Abstract
There is a lack of robust prevalence estimates of atopic dermatitis (AD) globally and trends over time due to wide variation of populations and age groups studied, different study methodologies and case definitions used. We sought to characterize 12-month AD prevalence across the life span and change over time in resource-rich countries focusing on population-based studies and using a standardized AD case definition. This systematic review was conducted according to PRISMA guidelines. Medline (Ovid), Embase, WOS core collection, Cinahl, and Popline were searched for studies published since inception through August 15, 2016. Studies were synthesized using random effects meta-analysis. Sources of heterogeneity were investigated using subgroup analyses and meta-regression. From 12,530 records identified, 45 studies met the inclusion criteria. Meta-analysis with random effects revealed the 12-month period prevalence of 9.2% (95% confidence interval 8.4–10.1%). The prevalence was significantly higher among 0–5-year-old children (16.2%; 95% confidence interval 14.2–18.7%) than in older age groups. Studies using a random sampling strategy yielded lower prevalence estimates than studies relying on other sampling methods. There was no clear time trend in AD prevalence over the period of 1992–2013.
Subject terms: Epidemiology, Skin diseases
Introduction
Atopic dermatitis (AD) is a common inflammatory skin disease that has been shown to mount a substantial psychological, social and economic charge to patients, their families and society1–4. The clinical concept of AD encompasses a wide spectrum of phenotypes regarding clinical features, severity, course, patient’s age and ethnicity as well as the development of comorbid disease and response to treatment5,6. Several genetic, immunologic and environmental factors contribute to the complex pathophysiology of AD, but the key driver is a subject of debate7,8.
The onset of AD is usually in early childhood, but the natural course of the disease has not been easy to predict9–13. Perhaps not surprisingly, most epidemiological studies have been conducted in children although nowadays it is increasingly recognized to persist into, or to begin also in, adulthood or even the elderly14–18.
In high-income countries, AD is considered one of the most common cutaneous inflammatory disorders3,4,7. Yet the studies on AD prevalence have been difficult to interpret because they differ in methodology—in terms of disease definition, sampling frame and methods, regions, or age groups19,20. Partly arising from the same methodological problems, literature is remarkably scant on time trends. We retrieved two studies estimating worldwide time trends of AD prevalence. In a systematic review of sequential data Deckers at al.20 found that the prevalence of AD was increasing in some regions with no clear trends in others. Although the analysis was restricted to studies with validated instruments only, the authors admitted that assessing trends was complicated by the wide range of outcome measures and changes in diagnostic criteria over time. Another report of secular trends brought forth that AD prevalence was plateauing in some populations with previously high prevalence rates while still increasing in others21. The study engaged only children and data were drawn from two identically designed phases of a large international multi-site study. Thereby, there is a lack of global robust prevalence estimates of AD in all ages and trends over time.
The objective of this study was to systematically review research on 12-month AD prevalence in the general population of resource-rich countries. Unlike Deckers et al20 we limited our search to population-based surveys and accepted somewhat wider criteria of explicit AD. In addition, we explored variations in prevalence based on age, gender, period, study design, region, and AD case definition.
Material and methods
We systematically reviewed population-based studies from EU/EEA and other high-income countries published between 1991 and 2016 where AD prevalence in the previous 12 months was presented or could be calculated from available data. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines22.
Eligibility criteria
To be included, studies had to be published in English and report original research. Eligible study designs were cross-sectional surveys or baseline evaluation in cohort studies that used population-based sampling methods and assessed 12-month period prevalence of AD. Studies with the following characteristics were excluded: narrative and systematic reviews; case control studies; birth cohort studies; experimental studies; and data published in letters, commentaries, and editorials.
We limited our inclusion to nationally representative samples of general population. We considered a well-defined general population based sampling strategy would apply to population-based studies23. Such studies encompass those that are defined by national and sub-national geographic boundaries of a country (including school or kindergarten if the sampling frame included all the institutions in the region) as well those defined by membership in health maintenance organizations23. We focused on AD prevalence in resource-rich countries from the EU/EEA24, and non-European high-income countries (as defined by the Organization for Economic Cooperation and Development25). Studies where the AD diagnosing criteria did not match our AD case definition (see below) were excluded from this review.
Information sources and search strategy
We searched Medline (Ovid), Embase, WOS core collection, Cinahl, and Popline from their inception until 15th August 2016. Search strategies, adapted for each search engine, included terms for “atopic dermatitis”, “atopic eczema” and “prevalence” and individual names of EU/EEA countries (and Switzerland), or “Europe”, or the non-European high-income countries (Australia, Canada, Chile, Israel, Japan, Korea, New Zealand, USA) (see Supplementary material 1). In addition, we searched reference lists for eligible studies. The full electronic search strategy for Medline (Ovid) is presented in Supplementary table 1.
Selection process
Pairs of qualified reviewers independently screened the titles and abstracts, then by full text to determine eligibility for final inclusion (following the predefined inclusion criteria) and recorded the results onto standardized forms of a preformatted data collection template. At both stages of screening, any differences between reviewers were discussed and a consensus decision for eligibility and inclusion was made for all articles; a third reviewer resolved differences between reviewers if necessary.
Data collection process
A data extraction sheet was developed based on the Cochrane Consumers and Communication Review Group's data extraction template26, pilot-tested on eight randomly selected but included studies and refined accordingly. If multiple publications reported one study, we extracted data from the primary publication (assigned as the publication with the most detailed description of the methods and the most data on specified prevalence measures). Data reported in the primary publication were used in case of inconsistencies between the publications based on a same source study. The two reviewers compared the extracted data and resolved differences by discussion. If there was still a discrepancy, a third reviewer adjudicated. We did not contact authors for additional information.
Data items
The following information was extracted: study design; country; setting (national or sub-national); demographic characteristics (age, gender); sampling method (random—simple, stratified, multi-staged, cluster; or convenience sampling); numbers of eligible, invited and participating subjects; number of subjects excluded and those with detected AD; definition of AD used; estimated prevalence and 95% confidence intervals (CI) reported in the study.
Study risk of bias assessment
We used published guidelines for cross-sectional prevalence studies by Boyle27 to assess the risk of bias related to methodological aspects of included studies and disagreement at any stage was solved by consensus or arbitration. The items assessed included: representativeness of the target and source populations; similarity of responders and non-responders; attained sample size; use of standardized/valid AD measurement/definition; appropriateness of statistical methods; and response rate. We pre-specified criteria to determine whether each of the features in a specific study could be rated as attributing a low or high risk of bias, or if there was insufficient information to decide (unclear). The overall risk of bias generally corresponded to the highest risk of bias in any of the items. However, if a study was judged to have ‘unclear’ risk of bias for multiple (two or more) items, it was regarded as at high risk of bias overall. Publication bias was assessed qualitatively using funnel plot symmetry as a surrogate for low risk of publication bias.
Data analysis and synthesis
We estimated the 12-month AD prevalence using the number of individuals with AD and the number of people tested (confidence intervals (CI) are based on the Clopper-Pearson method). Where authors reported stratified sampling methods, the published point estimate and 95% CI were used. Where simple random sampling has been used and data were available, we calculated AD prevalence with binomial 95% CI. We examined time trends in the AD prevalence estimates using meta-regression regression models with the prevalence estimates as the outcome variable and the midpoint of the data collection years as the predictor.
We calculated a response rate for each study using an algorithm to define numerators and denominators consistent with the recommendations of the American Association for Public Opinion Research28. Where available, the numerator was the number of people having AD and the denominator was the number of eligible subjects asked to participate, able to participate, or sent an invitation for study participation. If the study report did not include these figures, we used the number of people studied, followed by the number of study subjects used in the analysis as the numerator and the number of eligible people as the denominator. We used the published response rate in studies that used complex sampling methods and post-stratification weighting.
Prevalence estimates were pooled using random effects meta-analysis (generalized linear mixed model) to derive the average of the study estimates and their 95% CI, as suggested by Schwarzer et al.29. We assessed statistical heterogeneity using the Q-test and I2 statistic. The I2 statistic was interpreted according to the recommended thresholds30. Sensitivity analysis was undertaken to explore whether the results were sensitive to restriction of studies with low risk of bias.
Clinical and methodological heterogeneity was assessed in subgroup analysis using meta-regression. Variables for subgroup analysis were selected a priori: age (when study source population could be designated into age groups of 0–5, 6–12, 13–18 or over 18 years); geographic coverage (Asia versus elsewhere); study response rate (< 70%,70–79%, 80% +); time period of data collection (1991–2000, 2001–2010, 2011–2016); population setting (health care institution, elsewhere); sampling (simple random sampling, other); type of AD measure (patient self-report of symptoms; self-report of AD diagnosed by a doctor; other). Calculations were performed with R function metaprop from package meta31.
Outcomes of the study
The primary outcome was 12-month prevalence of AD. Secondary outcomes included the prevalence of AD across age, sex, study decade, AD case definition and country/region. For the current review, AD was pre-defined as (1) an itchy skin condition with a chronic and/or relapsing course and affecting the folds of the elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears or eyes32,33, or (2) diagnosed by a physician: (i) based on the self/parent report on atopic dermatitis/eczema diagnosis; (ii) observed in the study; or, (iii) extracted from a healthcare maintenance/administrative database according to the International Statistical Classification of Diseases and Related Health Problems (ICD-9/10) AD-specific diagnosis codes. Institutional review board approval was not sought as only data from already published studies was used.
Results
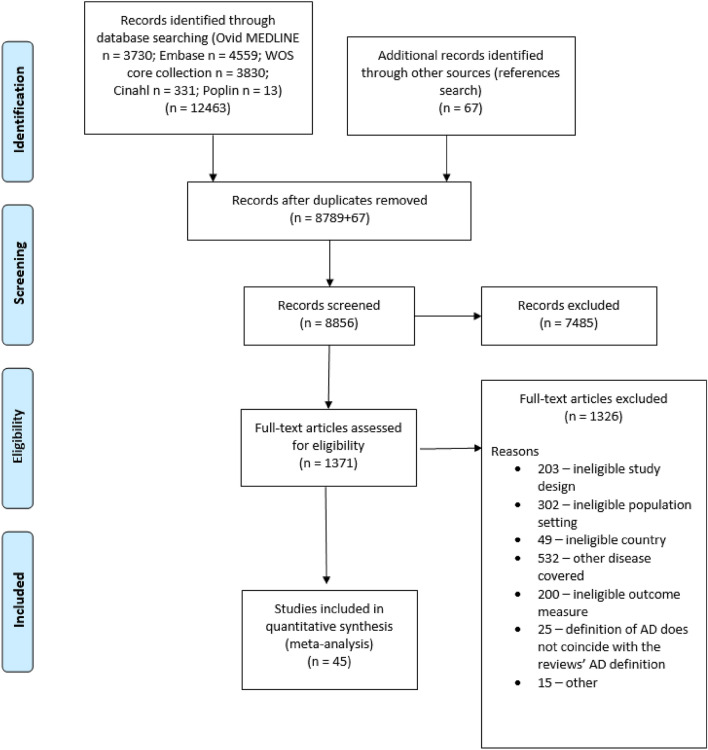
After removing duplicates, the search retrieved 8,856 records. After title and abstract screening, 7485 records were excluded. The remaining 1371 articles were read in full and screened for eligibility. After excluding 1326 articles for ineligibility, 45 studies remained for analysis. Expressly, out of the 532 studies excluded for covering other diseases, in 432 cases the disease investigated was defined as “eczema” (e.g., eczema or patient or doctor reported eczema or childhood eczema or food-sensitized and non-sensitized eczema or hand eczema) with no details allowing further clarification. In the rest of excluded studies hay fever (n = 39), food allergy (n = 28), asthma (n = 14), allergic rhinitis (n = 12) or atopy (n = 7) were studied. The flow chart of the selection process and reasons for excluding studies is detailed in Fig. 1.
Figure 1.
The study selection process for the systematic review and meta-analysis.
Included studies
The data of the 45 included studies had been collected from 27 countries between 1992 and 2013 and published during the period from 1998 to 2016. Altogether, AD prevalence was assessed in 75,203,859 individuals (3,494,054 when excluding individuals whose data was obtained from healthcare databases) (Supplementary Fig. 1). There were eight studies from South Korea34–41, seven from Japan42–48, five from Germany49–53, three from the UK54–56, two studies from Denmark57,58 and the USA59,60 and one from Italy61, Canada62, Croatia63, Cyprus64, Finland65, Lithuania66, New Zealand67, Poland68, the Netherlands69, Norway70, Spain71, Sweden72 and Switzerland73, respectively. Five studies reported data from multiple countries74–78, providing information also from France, Greece, Estonia, Latvia, Iceland, Belgium, Portugal and Hungary in addition to the aforementioned states. Table 1 summarizes the characteristics of each study.
Table 1.
Characteristics of included studies.
| Source | Country | Data collection period | Sample size | Study subjects’ age (in years) | Response rate (reported by authors) | Sampling strategy | Sampling unit | AD definition |
|---|---|---|---|---|---|---|---|---|
| Choi34 | South-Korea | 2008 | 6453 | 0–6 | 70–79 | Convenience | School | ISAAC |
| Cibella61 | Italy | 2005–2006 | 2150 | 10–17 | 80 + | Unclear | School | Modified ISAAC |
| Duhme49 | Germany | 1994–1995 | 13,123 | 5–8; 12–15 | 80 + | Random | School | ISAAC |
| Emerson54 | UK | 1995–1996 | 1761 | 1–5 | 80 + | Convenience | General practice | UK |
| Flohr76 | France, Greece, Italy, Netherlands, Norway, Spain, UK, Latvia, New Zealand | 1998–2004 | 20,049 | 8–12 | NR | Random | School | ISAAC |
| Flohr75 | Greece, Norway, Spain, Latvia, Iceland, New Zealand | 1998–2004 | 11,241 | 8–12 | NR | Random | School | ISAAC |
| Flohr74 | France, Greece, Netherlands, Norway, Spain, Latvia, Estonia, New Zealand | 1998–2004 | 11,587 | 8–12 | NR | Random | School | ISAAC |
| Garcia-Marcos77 | Estonia, Hungary, Lithuania, Poland, New Zealand, Belgium, Portugal, Spain, Japan, Canada, USA, Finland | 2001–2003 | 142,085 | 6–7; 13–14 | NR | Random | School | ISAAC |
| Anderson55 | UK | 1995, 2002 | 30,838 | 12–14 | 80 + | Random | School | ISAAC |
| Grize 200673 | Switzerland | 1992, 1995, 1998, 2001 | 5446 | 5–7 | 70 + | Convenience | School | ISAAC |
| Guiote-Domínguez71 | Spain | 2005 | 381 | 6–7; 13–14 | NR | Random | School | ISAAC |
| Hong35 | South-Korea | 2008 | 10,383 | 0–13 | NR | Convenience | School | ISAAC |
| Kudzyte66 | Lithuania | 1994–1995 | 1879 | 6–7 | 80 + | random | school | ISAAC |
| Kurosaka42 | Japan | 2005–2006 | 11,116 | 6 | 80 + | Convenience | School | ISAAC |
| Lee36 | South-Korea | 2005 | 8631 | 0–19 | NR | Random | Individual | Self-report of physician diagnosis |
| Augustin50 | Germany | 2009 | 293,181 | 0–18 | NA | Random | Health insurance data | ICD-10 |
| Miyake43 | Japan | 2004–2005 | 23,338 | 6–15 | NR | Random | School | ISAAC |
| Miyake44 | Japan | 2001 | 5539 | 12–15 | NR | Random | School | ISAAC |
| Mortz57 | Denmark | 1995–1996 | 1501 | 12–16 | NR | Random | School | Hanifin & Rajka |
| Radtke 201451 | Germany | 2009 | 1,349,671 | 18–100 | NA | Random | Health insurance data | ICD-10 |
| Sasaki45 | Japan | 2012 | 28,343 | 6–12 | NR | Unclear | Individual | ISAAC |
| Saunes70 | Norway | 1995–1997 | 8393 | 13–19 | 80 + | Random | Individual | ISAAC |
| Silverberg59 | USA | 2005–2006 | 4970 | 20- | 70–79 | Random | Individual | Modified ISAAC |
| Ukawa46 | Japan | unclear | 4254 | 6–12 | < 70 | Convenience | School | ISAAC |
| Kim72 | Sweden | 2000, 2008 | 17,946 | 15 | < 70 | Random | Individual | ISAAC |
| Asher67 | New Zealand | 1992–1993 | 31,083 | 6–7; 13–14 | 80 + | Random | School | ISAAC |
| Asher78 | UK, Spain, Canada | 2002–2003 | 67,414 | 6–7; 13–14 | NR | Random | School | ISAAC |
| Kolokotroni64 | Cypros | 1999–2000; 2007–2008 | 7160 | 7–8 | 80 + | Random | School | ISAAC |
| Austin56 | UK | 1995 | 27,507 | 12; 13; 14 | 80 + | Random | School | ISAAC |
| Lee37 | South-Korea | 2008 | 8644 | 6–11; 12–14 | 80 + | Random | School | Modified ISAAC |
| Lee38 | South-Korea | 2012–2013 | 1820 | 6–12; 12–15; 15–18 | NR | Random | School | Modified ISAAC |
| Banac63 | Croatia | 2001–2002, 2009–2010 | 6060 | 6–7; 13–14 | 80 + ; 70–79 | Random | School | ISAAC |
| Oh41 | South-Korea | 1995; 2000 | 82,631 | 6–12; 12–15 | 80 + | Random | School | ISAAC |
| Remes65 | Finland | 1994–1995 | 11,607 | 13–14 | 80 + | Random | School | ISAAC |
| Silverberg60 | USA | 2005–2006 | 3049 | 8–11; 12–15; 16–19 | 80 + | Random | Individual | Modified ISAAC |
| Sugiyama47 | Japan | 1995–1996 | 4466 | 13–14 | NR | Unclear | School | ISAAC |
| Sybilski68 | Poland | 2006–2008 | 18,617 | 6–7; 12–14; 20–44 | < 70 | Random | Individual | ISAAC |
| van de Ven69 | Netherlands | 2003 | 9713 | 12; 13; 14 | 80 + | Random | School | modified ISAAC |
| Wang62 | Canada | 2003 | 8334 | 13–14 | NR | Random | School | ISAAC |
| Yura48 | Japan | 1993–2006 | 2,802,403 | 7–12 | 80 + | Random | School | Self-report of physician diagnosis |
| Zutavern52 | Germany | 1995–1996 | 11,904 | 5–11 | 70–79 | Random | School | modified ISAAC |
| Worm53 | Germany | 1998–2000 | 1739 | 18–65 | < 70 | Random | Individual | Hanifin & Rajka |
| Vinding58 | Denmark | 2010–2013 | 16,507 | 30–39; 40–49; 50–59; 60–69; 70–79; 80–89 | < 70 | Random | Individual | Self-report by modified UK diagnostic criteria |
| Park39 | South-Korea | 2005 | 1989 | 20–39; 40–59; 60- | NR | Random | Individual | Self-report of physician diagnosis |
| Yu40 | South-Korea | 2003–2008 | NA | < 2; 2–5, 6–18 | NA | Random | Health insurance data | ICD-10 |
AD—atopic dermatitis; NR—not reported; NA—not applicable; ISAAC—criteria of the International Study of Asthma and Allergies in Childhood; Hanifin & Rajka—self-reported score of symptoms based on the Hanifin and Rajka criteria; UK—physician’s diagnosis based on UK refinement of diagnostic criteria; ICD-10—physician’s diagnosis according to ICD-10.
Risk of bias assessment and response rate
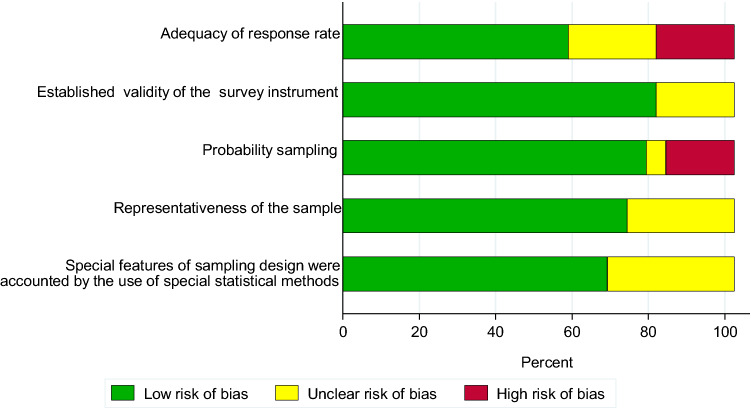
The assessments of risk of bias for each included study are presented in Supplementary table 2. Given the very limited information on data collection methods and response rates provided by the five studies of multiple countries74–78, formal risk of bias could not be assessed in these studies. Of note is that all of these studies compiled data from an international multisite collaboration research project based on a standardized study design32,33. From the remaining 40 studies, twelve (27%) were considered to have high risk of bias, and in 15 (33%) the risk was unclear, at least for one item assessed (Fig. 2). Thirty six studies out of the 45 (80%) utilized random sampling, six studies convenience sampling34,35,42,46,54,73 and in three45,47,61 the sampling strategy was unclear. In 24 of the studies utilizing random sampling, the sampling unit was a school36,37,40,42,43,47,48,52,54–56,61–66,68,70,74–78, in five the sampling unit was at an individual level39,53,58,70,72, four studies used multistage sampling (regional and individual)36,59,60,68, and three studies were based on health insurance data40,50,51. The studies using convenience sampling, or for which the sampling strategy was unclear, were regarded to be at high risk of bias in relation to the sampling from the source population.
Figure 2.
Risk of bias assessment.
The target population was assumed to represent the general population in 30/45 (67%) of studies. However, none of the studies provided a comparison between participants and non-participants and none of the studies gave enough information about the source population to determine whether or noth this was representative of the target population. Amongst the 40 studies with some site or country specific data available, only 26 (65%) reported a response rate or data allowing estimations to be made from them. From these, 17 studies had a response rate above 80%37,41,42,48,49,54–56,60,61,63–67,69,70, four had a response rate of between 70 and 80%34,52,59,63, and five below 70%46,53,58,68,72. The potential for publication bias assessed through funnel plots (stratified by age group) did not suggest a significant bias (Supplementary Fig. 2).
Measurement of AD in the studies
In most of the studies (38/45, 84%), AD case definition was based on the self-report of symptoms. Among them, in 28 studies34,35,41–47,49,55,56,62–68,70–78 the questionnaire of the International Study of Asthma and Allergies in Childhood (ISAAC)32, and in 7 studies37,38,52,59–61,69 modifications of the ISAAC instrument, were used. In the remaining two studies53,57, the self-reported score of symptoms were guided by criteria suggested by Hanifin and Rajka79, and in one study58 by modified UK diagnostic criteria80. In three studies36,39,48, a self-report of physician diagnosis of AD was used and in one study54 AD was diagnosed by a physician based on a UK refinement of diagnostic criteria81. In three of the included studies40,50,51 assessment was based on ICD-10 diagnostic codes for AD in administrative health data.
12-month prevalence of AD
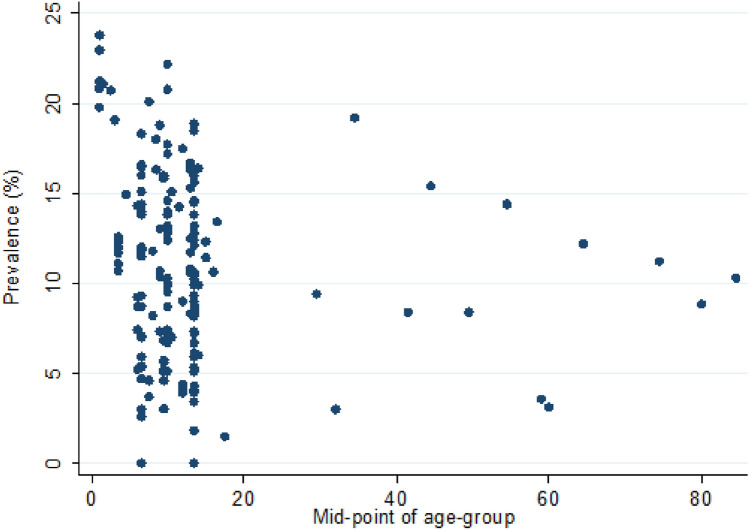
AD prevalence estimates ranged from 0 to 24% (Fig. 3). Meta-analysis identified overall 12-month period pooled prevalence of AD across all included studies of 9.2% (95% CI 8.4–10.1%) with a high level of heterogeneity. Meta-regression was used to explore potential variables that may have accounted for the observed high heterogeneity (Table 2). Female gender predicted higher AD prevalence (11.8 vs 8.2%; p = 0.0063). Then the analysis was stratified by age groups. Altogether, there were 17 prevalence estimates available for children aged 0 to five years (3 studies)34,40,54, 81 for children aged 6–12 years (21 studies)37,38,41,42,45,46,48,56,60,61,63,64,66,68,69,71,74–78, 41 for children aged 13–18 years (13 studies)38,47,56,61–63,65,68,69,71,72,77,78 and 11 prevalence estimates for adults (4 studies)39,58,59,68 in the included studies. Quantitative analysis yielded a pooled AD prevalence of 16.3% among children aged 0–5 years (95% CI 14.2–18.8%; 18,573,027 participants), 9.4% among 6–12-year-olds (95% CI 8.2–10.8%; 3,071,305 participants), 8.3% among 13–18-year-olds (95% CI 6.6–10.4%; 222,021 participants), and 9.3% among adults (95% CI 6.6–13.0%; 32,866 participants). For the youngest age group (0–5 years), the data could be drawn from just two countries, South Korea and the UK. Sensitivity analysis of age grouping did not reveal significant bias (Supplementary table 2).
Figure 3.
12-month prevalence of AD across age groups.
Table 2.
Subgroup analyses of factors associated with 12-month AD prevalence.
| Variable | Category | No of prevalence estimates | Mean prevalence, % (95% CI) | p-value |
|---|---|---|---|---|
| Individual characteristics | ||||
| Age | 0–5 | 17 | 16.3 (14.2; 18.8) | Ref |
| 6–12 | 81 | 9.4 (8.2; 10.8) | 0.0005 | |
| 13–18 | 41 | 8.3 (6.6; 10.4) | < 0.0001 | |
| 19 + | 11 | 9.3 (6.6; 13.0) | 0.0140 | |
| Gender | Female | 26 | 11.8 (9.9; 14.0) | Ref |
| Male | 26 | 8.2 (6.8; 9.9) | 0.0063 | |
| Study characteristics | ||||
| Sampling unit | Health insurance data | 19 | 10.4 (7.7; 13.9) | Ref |
| Pre/school | 82 | 10.1 (9.1; 11.2) | 0.8304 | |
| Individual | 22 | 7.7 (5.7; 10.3) | 0.0847 | |
| Other | 4 | 17.0 (13.7; 20.8) | 0.0925 | |
| Sampling | Random sampling | 164 | 8.9 (8.0; 9.8) | 0.0050 |
| Other | 17 | 13.9 (11.6; 16.5) | Ref | |
| Response rate | < 70% | 19 | 11.0 (8.6; 13.9) | Ref |
| 70–79% | 9 | 7.9 (5.7; 10.8) | 0.1475 | |
| ≥ 80% | 59 | 9.5 (8.1; 11.0) | 0.3297 | |
| Outcome assessment measure | Self-reported symptoms | 156 | 9.2 (8.3; 10.1) | Ref |
| Self-reported ADa | 10 | 7.0 (5.5; 8.8) | 0.2066 | |
| Other | 24 | 10.8 (8.3; 14.1) | 0.2247 | |
| Study configuration | ||||
| Region | Asia | 53 | 9.0 (6.9; 11.6) | 0.9724 |
| Non-Asia | 137 | 9.3 (8.5; 10.1) | Ref | |
| Study period | 1991–2000 | 85 | 10.4 (9.5; 11.5) | Ref |
| 2001–2010 | 88 | 7.4 (6.2; 8.8) | 0.0004 | |
| 2011–2016 | 10 | 14.1 (12.5; 15.9) | 0.1377 | |
aDiagnosed by a health care practitioner.
Further, the effect of study quality on the primary outcome was tested. Limiting the analysis to studies with low risk of bias (10 studies with 34 prevalence estimates48–51,56,57,60,65,67,70) gave an AD prevalence of 8.9% (95% CI 7.2–11.0). This did not differ significantly from the prevalence estimate based on all included studies. Of note is that no studies with low risk of bias were detected within populations designated to age limits of either 0–5 or over 18 years.
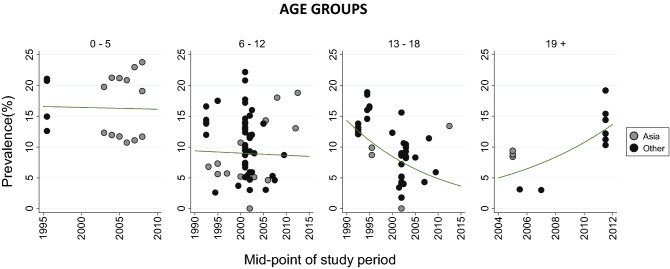
Besides age and gender, the methodological characteristics of studies and the period of data collection were significantly associated with AD prevalence in meta-regression. The prevalence of AD was lower in studies reporting data from 2001–2010 compared to 1991–2000 (7.4 vs. 10.4%; p = 0.0004). Next, we stratified the time trend analysis by age group. There was a slight decrease in 12-month prevalence among 0–5-year-old and 6–12-year-old children from mid-1990s to the late 2000s (not statistically significant, p = 0.9119, and p = 0.8259, respectively; Fig. 4). Among individuals aged 13–18 years, a significant downtrend in predicted AD prevalence was observed over the same time (p = 0.0122), from 12.3% (95% CI 8.5–17.4%) in 1993 to 4.3% (95% CI 2.5–7.6%) in 2012. In the adult group, we saw an increase in AD prevalence over the past decades (p = 0.0036), from 5.7% (95% CI 4.0–8.7%) in 2005 to 13.7% (95% CI 9.6–19.2%) in 2012 (Fig. 4).
Figure 4.
12-month prevalence of AD by age groups (0–5, 6–12, 13–18 and 19 + years) and region over time.
Studies using random sampling yielded lower AD prevalence than studies of non-random sampling (8.9 vs 13.9%; p = 0.005). There were no differences between Asia and other regions either in overall AD prevalence (Table 2) or in trends across age groups (Fig. 4). We further explored the effects of the case definition of AD through sensitivity analysis. AD prevalence was 9.2% (95% CI 8.3–10.1%) in studies using self-report on symptoms, 7.0% (95% CI 5.5–8.8%) in studies using self-report on physician diagnosis (p = 0.2066) and 10.8% (95% CI 8.7–14.9%) in studies using other measurement methods (p = 0.2247).
Discussion
Our study describes the prevalence and trends of AD over the past three decades in resource-rich countries. Drawn from pooled data from countries of Europe, North America, East Asia and Oceania, we have come to two main findings. Firstly, it ascertains that nearly one-tenth (9.2%) of all people have experienced AD during last 12 months. Secondly, the prevalence of the disease has remained stable during the last decades.
We saw the highest 12-month AD prevalence of 16.3% in the youngest age group (0–5 years old), being almost twice as high as in older age groups. Our finding of higher AD prevalence among the youngest children corroborates previously published evidence that AD occurs more frequently in early life3,4,7,82. Also, longitudinal cohort studies revealing distinct disease trajectories in childhood and adolescence have depicted that the most prevalent subphenotypes are the ‘early-onset-early-resolving’ ones9,10.
In our analysis, the prevalence of AD in children aged 6–18 years and adults did not differ. This is concordant with the finding from a systematic review of longitudinal studies from Northern European countries of similar AD prevalence in the age groups of up to 12 years and older83. Likewise, in two British birth cohorts with a longer follow-up period, the annual period prevalence of AD at the age of 5 years and onwards ranged from 5 to 14% with no clear trend across ages16. The steady prevalence across ages older than 5 years probably reflects a balance of different disease trajectories, i.e., persistent disease as well as the phenotypes of AD that have resolved or relapsed for that period and later-onset disease. The observed 12-month prevalence of 9.3% among adults is echoed in the latest study from Finland where the prevalence an AD of 10.1% in the adult population was detected84.
Previous studies on gender differences in AD prevalence have come to conflicting results85. We found the overall female preponderance of 1.4:1.0. This finding is in agreement with the recent systematic review documenting a higher burden of AD among women throughout all age groups and geographic regions3. It has been speculated that skin care practices, occupational exposures, higher awareness or disease misclassification can play a role in this phenomenon, but to our knowledge these factors have not been formally studied. In a recent analysis, AD was not associated with endogenous sex hormones, neither in adolescents nor adults86.
Although there was a transient decrease of reported AD prevalence in the period 2001–2010, no convincing time trend was disclosed across the three decades. This observation is in line with the findings of the collaborative research looking at the secular trends in childhood AD and documenting the levelling off or decreasing prevalence of AD in some formerly high prevalence sites from high-income countries21. The nature of current study precludes assessment of causes of the drop in AD prevalence witnessed among 13–18-year-olds over time. Neither does it discriminate whether children born at late 1980s and early 1990s were less likely to have persistent AD or to develop AD later at school age, or both. So far, no age-group specific individual or environmental risk factors have been identified in the pathogenesis of AD87. Across studies, the most important predictors of AD persistence beyond childhood have been earlier age of onset, disease severity, allergic multimorbidity, family history of atopy, filaggrin gene mutations, and urban environment88. None of these appear to be modified easily. We propose that there could be a cohort effect attributable to not yet known changes in exposome since 1990s (e.g., more stringent use of antibiotics or promotion of breastfeeding). Another way to explain the decline would be more efficient treatment of AD (e.g., liberal use of emollients or proactive therapy) leading to changes in disease course. Interventional or prospective cohort studies of longer duration than usual are needed to clarify this. Concomitantly, we saw some increase of AD prevalence among people aged 19 years and older over time. One can speculate that there is no real downtrend of prevalence in adolescents, but the manifestation of AD is postponed into adulthood due to environmental or behavioural changes. Nevertheless, considering that late-onset AD was largely ignored until the 1990s14,89, it cannot be excluded that the increase of AD prevalence found in this age group is due simply to a rise in awareness of AD in adults. It has been debated whether anyone could develop clinical syndrome of AD if exposed to enough key risk factors or a finite number of only genetically predisposed individuals are susceptible to it90,91. On a large scale, stable AD prevalence throughout time and ages gives support to the latter hypothesis and reassures that the epidemic is not increasing infinitely.
In our review, the sampling method was the only study design item associated with AD prevalence. Most of the included studies used a random sampling strategy and the remainder were mainly based on convenience samples. The latter yielded significantly higher prevalence estimates. This could have been anticipated since non-probability sampling based studies are known to have disadvantages and often oversample individuals with the condition studied due to overrepresentation either by respondents’ self-selection, membership bias or non-responding92–94.
To-date, there is no consensus on how to capture AD at population level. Without a pathognomonic biomarker available, history taking and clinical signs are needed for diagnosis of AD and a clinician's assessment is considered the gold standard7,95–97. The fluctuating course of the disease further complicates collecting reliable data in population settings91. Previously, higher prevalence of AD has been reported in studies using participants self-report of AD compared to health care practitioner’s assessment98. Interestingly, in the current analysis, we saw no difference in AD prevalence whether the outcome was measured by the diagnosis made by a health care practitioner or by a participant’s self-report. Likewise, we did not reveal any differences across Asia vs other regions that have been described in some previous studies3,12. Despite the regional variations in single features of AD99 our results indicate that the entity of AD might be the same in the setting of resource-rich countries. Thus, a standardized set of self-reported items delineating pruritic, inflammatory skin condition, characterized by a chronic and relapsing dermatitis in typical anatomical sites100 could reliably detect AD in population-based research.
There are several strengths to this review. To our knowledge, this is the largest systematic review of AD prevalence with a comprehensive literature search, succinct focus on source studies’ design validity (population-based studies, delineating sampling strategies) and using a pre-defined standardized AD definition as an inclusion criterion. Importantly, research findings may differ substantially if different definitions of AD are used19,101. In our study, despite within-sample heterogeneity, the AD prevalence estimates were quite similar irrespective of the ascertainment method.
There are also limitations that should be considered in the appraisal of the evidence presented by this review. High heterogeneity was observed between studies both overall and across subgroups. We explored whether study design aspects, geographic variation, or study period could explain part of the heterogeneity. However, the remitting and relapsing nature of the disease and residual study-level differences could have contributed to unexplained heterogeneity in outcome estimates. Namely, source population and sampling characteristics were often not described in enough detail and most studies using multistage sampling disregarded the complex design when estimating AD prevalence. High heterogeneity may affect interpretation and generalization of the results. Secondly, high-income countries included in the current study were defined by their EU/EEA and/or OECD status. This has closed out data from some other high-income countries such as Singapore and Taiwan. Thirdly, as we aimed to involve only data from affluent countries, the results cannot be attributed directly to low-income regions of the world. And last, but not least, in the analysis we were able to reckon only with the factors available in the original studies. Therefore, it was not possible to assess the effect of risk factors, such as living environment, migration or filaggrin gene mutations. However, these potential limitations seem unlikely to have accounted for the clear patterns observed in this study and we believe that the results allow inferences to be made in terms of the prevalence and trends of AD in the resource-rich populations.
Conclusions
Determining prevalence is a crucial step in understanding the impact of AD on affected people and health systems. Our results confirm that in affluent countries one-tenth of the general population suffers from AD annually and suggest that AD prevalence has not increased over time.
Supplementary Information
Acknowledgements
The work was supported by Estonian Ministry of Education and Research grant IUT34-17.
Author contributions
"A.U., A.V. and K.-T.L. designed the study. All authors developed the search strategy, performed the literature review and data extraction. K.T., A.U. and A.V. conducted the statistical analysis and contributed to the interpretation of the results. A.V. and A.U. wrote the main manuscript text. All authors reviewed the manuscript".
Data availability
The data extracted from included studies and used for analyses are available upon reasonable request from the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-19508-7.
References
- 1.Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: Summary of a report for the National Eczema Association. J. Invest. Dermatol. 2017;137:26–30. doi: 10.1016/j.jid.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg JI, Gelfand JM, Margolis DJ, Simpson EL, Ong PY, Fuxench ZCC. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann. Allergy Asthma Immunol. 2018;121:340–347. doi: 10.1016/j.anai.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Urban K, Chu S, Giesey RL, Mehrmal S, Uppal P, Nedley N, et al. The global, regional, and national burden of atopic dermatitis in 195 countries and territories: An ecological study from the Global Burden of Disease Study 2017. JAAD Int. 2021;2:12–18. doi: 10.1016/j.jdin.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughter MR, Maymone MBC, Mashayekhi S, Arents BWM, Karimkhani C, Langan SL, et al. The global burden of atopic dermatitis: Lessons from the Global Burden of Disease Study 1990–2017. Br. J. Dermatol. 2021;184:304–309. doi: 10.1111/bjd.19580. [DOI] [PubMed] [Google Scholar]
- 5.Deleuran M, Vestergaard C. Clinical heterogeneity and differential diagnosis of atopic dermatitis. Br. J. Dermatol. 2014;170(Suppl 1):2–6. doi: 10.1111/bjd.12933. [DOI] [PubMed] [Google Scholar]
- 6.Bieber T, D’Erme AM, Akdis CA, Traidl-Hoffmann C, Lauener R, Schäppi G, et al. Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J. Allergy Clin Immunol. 2017;139(4S):S58–S64. doi: 10.1016/j.jaci.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345–360. doi: 10.1016/S0140-6736(20)31286-1. [DOI] [PubMed] [Google Scholar]
- 8.Thyssen JP, Rinnov MR, Vestergaard C. Disease mechanisms in atopic dermatitis: A review of aetiological factors. Acta Derm. Venereol. 2020;100:adv00162. doi: 10.2340/00015555-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roduit C, Frei R, Depner M, Karvonen AM, Renz H, Braun-Fahrländer C, et al. Phenotypes of atopic dermatitis depending on the timing of onset and progression in childhood. JAMA Pediatr. 2017;171:655–662. doi: 10.1001/jamapediatrics.2017.0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paternoster L, Savenije OEM, Heron J, Evans DM, Vonk JM, Brunekreef B, et al. Identification of atopic dermatitis subgroups in children from 2 longitudinal birth cohorts. J. Allergy Clin. Immunol. 2018;141:964–971. doi: 10.1016/j.jaci.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams HC, Strachan DP. The natural history of childhood eczema: Observations from the British 1958 birth cohort study. Br J Dermatol. 1998;139:834–839. doi: 10.1046/j.1365-2133.1998.02509.x. [DOI] [PubMed] [Google Scholar]
- 12.Hu C, Duijts L, Erler NS, Elbert NJ, Piketty C, Bourdes V, et al. Most associations of early-life environmental exposures and genetic risk factors poorly differentiate between eczema phenotypes: The Generation R Study. Br. J. Dermatol. 2019;181:1190–1197. doi: 10.1111/bjd.17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulick AR, Mansfield KE, Silverwood RJ, Budu-Aggrey A, Roberts A, Custovic A, et al. Four childhood atopic dermatitis subtypes identified from trajectory and severity of disease and internally validated in a large UK birth cohort. Br. J. Dermatol. 2021;185:526–536. doi: 10.1111/bjd.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bannister MJ, Freeman S. Adult-onset atopic dermatitis. Australas J. Dermatol. 2000;41:225–228. doi: 10.1046/j.1440-0960.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 15.Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, et al. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy. 2018;73:1284–1293. doi: 10.1111/all.13401. [DOI] [PubMed] [Google Scholar]
- 16.Abuabara K, Ye M, McCulloch CE, Sullivan A, Margolis DJ, Strachan DP, et al. Clinical onset of atopic eczema: Results from 2 nationally representative British birth cohorts followed through midlife. J. Allergy Clin. Immunol. 2019;144:710–719. doi: 10.1016/j.jaci.2019.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD): A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2016;75:681–687. doi: 10.1016/j.jaad.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HH, Patel KR, Singam V, Rastogi S, Silverberg JI. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J. Am. Acad. Dermatol. 2019;80:1526–1532. doi: 10.1016/j.jaad.2018.05.1241. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Haider S, Colicino S, Murray CS, Holloway J, Simpson A, et al. Different definitions of atopic dermatitis: Impact on prevalence estimates and associated risk factors. Br. J. Dermatol. 2019;181:1272–1279. doi: 10.1111/bjd.17853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deckers IAG, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: A systematic review of epidemiological studies. PLoS ONE. 2012;7:e39803. doi: 10.1371/journal.pone.0039803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. Is eczema really on the increase worldwide? J. Allergy Clin. Immunol. 2008;121:947–954. doi: 10.1016/j.jaci.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szklo M. Population-based cohort studies. Epidemiol. Rev. 1998;20:81–90. doi: 10.1093/oxfordjournals.epirev.a017974. [DOI] [PubMed] [Google Scholar]
- 24.Countries in the European Union (EU) and the European Economic Area (EEA). http://www.efta.int/eea/eea-agreement/eea-basic-features#2 and http://www.efta.int/about-efta/the-efta-states. Accessed 16 June 2016.
- 25.High-income OECD (non-EU/EEA) countries. http://www.oecd.org/trade/xcred/2015-ctryclass-as-of-16-july-2015.pdf. Accessed 17 May 2016.
- 26.Ryan R, Synnot A, Prictor M, Hill S. Cochrane Consumers and Communication Group Data extraction template for included studies. La Trobe University, Melbourne. 2018. https://doi:10.26181/5B57CFD711743
- 27.Boyle MH. Guidelines for evaluating prevalence studies. Evid. Based Ment. Health. 1998;1:37–40. doi: 10.1136/ebmh.1.2.37. [DOI] [Google Scholar]
- 28.American Association for Public Opinion Research. Response rates – an overview. https://www.aapor.org/Education-Resources/For-Researchers/Poll-Survey-FAQ/Response-Rates-An-Overview.aspx. Accessed 19 May 2020.
- 29.Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res. Synth. Methods. 2019;10:476–483. doi: 10.1002/jrsm.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0. Cochrane. 2019. https://training.cochrane.org/handbook/current
- 31.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: A practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International study of asthma and allergies in childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 33.Williams H, Robertson C, Stewart A, Aït-Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the international study of asthma and allergies in childhood. J. Allergy Clin. Immunol. 1999;103:125–138. doi: 10.1016/S0091-6749(99)70536-1. [DOI] [PubMed] [Google Scholar]
- 34.Choi WJ, Ko JY, Kim JW, Lee KH, Park CW, Kim KH, et al. Prevalence and risk factors for atopic dermatitis: A cross-sectional study of 6,453 Korean preschool children. Acta Derm. Venereol. 2012;92:467–471. doi: 10.2340/00015555-1252. [DOI] [PubMed] [Google Scholar]
- 35.Hong S, Choi WJ, Kwon HJ, Cho YH, Yum HY, Son DK. Effect of prolonged breast-feeding on risk of atopic dermatitis in early childhood. Allergy Asthma Proc. 2014;35:66–70. doi: 10.2500/aap.2014.35.3716. [DOI] [PubMed] [Google Scholar]
- 36.Lee H, Kim GS. Geographical and sociodemographic risk factors for allergic diseases in Korean children. Asian Nurs. Res. (Korean Soc Nurs Sci). 2011;5:1–10. doi: 10.1016/S1976-1317(11)60008-X. [DOI] [PubMed] [Google Scholar]
- 37.Lee JY, Seo JH, Kwon JW, Yu J, Kim BJ, Lee SY, et al. Exposure to gene-environment interactions before 1 year of age may favor the development of atopic dermatitis. Int. Arch. Allergy Immunol. 2012;157:363–371. doi: 10.1159/000328778. [DOI] [PubMed] [Google Scholar]
- 38.Lee SH, Seo JH, Cho HJ, Lee E, Ha M, Burm E, et al. The prevalence and risk factors of atopic dermatitis from nationwide study: Korean environmental health survey in children and adolescents (KorEHS-C) J. Allergy Clin. Immunol. 2016;137:146. [Google Scholar]
- 39.Park H, Kim K. Association of blood mercury concentrations with atopic dermatitis in adults: A population-based study in Korea. Environ. Res. 2011;111:573–578. doi: 10.1016/j.envres.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Yu JS, Lee CJ, Lee HS, Kim J, Han Y, Ahn K, et al. Prevalence of atopic dermatitis in Korea: Analysis by using national statistics. J. Korean Med. Sci. 2012;27:681–685. doi: 10.3346/jkms.2012.27.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh JW, Pyun BY, Choung JT, Ahn K, Kim CH, Song SW, et al. Epidemiological change of atopic dermatitis and food allergy in school-aged children in Korea between 1995 and 2000. J. Korean Med. Sci. 2004;19:716–723. doi: 10.3346/jkms.2004.19.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurosaka F, Terada T, Tanaka A, Nakatani Y, Yamada K, Nishikawa J, et al. Risk factors for wheezing, eczema and rhinoconjunctivitis in the previous 12 months among six-year-old children in Himeji City, Japan: Food allergy, older siblings, day-care attendance and parental allergy history. Allergol. Int. 2011;60:317–330. doi: 10.2332/allergolint.10-OA-0246. [DOI] [PubMed] [Google Scholar]
- 43.Miyake Y, Tanaka K, Sasaki S, Arakawa M. Polyunsaturated fatty acid intake and prevalence of eczema and rhinoconjunctivitis in Japanese children: The Ryukyus Child Health Study. BMC Public Health. 2011;11:358–366. doi: 10.1186/1471-2458-11-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyake Y, Yura A, Iki M. Cross-sectional study of allergic disorders in relation to familial factors in Japanese adolescents. Acta Paediatr. 2004;93:380–385. doi: 10.1080/08035250410022819. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki M, Yoshida K, Adachi Y, Furukawa M, Itazawa T, Odajima H, et al. Environmental factors associated with childhood eczema: Findings from a national web-based survey. Allergol. Int. 2016;65:420–424. doi: 10.1016/j.alit.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Ukawa S, Araki A, Kanazawa A, Yuasa M, Kishi R. The relationship between atopic dermatitis and indoor environmental factors: A cross-sectional study among Japanese elementary school children. Int. Arch. Occup. Environ. Health. 2013;86:777–787. doi: 10.1007/s00420-012-0814-0. [DOI] [PubMed] [Google Scholar]
- 47.Sugiyama K, Sugiyama T, Toda M, Yukawa T, Makino S, Fukuda T. Prevalence of asthma, rhinitis and eczema among 13–14-year-old schoolchildren in Tochigi, Japan. Allergol. Int. 2000;49:205–211. doi: 10.1046/j.1440-1592.2000.00180.x. [DOI] [Google Scholar]
- 48.Yura A, Kouda K, Iki M, Shimizu T. Trends of allergic symptoms in school children: Large-scale long-term consecutive cross-sectional studies in Osaka Prefecture, Japan. Pediatr. Allergy Immunol. 2011;22:631–637. doi: 10.1111/j.1399-3038.2011.01159.x. [DOI] [PubMed] [Google Scholar]
- 49.Duhme H, Weiland SK, Rudolph P, Wienke A, Kramer A, Keil U. Asthma and allergies among children in West and East Germany: A comparison between Munster and Greifswald using the ISAAC phase I protocol. Eur. Respir. J. 1998;11:840–847. doi: 10.1183/09031936.98.11040840. [DOI] [PubMed] [Google Scholar]
- 50.Augustin M, Radtke MA, Glaeske G, Reich K, Christophers E, Schaefer I, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35–40. doi: 10.1159/000381913. [DOI] [PubMed] [Google Scholar]
- 51.Radtke MA, Schafer I, Jacobi A, Augustin M, Glaeske G. Prevalence and comorbidities in adults with psoriasis compared with atopic dermatitis: Analysis of health insurance data in Germany. Br. J. Dermatol. 2014;171:e117–e118. [Google Scholar]
- 52.Zutavern A, Hirsch T, Leupold W, Weiland S, Keil U, Von Mutius E. Atopic dermatitis, extrinsic atopic dermatitis and the hygiene hypothesis: Results from a cross-sectional study. Clin. Exp. Allergy. 2005;35:1301–1308. doi: 10.1111/j.1365-2222.2005.02350.x. [DOI] [PubMed] [Google Scholar]
- 53.Worm M, Forschner K, Lee HH, Roehr CC, Edenharter G, Niggemann B, et al. Frequency of atopic dermatitis and relevance of food allergy in adults in Germany. Acta Derm. Venereol. 2006;86:119–122. doi: 10.2340/00015555-0028. [DOI] [PubMed] [Google Scholar]
- 54.Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children? Br. J. Dermatol. 2001;144:514–522. doi: 10.1046/j.1365-2133.2001.04077.x. [DOI] [PubMed] [Google Scholar]
- 55.Anderson HR, Ruggles R, Strachan DP, Austin JB, Burr M, Jeffs D, et al. Trends in prevalence of symptoms of asthma, hay fever, and eczema in 12–14 year olds in the British Isles, 1995–2002: questionnaire survey. BMJ. 2004;328:1052–1053. doi: 10.1136/bmj.38057.583727.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Austin JB, Kaur B, Anderson HR, Burr M, Harkins LS, Strachan DP, et al. Hay fever, eczema, and wheeze: A nationwide UK study (ISAAC, international study of asthma and allergies in childhood) Arch. Dis. Child. 1999;81:225–230. doi: 10.1136/adc.81.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mortz CG, Lauritsen JM, Bindslev-Jensen C, Andersen KE. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents. The Odense adolescence cohort study on atopic diseases and dermatitis. Br. J. Dermatol. 2001;144:523–532. doi: 10.1046/j.1365-2133.2001.04078.x. [DOI] [PubMed] [Google Scholar]
- 58.Vinding GR, Zarchi K, Ibler KS, Miller IM, Ellervik C, Jemec GBE. Is adult atopic eczema more common than we think? - A population-based study in Danish adults. Acta Derm. Venereol. 2014;94:480–482. doi: 10.2340/00015555-1761. [DOI] [PubMed] [Google Scholar]
- 59.Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy. 2015;70:1300–1308. doi: 10.1111/all.12685. [DOI] [PubMed] [Google Scholar]
- 60.Silverberg JI. Association between childhood atopic dermatitis, malnutrition, and low bone mineral density: A US population-based study. Pediatr. Allergy Immunol. 2015;26:54–61. doi: 10.1111/pai.12315. [DOI] [PubMed] [Google Scholar]
- 61.Cibella F, Cuttitta G, La Grutta S, Melis MR, Lospalluti ML, Uasuf CG, et al. Proportional Venn diagram and determinants of allergic respiratory diseases in Italian adolescents. Pediatr. Allergy Immunol. 2011;22:60–68. doi: 10.1111/j.1399-3038.2010.01097.x. [DOI] [PubMed] [Google Scholar]
- 62.Wang HY, Pizzichini MMM, Becker AB, Duncan JM, Ferguson AC, Greene JM, et al. Disparate geographic prevalences of asthma, allergic rhinoconjunctivitis and atopic eczema among adolescents in five Canadian cities. Pediatr. Allergy Immunol. 2010;21:867–877. doi: 10.1111/j.1399-3038.2010.01064.x. [DOI] [PubMed] [Google Scholar]
- 63.Banac S, Rožmanić V, Manestar K, Korotaj-Rožmanić Z, Lah-Tomulić K, Vidović I, et al. Rising trends in the prevalence of asthma and allergic diseases among school children in the north-west coastal part of Croatia. J. Asthma. 2013;50:810–814. doi: 10.3109/02770903.2013.803115. [DOI] [PubMed] [Google Scholar]
- 64.Kolokotroni O, Middleton N, Nicolaou N, Pipis S, Priftis KN, Milton DK, et al. Temporal changes in the prevalence of childhood asthma and allergies in urban and rural areas of Cyprus: Results from two cross sectional studies. BMC Public Health. 2011;11:858. doi: 10.1186/1471-2458-11-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Remes ST, Korppi M, Kajosaari M, Koivikko A, Soininen L, Pekkanen J. Prevalence of allergic rhinitis and atopic dermatitis among children in four regions of Finland. Allergy. 1998;53:682–689. doi: 10.1111/j.1398-9995.1998.tb03954.x. [DOI] [PubMed] [Google Scholar]
- 66.Kudzyte J, Griška E, Bojarskas J. Time trends in the prevalence of asthma and allergy among 6–7-year-old children. Results from ISAAC phase I and III studies in Kaunas, Lithuania. Medicina (Kaunas). 2008;44:944–952. doi: 10.3390/medicina44120118. [DOI] [PubMed] [Google Scholar]
- 67.Asher MI, Barry D, Clayton T, Crane J, D'Souza W, Ellwood P, et al. The burden of symptoms of asthma, allergic rhinoconjunctivitis and atopic eczema in children and adolescents in six New Zealand centres: ISAAC Phase One. N Z Med J. 2001;114:114–120. [PubMed] [Google Scholar]
- 68.Sybilski AJ, Raciborski F, Lipiec A, Tomaszewska A, Lusawa A, Samel-Kowalik P, et al. Atopic dermatitis is a serious health problem in Poland. Epidemiology studies based on the ECAP study. Postep. Dermatol. Alergol. 2015;32:1–10. doi: 10.5114/pdia.2014.40935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van de Ven MOM, Van Den Eijnden RJJM, Engels RCME. Atopic diseases and related risk factors among Dutch adolescents. Eur. J. Public Health. 2006;16:549–558. doi: 10.1093/eurpub/ckl022. [DOI] [PubMed] [Google Scholar]
- 70.Saunes M, Smidesang I, Holmen TL, Johnsen R. Atopic dermatitis in adolescent boys is associated with greater psychological morbidity compared with girls of the same age: The Young-HUNT study. Br. J. Dermatol. 2007;156:283–288. doi: 10.1111/j.1365-2133.2006.07688.x. [DOI] [PubMed] [Google Scholar]
- 71.Guiote-Domínguez MV, Muñoz-Hoyos A, Gutiérrez-Salmerón MT. Prevalence of atopic dermatitis in schoolchildren in Granada, Spain. Actas Dermo-Sifiliográficas. 2008;99:628–638. doi: 10.1016/s1578-2190(08)70330-5. [DOI] [PubMed] [Google Scholar]
- 72.Kim JL, Brisman J, Al ÅM, Forslund HB, Winkvist A, Torén K. Trends in the prevalence of asthma, rhinitis, and eczema in 15 year old adolescents over an 8 year period. Respir. Med. 2014;108:701–708. doi: 10.1016/j.rmed.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 73.Grize L, Gassner M, Wüthrich B, Bringolf-Isler B, Takken-Sahli K, Sennhauser FH, et al. Trends in prevalence of asthma, allergic rhinitis and atopic dermatitis in 5–7-year old Swiss children from 1992 to 2001. Allergy. 2006;61:556–562. doi: 10.1111/j.1398-9995.2006.01030.x. [DOI] [PubMed] [Google Scholar]
- 74.Flohr C, Weinmayr G, Weiland SK, Addo-Yobo E, Annesi-Maesano I, Björksten B, et al. How well do questionnaires perform compared with physical examination in detecting flexural eczema? Findings from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. Br. J. Dermatol. 2009;161:846–853. doi: 10.1111/j.1365-2133.2009.09261.x. [DOI] [PubMed] [Google Scholar]
- 75.Flohr C, Nagel G, Weinmayr G, Kleiner A, Williams HC, Aït-Khaled N, et al. Tuberculosis, bacillus Calmette-Guérin vaccination, and allergic disease: Findings from the International Study of Asthma and Allergies in Childhood Phase Two. Pediatr. Allergy Immunol. 2012;23:324–331. doi: 10.1111/j.1399-3038.2011.01248.x. [DOI] [PubMed] [Google Scholar]
- 76.Flohr C, Nagel G, Weinmayr G, Kleiner A, Strachan DP, Williams HC, et al. Lack of evidence for a protective effect of prolonged breastfeeding on childhood eczema: Lessons from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. Br. J. Dermatol. 2011;165:1280–1289. doi: 10.1111/j.1365-2133.2011.10588.x. [DOI] [PubMed] [Google Scholar]
- 77.Garcia-Marcos L, Robertson CF, Anderson HR, Ellwood P, Williams HC, Wong GWK. Does migration affect asthma, rhinoconjunctivitis and eczema prevalence? Global findings from the international study of asthma and allergies in childhood. Int. J. Epidemiol. 2014;43:1846–1854. doi: 10.1093/ije/dyu145. [DOI] [PubMed] [Google Scholar]
- 78.Asher I, Montefort S, Björkstén B, Lai CKW, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 79.Hanifin RG. Diagnostic features of atopic dermatitis. Acta Derm. Venereol. 1980;Suppl 92:44–47. doi: 10.2340/00015555924447. [DOI] [Google Scholar]
- 80.Williams HC, Burney PGJ, Pembroke AC, Hay RJ. Validation of the U.K. diagnostic criteria for atopic dermatitis in a population setting. Br. J. Dermatol. 1996;135:12–17. doi: 10.1111/j.1365-2133.1996.tb03599.x. [DOI] [PubMed] [Google Scholar]
- 81.Williams HC, Forsdyke H, Boodoo G, Hay RJ, Burney PGJ. A protocol for recording the sign of flexural dermatitis in children. Br. J. Dermatol. 1995;133:941–949. doi: 10.1111/j.1365-2133.1995.tb06930.x. [DOI] [PubMed] [Google Scholar]
- 82.Abuabara K, Margolis DJ, Langan SM. The long-term course of atopic dermatitis. Dermatol Clin. 2017;35:291–297. doi: 10.1016/j.det.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abuabara K, Yu AM, Okhovat JP, Allen IE, Langan SM. The prevalence of atopic dermatitis beyond childhood: A systematic review and meta-analysis of longitudinal studies. Allergy. 2018;73:696–704. doi: 10.1111/all.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kiiski V, Salava A, Susitaival P, Barnhill S, Remitz A, Heliovaara M. Atopic dermatitis in adults: A population-based study in Finland. Int. J. Dermatol. 2022;61:324–330. doi: 10.1111/ijd.15912. [DOI] [PubMed] [Google Scholar]
- 85.Chen W, Mempel M, Schober W, Behrendt H, Ring J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy. 2008;63:1418–1427. doi: 10.1111/j.1398-9995.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 86.Kische H, Hannemann A, Voss C, Nauck M, Völzke H, Pieper L, et al. Lack of significant association between sex hormone concentrations and atopic dermatitis in adolescents and adults in two population-based studies. J. Invest. Dermatol. 2022;142:486–489. doi: 10.1016/j.jid.2021.07.133. [DOI] [PubMed] [Google Scholar]
- 87.Rutter CE, Silverwood RJ, Williams HC, et al. Are environmental factors for atopic eczema in ISAAC Phase Three due to reverse causation? J. Invest. Dermatol. 2019;139:1023–1036. doi: 10.1016/j.jid.2018.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chovatiya R, Silverberg JI. Evaluating the longitudinal course of atopic dermatitis: A review of the literature. J. Am. Acad. Dermatol. 2022;23:459–468. doi: 10.1016/j.jaad.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mackenzie-Wood AR, Freeman S. Unclassified endogenous eczema. Contact Dermat. 1999;41:18–21. doi: 10.1111/J.1600-0536.1999.TB06202.X. [DOI] [PubMed] [Google Scholar]
- 90.Diepgen TL. Atopic dermatitis: The role of environmental and social factors, the European experience. J. Am. Acad. Dermatol. 2001;45:S44–S48. doi: 10.1067/MJD.2001.117016. [DOI] [PubMed] [Google Scholar]
- 91.Williams HC. What is atopic dermatitis and how should it be defined in epidemiological studies? In: Williams HC, editor. Atopic Dermatitis. The Epidemiology, Causes and Prevention of Atopic Eczema. Cambridge University Press; 2000. [Google Scholar]
- 92.Tyrer S, Heyman B. Sampling in epidemiological research: Issues, hazards and pitfalls. BJPsych Bull. 2016;40:57–60. doi: 10.1192/pb.bp.114.050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Low N, Redmond S, Uusküla A, van Bergen J, Ward H, Andersen B, et al. Screening for genital chlamydia infection. Cochrane Database Syst. Rev. 2016;9:CD010866. doi: 10.1002/14651858.CD010866.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Low N, Macleod J, Salisbury C, Egger M. Bias in chlamydia prevalence surveys. Lancet. 2003;362:1157–1158. doi: 10.1016/S0140-6736(03)14480-7. [DOI] [PubMed] [Google Scholar]
- 95.Dizon MP, Yu AM, Singh RK, Wan J, Chren M-M, Flohr C, et al. Systematic review of atopic dermatitis disease definition in studies using routinely collected health data. Br. J. Dermatol. 2018;178:1280–1287. doi: 10.1111/bjd.16340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bieber T. How to define atopic dermatitis? Dermatol. Clin. 2017;35:275–281. doi: 10.1016/j.det.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 97.Andersen RM, Thyssen JP, Maibach HI. Qualitative vs. quantitative atopic dermatitis criteria - in historical and present perspectives. J. Eur. Acad. Dermatol. Venereol. 2016;30:604–618. doi: 10.1111/jdv.13442. [DOI] [PubMed] [Google Scholar]
- 98.Pols DHJ, Wartna JB, Moed H, van Alphen EI, Bohnen AM, Bindels PJE. Atopic dermatitis, asthma and allergic rhinitis in general practice and the open population: A systematic review. Scand. J. Prim. Health Care. 2016;34:143–150. doi: 10.3109/02813432.2016.1160629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J. Am. Acad. Dermatol. 2019;80:390–401. doi: 10.1016/j.jaad.2018.09.035. [DOI] [PubMed] [Google Scholar]
- 100.Silverberg JI, Thyssen JP, Paller AS, Drucker AM, Wollenberg A, Lee KH, et al. What’s in a name? Atopic dermatitis or atopic eczema, but not eczema alone. Allergy. 2017;72:2026–2030. doi: 10.1111/all.13225. [DOI] [PubMed] [Google Scholar]
- 101.Andersen YMF, Egeberg A, Hamann CR, Skov L, Gislason GH, Skaaby T, et al. Poor agreement in questionnaire-based diagnostic criteria for adult atopic dermatitis is a challenge when examining cardiovascular comorbidity. Allergy. 2018;73:923–931. doi: 10.1111/all.13360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data extracted from included studies and used for analyses are available upon reasonable request from the authors.