Abstract
Background/Aims
Recent epidemiologic studies have shown a continued increase in colorectal cancer incidence among younger adults. Little is known about the factors that contribute to the development of young-onset colorectal neoplasia (CRN).
Methods
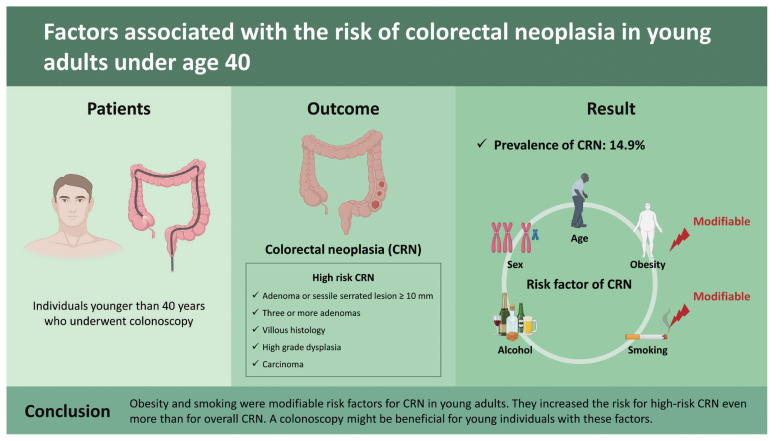
A cross-sectional analysis was performed for individuals younger than 40 years who underwent colonoscopy in Seoul St. Mary’s Hospital and its affiliated health screening center. High-risk CRN was defined as adenoma or sessile serrated lesion ≥ 10 mm, with three or more adenomas, villous histology, high grade dysplasia, or carcinoma.
Results
Of these 13,621 included participants, 2,023 (14.9%) had one and more CRN. Young patients with CRN tended to be elderly, male, obese, smoker, having a habit of drinking, and having comorbidities such as hypertension, dyslipidemia, diabetes, and chronic kidney disease. In a multivariate analysis adjusted for age, sex, obesity, smoking status, and alcohol intake, old age (odds ratio [OR], 1.086; 95% confidence interval [CI], 1.054 to 1.119), male sex (OR, 1.748; 95% CI, 1.247 to 2.451), obesity (OR, 1.439; 95% CI, 1.133 to 1.828), and smoking (OR, 1.654; 95% CI, 1.287 to 2.127) were independent risk factors for overall CRN. Obesity and smoking as two modifiable factors increased the risk for high-risk CRN even more than for overall CRN (OR, 1.734; 95% CI, 1.168 to 2.575 and OR, 1.797; 95% CI, 1.172 to 2.753, respectively).
Conclusions
Obesity and smoking were modifiable risk factors for CRN in young adults. They increased the risk for high-risk CRN even more than for overall CRN. A colonoscopy might be beneficial for young individuals with these factors.
Keywords: Colonoscopy, Colorectal neoplasms, Early detection of cancer, Risk factors
Graphical abstract
INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second leading cause of cancer mortality, with an incidence of 1.88 million and 915,880 deaths worldwide in 2020 [1]. Regular screening colonoscopy beginning at age 50 has reduced CRC incidence and mortality by removing precancerous adenoma and detecting early-stage CRC [2,3]. However, young adults who are outside of this age range for screening show increasing CRC incidence and mortality globally [3–5]. In particular, adults under the age of 50 who were born in 1990 have double the risk of colon cancer and quadruple the risk of rectal cancer compared with their age-matched counterparts born in 1950 in the United States [3]. Based on this strong epidemiologic evidence, the American Cancer Society has recommended lowering the age of screening to be 45 years old [6].
As the utilization of colonoscopy increased, the detection of sporadic colorectal adenomas in young people is becoming more common recently [3]. A recent systematic review has demonstrated that the pooled overall prevalence of young-onset adenoma diagnosed at ages between 18 and 49 years old is 9% [7]. Considering that most CRCs arise from precancerous adenomas [8], early detection and removal of which are also the best way to reduce the CRC incidence and mortality in young adults. However, in terms of cost-effectiveness, it is not easy to adopt a screening strategy covering all young adults. If risk factors for young-onset colorectal neoplasia (CRN) are clearly identified, it might be a more reasonable approach to conduct a selective screening program only for high-risk populations.
Risk factors associated with sporadic young-onset CRN have not been well-characterized yet. Previous studies have reported that risk factors for young-onset CRN include increasing age, male gender, obesity, smoking, alcohol intake, metabolic syndrome, elevated fasting blood glucose, hyper-triglyceridemia, and prior abdominopelvic radiotherapy for childhood cancers [9–13]. However, most studies were focused on the population over the age of 40. Data on the population under the age of 40 were very limited.
Therefore, the objective of this study was to investigate the prevalence and risk factors for CRN in a large cohort of the population under the age of 40 who underwent screening or diagnostic colonoscopy. In addition, we evaluated the degree of identified risk factors in high-risk CRN as well as in overall CRN.
METHODS
Study population
This cross-sectional, retrospective study reviewed 14,338 individuals aged 18 to 40 years who underwent screening or diagnostic colonoscopy between 2008 and 2018 at Seoul St. Mary’s Hospital and its affiliated health screening center. Subjects were excluded if they met the following exclusion criteria: (1) had a documented inflammatory bowel disease; (2) had a history of prior colon surgery; (3) had a first-degree family history of CRC; or (4) incomplete procedures due to poor preparation or failed cecal intubation. The study design was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (approval number: KC19RESI0337). Written informed consent by the patients was waived due to a retrospective nature of our study.
Measurements
All subjects who received a health check-up were demanded to complete a self-administered questionnaire, which included questions on medical history, medication use, health-related behaviors (smoking status [current, former, and never], alcohol consumption [less than once a fortnight, no; at least once a fortnight or more, yes], and physical activity) and family history of CRC. A family history of CRC was defined as CRC in at least one first-degree relative(s) of any age. Physical characteristics were measured by trained nurses. Body weight was measured with subjects wearing light clothes without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Subjects with a BMI ≥ 25 kg/m2 were defined as obese based on Asia-Pacific classification of BMI [9–11]. Abdominal obesity was defined as waist circumference ≥ 90 cm in men or ≥ 80 cm in women. Other covariates were defined as follows: elevated fasting blood glucose level ≥ 100 mg/dL, elevated total cholesterol ≥ 200 mg/dL, reduced high density lipoprotein cholesterol (HDL-C) < 40 mg/dL for men and < 50 mg/dL for women, elevated triglyceride (TG) ≥ 150 mg/dL, elevated systolic blood pressure ≥ 130 mmHg, elevated diastolic blood pressure ≥ 85 mmHg [12], and elevated carcinoembryonic antigen (CEA) ≥ 3 ng/mL. Fatty liver and chronic kidney disease were identified with a questionnaire or medical record review.
Colonoscopy
All colonoscopies were conducted by experienced endoscopists. Either endoscopic removal or biopsy was done for any suspicious neoplastic lesion during colonoscopies. All specimens were examined by experienced pathologists. Overall CRN refers to all neoplastic lesions including any adenoma and cancer. Sessile serrated lesions were also included in neoplastic lesions, while hyperplastic polyps were not. High-risk CRN was defined as adenoma with size ≥ 10 mm, sessile serrated lesion with size ≥ 10 mm, 3 or more adenomas in number, villous histology, high grade dysplasia, or carcinoma in pathology [13].
Statistical analysis
Continuous variables were described as mean±standard deviation or median (interquartile range) and compared using Student’s t test. Categorical variables were expressed as numbers (percentage) and analyzed using the chi-square test and trend test as appropriate. We used multiple logistic regression analysis to determine the odds ratios (OR) for developing overall CRN and high-risk CRN. Variables showing p values < 0.10 after univariate analysis and those that were considered clinically relevant were included in a multivariate logistic regression model to identify independent factors associated with CRN. Multivariate models were adjusted for age, sex, obesity, smoking status, and alcohol intake. p values < 0.05 were considered statistically significant. All data analyses were performed using SAS software package version 8.02 (SAS Institute, Cary, NC, USA). The present study was under the statistical consultation supported by the Department of Biostatistics of the Catholic Research Coordinating Center.
RESULTS
Among 14,338 eligible subjects, 717 were excluded because of documented inflammatory bowel disease (n = 22), a history of prior colon surgery (n = 46), first-degree family history of CRC (n = 298), or incomplete procedures due to poor preparation or failed cecal intubation (poor preparation, n = 247; failed cecal intubation, n = 104). As a result, 13,621 participants were included in this study. The mean age of study participants was 35.2 ± 4.0 years. The proportion of males was 62.5%.
Of these 13,621 included participants, 2,023 (14.9%) had one or more CRN. Table 1 shows the demographic characteristics of study participants according to the existence of CRN. Subdivided by age group, the prevalence of CRN was 9.8% (127/1291) in the age group of under 30 years and 15.4% (1,896/12,330) in the age group of 30 to 39 years. The prevalence of CRN was statistically significantly higher in subjects with the following characteristics: old age, male sex, obesity, fatty liver, chronic kidney disease, elevated FBG levels, elevated total cholesterol levels, reduced HDL-C levels, high low density lipoprotein cholesterol levels, elevated TG levels, elevated CEA levels, elevated systolic blood pressure, elevated diastolic blood pressure, drinker, and smoker. The characteristics of CRN were summarized in Table 2.
Table 1.
Demographic characteristics of study participants according to the existence of colorectal neoplasia
| Characteristic | Total (n = 13,621) | Without CRN (n = 11,598) | With CRN (n = 2,023) | p value |
|---|---|---|---|---|
| Age, yr | 35.2 ± 4.0 | 35.1 ± 4.1 | 35.7 ± 3.8 | < 0.001 |
| Male sex | 8,424 (61.8) | 6,901 (59.5) | 1,523 (75.3) | < 0.001 |
| Obesitya | 4,162 (30.6) | 3,329 (28.7) | 833 (41.2) | < 0.001 |
| Abdominal obesityb | 3,896 (28.6) | 3,305 (28.5) | 591 (29.2) | 0.556 |
| Fatty liver | 1,144 (8.4) | 928 (8.0) | 216 (10.7) | 0.020 |
| Chronic kidney disease | 114 (0.8) | 69 (0.6) | 45 (2.2) | < 0.001 |
| Elevated FBG levelsc | 1,408 (10.3) | 1,113 (9.6) | 295 (14.6) | < 0.001 |
| Elevated TC levelsd | 6,186 (45.4) | 5,138 (44.3) | 1,048 (51.8) | < 0.001 |
| Reduced HDL-C levelse | 2,970 (21.8) | 2,296 (19.8) | 674 (33.3) | < 0.001 |
| LDL-C levels, mg/dL | 122.5 ± 31.7 | 121.4 ± 31.2 | 127.8 ± 33.4 | < 0.001 |
| Elevated TG levelsf | 2,791 (20.5) | 2,192 (18.9) | 599 (29.6) | < 0.001 |
| Elevated CEA levelsg | 305 (2.4) | 243 (2.2) | 62 (3.7) | < 0.001 |
| Elevated systolic BPh | 2,136 (15.7) | 1,705 (14.7) | 431 (21.3) | < 0.001 |
| Elevated diastolic BPi | 1,202 (8.8) | 951 (8.2) | 251 (12.4) | < 0.001 |
| Alcohol consumptionj | 9,613 (70.6) | 8,084 (69.7) | 1,529 (75.6) | < 0.001 |
| Current or former smokerk | 6,503 (47.7) | 5,289 (45.6) | 1,214 (60.0) | < 0.001 |
| Daily coffee consumptionl | 4,753 (89.2) | 3,899 (89.1) | 854 (90.1) | 0.356 |
| Regular exercisem | 1,049 (40.8) | 883 (41.7) | 166 (36.8) | 0.057 |
Values are presented as mean ± standard deviation or number (%).
CRN, colorectal neoplasia; FBG, fasting blood glucose; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TG, triglyceride; CEA, carcinoembryonic antigen; BP, blood pressure.
Body mass index ≥ 25 kg/m2.
A waist circumference ≥ 90 cm for men and ≥ 80 cm for women.
Fasting blood glucose level of ≥ 100 mg/dL.
Total cholesterol ≥ 200 mg/dL.
HDL-C < 40 mg/dL for men and < 50 mg/dL for women.
Triglyceride ≥ 150 mg/dL.
CEA ≥ 3 ng/mL. CEA was evaluated in 11,038 and 1,684 participants in the without and with CRN group, respectively.
Systolic BP ≥ 130 mmHg.
Diastolic BP ≥ 85 mmHg.
One glass of liquor at least once a month.
Any amount of cigarette smoking history.
At least one cup of coffee a day. Daily coffee consumption was evaluated in 4,378 and 948 participants in the without and with CRN group, respectively.
At least once a fortnight. Regular exercise was evaluated in 2,120 and 451 participants in the without and with CRN group, respectively.
Table 2.
Characteristics of colorectal neoplasia
| Characteristic | Colorectal neoplasia group (n = 2,023) |
|---|---|
| Number | 1.3 ± 0.8 |
| 1 | 1,637 (80.9) |
| 2 | 281 (13.9) |
| ≥ 3 | 105 (5.2) |
| Size, mma | 4.5 ± 5.2 |
| < 5 | 1,675 (82.8) |
| 5–9 | 201 (9.9) |
| ≥ 10 | 147 (7.3) |
| Histology | |
| Tubular adenoma, low grade dysplasia | 1,567 (77.5) |
| Tubular adenoma, high grade dysplasia | 332 (16.4) |
| Tubulovillous/villous adenoma | 14 (0.7) |
| Sessile serrated lesion | 100 (4.9) |
| Adenocarcinoma | 10 (0.5) |
| Location | |
| Proximal colonb | 851 (42.1) |
| Distal colonc | 998 (49.3) |
| Both proximal and distal colon | 174 (8.6) |
Values are presented as mean ± standard deviation or number (%).
In cases of two or more adenomas, the largest adenoma or the adenoma with more advanced pathology was evaluated.
Cecum, ascending colon, and transverse colon.
Splenic flexure, descending colon, sigmoid colon, and rectum.
Results of univariate and multivariate analyses for risk factors of overall CRN are shown in Table 3. All significant univariate variables were chosen for multivariate analysis. In a multivariate analysis adjusted for age, sex, obesity, smoking status, and alcohol intake, old age (OR, 1.086; 95% confidence interval [CI], 1.054 to 1.119), male sex (vs. female: OR, 1.748; 95% CI, 1.247 to 2.451), obesity (OR, 1.439; 95% CI, 1.133 to 1.828), and smoking (OR, 1.654; 95% CI, 1.287 to 2.127) were independent risk factors for overall CRN.
Table 3.
Univariate and multivariate analyses of risk factors associated with overall colorectal neoplasia
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age, yr | 1.045 (1.031–1.058) | < 0.001 | 1.085 (1.054–1.119) | < 0.001 |
|
| ||||
| Male sex | 2.083 (1.866–2.326) | < 0.001 | 1.748 (1.247–2.451) | 0.001 |
|
| ||||
| Obesitya | 1.738 (1.571–1.922) | < 0.001 | 1.439 (1.133–1.828) | 0.003 |
|
| ||||
| Abdominal obesityb | 1.033 (0.928–1.149) | 0.554 | ||
|
| ||||
| Fatty liver | 1.373 (1.051–1.793) | 0.020 | 1.199 (0.752–1.911) | 0.446 |
|
| ||||
| Chronic kidney disease | 3.740 (1.935–7.230) | < 0.001 | 0.967 (0.259–3.609) | 0.960 |
|
| ||||
| Elevated FBG levelsc | 1.611 (1.390–1.867) | < 0.001 | 1.002 (0.205–4.886) | 0.999 |
|
| ||||
| Elevated TC levelsd | 1.354 (1.221–1.501) | < 0.001 | 0.974 (0.747–1.269) | 0.843 |
|
| ||||
| Reduced HDL-C levelse | 2.023 (1.818–2.250) | < 0.001 | 1.069 (0.850–1.345) | 0.569 |
|
| ||||
| LDL-C levels | 1.006 (1.005–1.008) | < 0.001 | 1.002 (0.998–1.007) | 0.243 |
|
| ||||
| Elevated TG levelsf | 1.811 (1.612–2.034) | < 0.001 | 1.190 (0.945–1.497) | 0.139 |
|
| ||||
| Elevated CEA levelsg | 1.692 (1.262–2.268) | < 0.001 | 0.848 (0.491–1.464) | 0.554 |
|
| ||||
| Elevated systolic BPh | 1.569 (1.378–1.785) | < 0.001 | 1.178 (0.745–1.861) | 0.483 |
|
| ||||
| Elevated diastolic Bpi | 1.587 (1.350–1.866) | < 0.001 | 1.064 (0.740–1.531) | 0.737 |
|
| ||||
| Alcohol consumptionj | 1.353 (1.175–1.558) | < 0.001 | 0.836 (0.630–1.109) | 0.213 |
|
| ||||
| Current or former smokerk | 1.790 (1.549–2.070) | < 0.001 | 1.654 (1.287–2.127) | < 0.001 |
|
| ||||
| Daily coffee consumptionl | 1.116 (0.884–1.409) | 0.356 | ||
|
| ||||
| Regular exercisem | 0.816 (0.661–1.007) | 0.058 | 0.804 (0.638–1.014) | 0.066 |
Odds ratios were calculated by using logistic regression.
OR, odds ratio; CI, confidence interval; FBG, fasting blood glucose; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TG, triglyceride; CEA, carcinoembryonic antigen; BP, blood pressure.
Body mass index ≥ 25 kg/m2.
A waist circumference ≥ 90 cm for men and ≥ 80 cm for women.
Fasting blood glucose level of ≥ 100 mg/dL.
Total cholesterol ≥ 200 mg/dL.
HDL-C < 40 mg/dL for men and < 50 mg/dL for women.
Triglyceride ≥ 150 mg/dL.
CEA ≥ 3 ng/mL.
Systolic BP ≥ 130 mmHg.
Diastolic BP ≥ 85 mmHg.
One glass of liquor at least once a month.
Any amount of cigarette smoking history.
At least one cup of coffee a day.
At least once a fortnight.
The prevalence of high-risk CRN was 3.8% (518/13,621). Results of univariate and multivariate analyses for risk factors of high-risk CRN are shown in Table 4. In a multivariate analysis adjusted for age, sex, obesity, smoking status, and alcohol intake, old age (OR, 1.090; 95% CI, 1.036 to 1.147), male sex (OR, 1.946; 95% CI, 1.052 to 3.597), obesity (OR, 1.734; 95% CI, 1.168 to 2.575), and smoking (OR, 1.797; 95% CI, 1.172 to 2.753) were independent risk factors for high-risk CRN. Those with two modifiable factors, obesity and smoking, had more significant risk for high-risk CRN than for overall CRN.
Table 4.
Univariate and multivariate analyses of risk factors associated with high-risk colorectal neoplasia
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 1.052 (1.027–1.078) | < 0.001 | 1.090 (1.036–1.147) | 0.001 |
|
| ||||
| Sex | 1.821 (1.495–2.222) | < 0.001 | 1.946 (1.052–3.597) | 0.034 |
|
| ||||
| Obesitya | 1.733 (1.442–2.082) | < 0.001 | 1.734 (1.168–2.575) | 0.006 |
|
| ||||
| Abdominal obesityb | 0.929 (0.761–1.134) | 0.469 | ||
|
| ||||
| Fatty liver | 1.233 (0.794–1.914) | 0.351 | ||
|
| ||||
| Chronic kidney disease | 1.921 (0.565–6.535) | 0.296 | ||
|
| ||||
| Elevated FBG levelsc | 1.438 (1.088–1.900) | 0.011 | 0.812 (0.466–1.414) | 0.462 |
|
| ||||
| Elevated TC levelsd | 1.344 (1.105–1.634) | 0.003 | 1.296 (0.885–1.900) | 0.183 |
|
| ||||
| Reduced HDL-C levelse | 2.430 (2.018–2.926) | < 0.001 | 0.898 (0.551–1.462) | 0.664 |
|
| ||||
| LDL-C levels | 1.005 (1.002–1.008) | < 0.001 | 1.005 (0.999–1.011) | 0.134 |
|
| ||||
| Elevated TG levelsf | 1.938 (1.566–2.398) | < 0.001 | 1.124 (0.730–1.730) | 0.597 |
|
| ||||
| Elevated CEA levelsg | 2.129 (1.314–3.452) | 0.002 | 1.043 (0.400–2.719) | 0.931 |
|
| ||||
| Elevated systolic BPh | 1.508 (1.184–1.921) | 0.001 | 1.422 (0.908–2.228) | 0.124 |
|
| ||||
| Elevated diastolic BPi | 1.382 (1.011–1.890) | 0.042 | 1.516 (0.863–2.664) | 0.148 |
|
| ||||
| Alcohol consumptionj | 1.252 (0.963–1.627) | 0.093 | 0.699 (0.441–1.108) | 0.128 |
|
| ||||
| Current or former smokerk | 1.581 (1.244–2.009) | < 0.001 | 1.797 (1.172–2.753) | 0.007 |
|
| ||||
| Daily Coffee consumptionl | 1.356 (0.883–2.081) | 0.164 | ||
|
| ||||
| Regular exercisem | 1.046 (0.732–1.494) | 0.805 | 0.932 (0.635–1.368) | 0.720 |
Odds ratios were calculated by using logistic regression.
OR, odds ratio; CI, confidence interval; FBG, fasting blood glucose; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TG, triglyceride; CEA, carcinoembryonic antigen; BP, blood pressure.
Body mass index ≥25 kg/m2.
A waist circumference ≥ 90 cm for men and ≥ 80 cm for women.
Fasting blood glucose level of ≥ 100 mg/dL.
Total cholesterol ≥200 mg/dL.
HDL-C < 40 mg/dL for men and < 50 mg/dL for women.
Triglyceride ≥ 150 mg/dL.
CEA ≥ 3 ng/mL.
Systolic BP ≥ 130 mmHg.
Diastolic BP ≥ 85 mmHg.
One glass of liquor at least once a month.
Any amount of cigarette smoking history.
At least one cup of coffee a day.
At least once a fortnight.
DISCUSSION
In this study conducted on a large population of young adults without a first-degree family history of CRC, we found a relatively high prevalence of CRN at 19.5% in this young cohort of both diagnostic and screening settings. By examining as many factors as possible known as risk factors for CRN at a young age, it was verified again that previously reported factors including old age, male sex, obesity, and smoking were independent risk factors for CRN in young adults. Additionally, the ORs of those with obesity and smoking as two modifiable risk factors were more prominent for high-risk CRN.
Although some previous studies have reported the prevalence of CRN in young adults, so-called individuals younger than 50, only a few studies have investigated the prevalence by limiting the study to young adults under 40 years of age. In a meta-analysis of 24 studies having data of 20,933 individuals younger than 50, the pooled prevalence of CRN was 9.0% [7]. Limiting studies to those about young adults under 40 years of age, the prevalence of CRN varied from 8.6 to 12.6% [14–19]. Subdivided by age group, the prevalence of CRN was 1.3% to 9.4% in the age group of 20 to 29 years and 9.5% to 13.4% in the age group of 30 to 39 years. In our study, the prevalence of CRN was 14.9% in all participants, 9.8% in those under 30 years of age, and 15.4% in those aged 30 to 39 years. Our results were somewhat higher than those reported in previous studies. The possible explanation of this might be because participants in our study were recruited together in a university hospital and an affiliated health screening center whereas other studies recruited asymptomatic subjects who were undergoing colonoscopy as a screening procedure. In addition, our study was conducted on a population who underwent colonoscopy most recently. The more recent the study, the higher the CRN prevalence [7]. The reason for such observation is that the actual CRN prevalence is increasing due to various risk factors. In addition, the detection rate of CRN is increasing due to improvement in the quality of examinations such as the introduction of high definition colonoscopies [20–22].
Our results verified four risk factors (old age, male sex, obesity, and smoking) for CRN in young adults aged under 40. Although several studies have explored risk factors for CRN in young adults under 50 years of age [16,23–26], studies on those under 40 years of age are very rare [16,18,19]. Age is the most well-known risk factor for CRC. Even in young adults under 40 years of age, age still appears to be a strong risk factor for CRN. In two previous studies on young adults aged under 40, age was an independent risk factor for CRN [18,19], consistent with the result of our study. However, the results of previous studies on whether sex is a risk factor for CRN are inconsistent. While male sex was an independent risk factor for CRN in two previous studies conducted on adults aged 30 to 39 years [16,19], it was not in another study on adults aged 20 to 29 years [18].
Two modifiable factors, obesity and smoking, were also revealed as risk factors for CRN of young adults in previous studies [16,19]. Interestingly, the OR of these two risk factors were greater for high-risk CRN than for overall CRN in our study. Overweight and obesity are well-known risk factors for CRC. A recent study has reported that CRC mortality in young adults is rising in the United States, Canada, United Kingdom, and Australia, but not in Europe or Asia [27]. Such difference could be explained by the highest and fastest growing rates of overweight/obesity in countries with high mortality rates [27,28]. Similar to our results, a Korean study has demonstrated that abdominal obesity and metabolic syndrome as well as obesity are independent risk factors for CRN [19].
It is currently unclear which anthropometric measure among BMI, waist circumference, and waist-hip ratio is the most important predictor of the risk of CRN. Previous studies have reported that abdominal obesity or visceral fat has a more positive association with CRN risk [29–31]. However, our study revealed that abdominal obesity by waist circumference showed no significant association with CRN even in the univariate analysis. From late middle age till age of 80s, decease in the volume of subcutaneous fat and redistribution of fat from subcutaneous to visceral depots have been reported [32]. Such age-associated decrease in the size of adipose depots is accompanied by the accumulation of fat outside adipose tissues and loss of lean body mass [32]. Data from the Korea National Health Insurance Service have shown that the prevalence of abdominal obesity by waist circumference increases with age, peaking at the age of 70 years [33], although the prevalence of general obesity by BMI peaks in the later part of 30s in men [33]. This was thought to be the reason why general obesity was more associated with CRN than abdominal obesity in our study.
Smoking has been consistently identified as a risk factor in previous studies, with two studies reporting current smokers and one reporting current or former smokers as risk factors [16,18,19]. Consequently, it seems clear that obesity and smoking can contribute to the occurrence of CRN in young adults. Thus, it is important to try to prevent CRC by controlling these factors as early as possible.
This study has several limitations. First, our study had a cross-sectional study design, making it difficult to judge the causal relationship. However, accumulating experimental evidence is showing that social factors such as obesity and smoking can promote the occurrence of colon cancer [34–39]. Second, participants in our study were recruited from a single center. They might not represent the general population of young adults. However, we collected and analyzed a sufficiently large number of participants of 10,000 or more. Third, because it was impossible to investigate whether the examination performed during the study period was index colonoscopy in all study subjects, some of them might have a history of CRN resection. However, the overall adenoma detection rate of our study was 14.9%, which was not less than those in previous studies [14–19]. Because of their young age, it could be assumed that most subjects received their first colonoscopy. Lastly, rather than analyzing only screening endoscopy in an asymptomatic population, we included diagnostic endoscopy in symptomatic patients because some advanced CRN might be accompanied by symptoms such as hematochezia and referred for resection after being diagnosed at a primary institution.
In conclusion, the present study investigated the prevalence and risk factors of CRN in a large group of adults under 40 years of age. The prevalence of CRN was slightly higher than previously reported, reflecting the recent increase of young onset CRC. Two preventable causes of CRC, obesity and smoking, were confirmed as risk factors for CRN in young adults. Their risks were more pronounced for high-risk CRNs. To reduce the incidence of CRC in young adults, intensive efforts are needed to reduce these risk factors. Early screening with colonoscopy might be beneficial for young individuals with these risk factors.
KEY MESSAGE
1. Of 13,621 young individuals aged 18 to 40 years who underwent screening or diagnostic colonoscopy, the prevalence of colorectal neoplasia was 14.9%.
2. The independent risk factors for colorectal neoplasia in young adults were identified as old age, male sex, obesity, and smoking.
3. Two modifiable factors, obesity and smoking, increased the risk for high-risk colorectal neoplasia even more than for overall colorectal neoplasia.
Acknowledgments
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (NRF-2018M3A9E8021507) and by grant of the Institute of Clinical Medicine Research in the Yeouido St. Mary’s hospital, Catholic University of Korea.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109:djw322.. doi: 10.1093/jnci/djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung JJY, Chiu HM, Jung KW, et al. Increasing trend in young-onset colorectal cancer in Asia: more cancers in men and more rectal cancers. Am J Gastroenterol. 2019;114:322–329. doi: 10.14309/ajg.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 5.Vuik FE, Nieuwenburg SA, Bardou M, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68:1820–1826. doi: 10.1136/gutjnl-2018-317592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250–281. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 7.Enwerem N, Cho MY, Demb J, et al. Systematic review of prevalence, risk factors, and risk for metachronous advanced neoplasia in patients with young-onset colorectal adenoma. Clin Gastroenterol Hepatol. 2021;19:680–689. doi: 10.1016/j.cgh.2020.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 9.Kim BY, Kang SM, Kang JH, et al. 2020 Korean Society for the Study of Obesity guidelines for the management of obesity in Korea. J Obes Metab Syndr. 2021;30:81–92. doi: 10.7570/jomes21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen CP, David Cheng TY, Tsai SP, et al. Are Asians at greater mortality risks for being overweight than Caucasians?: redefining obesity for Asians. Public Health Nutr. 2009;12:497–506. doi: 10.1017/S1368980008002802. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney (AU): Health Communications Australia; 2000. Regional Office for the Western Pacific. [Google Scholar]
- 12.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Choe JW, Chang HS, Yang SK, et al. Screening colonoscopy in asymptomatic average-risk Koreans: analysis in relation to age and sex. J Gastroenterol Hepatol. 2007;22:1003–1008. doi: 10.1111/j.1440-1746.2006.04774.x. [DOI] [PubMed] [Google Scholar]
- 15.Park HW, Byeon JS, Yang SK, et al. Colorectal neoplasm in asymptomatic average-risk Koreans: the KASID Prospective Multicenter Colonoscopy Survey. Gut Liver. 2009;3:35–40. doi: 10.5009/gnl.2009.3.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung SJ, Kim YS, Yang SY, et al. Prevalence and risk of colorectal adenoma in asymptomatic Koreans aged 40–49 years undergoing screening colonoscopy. J Gastroenterol Hepatol. 2010;25:519–525. doi: 10.1111/j.1440-1746.2009.06147.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Baik SJ, Kim KH, Oh CR, Lee SI. Prevalence and risk factors of colorectal adenoma in 14,932 Koreans undergoing screening colonoscopy. Korean J Gastroenterol. 2013;62:104–110. doi: 10.4166/kjg.2013.62.2.104. [DOI] [PubMed] [Google Scholar]
- 18.Kwak JY, Kim KM, Yang HJ, et al. Prevalence of colorectal adenomas in asymptomatic young adults: a window to early intervention? Scand J Gastroenterol. 2016;51:731–738. doi: 10.3109/00365521.2015.1130163. [DOI] [PubMed] [Google Scholar]
- 19.Kim NH, Jung YS, Yang HJ, et al. Prevalence of and risk factors for colorectal neoplasia in asymptomatic young adults (20–39 years old) Clin Gastroenterol Hepatol. 2019;17:115–122. doi: 10.1016/j.cgh.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian V, Mannath J, Hawkey CJ, Ragunath K. High definition colonoscopy vs. standard video endoscopy for the detection of colonic polyps: a meta-analysis. Endoscopy. 2011;43:499–505. doi: 10.1055/s-0030-1256207. [DOI] [PubMed] [Google Scholar]
- 21.Adler A, Aminalai A, Aschenbeck J, et al. Latest generation, wide-angle, high-definition colonoscopes increase adenoma detection rate. Clin Gastroenterol Hepatol. 2012;10:155–159. doi: 10.1016/j.cgh.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Waldmann E, Britto-Arias M, Gessl I, et al. Endoscopists with low adenoma detection rates benefit from high-definition endoscopy. Surg Endosc. 2015;29:466–473. doi: 10.1007/s00464-014-3688-2. [DOI] [PubMed] [Google Scholar]
- 23.Gupta AK, Samadder J, Elliott E, Sethi S, Schoenfeld P. Prevalence of any size adenomas and advanced adenomas in 40- to 49-year-old individuals undergoing screening colonoscopy because of a family history of colorectal carcinoma in a first-degree relative. Gastrointest Endosc. 2011;74:110–118. doi: 10.1016/j.gie.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SE, Jo HB, Kwack WG, Jeong YJ, Yoon YJ, Kang HW. Characteristics of and risk factors for colorectal neoplasms in young adults in a screening population. World J Gastroenterol. 2016;22:2981–2992. doi: 10.3748/wjg.v22.i10.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong SN, Kim JH, Choe WH, et al. Prevalence and risk of colorectal neoplasms in asymptomatic, average-risk screenees 40 to 49 years of age. Gastrointest Endosc. 2010;72:480–489. doi: 10.1016/j.gie.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Koo JE, Kim KJ, Park HW, et al. Prevalence and risk factors of advanced colorectal neoplasms in asymptomatic Korean people between 40 and 49 years of age. J Gastroenterol Hepatol. 2017;32:98–105. doi: 10.1111/jgh.13454. [DOI] [PubMed] [Google Scholar]
- 27.Santucci C, Boffetta P, Levi F, La Vecchia C, Negri E, Malvezzi M. Colorectal cancer mortality in young adults is rising in the United States, Canada, United Kingdom, and Australia but not in Europe and Asia. Gastroenterology. 2021;160:1860–1862. doi: 10.1053/j.gastro.2020.12.070. [DOI] [PubMed] [Google Scholar]
- 28.GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Im JP, Kim D, Chung SJ, et al. Visceral obesity as a risk factor for colorectal adenoma occurrence in surveillance colonoscopy. Gastrointest Endosc. 2018;88:119–127. doi: 10.1016/j.gie.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 30.Nagata N, Sakamoto K, Arai T, et al. Visceral abdominal fat measured by computed tomography is associated with an increased risk of colorectal adenoma. Int J Cancer. 2014;135:2273–2281. doi: 10.1002/ijc.28872. [DOI] [PubMed] [Google Scholar]
- 31.Kang HW, Kim D, Kim HJ, et al. Visceral obesity and insulin resistance as risk factors for colorectal adenoma: a cross-sectional, case-control study. Am J Gastroenterol. 2010;105:178–187. doi: 10.1038/ajg.2009.541. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; Geneva. 8–11 December 2008; Geneva (CH): World Health Organization; 2011. [Google Scholar]
- 33.Seo MH, Kim YH, Han K, et al. Prevalence of obesity and incidence of obesity-related comorbidities in Koreans based on National Health Insurance Service health checkup data 2006–2015. J Obes Metab Syndr. 2018;27:46–52. [Google Scholar]
- 34.Tie G, Yan J, Khair L, et al. Hypercholesterolemia increases colorectal cancer incidence by reducing production of NKT and γδ T cells from hematopoietic stem cells. Cancer Res. 2017;77:2351–2362. doi: 10.1158/0008-5472.CAN-16-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsui S, Okabayashi K, Tsuruta M, et al. Interleukin-13 and its signaling pathway is associated with obesity-related colorectal tumorigenesis. Cancer Sci. 2019;110:2156–2165. doi: 10.1111/cas.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Franco S, Bianca P, Sardina DS, et al. Adipose stem cell niche reprograms the colorectal cancer stem cell metastatic machinery. Nat Commun. 2021;12:5006. doi: 10.1038/s41467-021-25333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye YN, Liu ES, Shin VY, Wu WK, Luo JC, Cho CH. Nicotine promoted colon cancer growth via epidermal growth factor receptor, c-Src, and 5-lipoxygenase-mediated signal pathway. J Pharmacol Exp Ther. 2004;308:66–72. doi: 10.1124/jpet.103.058321. [DOI] [PubMed] [Google Scholar]
- 38.Ye YN, Wu WK, Shin VY, Cho CH. A mechanistic study of colon cancer growth promoted by cigarette smoke extract. Eur J Pharmacol. 2005;519:52–57. doi: 10.1016/j.ejphar.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Wong HP, Li ZJ, Shin VY, et al. Effects of cigarette smoking and restraint stress on human colon tumor growth in mice. Digestion. 2009;80:209–214. doi: 10.1159/000231898. [DOI] [PubMed] [Google Scholar]