Abstract
Objective:
Amyotrophic lateral sclerosis (ALS) is a multi-system disorder characterized primarily by motor neuron degeneration, but may be accompanied by cognitive dysfunction. Statistically appropriate criteria for establishing cognitive impairment (CI) in ALS are lacking. We evaluate quantile regression (QR), that accounts for age and education, relative to a traditional 2 standard deviation (SD) cut-off for defining CI.
Methods:
QR of cross-sectional data from a multi-center North American Control (NAC) cohort of 269 healthy adults was used to model the 5th percentile of cognitive scores on the Edinburgh Cognitive and Behavioral ALS Screen (ECAS). The QR approach was compared to traditional 2 SD cut-off approach using the same NAC cohort (2SD-NAC) and to existing UK-based normative data derived using the 2SD approach (2SD-UK) to assess the impact of cohort selection and statistical model in identifying CI in 182 ALS patients.
Results:
QR-NAC models revealed that age and education impact cognitive performance on the ECAS. Based on QR-NAC normative cutoffs, the frequency of CI in the 182 PENN ALS patients was 15.9% for ALS Specific, 12.6% for ALS Non-Specific and 15.4% for ECAS Total. This frequency of CI is substantially more conservative in comparison to the 2SD-UK (20.3%−34.6%) and modestly more conservative to the 2SD-NAC (14.3%−16.5%) approaches for estimating CI.
Conclusions:
The choice of normative cohort has a substantial impact and choice of statistical method a modest impact on defining CI in ALS. This report establishes normative ECAS thresholds to identify whether ALS patients in the North American population have CI.
Amyotrophic lateral sclerosis (ALS) is a multi-system disorder primarily characterized by progressive lower motor neuron (LMN) and upper motor neuron (UMN) degeneration1, but there is also increasing evidence for cognitive and behavioral impairment2. Indeed, current international consensus criteria for ALS frontotemporal spectrum disorder (ALS-FTSD) recommend two axes for the diagnosis of ALS including (I) defining the motor neuron syndrome and (II) defining neuropsychological deficits3. Cognitive impairment in ALS is an important clinical consideration given its association with poor prognosis including reduced survival and functional decline4.
The Edinburgh Cognitive and Behavioral Assessment Screen (ECAS) is an established brief neuropsychological assessment designed specifically for ALS that accommodates physical challenges (e.g., limited ability to speak or write) that potentially confound traditional neuropsychological testing in this population5. It is recommended as one of the principal tools for assessing cognition in ALS-FTSD3. Administration of the ECAS takes approximately 25 minutes to complete and uses a 136-point scale to evaluate several domains of cognition including language, executive functioning, and verbal fluency that are commonly believed to be most often affected in ALS, collectively contributing to an “ALS Specific” composite score; as well as visuospatial and memory domains that are thought to be less frequently affected in ALS and contribute to an “ALS Non-Specific” score. The ECAS has been validated against more extensive neuropsychological tests6,7 and multimodal studies, suggesting that ECAS performance relates to biologically plausible regional neuroimaging8 and neuropathological burden9 in ALS patients. While the ECAS has been translated into several languages10–13, normative data to empirically define cognitive impairments in North American ALS patients are lacking, and only rare prior studies have considered age and education when defining thresholds of cognitive impairment14,15. Moreover, prior approaches to defining impairment cutoffs on the ECAS have typically been based on data from relatively small series of healthy adults and have been based on statistical models that may violate important assumptions about the psychometric properties of ECAS scores.
In this report we present and compare 3 sets of normative thresholds for defining cognitive impairment, calculated based on different reference populations (N=40 single-center United Kingdom controls vs. N=269 multi-center North American Controls (NAC)) and statistical methods: the most common approach of “2 standard deviations below the mean” (2SD) vs. quantile regression with covariate adjustment (QR), as summarized in Table 1. Moreover, we illustrate how the choice of normative cutoffs impacts the classification of impairment (or not) in a cohort of 182 ALS patients.
Table 1.
Study populations from which normative data were collected, statistical methods, and threshold strategies used to define cognitive impairment on the ECAS
| 2SD-UK | 2SD-NAC | QR-NAC | |
|---|---|---|---|
| Study population | N=40 UK Controls |
N=269 North American Controls (CALSNIC, CRiALS, PENN) |
|
| Statistical method | Mean ± 2 SD | Mean ± 2 SD | Quantile Regression |
| Threshold strategy | 2 SDs below the mean* | 2 SDs below the mean | 5th percentile, adjusting for age and education |
Note. SD = Standard deviation;
Cutoff values previously published5; NAC=North American Controls.
Methods
Control Participants and Sources of Normative Data
Data to establish normative ECAS performance were pooled together from three North American sources which we collectively refer to as the North American Control (NAC) cohort: the research cohort at the Penn Frontotemporal Degeneration Center and Comprehensive ALS Center at the University of Pennsylvania (PENN); the multi-center study conducted by the Clinical Research in ALS study at the University of Miami (CRiALS); and the multi-center study conducted by the Canadian ALS Neuroimaging Consortium (CALSNIC)16. This resulted in a total of 269 healthy adults; each source of data is described in detail below and demographic characteristics are summarized in Table 2.
Table 2.
Summary demographics and ECAS performance from three cohort studies of healthy adults, constituting our North American Controls (N=269).
| Demographics | Overall | CALSNIC | CRiALS | PENN | ||||
|---|---|---|---|---|---|---|---|---|
| ≥ College Education, % | 60% | 50% | 80% | 70% | ||||
| ECAS Performance | Mean (SD) |
Median [25–75 %tile] |
Mean (SD) |
Median [25–75 %tile] |
Mean (SD) |
Median [25–75 %tile] |
Mean (SD) |
Median [25–75 %tile] |
| Language (Max score = 28) |
26.5 (1.8) |
27 [26, 28] |
26.4 (2.0) |
27 [26, 28] |
26.6 (1.7) |
27 [26, 28] |
26.7 (1.5) |
27 [26, 28] |
| Fluency (Max score = 24) |
18.6 (4.0) |
20 [16, 20] |
18.0 (4.3) |
20 [16, 20] |
18.9 (3.0) |
20 [18, 20] |
19.5 (3.8) |
20 [18, 22] |
| Executive (Max score = 48) |
39.8 (4.5) |
41 [38, 43] |
39.3 (4.9) |
41 [38, 43] |
39.9 (4.4) |
41 [38, 43] |
40.7 (3.6) |
42 [38, 43] |
| Memory (Max score = 24) |
17.1 (2.9) |
17 [15, 19] |
16.8 (3.0) |
17 [15, 19] |
18.2 (2.4) |
19 [16.75, 20] |
17.1 (2.7) |
17 [15, 18.75] |
| Visuospatial (Max score = 12) |
11.8 (0.5) |
12 [12, 12] |
11.8 (0.4) |
12 [12, 12] |
11.8 (0.5) |
12 [12, 12] |
11.8 (0.6) |
12 [12, 12] |
| ALS Specific (Max score = 100) |
84.9 (8.1) |
87 [81, 90] |
83.7 (8.9) |
86 [80, 89] |
85.4 (6.6) |
87 [81, 9] |
86.9 (6.7) |
89 [84, 91] |
| ALS Non-Specific (Max score = 36) |
29.0 (3.0) |
29 [27, 31] |
28.7 (3.1) |
29 [27, 31] |
30.0 (2.4) |
31 [28, 32] |
29.0 (2.8) |
29 [27, 30.75] |
| Total (Max score = 136) |
113.9 (9.3) |
115 [110, 120] |
112.4 (10.5) |
114 [108, 120] |
115.4 (7.6) |
117 [110, 121] |
115.9 (7.3) |
116 [112, 122] |
Education was recorded differently across studies, and for simplicity in interpretation, we categorically defined education for statistical models as individuals who completed a four-year college degree or higher level of education (≥ College) or did not (< College).
CALSNIC:
147 healthy adults recruited across Canada and the U.S. and with no history of neurological disease, head trauma, or significant psychiatric history. The CALSNIC research protocol was approved by a Health Research Ethics Board convened at each of the participating universities including University of Alberta, University of British Columbia, Université Laval, University of Calgary, the Montreal Neurological Institute and Hospital at McGill University, Western University, University of Toronto and University of Miami. All participants at each of these institutions provided written consent following an approved informed consent procedure.
CRiALS:
40 healthy adults recruited from across the U.S. with no history of neurological disease, no significant psychiatric history, and either have no family history of ALS or FTD or do not carry the genetic mutation(s) known to cause ALS/FTD in their family. The Clinical Research in ALS (CRiALS) research protocol was approved by an Institutional Review Board convened at the University of Miami and all participants provided written consent following an approved informed consent procedure.
PENN:
82 community-dwelling healthy adults who self-report no history of neurological or significant psychiatric condition and were screened to have a Mini Mental Status Examination score of 27–30. The PENN research protocol was approved by an Institutional Review Board convened at the University of Pennsylvania and all participants provided written consent following an approved informed consent procedure.
ALS Participants
To illustrate the impact of different ECAS normative cutoffs on identifying cognitive dysfunction in ALS, we evaluated 182 ALS patients at PENN who were diagnosed by a board-certified neurologist according to published consensus criteria1. Demographic and clinical characteristics are described in Online Supplementary Table 1. In addition to a clinical diagnosis of ALS, further inclusion criteria required completion of the ECAS along with three clinical assessments comprised of (1) a seated forced vital capacity (FVC) assessment of respiratory function; (2) the Revised ALS Functional Rating Scale (ALSFRS-R); and (3) Penn Upper Motor Neuron Score (PUMNS). All patients provided informed consent under a protocol approved by an Institutional Review Board convened at the University of Pennsylvania.
ECAS
All healthy adult control participants and ALS patients completed the North American ECAS (Version 1). Administration forms, published guidelines and instructions are available online (https://ecas.psy.ed.ac.uk/ecas-international/#American). The North American ECAS was lightly adapted from the original version, as described in detail elsewhere5, using the established Guidelines for Translation (https://ecas.psy.ed.ac.uk/wp-content/uploads/2017/11/Guidelines-for-Translation.pdf) that include using more culturally appropriate language materials in the memory recall (e.g., “shopping trolleys” → “shopping carts”, “Primose Woods” → “Marigold Woods”) and executive sentence completion (e.g., “postman” → “mailman”) tests. Scoring was also adapted to allow responses consistent with North American cultures. For example, in the naming test we accepted “coyote” for fox, “vest” for waistcoat, “camper” or “RV” for trailer, and “porcupine” for “hedgehog”. All evaluators were trained in the administration and scoring of the ECAS, which assesses the participants’ oral response performance in each of the 5 domains (language, fluency, executive, memory, and visuospatial function).
Statistical Approaches
All statistical analyses were performed using R software (Version 3.5.1). Visual assessment of histograms and Q-Q plots for each domain as well as the composite scores on the ECAS suggested that most scores are not normally distributed (see Online Supplementary Figure 1) and Kolmogorov-Smirnov tests relative to a normal distribution centered on the mean of each score confirmed lack of normality for each domain (all p<0.1e−8). Therefore, we compare the most common approaches for defining cutoffs based on 2 standard deviations (2SD) below the mean across both sources of normative data relative to a Quantile Regression (QR) approach that does not make Gaussian assumptions and further accommodates adjustments for age and education.
We additionally summarize descriptive results using both parametric (e.g., mean) and non-parametric (e.g., median) statistics to facilitate comparison to previous reports.
Methods for Defining Thresholds of Cognitive Impairment
Parametric UK Norms (2SD-UK):
The most widely applied approach for defining cognitive impairment on the ECAS is based on a previously established < −2 standard deviation cutoff relative to the mean in each domain and composite subscore from a sample of 40 Edinburgh-based healthy adults5.
Parametric North American (2SD-NAC):
For comparison purposes we also implemented a < −2 standard deviation cutoff relative to the mean in each domain and the composite subscores, but using our sample of 269 North American healthy adults.
Quantile Regression North American (QR-NAC):
Quantile regression has one key benefit over traditional parametric approaches for defining normative cutoffs. It makes no assumption about the data distribution and is, therefore, more appropriate for skewed data distributions that may have floor or ceiling effects. In addition, it can account for heteroscedasticity in which the variance of an outcome (e.g., ECAS score) is not uniform across a factor (e.g., age or education), whereas the 2SD method does not allow for covariate adjustments except through stratification17. QR-NAC cutoffs were calculated using the R quantregGrowth package18 for the 5th percentile of performance in each ECAS domain and composite subscores for the 269 healthy adults.
Results
Normative ECAS Performance in the North American Control Cohort
The NAC study population comprised 269 healthy adults, including 150 (56%) females. An assessment of demographics revealed, on average, that 60% of adults completed college or a higher degree (range 50–70% across studies) and that participants ranged in age from 24 to 83, with a mean ± SD of 55.5 ± 11.5 years (Table 2). CALSNIC healthy adults were significantly less educated than CRiALS and PENN healthy adults (X2=11.0; p=0.004), and CRiALS healthy adults (ß=−6.1; p=0.001) were significantly younger than CALSNIC and PENN healthy adults. To further evaluate heterogeneity across cohorts we compared performance across ECAS subtest using Kruskal-Wallis tests and only observed differences in the CALSNIC healthy adults having lower S-Word verbal fluency, and the CRiALS cohort having higher delay recall and recognition performance (see Online Supplementary Table 2). These observations highlight the importance for considering age and education in statistical models and are consistent with demographic differences across studies with the proportionally less educated cohort (CALSNIC) having lower verbal fluency and the younger cohort (CRiALS) having higher memory performance.
An evaluation of ECAS performance revealed median [25th – 75th percentile] of ECAS Total, ALS Specific and ALS Non-Specific scores of 115 [110–120], 87 [81–90] and 29 [27–31], with maximum possible scores of 136, 100 and 36 respectively (Table 2). Age- and education-adjusted estimates of 5th percentile thresholds for cognitive impairment are summarized for all ECAS scores, including each cognitive domain, in Table 3 (see also Figure 1 and Online Supplementary Figure 2). Importantly, the QR-NAC approach demonstrates a significant decline in healthy adult performance associated with age on each composite score (Figure 1, Table 3), with higher educational attainment (completing college) increasing the threshold for cognitive impairment by 1–4 points. Moreover, for individual cognitive domains, the 5th percentile of performance also declines with age for memory, fluency, executive and language performance, but not the visuospatial domain in which performance approached ceiling across all ages (Online Supplementary Figure 2).
Table 3.
Comparison of ECAS cutoffs determined using two standard deviation approach with published United Kingdom values (2SD-UK)5 and, in the North American Controls, using a two standard deviation (2SD-NAC) and Quantile Regression (QR-NAC) statistical approaches.
| ECAS Score | 2SD-UK | 2SD-NAC | Education | QR-NAC (Age) 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 35 | 40 | 45 | 50 | 55 | 60 | 65 | 70 | 75 | ||||
| Language (Max value = 28) |
26 | 23 | < College | 24 | 24 | 24 | 24 | 24 | 23 | 23 | 22 | 22 |
| ≥ College | 24 | 24 | 24 | 24 | 24 | 23 | 23 | 22 | 22 | |||
| Fluency (Max value = 24) |
14 | 11 | < College | 12 | 12 | 12 | 11 | 11 | 9 | 8 | 6 | 5 |
| ≥ College | 14 | 14 | 14 | 13 | 13 | 11 | 10 | 8 | 7 | |||
| Executive (Max value = 48) |
33 | 31 | < College | 33 | 32 | 32 | 31 | 31 | 30 | 30 | 29 | 29 |
| ≥ College | 32 | 31 | 31 | 30 | 30 | 29 | 29 | 29 | 28 | |||
| Memory (Max value = 24) |
13 | 11 | < College | 14 | 13 | 13 | 12 | 11 | 11 | 10 | 9 | 9 |
| ≥ College | 15 | 15 | 14 | 13 | 13 | 12 | 11 | 11 | 10 | |||
| Visuospatial (Max value = 12) |
10 | 11 | < College | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 |
| ≥ College | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | 11 | |||
| ALS Specific (Max value = 100) |
77 | 69 | < College | 74 | 73 | 72 | 71 | 70 | 68 | 67 | 66 | 65 |
| ≥ College | 77 | 76 | 75 | 73 | 72 | 71 | 70 | 69 | 67 | |||
| ALS Non-Specific (Max value = 36) |
24 | 23 | < College | 26 | 25 | 25 | 24 | 23 | 23 | 22 | 22 | 21 |
| ≥ College | 27 | 26 | 25 | 25 | 24 | 24 | 23 | 23 | 22 | |||
| Total Max value = 136) |
105 | 95 | < College | 102 | 101 | 99 | 98 | 96 | 94 | 92 | 90 | 87 |
| ≥ College | 106 | 105 | 103 | 101 | 100 | 98 | 96 | 93 | 91 | |||
Quantile regression technique used to estimate the 5th percentile values adjusted for age and education
Figure 1.
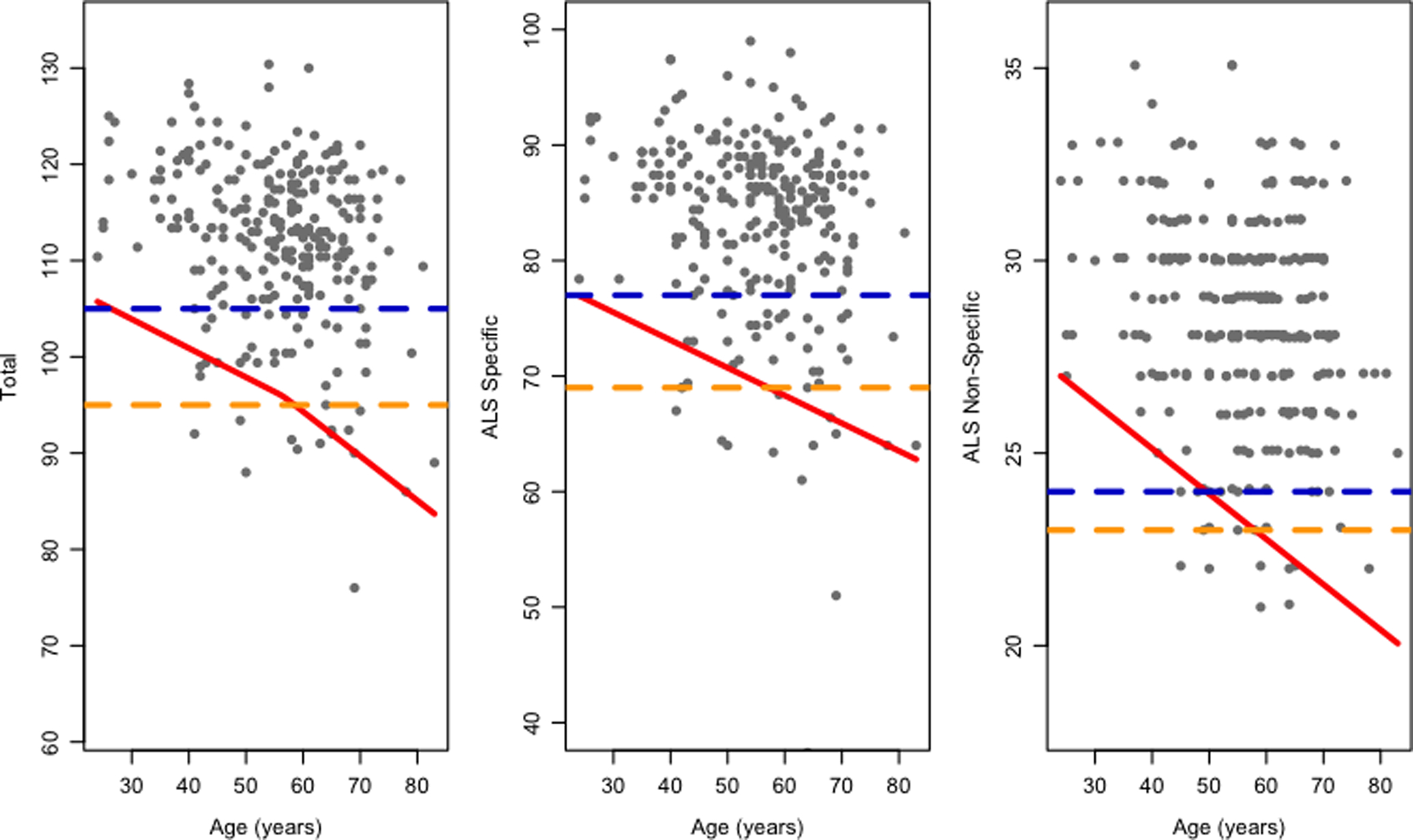
Quantile regression model for ECAS Total, ALS Specific, and ALS Non-Specific scores across age, including 5th percentile cutoff (red) as well as for comparison the two standard deviation approach for North America (2SD-NAC; orange) and United Kingdom (2SD-UK; blue).
Prevalence of Cognitive Impairments in PENN ALS Cohort
The prevalence of cognitive impairment in ALS varies depending on the population used to generate normative data as well as the approach used to determine thresholds for defining cognitive impairment (Figure 2 and Online Supplementary Table 3). When comparing the statistical approaches (2SD and QR) developed across NAC and UK cohorts, the 2SD-NAC and QR-NAC cutoffs suggest that ~16% of PENN ALS patients exhibit cognitive impairment based on the ALS-Specific and ECAS Total composite scores, substantially fewer than that estimated using the 2SD-UK approach for ALS-Specific (32.4% impaired) or ECAS Total (34.6% impaired). The discordance between cutoff approaches is even more apparent in the Language domain with an estimated ~63% impairment relative to ~19% and ~20% impaired in the PENN ALS cohort based on the 2SD-NAC and QR-NAC approaches, respectively. Fluency and Executive domains also suggest a lower frequency of cognitive impairment in the PENN ALS cohort using the 2SD-NAC and QR-NAC approach relative to the 2SD-UK approach.
Figure 2.
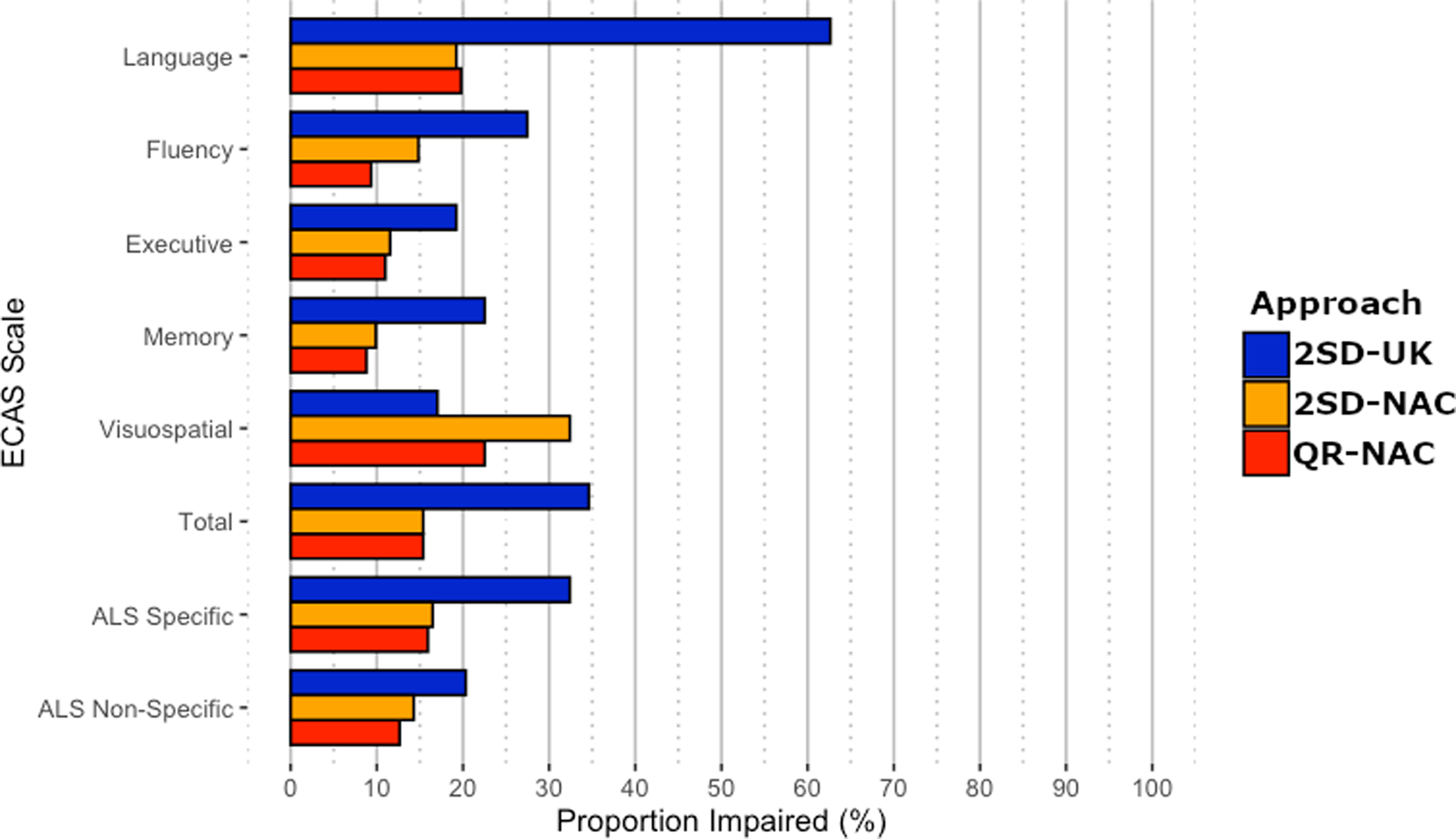
Prevalence of impairment on the ECAS in 182 ALS patients using three different sets of normative cutoffs.
Note. QR-NAC=Quantile regression North American Controls; 2SD-NAC=2 standard deviation cutoff North American; 2SD-UK= 2 standard deviation cutoff United Kingdom
In contrast to the NAC vs. UK comparisons above, the differences between the QR and parametric statistical approaches yield relatively similar estimates when evaluating the prevalence of cognitive impairment in the PENN ALS cohort using NAC reference populations. These include the ALS Specific score - 15.9% (QR-NAC) vs. 16.5% (2SD-NAC) and the individual cognitive domains that contribute to this score: Language - 19.8% (QR-NAC) vs. 19.2% (2SD-NAC); Executive - 11.0% (QR-NAC) vs. 11.5% (2SD-NAC); and Fluency - 9.3% (QR-NAC) vs. 14.8% (2SD-NAC). The greatest divergence between QR-NAC and 2SD-NAC approaches was for Visuospatial impairment, 22.5% (QR-NAC) vs. 32.4% (2SD-NAC), that together with Memory (QR-NAC=8.8% vs. 2SD-NAC=9.9%) contributes to a discrepancy in rates of impairment on the ALS Non-Specific score - 12.6% (QR-NAC) vs. 14.3% (2SD-NAC).
Discussion
Increasing interest in frontotemporal spectrum impairment in ALS and the overlap between ALS and FTD, have necessitated development of tools to quantify the nature and frequency of cognitive dysfunction in ALS. While the ECAS, a brief assessment tool, has emerged as the dominant instrument for evaluating cognition and behavior in ALS19, limited reference data (at least within North America) has hampered progress. In particular, efforts are underway to better understand genotype-phenotype relationships, including predictors of cognitive impairment20. Indeed, such large-scale studies principally rely on the ECAS for quantifying cognitive impairment since longitudinal assessment using a full neuropsychological battery may be impractical, especially as accrual of motor deficits with disease progression undermines the validity of established pen-and-paper neuropsychological tests. Without appropriate normative data for the ECAS, these efforts may be less successful due to potentially significant misclassification of cognitive impairment. Moreover, the recently revised Airlie House guidelines for the design and implementation of ALS clinical trials recommend that investigators stratify for cognitive impairments21; it is therefore essential to appropriately and accurately define whether individual patients are impaired or not.
Here we present normative data for the North American ECAS based on a moderately-sized, multi-center dataset and recommend (a) that it is essential to have culturally appropriate healthy control data for categorizing cognitive impairments; (b) the use of quantile regression as appropriate for estimating normative thresholds (e.g. 5th percentile) of data that are not normally distributed, and (c) adjusting for age and education, factors known to associate with cognitive function. Applying these findings to the ALS patient cohort at PENN, we have estimated the prevalence of cognitive impairment in ALS to be ~16%. This is substantially less frequent than what we and others have previously estimated based on published norms, which suggested typically up to 50%, and one estimate as high as 70%, of ALS patients have cognitive impairment. Of note, prior estimates that are inflated in comparison to our observations were derived from neuropsychological tests that, unlike ECAS, do not control for motor confounds. Future work will be necessary to validate the QR-NAC approach by comparing these suggested cutoffs relative to other more extensive “gold-standard” neuropsychological assessments. However, while such validation approaches are desirable they are also highly challenging because the vast majority of “gold-standard” neuropsychological assessments are typically confounded by motor features. Critically, our observation of a reduced frequency of cognitive impairment in ALS appears to be less related to the implementation of a QR statistical approach than to the selection of a culturally and demographically-comparable normative healthy control cohort. We discuss the interpretation of each of these factors in detail below.
While there is increasing evidence for language impairment in ALS, the current findings suggest that estimates of the rate of language impairment varies widely depending on the statistical approach and control population employed to generate normative data, ranging from ~19% for the 2SD-NAC and QR-NAC approaches to as high as ~63% of PENN ALS patients using the 2SD-UK approach. These findings therefore emphasize that sociodemographic differences across cultures are a critical consideration when defining normal language performance. As the ECAS becomes increasingly used internationally, it may be valuable to account for potential cultural and regional confounds in subsequent versions of the test, such as including cross-linguistic naming of objects24, differences in orthographic-phonemic mapping that may influence spelling across languages, and implementing norms across several languages25. While letter-guided verbal fluency constitutes a “domain” distinct from language in the ECAS, it is linguistically-mediated and thus susceptible to cross-linguistic differences, such as variable letter frequency26. It will, therefore, likely be important to re-calibrate the “verbal fluency indices” across ECAS versions. Nevertheless, it is valuable to note the overall relatively high rate of impairment on language skills probed by ECAS. This has been noted by other investigators27, but may be underappreciated. In an exploratory comparison of coefficient of variation across language domain subtests it appears that spelling has a disproportionately high level of dispersion in both the NAC cohort (14%) and the ALS cohort (23%) in comparison to naming (9% NAC; 16% ALS) and comprehension tests (3% NAC; 6% ALS). While this suggests that spelling may be a driver of poor performance in the language domain for both healthy adult controls and ALS patients it is not clear why this is the case. Future item analyses may help elucidate the contribution of this domain for defining language impairment and provide insight into the reliability of spelling for identifying language impairment. Lastly, while semantic deficits have been reported in ALS28, there is increasing evidence for grammatical and discourse deficits in ALS29–31. Supplementing the current language domain with tests of grammar and discourse in subsequent ECAS versions may provide a more accurate picture of language impairment in ALS.
Our use of the QR-NAC approach, in addition to accounting for non-normality, benefits by also accounting for cognitive performance across age. Importantly, ECAS Total score cutoffs range from 106 to 87 points depending on the age of the individual, highlighting the importance of considering age in the determination of impairment by using a dynamic rather than static cutoff strategy. Memory appears to be the domain most affected by age, as well as the ALS Non-Specific score, which suggests that these components are more sensitive to age-related or non-FTD contributors to cognitive decline. Indeed, memory decline in aging may reflect mild cognitive impairment or prodromal Alzheimer disease (AD), and AD co-pathology has been noted in autopsy-confirmed series of ALS patients sensitive to cognitive impairment33. In this context, it is important to consider that ALS cognitive impairment (ALSci), as defined using current clinical criteria3, may be associated with the ALS-FTSD spectrum as a prodromal phase of ALS-FTD or ALS-Dementia. Importantly, the QR-NAC approach suggests that the highest frequency of impairment based on composite scores (e.g., ALS Specific, ALS Non-Specific, Total) was the ALS Specific composite, which comprises the more predominantly affected executive, fluency, and language domains.
Beyond adjusting for age, the level of educational attainment has a modest impact on normative cutoff values. In particular, attainment of a college degree appears to have the greatest impact on verbal fluency and executive domains that contribute to the ALS Specific score, and only a 1-point impact on memory domains, while language and visuospatial performance remain constant across education groups. There are at least two possible reasons for this observation. One possibility for a lack of contribution of education to language and visuospatial domains may simply be a function of these domains having the most limited statistical variance. Indeed, we observed the highest proportion of cognitively impaired ALS patients in the language (particularly spelling) and visuospatial and domains and, while the QR-NAC approach does not have normality assumptions like the SD approaches, a lack of variance on these subtests may inflate estimates of impairment and underestimate the contribution of other factors like education. Another possibility, mentioned above, is that the limited range of probed language areas may be relatively insensitive to education. Yet an alternative possibility is that verbal fluency, executive, and memory domains are indeed preferentially impacted by educational status. For example, models of cognitive reserve suggest that factors like education may help stave off the clinical manifestations of underlying pathologies in aging individuals34 and evidence suggests that reserve may selectively impact executive functions but not visuospatial functions in healthy aging35 and frontotemporal degeneration36.
There are several caveats to consider. First, behavior is an important component of ALS-FTSD and, while this report establishes the frequency of cognitive impairment in ALS, it does not establish normative data for defining behavioral impairment in healthy adults or ALS patients. Second, our estimates of normative cognitive performance are based on a cross-sectional evaluation, which represents a snapshot of a progressive disease in which cognitive impairment may only emerge as disease unfolds37. While initial efforts have parametrically defined longitudinal change on the ECAS for healthy adults38, it is critical for future longitudinal investigations to define age-related rate of cognitive decline in healthy adults and in a manner that accounts for sociodemographic and cultural differences, in order to determine how the tempo of disease progression in ALS differs from normal age-related cognitive decline. Third, a limitation of pooling normative healthy adult data across cohorts is that educational attainment was not captured in a harmonized manner and therefore we were limited to dichotomizing education as college completion vs. less than a college education. However, cognitive performance may be more sensitive to finer-grained educational differences (e.g., high school, graduate degree) and this is an important consideration for future investigations. Likewise, our control cohort had a greater frequency of college completion (~65%) than the general population; although the current study is the largest ECAS normative study to date, future studies with increased sampling of the general population may better capture the influence of education on ECAS performance. Fourth, the visuospatial domain is the only domain in which we observed a higher rate of cognitive impairment in ALS patients relative to the 2SD-UK approach. While this is less exaggerated using the QR-NAC approach, which is less susceptible to ceiling effects than the 2SD-NAC approach, this observation highlights the importance for considering the psychometric properties of the ECAS and subsequent ECAS versions may benefit by revising this domain to lower the current ceiling properties. Lastly, we defined thresholds of cognitive impairment using a commonly implemented 5th percentile cutoff for the QR approach and 2SD cutoff for the other approaches, but it is possible that alternative less conservative thresholds may be more appropriate.
In summary, estimates of the frequency of cognitive impairment in ALS vary widely depending on the choice of cohort and statistical approach used to define thresholds for defining cognitive impairment. While our estimates are based on application of norms to ALS patients in the PENN cohort, we suggest that the use of quantile regression models, as employed in our QR-NAC approach, provides a robust strategy to define cognitive impairments that is statistically appropriate and accounts for potential confounding factors such as age and education.
Supplementary Material
Acknowledgments
We extend thanks to research coordinators at the University of Pennsylvania including Laura Baehr, Laura Hennessy, and Hayley Donaldson for their efforts with participant recruitment and evaluation. CALSNIC additionally thanks the research team for participant recruitment and evaluations. Additionally, CRiALS extends thanks to the research team at the University of Miami for participant recruitment and evaluation (Anne-Laure Grignon, Danielle Dauphin, Danielle Sheldon, Sumaira Hussain, Anne Cooley, Jessica Medina, Ashley Manso).
Dr. McMillan reports grants from the National Institutes of Health (NIH; AG017586; AG066597).
Dr. Kalra reports grants that support CALSNIC including the Canadian Institutes of Health Research, ALS Society of Canada, Brain Canada Foundation, and Shelly Mrkonjic ALS Research Fund.
Dr. Benatar reports grants from Muscular Dystrophy Association (Grants #4365 and #172123), the ALS Association (Grant #2015), and the National Institutes of Health (NIH; NS105479); philanthropic support from the ALS Recovery Fund and the Kimmelman Estate during the conduct of the study.
Declaration of Conflicts
Dr. McMillan receives research funding outside the submitted work from Biogen, Inc and provides consulting services for personal fees from Invicro and Axon Advisors on behalf of Translational Bioinformatics, LLC. He also receives an honorarium as Associate Editor of NeuroImage: Clinical.
Dr. Johnston receives research funding from Annexon, Alexion, ALS-Pharma, Biogen, Cytokinetics, Mallinckrodt, Mitsubishi-Tanabe Canada, Orion, and serves as a paid consultant to Biogen and Mitsubishi-Tanabe Canada.
Dr. Genge provides consulting services for the following companies: AB Sciences, Akcea, Alexion, AL-S Pharma, Anavex, Avexis, Bayshore, Biogen, Clene, CSL Behring, Cytokinetics, QurAlis, Mitsubishi Tenabe Pharma America, Novartis, Orion, Revalesio, Roche, Sanofi Genzyme, and Wave life sciences. She is also CRU Medical Director, PI or sub-PI on trials sponsored by the following companies: AB Sciences, AL-S Pharma, Acceleron, Amicus, Alnylam, Bioblast, Biogen, BMS, Boston Biomedical, Cytokinetics, Sanofi Genzyme, Grifols, Ionis, Lily, Mallinckrodt, Medimmune, Novartis, Orion, Orphazyme, Pfizer, Ra Pharmaceuticals, Roche, Teva, and UCB.
Dr. Benatar reports personal fees from Roche and Biogen.
Drs. Wuu, Rascovsky, Cosentino, Grossman, Elman, Quinn, Rosario, Stark, Granit, Briemberg, Chenji, Dionne, Korngut, Shoesmith, Zinman report no conflicts of interest.
References
- 1.Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis and other motor neuron disorders. 2000. p. 293–299. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein LH, Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 2013;12:368–380. [DOI] [PubMed] [Google Scholar]
- 3.Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. Epub 2017 Jan 5.:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elamin M, Bede P, Byrne S, et al. Cognitive changes predict functional decline in ALS: a population-based longitudinal study. Neurology. 2013;80:1590–1597. [DOI] [PubMed] [Google Scholar]
- 5.Abrahams S, Newton J, Niven E, Foley J, Bak TH. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:9–14. [DOI] [PubMed] [Google Scholar]
- 6.Niven E, Newton J, Foley J, et al. Validation of the Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen (ECAS): A cognitive tool for motor disorders. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:172–179. [DOI] [PubMed] [Google Scholar]
- 7.De Icaza Valenzuela Mónica M, H BT, Suvankar P, Sharon A. The Edinburgh Cognitive and Behavioral ALS screen: relationship to age, education, IQ and the Addenbrooke’s Cognitive Examination-III. Amyotroph Lateral Scler Frontotemporal Degener. 2nd ed. Taylor & Francis; 2018;00:1–7. [DOI] [PubMed] [Google Scholar]
- 8.Trojsi F, Caiazzo G, Siciliano M, et al. Microstructural correlates of Edinburgh Cognitive and Behavioural ALS Screen (ECAS) changes in amyotrophic lateral sclerosis. Psychiatry Res Neuroimaging. 2019;288:67–75. [DOI] [PubMed] [Google Scholar]
- 9.Gregory JM, McDade K, Bak TH, et al. Executive, language and fluency dysfunction are markers of localised TDP-43 cerebral pathology in non-demented ALS. Journal of Neurology, Neurosurgery, and Psychiatry. BMJ Publishing Group Ltd; Epub 2019 Sep 12.:jnnp–2019–320807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakker LA, Schröder CD, Spreij LA, et al. Derivation of norms for the Dutch version of the Edinburgh cognitive and behavioral ALS screen. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:19–27. [DOI] [PubMed] [Google Scholar]
- 11.Lulé D, Burkhardt C, Abdulla S, et al. The Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen: a cross-sectional comparison of established screening tools in a German-Swiss population. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:16–23. [DOI] [PubMed] [Google Scholar]
- 12.Mora JS, Salas T, Fernández MC, et al. Spanish adaptation of the edinburgh cognitive and behavioral amyotrophic lateral sclerosis screen (ECAS). Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:74–79. [DOI] [PubMed] [Google Scholar]
- 13.Poletti B, Solca F, Carelli L, et al. Cognitive-behavioral longitudinal assessment in ALS: the Italian Edinburgh Cognitive and Behavioral ALS screen (ECAS). Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:387–395. [DOI] [PubMed] [Google Scholar]
- 14.Loose M, Burkhardt C, Aho-Özhan H, et al. Age and education-matched cut-off scores for the revised German/Swiss-German version of ECAS. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:374–376. [DOI] [PubMed] [Google Scholar]
- 15.Pinto-Grau M, Burke T, Lonergan K, et al. Screening for cognitive dysfunction in ALS: validation of the Edinburgh Cognitive and Behavioural ALS Screen (ECAS) using age and education adjusted normative data. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:99–106. [DOI] [PubMed] [Google Scholar]
- 16.Kalra S, Khan MU, Barlow L, et al. The Canadian ALS Neuroimaging Consortium (CALSNIC) - a multicentre platform for standardized imaging and clinical studies in ALS. medRxiv 2020. p. 1–19. [Google Scholar]
- 17.Benatar M, Wuu J, Peng L. Reference data for commonly used sensory and motor nerve conduction studies. Muscle Nerve. 2009;40:772–794. [DOI] [PubMed] [Google Scholar]
- 18.Muggeo VMR, Sciandra M, Tomasello A, Calvo S. Estimating growth charts via nonparametric quantile regression: a practical framework with application in ecology. Environ Ecol Stat. Springer US; 2013;20:519–531. [Google Scholar]
- 19.Hodgins F, Mulhern S, Abrahams S. The clinical impact of the Edinburgh Cognitive and Behavioural ALS Screen (ECAS) and neuropsychological intervention in routine ALS care. Amyotroph Lateral Scler Frontotemporal Degener. 2019;12:1–8. [DOI] [PubMed] [Google Scholar]
- 20.Placek K, Benatar M, Wuu J, et al. Machine learning suggests polygenic risk for cognitive dysfunction in amyotrophic lateral sclerosis. EMBO Mol Med. John Wiley & Sons, Ltd; 2020;15:e12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Berg LH, Sorenson E, Gronseth G, et al. Revised Airlie House consensus guidelines for design and implementation of ALS clinical trials. Neurology. 2019;92:e1610–e1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology; 2005;65:586–590. [DOI] [PubMed] [Google Scholar]
- 23.Frank B, Haas J, Heinze HJ, Stark E, Münte TF. Relation of neuropsychological and magnetic resonance findings in amyotrophic lateral sclerosis: evidence for subgroups. Clin Neurol Neurosurg. 1997;99:79–86. [DOI] [PubMed] [Google Scholar]
- 24.Ardila A Toward the development of a cross-linguistic naming test. Arch Clin Neuropsychol. 2007;22:297–307. [DOI] [PubMed] [Google Scholar]
- 25.Bates E, D’Amico S, Jacobsen T, et al. Timed picture naming in seven languages. Psychon Bull Rev. 2003;10:344–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosselli M, Ardila A, SALVATIERRA J, MARQUEZ M, Matos L, WEEKES VA. A cross-linguistic comparison of verbal fluency tests. Int J Neurosci. 2002;112:759–776. [DOI] [PubMed] [Google Scholar]
- 27.Taylor LJ, Brown RG, Tsermentseli S, et al. Is language impairment more common than executive dysfunction in amyotrophic lateral sclerosis? Journal of Neurology, Neurosurgery, and Psychiatry. 2013;84:494–498. [DOI] [PubMed] [Google Scholar]
- 28.Libon DJ, McMillan C, Avants B, et al. Deficits in concept formation in amyotrophic lateral sclerosis. 2012;26:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ash S, Olm C, McMillan CT, et al. Deficits in sentence expression in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ash S, Menaged A, Olm C, et al. Narrative discourse deficits in amyotrophic lateral sclerosis. Neurology. 2014;83:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nevler N, Ash S, McMillan C, et al. Automated analysis of natural speech in amyotrophic lateral sclerosis spectrum disorders. Neurology. 2020;95:e1629–e1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knopman DS, Caselli RJ. Appraisal of cognition in preclinical Alzheimer’s disease: a conceptual review. Neurodegener Dis Manag. 2012;2:183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brettschneider J, Libon DJ, Toledo JB, et al. Microglial activation and TDP-43 pathology correlate with executive dysfunction in amyotrophic lateral sclerosis. Acta Neuropathol. 2012;123:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stern Y Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavrencic LM, Churches OF, Keage HAD. Cognitive reserve is not associated with improved performance in all cognitive domains. Appl Neuropsychol Adult. 2017;25:473–485. [DOI] [PubMed] [Google Scholar]
- 36.Placek K, Massimo L, Olm C, et al. Cognitive reserve in frontotemporal degeneration: Neuroanatomic and neuropsychological evidence. Neurology. 2016;87:1813–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crockford C, Newton J, Lonergan K, et al. ALS-specific cognitive and behavior changes associated with advancing disease stage in ALS. Neurology. Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology; 2018;91:e1370–e1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crockford C, Newton J, Lonergan K, et al. Measuring reliable change in cognition using the Edinburgh Cognitive and Behavioural ALS Screen (ECAS). Amyotroph Lateral Scler Frontotemporal Degener. 2018;19:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


