Abstract
Rationale
Alpha lipoic acid is known to reverse NMDA receptor hypofunction in addition to dopamine receptor blockade activity. It also enhances neurotrophic factors and has antioxidant potential. These properties combined together may be beneficial for treatment-resistant schizophrenia (TRS).
Objectives
This study evaluates the effect of alpha lipoic acid (ALA) on psychopathological scores (positive, negative, cognitive), neurotrophic factors and oxidative stress in TRS.
Methods
A pilot randomized double-blind placebo-controlled parallel design trial was conducted in 20 patients with TRS. After initial screening, participants were randomized into test (add-on ALA) and control (add-on placebo) groups. After recruitment, clinical evaluations with scale for assessment of positive symptoms and negative symptoms (SAPS and SANS), schizophrenia cognitive rating scale (SCoRS), UKU side effect rating scale were done. Serum levels of BDNF, MDA, and GSH were estimated. Patients were followed up for 8 weeks, and clinical and biochemical evaluations were repeated. Adherence to medication was evaluated at follow-up.
Results
A significantly greater improvement was found in SANS score in the test group when compared to control (Mann–Whitney U = 17.0; p = 0.021), whereas there was no significant improvement in SAPS score (Mann–Whitney U = 41.5; p = 0.780). A significant increase in BDNF levels was observed in the control group when compared to ALA (U = 20.0; p = 0.041). No significant differences were found between the test and control groups in serum MDA (U = 30.0; p = 0.221), serum GSH (U = 40.0; p = 0.683), and medication adherence rating scale (MARS) scores (U = 44.0; p = 0.934).
Conclusions
ALA supplementation improved psychopathology and decreased oxidative stress in patients with TRS. This study thus shows the potential of adjunctive ALA in TRS.
Trial registration
The study was prospectively registered in Clinical Trial Registry of India (CTRI/2020/03/023707 dated 02.03.2020).
Keywords: Schizophrenia, Treatment-resistant; Alpha lipoic acid; Psychopathology; Oxidative stress markers
Introduction
The prevalence of schizophrenia is 3.3 per 1000 population globally with approximately 21 million people affected by this mental disorder (Bhugra 2005). Antipsychotics are the mainstay of treatment of schizophrenia, with second-generation antipsychotics being the first choice. Treatment-resistant schizophrenia (TRS) has been defined as patients who show symptoms despite at least two antipsychotic trials of adequate dose and duration and represents approximately 30% of cases of people diagnosed with schizophrenia (Correll et al. 2019; NICE 2014). Clozapine is the only FDA-approved agent indicated for TRS and only 60% of patients with TRS respond to clozapine (Bruton et al. 2018; Pickar et al. 1992).
The neurobiology of TRS is complex and not fully understood till date. Understanding the neurobiology of the TRS is the need of the hour for optimizing the therapeutic regimen in such patients. A number of hypotheses have been put forth for explaining the symptomatology and nonresponse in patients, such as super sensitivity of dopamine receptors, NMDA receptor hypofunction, inflammation, and dysregulated oxidative stress. Most of the time, these dysfunctional pathways converge to express refractoriness to antipsychotics (Potkin et al. 2020). The role of oxidative stress has been widely implicated in treatment-resistant cases compared to responders (Bitanihirwe and Woo 2011).
Alpha lipoic acid (ALA) is a naturally occurring antioxidant that plays an important role in the functioning of enzymes involved in mitochondrial oxidative metabolism (Salehi et al. 2019). ALA and its reduced form are considered natural free radical scavengers and thus act as antioxidant and anti-inflammatory agents (Goraca et al. 2011; Shay et al. 2009). ALA has also been reported to cause upregulation of NMDA receptors in addition to blockade of dopamine receptors (Molz and Schroder 2017). Although ALA was historically used as a therapeutic option in the 1950s for psychiatric conditions, researchers could not find any mechanistic explanation for its effects (Altschule et al. 1959). Some investigators generated developmental interneurons from induced pluripotent cells derived from patients with schizophrenia and co-cultured them with activated microglia leading to mitochondrial dysfunction. ALA has been shown to reverse these dysfunctions (Ni et al. 2020; Park et al. 2020). Other researchers have demonstrated that ALA potentiated the effect of antipsychotics in animal models of schizophrenia (Deslauriers et al. 2014; Sampaio et al. 2017, 2018; Vasconcelos et al. 2015).
The number of clinical studies exploring the effect of ALA on clinical symptoms in schizophrenia are limited. Moreover, even for studies conducted so far, there are variations in the dose and duration of treatment. Sanders et al. studied the effect of ALA in a dose of 100 mg in patients with schizophrenia and found a remarkable response in psychopathology, neurocognition, and alleviation of extrapyramidal symptoms (Sanders et al. 2017). Moreover, ALA supplementation has been shown to decrease lipid peroxidation in healthy controls but not in patients with schizophrenia (Vidovic et al. 2014). In a study by Emsley et al., omega-3 fatty acid and ALA were not found to be a suitable alternative for treatment of schizophrenia after antipsychotic discontinuation (Emsley et al. 2014). Thus, conflicting results have been observed regarding the efficacy of ALA in different trial settings to date.
Schizophrenia has been one of the few medical conditions that has been investigated with such vigor over the past century and still remains an unsolved puzzle for clinicians and researchers, with evolving theories that can neither be outwardly rejected nor unequivocally approved. Resistance to treatment, which has been defined differently in different guidelines, and response only to 60% even to clozapine therapy, makes the picture grimmer. Despite undisputable superiority over other antipsychotics, 40–70% of patients with TRS on clozapine continue to have incomplete remission [< 20–30% reduction in BPRS (Baseline Brief Psychiatric Rating Scale) or PANSS score (Positive and Negative Syndrome Scale)] (Porcelli et al. 2012). Monitoring of patients for hematological adverse events like agranulocytosis becomes mandatory with clozapine therapy. It has higher affinity for M1, H1, and α1-adrenergic receptors which leads to sedation, weight gain, and anticholinergic adverse effects. Metabolic adverse effects and sialorrhea are other adverse events associated with clozapine. A previous meta-analysis reported an improvement in schizophrenia symptoms and side effects of the antipsychotic drug with ALA (de Sousa et al. 2019). Additionally, ALA has also been shown to improve adiponectin levels, prevent weight gain or weight loss, and reverse metabolic risk factors in patients with schizophrenia (Kim et al. 2008, 2016; Ratliff et al. 2015; Vidovic et al. 2017). However, these studies did not show improvement in psychotic symptoms.
Reviewing the available literature and the mechanistic role of ALA, it appears to give some hope to patients with TRS. Therefore, this study was planned to generate some preliminary evidence for the effect of add-on ALA on psychopathology, neurotrophic, and oxidative markers in patients with TRS.
Material and methods
Study design
The present study was a randomized, double-blind, placebo-controlled, parallel design pilot trial which was conducted in a single tertiary care centre at All India Institute of Medical Sciences (AIIMS), New Delhi, India. Written approval from Institute ethics committee (IECPG-674/19.12.2019, RT-04/30.01.2020) was obtained before commencement of the study. The study was carried out according to the ICMR’s ethical guidelines for biomedical research on human subjects (2017) and was prospectively registered in Clinical Trial Registry of India (CTRI/2020/03/023707 dated 02.03.2020). Written informed consent was obtained from participants before enrolment. In patients not able to provide consent due to the nature of their illness, informed consent was sought from the primary care giver of the patient and assent was taken from the patient.
Study population and eligibility
Patients aged 18–65 years, of either sex, attending the outpatient psychiatry department of AIIMS, New Delhi, India with schizophrenia diagnosis according to Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM 5) verified according to MINI version 7.0.2 by the clinician and who were resistant to treatment according to modified Kane’s criteria were screened for the study (Kane et al. 2019). Patients with stable dose of antipsychotics for 4 weeks were enrolled. Patients with other major psychiatric co-morbidities that could confound the evaluation of biomarkers, including schizoaffective, mood disorders, and substance use dependence; severe neurological illnesses; significant intellectual disability and sensory impairment; and severe medical illnesses that prevent participation in the study were excluded. Pregnant and lactating women and those patients who were already on ALA or had received the same in past or who were not willing to give informed consent, were also excluded.
Randomization, blinding and allocation concealment
Randomization was done using computer generated random block randomization codes in an allocation ratio of 1:1 into add-on drug and add-on placebo groups by a third person, not involved in this study. Control group received placebo matched to ALA to rule out the psychobiogical effect due to administration of an add-on intervention. Allocation concealment was achieved by labeling similar-looking opaque white containers of drug and placebo with consecutive numbers.
Study intervention(s)
Drug group received ALA 300 mg capsules and control group received placebo (matched for color, shape, and odor) for 8 weeks in addition to antipsychotics prescribed by the treating psychiatrist. Participants were advised to take the study intervention(s) at bedtime to ensure uniform intake. We used a dose of 300 mg as ALA is known to affect pathophysiological systems in a dose-dependent manner and a dose of 300 mg is reported to be well tolerated (Mahmoudinezhad and Farhangi 2021). From the literature, a dose of 300 mg of ALA is known to reverse dysfunctional oxidative states as well as depressive symptoms in patients with various health conditions (Mandani et al. 2021; Rezaei Kelishadi et al. 2021).
Study procedure and data collection
Patients with diagnosis of TRS were screened and recruited following inclusion and exclusion criteria. After enrolment, detailed history was taken and clinical evaluations were done. Psychopathological evaluation for positive, negative, and cognitive symptoms was performed in patients by investigators using the scale for the assessment of positive symptoms (SAPS), the scale for the assessment of negative symptoms (SANS), and the schizophrenia cognition rating scale (SCoRS), respectively. Blood samples were collected for the evaluation of serum concentrations of brain-derived neurotrophic factor (BDNF), malondialdehyde (MDA), and reduced glutathione (GSH). Udvalg for Kliniske Undersøgelse (UKU) side effects rating scale for the registration of unwanted effects of psychotropics was used for evaluation of adverse effects. All patients were followed up and all the clinical and biochemical assessments were repeated at the end of 8 weeks. Compliance with treatment was assessed with the help of the medication adherence rating scale at the end of 8 weeks.
Outcome measures
The effect of ALA on patients with TRS was assessed by SAPS and SANS score for evaluation of change in psychopathology of the disease as primary outcome measure. Apart from this, SCoRS for cognitive improvement and UKU side effect rating scale for registration of unwanted effects of psychotropics was used for evaluation of adverse effects as secondary outcome. In addition to clinical outcome measures, serum BDNF, MDA, GSH were estimated and medication adherence rating scale was administered.
Efficacy assessment
Clinical outcome measures
The evaluation of psychopathology as assessed by the change in positive and negative symptoms in patients was the primary outcome measure of the study. SAPS and SANS were used to measure the severity of symptoms (Andreasen et al. 1991). A composite score for SAPS (0–170) and SANS (0–125) was calculated separately along with domain-specific scores. A schizophrenia cognition rating scale was used to assess cognition deficits and their impact on the normal functioning of patients (Keefe et al. 2006). Medication adherence rating scale (MARS) was used to capture “medication adherence behavior” (items 1–4), “attitude toward taking medication” (items 5–8), and “negative side effects and attitudes towards psychotropic medications” (items 9, 10) (Thompson et al. 2000).
Biochemical outcome measures
Serum BDNF, malondialdehyde (MDA), and reduced glutathione (GSH) levels estimation: 5 ml of venous blood was collected and allowed to clot by leaving it undisturbed at room temperature. The clotted blood was then centrifuged, serum was separated, collected in microcentrifuge tubes, and stored in a − 20 °C refrigerator. Serum BDNF was estimated by ELISA using commercially available human BDNF ELISA kits from (Cloude-clone Corp., USA) based on standard sandwich enzyme-linked immunosorbent assay technology. Estimations for serum MDA and GSH were made using spectrophotometer using commercially available kit (Cayman Chemical, USA). All standards and samples were estimated in duplicate. The average of the two values was taken for further analysis.
Safety assessment
Patients had free access to investigators for reporting of any adverse events anytime during the study. Adverse events were studied with the help of UKU side effect rating scale at baseline and after 8 weeks of study (Lingjaerde et al. 1987).
Statistical analysis
Continuous variables were represented as median (range) and categorical variables as percentage. Comparison of medians of continuous variables (non-parametric) was done using a two-sided Mann–Whitney U test for in between group comparisons and Wilcoxon signed rank test in case of within group comparisons. The Chi-square test/Fisher’s exact test was used for categorical variables. Statistical analyses were performed using statistical software SPSS 23.0 (IBM, NY USA) considering a significance level of p < 0.05. A receiver operating characteristics (ROC) analysis was performed to obtain cut-off value of serum MDA levels at baseline to differentiate between responders and non-responders as determined by changes in psychopathology. The R software package “pROC” was used for ROC analysis.
Sample size
Due to time-bound nature of this study and containment strategies imposed due to COVID-19, this study was conducted in only twenty patients with treatment-resistant schizophrenia for obtaining a preliminary idea about the effect of alpha lipoic acid in patients with treatment-resistant schizophrenia.
Results
Patients
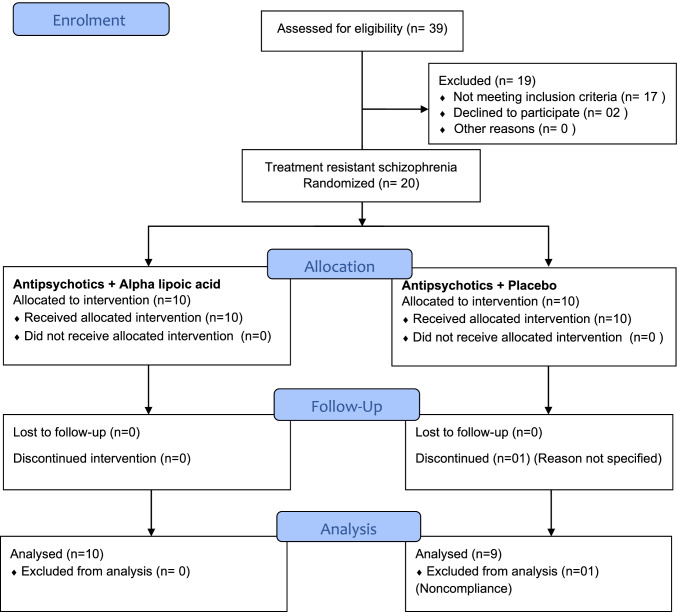
The screening of participants was started in March 2020 but first participant was recruited in October 2020. The follow-up of the participants ended in November 2021. Of the 39 patients selected, 17 patients did not meet the inclusion criteria and 02 (two) participants declined to participate. A total of 20 participants were recruited for the study. All participants met modified Kane’s criteria. After recruitment, the participants were randomized to add-on alpha lipoic acid group and add-on placebo group. Each group had 10 participants.
One participant discontinued treatment and follow-up data was not taken for noncompliance. The CONSORT flow chart for the study is given in Fig. 1. No statistically significant difference was found between the groups at baseline. Therefore, at recruitment, the characteristics of the patients were homogeneous. The mean age of participants in the study was 36 years and male:female ratio was 60:40. The baseline characteristics of the participants across the groups is depicted in Table 1.
Fig. 1.
CONSORT flow diagram
Table 1.
Baseline characteristics
| Parameter | Placebo group | Alpha lipoic acid group | P value |
|---|---|---|---|
| Number recruited | 10 | 10 | |
| Age (years) | 34.60 ± 7.94 | 37.40 ± 12.81 | 0.564$ |
| Sex (male: female) | 4:6 | 8:2 | 0.170a |
|
Marital status (Never married: married: separated: divorced) |
8:1:0:1 | 8:1:1:0 | 0.428 a |
|
Education (Matric: intermediate: graduate: post-graduate: professional) |
1:1:7: 0:1 | 1:2:5: 2:0 | 0.657 a |
|
Occupation (professional: unemployed: housewife: retired: student: business: not known) |
0:7:1: 0:2:0:0 | 1:5:0: 1:1:1:1 | 0.342 a |
|
Monthly income (in Rupees) (< 5000: 5000–10,000: 10,000–15,000: 15,000–20,000: > 20,000) |
10:0:0:0 | 7:0:0:03 | 0.211 a |
|
Employment (employed: unemployed) |
2:8 | 1:9 | 0.528 a |
| Age of illness onset (years) | 21.50 ± 8.37 | 21.20 ± 3.26 | 0.917$ |
| Total duration of illness (years) | 13.40 ± 6.85 | 15.80 ± 8.11 | 0.484$ |
| SAPS | 60.5 (9 to 79) | 61.50 (37 to 89) | 0.850# |
| SANS | 63.5 (40 to 91) | 60.5 (32 to 67) | 0.405# |
| SCoRS_Patient | 39 (29 to 62) | 41 (30 to 61) | 0.971# |
| SCoRS_Informant | 42 (33 to 64) | 43 (33 to 64) | 0.796# |
| SCoRS_Interviewer | 42 (33 to 64) | 42.5 (29 to 64) | 0.853# |
| SCoRS_Global Assessment | 5.5 (4 to 8) | 6 (4 to 8) | 0.853# |
| Serum BDNF (ng/mL) | 5.06 (2.30 to 7.29) | 5.99 (2.48 to 8.41) | 0.406# |
| Serum MDA (μΜ/L) | 4.45 (2.31 to 7.56) | 5.72 (1.97 to 7.24) | 0.257# |
| Serum GSH (μΜ/L) | 34.76 (31.19 to 47.04) | 32.49 (10.81 to 56.66) | 0.597# |
BDNF, brain-derived neurotrophic factor; MDA, malondialdehyde; GSH, reduced glutathione; SAPS, scale for the assessment of positive symptoms; SANS, scale for the assessment of negative symptoms; SCoRS, schizophrenia cognition rating scale; UKU, Udvalg for Kliniske Undersøgelse
$Independent sample t-test (data in mean ± SD); #Mann–Whitney U test (data in median and range); aFischer exact test
Outcome measures
Change in psychopathology scores
The median total SAPS scores decreased from 60.5 to 56.0 in the placebo group and from 61.50 to 56.0 in the ALA group. Wilcoxon signed rank test was carried out and there was a statistically significant decrease in scores in both groups. The change in scores in placebo over ALA group was not statistically significant (p = 0.780) (Table 2). The median total SANS scores significantly decreased from 63.5 to 57.0 in add-on placebo group (p = 0.007). The scores decreased to 48.0 from a baseline value of 60.50 in the ALA group, which was statistically significant (p = 0.005). The median change of the ALA group over the placebo group was found to be statistically significant (U = 17.0; z = − 2.307; p = 0.021) (Table 2).
Table 2.
Change in outcome measures
| Variable | Placebo group (n = 9) |
ALA group (n = 10) |
Difference between groups Δ placebo vs. Δ ALA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Mean diff Δ (95%CI)/ z statistic |
P value$ | Baseline | Follow-up | Mean diff Δ (95%CI)/ z statistic |
P value$ | Mean diff Δ (95%CI)/ Mann–Whitney U | P value# | |
|
SAPS (Total) |
60.5 (9 to 79) |
56 (9 to 74) |
− 2.53 | 0.011 |
61.5 (37 to 89) |
56 (33 to 79) |
− 2.83 | 0.005 | 41.5 | 0.780 |
|
SANS (Total) |
63.5 (40 to 91) |
57.0 (33 to 79) |
− 2.68 | 0.007 |
60.5 (32 to 67) |
48 (25 to 57) |
− 2.81 | 0.005 | 17.0 | 0.021 |
| Serum BDNF (ng/mL) | 5.06 (2.30 to 7.29) | 5.50 (2.39 to 7.52) | − 0.415 | 0.678 |
5.99 (2.48 to 8.41) |
6.0 (2.94 to 8.77) |
− 1.784 | 0.074 | 20.0 | 0.041 |
|
Serum MDA (μM/L) |
4.45 (2.3 to 7.52) | 4.58 (3.3 to 7.15) | − 1.244 | 0.214 | 5.72 (1.97 to 7.24) | 5.65 (1.71 to 7.26) | − 2.090 | 0.037 | 30.0 | 0.221 |
|
Serum GSH (μM/L) |
34.76 (28.75 to 55.82) |
38.43 (27.99 to 57.06) |
− 1.007 | 0.314 |
32.49 (10.81 to 56.66) |
35.59 (16.07 to 58.93) |
− 1.070 | 0.285 | 40.0 | 0.683 |
| MARS | - |
6 (1 to 8) |
- | - | - |
5.5 (3 to 8) |
- | - | 44.0 | 0.934 |
BDNF, brain-derived neurotrophic factor; MARS, medication adherence rating scale; MDA, malondialdehyde; GSH, reduced glutathione; SAPS, scale for the assessment of positive symptoms; SANS, scale for the assessment of negative symptoms; SCoRS, schizophrenia cognition rating scale; UKU, Udvalg for Kliniske Undersøgelse
Data represented in median (range): $Wilcoxon signed rank test for within group comparisons; #Mann–Whitney U test for comparison of change from baseline between groups
Change in cognitive functions
There was no significant difference between placebo and the ALA groups in scores of cognitive functions. The correlation between self-reported scores by patient, informant, and interviewer was strong. The scores between patient and informant as well as patient and interviewer are correlated significantly (Tau = 0.793; p < 0.001). Scores between informant and interviewer show a near perfect correlation (Tau = 0.995; p < 0.001). There was no significant difference between placebo and ALA as determined by the global rating given by the interviewer. There was no change in cognitive function at follow-up.
Change in serum BDNF levels
The median BDNF levels increased from 5.06 to 5.50 ng/mL in add-on placebo group and 5.99 to 6.0 ng/mL in add-on ALA group, which were not significant statistically. The change in score in placebo group over ALA group was statistically significant (p = 0.041) (Table 2).
Change in oxidative stress markers
The median serum MDA increased from 4.45 to 4.58 μM /L in add-on placebo group, which was not found to be statistically significant (p = 0.214). The scores decreased to 5.65 μM /L from a baseline value of 5.72 μM/L in the ALA group which was statistically significant (p = 0.037). The change in ALA group over placebo group was not found to be statistically significant (U = 30.0; p = 0.021) (Table 2). The median serum GSH increased from 34.76 to 38.43 μM/L in add-on placebo group which was not found to be statistically significant (p = 0.314). The scores increased to 35.59 μM/L from a baseline value of 32.49 μM/L in ALA group which not was statistically significant (p = 0.285). The change in ALA group over placebo group was not found to be statistically significant U = 40.0; p = 0.683) (Table 2).
Change in UKU side effect rating scale
All participants included in the study suffered at least one adverse event in the psychic domain at baseline. Fifty percent of the participants in the ALA group and 60% in the placebo group suffered from at least one adverse event in the neurologic domain. Nine out of 10 in the ALA group and eight out of 10 in the placebo group suffered from at least one adverse event in the autonomic domain. For other adverse events, 7 participants out of 10 in each group experienced at least one out of those listed in the scale. The most common adverse effects experienced by patients were sleep disturbances, rigidity, gastrointestinal disturbances (constipation), weight gain, and headache. The severity of adverse events was mild in most patients and moderate in some. No action or frequent monitoring was required for the participants. No stopping the drug or dose changes was advised to any of the participants. There was no change in the occurrence of adverse event at follow-up visit.
Medication adherence rating scale
The median adherence score to the medication in the placebo group was not significantly different from that of the ALA group (p = 0.934). The scores were classified into low adherence and high adherence, based on scores of < 7 and ≥ 8, respectively. There was no statistically significant difference in the degree of treatment adherence between the two study groups on Fischer’s exact test.
Response rate
Response is defined at ≥ 20% decrease in either SAPS or SANS scores for a participant. Five out of 10 participants achieved response in ALA group when evaluated on SANS scale, whereas none of the participants in placebo group achieved response. A 2 × 2 contingency table was made and a Fischer exact test was performed. There was a significant statistical difference between the groups in terms of responders to the study medication (Fischer exact value = 6.107; p = 0.033).
ROC analysis
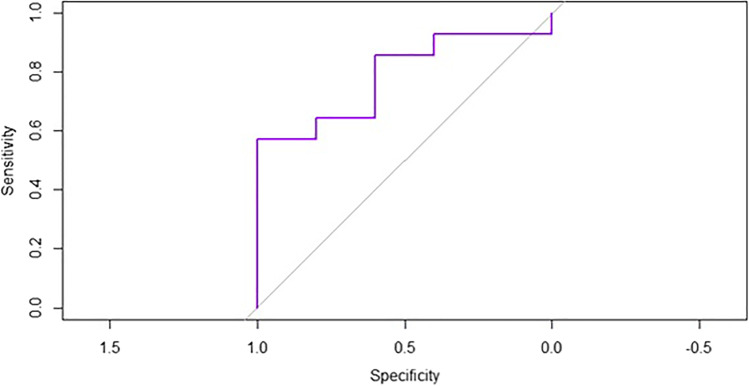
ROC curve analysis was performed to illustrate the diagnostic ability for responders and non-responders as the discrimination threshold of baseline serum MDA levels was varied. The area under the curve was 0.786 for a threshold serum MDA level of 5.159 μΜ/L to differentiate responders from non-responders. This threshold value has a sensitivity of 100% and specificity of 54.17% (Fig. 2).
Fig. 2.
ROC analysis. The area under the curve is 0.786 for a threshold serum MDA level of 5.159 μΜ/L to differentiate responders from non-responders. This threshold value has a sensitivity of 100% and specificity of 54.17%
Discussion
Patients with TRS have an early age of onset, altered levels of oxidative stress markers, and differential expression of serotonergic and dopaminergic pathways and receptors, which are different from treatment-responsive patients. The present study reported an improvement in negative symptoms in TRS patients with add-on ALA. Given the response criteria of > 20% improvement, a significantly higher number of participants achieved response with ALA when compared to placebo. There was an increasing trend in serum BDNF but add-on placebo had better effect when compared with ALA, though change in neither of the groups was significant individually. The addition of ALA significantly decreased lipid peroxidation as measured by serum MDA, but the increase in reduced glutathione was not significant. The improvement in psychopathology from baseline was significant for both groups due to the antipsychotics administered to all study participants. This effect was further enhanced by the addition of ALA in the test group for negative symptoms.
Iasevoli et al. (Iasevoli et al. 2021) studied relationship between age at onset and treatment refractoriness and concluded that treatment-resistant status can be strongly linked to early age of onset of psychotic symptoms. This finding of an earlier age of onset is consistent with the findings of our study. ALA has historically been used for psychiatric therapy before antipsychotics became popular (Altschule et al. 1959; Giamattei 1957). Our study has shown results similar to studies conducted seven decades earlier, wherein almost half of the patients with ALA showed improvement at lower doses. However, further studies with higher doses of ALA did have a beneficial effect on metabolic factors but did not affect psychopathology of patients. ALA combined with omega-3-fatty acids did not prevent relapse nor decreased severity of symptoms in schizophrenics, but it is noteworthy here that investigators followed the antipsychotic discontinuation design (Emsley et al. 2014). Antipsychotic discontinuation has already been reported to precipitate dysfunctional dopamine autoregulation or dopaminergic super sensitivity, and therefore psychotic relapse (Kim et al. 2021; Samaha et al. 2007). Thus, ALA cannot be used as monotherapy for maintenance in schizophrenia patients. Similar was the case with other studies where 1200 mg or more of ALA did not show any improvement in symptoms of schizophrenia, though it neutralized the effect of atypical antipsychotic-induced weight gain in other studies (Kim et al. 2016; Ratliff et al. 2015). A possible explanation of this effect may be that at higher doses of ALA, thiamine stores are depleted and thiamine deficiency is aggravated, resulting in further worsening of psychosis. But, recently, in a pilot study by Sanders et al. (Sanders et al. 2017) add-on ALA 100 mg has been shown to possess beneficial effect on psychopathology, oxidative stress parameters, and ameliorated adverse events.
Our study findings follow similar trend, though study population in the above study was not restricted to treatment-resistant cases as in our study. ALA has been observed to have more than one mechanism to alleviate the symptoms of schizophrenia. Like other antipsychotics, ALA has dopamine receptor blockade activity. Additionally, it also helps in reversing the NMDA receptor hypofunction (Stoll et al. 1993; Tang and Aizenman 1993). ALA is an antioxidant, inhibits formation or scavenges free radicals and lipid peroxidation products and enhances neurotrophic factors (Packer et al. 1995; Rezaei Zonooz et al. 2021). Oxidative stress and NMDA receptor hypofunction is known to give rise to negative symptoms and cognitive impairment predominantly and positive symptoms to a certain extent in TRS. Thus, ALA has been shown to potentiate the effect of antipsychotics in patients with TRS by modulating dysfunctional pathobiological targets. Mechanistically, ALA exerts an additive effect on antipsychotic drugs by activation of Akt/GSK-3β pathway of dopamine receptor activation through β-arrestin (Deslauriers et al. 2013). It further activates the mTOR pathway which potentiates neurorestorative functions. ALA is well known to decrease lipid peroxidation and reverse neurological and metabolic adverse effects of antipsychotic drugs (Thaakur and Himabindhu 2009).
The limitations of this study were inability to recruit large numbers of participants due to the pandemic-induced restrictions and the time-bound nature of this investigation. The duration of follow-up in the study was short and the evaluation of changes in cognitive symptoms and adverse effects reported in the literature could not be confirmed in this study. As the study was conducted in a tertiary care centre, there is a high probability of referral bias in the study and the study results may not be generalizable. Meanwhile, subjects were allowed to take concomitant antipsychotics if the dose has been stabilized for 4 weeks prior to the enrolment. It may be assumed that the types and dosage of these concomitant antipsychotics may have clinically significant impact on the outcome measurements for more than 4 weeks after their dose modification. But the study being randomized and evaluation against a placebo-controlled group could have balanced this effect.
The addition of ALA showed a trend of improvement in psychopathology, neurotrophic factors, and oxidative stress parameters.
Conclusion
In conclusion, ALA showed improvement on the psychopathology and decreased lipid peroxidation in patients with TRS. A multicentric, randomized trial with a larger sample size and longer duration is advocated for the evaluation of the true intervention effect of adjunctive ALA in TRS.
Acknowledgements
The authors gratefully acknowledge the financial assistance for the DM thesis from Indian Council of Medical Research (ICMR).
Author contribution
All authors made a substantial contribution to the conception of the study. A.M., S.C.S and M.S. contributed to the acquisition of data. A.M., K.R. and R.M. contributed to data analysis and interpretation. A.M., K.R. and S.C.S. drafted the manuscript. R.M. and M.S. critically revised the manuscript for important intellectual content. All authors approved the final manuscript.
Funding
The first author has received a DM thesis grant from Indian Council of Medical Research (ICMR) vide letter no. 3/2/Dec-2019/PG-Thesis-HRD (12). ICMR had no role in conduct of the research and/or preparation/submission of the article.
Data availability
The trial data are available on reasonable request from the first or corresponding author.
Declarations
Ethics approval and consent to participate
The study was carried out according to good clinical practice and the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from participants before enrolment. In patients not able to provide consent due to the nature of their illness, informed consent was sought from the primary care giver of the patient and assent was taken from the patient.
Conflict of interest
The authors declare no competing interests.
Footnotes
Chemical compound studied in this article
Alpha lipoic acid (PubChem CID 864)
The original version of this article was revised: This article was originally published with an added affiliation linking for the author Rituparna Maiti.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/14/2022
A Correction to this paper has been published: 10.1007/s00213-022-06237-y
Contributor Information
Archana Mishra, Email: archanapmv@gmail.com.
K. H. Reeta, Email: reetakh@gmail.com
Sudhir Chandra Sarangi, Email: scsarangi@gmail.com.
Rituparna Maiti, Email: pharm_rituparna@aiimsbhubaneswar.edu.in.
Mamta Sood, Email: soodmamta@gmail.com.
References
- Altschule MD, Goncz RM, Holliday PD. Carbohydrate metabolism in brain disease. XI. Effects of thioctic (alpha-lipoic) acid in chronic schizophrenia. AMA Arch Intern Med. 1959;103:726–729. doi: 10.1001/archinte.1959.00270050048008. [DOI] [PubMed] [Google Scholar]
- Andreasen NCFM, Arndt S, Alliger R, Swayze VW. Positive and negative symptoms: assessment and validity. Berlin, Heidelberg: Springer; 1991. [Google Scholar]
- Bhugra D. The global prevalence of schizophrenia. PLoS Med. 2005;2:e151. doi: 10.1371/journal.pmed.0020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Woo TU. Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev. 2011;35:878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton LL, Hilal-Dandan R, Knollman BC. Goodman & Gilman’s the pharmacological basis of therapeutics. 13. New York: McGraw-Hill; 2018. pp. 279–303. [Google Scholar]
- Correll CU, Brevig T, Brain C. Patient characteristics, burden and pharmacotherapy of treatment-resistant schizophrenia: results from a survey of 204 US psychiatrists. BMC Psychiatry. 2019;19:362. doi: 10.1186/s12888-019-2318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa CNS, da Silva Leite CMG, da Silva MI, Vasconcelos LC, Cabral LM, Patrocinio CFV, Patrocinio MLV, Mouaffak F, Kebir O, Macedo D, Patrocinio MCA, Vasconcelos SMM. Alpha-lipoic acid in the treatment of psychiatric and neurological disorders: a systematic review. Metab Brain Dis. 2019;34:39–52. doi: 10.1007/s11011-018-0344-x. [DOI] [PubMed] [Google Scholar]
- Deslauriers J, Desmarais C, Sarret P, Grignon S. Alpha-lipoic acid interaction with dopamine D2 receptor-dependent activation of the Akt/GSK-3beta signaling pathway induced by antipsychotics: potential relevance for the treatment of schizophrenia. J Mol Neurosci. 2013;50:134–145. doi: 10.1007/s12031-012-9884-4. [DOI] [PubMed] [Google Scholar]
- Deslauriers J, Racine W, Sarret P, Grignon S. Preventive effect of alpha-lipoic acid on prepulse inhibition deficits in a juvenile two-hit model of schizophrenia. Neuroscience. 2014;272:261–270. doi: 10.1016/j.neuroscience.2014.04.061. [DOI] [PubMed] [Google Scholar]
- Emsley R, Chiliza B, Asmal L, du Plessis S, Phahladira L, van Niekerk E, van Rensburg SJ, Harvey BH. A randomized, controlled trial of omega-3 fatty acids plus an antioxidant for relapse prevention after antipsychotic discontinuation in first-episode schizophrenia. Schizophr Res. 2014;158:230–235. doi: 10.1016/j.schres.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Giamattei L. Thioctic acid in therapy of schizophrenia. Osp Psichiatr. 1957;25:221–228. [PubMed] [Google Scholar]
- Goraca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid - biological activity and therapeutic potential. Pharmacol Rep. 2011;63:849–858. doi: 10.1016/S1734-1140(11)70600-4. [DOI] [PubMed] [Google Scholar]
- Iasevoli F, Razzino E, Altavilla B, Avagliano C, Barone A, Ciccarelli M, D'Ambrosio L, Matrone M, Milandri F, Notar Francesco D, Fornaro M, de Bartolomeis A (2022) Relationships between early age at onset of psychotic symptoms and treatment resistant schizophrenia. Early Interv Psychiatry 16(4):352–362 [DOI] [PMC free article] [PubMed]
- Kane JM, Agid O, Baldwin ML, Howes O, Lindenmayer JP, Marder S, et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry. 2019;80(2):18com12123. doi: 10.4088/JCP.18com12123. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Poe M, Walker TM, Kang JW, Harvey PD. The schizophrenia cognition rating scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am J Psychiatry. 2006;163:426–432. doi: 10.1176/appi.ajp.163.3.426. [DOI] [PubMed] [Google Scholar]
- Kim E, Park DW, Choi SH, Kim JJ, Cho HS. A preliminary investigation of alpha-lipoic acid treatment of antipsychotic drug-induced weight gain in patients with schizophrenia. J Clin Psychopharmacol. 2008;28:138–146. doi: 10.1097/JCP.0b013e31816777f7. [DOI] [PubMed] [Google Scholar]
- Kim NW, Song YM, Kim E, Cho HS, Cheon KA, Kim SJ, Park JY. Adjunctive alpha-lipoic acid reduces weight gain compared with placebo at 12 weeks in schizophrenic patients treated with atypical antipsychotics: a double-blind randomized placebo-controlled study. Int Clin Psychopharmacol. 2016;31:265–274. doi: 10.1097/YIC.0000000000000132. [DOI] [PubMed] [Google Scholar]
- Kim S, Shin SH, Santangelo B, Veronese M, Kang SK, Lee JS, Cheon GJ, Lee W, Kwon JS, Howes OD, Kim E. Dopamine dysregulation in psychotic relapse after antipsychotic discontinuation: an [(18)F]DOPA and [(11)C]raclopride PET study in first-episode psychosis. Mol Psychiatry. 2021;26:3476–3488. doi: 10.1038/s41380-020-00879-0. [DOI] [PubMed] [Google Scholar]
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- Mahmoudinezhad M, Farhangi MA (2021) Alpha lipoic acid supplementation affects serum lipids in a dose and duration-dependent manner in different health status. Int J Vitam Nutr Res [DOI] [PubMed]
- Mandani M, Badehnoosh B, Jalali-Mashayekhi F, Tavakoli-Far B, Khosrowbeygi A. Alpha-lipoic acid supplementation effects on serum values of some oxidative stress biomarkers in women with gestational diabetes. Gynecol Endocrinol. 2021;37:1111–1115. doi: 10.1080/09513590.2021.1963955. [DOI] [PubMed] [Google Scholar]
- Molz P, Schroder N. Potential therapeutic effects of lipoic acid on memory deficits related to aging and neurodegeneration. Front Pharmacol. 2017;8:849. doi: 10.3389/fphar.2017.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni P, Noh H, Park GH, Shao Z, Guan Y, Park JM, Yu S, Park JS, Coyle JT, Weinberger DR, Straub RE, Cohen BM, McPhie DL, Yin C, Huang W, Kim HY, Chung S. iPSC-derived homogeneous populations of developing schizophrenia cortical interneurons have compromised mitochondrial function. Mol Psychiatry. 2020;25:2873–2888. doi: 10.1038/s41380-019-0423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE (2014) Psychosis and schizophrenia in adults: treatment and management (Clinical Guideline 178) London: Royal College of Psychiatrists
- Packer L, Witt EH, Tritschler HJ (1995) Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med 19:227–250 [DOI] [PubMed]
- Park GH, Noh H, Shao Z, Ni P, Qin Y, Liu D, Beaudreault CP, Park JS, Abani CP, Park JM, Le DT, Gonzalez SZ, Guan Y, Cohen BM, McPhie DL, Coyle JT, Lanz TA, Xi HS, Yin C, Huang W, Kim HY, Chung S. Activated microglia cause metabolic disruptions in developmental cortical interneurons that persist in interneurons from individuals with schizophrenia. Nat Neurosci. 2020;23:1352–1364. doi: 10.1038/s41593-020-00724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickar D, Owen RR, Litman RE, Konicki E, Gutierrez R, Rapaport MH. Clinical and biologic response to clozapine in patients with schizophrenia. Crossover comparison with fluphenazine. Arch Gen Psychiatry. 1992;49:345–353. doi: 10.1001/archpsyc.1992.01820050009001. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Balzarro B, Serretti A. Clozapine resistance: augmentation strategies. Eur Neuropsychopharmacol. 2012;22(3):165–182. doi: 10.1016/j.euroneuro.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Kane JM, Correll CU, Lindenmayer JP, Agid O, Marder SR, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6:1. doi: 10.1038/s41537-019-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff JC, Palmese LB, Reutenauer EL, Tek C. An open-label pilot trial of alpha-lipoic acid for weight loss in patients with schizophrenia without diabetes. Clin Schizophr Relat Psychoses. 2015;8:196–200. doi: 10.3371/CSRP.RAPA.030113. [DOI] [PubMed] [Google Scholar]
- Rezaei Kelishadi M, Alavi Naeini A, Askari G, Khorvash F, Heidari Z. The efficacy of alpha-lipoic acid in improving oxidative, inflammatory, and mood status in women with episodic migraine in a randomised, double-blind, placebo-controlled clinical trial. Int J Clin Pract. 2021;75:e14455. doi: 10.1111/ijcp.14455. [DOI] [PubMed] [Google Scholar]
- Rezaei Zonooz S, Hasani M, Morvaridzadeh M, Beatriz Pizarro A, Heydari H, Yosaee S, et al. Effect of alpha-lipoic acid on oxidative stress parameters: a systematic review and meta-analysis. J Funct Foods. 2021;87:104774. doi: 10.1016/j.jff.2021.104774. [DOI] [Google Scholar]
- Salehi B, Berkay Yilmaz Y, Antika G, Boyunegmez Tumer T, Fawzi Mahomoodally M, Lobine D, et al. Insights on the use of alpha-lipoic acid for therapeutic purposes. Biomolecules. 2019;9(8):356. doi: 10.3390/biom9080356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Seeman P, Stewart J, Rajabi H, Kapur S. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27:2979–2986. doi: 10.1523/JNEUROSCI.5416-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio LRL, Borges LTN, Barbosa TM, Matos NCB, Lima RF, Oliveira MN, et al. Electroencephalographic study of chlorpromazine alone or combined with alpha-lipoic acid in a model of schizophrenia induced by ketamine in rats. J Psychiatr Res. 2017;86:73–82. doi: 10.1016/j.jpsychires.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Sampaio LRL, Cysne Filho FMS, de Almeida JC, Diniz DDS, Patrocinio CFV, de Sousa CNS, et al. Advantages of the alpha-lipoic acid association with chlorpromazine in a model of schizophrenia induced by ketamine in rats: behavioral and oxidative stress evidences. Neuroscience. 2018;373:72–81. doi: 10.1016/j.neuroscience.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Sanders LLO, de Souza Menezes CE, Chaves Filho AJM, de Almeida VG, Fechine FV, Rodrigues de Queiroz MG, et al. Alpha-lipoic acid as adjunctive treatment for schizophrenia: an open-label trial. J Clin Psychopharmacol. 2017;37:697–701. doi: 10.1097/JCP.0000000000000800. [DOI] [PubMed] [Google Scholar]
- Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790:1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll S, Hartmann H, Cohen SA, Muller WE. The potent free radical scavenger alpha-lipoic acid improves memory in aged mice: putative relationship to NMDA receptor deficits. Pharmacol Biochem Behav. 1993;46:799–805. doi: 10.1016/0091-3057(93)90204-7. [DOI] [PubMed] [Google Scholar]
- Tang LH, Aizenman E. Allosteric modulation of the NMDA receptor by dihydrolipoic and lipoic acid in rat cortical neurons in vitro. Neuron. 1993;11:857–863. doi: 10.1016/0896-6273(93)90115-8. [DOI] [PubMed] [Google Scholar]
- Thaakur S, Himabindhu G (2009) Effect of alpha lipoic acid on the tardive dyskinesia and oxidative stress induced by haloperidol in rats. J Neural Transm (Vienna) 116:807–814 [DOI] [PubMed]
- Thompson K, Kulkarni J, Sergejew AA. Reliability and validity of a new Medication Adherence Rating Scale (MARS) for the psychoses. Schizophr Res. 2000;42:241–247. doi: 10.1016/S0920-9964(99)00130-9. [DOI] [PubMed] [Google Scholar]
- Vasconcelos GS, Ximenes NC, de Sousa CN, Oliveira Tde Q, Lima LL, de Lucena DF, et al. Alpha-lipoic acid alone and combined with clozapine reverses schizophrenia-like symptoms induced by ketamine in mice: participation of antioxidant, nitrergic and neurotrophic mechanisms. Schizophr Res. 2015;165:163–170. doi: 10.1016/j.schres.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Vidovic B, Milovanovic S, Dordevic B, Kotur-Stevuljevic J, Stefanovic A, Ivanisevic J, et al. Effect of alpha-lipoic acid supplementation on oxidative stress markers and antioxidative defense in patients with schizophrenia. Psychiatr Danub. 2014;26:205–213. [PubMed] [Google Scholar]
- Vidovic B, Milovanovic S, Stefanovic A, Kotur-Stevuljevic J, Takic M, Debeljak-Martacic J, et al. Effects of alpha-lipoic acid supplementation on plasma adiponectin levels and some metabolic risk factors in patients with schizophrenia. J Med Food. 2017;20:79–85. doi: 10.1089/jmf.2016.0070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The trial data are available on reasonable request from the first or corresponding author.