Abstract
Introduction
Cleft lip and/or palate (CL/P) represent the most common congenital anomalies of the face.
Objective
To assess the relationship between maternal smoking, gender and CL/P.
Methods
This is an epidemiological cross-sectional study. We interviewed 1519 mothers divided into two groups: Cases: mothers of children with CL/P (n = 843) and Controls: mothers of children without CL/P (n = 676). All mothers were classified as smoker or non-smoker subjects during the first trimester of pregnancy. To determine an association among maternal smoking, gender, and CL/P, odds ratios were calculated and the adjustment was made by a logistic regression model.
Results
An association between maternal smoking and the presence of cleft was observed. There was also a strong association between male gender and the presence of cleft (OR = 3.51; 95% CI 2.83–4.37). By binary logistic regression analysis, it was demonstrated that both variables were independently associated with clefts. In a multivariate analysis, male gender and maternal smoking had a 2.5- and a 1.5-time greater chance of having a cleft, respectively.
Conclusion
Our findings are consistent with a positive association between maternal smoking during pregnancy and CL/P in male gender. The results support the importance of smoking prevention and introduction of cessation programs among women with childbearing potential.
Keywords: Cleft lip, Cleft palate, Smoking, Pregnancy
Resumo
Introdução
Fendas labiais e/ou palatinas (FL/P) representam as anomalias congênitas mais comuns da face.
Objetivo
Avaliar a relação entre tabagismo materno, gênero e FL/P.
Método
Realizou-se um estudo epidemiológico, de corte transversal. Foram entrevistadas 1.519 mães, divididas em dois grupos: Casos: mães de crianças com FL/P (n = 843); e Controles: mães de crianças sem FL/P (n = 676). Todas as mães foram classificadas como fumantes ou não fumantes durante o primeiro trimestre de gravidez. Para determinar a associação entre tabagismo materno, gênero e FL/P, odds ratios foram calculadas e o ajuste realizado pelo modelo de regressão logística.
Resultados
Observou-se associação entre tabagismo materno, e fendas. Houve também forte associação entre sexo masculino e presença de fendas (OR = 3,51; 95% IC 2,83–4,37). Regressão logística binária demonstrou que ambas as variáveis foram independentemente associadas com a ocorrência de fendas. Na análise multivariada, o sexo masculino teve 2,5 vezes mais chance de apresentar fendas e tabagismo materno teve 1,5 vez mais chance dessa ocorrência.
Conclusão
Os resultados são consistentes com a associação positiva entre tabagismo materno durante a gravidez e a ocorrência de FL/P no gênero masculino. Os resultados suportam a importância da prevenção do tabagismo e a aplicação de programas entre mulheres com potencial de gravidez.
Palavras-chave: Fenda labial, Fenda palatina, Hábito de fumar, Gravidez
Introduction
Nonsyndromic cleft lip and/or palate (NSCL/P, MIM #119530) represents the most frequent congenital malformation in the head and neck region, with a prevalence ranging from 1:500 to 1:2500 live births.1 Its prevalence varies according to ethnicity (Africans 0.3:1000; Europeans 1.3:1000; Asians 2.1:1000; Native Americans 3.6:1000) and socioeconomic level.2 In Brazil, the prevalence of NSCL/P ranges from 0.36 to 1.54:1000 live births.3, 4
NSCL/P is an immediately recognizable disruption of normal facial structure. Although not a major cause of mortality in developed countries, NSCL/P does cause considerable morbidity in affected children and imposes a substantial financial burden, especially for families of low socioeconomic status.5 Individuals with NSCL/P may experience problems with feeding, speaking, hearing, social integration, and cancer.1, 6
NSCL/P etiology, which involves both genetic and environmental factors, is highly complex; its molecular basis remains largely unknown.2, 7 The identification of modifiable risk factors for NSCL/P is the first step toward primary prevention. Risk factors such as maternal exposure to tobacco smoke, alcohol use, poor nutrition, gender, maternal age, viral infection, medicinal drugs, and teratogens in the workplace or at home in early pregnancy have previously been investigated.1, 8, 9, 10, 11
The association between maternal smoking and NSCL/P has been assessed in many studies, and a meta-analysis of these studies suggests a positive association.12, 13 Different studies have been conducted worldwide to evaluate NSCL/P distribution, often resulting in varying prevalence rates.14, 15 In a Brazilian population study, predominance of cleft lip and palate (52.6%) was demonstrated, followed by cleft lip (33.12%) and cleft palate (14.28%).16 The incidence of cleft lip and palate and cleft lip is greater in males, while cleft palate shows prevalence in females.16, 17
Very few studies have evaluated the influence of environmental factors in the Brazilian NSCL/P population. The purpose of this study was to evaluate the relationship between maternal smoking, gender, and NSCL/P in a Brazilian population.
Methods
The design of the study was an epidemiological, case-control, and single-center. All participants were recruited from the same institution (Centre for Rehabilitation of Craniofacial Anomalies and Dental Clinics, State of Minas Gerais, Brazil), between February 2009 and August 2012, in an attempt to select cases and control individuals with similar ethnicities and social-culture backgrounds.
The authors interviewed 1519 mothers, who were divided into two groups: cases: mothers of children with NSCL/P (n = 843); and controls: mothers of children without NSCL/P (n = 676). All mothers were further classified as smokers or non-smokers during the first trimester of pregnancy. All children with associated anomalies or syndromes or a family history of genetic diseases were excluded from this study. The mothers of children with NSCL/P were evaluated in the Center for Rehabilitation of Craniofacial Anomalies, while the mothers of children without NSCL/P were evaluated in dental clinics.
The clefts were categorized into three groups, with the incisive foramen as reference: (1) cleft lip (CL): includes complete or incomplete pre-foramen clefts, either unilateral or bilateral; (2) cleft lip and palate (CLP): includes unilateral or bilateral transforamen clefts and pre- or post-foramen clefts; (3) cleft palate (CP): includes all post-foramen clefts, complete or incomplete.18 For analysis purposes, CL and CLP (CL/P–cleft lip with or without cleft palate) were merged as one group, with CP as the other group.11, 19 The children were evaluated by professionals with considerable experience with oral cleft.
The information collected was stored in a database and analyzed using SPSS® version 18.0 (SPSS for Windows). The analysis was conducted in two steps. Statistical analysis was initially based on the presentation of descriptive data and the distribution of categorical variables. Prevalence odds ratio (OR) and 95% confidence intervals (95% CI) were used to determine associations among maternal smoking, gender, and occurrence of NSCL/P. The association between maternal smoking and clefts was tested in each gender separately. Next, the association between the gender and the occurrence of NSCL/P was tested separately among those whose mothers smoked and among those whose mothers did not smoke. Finally, a logistic regression model was applied to identify variables that were independently associated with occurrence of NSCL/P.
This study was approved by the Ethics Committee in Research of the University (#259-2010). Informed consent was obtained from subjects or guardians before participating in the study.
Results
A total of 1519 women were interviewed, including 680 mothers of infants with nonsyndromic CL/P, 163 mothers of infants with nonsyndromic CP, and 676 mothers of infants with no major birth defects (controls). Of 1519 children included in the analysis, there was a predominance of females (57%). Of these children, 843 presented clefts, including 680 born with CL/P and 163 with isolated CP. Of the 1519 mothers, 307 (20%) were smokers, with 212/843 (25%) in the case group and 95/676 (14%) in the control group. There was an association between maternal smoking and clefts (OR = 2.02; 95% CI 1.54–2.63). Maternal smoking was also associated with CL/P (OR = 2.08; 95% CI 1.58–2.75) and with CP (OR = 1.92; 95% CI 1.26–2.92) (Table 1). The majority of infants with CL/P were male (61%), and females predominated among children with CP (63.8%). There was also a strong association between male gender and the presence of clefts (OR = 3.51; 95% CI 2.83–4.37). This association was consistent among the different types of clefts (CL/P: OR = 4.28; 95% CI 3.40–5.39; CP: OR = 1.55; 95% CI 1.08–2.23) (Table 1).
Table 1.
Univariate analysis of the distribution of cleft lip with or without cleft palate (CL/P), cleft palate (CP), and all clefts according to maternal smoking status and infant gender.
| CL/P | p-Value | CP | p-Value | All clefts | p-Value | |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Maternal smoking | <0.001 | |||||
| Absent | Ref | <0.001 | Ref | 0.001 | Ref | |
| Present | 2.08 (1.58–2.75) | 1.92 (1.26–2.92) | 2.02 (1.54–2.63) | |||
| Gender | <0.001 | |||||
| Female | Ref | <0.001 | Ref | 0.017 | Ref | |
| Male | 4.28 (3.40–5.39) | 1.55 (1.08-2.23) | 3.51 (2.83–4.37) |
Association between maternal smoking and clefts was assessed in each gender separately. Although maternal smoking increased the risk of clefts in both genders, this increase was significant only for females (OR = 2.48; 95% CI 1.73–3.54) (Table 2). By contrast, there was an association between male gender and clefts among those whose mother smoked and among those whose mothers did not smoke. It was found that the occurrence of cleft was significantly higher in males both among those whose mother smoked and among those whose mothers did not smoke (mother smokes: OR = 2.10; 95% CI 1.28–3.45 and non-smokers mother: OR = 3.89; 95% CI 3.05–4.97).
Table 2.
Analyses of the association between maternal smoking and the occurrence of nonsyndromic cleft lip and/or palate in each gender separately, as well as association between gender and the occurrence of clefts in relation to maternal smoking.
| Variable | Case | Control | OR | 95% CI | p |
|---|---|---|---|---|---|
| Female | |||||
| Non-smoking mother | 275 | 435 | 1.00 | ||
| Mother smokes | 94 | 60 | 2.48 | 1.73–3.54 | <0.001 |
| Male | |||||
| Non-smoking mother | 356 | 146 | 1.00 | ||
| Mother smokes | 118 | 35 | 1.38 | 0.90–2.12 | 0.149 |
| Non-smoking mother | |||||
| Female | 275 | 435 | 1.00 | ||
| Male | 356 | 146 | 3.85 | 3.02–4.92 | <0.001 |
| Mother smokes | |||||
| Female | 94 | 60 | 1.00 | ||
| Male | 118 | 35 | 2.15 | 1.30–3.53 | 0.003 |
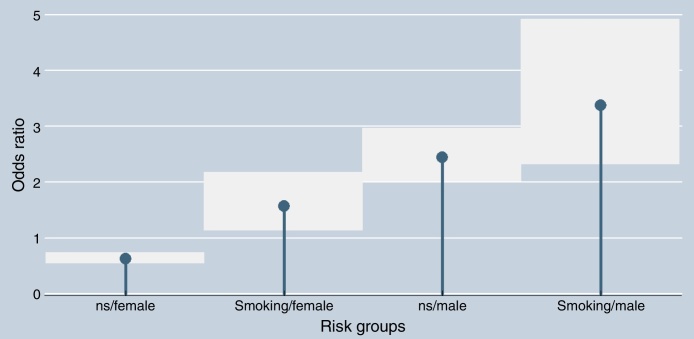
By binary logistic regression analysis, it was demonstrated that both variables (gender and maternal smoking) were independently associated with clefts. In this multivariable analysis, the male gender had approximately 2.5-fold greater chance of clefts (OR = 2.39; 95% CI 2.0–2.87; p < 0.001) and maternal smoking had 1.5 times greater chance (OR = 1.49; 95% CI 1.15–1.93; p = 0.002). Of note, there was an increasing odds ratio across subgroups stratified by gender of the children and maternal smoking status: female/non-smoking mother (OR = 0.63; 95% CI 0.54–0.73), female/smoking mother (OR = 1.56; 95% CI 1.13–2.16), male/non-smoking mother (OR = 2.43; 95% CI 2.01–2.95), and male/smoking mother (OR = 3.37; 95% CI 2.31–4.91) (x2 = 153.5; p < 0.001). Fig. 1 illustrates the difference of the simultaneous effects of both variables stratified into these four subgroups.
Figure 1.
Association between maternal smoking status and gender with the presence of facial cleft deformities (ns, non-smoking). The squares represent the 95% confidence intervals.
Discussion
The patients described in this study were recruited from the Center for Rehabilitation of Craniofacial Anomalies, Minas Gerais State, Brazil. This Service is considered one of the largest cleft repair centers in Brazil, and performs all procedures of rehabilitation that pass through the Brazilian Public Health System.9, 11 With a population exceeding 190 million and 3 million births every year, NSCL/P is an important public health problem in Brazil, with approximately 4000 new cases of NSCL/P every year.11
Although the environmental and genetic risk factors associated with NSCL/P remain unclear, the understanding of the mechanisms involved in this malformation is evolving.1, 2 Maternal smoking has been repeatedly associated with increased risk of NSCL/P, and meta-analysis supports an overall OR for having clefts of ∼1.3 among offspring of mothers who smoke.12, 20, 21 The present findings confirm the literature data. In this analysis, the chance of cleft in the children whose mother smoked was 2-fold greater compared with children of non-smoking mothers. Little et al.12 performed a meta-analysis of the association between maternal smoking during pregnancy and oral clefts using data from 24 case-control and cohort studies. A relative risk of 1.34 (95% CI 1.25–1.34) was found between maternal smoking and CL/P and a relative risk of 1.22 (95% CI 1.10–1.35) between maternal smoking and CP. Similarly, Honei et al.13 have shown that periconceptional smoking was associated with CLP (OR = 1.3; 95% CI 1.0–1.6), and more strongly associated with bilateral CLP (OR = 1.7; 1.2–2.6), with a weaker association observed for CP.
Cigarette smoke contains nicotine, polycyclic aromatic hydrocarbons, tar, carbon particles, and carbon monoxide. The exposure of embryonic tissues depends on the number of cigarettes smoked, frequency of puffing, depth of inhalation, and maternal embryonic transfer and metabolism. The mechanisms by which cigarette smoke detrimentally affects pregnancy outcome are inadequately understood.22 Increased risks from exposure to maternal smoking during periconceptional period raise the possibility that genes in certain metabolic pathways may play a role in the development of NSCL/P. Specifically, markers in the GSTT1 (glutathione S-transferase theta) or NOS3 (nitric oxide synthase 3) genes appear to influence the risk of NSCL/P in the presence of maternal smoking.20, 21, 23 The GSTT1 markers are gene deletion variants, which suggest deficiencies in detoxification pathways may underlie some of the susceptibility. Smoking has also recently been associated with variants in the IRF6 gene.17
With respect to the distribution of clefts in the two groups (CL/P and CP), it was found that of the 843 NSCL/P, 680 (80.7%) were CL/P and 163 (19.3%) were isolated CP. In most published studies, the percentage of subjects with CL/P was higher compared to that of CP alone, including the Brazilian studies.24, 25 The present authors group, in another study, found similar results in the distribution of NSCL/P (CL/P = 81.61% and CP = 18.37%).11 Among the 843 children with clefts evaluated, 474 (56.2%) were male and 369 (43.8%) were female. In another study, it was demonstrated that CP was more frequent in females (28.7% vs. 13.6%; 1.77:1), while CLP (59.8% vs. 45.5%; 1.56:1) and CL (25.7% vs. 26.6%; 1.23:1) predominated in males (p = 0.001).17 It was also determined, by multinomial logistic regression, that the chance of occurrence of CL in males was 2.19-fold higher in relation to CP in females, while the risk of CLP in males was 2.78-fold higher than the risk of CP in females.16 In white populations, this majority of males with CL/P becomes more evident with the increasing severity of the cleft and less evident when more than one sibling in the family is affected.26
Recently, Lei et al.27 have shown, in a population-based epidemiological study in Taiwan, that higher prevalence of CL/P or CP was observed with multiple pregnancies, being male for CL/P, being female for CP, gestational age ≤37 weeks, and lower birth weight (<1.5 kg). According to their findings, male newborns and female newborns were strongly associated with CL/P and CP, respectively (both p < 0.0001). In the present analysis, maternal smoking increased the chance of clefts in both genders; however, this increase was significant only for females (OR = 2.48; 95% CI 1.73–3.54). Possibly, this finding is related with the fact that there was an association between male gender and clefts among those whose mother smoked and among those whose mothers did not smoke. Of particular interest, when this sample was stratified by maternal smoking status and child's gender, there was a consistent increas in odds ratio across the subgroups, from the maternal non-smoking/female gender to the maternal smoking/male gender pairs.
Concerning the limitations of this study, there was no quantitative assessment of maternal exposure to passive smoking, quantification of the number of cigarettes consumed by the mother, and the duration of the smoking habit. In addition, the database did not provide information on maternal conditions and perinatal features (such as birth weight and gestational age), which clearly may play a role in development of facial cleft deformities. Limitations still exist between the pairings made between the case and control groups, even though conducted at the same institution.
Conclusion
In summary, these findings are consistent with a positive association between maternal smoking during pregnancy and NSCL/P in the offspring. It is also noted that male children have a 3.5-fold greater chance of being born with clefts compared with females. The identification of modifiable risk factors for NSCL/P, such as maternal smoking, is the first step toward primary prevention. The consistency of the findings for NSCL/P and maternal smoking suggests an opportunity for prevention of these serious defects. The results presented here support the importance of smoking prevention and cessation programs among all women with childbearing potential.
Funding
This study was supported the by Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG Procad/Casadinho – CNPq-Capes.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by grants from the Minas Gerais State Research Foundation – FAPEMIG, Brazil; the National Council for Scientific and Technological Development – CNPq, Brazil; the Coordination for Improvement of Higher Education Personnel (CAPES), Brazil; and Procad/Casadinho – Capes/CNPq.
Footnotes
Please cite this article as: Martelli DRB, Coletta RD, Oliveira EA, Swerts MSO, Rodrigues LAM, Oliveira MC, et al. Association between maternal smoking, gender, and cleft lip and palate. Braz J Otorhinolaryngol. 2015;81:514–9.
Institution: Programa de Pós-graduação em Ciências da Saúde da Universidade Estadual de Montes Claros (Unimontes), Montes Claros, MG, Brazil.
References
- 1.Dixon M.J., Marazita M.L., Beaty T.H., Murray J.C. Cleft lip and palate: understanding genetic and environmental influences. Nat Rev Genet. 2011;12:167–178. doi: 10.1038/nrg2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahimov F., Jugessur A., Murray J.C. Genetics of nonsyndromic orofacial clefts. Cleft Palate Craniofac J. 2012;49:73–91. doi: 10.1597/10-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martelli-Junior H., Porto L.V., Martelli D.R., Bonan P.R., Freitas A.B., Coletta R. Prevalence of nonsyndromic oral clefts in a reference hospital in the state of Minas Gerais, Brazil, between 2000–2005. Braz Oral Res. 2007;21:314–317. doi: 10.1590/s1806-83242007000400006. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues K., Sena M.F., Roncalli A.G., Ferreira M.A. Prevalence of orofacial clefts and social factors in Brazil. Braz Oral Res. 2009;23:38–42. doi: 10.1590/s1806-83242009000100007. [DOI] [PubMed] [Google Scholar]
- 5.Wehby G., Cassell C.H. The impact of orofacial clefts on quality of life and healthcare use and costs. Oral Dis. 2010;16:3–10. doi: 10.1111/j.1601-0825.2009.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vieira A.R., Khaliq S., Lace B. Risk of cancer in relatives of children born with isolated cleft lip and palate. Am J Med Genet A. 2012;158:1503–1504. doi: 10.1002/ajmg.a.35359. [DOI] [PubMed] [Google Scholar]
- 7.Brito L.A., Paranaiba L.M.R., Bassi C.F.S., Massoti C., Malcher C., Schesinger D., et al. Region 8q24 is a susceptibility locus for nonsyndromic oral clefting in Brazil. Birth Defects Res A: Clin Mol Teratol. 2012;94:464–468. doi: 10.1002/bdra.23011. [DOI] [PubMed] [Google Scholar]
- 8.Boyles A.L., DeRoo L.A., Lie R.T., Taylor J.A., Jugessur A., Murray J.C., et al. Maternal alcohol consumption, alcohol metabolism genes, and the risk of oral clefts: a population-based case-control study in Norway,1996–2001. Am J Epidemiol. 2010;172:924–931. doi: 10.1093/aje/kwq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bufalino A., Paranaíba L.M.R., Aquino S.N., Martelli-Júnior H., Swerts M.S.O., Coletta R.D. Maternal polymorphisms in folic acid metabolic genes are associated with nonsyndromic cleft lip and/or palate in the Brazilian population. Birth Defects Res A: Clin Mol Teratol. 2010;88:980–986. doi: 10.1002/bdra.20732. [DOI] [PubMed] [Google Scholar]
- 10.Jentink J., Loane M.A., Dolk H., Barisic I., Garne E., Morris J.K., et al. Valproic acid monotherapyin pregnancy and major congenital malformations. N Engl J Med. 2010;362:2185–2193. doi: 10.1056/NEJMoa0907328. [DOI] [PubMed] [Google Scholar]
- 11.Martelli D.R.B., Cruz K.M., Barros L.M., Silveira M.F., Swerts M.S.O., Maternal Martelli-Júnior H. paternal age, birth order and interpregnancy interval evaluation for cleft lip-palate. Braz J Otorhinolaryngol. 2010;76:107–112. doi: 10.1590/S1808-86942010000100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Little J., Cardy A., Munger R.G. Tobacco smoking and oral clefts: a meta analysis. Bull World Health Organ. 2004;82:213–218. [PMC free article] [PubMed] [Google Scholar]
- 13.Honein M.A., Rasmussen S.A., Reefhuis J., Romitti P.A., Lammer E.J., Correa A. Maternal smoking and environmental tobacco smoke exposure and the risk of orofacial clefts. Epidemiology. 2007;18:226–233. doi: 10.1097/01.ede.0000254430.61294.c0. [DOI] [PubMed] [Google Scholar]
- 14.Christensen K., Juel K., Herskind A.M., Murray J.C. Long term follow up study of survival associated with cleft lip and palate at birth. BMJ. 2004;328:1405. doi: 10.1136/bmj.38106.559120.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derijcke A., Eerens A., Carels C. The incidence of oral clefts: a review. Br J Oral Maxillofac Surg. 1996;34:488–494. doi: 10.1016/s0266-4356(96)90242-9. [DOI] [PubMed] [Google Scholar]
- 16.Martelli D.R., Machado R.A., Swerts M.S., Rodrigues L.A., Aquino S.N., Martelli-Júnior H. Non-syndromic cleft lip and palate: relationship between sex and clinical extension. Braz J Otorhinolaryngol. 2012;78:116–120. doi: 10.5935/1808-8694.20120018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu T., Liang K.Y., Hetmanski J.B., Ruczinski I., Fallin M.D., Ingersoll R.G., et al. Evidence of gene environment interaction for the IRF6 gene and maternal multivitamin supplementation in controlling the risk of cleft lip with/without cleft palate. Hum Genet. 2010;128:401–410. doi: 10.1007/s00439-010-0863-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spina V., Psillakis J.M., Lapa F.S., Ferreira M.C. Classification of cleft lip and cleft palate. Suggested changes. Rev Hosp Clin Fac Med Sao Paulo. 1972;27:5–6. [PubMed] [Google Scholar]
- 19.Christensen K., Fogh-Andersen P. Cleft lip (+/− cleft palate) in Danish twins, 1970–1990. Am J Med Genet. 1993;47:910–916. doi: 10.1002/ajmg.1320470620. [DOI] [PubMed] [Google Scholar]
- 20.Shi M., Christensen K., Weinberg C.R., Romitti P., Bathum L., Lozada A., et al. Orofacial cleft risk is increased with maternal smoking and specific detoxification gene variants. Am J Hum Genet. 2007;80:76–90. doi: 10.1086/510518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi M., Christensen K., Weinberg C.R., Romitti P., Bathum L., Lozada A., et al. Review on genetic variants and maternal smoking in the etiology of oral clefts and other birth defects. Birth Defects Res C: Embryo Today. 2008;84:16–29. doi: 10.1002/bdrc.20117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Rooij I.A., Wegerif M.J., Roelofs H.M., Peters W.H., Kuijpers-Jagtman A.M., Zielluis G.A., et al. Smoking, genetics polymorphisms in biotransformation enzymes, and nonsyndromic oral clefting: a gene-environment interaction. Epidemiology. 2001;12:502–507. doi: 10.1097/00001648-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H., Kartiko S., Finnell R.H. Importance of gene-environment interactions in the etiology of selected birth defects. Clin Genet. 2009;75:409–420. doi: 10.1111/j.1399-0004.2009.01174.x. [DOI] [PubMed] [Google Scholar]
- 24.Freitas J.A., Dalben Gda S., Santamaria M., Jr., Freitas P.Z. Current data on the characterization of oral clefts in Brazil. Braz Oral Res. 2004;18:128–133. doi: 10.1590/s1806-83242004000200007. [DOI] [PubMed] [Google Scholar]
- 25.Martelli D.R., Bonan P.R., Soares M.C., Paranaíba L.R., Martelli-Júnior H. Analysis of familial incidence of non-syndromic cleft lip and palate in a Brazilian population. Med Oral Patol Oral Cir Bucal. 2010;15:898–901. [PubMed] [Google Scholar]
- 26.Niswander J.D., MacLean C.J., Chung C.S., Dronamraju K. Sex ratio and cleft lip with or without cleft palate. Lancet. 1972;2:858–860. doi: 10.1016/s0140-6736(72)92217-9. [DOI] [PubMed] [Google Scholar]
- 27.Lei R.-L., Chen H.-S., Huang B.-Y., Chen Y.-C., Chen P.K.-T., Lee H.Y., et al. Population-based study of birth prevalence and factors associated with cleft lip and/or palate in Taiwan 2002–2009. PLOS ONE. 2013;8:e58690. doi: 10.1371/journal.pone.0058690. [DOI] [PMC free article] [PubMed] [Google Scholar]