Abstract
Introduction
Diseases of the maxillary sinus have been associated with dental roots near the maxillary sinus that have undergone endodontic treatment.
Objective
To investigate the presence of filamentous fungi in patients with dental roots near the maxillary sinus who had apical periodontitis treated endodontically, and to alert practitioners that this could be a possible avenue of contamination of the sinus in patients who develop maxillary sinus infection.
Methods
Cross-sectional study in 60 palatal roots of the first maxillary molars near the maxillary sinus, that underwent endodontic treatment for apical periodontitis. After removal of the filling material, dentin shavings were collected and placed in test tubes containing Sabouraud dextrose agar and chloramphenicol. The phenotype was determined by macroscopic and microscopic examination of the colonies. For polymerase chain reaction, the primers ITS-5 and ITS-4 were used. The sequences obtained were compared with those deposited at GenBank using the Basic Local Alignment Search Tool program.
Results
Filamentous fungi were isolated from 6 of 60 canals (10%): Aspergillus niger (6.7%), Aspergillus versicolor (1.6%), and Aspergillus fumigatus (1.6%).
Conclusion
Root canals near the maxillary sinus with endodontic treatment and apical periodontitis may exhibit positive cultures for filamentous fungi. Interested professionals should be alert, because these microorganisms have pathogenic characteristics that can cause disease of odontogenic origin in the maxillary sinus.
Keywords: Aspergillus, Endodontics, Infection, Maxillary sinus
Resumo
Introdução
Doenças do seio maxilar têm sido associadas à raízes com tratamento endodôntico próximas ao seio maxilar.
Objetivo
Investigar a presença de fungos filamentosos em raízes com tratamento endodôntico e lesão periapical, próximas ao seio maxilar, alertando para uma possível contaminação do seio maxilar por via odontogênica.
Método
Estudo transversal em sessenta raízes palatinas de primeiros molares superiores próximas ao seio maxilar, com tratamento endodôntico e lesão periapical. Após remoção do material obturador, raspas de dentina foram coletadas e inseridas em tubos de ensaio contendo Agar Sabouraud Dextrose e Clorafenicol. O fenótipo foi determinado pela análise macroscópica e microscópica das colônias. Para o PCR utilizou-se iniciadores ITS-5 e ITS-4. As sequencias obtidas foram comparadas as disponíveis no GenBank utilizando Basic Local Alignment Search Tool.
Resultados
Fungos filamentosos foram isolados de 6 dos 60 canais (10%): Aspergillus niger (6,7%), Aspergillus versicolor (1,6%) e Aspergillus fumigatus (1,6%).
Conclusão
Raízes próximas ao seio maxilar com tratamento endodôntico e lesão periapical, podem apresentar cultura positiva para fungos filamentosos. Profissionais afins devem estar alerta, pois este micro-organismo possuem características de patogenicidade podendo causar doenças no seio maxilar de origem odontogênica.
Palavras-chave: Aspergillus, Endodontia, Infecção, Seio maxilar
Introduction
Approximately 10–12% of cases of maxillary sinusitis are caused by odontogenic infection, due to the proximity of the roots of posterior maxillary teeth with the maxillary sinus cavities.1 Apical and marginal periodontitis constitute 83% of odontogenic factors that may cause signs in maxillary sinuses.2, 3 Lu et al.3 showed a direct relationship between presence of apical periodontitis and maxillary sinus membrane thickening, at a rate of 100%. The authors believe that microorganisms and toxins present in endodontic infections can seep into the maxillary sinus directly or through their countless vascular anastomoses, alveolar bone marrow, and lymph vessels.
Although bacteria are the most extensively studied etiological agents in endodontic infections, fungi also can be isolated from root canals.4, 5, 6, 7, 8, 9 The presence of filamentous fungi in root channels of teeth with pulp necrosis and apical periodontitis was first detected by Gomes et al. in 2010.10
There are several articles published in the medical literature that link the presence of filamentous fungi in the maxillary sinus with endodontically treated root canals in close contact with the maxillary sinus.11, 12, 13, 14, 15, 16 Recent studies have reported that endodontic treatment in posterior maxillary teeth is a strong risk factor for onset of a fungus ball within the maxillary sinus.12, 13
However, the role of endodontic infections in maxillary sinuses is not very clear. Based on these reports, the aim of this study was to investigate the presence of filamentous fungi in endodontically treated root canals with apical periodontitis, located near the maxillary sinus.
Methods
This research was approved by the Research Ethics Committee (CEP CMM/HUAP 134/08 CAAE 0101.0.258.000-08). All participants completed a health questionnaire and signed an informed consent. For this study, 60 (24 female and 36 male) patients aged 18–65 years were selected; these patients were under treatment at the School of Dentistry.
Study design
This was a cross-sectional study of a contemporary cohort.
Criteria for inclusion
Palatine roots of first maxillary molars that were radiographically in close contact with maxillary sinus, with endodontic treatment performed over a 3-year period prior to the study and with evidence of apical periodontitis.
Exclusion criteria
Teeth with pulp chamber exposed to oral cavity, with symptoms of pain, presence of mobility, and periodontal pocket. Patients treated with oral antibiotics and/or antifungals in the last six months and those with clinical manifestations of oral candidiasis were also excluded.
Sample collection
The selected teeth were accessed after receiving absolute isolation, antisepsis with 5.25% sodium hypochlorite, and neutralization with 5% sodium thiosulfate. Access to root canals was performed with the use of a high-speed, carbide, round drill bit, and then the operative field was again cleaned with the aforementioned solutions. The palatine root canal was irrigated with sterile saline; according to a pre-established length calculated in an initial radiography, gutta-percha was removed without using solvent, using Gates-Glidden burs #2 and #3 and Hedströen files #25, #30 and #40. Then, a Kerr file of compatible diameter and length for the root canal was passively introduced, and the odontometry was established with the use of an apex locator, with radiographic confirmation.
Following the protocol recommended by Gomes et al.,10 the channel was irrigated with sterile saline and a Hedströen file #40 was used to remove dentin shavings. Then, three sterile absorbent paper points were inserted into the root canal, one by one, 1 mm short of the root apex, and maintained there for one minute. These paper points were then transferred to a test tube containing Sabouraud agar (Difco Laboratories, Detroit, MI, United States) with addition of 0.5% chloramphenicol (Medleyk Campinas, SP, Brazil), in a two-lamp isolated field. An open Petri dish containing the same culture medium (Sabouraud agar plus 0.5% chloramphenicol) was placed within the field isolated by the two lamps in order to verify the accuracy of isolation of the operative field (negative control). At the same time, a positive control, including a Petri dish containing the same culture medium but which remained open outside the isolated field during the collection of samples, was conducted, in order to check the growth of potential environmental fungi, verifying whether these organisms coincided with those isolated from root canals. Petri dishes and test tubes were kept at room temperature for 7–14 days; during this period, mycelial growth was observed for subsequent isolate analysis at the Fungal Culture Collection Laboratory.
Direct examination of colonies
From a monosporic culture, a colony fragment was collected and placed on a slide. A drop of Amann's lactophenol-cotton blue cytoplasmic stain was added (Difco Laboratories, Detroit, MI, United States) and an optical microscope (YS 100; Nikon Corporation, Japan) was used to observe vegetative and reproductive mycelium of the fungi.
Macroscopic identification
After checking the characteristics of the fungi, the strains were subcultured with the aid of a sterile platinum loop, and one fragment of fungus was inoculated on a Petri dish containing 20 mL of a specific medium to facilitate its growth and sporulation. Depending on the characteristics presented, a specific culture medium was used, including malt extract agar, Czapek agar, or potato agar (Difco Laboratories, Detroit, MI, United States). The colonies were incubated at room temperature and observed every 24 h, being aware that each strain has a specific growth time, depending on its taxonomic group. Then, the colonies were analyzed and classified macroscopically with respect to texture, color (front and back side), diameter, pigment production, growth time, and production or nonproduction of exudate.
Microscopic identification
Mycelial fragments of each isolate were carefully examined in preparations obtained from microcultures in a specific medium, according to the Rivalier and Seydel technique.17 This methodology shows the arrangement of conidia in situ, allowing the morphological visualization of reproductive and/or propagation structures under optical microscopy (YS 100; Nikon Corporation, Japan), after slide preparation and application of a cytoplasmic stain, Amann's lactophenol-cotton blue (Difco Laboratories, Detroit, MI, United States). From morphological macroscopic and microscopic studies and with the aim of the specific literature, the taxonomic identification of isolated fungi was performed.18, 19, 20
Molecular identification – DNA extraction
Aiming to confirm the taxonomic identification, sequencing of isolates was performed. DNA extraction was performed according Yamakami et al.21 Approximately 50 mL of culture medium were inoculated with a conidium suspension and incubated at 30 °C × 72 h. The mycelium was transferred to a 50-mL polypropylene vial containing six glass spheres (diameter 4 mm). The tube was immersed in liquid nitrogen for 10 s and agitated in a vortex for 20 s. Then, 2 mL of DNA extraction buffer (containing 10 mM Tris–HCl [pH 8.0], 10 mM EDTA, 0.15 M NaCl, 2% sodium dodecyl sulfate [SDS], and 0.5 mg proteinase K per mL [Sigma Chemical Co., St. Louis, MO, United States]) were added. Then, the mixture was allowed to stand for thawing. This mixture was incubated at 55 °C × 2 h. Proteinase K was inactivated by heating the mixture at 95 °C × 10 min, and a volume of 0.7 mL was transferred to a 1.5-mL microcentrifuge tube; and an equal volume of phenol–chloroform (1:1) was added. The mixture was centrifuged at 10,000 g at 4 °C × 5 min. The supernatant was transferred to another tube and the same procedure was repeated with chloroform–isoamyl alcohol (24:1). The DNA was precipitated with two volumes of ethanol at −20 °C and centrifuged at 12,000 × g at 4 °C × 20 min. Thus, the pellet obtained was dried. After washing with 70% ethanol at 4 °C, the extracted DNA was dissolved into 50 μL of distilled water and 5 μL of suspension, and used for polymerase chain reaction (PCR).
Amplification of ITS region by PCR
Amplification reactions were performed in a final volume of 25 μL containing 10 pmol of each primer; 1.5 mL of MgCl2 (25 mM); 1.0 μL of dNTPs (5 mM); 2.5 μL of 10× buffer (Taq); 1 unit of Taq DNA polymerase, and 20 ng of DNA (Fermentas Inc.). Primers ITS5 and ITS4 (ITS5 – 5′-GGA AGT AAA AGT CGT AAG AAC G-3′, ITS4 – 5′-TCC TCC GCT TAT TGA TAT GC-3′) were used. PCRs were performed in a T100 thermocycler (Bio-Rad Laboratories Brasil Ltda.), programmed with the following steps: pre-denaturation at 94 °C × 5 min, 30 cycles (denaturation at 94 °C × 30 s), 40–55 °C with an increase of 0.5 °C/s (totaling 30 s) (annealing), and extension (72° C/1 min × 30 s). The final extension was carried out at 72 °C × 4 min (29). The amplification reaction products were detected using agarose gel electrophoresis in 1% 1× TBE buffer (Tris–borate EDTA), and an electrophoresis analysis was performed at 36 V × 2 h. A 1-Kb DNA molecular marker was used. The gel was stained with ethyl bromide 0.5 μg/mL for 15 min, visualized with the aid of an ultraviolet (UV) transilluminator, and photographed with an image detection and analysis automatic system (Gel Doc XR + System with Software; ImageLab, Bio-Rad Laboratories Brasil Ltda.).
Sequencing
RCP products (6 μL) were treated with the enzymes exonuclease I (USB; 3.3 L) and shrimp alkaline phosphatase (SAP, USB; 0.66 L) in order to eliminate remnants of primers and of dNTPs. The tubes containing the mixture were maintained at 37 °C × 45 min and then at 80 °C × 15 min. In the sequencing reaction, fluorescent dideoxynucleotide was used with an ABI Prism 377 automatic sequencer model. The reactions were performed in a final volume of 10 μL containing 0.5 mL of primers DYEnamic ET Terminator Sequence Premix (DYEnamic ET Dye Terminator Cycle Sequencing Kit; Amersham Bioscience). The T100 thermocycler (Bio-Rad Laboratories Brasil Ltda.) was programmed in 30 cycles of 96 °C × 30 s, 40 °C × 30 s, and 60 °C × 1 min. An aliquot (20 μL) of the sequencing reaction was transferred to a sterile tube, with addition of 20 μL of MilliQ® water and 60 μL of isopropanol. The mixture was homogenized by vortex, left for 20 min at room temperature, and then centrifuged. The supernatant was removed and the precipitate washed twice with 250 μL of 70% ethanol and dried in an oven at 37 °C. The DNA was dissolved in 4 μL of Formanide Loading Dye and subjected to electrophoresis in an ABI PRISM 377 automatic sequencer (Applied Biosystems). The obtained sequences were analyzed using the Basic Local Alignment Search Tool program (BLASTn) and the sequences obtained were compared with those deposited at GenBank.
Results
The growth of filamentous fungi was observed in six out of 60 analyzed palatine roots (10%). Macroscopic examination of isolates showed growth of colonies compatible with Aspergillus spp. (Fig. 1). Microscopic examination of isolates showed growth of hyaline, septate, and globous conidiophore hyphae, characteristics of Aspergillus genus (Fig. 2). The homology with sequences of filamentous fungi, analyzed by BLAST and compared with sequences deposited at GenBank, was: 100% for Aspergillus niger (GenBank No. AJ223852) in four samples, 100% for Aspergillus versicolor (GenBank No. JX232271) in one sample, and 99% for Aspergillus fumigatus (GenBank No. AB025434) in one sample.
Figure 1.

Aspergillus spp. macroscopic image in malt extract agar medium at 37 °C.
Figure 2.
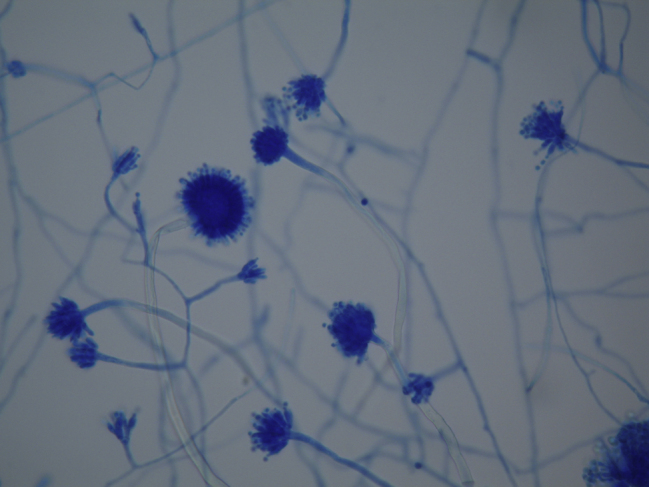
Aspergillus spp. – image under optical microscopy.
After phenotypic and genotypic analyzes, the filamentous fungi isolated were classified as A. niger (6.7%), A. versicolor (1.6%), and A. fumigatus (1.6%).
Discussion
Although several studies have already pointed to the presence of yeast4, 5, 6, 7, 8, 9, 22, 23, 24 and filamentous fungi10 in endodontic infections, no study so far had checked the presence of filamentous fungi in root canals with endodontic treatment and apical periodontitis, although there are reports in the literature showing that low zinc concentrations, as found in most endodontic sealers, provide an ideal environment for the development of these pathogens.25
The present study resorted to palatine roots of first maxillary molars because the apexes of these roots are anatomically closer to the floor of the maxillary sinus.3, 26, 27 Thus, the hypothesis was that teeth with endodontic treatment, with apical periodontitis and in close contact with the maxillary sinus, could result in a positive culture for filamentous fungi, since these pathogens are commonly isolated from the maxillary sinus.
The results of this study may serve as a warning to dentists and otolaryngologists, since Aspergillus organisms are also isolated from the maxillary sinus in cases of chronic sinusitis, and aerogenic and odontogenic pathways are described as the main routes of entry.26 Several studies have emphasized that the low concentration of zinc found in root canal sealers, based on zinc oxide plus eugenol, can facilitate the fungus installation in the maxillary sinus membrane.11, 12, 13, 15, 16 Leakage of filling material into the maxillary sinus during endodontic treatment has also been suggested as a cause of chronic sinusitis by foreign body.2, 11, 12, 13 Moreover, the amount of eugenol used in endodontic sealers has been directly related to the growth of the Aspergillus organism, causative of mycetoma within the maxillary sinus.15, 16
This proximity of the palatal roots of upper molars could encourage the migration of these filamentous fungi isolated from root canals to the maxillary sinus; on the other hand, existing fungi in the maxillary sinus could have their growth facilitated by a propitious environment in the root canal, with the presence of nutrients and sealing materials essential for growth and spreading of these pathogens. Interested professionals should be aware of this scenario, as these organisms exhibit characteristics of pathogenicity that may cause maxillary sinus disease from odontogenic origin, or vice versa. Thus, further studies should be conducted to correlate the presence of filamentous fungi in root canals and the possibility of infiltration of these pathogens toward the maxillary sinus, also to investigate the possibility that the maxillary sinus can act as a pathway for contamination that may lead to endodontic treatment failure.
Conclusion
Under the experimental conditions in which this research was conducted, and based on these results, it can be concluded that dental roots in close proximity to maxillary sinuses undergoing endodontic treatment for apical periodontitis may exhibit positive cultures for filamentous fungi.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Please cite this article as: Gomes CC, Pinto LCC, Victor FL, da Silva EAB, Ribeiro AA, Sarquis MIM, et al. Aspergillus in endodontic infection near the maxillary sinus. Braz J Otorhinolaryngol. 2015;81:527–32.
Institution: School of Dentistry, Universidade Federal Fluminense (UFF), Campus Nova Friburgo, Nova Friburgo, RJ, Brazil.
References
- 1.Brock I. Sinusitis of odontogenic origin. Otolaryngol Head Neck Surg. 2006;135:349–355. doi: 10.1016/j.otohns.2005.10.059. [DOI] [PubMed] [Google Scholar]
- 2.Melen I., Lindahl L., Andreasson L., Rundcrantz H. Chronic maxillary sinusitis. Definition, diagnosis and relation to dental infections and nasal polyposis. Acta Otolaryngol. 1986;101:320–327. doi: 10.3109/00016488609132845. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y., Liu Z., Zhang L., Zhou X., Zheng Q., Duan X., et al. Associations between maxillary sinus mucosal thickening and apical periodontitis using cone-beam computed tomography scanning: a retrospective study. J Endod. 2012;38:1069–1074. doi: 10.1016/j.joen.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 4.Nair P.N.R., Sjogren U., Krey G., Kahnberg K., Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod. 1990;16:580–588. doi: 10.1016/S0099-2399(07)80201-9. [DOI] [PubMed] [Google Scholar]
- 5.Sen B.H., Piskin B., Demirci T. Observation of bacteria and fungi in infected root canals and dentinal tubules by SEM. Endod Dent Traumatol. 1995;11:6–9. doi: 10.1111/j.1600-9657.1995.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 6.Waltimo T.M.T., Siren E.K., Torkko H.L., Olsen I., Hapassalo M.P. Fungi in therapy-resistant in apical periodintitis. Int Endod J. 1997;30:96–101. doi: 10.1046/j.1365-2591.1997.00058.x. [DOI] [PubMed] [Google Scholar]
- 7.Waltimo T.M.T., Sirén E.K., Orstavik D., Haapasalo M.P. In vitro susceptibility of Candida albicans to four desinfectants and their combinations. Int Endod J. 1999;32:421–429. doi: 10.1046/j.1365-2591.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner J.C., Watss C.M., Xia T. Occurrence of Candida albicans in infections of endodontic origin. J Endod. 2000;26:695–698. doi: 10.1097/00004770-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Siqueira J.F., Jr., Sen B.H. Fungi in endodontics infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:632–641. doi: 10.1016/S1079210404000046. [DOI] [PubMed] [Google Scholar]
- 10.Gomes C., Fidel S., Fidel R., de Mora Sarquis M.I. Isolation and taxonomy of filamentous fungi in endodontics infections. J Endod. 2010;36:626–629. doi: 10.1016/j.joen.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Legent F., Billet J., Beauvillain C., Bonnet J., Miegeville M. The role of dental canal fillings in the development of Aspergillus sinusitis: a report of 85 cases. Acta Otolaryngol. 1989;246:318–320. doi: 10.1007/BF00463584. [DOI] [PubMed] [Google Scholar]
- 12.Mensi M., Piccioni M., Marsili F., Nicolai P., Sapelli C.L., Latronico N. Risk of maxillary fungus ball in patients with endodontic treatment on maxillary teeth: a case–control study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:433–436. doi: 10.1016/j.tripleo.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Park G.Y., Kim H.Y., Young Min J., Dhong H.J., Chung S.K. Endodontic treatment: a significant risk factor for the development of maxillary fungal ball. Clin Exp Otorhinolaryngol. 2010;3:136–140. doi: 10.3342/ceo.2010.3.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh T.J., Wissel M.C., Grantham K.J., Petraitiene R., Petraitis V., Kasai M., et al. Molecular detection and species-specific identification of medically important Aspergillus species by real-time PCR in experimental invasive pulmonary Aspergillosis. J Clin Microbiol. 2011;49:4150–4157. doi: 10.1128/JCM.00570-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa F., Polini F., Zerman N., Robiony M., Toro C., Politi M. Surgical treatment of Aspergillus mycetomas of the maxillary sinus: review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:23–29. doi: 10.1016/j.tripleo.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Mensi M., Salgarello S., Pinsi G., Piccioni M. Mycetoma of the maxillary sinus: endodontic and microbiological correlations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:119–123. doi: 10.1016/j.tripleo.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Rivalier E., Seydel S. New method for the cultivation of blades lose ge es applied to the study of microscopic fungi of tinea. Ann Parasitol. 1932;10:444–452. [Google Scholar]
- 18.Raper K.B., Fennel D.I. Williams & Wilkins Company; Baltimore: 1965. The genus Aspergillus; pp. 640–686. [Google Scholar]
- 19.Samson R.A., Hong S., Frisvad J.C. Old and new concepts of species differentiation in Aspergillus. Med Mycol. 2006;44:133–148. doi: 10.1080/13693780600913224. [DOI] [PubMed] [Google Scholar]
- 20.Samson R.A., Varga J., Witiak S.M., Geiser D.M. The species concept in Aspergillus: recommendations of an international panel. Stud Mycol. 2007;59:71–73. doi: 10.3114/sim.2007.59.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamakami Y., Hashimoto A., Tokimatsu I., Nasu M. PCR detection of DNA specific for Aspergillus species in serum of patients with invasive Aspergillosis. J Clin Microbiol. 1996;34:2464–2468. doi: 10.1128/jcm.34.10.2464-2468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waltimo T.M.T., Kuusinen M., Järvensivu A., Nyber G.P., Vänänen A., Richardson M., et al. Examination on Candida spp. in refractory periapical granulomas. Int Endod J. 2003;36:643–647. doi: 10.1046/j.1365-2591.2003.00707.x. [DOI] [PubMed] [Google Scholar]
- 23.Siqueira J.F., Jr., Rôças I.N. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:85–94. doi: 10.1016/s1079-2104(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 24.Siqueira J.F., Jr. Endodontics infections: concepts paradigms, and perspectives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:281–293. doi: 10.1067/moe.2002.126163. [DOI] [PubMed] [Google Scholar]
- 25.Soll D.R., Bendell G.W., Brummel M. Zinc and regulation of growth and phenotype in the infectious yeast Candida albicans. Infect Immun. 1991;32:1139–1147. doi: 10.1128/iai.32.3.1139-1147.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberhardt J.A., Torabinejad M., Christiansen E.L. A computed tomographic study of the distances between the maxillary sinus floor and the apices of the maxillary posterior teeth. Oral Surg Oral Med Oral Pathol. 1992;73:345–346. doi: 10.1016/0030-4220(92)90133-b. [DOI] [PubMed] [Google Scholar]
- 27.Kilic C., Kamburoglu K., Yuksel S.P., Ozen T. An assessment of the relationship between the maxillary sinus floor and the maxillary posterior teeth root tips using dental cone-beam computerized tomography. Eur J Dent. 2010;4:462–467. [PMC free article] [PubMed] [Google Scholar]


