cirrhosis (p for interaction 0.12, supplementary table 3).
Table 2.
Crude and adjusted odds ratios for worse COVID-19 outcome by vaccination status.
| Crude | Age and sex adjusted | Age, sex, comorbid diseases and cirrhosis adjusted | |
|---|---|---|---|
| Vaccine (any) doses | 0.28 (0.16–0.46) | 0.27 (0.16–0.44) | 0.18 (0.10–0.31) |
| Vaccine (1 or 2) doses | 0.31 (0.17–0.52) | 0.30 (0.17–0.51) | 0.20 (0.11–0.36) |
| Vaccine (3) doses | 0.18 (0.04–0.51) | 0.16 (0.04–0.48) | 0.11 (0.02–0.34) |
4. Discussion
SARS-CoV-2 vaccination has shown an excellent safety profile and significantly reduces the risks of COVID-19 and its complications in the general population [9,10,17]. The current hepatology guidelines strongly recommend full vaccination for patients with CLD and LT recipients while data regarding the clinical effectiveness of vaccination in these patient groups has been little documented [11]. In this study, we investigated the impact of SARS-CoV-2 vaccination on the clinical severity of COVID-19 in an international multi-center dataset of patients with AIH. The rates of hospitalization, need for supplemental oxygen and mortality were significantly lower among vaccinated patients compared to unvaccinated patients with AIH. Overall, our data showed that receiving at least one vaccine dose significantly reduced the risk of worse COVID-19 outcome and death in patients with AIH.
A recent study suggested that vaccination against SARS-CoV-2 was associated with favorable COVID-19 outcomes among 21 vaccinated patients with CLD, as assessed by the absence of mechanical ventilation and death [13]. The limited number of patients precluded more rigorous analyses. In another study [12], 83 patients with cirrhosis who received mRNA-based vaccine (Pfizer-BioNTech or Moderna) were compared with a propensity-matched control group of 105 patients at similar risk of COVID-19. In that cohort, SARS-CoV-2 vaccine significantly reduced the risk of COVID-19 related hospitalization and death. Similarly, another study from USA showed reduced mortality of COVID-19 in vaccinated patients with cirrhosis [18]. In our AIH cohort, vaccination status and mortality did not show a significant heterogeneity between patients with or without cirrhosis.
Among 155 AIH patients with breakthrough infections, four (2.6%) patients were admitted ICU and one (0.6%) died. Several studies have reported that vaccine immunogenicity may not be robust for patients with CLD, particularly those with advanced liver disease [19,20]. Similarly, patients who are under immunosuppressive medications, such as liver transplant recipients, have blunted spike antibody response following SARS-CoV-2 vaccination [20,21]. A recent study evaluated the immune response to SARS-CoV-2 vaccination in 96 AIH patients [22]. Ten (10%) of these patients had low or no response to vaccination. AIH patients had significantly lower antibody levels than healthy controls and an early third booster dose was recommended for patients with AIH. We evaluated the clinical efficacy of a third vaccine dose in our AIH population. The relative risk of worse COVID-19 severity was numerically lower in patients who received third dose booster than in those who received one or two vaccine doses. Three patients who received three doses were still hospitalized, suggesting that patients with AIH are still at risk for severe COVID-19 outcome despite vaccination. Additional preventive health measures such as social distancing and mask-wearing should be implemented in this vulnerable population.
We acknowledge that the retrospective nature of our study is the main limitation. Our study group is mainly interested in the outcome of COVID-19 in AIH and all members rigorously collected data. We used the WHO clinical progression scale of COVID-19 which includes very clear endpoints. We therefore believe that the risk of major bias is limited in our main study results. Another limitation is the possibility of selection bias since patients who had COVID-19 vaccination may be less likely to receive COVID-19 PCR testing in the presence of symptoms, and asymptomatic patients or those with mild disease were probably not identified and therefore not included in our study. Importantly, in case of such a bias, vaccine effectiveness would likely be stronger than reported here. We also did not collect data on virus genotypes or spike protein antibody titers and could not evaluate effect of different variants on outcome of AIH patients with COVID-19. The comparison of time periods, before and after December 2021 when the omicron variant became predominant, did not show any association with COVID-19 outcome (supplementary table 4). Evaluation of any virus variant effects on COVID-19 outcome would require large population-based studies. We aimed to study the clinical outcome of COVID-19 in vaccinated patients with AIH and we believe that our study findings provide valuable insights into the clinical effectiveness of vaccination in AIH patients and that they may be generalized to patients with other etiologies of CLD.
In conclusion, this large multi-center study found that vaccination against SARS-CoV-2 (mRNA or vector based) significantly reduced the risk of a worse COVID-19 disease course and death in patients with AIH.
Author statement
CE, KT, SW, PI and ER conceptualized the study. CE, KT FB, NKG and ER collected and analyzed data. KT performed statistical analysis. CE, CL, BE, AFS, MC, DTG, AG, MP, HM, PM, EC, CR, SY, GA, ARÇ, YB, FE, TE, SB, EL, GMZ, MAK, AHB, EDM, AY, MB, GCN, NH, NKG, JA, RI, MS, MM, KA, FG, RD, AJML, ER, SD, GND, PI, and SW contributed data and approved final manuscript. CE, KT, RD, ER, PI and SW interpreted data and prepared manuscript for the final submission.
Declaration of competing interest
None.
Acknowledgement
Turkish Association for the Study of Liver (TASL) organized and supported data collection of Turkish patients.
A. Gerussi, and P. Invernizzi acknowledge that this research was partially supported by the Italian Ministry of University and Research (MIUR)-Department of Excellence project PREMIA (PREcision MedIcine Approach: bringing biomarker research to clinic).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaut.2022.102906.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;7(323):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 12;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 5.Yapalı S. What hepatologists need to know about COVID-19? Hepatol. Forum. 2020;2:41–43. doi: 10.14744/hf.2020.2020.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pascolini S., Vannini A., Deleonardi G., Ciordinik M., Sensoli A., Carletti I., et al. COVID-19 and immunological dysregulation: can autoantibodies be useful? Clin. Transl. Sci. 2021;14:502–508. doi: 10.1111/cts.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efe C., Lammert C., Taşçılar K., Dhanasekaran R., Ebik B., Higuera-de la Tijera F., Calışkan A.R., et al. Effects of immunosuppressive drugs on COVID-19 severity in patients with autoimmune hepatitis. Liver Int. 2022;42:607–614. doi: 10.1111/liv.15121. [DOI] [PubMed] [Google Scholar]
- 8.Khandker S.S., Godman B., Jawad M.I., Meghla B.A., Tisha T.A., Khondoker M.U., et al. A systematic review on COVID-19 vaccine strategies, their effectiveness, and issues. Vaccines (Basel) 2021 24;9:1387. doi: 10.3390/vaccines9121387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021 4;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fix O.K., Blumberg E.A., Chang K.M., Chu J., Chung R.T., Goacher E.K., et al. AASLD COVID-19 vaccine working group. American association for the study of liver diseases expert panel consensus statement: vaccines to prevent coronavirus disease 2019 infection in patients with liver disease. Hepatology. 2021;74:1049–1064. doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John B.V., Deng Y., Scheinberg A., Mahmud N., Taddei T.H., Kaplan D., et al. Association of BNT162b2 mRNA and mRNA-1273 vaccines with COVID-19 infection and hospitalization among patients with cirrhosis. JAMA Intern. Med. 2021 1;181:1306–1314. doi: 10.1001/jamainternmed.2021.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon A.M., Webb G.J., García-Juárez I., Kulkarni A.V., Adali G., Wong D.K., et al. SARS-CoV-2 infections among patients with liver disease and liver transplantation who received COVID-19 vaccination. Hepatol. Commun. 2022;6:889–897. doi: 10.1002/hep4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efe C., Dhanasekaran R., Lammert C., Ebik B., Higuera-de la Tijera F., Aloman C., et al. Outcome of COVID-19 in patients with autoimmune hepatitis: an international multicenter study. Hepatology. 2021;73:2099–2109. doi: 10.1002/hep.31797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabaçam G., Wahlin S., Efe C. Autoimmune hepatitis triggered by COVID-19: a report of two cases. Liver Int. 2021;41:2527–2528. doi: 10.1111/liv.15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . 2020. COVID-19 Therapeutic Trial Synopsis.https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis [Google Scholar]
- 17.Efe C., Kulkarni A.V., Terziroli Beretta-Piccoli B., Magro B., Friedrich Stättermayer A., Cengiz M., et al. Liver injury after SARS-CoV-2 vaccination: features of immune-mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology. 2022 May 14 doi: 10.1002/hep.32572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John B.V., Deng Y., Schwartz K.B., Taddei T.H., Kaplan D.E., Martin P., et al. Post-vaccination COVID-19 infection is associated with reduced mortality in patients with cirrhosis. Hepatology. 2022;76:126–138. doi: 10.1002/hep.32337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakasis A.D., Bitzogli K., Mouziouras D., Pouliakis A., Roumpoutsou M., Goules A.V., et al. Antibody responses after SARS-CoV-2 vaccination in patients with liver diseases. Viruses. 2022 21;14:207. doi: 10.3390/v14020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chauhan M., Nzeako I., Li F., Thuluvath P.J. Antibody response after a booster dose of SARS-CoV-2 vaccine in liver transplant recipients and those with chronic liver diseases. Ann. Hepatol. 2022 23;27 doi: 10.1016/j.aohep.2022.100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galmiche S., Luong Nguyen L.B., Tartour E., de Lamballerie X., Wittkop L., Loubet P., et al. Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: a systematic review. Clin. Microbiol. Infect. 2022;28:163–177. doi: 10.1016/j.cmi.2021.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Düngelhoef M.P., Hartl J., Rüther D.F., Steinmann S., Kaur M., Glaser F.V., et al. Low antibody titers after second SARS-CoV-2 vaccination in patients with autoimmune hepatitis. Z. Gastroenterol. 2022;60:e24. doi: 10.1002/ueg2.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.