Abstract
Introduction
Mucosal leishmaniosis (ML) is a severe clinical form of leishmaniosis. Complex factors related to the parasite and the host are attributed to the development of mucosal lesions. Leishmania RNA virus 1 (LRV1) can disrupt immune response, and may be the main determinant of severity of the disease; it should be investigated.
Objective
To study the existence of clinical differences between patients with ML with endosymbiosis by LRV1 and. those without it.
Methods
A cross-sectional cohort study with clinical evaluation, polymerase chain reaction (PCR) detection of Leishmania, species classification, and search of LRV1 was performed. Only patients with confirmed diagnosis of ML by positive PCR and with nasal mucosa injuries were included in this analysis.
Results
Out of 37 patients, 30 (81.1%) were diagnosed with Leishmania braziliensis, five (13.5%) with Leishmania guyanensis, and two (5.4%) with mixed infection of L. braziliensis and L. guyanensis. LVR1 virus was present in 26 (70.3%) of the cases.
Conclusion
Correlation between clinical phenotype and presence of LRV1 was not observed, although the frequency of the virus is two-fold higher in mucosal lesions than that found in the literature on skin lesions in the same geographical area.
Keywords: Leishmaniosis mucocutaneous, Leishmaniosis, Leishmania braziliensis, Leishmania guyanensis, Leishmaniavirus
Resumo
Introdução
A leishmaniose de mucosa (LM) é uma forma clínica grave da leishmaniose. Fatores complexos ligados ao parasita e ao hospedeiro são atribuídos ao desenvolvimento das lesões de mucosa. Leishmania RNA Vírus 1 (LRV1) pode subverter a resposta imune, podendo ser o principal determinante da gravidade da doença e deve ser pesquisado.
Objetivo
Estudar a existência de diferenças clínicas entre pacientes portadores de LM com endosimbiose por LRV1 e as que não possuem.
Método
Foi realizado um estudo de coorte histórica com corte transversal com avaliação clínica, detecção da Leishmania por técnica de PCR, classificação da espécie e pesquisa de LRV1. Foram incluídos na análise da pesquisa somente os pacientes com diagnóstico confirmado de LM com PCR positivo, com lesão de mucosa nasal. Resultados: Dos 37 pacientes, 30 (81,1%) foram diagnosticados com L. braziliensis, 5 (13,5%) com L. guyanensis e 2 (5,4%) com infecção mista de L. braziliensis e L. guyanensis. O vírus LVR1 estava presente em 26 casos (70,3%).
Conclusão
A correlação entre o fenótipo clínico e a presença do LRV1 não foi constatada, porém a frequência do vírus é duas vezes maior em lesão de mucosa do que encontrado em trabalho, da mesma região, sobre lesão cutânea.
Palavras-chave: Leishmaniose mucocutânea, Leishmaniose, Leishmania braziliensis, Leishmania guyanensis, Leishmaniavírus
Introduction
Leishmaniosis is a neglected tropical disease that is largely ignored in the discussion of important tropical diseases. Contributing to this neglect are a complex epidemiology, ecology, lack of simple management tools, and a lack of data.1 In 2010, the World Health Organization (WHO) estimated there were1 19,600 cases of American cutaneous leishmaniosis (ACL) in Brazil; they employed a 2.8–4.6 fold underreporting grade that is considered mild.1 Vega et al. calculated the actual per capita cost (medical and non-medical expenditure) of cutaneous leishmaniosis (CL) treatment in Colombia to be US$ 345. Projecting the same cost to Brazil, the mean expenditure for ACL would be US$ 7,586,490 per year for new cases. The estimated cost of years of life lost to disease (DALY-WHO) per patient in Colombia was US$ 15,000.2 If only mucosal leishmaniosis (ML) is considered, treatment and DALY costs would be much higher.
Mucosal leishmaniosis (ML) is an important and severe clinical form of leishmaniosis, due to the destructive potential of its injuries. ML is caused by a protozoan of the genus Leishmania that features an extranuclear DNA and a mitochondrial organelle, the kinetoplast. ML has two developmental forms during its life cycle: amastigote, which is a mandatory intracellular parasite in vertebrates, and promastigote, existing in invertebrate vectors (phlebotomines).3
There are indications that leishmaniosis may be native to the Amazon region. The Spanish chronicler Pedro Pizarro reported that people living in hot valleys of Peru were decimated by a nose disease on the Amazon side. The Andean theory, formulated by Rabello, has its origin from Peruvian huacos (pieces of pre-Columbian ceramics) discovered, depicting people with nose deformities. Based on epidemiological studies of Leishmania braziliensis, Marzochi and Marzochi proposed that leishmaniosis has its origin in the western Amazon.4, 5
Leishmania are divided into two subgenera, Viannia and Leishmania. In Brazil, at least seven species that cause disease are recognized; cutaneous leishmaniosis is caused mainly by L. (V.) braziliensis, Leishmania (V.) guyanensis, and L. (L.) amazonensis, and, more rarely, by L. (V.) laisoni, L. (V.) naiffi, L. (V.) shawi, and L. (V.) lindenbergi, all of interest to the Amazon region. The first three species are involved in mucosal leishmaniosis, while L. (L.) chagasi is the causal agent of visceral disease.3, 6, 7, 8 L. braziliensis is the main cause of ML; however, a recently published study revealed significant prevalence of L. guyanensis, mainly north of the Amazon river.9 L. amazonensis may also cause ML.3 No case of ML by L. (V.) panamensis was reported in Brazil.
ML can manifest itself with nasal obstruction, epistaxis associated with crust production, rhinorrhea, and mild pain. At the initial stage, there is edema and anterior septal mucosa hyperemia, presence of nodules, and subsequent formation of a granulomatous lesion, which can evolve to septal perforation, nasal edema with skin thickening, nasal septum collapse (tapir nose), and bulky nasal pyramid.8, 10 More than 90% of mucosal injuries affect only the anterior nasal septum.8, 9, 11 This condition can severely compromise the nose, palate, gums, pharynx, and larynx, causing deformities that impair phonation, breathing, swallowing, and self-esteem.8, 10, 11
Factors related to the parasite, host, and magnitude of the immune response are relevant to mucosal damage. Metastases of the parasite to the upper aerodigestive tract mucosa can occur through lymphatic or hematogenous routes.3, 8, 12 The development of mucosal injuries is attributed to complex and poorly understood factors (socioeconomic, environmental, and those of the host and parasite). Mucosal lesions by contiguity may also occur.3, 8
A common pathway for ML development is associated with a lasting immune response of the host against the parasite, with increases in inflammatory mediators, such as TNF-α, CXCL10, and CCL4, and in T-cell-mediated cytotoxicity activity; higher numbers of CD4+ and CD8+ cells, increases in IFNγ, IL-2 and IL-5, lower production of IL-1013-15, and also polymorphism of the genes encoding inflammatory mediators, such as TNF-α and IL-6.3, 13, 14, 15
Type 1 helper (Th1) T cells produce lymphokines that activate macrophages (IL-2, IFN-γ, TNF-α, and IL-12) to fight these parasites. Type 2 helper (Th2) T cells produce IL-4 and IL-10, which inhibit macrophages, leaving the host susceptible to infection. Leishmania is able to facilitate the differentiation of T cells into a Th2-type response, characterized by persistent infection.14, 16, 17 The parasite must adapt its metabolism to the intracellular oxidative stress into the phagolysosome of macrophages.16 Paradoxically, ML is characterized by an exaggeration in response to Leishmania antigens and by a scarcity of parasites. The exaggeration in Th1 response causes destruction of soft tissues where the antigenic particles are located.14, 18, 19
Recently, Ives et al. demonstrated that the parasitism of Leishmania by the Leishmania RNA virus 1 (LRV1), a double-stranded RNA virus of Totviridae family, increases the concentration of cytokines and chemokines (TNF-α, CXCL10, CCL5, IL-6) in TLR3/TRIF-mediated macrophages of L. guyanensis clones. In this study there was a high potential for metastasis in guinea pigs, indicating that the nucleic acids of the endosymbiotic virus function as strong immunogens, and cause destructive mucosal inflammation.13, 16 Although research on LRV1 has been conducted since the original description of the virus 20 years ago, the role of LRV1 in leishmaniosis remains unknown. No major studies were published on the impact of the virus until the publication of the study by Ives et al.
In the phylogenetic study of LRV, with the sole exception of one strain of L. major infected with Leishmania RNA virus 2 (LRV2) from a skin lesion in former Soviet Union, all LRV strains have their origin in South America. In the assessment of the genetic evolution among LRV types and among Leishmania species infected with these viruses, there is evidence of a parallelism in the evolution of Leishmania and LRV.19, 20
The model suggests that innate recognition of LRV1 occurs in the first hours of infection. Viral dsRNA release occurs from dead parasites; then the particle binds to the Toll-like receptor 3 (TLR3) and triggers the inflammatory cascade that aggravates the disease, perhaps representing the main determinant of its severity.21 Thus, the detection of LRV can have clinical importance, guiding therapy and prognosis.13, 16, 19, 21
Despite the detection of LRV1 in large metastatic strains of L. braziliensis and L. guyanensis, metastases may occur in its absence.19, 22 LRV discovery as an innate immunogen, changing the course of leishmaniosis, should motivate further research on such viral hyperpathogens in other infections.19
The methods routinely used in the diagnosis of cutaneous leishmaniosis are limited for mucosal lesions.8, 9, 11, 12 Montenegro's reaction is not suitable because the mucosal injuries typically occur secondary to cutaneous lesions. In these lesions, the low parasitaemia significantly reduces the diagnostic accuracy of biopsy, as well as the direct evaluation with smears obtained from injuries.8, 12, 23 The low level of antibodies reduces the effectiveness of serological tests.11, 23 With material collected from mucosal lesions, identification under optical microscopy or by culture rarely is successful.24 Polymerase chain reaction (PCR) stands out as an excellent test (i.e. gold standard),12 because of its sensitivity and specificity, especially to establish parasite species24 and also to detect LRV1.22, 25
Given the relevant research on LRV1 conducted by Ives13 on the definition of the mucosal leishmaniosis phenotype in mice, and also considering also scarcity of information on the presence of this virus and clinical manifestations in patients, it was decided to study the existence of clinical differences in patients with ML with or without LRV1 endosymbiosis. We hope that this will contribute to the continuing education process for health professionals working with this very challenging disease, that still represents a major public health problem in Brazil. A better understanding of this pathology can guide a more pragmatic and workable protocol, in an attempt to reduce the destructive effects of this disease.
Methods
The research was a cross sectional study of a historical cohort of patients seen at the otorhinolaryngology outpatient clinic, referred with suspected ML for evaluation by a single otorhinolaryngologist responsible for these cases, in order to obtain a standardization of clinical information in a period from December 2012 to December 2013.
After confirming the clinical suspicion of ML by the specialist, patients were invited to participate and, after signing a written informed consent, underwent biopsy for histopathology with topical instillation of 10% lidocaine spray into the lesion, with preservation in 10% buffered formaldehyde. Soon after, material from the biopsy bed was collected with a cervical brush for Pap smear to determine PCR in two samples; to accomplish this, the surgeon made a slight rotation on the wound biopsy with two brushes; one sample was allocated to the Leishmania test and determination of the species, and the other to the LVR1 search, with preservation in RNALater™ (Ambion®) until the time of DNA and RNA extraction. For these purposes, commercial kits were used. Patients with no apparent lesions were not biopsied, but samples were collected for molecular research with anesthetic instillation (as above), and again a brush rotation was made on the septal mucosa until a slight bruise was produced.
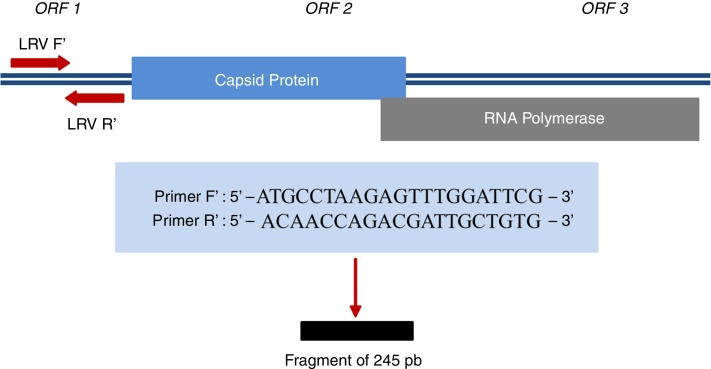
For Leishmania DNA detection, three pairs of primers (ITS1, kDNA, and hsp70) (Table 1) were used; for LRV1, a pair of primers that amplify a small region of ORF1 (Fig. 1) was used; for all of them, positive controls were provided. LVR1 search was performed only in samples positive for Leishmania, since this is an intracellular virus of the parasite.
Table 1.
Description of primers used for DNA detection of Leishmania.
| Primer | Molecular target |
|---|---|
| LITSR 5′-CTGGATCATTTTCCGATG-3′ L5.8S 5′-TGATACCACTTATCGCACTT-3′ |
Internal Transcribed Spacer 1 (ITS1) |
| kDNA F 5′-GAACGGGGTTTCTGTATGC-3′ kDNA R 5′-TACTCCCCGACATGCCTCTG-3′ |
Kinetoplast DNA minicircle (kDNA) |
| Hsp70cF 5-GGACGAGATCGAGCGCATGGT 3′ Hsp70cR 5′-TCCTTCGACGCCTCCTGGTTG-3′ |
Heat shock protein 70 (hsp70) |
Figure 1.
Region amplified for detection of LRV1 and sequences of primers used.
Source: Cantanhêde.30
Only patients with confirmed diagnosis of ML with a positive CPR test and with nasal mucosal injury (in order to get lesion standardization), and who signed the free and informed consent were included. Cases not native to the Brazilian North Region were excluded.
To compare the severity of lesions associated with presence of virus, the staging system proposed by Lessa10 for nasal mucosal leishmaniosis was used. Stage 0 (no apparent injury) was included for patients with latent metastases, with the parasite present in apparently normal mucosa (Table 2).12
Table 2.
Staging of nasal mucosa lesions.
| Stage | Clinical observations of nasal mucosa lesion |
|---|---|
| 0 | No apparent lesions |
| I | Nodulation without ulceration |
| II | Superficial ulceration |
| III | Deep ulceration |
| IV | Septal perforation |
| V | Destruction of nasal architecture and changes in facial structure |
Staging system proposed by Lessa,10 modified by Ito.
Statistical analyzes were conducted with SPSS software v. 19, EpiData® v. 3.1, and EpiData Analysis v. 1.1. Descriptive analyzes of absolute and relative frequencies (with a 95% confidence interval) for clinical signs detected and for species of Leishmania were carried out. Relative risk (RR) of presence of LRV1 in patients with mucosal vs. skin lesion, obtained in a study by Catanhêde (2011), was calculated.
The collected samples met the criteria of Resolution CNS 441/2011. The research project was approved by the Research Ethics Committee under No. CAAE 10215912.1.0000.5300.
Results
This study evaluated 44 patients; 6 were excluded for not having a diagnosis confirmed by PCR. Thus, 37 patients from 13 municipalities of Rondonia and two of Amazonas were included (Table 3 and Fig. 2).
Table 3.
Epidemiological data of study participants in POC, city of Porto Velho, AC, Brazil.
| City | n | M | SCH |
MA | RUR | RAct | OPL | TL |
TNS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | C | I | N | ||||||||
| Alto A. Parecis | 2 | 2 | – | 2 | – | 54.0 | 2 | 2 | 13.0 | 1 | 1 | – | 6.4 |
| Buritis | 2 | 2 | – | 2 | – | 51.0 | 2 | 2 | 24.0 | 1 | – | 1 | 13.0 |
| Candeias | 1 | 1 | – | 1 | – | 32.0 | – | – | 14.0 | – | 1 | – | 0.7 |
| Costa Marques | 1 | 1 | – | – | 1 | 24.0 | – | 1 | 13.0 | 1 | – | – | 3.0 |
| Guajará Mirim | 2 | 2 | 1 | 1 | – | 68.5 | 2 | 2 | 34.5 | – | 1 | 1 | 11.5 |
| Humaitá | 2 | 2 | – | 1 | 1 | 35.5 | 1 | 1 | 25.5 | 1 | – | 1 | 0.5 |
| Jaru | 2 | 1 | – | 1 | 1 | 49.0 | 1 | 1 | 11.0 | 1 | – | 1 | 11.0 |
| Manicoré | 1 | 1 | – | – | 1 | 35.0 | 1 | 1 | 28.0 | – | – | 1 | 22.0 |
| Nova Aripuanã | 1 | 1 | – | 1 | – | 40.0 | – | 1 | 8.0 | – | – | 1 | 0.4 |
| Nova Brasilândia | 1 | 1 | – | 1 | – | 47.0 | 1 | 1 | 10.0 | – | – | 1 | 2.0 |
| Ouro P. do Oeste | 2 | 2 | – | 2 | – | 68.5 | 2 | 2 | 22.5 | – | 2 | – | – |
| Porto Velho | 15 | 13 | 1 | 12 | 2 | 54.5 | 6 | 9 | 13.4 | 5 | 3 | 7 | 4.5 |
| Rio Crespo | 1 | 1 | – | 1 | – | 58.0 | – | 1 | – | – | – | 1 | 10.0 |
| São M. Guaporé | 1 | – | – | 1 | – | 72.0 | 1 | 1 | 10.0 | 1 | – | – | 3.0 |
| Theobroma | 3 | 2 | – | 3 | – | 53.0 | 3 | 3 | 12.7 | – | – | 2 | 1.5 |
| Total | 37 | 32 | 2 | 29 | 6 | 52.4 | 22 | 28 | 16.6 | 11 | 8 | 17 | 5.9 |
n, number of participants; M, males; SCH, schooling; 0, illiterate; 1, primary education; 2, high school; MA, mean age; RUR, living in rural area; RAct, number of individuals with rural activities; OPL, mean of onset of primary lesion in years; TL, treatment for leishmaniosis; C, complete; I, incomplete; N, not treated; TNS, mean time of onset of nasal symptoms in years.
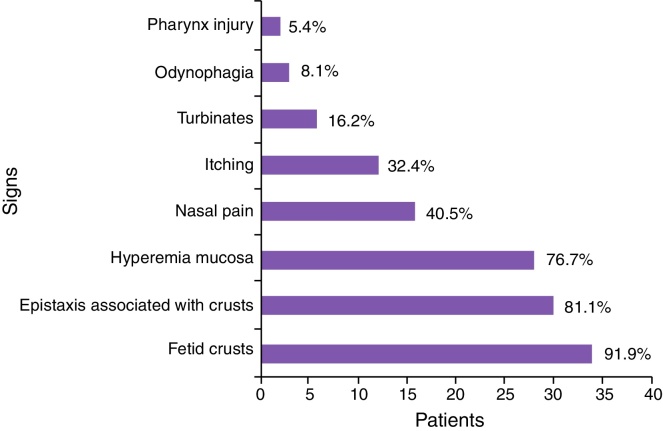
Figure 2.
Main signs and symptoms presented by patients evaluated in the survey.
Twenty-nine (78.3%) patients reported having CL with presence of a compatible scar on their skin; three (8.1%) patients had a history of CL without a compatible scar; and five (13.5%) patients had neither previous history nor CL scar.
According to Table 4, the most common symptoms were: production of fetid crusts, chronic fetid rhinorrhea, slight epistaxis associated with crust removal, nasal obstruction and, at the time of physical examination, granulomatous ulcer, presence of crusts, mucosal hyperemia, and septal perforation.
Table 4.
Leishmania species and presence of LRV1 virus.
| Municipality | RCP |
LRV1 | ||
|---|---|---|---|---|
| Lb | Lg | Lb + Lg | ||
| Alto Alegre dos Parecis | 2 | – | – | 2 |
| Buritis | 2 | – | – | 2 |
| Candeias do Jamari | – | – | 1 | – |
| Costa Marques | 1 | – | – | 1 |
| Guajará Mirim | 2 | – | – | – |
| Humaitá | 1 | – | – | 1 |
| Jaru | 2 | – | – | 2 |
| Manicoré | 2 | – | – | 2 |
| Nova Brasilândia D’Oeste | 1 | – | – | 1 |
| Novo Aripuanã | 1 | – | – | 1 |
| Ouro Preto do Oeste | 1 | 1 | – | – |
| Porto Velho | 11 | 3 | 1 | 11 |
| Rio Crespo | – | 1 | – | – |
| São Miguel do Guaporé | 1 | – | – | 1 |
| Theobroma | 3 | – | – | 2 |
| Total | 30 | 5 | 2 | 26 |
Lb, Leishmania braziliensis; Lg, Leishmania guyanensis; LRV1, Leishmania RNA Virus 1.
Of this group of 37 patients, 30 (81.1%) were diagnosed with L. braziliensis, five (13.5%) with L. guyanensis, and two (5.4%) had mixed infection with L. braziliensis and L. guyanensis. LVR1 virus was present in 26 cases (70.3%), with 23 of them associated with L. braziliensis, two with L. guyanensis, and one with a mixed infection.
The mean time for onset of the primary lesion was 16.6 years (95% CI 11.1–22.1; SD = 16.4), ranging from 0 to 66 years. When the groups were compared with respect to presence or absence of LRV1, the mean time for onset of the primary lesion was 16.2 years (CI 95% 10.2–22.2; SD = 14.9) for LRV1+ and 17.7 years (95% CI 4.0–31.4; SD = 20.4) for LRV1−. There was no difference between LRV1+ and LRV1− groups (p = 0.790).
The mean time for the onset of nasal symptoms was 5.9 years (95% CI 3.5–8.3, SD = 7.3), ranging from 0 to 22 years. In the comparison of presence vs. absence of LRV1 virus, the following mean times were obtained: LRV1+: 5.7 years (95% CI 2.88–8.50; SD = 6.95); LRV1−: 4.7 years (95% CI 0.04–9.43, SD = 6.99). No difference was observed between LRV1+ and LRV1− groups (p = 0.351). One patient with HIV developed CL and ML almost simultaneously.
To compare whether patients with LRV1 had more severe injuries vs. patients without the virus, lesion staging was used in this analysis (Table 5).
Table 5.
Clinical staging system for patients’ nasal mucosa.
| Staging | Number of patients | LRV+ | LRV− |
|---|---|---|---|
| 0 | 1 | 1 | 0 |
| I | 5 | 2 | 3 |
| II | 4 | 3 | 1 |
| III | 14 | 11 | 3 |
| IV | 6 | 5 | 1 |
| V | 7 | 4 | 3 |
| Total | 37 | 26 | 11 |
Table 5 shows that the majority of the lesions presented in stages III, IV, and V (73%). Of those LRV1+ patients, 20 (76.9%) were in most advanced stages (III, IV, and V), while of those LRV− patients, seven (63.6%) were in these stages. However, there was no significant difference in clinical staging between LRV1+ vs. LRV1− patients (p = 0.09).
For technical reasons, only 21 of all histopathology results were received: six diagnosed with leishmaniosis, seven with compatibility (one of these with PCR negative for leishmaniosis), six considered nonspecific, one with chronic rhinitis, and one with paracoccidioidomycosis.
Discussion
Leishmaniosis is an endemic disease in North Region of Brazil, which has the country's largest detection rate.9, 26, 27 Based on the Sistema de Informação de Agravos de Notificação (SINAN) database28 concerning ACL in Brazil, until 2003–2004, there was an increase in notifications of cutaneous leishmaniosis (CL) in Brazil, mainly in the North Region, with a peak close to 30,000 cases/year, but from 2006 onwards there has been a decrease and stabilization to approximately 21,000 notifications per year. ML follows this trend, falling from 2000 to 1400 cases in the same period. The state of Rondonia experienced the steepest decline of CL, decreasing from 1981 cases reported in 2004 to 859 cases in 2010. ML followed a similar curve, with a peak of 196 cases in 2005 that diminished to 118 cases in 2010. For the same year, the detection coefficients for CL and ML were 46.33 and 5.7 per 10,000 inhabitants, respectively.
The epidemiological profile of the studied group was as follows: men (86%) with a mean age of 52 years, living in a rural area, with a history of CL for a mean of 18 years, with no treatment or only incompletely treated, and with a mean of 5.4 years of nasal symptoms. This profile is similar to that of Guerra's9 study in Manaus on 47 patients with ML; little difference in mean age (47 years) and in duration of nasal lesion (8.3 years) was observed. In this study, specific epidemiological profiles for LRV1+ and LRV1− exhibited no significant difference.
The predominant species in cases of ML was L. braziliensis (81%); corroborating the findings of Guerra9 and contrary to other published data, the present study found five (13.5%) patients infected with L. guyanensis. To the authors’ knowledge, this is the first report of nasal mucosa mixed infection with L. braziliensis and L. guyanensis, with two (5.6%) cases: one with the virus, and both cases involving palate and pharynx mucosa. This finding increased the rate of L. guyanensis infections to 19%, confirming the significant prevalence of this species. There are few studies on the prevalence of Leishmania species in the region; it is believed that this species (i.e. L. guyanensis) has always been present, in part because it was detected in the present and also in Guerra's study.
The presence of mixed infections highlights the lack of cross-protection, which a particular species can elicit relative to another species. However, this dual infection could constitute a large source of antigens, generating a Th1-type hyperimmune response and causing a more extensive involvement of aerodigestive mucosa, a finding observed in these two patients.
Of the 37 cases, LRV1 was detected in 26 (70.3%). LRV1 was detected in 23/30 cases of L. braziliensis, 2/5 cases of L. guyanensis, and 1/2 cases of mixed infections. These findings reveal a high frequency of this virus in mucosal infections by both Leishmania species; however, one-third of patients had ML and did not harbor the virus, indicating that metastatic lesions exhibit other factors associated with this clinical form. Pereira et al. found LRV1 in two of five cases (40%) of CL from the North Region of Brazil, and no cases with LRV1 in 40 ACL patients (nine with ML) in Rio de Janeiro (Southeastern Region); Ogg25 detected 25.5% LRV1+ in 47 samples of ACL (two of ML). Hartley19 reported that LRV may contribute variably to ML, acting in isolation or together with other factors.
In Pereira's study, the absence of LRV1 in Rio de Janeiro supports theories proposing that Leishmania is native to the Amazon region, while the species that predominate in South and Southeast Regions of this country may have different origins (Mediterranean theory).4, 5 The presence of LRV1 can serve as phylogenetic marker for the origin of parasites. Leishmania from Amazon region have higher genetic diversity than Southeast Leishmaniae.29
Cantanhêde,30 in his work on LRV1 detection in ACL patients, which is part of a larger research group on ACL in the Amazon region (and of which the present work is also part), detected 35.9% LRV1 positivity in 78 CL patients (RR = 0.63; 95% CI 0.10–0.55). In the present assessment, LRV1 detection in ML was 70.3%, showing a significant association of virus involvement with ML (RR = 2.67; 95% CI 1.82–9.81). This association may result from a change in the immune response caused by this virus.13, 16, 19, 21
For the first time, a correlation study between presence of LRV1 and clinical phenotype of ML was carried out. This study found no significant difference between LRV1+ vs. LRV1- groups based on the staging system proposed for mucosal lesions,10 with regard to the severity of injuries. Similarly, epidemiological differences between groups were not found, nor differences between signs and symptoms exhibited by such groups.
Faced with the possibility of using the virus to determine more severe lesions or lesions with more rapid onset, and considering that this would cause the patient to seek medical care more quickly, the authors evaluated the elapsed interval to the onset of symptoms of nasal injury for each group (LRV+/LRV−) after the onset of the skin injury, and also the time spent by each group to seek medical advice after the onset of nasal symptoms. These two pieces of information could represent the precocity of the onset of the injuries and the severity of their clinical symptoms, respectively. There was no significance in the time to onset of symptoms (p = 0.13), or in the elapsed time to seek medical advice (p = 0.35) between groups.
To compare the degree of mucosal compromise between the two groups, staging of septal injuries was performed, as proposed by Lessa,10 with the inclusion of stage 0 for cases where the mucosa shows normal appearance, but contains parasites.12 This staging was done quite timely, considering that this study only included nasal injury, which comprises more than 90% of mucosal injuries.8, 9, 11 Among LRV1+ patients, four (15.38%) were in the most advanced stage; and among LRV− patients, three (27.3%) were also in this stage. There was no difference in group staging (p = 0.09).
Considering that these patients visit the doctor with injuries that are already very advanced, these lesions would probably be at a stage where a differentiation between both groups, with respect to clinical phenotype, can no longer be made. Whether there are differences was not found in this study; but undoubtedly, it was ascertained that strongly destructive lesions are virus-independent.
In an ideal scenario, CL patients with and without LRV1 would be followed-up, so that one could compare the development of mucosal lesions of both groups. However, in daily routine this appears impractical, because the appearance of these lesions can take a very long time; and as Figueroa et al.12 showed in their study, the parasite may be present in healthy mucosa without indicating disease. These authors point out that this condition is more the rule rather than exception.
ML patients are diagnosed late, and most present with advanced lesions, with a great potential of occurrence of sequel after treatment. This scenario may reflect the difficulty of accessing the medical care system,9, 27 diagnostic difficulty,8, 9, 11, 12 and/or poor knowledge of the disease.
It is common that in his/her first contact with a patient clinically diagnosed with ML, the physician (general practitioner, dermatologist, or infectious disease specialist) is unfamiliar with clinical evaluation of nasal mucosa, which is the most common site for this disease. Thus, this professional ends up referring this patient to the otolaryngologist, who also is not used to mucosal granulomatous diseases, due to the difficulty for confirmation of the diagnosis of this disease. The diagnostic means available in health facilities are insufficient and inadequate to confirm an ML case, due to natural difficulties of the disease (low parasitemia in lesions, previous skin infection). Thus, one would depend on more complex tests such as PCR that are not available in endemic areas.
LRV1 exists in many species of Leishmania, in the form of stable infection. The virus has been detected throughout South America in patients with cutaneous leishmaniosis, often complicated by the presence of infectious metastasis accompanied by an underlying hyperinflammatory response.13 LRV1 detection may represent clinical benefits by guiding treatment and prognosis, due to its potential in determining the clinical forms of leishmaniosis. Additionally, it can function as a target for development of new treatments, for example, the production of vaccines or other pharmacologic agents. LRV1 seems not to be the last frontier in elucidating the pathophysiology of ML, but it represents another strong factor involved in the natural history of this disease.
Conclusion
Despite the demonstration of an association between presence of LRV1 virus and a change in immune response, this study found no correlation among clinical features and presence of the virus in patients with mucosal leishmaniosis. Nonetheless, the frequency of the virus in mucosal injuries is twice that in skin lesions, demonstrating the need for a better understanding.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Please cite this article as: Ito MM, Catanhêde LM, Katsuragawa TH, da Silva Junior CF, Aranha Camargo LM, Mattos RG, et al. Correlation between presence of Leishmania RNA virus 1 and clinical characteristics of nasal mucosal leishmaniosis. Braz J Otorhinolaryngol. 2015;81:533–40.
Institution: Universidade Federal de Rondônia (UNIR), Porto Velho, RO, Brazil.
References
- 1.Alvar J., Velez I.D., Bern C., Herrero M., Desjeux F., Cano J., et al. The WHO Leishmaniasis Control Team Leishmaniasis Worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vega J.C., Sanchez B.F., Montero L.M., Montaña R., Mahecha M.P., Dueñes B., et al. The cost-effectiveness of cutaneous leishmaniasis patient management during an epidemic in Chaparral, Colombia in 2004. Trop Med Int Health. 2007;12:1540–1544. doi: 10.1111/j.1365-3156.2007.01962.x. [DOI] [PubMed] [Google Scholar]
- 3.BRASIL – Ministério da Saúde . Editora do Ministério da Saúde; Brasília: 2010. Manual de Vigilância da Leishmaniose Tegumentar Americana. Segunda Edição Atualizada; pp. 17–31. [Google Scholar]
- 4.Vale E., Furtado T. Leishmaniose tegumentar no Brasil: revisão histórica da origem, expansão e etiologia. An Bras Dermatol. 2005;80:421–428. [Google Scholar]
- 5.Altamirano-Enciso A.J., Marzochi M.C.A., Moreira J.S., Schubach A.O., Marzochi K.B.F. On the origin and spread of cutaneous and mucosal leishmaniasis, based on pre- and post-Colombian historical sources. Hist Cienc Saúde-Manguinhos. 2003;10:853–882. [PubMed] [Google Scholar]
- 6.Lainson R. The Neotropical Leishmania species: a brief historical review of their discovery, ecology and taxonomy. Rev Pan-Amaz Saúde. 2010;1:13–32. [Google Scholar]
- 7.Cupolillo E., Grimaldi G., Jr., Momen H. A general classification of New World Leishmania using numerical zymotaxonomy. Am J Trop Med Hyg. 1994;50:296–311. doi: 10.4269/ajtmh.1994.50.296. [DOI] [PubMed] [Google Scholar]
- 8.Lessa M.M., Lessa H.A., Castro T.W.N., Oliveira A., Scheifer A., Machado P., et al. Mucosal leishmaniasis: epidemiological and clinical aspects. Braz J Otorhinolaryngol. 2007;73:843–847. doi: 10.1016/S1808-8694(15)31181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerra J.A.O., Prestes S.R., Silveira H., Coelho LIARC, Gama P., Moura A., et al. Mucosal Leishmaniasis caused by Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis in the Brazilian Amazon. PLoS Negl Trop Dis. 2011;8:e980. doi: 10.1371/journal.pntd.0000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lessa H.A., Lessa M.M., Guimarães L.H., Lima C.M., Arruda S., Machado P.R., et al. A proposed new clinical staging system for patients with mucosal leishmaniasis. Trans R Soc Trop Med Hyg. 2012;106:376–381. doi: 10.1016/j.trstmh.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diniz J.L.C., Costa M.O.R., Gonçalves D.U. Mucocutaneous Leishmaniasis: clinical markers in presumptive diagnosis. Braz J Otorhinolaryngol. 2011;77:380–384. doi: 10.1590/S1808-86942011000300018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueroa R., Lozano L.E., Romero I.C., Cardona M.T., Prager M., Pacheco R., et al. Detection of Leishmania in unaffected mucosal tissues of patients with cutaneous Leishmaniasis caused by Leishmania (Viannia) Species. J Infect Dis. 2009;200:638–646. doi: 10.1086/600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ives A., Ronet C., Prevel F., Ruzzante G., Fuertes-Marraco S., Schutz F., et al. Leishmania RNA virus controls the severity of mucocutaneous. Science. 2011;331:775–778. doi: 10.1126/science.1199326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reis L.C., Brito M.E.F., Souza M.A., Pereira M.R.A. Mecanismos imunológicos na resposta celular e humoral na leishmaniose tegumentar americana. RPT. 2006;35:103–115. [Google Scholar]
- 15.Ronet C., Beverley S.M., Fasel1F N. Muco-cutaneous leishmaniasis in the New World: the ultimate subversion. Virulence. 2011;2:547–552. doi: 10.4161/viru.2.6.17839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.BRASIL – Ministério da Saúde . Editora do Ministério da Saúde; Brasília: 2010. Manual de Vigilância da Leishmaniose Tegumentar Americana. Segunda Edição Atualizada; pp. 33–41. [Google Scholar]
- 17.Carvalho L.P., Passos S., Bacellar O., Lessa M., Almeida R.P., Magalhães A., et al. Differential Immune regulation of activated T cells between cutaneous and mucosal leishmaniasis as a model for pathogenesis. Parasite Immunol. 2007;29:251–258. doi: 10.1111/j.1365-3024.2007.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacellar O., Lessa H., Schriefer A., Machado P., Jesus A.R., Dutra W.O., et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–6740. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartley M.A., Ronet C., Zangger H., Beverley S.M., Fasel N. Leishmania RNA virus: when the host pays the toll. Front Cell Infect Microbiol Microbiol. 2012;2:1–15. doi: 10.3389/fcimb.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Widmer G., Dooley S. Phylogenetic analysis of Leishmania RNA virus and Leishmania suggests ancient virus-p3arasite association. Nucleic Acids Res. 1995;23:2300–2304. doi: 10.1093/nar/23.12.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zangger H., Ronet C., Desponds C., Kuhlmann F.M., Robinson J., Hartley M.A., et al. Detection of Leishmania RNA virus in Leishmania. Parasites PLoS Negl Trop Dis. 2013;7:e-2006. doi: 10.1371/journal.pntd.0002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira L.O.R., Maretti-Mira A.C., Rodrigues K.M., Lima R.B., Oliveira-Neto M.P., Cupolillo E., et al. Severity of tegumentary leishmaniasis is not exclusively associated with Leishmania RNA virus 1 infection in Brazil. Mem Inst Oswaldo Cruz. 2013;108:665–667. doi: 10.1590/0074-0276108052013021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medeiros A.C.R., Rodrigues S.S., Roselino A.M.F. Comparação entre a especificidade do PCR e detecção histopatológico de leishmania para o diagnóstico da leishmaniose cutânea americana. Braz J Med Biol Res. 2002;35:421–424. doi: 10.1590/s0100-879x2002000400002. [DOI] [PubMed] [Google Scholar]
- 24.Weirather J.L., Jeronimo S.M.B., Gautam S., Sundar S., Kang M., Kurtz M.A., et al. Serial quantitative PCR assay for detection, species discrimination and quantification of Leishmania spp. in Human Samples. J Clin Microbiol. 2011;49:3892–3904. doi: 10.1128/JCM.r00764-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogg M.M., Carrion R., Botelho J.R.A.C.C., Mayrink W., Correa-Oliveira R., Patterson J.L. Short report: quantification of leishmaniavirus RNA in clinical Samples and its possible role in pathogenesis. Am J Trop Med Hyg. 2003;69:309–313. [PubMed] [Google Scholar]
- 26.Silva N., Muniz V. Epidemiologia da leishmaniose tegumentar americana no Estado do Acre, Amazônia brasileira. Cad Saude Publica. 2009;25:1325–1336. doi: 10.1590/s0102-311x2009000600015. [DOI] [PubMed] [Google Scholar]
- 27.Basano S.A., Camargo L.M.A. Leishmaniose tegumentar americana: histórico, epidemiologia e perspectivas de controle. Rev Bras Epidemiol. 2004;7:328–337. [Google Scholar]
- 28.Brasil. Sistema de Informação de Agravos de Notificação (SINAN). Ministério da Saúde. Available from: http://dtr2004.saude.gov.br/sinamweb [accessed: 07.01.14].
- 29.Cupolillo E., Brahim L.R., Toaldo C.B., Oliveira-Neto M.P., Brito M.E., Falqueto A. Genetic polymorphism and molecular epidemiology of Leishmania (Viannia) braziliensis from different hosts and geographic areas in Brazil. J Clin Microbiol. 2003;41:3126–3132. doi: 10.1128/JCM.41.7.3126-3132.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantanhêde L.M. UNIR – Universidade Federal de Rondônia; Porto Velho-RO: 2013. Detecção de leishmaniavírus em amostras de pacientes com leishmaniose tegumentar americana atendidos no centro de medicina tropical de Rondônia. CEMETRON. [dissertação] [Google Scholar]