Abstract
Accurate measurement of cortisol is critical in adrenal insufficiency as it reduces the risk associated with misdiagnosis and supports the optimization of stress dose. Comprehensive assays have been developed to determine the levels of bioactive free cortisol and their clinical and analytical efficacies have been extensively discussed because the level of total cortisol is affected by changes in the structure or circulating levels of corticoid-binding globulin and albumin, which are the main reservoirs of cortisol in the human body. Antibody-based immunoassays are routinely used in clinical laboratories; however, the lack of molecular specificity in cortisol assessment limits their applicability to characterize adrenocortical function. Improved specificity and sensitivity can be achieved by mass spectrometry coupled with chromatographic separation methods, which is a cutting-edge technology to measure individual as well as a panel of steroids in a single analytical run. The purpose of this review is to introduce recent advances in free cortisol measurement from the perspectives of clinical specimens and issues associated with prospective analytical technologies.
Keywords: Cortisol, Mass spectrometry, Immunoassay, Saliva, Adrenal insufficiency
GRAPHICAL ABSTRACT
INTRODUCTION
The adrenal gland consists of the mesodermal cortex and neuroectodermal medulla, which produce different signaling hormones [1,2]. In contrast to medullary catecholamines, which instantly respond to biochemical stress, the secretion of adrenal steroids, including mineralocorticoids and glucocorticoids, controls prolonged responses in the cortex [3]. Cortisol, the major glucocorticoid, is synthesized in the zona fasciculata of the human adrenal cortex, and its biological concentration reflects acute, chronic, and diurnal changes in physiological and psychological events [4,5].
In addition to its biological role as a stress mediator, homeostatic regulation of cortisol in the hypothalamus–pituitary–adrenal (HPA) axis maintains its levels within the physiological range, and alteration of this dynamic equilibrium may result in functional changes in cells and organs during diseased conditions [6,7]. Optimization of the stress dose in managing patients with glucocorticoid-induced adrenal insufficiency during glucocorticoid treatment and after withdrawal is necessary based on the rate of endogenous cortisol production in unstressed conditions [8]. However, whether and when the blood concentrations of cortisol return to physiological levels has not yet been established in critical illness although dynamic alterations in the blood levels of adrenocorticotropic hormone (ACTH), cortisol, and cortisol-binding globulin (CBG) were described in the acute and chronic phases [9].
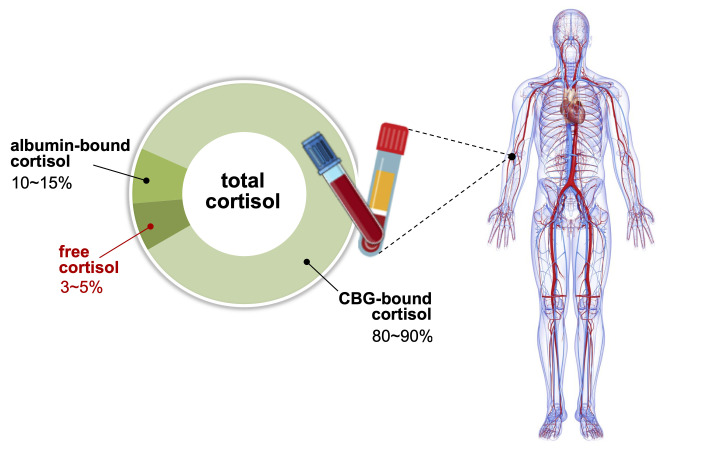
Comprehensive evaluation of cortisol is a prerequisite in diagnosing adrenal disorders and in ensuring optimal therapeutic effects of glucocorticoid replacement; however, clinical laboratories provide limited information on cortisol concentration in the blood [10,11]. Blood cortisol circulates in bound forms with CBG and albumin, which prevents glucocorticoids from penetrating the membrane of target cells. Although CBG plays critical roles in regulating the bioavailability and metabolic clearance of glucocorticoids, only 3% to 5% of total blood cortisol exists in its bioactive form as unbound free cortisol (Fig. 1). As enzyme-linked immunoassays are routinely used in laboratory medicine, free cortisol has not been properly focused upon in clinical practice. Direct measurement of free cortisol has been applied with additional sample preparation techniques; however, these methods may result in inaccurate quantification [10]. This review introduces and discusses the current status of immune-affinity-based technical developments in the measurement of free cortisol in clinical laboratories and the clinical significance of free cortisol, followed by a prospect on addressing technical issues using immunoassay and mass spectrometry.
Fig. 1.
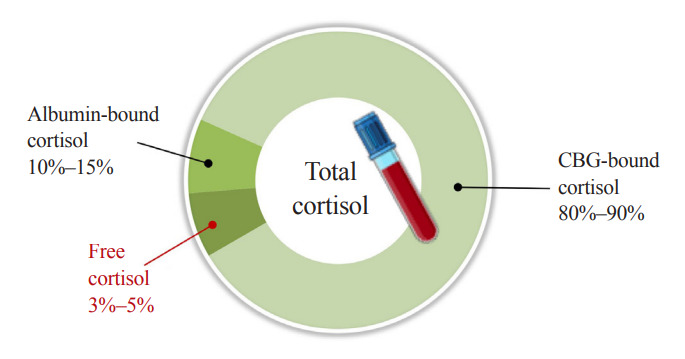
Distribution of cortisol in human blood. Corticoid-binding globulin (CBG) and albumin are main reservoirs of cortisol, binding 80%–90% and 10%–15% of total cortisol, respectively. Approximately 3%–5% of total cortisol is present as biologically active cortisol. States of altered CBG and albumin concentrations may lead to misrepresentation of cortisol action based on total cortisol levels.
DYNAMIC EQUILIBRIUM OF CORTISOL
Bound and free cortisol in the blood remain in a dynamic equilibrium. Owing to its sensitivity to temperature, CBG releases cortisol in response to fever and external heat. The binding affinity of cortisol to CBG declines approximately 16-fold with the increase in temperature from 35°C to 42°C [12,13]. Systemic inflammation-driven proteolysis can alter CBG and albumin levels [14]. Cleavage of human CBG by neutrophil elastase results in a conformational change and substantial loss of cortisol binding affinity of CBG, thereby, regulating cortisol bioavailability [15]. Prereceptor regulation of cortisol involves affinity of binding with CBG and cortisol–cortisone interconversion catalyzed by 11β-hydroxysteroid dehydrogenase (11β-HSD); both these mechanisms affect the bioavailability of cortisol for binding to the glucocorticoid receptor [16]. The 11β-HSD modulates cortisol bioavailability through activation or inactivation at both systemic and localized tissue levels. Besides physiological conditions, medication can also affect circulating CBG levels by directly regulating its production and clearance.
Estrogens accelerate hepatic CBG production and cortisol level is also frequently assessed in adrenocortical carcinomas, treated with mitotane, an adrenolytic drug, as an estrogen-like effect [17]. In addition, CBG levels increased by oral contraceptive drugs lead to the overestimation of cortisol action based on total cortisol levels and underdiagnosis of adrenal insufficiency [18]. Acute decreases in CBG levels [15] and hypoproteinemia [19] have been observed in sepsis and acute illness, while patients had subnormal total cortisol levels in their sera even though their adrenal function was normal. Therefore, the measurement of free cortisol, rather than total cortisol, is recommended for better classification of hypoadrenal patients and may help prevent unnecessary glucocorticoid therapy [19,20]. Some SERPINA6 mutations show low or no circulating CBG and some polymorphisms only affect the cortisol-binding site structurally, with normal levels of CBG. These patients commonly present with low or undetectable total serum cortisol with normal ACTH levels, whereas free cortisol levels in serum and saliva are consistently normal [16].
SERUM SAMPLING WITH PRETREATMENT METHODS
Accurate measurement of HPA function is a key factor in supporting clinical decisions regarding the controversies associated with the diagnosis and treatment of hypoadrenalism [6]. An inadequate stress response is demonstrated by a subnormal adrenal stimulation; however, its diagnosis is based on total serum cortisol levels [21]. Reduced cortisol breakdown, which is related to the suppression of cortisol-metabolizing enzymes, is associated with critical illness and results in hypercortisolemia [22]. Sepsis and critical illness show decreased levels of CBG and assays for free cortisol might underestimate the levels in febrile patients as measurement is usually done at 37°C, which is considered not to be the effect of increased body temperature on the binding equilibrium [12,13].
Free cortisol levels in serum have been directly measured after removing bound fractions with ultrafiltration or equilibrium dialysis, which show acceptable inter-assay coefficients of variation <10% [23]. However, labor-intensive procedures may result in uncertainty in quantitative outcomes, which should be addressed by both technical standardization and quality control in measuring free cortisol levels in serum. Free cortisol can also be calculated based on both total cortisol and CBG levels, and the free cortisol index (FCI) shows a good correlation with free cortisol levels in serum measured using a steady-state gel-filtration method [24]. FCI could be a better tool in evaluating the HPA status than total cortisol levels in serum, in conditions where CBG changes significantly under acute-phase stress [25]. However, an accurate quantification of the levels of CBG is necessary as its tertiary structure leads to individual variations with different binding affinities for cortisol [26].
Zinc sulfate is used to precipitate large proteins, such as immunoglobulins and albumin, at 2:1 or higher ratios (v/v) with serum. Acetonitrile or methanol then precipitates relatively smaller proteins and the remaining zinc sulfate [27]; however, organic solvents are not directly compatible with immunoassay. Unbound cortisol levels in serum can be determined from deproteinized serum samples with zinc sulfate/methanol, with or without further purification steps prior to liquid chromatography-mass spectrometry (LC-MS) analysis, which can be implemented in routine clinical laboratories [28,29]. As zinc ions and acetonitrile/methanol denature antibodies, these methods cannot be used in immunoassays; however, precipitation of proteins with zinc sulfate followed by further precipitation of abundant remaining zinc ions may be suitable for mass spectrometry and immunoassay to measure free cortisol (Fig. 2).
Fig. 2.
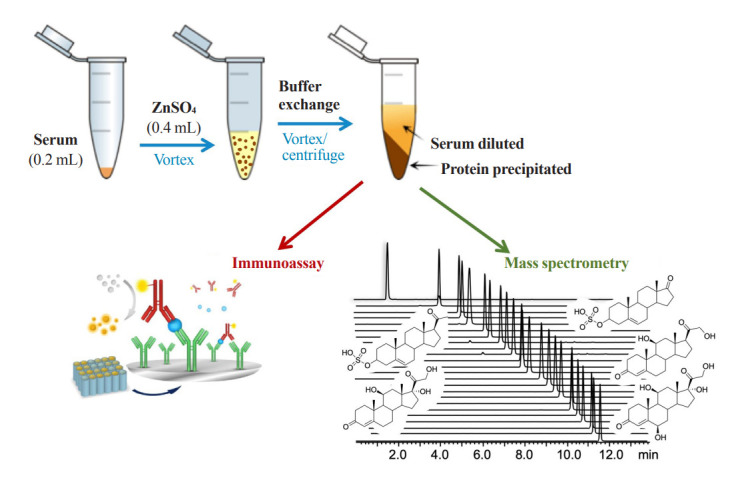
A schematic diagram of free cortisol analysis in serum. Zinc sulfate is used to precipitate large proteins in serum and an aqueous buffer exchange is then performed to further precipitate the remaining small proteins and zinc ions in sample solution. Samples are vortexed and centrifuged before analysis using mass spectrometry or immunoassay, and only the supernatant is used.
SALIVARY CORTISOL
Most salivary compounds are derived from the blood via passive diffusion or active transport. Cortisol diffuses into the salivary gland independently of the salivary flow rate and reflects the circadian rhythm and early morning peak [30]. Because no bound cortisol exists in saliva, its concentration may be independent of cortisol levels in the blood. Saliva sampling is noninvasive, and therefore, minimizes stress-induced overestimation observed in case of blood sampling; moreover, the inability to assess rapid changes in urinary levels can be compensated [10]. Salivary cortisol has great potential for diagnostic work-up on adrenal disorders; it can facilitate longitudinal studies on the HPA axis following stimulation by ACTH and corticotropin releasing hormone, providing an alternative to the evaluation of free cortisol in human serum [31-33]. The results of a previous study were discouraging [31], but in that study salivary cortisol was compared with total serum cortisol and not free cortisol. The oral hydrocortisone protocol may not be reliable because ingestion of drugs may increase cortisol levels in saliva [32]. Basal and stimulated salivary cortisol concentrations have been correlated with total cortisol concentrations in serum during the short Synacthen test, and an optimal cutoff value of 13.2 nM for stimulated salivary cortisol has been suggested to be diagnostic of adrenal insufficiency [33].
The single salivary test at midnight showed 100% sensitivity (95% class interval [CI], 62.9 to 100) and >96% specificity (95% CI, 92.8 to 100) considering a cut-off >6.1 nM in diagnosing Cushing’s syndrome (CS); therefore, it could be an alternative assay to the 24-hour urinary free cortisol as the first-line outpatient screening test for CS [34,35]. In another night-time salivary cortisol (NSC) assay, high salivary cut-off values of 11.5 to 12.6 nM detected with 100% specificity and 93% sensitivity (CI, 89% to 98%) were diagnostic of CS [36]. However, the NSC assay should be validated at larger scales, and its reference range and cut-off values should be established to confirm suitability in clinical practice. In the overnight 1 mg dexamethasone test, salivary cortisol could be an acceptable biomarker with a cut-off of 50 ng/mL in overt CS; however, identifying mild autonomous cortisol secretion in adrenal incidentalomas with lower cut-off values of 18 to 25 ng/mL is not advisable [37]. In addition, salivary cortisol is not a good indicator of hydrocortisone replacement in Addison’s disease, which is measurable at low concentrations [38].
Different devices for salivary sampling are available, and various absorbent materials, such as swabs, are used to facilitate saliva collection. Sterile cotton dental rolls, such as Salivette (Sarsted, Newton, NC, USA), are the most commonly used; however, non-cotton-based swabs, such as polyester Salivette, have often been recommended because cortisol may bind to cotton wool [39]. Saliva samples are collected by centrifugation of the swab, which is a critical step in obtaining reproducible quantities of salivary analytes. Several factors, including the distribution of mucosal transudate, affect salivary flux and composition, and whole-oral fluid collection may be the most feasible method. However, there are practical challenges in obtaining saliva samples from patients with sepsis and critical illness and it is not always possible to collect adequate volumes. A cotton bud may be an alternative tool for salivary sampling. Salivary cortisol levels could be proportionally calculated with the results obtained using Salivette swab in our preliminary experiment (unpublished).
Storing samples at room temperature is not recommended owing to the onset of bacterial growth in saliva in a few hours. Although cortisol and dehydroepiandrosterone (DHEA) levels are not affected, the salivary concentration of testosterone is changed [40]. Salivary cortisol in intact and centrifuged samples has been stable for 3 months at 4°C and for at least 1 year at –20°C. Long-term storage can be desirable at –80°C, and repeated freezing/thawing (F/T) cycles may not affect its concentration [41,42]. Repeated F/T cycles of saliva are necessary prior to cortisol measurement to break the viscous salivary materials, such as mucin, and disperse all salivary proteins/lipids to efficiently extract steroids [43].
METABOLIC RATIO OF CORTISOL TO CORTISONE
The intracellular level of cortisol can be balanced by synthesis and metabolism, and two isoforms of 11β-HSD regulate the physiological concentrations of biologically inactive cortisone and cortisol [44]. In clinical practice, measurement of free cortisol has been established, but localized levels in target tissues may not be sufficient to indicate metabolic phenotypes altered by cellular and physiological functions [45,46]. Of the type 1 and 2 11β-HSDs, 11β-HSD1 converts inactive cortisone to active cortisol, and the production rate of cortisol to cortisone (F/E) reflects the enzymatic activity of 11β-HSD1 [47]. Increased F/E ratios in serum and plasma have been observed in hypertension, obesity, and metabolic syndrome, implicating the pathogenic effects of 11β-HSD [48-50].
Excessive and deficient production of glucocorticoids is a clinical feature of CS and Addison’s disease, respectively, and metabolic changes in glucocorticoids contribute to the pathophysiology of adrenal defects in addition to hypertension, obesity, and metabolic syndrome [7]. Increased corticoid production is a protective process against stress to meet the physiological needs, and inappropriate responses caused by adrenal insufficiency are common in critical illness with decreased levels of serum cortisol, which may lead to a poor prognostic value [51]. Although the suitability of 11β-HSD as a biomarker of stress response has not been assessed, an increased serum F/E ratio was observed in a heterogeneous population of critical illness, leading to the speculation of an increased hemodynamic stability and preserved tissue perfusion conferring survival potential [52].
Because 11β-HSD2 activity is found in the parotid gland, salivary cortisone can be measured and its concentrations are higher than those of salivary cortisol [53,54]. It may be a better biomarker than salivary cortisol to reflect the serum levels of free cortisol under physiological conditions and after adrenal stimulation and hydrocortisone administration, because it is less affected by CBG; its levels may increase with the use of longstanding glucocorticoids [33-37]. Both cortisol and cortisone in saliva could be reliable biomarkers in febrile patients because their levels are not affected by temperature. Cortisol, cortisone, and F/E ratio in saliva showed good intraindividual stability as single-point and single-day biomarkers, whereas reliability during a 2-week period was not found [55]. In reference to the individual stability of biological specimens, hair biomarkers have no absolute stability, but show moderate results throughout a year. The time-dependent alteration in hair cortisol levels could be attributed to seasonal variation, which may be reduced in summer and elevated in winter [56]. However, further information regarding hair cortisol is not provided because it reflects chronic stress [3].
CONSIDERING FURTHER TECHNICAL DEVELOPMENT
Currently, cortisol is assessed only in the laboratory; therefore, urgent detection of abnormal cortisol levels for diagnosis, treatment, and prevention of hypocortisolism is restricted. It has also been challenging to assess state-related cortisol levels in the blood, saliva, and sweat using ambulatory sensing and wearable devices [57-60]. These novel analytical techniques, based on either affinity to immobilized antibodies or electrical sensing, are promising tools for real-time monitoring and self-testing devices. In principle, immunoassay and mass spectrometry could be complementary, and further development is necessary to establish diagnostic criteria for different types of assays.
Immunoassay
Because cortisol is less soluble in water than most of its metabolites and conjugates with proteins, extraction with an organic solvent prior to the immunoassay may be useful for quantifying low levels of serum cortisol in clinical laboratories [61]. However, the organic extract must be removed because the antibodyimmobilized in immunoassay is denatured and this step requires technical expertise. Immunoassays are susceptible to interference because of the complexities of the antigen–antibody interaction occurring in a complex matrix [62]. The lack of specificity is also derived from different properties of various antibodies used in immunoassays, which leads to variability between different commercially available platforms [63,64]. A multicenter comparative study on cortisol measurement showed a good correlation coefficient between laboratories (CI, 0.97 to 0.99) using a kit from the same manufacturer, whereas a CI of 0.54 to 0.65 was obtained for different immunoassay platforms [65]. In addition, heterophilic antibodies administered through transfusion or auto-antibodies to antigens interfere with the immunoassay [14,66]. A significant variation between immunoassays after corticotropin stimulation was observed because corticotropin stimulates the release of other corticosteroids [66].
While measuring cortisol levels, significant cross-reactivity with endogenous cortisol precursors and metabolites, such as 21-deoxycortisol, 5α-dihydrocortisol, and 5β-dihydrocortisol, as well as with a synthetic corticosteroid, prednisolone, and its 6α-methylated metabolite have been observed (11% to 84.3%); however, tetrahydrocortisol and corticosterone showed low cross-reactivities [67,68]. This cross-reactivity with structurally similar steroids and the low affinity of steroid antibodies result in overestimation in both serum and saliva samples [64,69]. In particular, 21-deoxycortisol can accumulate to high levels in certain disease conditions, such as congenital adrenal hyperplasia with 21-hydroxylase deficiency [70]. A comparison of cortisol quantification using immunoassay and mass spectrometry revealed correlation coefficients ranging from 0.43 to 0.97. These inter-assay variations were reported to complicate the diagnosis in critical illness-related corticosteroid insufficiency [66].
Mass spectrometry
In clinical steroid analysis, mass spectrometry is not performed alone, and chromatography-based separation prior to mass spectrometric detection makes the analysis unique, with high analytical sensitivity and selectivity over immunoassays [71]. Gas chromatographic separation combined with mass spectrometry is an old technique that requires complex steps of sample pretreatment, including deconjugation and vaporization of intact steroid molecules; however, it is still a promising tool in clinical applications [72]. However, LC-MS has been extensively used in clinical laboratories for routine measurement of steroid hormones [73]. In recent, mass spectrometry has been successfully used as a discovery tool to identify novel steroidogenic pathways and to obtain new insights into adrenal pathophysiology. Its development together with machine learning algorithms can facilitate diagnosis [74-76].
Considering the cross-reactivity in immunoassays, improved analytical selectivity with mass spectrometry is currently preferred for trace analysis of cortisol and for profiling of adrenal steroids, including cortisol and its inactive metabolite cortisone [47,53-55,74-76]. In particular, salivary cortisol is present at low concentrations, but one of the endogenous interfering cortisones, which has a cross-reactivity of 0.3% to 31.1% in cortisol immunoassays, is commonly detected [53,54]. In contrast, mass spectrometry can determine accurate quantities and considerably reduces variation across laboratories. Moreover, exogenous corticosteroids can be assessed simultaneously with target endogenous adrenal steroids [74,77], which can provide new insights into the pathophysiology of adrenal steroidogenesis [78].
CONCLUSIONS
This review discusses the analytical and diagnostic properties of free cortisol. Clinical mass spectrometry is undoubtedly the gold standard for steroid assessment and exhibits improved diagnostic specificity. Mass spectrometry coupled with chromatographic separation will be more reliable for determining multiplexed steroid panels to support diagnosis and monitoring in different clinical settings. However, the simplicity and practical availability of the immunoassay is still in demand, and it should be driven by the assay performance in single-component screening. Immunoassay and mass spectrometry are orthogonal, but should be complementary to address the unmet clinical needs. The limitations of one of these techniques are overcome in the other (Table 1).
Table 1.
Comparison of Free Cortisol Analytics Using Immunoassay and Mass Spectrometry
| Function | Immunoassay | Mass spectrometry |
|---|---|---|
| Free cortisol measurement | Needs a prefractionation technique (blood) | Feasible (blood and saliva) |
| Feasible (saliva) | ||
| Total cortisol measurement | Feasible | Impractical |
| Detection specificity | Limited | Excellent |
| Reproducibility in quantification | Limited | Excellent |
| Inter-laboratory repeatability | Limited | Excellent |
| Sample preparation | Simple | Expertise required |
| Analytical process | Simple | Expertise required |
| Automation | Good | Excellent |
| Multiplexed screening | Poor | Excellent |
In addition to the measurement of hair cortisol levels for evaluating chronic stress, salivary assays have been extensively considered owing to easy sample availability and the ability to reflect the serum levels of biologically active free cortisol after adrenal stimulation and hydrocortisone replacement. However, optimized thresholds for individual specimens and sampling protocol are also required in large-scale population studies for clinical application of these assays under various conditions, such as the type of analytics and sample collection time, in different pathologies. Inter-laboratory accreditation is a perennial challenge for clinical quality control, and the selection of analytical methods should be dictated by clinical needs, not by assay technology.
Acknowledgments
This study was supported by a grant from the Korea Institute of Science and Technology Institutional Program (Project No. 2E3161C), and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare of the Republic of Korea (Project No. HI21C0032).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Ross IL, Louw GJ. Embryological and molecular development of the adrenal glands. Clin Anat. 2015;28:235–42. doi: 10.1002/ca.22422. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Choi MH. Embryonic development and adult regeneration of the adrenal gland. Endocrinol Metab (Seoul) 2020;35:765–73. doi: 10.3803/EnM.2020.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee DY, Kim E, Choi MH. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015;48:209–16. doi: 10.5483/BMBRep.2015.48.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staab CA, Maser E. 11beta-Hydroxysteroid dehydrogenase type 1 is an important regulator at the interface of obesity and inflammation. J Steroid Biochem Mol Biol. 2010;119:56–72. doi: 10.1016/j.jsbmb.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Lightman SL, Birnie MT, Conway-Campbell BL. Dynamics of ACTH and cortisol secretion and implications for disease. Endocr Rev. 2020;41:bnaa002. doi: 10.1210/endrev/bnaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seckl JR, Walker BR. Minireview: 11beta-hydroxysteroid dehydrogenase type 1-a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142:1371–6. doi: 10.1210/endo.142.4.8114. [DOI] [PubMed] [Google Scholar]
- 8.Borresen SW, Klose M, Glintborg D, Watt T, Andersen MS, Feldt-Rasmussen U. Approach to the patient with glucocorticoid-induced adrenal insufficiency. J Clin Endocrinol Metab. 2022;107:2065–76. doi: 10.1210/clinem/dgac151. [DOI] [PubMed] [Google Scholar]
- 9.Teblick A, Peeters B, Langouche L, Van den Berghe G. Adrenal function and dysfunction in critically ill patients. Nat Rev Endocrinol. 2019;15:417–27. doi: 10.1038/s41574-019-0185-7. [DOI] [PubMed] [Google Scholar]
- 10.Gatti R, Antonelli G, Prearo M, Spinella P, Cappellin E, De Palo EF. Cortisol assays and diagnostic laboratory procedures in human biological fluids. Clin Biochem. 2009;42:1205–17. doi: 10.1016/j.clinbiochem.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 11.El-Farhan N, Rees DA, Evans C. Measuring cortisol in serum, urine and saliva: are our assays good enough? Ann Clin Biochem. 2017;54:308–22. doi: 10.1177/0004563216687335. [DOI] [PubMed] [Google Scholar]
- 12.Cameron A, Henley D, Carrell R, Zhou A, Clarke A, Lightman S. Temperature-responsive release of cortisol from its binding globulin: a protein thermocouple. J Clin Endocrinol Metab. 2010;95:4689–95. doi: 10.1210/jc.2010-0942. [DOI] [PubMed] [Google Scholar]
- 13.Vogeser M, Briegel J. Effect of temperature on protein binding of cortisol. Clin Biochem. 2007;40:724–7. doi: 10.1016/j.clinbiochem.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Diamandis EP. Immunoassay interference: a relatively rare but still important problem. Clin Biochem. 2004;37:331–2. doi: 10.1016/j.clinbiochem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Hammond GL, Smith CL, Paterson NA, Sibbald WJ. A role for corticosteroid-binding globulin in delivery of cortisol to activated neutrophils. J Clin Endocrinol Metab. 1990;71:34–9. doi: 10.1210/jcem-71-1-34. [DOI] [PubMed] [Google Scholar]
- 16.Perogamvros I, Ray DW, Trainer PJ. Regulation of cortisol bioavailability: effects on hormone measurement and action. Nat Rev Endocrinol. 2012;8:717–27. doi: 10.1038/nrendo.2012.134. [DOI] [PubMed] [Google Scholar]
- 17.Nader N, Raverot G, Emptoz-Bonneton A, Dechaud H, Bonnay M, Baudin E, et al. Mitotane has an estrogenic effect on sex hormone-binding globulin and corticosteroid-binding globulin in humans. J Clin Endocrinol Metab. 2006;91:2165–70. doi: 10.1210/jc.2005-2157. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi AC, Bahri A, Breen LA, Barnes SC, Powrie JK, Thomas SM, et al. The influence of the route of oestrogen administration on serum levels of cortisol-binding globulin and total cortisol. Clin Endocrinol (Oxf) 2007;66:632–5. doi: 10.1111/j.1365-2265.2007.02784.x. [DOI] [PubMed] [Google Scholar]
- 19.Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629–38. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]
- 20.Ho JT, Al-Musalhi H, Chapman MJ, Quach T, Thomas PD, Bagley CJ, et al. Septic shock and sepsis: a comparison of total and free plasma cortisol levels. J Clin Endocrinol Metab. 2006;91:105–14. doi: 10.1210/jc.2005-0265. [DOI] [PubMed] [Google Scholar]
- 21.Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–71. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 22.Boonen E, Vervenne H, Meersseman P, Andrew R, Mortier L, Declercq PE, et al. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013;368:1477–88. doi: 10.1056/NEJMoa1214969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogeser M, Mohnle P, Briegel J. Free serum cortisol: quantification applying equilibrium dialysis or ultrafiltration and an automated immunoassay system. Clin Chem Lab Med. 2007;45:521–5. doi: 10.1515/CCLM.2007.104. [DOI] [PubMed] [Google Scholar]
- 24.le Roux CW, Sivakumaran S, Alaghband-Zadeh J, Dhillo W, Kong WM, Wheeler MJ. Free cortisol index as a surrogate marker for serum free cortisol. Ann Clin Biochem. 2002;39(Pt 4):406–8. doi: 10.1258/000456302760042182. [DOI] [PubMed] [Google Scholar]
- 25.le Roux CW, Chapman GA, Kong WM, Dhillo WS, Jones J, Alaghband-Zadeh J. Free cortisol index is better than serum total cortisol in determining hypothalamic-pituitary-adrenal status in patients undergoing surgery. J Clin Endocrinol Metab. 2003;88:2045–8. doi: 10.1210/jc.2002-021532. [DOI] [PubMed] [Google Scholar]
- 26.Pemberton PA, Stein PE, Pepys MB, Potter JM, Carrell RW. Hormone binding globulins undergo serpin conformational change in inflammation. Nature. 1988;336:257–8. doi: 10.1038/336257a0. [DOI] [PubMed] [Google Scholar]
- 27.Polson C, Sarkar P, Incledon B, Raguvaran V, Grant R. Optimization of protein precipitation based upon effectiveness of protein removal and ionization effect in liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;785:263–75. doi: 10.1016/s1570-0232(02)00914-5. [DOI] [PubMed] [Google Scholar]
- 28.Vogeser M, Briegel J, Jacob K. Determination of serum cortisol by isotope-dilution liquid-chromatography electrospray ionization tandem mass spectrometry with on-line extraction. Clin Chem Lab Med. 2001;39:944–7. doi: 10.1515/CCLM.2001.151. [DOI] [PubMed] [Google Scholar]
- 29.Owen LJ, Adaway JE, Davies S, Neale S, El-Farhan N, Ducroq D, et al. Development of a rapid assay for the analysis of serum cortisol and its implementation into a routine service laboratory. Ann Clin Biochem. 2013;50(Pt 4):345–52. doi: 10.1177/0004563212473448. [DOI] [PubMed] [Google Scholar]
- 30.Vining RF, McGinley RA. The measurement of hormones in saliva: possibilities and pitfalls. J Steroid Biochem. 1987;27:81–94. doi: 10.1016/0022-4731(87)90297-4. [DOI] [PubMed] [Google Scholar]
- 31.Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983;20(Pt 6):329–35. doi: 10.1177/000456328302000601. [DOI] [PubMed] [Google Scholar]
- 32.Thomson AH, Devers MC, Wallace AM, Grant D, Campbell K, Freel M, et al. Variability in hydrocortisone plasma and saliva pharmacokinetics following intravenous and oral administration to patients with adrenal insufficiency. Clin Endocrinol (Oxf) 2007;66:789–96. doi: 10.1111/j.1365-2265.2007.02812.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim YJ, Kim JH, Hong AR, Park KS, Kim SW, Shin CS, et al. Stimulated salivary cortisol as a noninvasive diagnostic tool for adrenal insufficiency. Endocrinol Metab (Seoul) 2020;35:628–35. doi: 10.3803/EnM.2020.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaneva M, Mosnier-Pudar H, Dugue MA, Grabar S, Fulla Y, Bertagna X. Midnight salivary cortisol for the initial diagnosis of Cushing’s syndrome of various causes. J Clin Endocrinol Metab. 2004;89:3345–51. doi: 10.1210/jc.2003-031790. [DOI] [PubMed] [Google Scholar]
- 35.Viardot A, Huber P, Puder JJ, Zulewski H, Keller U, Muller B. Reproducibility of nighttime salivary cortisol and its use in the diagnosis of hypercortisolism compared with urinary free cortisol and overnight dexamethasone suppression test. J Clin Endocrinol Metab. 2005;90:5730–6. doi: 10.1210/jc.2004-2264. [DOI] [PubMed] [Google Scholar]
- 36.Papanicolaou DA, Mullen N, Kyrou I, Nieman LK. Nighttime salivary cortisol: a useful test for the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 2002;87:4515–21. doi: 10.1210/jc.2002-020534. [DOI] [PubMed] [Google Scholar]
- 37.Vieira-Correa M, Giorgi RB, Oliveira KC, Hayashi LF, Costa-Barbosa FA, Kater CE. Saliva versus serum cortisol to identify subclinical hypercortisolism in adrenal incidentalomas: simplicity versus accuracy. J Endocrinol Invest. 2019;42:1435–42. doi: 10.1007/s40618-019-01104-8. [DOI] [PubMed] [Google Scholar]
- 38.Ross IL, Lacerda M, Pillay TS, Blom DJ, Johannsson G, Dave JA, et al. Salivary cortisol and cortisone do not appear to be useful biomarkers for monitoring hydrocortisone replacement in Addison’s disease. Horm Metab Res. 2016;48:814–21. doi: 10.1055/s-0042-118182. [DOI] [PubMed] [Google Scholar]
- 39.Chiappin S, Antonelli G, Gatti R, De Palo EF. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clin Chim Acta. 2007;383:30–40. doi: 10.1016/j.cca.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Whembolua GL, Granger DA, Singer S, Kivlighan KT, Marguin JA. Bacteria in the oral mucosa and its effects on the measurement of cortisol, dehydroepiandrosterone, and testosterone in saliva. Horm Behav. 2006;49:478–83. doi: 10.1016/j.yhbeh.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Garde AH, Hansen AM. Long-term stability of salivary cortisol. Scand J Clin Lab Invest. 2005;65:433–6. doi: 10.1080/00365510510025773. [DOI] [PubMed] [Google Scholar]
- 42.Lewis JG. Steroid analysis in saliva: an overview. Clin Biochem Rev. 2006;27:139–46. [PMC free article] [PubMed] [Google Scholar]
- 43.Wood P. Salivary steroid assays: research or routine? Ann Clin Biochem. 2009;46(Pt 3):183–96. doi: 10.1258/acb.2008.008208. [DOI] [PubMed] [Google Scholar]
- 44.Tomlinson JW, Stewart PM. Cortisol metabolism and the role of 11beta-hydroxysteroid dehydrogenase. Best Pract Res Clin Endocrinol Metab. 2001;15:61–78. doi: 10.1053/beem.2000.0119. [DOI] [PubMed] [Google Scholar]
- 45.Vogeser M, Groetzner J, Kupper C, Briegel J. The serum cortisol:cortisone ratio in the postoperative acute-phase response. Horm Res. 2003;59:293–6. doi: 10.1159/000070628. [DOI] [PubMed] [Google Scholar]
- 46.Morita H, Isomura Y, Mune T, Daido H, Takami R, Yamakita N, et al. Plasma cortisol and cortisone concentrations in normal subjects and patients with adrenocortical disorders. Metabolism. 2004;53:89–94. doi: 10.1016/j.metabol.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Schiffer L, Barnard L, Baranowski ES, Gilligan LC, Taylor AE, Arlt W, et al. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: a comprehensive review. J Steroid Biochem Mol Biol. 2019;194:105439. doi: 10.1016/j.jsbmb.2019.105439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mariniello B, Ronconi V, Sardu C, Pagliericcio A, Galletti F, Strazzullo P, et al. Analysis of the 11beta-hydroxysteroid dehydrogenase type 2 gene (HSD11B2) in human essential hypertension. Am J Hypertens. 2005;18:1091–8. doi: 10.1016/j.amjhyper.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Duclos M, Marquez Pereira P, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes Res. 2005;13:1157–66. doi: 10.1038/oby.2005.137. [DOI] [PubMed] [Google Scholar]
- 50.Kim SH, Kim SE, Choi MH, Park MJ. Altered glucocorticoid metabolism in girls with central obesity. Mol Cell Endocrinol. 2021;527:111225. doi: 10.1016/j.mce.2021.111225. [DOI] [PubMed] [Google Scholar]
- 51.Wu JY, Hsu SC, Ku SC, Ho CC, Yu CJ, Yang PC. Adrenal insufficiency in prolonged critical illness. Crit Care. 2008;12:R65. doi: 10.1186/cc6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Venkatesh B, Cohen J, Hickman I, Nisbet J, Thomas P, Ward G, et al. Evidence of altered cortisol metabolism in critically ill patients: a prospective study. Intensive Care Med. 2007;33:1746–53. doi: 10.1007/s00134-007-0727-7. [DOI] [PubMed] [Google Scholar]
- 53.Perogamvros I, Keevil BG, Ray DW, Trainer PJ. Salivary cortisone is a potential biomarker for serum free cortisol. J Clin Endocrinol Metab. 2010;95:4951–8. doi: 10.1210/jc.2010-1215. [DOI] [PubMed] [Google Scholar]
- 54.Debono M, Harrison RF, Whitaker MJ, Eckland D, Arlt W, Keevil BG, et al. Salivary cortisone reflects cortisol exposure under physiological conditions and after hydrocortisone. J Clin Endocrinol Metab. 2016;101:1469–77. doi: 10.1210/jc.2015-3694. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Q, Chen Z, Chen S, Xu Y, Deng H. Intraindividual stability of cortisol and cortisone and the ratio of cortisol to cortisone in saliva, urine and hair. Steroids. 2017;118:61–7. doi: 10.1016/j.steroids.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 56.Walker BR, Best R, Noon JP, Watt GC, Webb DJ. Seasonal variation in glucocorticoid activity in healthy men. J Clin Endocrinol Metab. 1997;82:4015–9. doi: 10.1210/jcem.82.12.4430. [DOI] [PubMed] [Google Scholar]
- 57.Choi S, Kim S, Yang JS, Lee JH, Joo C, Jung HI. Real-time measurement of human salivary cortisol for the assessment of psychological stress using a smartphone. Sens Bio-Sens Res. 2014;2:8–11. [Google Scholar]
- 58.Sanghavi BJ, Moore JA, Chavez JL, Hagen JA, Kelley-Loughnane N, Chou CF, et al. Aptamer-functionalized nanoparticles for surface immobilization-free electrochemical detection of cortisol in a microfluidic device. Biosens Bioelectron. 2016;78:244–52. doi: 10.1016/j.bios.2015.11.044. [DOI] [PubMed] [Google Scholar]
- 59.Pinto V, Sousa P, Catarino SO, Correia-Neves M, Minas G. Microfluidic immunosensor for rapid and highly-sensitive salivary cortisol quantification. Biosens Bioelectron. 2017;90:308–13. doi: 10.1016/j.bios.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 60.Parlak O, Keene ST, Marais A, Curto VF, Salleo A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci Adv. 2018;4:eaar2904. doi: 10.1126/sciadv.aar2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davison SL, Bell R, Montalto JG, Sikaris K, Donath S, Stanczyk FZ, et al. Measurement of total testosterone in women: comparison of a direct radioimmunoassay versus radioimmunoassay after organic solvent extraction and celite column partition chromatography. Fertil Steril. 2005;84:1698–704. doi: 10.1016/j.fertnstert.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 62.Ghazal K, Brabant S, Prie D, Piketty ML. Hormone immunoassay interference: a 2021 update. Ann Lab Med. 2022;42:3–23. doi: 10.3343/alm.2022.42.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts RF, Roberts WL. Performance characteristics of five automated serum cortisol immunoassays. Clin Biochem. 2004;37:489–93. doi: 10.1016/j.clinbiochem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 64.El-Farhan N, Pickett A, Ducroq D, Bailey C, Mitchem K, Morgan N, et al. Method-specific serum cortisol responses to the adrenocorticotrophin test: comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clin Endocrinol (Oxf) 2013;78:673–80. doi: 10.1111/cen.12039. [DOI] [PubMed] [Google Scholar]
- 65.Briegel J, Sprung CL, Annane D, Singer M, Keh D, Moreno R, et al. Multicenter comparison of cortisol as measured by different methods in samples of patients with septic shock. Intensive Care Med. 2009;35:2151–6. doi: 10.1007/s00134-009-1627-9. [DOI] [PubMed] [Google Scholar]
- 66.Cohen J, Ward G, Prins J, Jones M, Venkatesh B. Variability of cortisol assays can confound the diagnosis of adrenal insufficiency in the critically ill population. Intensive Care Med. 2006;32:1901–5. doi: 10.1007/s00134-006-0389-x. [DOI] [PubMed] [Google Scholar]
- 67.Jonsson BA, Malmberg B, Amilon A, Helene Garde A, Orbaek P. Determination of cortisol in human saliva using liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784:63–8. doi: 10.1016/s1570-0232(02)00753-5. [DOI] [PubMed] [Google Scholar]
- 68.Wood L, Ducroq DH, Fraser HL, Gillingwater S, Evans C, Pickett AJ, et al. Measurement of urinary free cortisol by tandem mass spectrometry and comparison with results obtained by gas chromatography-mass spectrometry and two commercial immunoassays. Ann Clin Biochem. 2008;45(Pt 4):380–8. doi: 10.1258/acb.2007.007119. [DOI] [PubMed] [Google Scholar]
- 69.Miller R, Plessow F, Rauh M, Groschl M, Kirschbaum C. Comparison of salivary cortisol as measured by different immunoassays and tandem mass spectrometry. Psychoneuroendocrinology. 2013;38:50–7. doi: 10.1016/j.psyneuen.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 70.Miller WL. Congenital adrenal hyperplasia: time to replace 17OHP with 21-deoxycortisol. Horm Res Paediatr. 2019;91:416–20. doi: 10.1159/000501396. [DOI] [PubMed] [Google Scholar]
- 71.Choi MH. Mass spectrometry-based steroid profiling: meeting unmet clinical needs. Tohoku J Exp Med. 2021;253:171–80. doi: 10.1620/tjem.253.171. [DOI] [PubMed] [Google Scholar]
- 72.Choi MH, Chung BC. Bringing GC-MS profiling of steroids into clinical applications. Mass Spectrom Rev. 2015;34:219–36. doi: 10.1002/mas.21436. [DOI] [PubMed] [Google Scholar]
- 73.Wudy SA, Choi MH. Steroid LC-MS has come of age. J Steroid Biochem Mol Biol. 2016;162:1–3. doi: 10.1016/j.jsbmb.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 74.Storbeck KH, Schiffer L, Baranowski ES, Chortis V, Prete A, Barnard L, et al. Steroid metabolome analysis in disorders of adrenal steroid biosynthesis and metabolism. Endocr Rev. 2019;40:1605–25. doi: 10.1210/er.2018-00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahn CH, Lee C, Shim J, Kong SH, Kim SJ, Kim YH, et al. Metabolic changes in serum steroids for diagnosing and subtyping Cushing’s syndrome. J Steroid Biochem Mol Biol. 2021;210:105856. doi: 10.1016/j.jsbmb.2021.105856. [DOI] [PubMed] [Google Scholar]
- 76.Lee C, Kim JH, Moon SJ, Shim J, Kim HI, Choi MH. Selective LC-MRM/SIM-MS based profiling of adrenal steroids reveals metabolic signatures of 17α-hydroxylase deficiency. J Steroid Biochem Mol Biol. 2020;198:105615. doi: 10.1016/j.jsbmb.2020.105615. [DOI] [PubMed] [Google Scholar]
- 77.Casals G, Hanzu FA. Cortisol measurements in Cushing’s syndrome: immunoassay or mass spectrometry? Ann Lab Med. 2020;40:285–96. doi: 10.3343/alm.2020.40.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamrath C, Hochberg Z, Hartmann MF, Remer T, Wudy SA. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: evidence from urinary steroid hormone analysis. J Clin Endocrinol Metab. 2012;97:E367–75. doi: 10.1210/jc.2011-1997. [DOI] [PubMed] [Google Scholar]