Abstract
Purpose
Serum electrolyte imbalances are highly prevalent in COVID-19 patients. However, their associations with COVID-19 outcomes are inconsistent, and of unknown prognostic value. We aim to systematically clarify the associations and prognostic accuracy of electrolyte imbalances (sodium, calcium, potassium, magnesium, chloride and phosphate) in predicting poor COVID-19 clinical outcome.
Methods
PubMed, Embase and Cochrane Library were searched. Odds of poor clinical outcome (a composite of mortality, intensive-care unit (ICU) admission, need for respiratory support and acute respiratory distress syndrome) were pooled using mixed-effects models. The associated prognostic sensitivity, positive and negative likelihood ratios (LR + , LR-) and predictive values (PPV, NPV; assuming 25% pre-test probability), and area under the curve (AUC) were computed.
Results
We included 28 observational studies from 953 records with low to moderate risk-of-bias. Hyponatremia (OR = 2.08, 95% CI = 1.48–2.94, I2 = 93%, N = 8), hypernatremia (OR = 4.32, 95% CI = 3.17–5.88, I2 = 45%, N = 7) and hypocalcemia (OR = 3.31, 95% CI = 2.24–4.88, I2 = 25%, N = 6) were associated with poor COVID-19 outcome. These associations remained significant on adjustment for covariates such as demographics and comorbidities. Hypernatremia was 97% specific in predicting poor outcome (LR + 4.0, PPV = 55%, AUC = 0.80) despite no differences in CRP and IL-6 levels between hypernatremic and normonatremic patients. Hypocalcemia was 76% sensitive in predicting poor outcome (LR- 0.44, NPV = 87%, AUC = 0.71). Overall quality of evidence ranged from very low to moderate.
Conclusion
Hyponatremia, hypernatremia and hypocalcemia are associated with poor COVID-19 clinical outcome. Hypernatremia is 97% specific for a poor outcome, and the association is independent of inflammatory marker levels. Further studies should evaluate if correcting these imbalances help improve clinical outcome.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40618-022-01877-5.
Keywords: Electrolytes, Severe acute respiratory syndrome, Hypernatremia, Death risk, Intensive care, Respiratory medicine
Introduction
Since the first case of the coronavirus disease 2019 (COVID-19) in December 2019 [1], more than 400 million people have been diagnosed and cumulative deaths have exceeded 6 million as of 11th February [2]. Biochemical markers associated with risk of deterioration and poor outcome (such as C-reactive protein, ferritin, lactate dehydrogenase) were used to triage patients and allocate hospital resources as healthcare systems became overwhelmed. [3–9] Recent studies have reported high prevalence of electrolyte imbalances in COVID-19 patients and associated these imbalances with more severe infection. [10, 11] Tzoulis et al. reported that dysnatremia was associated with a higher risk for mechanical ventilation and mortality [12]. Several hypotheses exist that explain this prevalence, such as the involvement of cell entry receptor angiotensin-converting enzyme 2 (ACE2), a key enzyme in the renin-angiotensin system (RAS) [13, 14]. As serum electrolytes tests are readily available in laboratories, they are useful as prognostic markers in COVID-19 to help risk stratify patients.
While previous meta-analyses have reported associations of hypocalcemia and hyponatremia with COVID-19 severity [15, 16], recent published studies have also suggested associations of other electrolyte imbalances such as dysnatremia, dyskalemia, dysmagnesemia and dyschloremia with COVID-19 severity [12, 17–21]. However, these associations are varied and no pooled prognostic value was reported. Furthermore acute kidney injury (AKI) and acute respiratory distress syndrome (ARDS), which are common complications in severe COVID-19 infections, were not included as outcomes when evaluating these associations [22, 23]. Hence, we sought to conduct a systematic review and meta-analysis to investigate the association of electrolyte imbalances with COVID-19 outcomes. Given the disruption this pandemic has brought to daily lives [24, 25], coupled with the immense toll on some healthcare systems [26], this review is both timely and clinically relevant to help improve risk stratification and resource allocation.
Methods
This review is registered on PROSPERO (CRD42021257711) and reported according to Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (Supplementary Table S1, Online Resource) [27].
Search strategy
We searched three databases (PubMed, Embase and Cochrane Library) from inception till 22nd May 2021 using search terms related to COVID-19 and electrolyte imbalances concerning the electrolytes sodium, calcium, potassium, magnesium, chloride and phosphate (Supplemental Methods, Online Resource). We also hand-searched the bibliography of included articles and relevant reviews but included no additional studies.
Study selection, data extraction, risk of bias assessment and quality of evidence
Two authors independently selected relevant studies, extracted key data and assessed risk of bias in a blinded manner using the online platform Rayyan [28]. We accepted observational studies published as full-length articles in peer-reviewed journals that reported the associations of electrolyte imbalances in patients diagnosed with COVID-19 that were either higher (e.g. hypernatremia) or lower (e.g. hyponatremia) than the normal physiological range. Outcomes of interest included mortality, intensive care unit (ICU) admissions, respiratory support, acute respiratory distress syndrome (ARDS) and/or acute kidney injury (AKI). We also included articles that reported the laboratory parameters of serum creatinine (SCr), C-reactive protein (CRP) and interleukin-6 (IL-6) at admission. We excluded case reports, reviews and letters, as well as articles published in languages other than English. We extracted key data and assessed the risk of bias using the Newcastle–Ottawa Scale (Supplemental Methods, Online Resource). Overall quality of evidence was assessed using the GRADE framework [29].
Statistical analyses
We did separate meta-analyses for each type of electrolyte imbalance to compute a summary estimate of the association of the electrolyte imbalance with the above specified clinical outcomes using an inverse variance-weighted mixed-effects model (Supplemental Methods, Online Resource). We pooled odds ratios for dichotomous outcomes and mean differences for continuous outcomes including laboratory parameters and hospitalization time (Supplemental Methods, Online Resource). We defined poor outcome as a composite of mortality, ICU admission, respiratory support (oxygen supplementation, invasive and/or non-invasive ventilation) and ARDS due to their resource-intensive nature, in line with previous landmark studies on severe COVID [30, 31]. If available, we also pooled odds ratios that adjusted for potential confounders such as sex, age and comorbidities such as diabetes, cardiovascular diseases and chronic liver disease. We generated a summary receiver operator characteristic curve (SROC), Fagan’s nomogram, coupled funnel plots and calculated the area under the curves (AUC), sensitivity, specificity, positive (LR +) and negative likelihood ratios (LR-), and positive (PPV) and negative predictive values (NPV) to evaluate the performance and prognostic value of each type of electrolyte imbalance in predicting the unadjusted odds of poor outcome. We assessed and considered between-study heterogeneity as significant if the I2 statistic was ≥ 50% and the p-value for the Q-test was < 0.10 [32]. To investigate potential sources of heterogeneity, we pre-specified various study-level characteristics (Supplemental Methods, Online Resource) to perform subgroup or sensitivity analyses. We conducted all analyses using RevMan (version 5.4), Stata (version 17) and RStudio (version 1.4) using the meta package (version 4.18).
Results
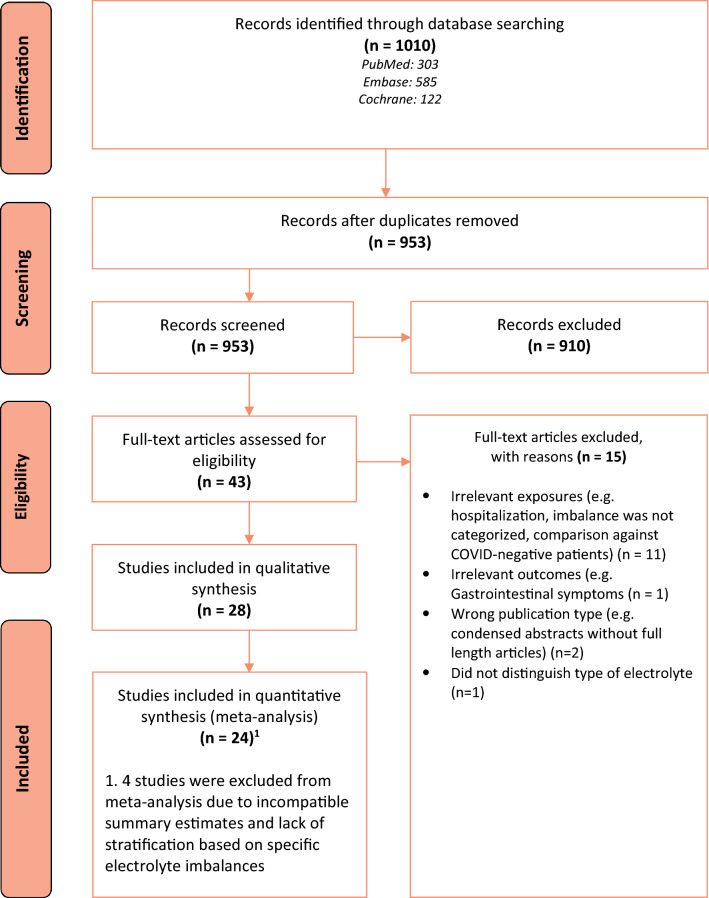
We screened 953 records after removing duplicates and subsequently identified 28 studies for inclusion after screening based on the title and abstract, followed by screening based on full-text (Fig. 1) [12, 17–21, 33–54]. Twenty-four studies were included in our various meta-analyses [12, 17–20, 33–40, 42, 44–53].
Fig. 1.
PRISMA flow diagram of the study selection process
Study characteristics
Of the 28 included studies (Table 1), 21 were retrospective cohorts [12, 17, 18, 20, 21, 36–44, 46, 48, 50–54], four were prospective cohorts [19, 33–35], two were cross-sectional[45, 49] and one was a case–control study [47]. Sensitivity analyses excluding non-cohort studies did not change our findings substantially. A total of 14, 10, two studies and one study were conducted in Asia [21, 33, 37, 40–43, 45–47, 49, 50, 53, 54], Europe [12, 17, 19, 20, 34, 36, 38, 44, 51, 52], America [18, 39] and Africa[35], respectively. One study spanned across America, Asia and Europe [48]. When assessed using the Newcastle–Ottawa Scale, twenty and eight studies had a moderate and low risk of bias, respectively. Overall quality of evidence ranged from very low to moderate.
Table 1.
Summary of included studies
| First author, year country | Study design | Total sample size % male average age | COVID-19 diagnosis method | COVID-19 baseline severity | Electrolyte imbalances studied | Definition of electrolyte imbalance | Timepoint of electrolyte measurement | Outcomes studied | Covariates | NOS score (out of 9) |
|---|---|---|---|---|---|---|---|---|---|---|
| Alfano, 2020 Italy | Retrospective cohort |
320 65.9 64.8 (Mean) |
WHO interim guidelines | N.S | Hypokalemia | < 3.5 mmol/L | At any time during hospitalization | 1, 2, 5, 6, 7 | Sex, age and SOFA score | 7 |
| Asghar, 2020 Pakistan | Prospective cohort |
373 67.0 52.9 (Mean) |
RT-qPCR | N.S | Hypernatremia | > 145 mmol/L | At admission | 1, 3 | N.A | 7 |
| Atila, 2021 Switzerland | Prospective cohort |
172 55.8 60.0 (Mean) |
RT-qPCR | N.S | Hyponatremia, Hypernatremia | Hyponatremia: < 135 mmol/L Hypernatremia: > 145 mmol/L | At admission | 1, 2, 3, 4, 7, 8 | Sex, age, no. of comorbidities (presence of coronary heart disease, heart failure, arterial hypertension, pneumopathy, renal failure, hepatopathy, obesity, rheumatological disease, immunosuppression inclusive HIV infection, cerebrovascular disease, active neoplastic disease and diabetes mellitus) | 9 |
| Bennouar, 2020 Algeria | Prospective cohort |
120 69.2 62.3 (Mean) |
According to WHO criteria | 100% Severe according to WHO guidelines | Hypocalcemia | < 2.20 mmol/L | At admission | 1 | Sex, age, acute kidney injury, cardiac injury, blood glucose, C-reactive protein levels, neutrophil–lymphocyte-ratio, lactate dehydrogenase, albumin and total cholesterol | 7 |
| Berni, 2021 Italy | Retrospective cohort |
380 61.6 67.5 (Median) |
Laboratory confirmed (details N.S.) | N.S | Hyponatremia, Hypernatremia | Hyponatremia: < 135 mmol/L Hypernatremia: > 145 mmol/L | At admission | 1, 2, 3, 6, 8 | Sex and age | 8 |
| De Carvalho, 2021 France | Retrospective cohort |
296 53.7 68.4 (Mean) |
RT-qPCR | N.S | Hyponatremia | < 135 mmol/L | Within 24 h of COVID-19 suspicion | 1, 2, 3, 6, 7 | Sex, age, tympanic temperature, diabetes, serum creatinine, ALT, lymphocyte count and oxygen flow rate at admission | 7 |
| Chen, 2020 China | Retrospective cohort |
179 50.3 45 (Mean) |
According to the criteria by National Health Commission of China | 21% severe, 2% critical according to WHO guidelines | Hypokalemia | Mild hypokalemia: 3—3.5 mmol/L Severe hypokalemia: < 3 mmol/L | N.S | 6, 7 | N.A | 6 |
| Frontera, 2020 USA | Retrospective cohort |
4645 62.9 62.5 |
RT-qPCR | N.S | Hyponatremia | Mild hyponatremia: 130 – 134 mmol/L moderate hyponatremia: 121 – 129 mmol/L Severe hyponatremia: < 120 mmol/L | At admission | 1, 3, 5, 6, 8 | Sex, age, race, BMI, past medical history, admission laboratory abnormalities, admission SOFA score, renal failure, encephalopathy and mechanical ventilation | 8 |
| Hirsch, 2021 USA | Retrospective cohort |
9946 59.4 66.4 (Mean) |
RT-qPCR | N.S | Hyponatremia, Hypernatremia | Mild hyponatremia: 130 – 135 mmol/L Severe hyponatremia: < 130 mmol/L Mild hypernatremia: 145 – 149 mmol/L Severe hypernatremia: ≥ 150 mmol/L | At admission | 1, 6, 7 | Sex, age, race, BMI, diabetes, hypertension, cardiovascular diseases, respiratory diseases, chronic kidney disease, chronic liver disease, cancer, oxygen saturation, systolic blood pressure, hemoglobin, lymphocyte, red cell distribution width, platelet, serum creatinine, bilirubin and albumin, CRP, serum ferritin | 8 |
| Hu, 2020 China | Retrospective cohort |
1254 51.1 56 (Median) |
According to the criteria by National Health Commission of China | 15.9% severe, 6.7% critical, according to National Health Commission of China guidelines | Hyponatremia, Hypernatremia | Hyponatremia: < 135 mmol/L Hypernatremia: > 145 mmol/L | N.S | 1, 3, 5, 6 | N.A | 5 |
| Hu, 2021* China | Retrospective cohort |
206 48.1 53.7 (Mean) |
RT-qPCR | 4.9% severe, 2.4% critical, according to National Health Commission of China guidelines | Hyponatremia, Hypokalemia | Hyponatremia: < 135 mmol/L Hypokalemia: < 3.5 mmmol/L | At admission | Prolonged hospitalization | N.A | 6 |
| Liu, 2020 China | Retrospective cohort |
107 48.6 68 (Median) |
According to WHO interim guidance criteria | 100% severe according to National Health Commission of China guidelines | Hypocalcemia | < 2.15 mmol/L | Within 24 h of admission | A composite of 1, 2 and 3, as well as 6 and 7 | Sex, age, hypertension, diabetes, C-reactive protein, procalcitonin, interleukin-6 and D-dimer | 7 |
| Ma, 2020* China | Retrospective cohort |
1160 52.2 46 (Median) |
Laboratory confirmed (details N.S.) | N.S | Hyponatremia, Hypokalemia | N.S | N.S | Unfavourable outcome defined as mortality or disease progression from moderate to severe illness | Sex, age, BMI and first onset COVID-19 symptoms | 8 |
| Moreno, 2020 Spain | Retrospective cohort |
306 57.8 65 (Median) |
RT-qPCR | N.S | Hypokalemia | Mild hypokalemia: 3–3.5 mmol/L Severe hypokalemia: < 3 mmol/L | Within 72 h of hospital admission | 1, 2, 3, 8 | Age, sex, dyspnea, PaO2, lactate dehydrogenase, procalcitonin, CRP, BNP, lymphocyte count, opacity of lung x ray | 9 |
| Nasomsong, 2021 Thailand | Cross-sectional |
36 63.9 42.6 (Mean) |
RT-qPCR | N.S | Hypokalemia | < 3.5 mmol/L | At COVID-19 diagnosis | 3, 6, 8 | N.A | 5 |
| Osman, 2021 Oman | Retrospective cohort |
445 62 50.8 (Mean) |
N.S | 33.6% had an admission score of 5–8 based on the WHO Ordinal Scale for Clinical Improvement | Hypocalcemia | < 2.1 mmol/L | At admission | 1, 2, 3, 4, 7, 8 | N.A | 5 |
| Quilliot, 2020 France | Prospective cohort |
300 60.7 68 (Median) |
RT-qPCR and/or chest CT scans | 36% were severe, 49.7% were critical according to WHO guidelines | Hypomagnesemia | < 0.75 mmol/L | 5.29 ± 5.02 days after admission | 2, 3 | N.A | 5 |
| Raesi, 2021 Iran | Case–control |
91 60.4 55.4 (Mean) |
RT-qPCR | 55.9% were severe according to WHO guidelines | Hypocalcemia | < 2.15 mmol/L | Within 24 h of admission | 1, 2, 8 | N.A | 5 |
| Ruiz-Sánchez, 2020 Canada, Germany, China, Ecuador, Cuba, Italy, Spain | Retrospective cohort |
4464 58 66 (Median) |
RT-qPCR | All had pneumonia | Hyponatremia, Hypernatremia | Hyponatremia: < 135 mmol/L Hypernatremia: > 145 mmol/L | At admission | 1, 8 | Sex, age, hypertension, dyslipidemia, diabetes, obesity, smoking, chronic kidney disease, chronic liver disease, cardiovascular disease, cerebrovascular disease, chronic lung disease, cancer, immunosuppression, use of angiotensin-converting enzyme inhibitors/angiotensin-2-receptor antagonists, oxygen saturation, serum creatinine and type of pneumonia | 9 |
| Sarvazad, 2020 Iran | Cross-sectional |
58 56.9 62 (Median) |
RT-qPCR and/or chest CT scans | N.S | Hyponatremia, Hypernatremia, Hypokalemia, Hyperkalemia, Hypomagnesemia, Hypermagnesemia | Hyponatremia: 121—134 mmol/L Hypernatremia: > 146 mmol/L Mild hypokalemia: 3—3.4 mmol/L Severe hypokalemia: < 3 mmol/L Hyperkalemia: > 5.5 mmol/L Mild hypomagnesemia: 0.52—0.7 mmol/L Severe hypomagnesemia: < 0.51 mmol/L Hypermagnesemia: > 1.07 mmol/L | At admission | 2 | 5 | |
| Sun, 2020 China | Retrospective cohort |
241 46.5 65 (Median) |
RT-qPCR | 69.3% severe, 10.5% critical, according to National Health Commission of China guidelines | Hypocalcemia | Mild hypocalcemia: 2.0—2.2 mmol/L Severe hypocalcemia: < 2.0 mmol/L | At admission | 1, 3, 4, 5, 6, 7 | N.A | 6 |
|
Tezcan, 2020 Turkey |
Retrospective cohort |
408 46.1 54.3 (Mean) |
RT-qPCR or according to Turkey’s national guidelines | N.S | Hypocalcemia, Hyponatremia, Hypokalemia, Hypochloremia | N.S | At admission | 1 | Sex, age, disease severity, time between disease onset and hospitalization, co-morbidities, pulmonary infiltrations, fever and hypoxemia during hospitalization | 8 |
| Torres, 2021 Spain | Retrospective cohort |
316 65 65 (Median) |
RT-qPCR or clinical, radiologic and lab findings that are consistent with other COVID-19 patients | N.S | Hypocalcemia | < 2.12 mmol/L | Within 72 h of hospital admission | 1, 2, 3, 6, 7 | Sex, advanced life support, SpO2/FiO2, lymphocyte count, C-reactive protein, D dimer and potassium levels | 7 |
|
Trecarichi, 2020 Italy |
Retrospective cohort |
50 57.1 80 (Mean) |
Positive SARS-CoV-2 molecular test conducted on nasopharyngeal swab | 52% severe, according to Italy National Institute of Health criteria | Hypernatremia | > 145 mmol/L | At admission | 1 | Lymphocyte count, cardiovascular disease excluding hypertension, interleukin-6 levels | 6 |
| Tzoulis, 2021 UK | Retrospective cohort |
488 56.8 68 (Median) |
RT-qPCR | N.S | Hyponatremia, Hypernatremia | Hyponatremia: < 135 mmol/L Hypernatremia: > 145 mmol/L | First 5 days of admission | 1, 3 | Sex, age, ethnicity, smoking status, number of co-morbidities, urea and C-reactive protein levels | 8 |
| Wu, 2020* China | Retrospective cohort |
125 52.8 55 (Median) |
Detection of SARS-CoV-2 RNA | 1.6% were severe, defined as dyspnea, hypoxemia and/or lung infiltrates > 50% | Hyponatremia, Hypernatremia, Hypocalcemia, Hypokalemia, Hyperkalemia, Hypochloremia | Hyponatremia: < 136 mmol/L Hypernatremia: > 145 mmol/L Hypocalcemia: < 2.2 mmol/L Hypokalemia: < 3.5 mmol/L Hyperkalemia: > 5.1 mmol/L Hypochloremia: < 99 mmol/L | At admission | Prolonged hospitalization | Age and comorbidities | 7 |
| Zheng, 2021 China | Retrospective cohort |
161 62.8 64 (Median) |
RT-qPCR | All were ICU patients | Hypocalcemia | < 1.8 mmol/L | At admission | 1 | N.A | 5 |
| Zhou, 2020* China | Retrospective cohort |
127 N.S N.S |
RT-qPCR | All had pneumonia | Hypocalcemia | < 2.2 mmol/L | Within 24 h of admission | Progression from mild/moderate infection to severe/critical infection | N.A | 5 |
*Not included in meta-analyses; NS not stated; NA not applicable; 1, Mortality; 2, ICU admission; 3, Respiratory support; 4, Acute respiratory distress syndrome; 5, Acute kidney injury; 6, Serum creatinine; 7, C-reactive protein; 8, Hospitalization time
Definitions of electrolyte imbalances
Studies measured the respective electrolyte levels at hospital admission (15 studies), within 24 h (4 studies), 72 h (2 studies) or beyond 72 h of admission (2 studies). One study measured electrolyte levels at COVID-19 diagnosis and one study recorded the imbalance at any time during hospitalization [17, 45]. As some studies measured electrolyte levels beyond 24 h, [12, 19, 44, 51] we excluded them in sensitivity analyses. This did not did not alter our conclusions. We searched for but found no studies investigating dysphosphatemia in relation to COVID-19 outcomes.
Dysnatremia
Thirteen and ten studies investigated the association of hyponatremia and hypernatremia with the above specified COVID-19 clinical outcomes, respectively (Table 1) [12, 18, 20, 21, 33, 34, 36, 38–41, 43, 48, 49, 52]. Majority of studies defined hyponatremia and hypernatremia as having a serum sodium level of < 135 mmol/L or > 145 mmol/L, respectively. Two studies further stratified their sample based on dysnatremia severity [18, 39]. Three studies corrected their sodium measurements with glucose levels [12, 18, 36].
Dyskalemia
Nine and two studies investigated the association of hypokalemia and hyperkalemia with the same COVID-19 outcomes, respectively (Table 1) [17, 20, 21, 37, 41, 43–45, 49]. Majority of studies defined hypokalemia and hyperkalemia as having a serum potassium level of < 3.5 mmol/L or > 5.1 mmol/L, respectively. Three studies further stratified their sample based on hypokalemia severity [37, 44, 49].
Dyscalcemia
Ten studies investigated the association of hypocalcemia with the same COVID-19 clinical outcomes (Table 1) [20, 21, 35, 42, 46, 47, 50, 51, 53, 54]. Majority of the studies defined hypocalcemia as having a serum calcium level of < 2.20 mmol/L, with the exception of one study that defined it as < 1.8 mmol/L [53]. There were no studies that investigated hypercalcemia.
Dysmagnesemia
Two studies investigated the association of dysmagnesemia including hypomagnesemia (2 studies) and hypermagnesemia (1 study) with ICU admission and respiratory support (Table 1). Hypomagnesemia and hypermagnesemia were defined as having a serum magnesium level of < 0.75 mmol/L or > 1.07 mmol/L, respectively [19, 49].
Dyschloremia
Two studies investigated the association of hypochloremia, defined as a serum chloride level of < 99 mmol/L, with prolonged hospitalization and mortality, respectively [20, 21].
Association of electrolyte imbalances with COVID-19 poor outcome
Overall poor outcome
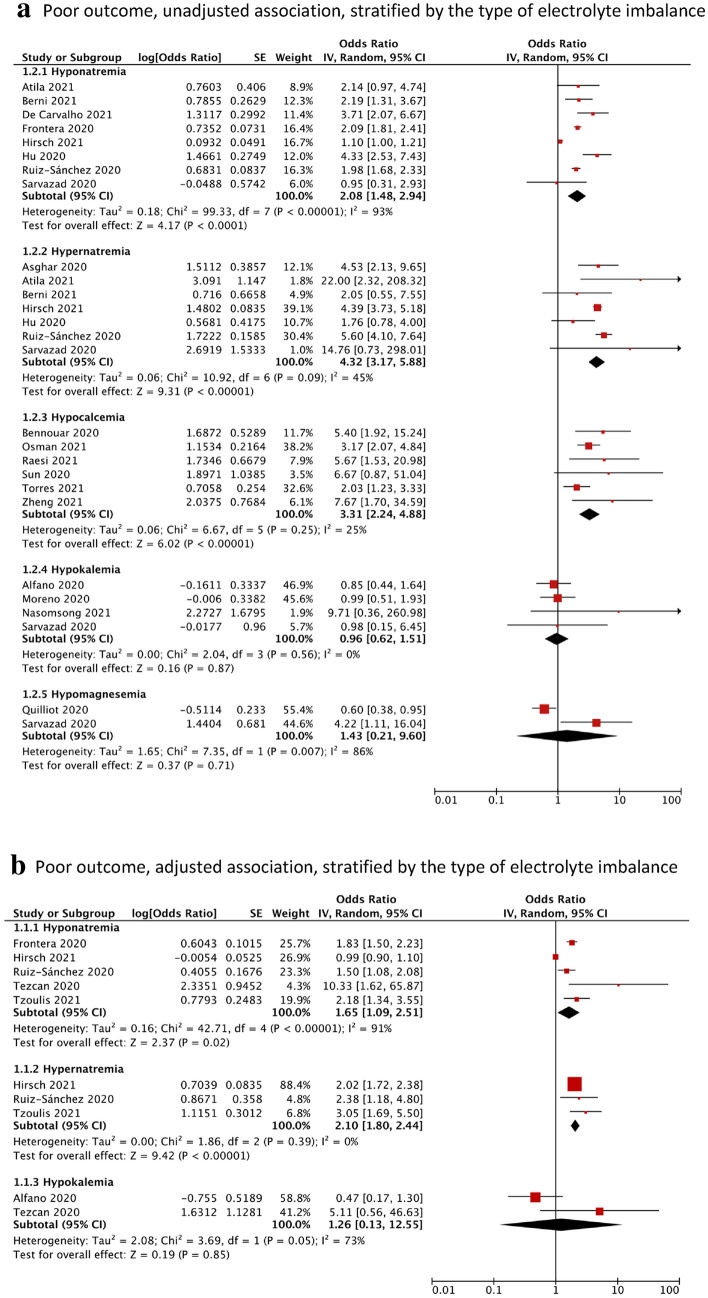
Compared to the control group, participants with hyponatremia (OR = 2.08, 95% CI = 1.48–2.94, I2 = 93%, N = 8), hypernatremia (OR = 4.32, 95% CI = 3.17–5.88, I2 = 45%, N = 7) or hypocalcemia (OR = 3.31, 95% CI = 2.24–4.88, I2 = 25%, N = 6) had, on average, significantly higher pooled odds of poor outcome, defined as a composite of mortality, ICU admission, respiratory support and ARDS (Fig. 2a). After adjustment, the associations were attenuated but remained significant for hyponatremia (aOR = 1.65, 95% CI = 1.09–2.51, I2 = 91%, N = 5) and hypernatremia (aOR = 2.10, 95% CI = 1.80–2.44, I2 = 0%, N = 3) (Fig. 2b). There was no significant association found for participants with hypokalemia (OR = 0.96, 95% CI = 0.62–1.51, I2 = 0%, N = 4) or hypomagnesemia (OR = 1.43, 95% CI = 0.21–9.60, I2 = 86%, N = 2) (Fig. 2a). Between-study heterogeneity was significant for hyponatremia (I2 = 91%) and hypomagnesemia (I2 = 86%) but expected due to the pooling of different clinical outcomes. As ICU admission criteria differ across countries, we performed sensitivity analyses excluding ICU admission, which did not change our findings. In studies excluded from meta-analysis, Ma et al. reported participants with hyponatremia and/or hypokalemia having an increased odds of unfavourable outcome, defined as mortality or disease progression from moderate to severe (OR = 19.44, 95% CI = 11.47–32.96), after adjusting for sex, age, BMI and first-onset COVID-19 symptoms [43]. Zhou et al. reported participants with low calcium levels tended to progress to a severe or critical infection [54].
Fig. 2.
Forest plot showing the (a) unadjusted and (b) adjusted association between electrolyte imbalances with poor outcome*, stratified by the type of electrolyte imbalance. Black diamonds are the estimated pooled odds ratios for each random-effects meta-analysis; red boxes reflect the relative weight apportioned to studies in the meta-analysis.*Poor outcome Is defined as a composite of mortality, ICU admission, respiratory support and acute respiratory distress syndrome
Mortality
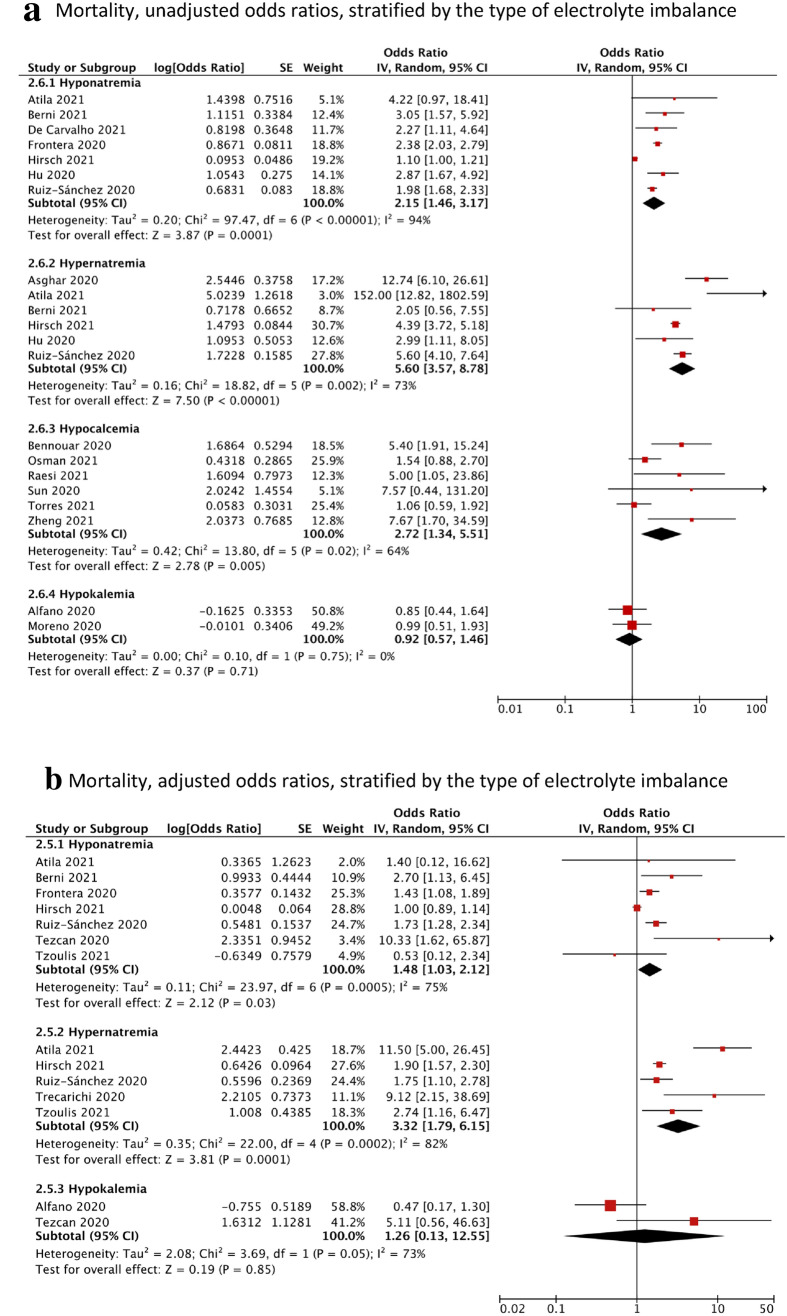
Looking at the specific poor outcome composites, participants with hyponatremia (OR = 2.15, 95% CI = 1.46–3.17, I2 = 94%, N = 7), hypernatremia (OR = 5.60, 95% CI = 3.57–8.78, I2 = 73%, N = 6) or hypocalcemia (OR = 2.72, 95% CI = 1.34–5.51, I2 = 64%, N = 6) had, on average, significantly higher pooled odds of mortality compared to the control group (Fig. 3a). The adjusted association remained significant for hyponatremia (aOR = 1.48, 95% CI = 1.03–2.12, I2 = 75%. N = 7) and hypernatremia (aOR = 3.32, 95% CI = 1.79–6.15, I2 = 82%, N = 5) (Fig. 3b). There were insufficient studies that calculated the adjusted association for hypocalcemia. There were no significant associations found for participants with hypokalemia (OR = 0.92, 95% CI = 0.57–1.46, I2 = 0%).
Fig. 3.
Forest plot showing the pooled unadjusted odds ratios (a) and adjusted odds ratios (b) of the association between electrolyte imbalances and mortality, stratified by the type of electrolyte imbalance. Black diamonds are the estimated pooled odds ratios for each random-effects meta-analysis; blue/red boxes reflect the relative weight apportioned to studies in the meta-analysis
ICU admission
Compared to the control group, participants with hyponatremia (OR = 2.19, 95% CI = 1.36–3.52, I2 = 44%, N = 4) or hypocalcemia (OR = 2.23, 95% CI = 1.60–3.11, I2 = 6%, N = 3) had on average, significantly higher odds of ICU admission. There were no significant associations for hypernatremia (OR = 3.72, 95% CI = 0.14–99.22, I2 = 82%, N = 3), hypokalemia (OR = 1.35, 95% CI = 0.25–7.30, I2 = 90%, N = 3) or hypomagnesemia (OR = 1.43, 95% CI = 0.21–9.61, I2 = 86%, N = 2) (Fig. 4).
Fig. 4.
Forest plot showing the unadjusted association between electrolyte imbalances with ICU admission, stratified by the type of electrolyte imbalance. Black diamonds are the estimated pooled odds ratios for each random-effects meta-analysis; blue boxes reflect the relative weight apportioned to studies in the meta-analysis
Respiratory support
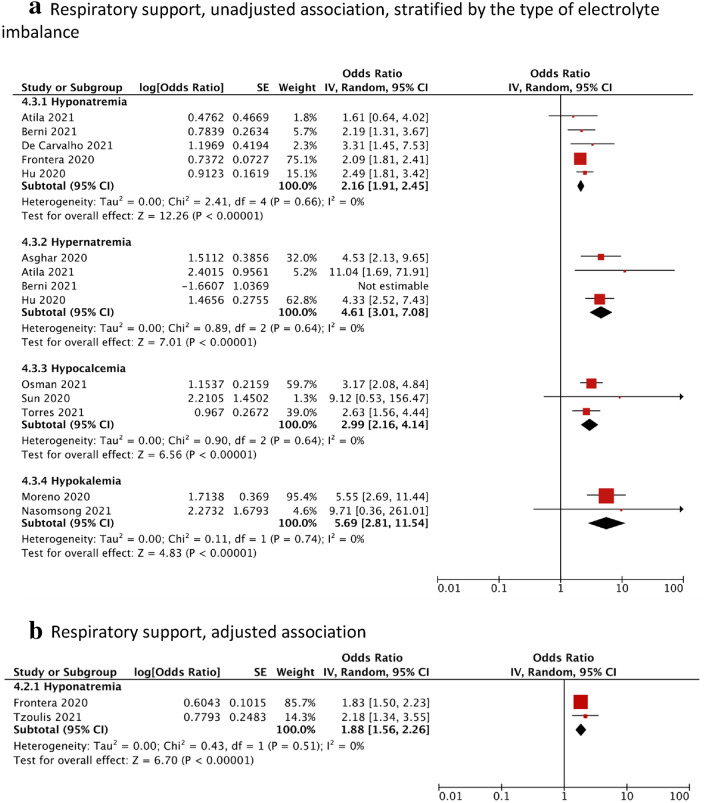
A total of 12 studies reported the use of respiratory support, defined as the need for either invasive ventilation or non-invasive ventilation [12, 33, 34, 36, 38–40, 44–46, 50, 51]. Compared to the control group, participants with hyponatremia (OR = 2.16, 95% CI = 1.91–2.45, I2 = 0%, N = 5), hypernatremia (OR = 3.24, 95% CI = 1.24–8.50, I2 = 70%, N = 4), hypocalcemia (OR = 2.99, 95% CI = 2.16–4.14, I2 = 0%, N = 3) or hypokalemia (OR = 5.69, 95% CI = 2.81–11.54, I2 = 0%, N = 2) had on average, a significantly higher odds of requiring respiratory support (Fig. 5a). The adjusted association remained significant for hyponatremia (aOR = 1.88, 95% CI = 1.56–2.26, I2 = 0%, N = 2) (Fig. 5b).
Fig. 5.
Forest plot showing the a unadjusted and b adjusted association between electrolyte imbalances and respiratory support, stratified by the type of electrolyte imbalance. Black diamonds are the estimated pooled odds ratios for each random-effects meta-analysis; red boxes reflect the relative weight apportioned to studies in the meta-analysis
Performance and prognostic value of electrolyte imbalances in predicting unadjusted odds of poor outcome
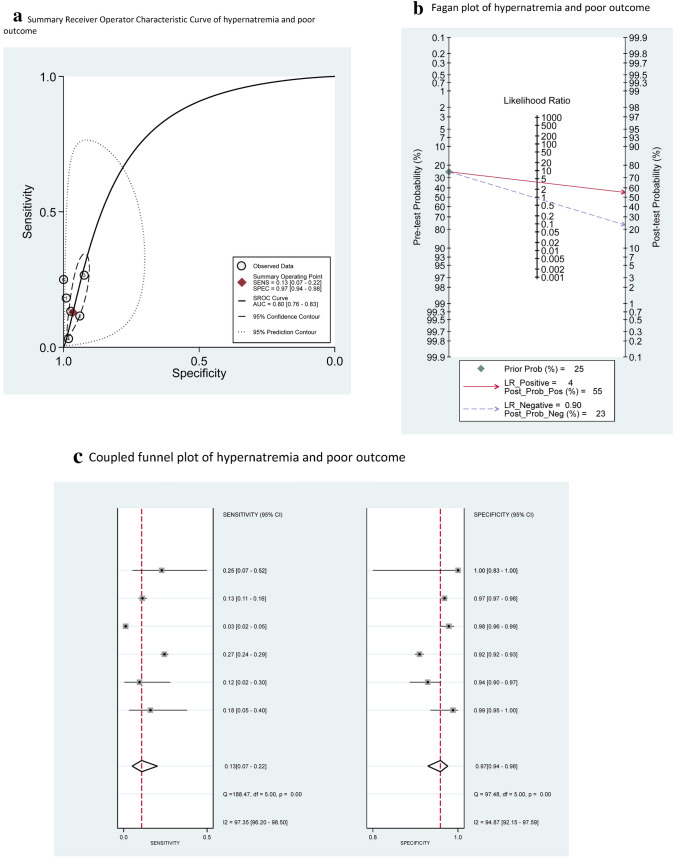
Based on the SROC curves generated for each electrolyte imbalance, hypernatremia (AUC = 0.80, 95% CI = 0.76–0.83) and hypocalcemia (AUC = 0.71, 95% CI = 0.67–0.75) performed adequately, with AUC > 0.70. In particular, hypernatremia was 97% specific (95% CI = 0.94–0.98; LR + 4.0) for a poor outcome with low sensitivity (0.13, 95% CI = 0.07–0.22; LR – 0.90), while hypocalcemia was 76% sensitive (95% CI = 0.53–0.90; LR – 0.44) for a poor outcome with low specificity (0.53, 95% CI = 0.26–0.78; LR + 2.0) (Fig. 6a and Fig. 7a). In contrast, hyponatremia and hypokalemia performed inadequately (AUC < 0.70) (Supplemental Results, Online Resource). Visual inspection of coupled funnel plots did not indicate a clear threshold effect (Fig. 6c and Fig. 7c).
Fig. 6.
a Summary receiver operator characteristic curve, b Fagan plot and c Coupled funnel plot of hypernatremia in predicting poor outcome
Fig. 7.
a Summary receiver operator characteristic curve, b Fagan plot and c Coupled funnel plot of hypocalcemia in predicting poor outcome
Assuming a 25% pre-test probability of progression to severe COVID based on published estimates in the general population infected with COVID [55–57], the presence of hypernatremia (LR + 4.0) would be associated with a PPV of 55%, or a 55% post-test probability of progression to severe COVID based on Fagan’s nomogram (Fig. 6b). Similarly, the absence of hypocalcemia (LR- 0.44) would be associated with a 13% post-test probability of severe COVID, or a NPV of 87% (Fig. 7b).
Association of electrolyte imbalances with Acute Kidney Injury (AKI) and serum creatinine levels
Participants with hyponatremia had a significantly increased odds ratio (OR = 1.63, 95% CI = 1.26–2.10, I2 = 13%, N = 2) (Supplemental Figure S4, Online Resource) compared to the controls. In studies excluded from the meta-analysis, Sun et al. and Alfano et al. reported no significant association for hypocalcemia (OR = 4.67, 95% CI = 0.59–36.47) and hypokalemia (OR = 0.88, 95% CI = 0.49–1.60), respectively [12, 17, 50]. Additionally, Tzoulis et al. concluded that sodium values were not associated with the risk for AKI, although sufficient data was not provided. Compared to the control group, there was no significant difference in serum creatinine levels for dysnatremia, hypocalcemia and hypokalemia (Supplemental Figure S5a, Online Resource).
Association of electrolyte imbalances with C-reactive protein (CRP) levels
While patients with hyponatremia (MD = 27.92 mg/L, 95% CI = 16.97–38.86 mg/L, I2 = 56%, N = 3), hypocalcemia (MD = 10.18 mg/L, 95% CI = 7.15–13.20 mg/L, I2 = 0%, N = 4) and hypokalemia (MD = 5.82 mg/L, 95% CI = 0.26–11.37 mg/L, I2 = 0%, N = 2) showed significantly higher CRP levels as compared to the control group, patients with hypernatremia (MD = 57.16 mg/L, 95% CI = – 27.12– 141.45 mg/L, I2 = 98%, N = 3) showed no significant difference (Supplemental Figure S5b, Online Resource).
Association of electrolyte imbalances with Interleukin-6 (IL-6) levels
In studies excluded from meta-analyses, Berni et al. reported significantly higher (p-value < 0.001) baseline IL-6 levels in hyponatremic participants as compared to the control group. There was no significant difference in IL-6 levels between hypernatremic and normonatremic participants (p-value = 0.395) [36]. Liu et al. reported a significantly higher IL-6 levels (p-value = 0.0276) in hypocalcemic participants as compared to the control group [42].
Discussion
In this systematic review and meta-analysis of 28 observational studies comprising a combined cohort of 26,897 participants with COVID-19, we found that hyponatremia, hypernatremia and hypocalcemia were associated with a twofold, fourfold and threefold increased odds of poor clinical outcome, defined as a composite of mortality, ICU admission, ARDS and respiratory support. Participants with hyponatremia had a 63% increased odds of AKI. Hypernatremia and hypocalcemia performed adequately with an AUC score of more than 0.7. Hypernatremia had a specificity of 97% and hypocalcemia had a sensitivity of 76%, suggesting their predictive utility for a poor clinical outcome. The association of hypernatremia and poor outcome could not be explained by differences in CRP and IL-6 compared to normonatremic controls, thus highlighting its potential use as a unique clinical indicator of disease progression. These associations were robust to pre-specified sensitivity analyses and attenuated but remained significant upon adjustment for covariates. Hypokalemia, hypomagnesemia and hypochloremia was not significantly associated with a poor outcome and AKI.
To the best of our knowledge, this is the first comprehensive systematic review and meta-analysis looking at multiple electrolyte imbalances and its associations with a poor clinical outcome. Our findings are consistent with recent meta-analyses that also reported significantly higher odds of poor outcome and severe infection amongst hyponatremic and hypocalcemic participants, respectively [15, 16]. We further add value to these studies by including ARDS and AKI as additional outcomes as well as by investigating additional electrolyte imbalances—hypernatremia, hypokalemia, hypochloremia and hypomagnesemia.
These associations may be confounded by the underlying disease process—either as part of a non-specific septic response or via mechanisms specific to COVID-19. Marked elevation of inflammatory cytokines have been described in COVID-19, manifesting in severe cases as cytokine storm [58]. This increase in cytokines such as IL-6 can result in syndrome of inappropriate secretion of antidiuretic hormone (SIADH) either through directly stimulating non-osmotic release of anti-diuretic hormone (ADH) or through injuring alveolar tissues which then triggers the hypoxic pulmonary vasoconstriction pathway [59–61]. Other potential mechanisms that can lead to increased ADH secretion include that of volume depletion from reduced oral intake or gastrointestinal losses. These processes lead to increased water retention, resulting in hyponatremia. In a small observational study of 26 COVID-19 patients by Berni et al. it was noted that IL-6 was inversely correlated with sodium levels and sodium was directly correlated with P/F ratio [59]. The correlation between active inflammatory processes and electrolyte imbalances is also observed in our results where hyponatremia, hypocalcemia and hypokalemia were significantly associated with higher baseline CRP levels, which itself is an established marker for inflammation and disease severity. [62] Electrolyte imbalances may also be a general indication of kidney impairment as AKI has been reported to be prevalent in patients hospitalized with COVID-19 [63].
However, in a number of the included primary studies, the association of some electrolyte imbalances with poorer outcome in COVID-19 remained significant even after adjusting for inflammatory biomarkers. Furthermore, our study found that CRP levels were not significantly different between hypernatremic and normonatremic patients, which could suggest that the poor outcome associated with hypernatremia may be unrelated to the systemic inflammatory response. It has been proposed that hypernatremia can be caused by increased angiotensin II activity secondary to SARS-CoV-2-induced down regulation of ACE2 receptors in the proximal tubule after viral entry. [64] While hypernatremia in COVID disease likely represents dehydration from insensible water losses such as fever and tachypnea, it is not clear if dehydration alone can explain the observed poor prognosis, as a recent case series documented persistent hypernatremia despite adequate infusion of free water in 6 patients. [65] This could be consistent with a COVID-19-specific mechanism for hypernatremia rather than simple dehydration.
Electrolyte imbalances are a manifestation of the physiological derangement caused by COVID-19 infection though they likely do not exacerbate the disease process. These findings suggest a role for hyponatremia, hypocalcemia and hypernatremia to be used in the risk stratification, prognostication and clinical decision-making in the treatment of patients with COVID-19. As conventional biomarkers like IL-6 and CRP are expensive to test especially in rural healthcare centres, the measurement of electrolytes which is cheaper and more readily available, can serve as a valuable tool to triage scarce healthcare in these areas. Hypernatremia may be a clinically useful indicator of progression to a poor outcome due to its high LR + of 4.0 resulting in a PPV of 55% in the general population of COVID patients.
The strengths of our study lie in the large number of studies analyzed looking at a broad range of electrolyte imbalances. None of our included studies had a high risk-of-bias according to the NOS scale, increasing the quality of findings. We employed a rigorous methodology according to international guidelines pre-specified in our protocol. Additionally, we pooled maximally adjusted estimates to account for potential confounders and assessed the prognostic value of each electrolyte imbalance by calculating their overall sensitivity, specificity, positive likelihood ratio, negative likelihood ratio and area the under curve scores. Our findings were also robust to pre-specified subgroup and sensitivity analyses.
Limitations
Firstly, there were insufficient studies looking at the same outcome and same electrolyte imbalance for a statistically-powered meta-regression and funnel plot for the assessment of publication bias. We were unable to conduct some meaningful subgroup analyses to explain heterogeneity, but this potentially could be explained by the differing impacts of the pandemic on different healthcare systems globally, as well as their varying management strategies. This may be confirmed using future studies for subgroup analyses stratified by country or region. Secondly, there is heterogeneity in the severity of disease at the time when data was collected in the studies. We are unable to separately analyse patients who were admitted with a severe disease requiring intensive care from those who deteriorate subsequently during hospitalization. This potentially introduces a source of bias as participants who had more severe disease from the start are more likely to have a poorer outcome. Nonetheless, we mitigated this by marking down the study’s representativeness on the NOS scale. Thirdly, our results do not allow us to interpret the causality of the association as it is unclear whether the electrolyte imbalances further aggravate participants with COVID-19 or whether it’s just a general indication of poor health. Furthermore, the temporal sequence between COVID-19 diagnosis and the presence of electrolyte imbalances is hard to establish. Studies also did not monitor the progression of these imbalances throughout the length of hospital stay. Additionally, we acknowledge that respiratory infections such as COVID-19 commonly result in dehydration because of pyrexia or tachypnea and that our association could be confounded by abnormalities such as blood volume and osmolarity. Not all our studies assessed the specific etiology of the electrolyte imbalance, whether it is hypovolemic, euvolemic or hypervolemic which could potentially influence management.
Conclusion
In this multi-adjusted observational meta-analysis of 26,897 participants with COVID-19, hyponatremia, hypernatremia and hypocalcemia were associated with a twofold, fourfold and threefold increased odds of poor clinical outcome, respectively. We also observed that hypernatremia had a specificity of 97% independent of CRP and IL-6 levels, highlighting its potential use as a unique clinical indicator of poor disease progression. Our findings are pertinent to triaging and risk assessment of COVID-19 patients, especially since severe COVID-19 patients continue to take up significant healthcare resources. Future interventional studies and randomized controlled trials should look at whether correcting for these electrolyte imbalances via resuscitation strategies, fluid replacement or supplements can mitigate the odds of poor outcome.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr Chan Yong Hiuak (Founding Mentor, NUS Medicine Biostatistics Unit) for his statistical review of our manuscript.
Author contributions
Concept and design (HJJMDS, AZQC, BKJT, CBT), data collection (HJJMDS, AZQC), data analysis (HJJMDS, AZQC, BKJT, CBT), data interpretation (all authors), manuscript writing (HJJMDS, AZQC), critical revision (all authors), overall supervision (AK), approval for publication (all authors).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Additional data may reasonably be requested from the corresponding author.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Research involving human participants and/or animals
This article is a systematic review and meta-analysis, and hence does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
H. J. J. M. D. Song and A. Z. Q. Chia are contributed equally and should be considered as joint first-authors.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Y, Zhou N, Zha W, Lv Y. Hypertension is a clinically important risk factor for critical illness and mortality in COVID-19: A meta-analysis. Nutr Metab Cardiovasc Dis. 2021;31:745–755. doi: 10.1016/j.numecd.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76:428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 7.Zhang XM, Jiao J, Cao J, Huo XP, Zhu C, Wu XJ, et al. Frailty as a predictor of mortality among patients with COVID-19: a systematic review and meta-analysis. BMC Geriatr. 2021;21:186. doi: 10.1186/s12877-021-02138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh AK, Gillies CL, Singh R, Singh A, Chudasama Y, Coles B, et al. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metab. 2020;22:1915–1924. doi: 10.1111/dom.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Cai Z, Zhang J. 2021. Hyperglycemia at admission is a strong predictor of mortality and severe/critical complications in COVID-19 patients: a meta-analysis. Biosci Rep. [DOI] [PMC free article] [PubMed]
- 10.Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19) Ann Clin Biochem. 2020;57:262–265. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malieckal DA, Uppal NN, Ng JH, Jhaveri KD, Hirsch JS. Northwell nephrology C-RC. electrolyte abnormalities in patients hospitalized with COVID-19. Clin Kidney J. 2021;14:1704–7. doi: 10.1093/ckj/sfab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzoulis P, Waung JA, Bagkeris E, Hussein Z, Biddanda A, Cousins J, et al. Dysnatremia is a predictor for morbidity and mortality in hospitalized patients with COVID-19. J Clin Endocrinol Metab. 2021;106:1637–1648. doi: 10.1210/clinem/dgab107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Carvalho H, Richard MC, Chouihed T, Goffinet N, Le Bastard Q, Freund Y, et al. Electrolyte imbalance in COVID-19 patients admitted to the emergency department: a case-control study. Intern Emerg Med. 2021;16(7):1–6. doi: 10.1007/s11739-021-02632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martha JW, Wibowo A, Pranata R. Hypocalcemia is associated with severe COVID-19: a systematic review and meta-analysis. Diabetes Metab Syndr. 2021;15:337–342. doi: 10.1016/j.dsx.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbar MR, Pranata R, Wibowo A, Irvan Sihite TA, Martha JW. The prognostic value of hyponatremia for predicting poor outcome in patients with COVID-19: a systematic review and meta-analysis. Front Med (Lausanne) 2021 doi: 10.3389/fmed.2021.666949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alfano G, Ferrari A, Fontana F, Perrone R, Mori G, Ascione E, et al. Hypokalemia in patients with COVID-19. Clin Exp Nephrol. 2021;25:401–409. doi: 10.1007/s10157-020-01996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch JS, Uppal NN, Sharma P, Khanin Y, Shah HH, Malieckal DA, et al. Prevalence and outcomes of hyponatremia and hypernatremia in patients hospitalized with COVID-19. Nephrol Dial Transplant. 2021;36(6):1135–1138. doi: 10.1093/ndt/gfab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quilliot D, Bonsack O, Jaussaud R, Mazur A. Dysmagnesemia in Covid-19 cohort patients: prevalence and associated factors. Magnes Res. 2020;33:114–122. doi: 10.1684/mrh.2021.0476. [DOI] [PubMed] [Google Scholar]
- 20.Tezcan ME, Dogan Gokce G, Sen N, Zorlutuna Kaymak N, Ozer RS. Baseline electrolyte abnormalities would be related to poor prognosis in hospitalized coronavirus disease 2019 patients. New Microbes New Infect. 2020;37:100753. doi: 10.1016/j.nmni.2020.100753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Hou B, Liu J, Chen Y, Zhong P. Risk factors associated with long-term hospitalization in patients with covid-19: a single-centered, retrospective study. Front Med (Lausanne) 2020;7:315. doi: 10.3389/fmed.2020.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24:516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y-T, Shao S-C, Hsu C-K, Wu IW, Hung M-J, Chen Y-C. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24:346. doi: 10.1186/s13054-020-03009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pak A, Adegboye OA, Adekunle AI, Rahman KM, McBryde ES, Eisen DP. Economic consequences of the COVID-19 outbreak: the need for epidemic preparedness. Front Public Health. 2020;8:241. doi: 10.3389/fpubh.2020.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller IF, Becker AD, Grenfell BT, Metcalf CJE. Disease and healthcare burden of COVID-19 in the United States. Nat Med. 2020;26:1212–1217. doi: 10.1038/s41591-020-0952-y. [DOI] [PubMed] [Google Scholar]
- 26.Usher AD. Medical oxygen crisis: a belated COVID-19 response. Lancet. 2021;397:868–869. doi: 10.1016/S0140-6736(21)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4 rating the quality of evidence–study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–15. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Soeroto AY, Soetedjo NN, Purwiga A, Santoso P, Kulsum ID, Suryadinata H, et al. Effect of increased BMI and obesity on the outcome of COVID-19 adult patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:1897–1904. doi: 10.1016/j.dsx.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ong SWX, Chiew CJ, Ang LW, Mak TM, Cui L, Matthias PHS, et al. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective study comparing B 1 1 7 (Alpha), B 1 315 (Beta), and B 1 617 2 (Delta) Lancet. 2021 doi: 10.2139/ssrn.3861566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 33.Asghar MS, Haider Kazmi SJ, Khan NA, Akram M, Jawed R, Rafaey W, et al. Role of biochemical markers in invasive ventilation of coronavirus disease 2019 patients: multinomial regression and survival analysis. Cureus. 2020;12:e10054. doi: 10.7759/cureus.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atila C, Sailer CO, Bassetti S, Tschudin-Sutter S, Bingisser R, Siegemund M, et al. Prevalence and outcome of dysnatremia in patients with COVID-19 compared to controls. Eur J Endocrinol. 2021;184:409–418. doi: 10.1530/EJE-20-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennouar S, Cherif AB, Kessira A, Bennouar DE, Abdi S. Vitamin D deficiency and low serum calcium as predictors of poor prognosis in patients with severe COVID-19. J Am Coll Nutr. 2021;40:104–110. doi: 10.1080/07315724.2020.1856013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berni A, Malandrino D, Corona G, Maggi M, Parenti G, Fibbi B, et al. Serum sodium alterations in sars cov2 (covid19) infection: impact on patient outcome. Eur J Endocrinol. 2021;185(1):137–144. doi: 10.1530/EJE-20-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen D, Li X, Song Q, Hu C, Su F, Dai J, et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou. China JAMA Netw Open. 2020;3:e2011122. doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Carvalho H, Letellier T, Karakachoff M, Desvaux G, Caillon H, Papuchon E, et al. Hyponatremia is associated with poor outcome in COVID-19. J Nephrol. 2021;34(4):1–8. doi: 10.1007/s40620-021-01036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frontera JA, Valdes E, Huang J, Lewis A, Lord AS, Zhou T, et al. Prevalence and impact of hyponatremia in patients with coronavirus disease 2019 in New York City. Crit Care Med. 2020;48:e1211–e1217. doi: 10.1097/CCM.0000000000004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu W, Lv X, Li C, Xu Y, Qi Y, Zhang Z, et al. Disorders of sodium balance and its clinical implications in COVID-19 patients: a multicenter retrospective study. Intern Emerg Med. 2020;16(4):1–10. doi: 10.1007/s11739-020-02515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu Z, Li S, Yang A, Li W, Xiong X, Hu J, et al. Delayed hospital admission and high-dose corticosteroids potentially prolong SARS-CoV-2 RNA detection duration of patients with COVID-19. Eur J Clin Microbiol Infect Dis. 2021;40:841–848. doi: 10.1007/s10096-020-04085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Han P, Wu J, Gong J, Tian D. Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. J Infect Public Health. 2020;13:1224–1228. doi: 10.1016/j.jiph.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Y, Zhu DS, Chen RB, Shi NN, Liu SH, Fan YP, et al. Association of overlapped and un-overlapped comorbidities with COVID-19 severity and treatment outcomes: a retrospective cohort study from nine provinces in China. Biomed Environ Sci. 2020;33:893–905. doi: 10.3967/bes2020.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreno PO, Leon-Ramirez JM, Fuertes-Kenneally L, Perdiguero M, Andres M, Garcia-Navarro M, et al. Hypokalemia as a sensitive biomarker of disease severity and the requirement for invasive mechanical ventilation requirement in COVID-19 pneumonia: a case series of 306 mediterranean patients. Int J Infect Dis. 2020;100:449–454. doi: 10.1016/j.ijid.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasomsong W, Ungthammakhun C, Phiboonbanakit D, Prapaso S, Luvira V, Dhitiwat C. Low serum potassium among patients with COVID-19 in Bangkok, Thailand: coincidence or clinically relevant? Trop Doct. 2021;51:212–215. doi: 10.1177/0049475520978174. [DOI] [PubMed] [Google Scholar]
- 46.Osman W, Al Fahdi F, Al Salmi I, Al Khalili H, Gokhale A, Khamis F. Serum calcium and vitamin D levels: correlation with severity of COVID-19 in hospitalized patients in royal hospital. Oman Int J Infect Dis. 2021;107:153–163. doi: 10.1016/j.ijid.2021.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raesi A, Saedi Dezaki E, Moosapour H, Saeidifard F, Habibi Z, Rahmani F, et al. Hypocalcemia in Covid-19: a prognostic marker for severe disease. Iran J Pathol. 2021;16:144–153. doi: 10.30699/ijp.2020.130491.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruiz-Sánchez JG, Núñez-Gil IJ, Cuesta M, Rubio MA, Maroun-Eid C, Arroyo-Espliguero R, et al. Prognostic impact of hyponatremia and hypernatremia in COVID-19 pneumonia A HOPE-COVID-19 (health outcome predictive evaluation for COVID-19) registry analysis. Front Endocrinol (Lausanne) 2020;11:599255. doi: 10.3389/fendo.2020.599255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarvazad H, Cahngaripour SH, Eskandari Roozbahani N, Izadi B. Evaluation of electrolyte status of sodium, potassium and magnesium, and fasting blood sugar at the initial admission of individuals with COVID-19 without underlying disease in Golestan Hospital. Kermanshah New Microbes New Infect. 2020;38:100807. doi: 10.1016/j.nmni.2020.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun JK, Zhang WH, Zou L, Liu Y, Li JJ, Kan XH, et al. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging (Albany NY) 2020;12:11287–11295. doi: 10.18632/aging.103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres B, Alcubilla P, González-Cordón A, Inciarte A, Chumbita M, Cardozo C, et al. Impact of low serum calcium at hospital admission on SARS-CoV-2 infection outcome. Int J Infect Dis. 2021;104:164–168. doi: 10.1016/j.ijid.2020.11.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trecarichi EM, Mazzitelli M, Serapide F, Pelle MC, Tassone B, Arrighi E, et al. Clinical characteristics and predictors of mortality associated with COVID-19 in elderly patients from a long-term care facility. Sci Rep. 2020;10:20834. doi: 10.1038/s41598-020-77641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng R, Zhou J, Song B, Zheng X, Zhong M, Jiang L, et al. COVID-19-associated coagulopathy: thromboembolism prophylaxis and poor prognosis in ICU. Exp Hematol Oncol. 2021;10(1):1–11. doi: 10.1186/s40164-021-00202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou X, Chen D, Wang L, Zhao Y, Wei L, Chen Z, 2020. Low serum calcium: a new, important indicator of COVID-19 patients from mild/moderate to severe/critical. Biosci Rep. [DOI] [PMC free article] [PubMed]
- 55.Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Who W. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) WHO-China Jt Mission Coronavirus Dis. 2020;2019:16–24. [Google Scholar]
- 58.Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm what we know so far. Front Immunol. 2020 doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berni A, Malandrino D, Parenti G, Maggi M, Poggesi L, Peri A. Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together? J Endocrinol Invest. 2020;43:1137–1139. doi: 10.1007/s40618-020-01301-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuesta M, Thompson CJ. The syndrome of inappropriate antidiuresis (SIAD) Best Pract Res Clin Endocrinol Metab. 2016;30:175–187. doi: 10.1016/j.beem.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Yousaf Z, Al-Shokri SD, Al-Soub H, Mohamed MFH. COVID-19-associated SIADH: a clue in the times of pandemic! Am J Physiol Endocrinol Metab. 2020;318:E882–E885. doi: 10.1152/ajpendo.00178.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kazemi E, Soldoozi Nejat R, Ashkan F, Sheibani H. The laboratory findings and different COVID-19 severities: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2021;20:17. doi: 10.1186/s12941-021-00420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Legrand M, Bell S, Forni L, Joannidis M, Koyner JL, Liu K, et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021 doi: 10.1038/s41581-021-00452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertens Res. 2020;43:648–654. doi: 10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zimmer MA, Zink AK, Weißer CW, Vogt U, Michelsen A, Priebe H-J, et al. Hypernatremia-a manifestation of COVID-19: a case series. A A Pract. 2020;14(9):e01295. doi: 10.1213/XAA.0000000000001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Yang Y, Cai Z, Zhang J. 2021. Hyperglycemia at admission is a strong predictor of mortality and severe/critical complications in COVID-19 patients: a meta-analysis. Biosci Rep. [DOI] [PMC free article] [PubMed]
- Zhou X, Chen D, Wang L, Zhao Y, Wei L, Chen Z, 2020. Low serum calcium: a new, important indicator of COVID-19 patients from mild/moderate to severe/critical. Biosci Rep. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Additional data may reasonably be requested from the corresponding author.