Abstract
The maintenance of poultry gut health is complex depending on the intricate balance among diet, the commensal microbiota, and the mucosa, including the gut epithelium and the superimposing mucus layer. Changes in microflora composition and abundance can confer beneficial or detrimental effects on fowl. Antibiotics have devastating impacts on altering the landscape of gut microbiota, which further leads to antibiotic resistance or spread the pathogenic populations. By eliciting the landscape of gut microbiota, strategies should be made to break down the regulatory signals of pathogenic bacteria. The optional strategy of conferring dietary fibers (DFs) can be used to counterbalance the gut microbiota. DFs are the non-starch carbohydrates indigestible by host endogenous enzymes but can be fermented by symbiotic microbiota to produce short-chain fatty acids (SCFAs). This is one of the primary modes through which the gut microbiota interacts and communicate with the host. The majority of SCFAs are produced in the large intestine (particularly in the caecum), where they are taken up by the enterocytes or transported through portal vein circulation into the bloodstream. Recent shreds of evidence have elucidated that SCFAs affect the gut and modulate the tissues and organs either by activating G-protein-coupled receptors or affecting epigenetic modifications in the genome through inducing histone acetylase activities and inhibiting histone deacetylases. Thus, in this way, SCFAs vastly influence poultry health by promoting energy regulation, mucosal integrity, immune homeostasis, and immune maturation. In this review article, we will focus on DFs, which directly interact with gut microbes and lead to the production of SCFAs. Further, we will discuss the current molecular mechanisms of how SCFAs are generated, transported, and modulated the pro-and anti-inflammatory immune responses against pathogens and host physiology and gut health.
Keywords: Dietary Fibers, G-protein-coupled Receptors, Gut Microbiota, Histone Deacetylases, Short-chain Fatty Acids
INTRODUCTION
The development of the gastrointestinal tract (GIT) starts at an early embryonic stage where certain factors including dam, diet, and environment affect its formation. During embryonic development, several species of microflora from the hen’s reproductive tract and eggshell moved to the embryo, and soon after birth, they start multiplying rapidly while certain other bacterial species and microorganisms from eggshells, environment, and feed also join existing microbiota and start colonization in certain parts of GIT [1]. Among the bacterial species, many acts as beneficial for the host and play important role in several physiological processes such as nutrient absorption, metabolism, tissue development, immune homeostasis, and maintaining overall health [2]. Microflora regulates these processes by the production of different metabolites such as short-chain fatty acids (SCFAs) which are mainly produced by colonic anaerobic bacterial fermentation of dietary fibers (DFs) [3]. The SCFAs are chemically composed of hydrocarbon chains and carboxylic acid moiety [4]. In poultry, the most frequently studied SCFAs are the acetate, propionate, and butyrate with two, three, and four carbon molecules in their chemical structure while their effect on several systems has been accentuated both at cellular and molecular levels [5].
In the past two decades, there has been immense research on optimizing poultry gut health by modulating intestinal microbiota through dietary interventions, and in this regard, DFs seemed important [6]. There is a difference between single and compound stomach animal species for the digestion of fibers. Unlike compound stomach animals, the microbial fermentation of DFs in single stomach animals like chicken mainly takes place in the hindgut though each part of GIT harbors its own specific microbiota. The DFs greatly affect the poultry’s gut physiology by modulating its microbiota and eliminating solid waste products [7]. Previously localization of different microbiome in GIT has been described in chicken [8] however the interaction between DFs and gut microbiota has received little attention [9]. Similarly, there is also a need to focus on interaction between DFs, mucus, and epithelial cells which play a central role in development of physicochemical and immunological barriers to restrain the microbiota and to halt invading pathogens, antigens, microbial populations, and toxins [10].
In this review, we have focused on clear and recent narrations of the microbially-derived SCFAs on host tissues as well as their cellular and molecular mechanisms. For this, we have mainly focused on different sources of DFs, their interrelationship with microbiota, and their influence on mucus. For better understanding, we have summarized the intestinal epithelial cell (IEC) polarity, generation mechanisms, and transportation of microbially-derived SCFAs, and their role in modulating pathophysiological changes in the gut, histone deacetylases (HDACs) inhibition, and pro-and anti-inflammatory consequences on immune cells.
TYPES OF DIETARY FIBERS AND THEIR IMPACT ON GASTROINTESTINAL TRACT
Dietary fibers are homogenous or heterogenous carbohydrate polymers with three or more monomeric units. They are resilient to digestion by host endogenous enzymes and belong to the following three categories: i) present naturally in the foods and available to the farm animals (i.e. cereals, legumes, etc.), ii) acquired from raw food materials via chemical, enzymatic, and physical means and iii) synthetic ones such as polydextrose. The DFs can also be defined based on their chemical composition and nutritional functions such that chemically they are the sum of lignin and non-starch polysaccharides while nutritionally they are the carbohydrates indigestible by host endogenous enzymes [11]. DFs are also categorized depending upon their primary food sources, water-solubility, chemical structure, and degree of fermentation. They are subdivided into resistant starch (RS), resistant oligosaccharides, polysaccharides, soluble and insoluble forms [12]. All types of fiber, however, do not exist in the same category of food such as RS only exists in cereals and legumes, while arabinoxylans and β-glucans are found only in cereals [13]. In the upper part of the GIT, RS withstands enzymatic digestion in pigs, so their digestion takes place in the hindgut where they yield butyrate-producing Bifidobacterium adolescentis, Parabacteroides distasonis, Faecalibacterium prausnitzii, and total SCFAs [14].
The most useful sources of DFs that modulate the poultry gut microbiota and contribute to poultry health are given in Table 1. Inulin supplementation had been shown to improve host gut inflammatory responses and gut health through promoting Lactobacilli and Bifidobacteria colonization and increasing SCFAs production largely butyrate, acetate, and propionate in broilers which exert antibacterial properties to combat Salmonella infection in chicks [15]. In addition, the intake of dietary fructooligosaccharide enhances the production of SCFAs and reduces the colonization of Salmonella spp., Clostridia perfringens, and Escherichia coli in broiler chickens [16]. Similarly, supplementation of cellulose affects the composition of microbiota at a level of phylum Bacteroidetes and genus Alistipes in the cecum of Saanen goats [17], whereas this genus may improve the performance of broilers by producing succinate as an end product [18]. Arabinoxylan utilization by ducks had increased the stimulation of Megamonas and Bifidobacterium spp., which further resulted in increased concentration of SCFAs (butyrate, acetate, and propionate) and branch-chain fatty acids (isobutyric acids) [19]. The fermentation of DFs in poultry depends on their physico-chemical properties and the matrix. Most of the DFs such as pectin does not cause fecal bulking effect and are gradually fermented by the gut microbes to produce SCFAs however, several insoluble forms such as cellulose, hemicellulose, and lignin cause fecal bulking effects and are either partly digested by the gut microbes in intestine or excreted as such. Similarly, some of the soluble non-starch polysaccharides polymers with high molecular weight (i.e. β-glucans, guar gum, psyllium, and pectin) are viscid and form a gel-like structure in the intestinal tract and influence the postprandial metabolism of lipids and delay glucose absorption in humans and pigs [12].
Table 1.
Sources, chemical composition, and mechanisms and action of dietary fibers
| Dietary fiber | Chemical composition | Fermentation bacteria | Mechanisms and action | Sources | |
|---|---|---|---|---|---|
|
| |||||
| Main chain | Branch chain | ||||
| Cellulose | β–(1–4)-D-glucose | - | Alistipes spp. and Bacteriodetes bacteria | The supplementation of cellulose changed the composition of microbiota at a level of phylum Bacteroidetes particularly the Alistipes genus in the cecum of broilers. This genus may improve the performance of broilers by producing succinate as an end product [18]. | All plants, particularly cotton, some bacteria, and algae |
| Chitin | β–(1–4)-N-acetyl-D-glucose | - | Oscillospira | Ingestion of chitin (1.02 g/d) by hens throughout 21 weeks of age, enhanced the SCFA production and decreased the triglyceride content in serum and cholesterol content in serum and egg yolks [97]. | Shells of crustaceans, insects, arthropods, yeast, and fungi |
| Chitosan | β-(1–4)-N-acetyl-D-glucosamine and β-(1–4)-D-glucosamine | - | Bifidobacterium spp. and Lactobacillus spp. | Chitosan oligosaccharides ingestion in mice increased SCFAs production that resulted in decreased S. aureus, E.coli, non-typhoidal Salmonella, Listeria spp., Vibrio spp., B. Cereus, and Campylobacter spp. [98]. | The exoskeleton of crustaceans and cell walls of fungi but mostly produced by the deacetylation of chitin |
| Lignin | Polyphenols: p-coumaryl, coniferyl, sinapyl, syringyl, and guaiacyl alcohols | - | Bifidobacteria | Application of lignin in in vitro model has resulted in phenolic metabolites production along with stable Bifidobacteria. Furthermore, lignin improved the performance by reducing the colonization of E. coli, and Enterobacteriaceae in piglets [99]. | Seeds (flax, pumpkin, sunflower, poppy, sesame), whole grains (rye, oats, barley), bran (wheat, oat, rye), beans, fruit (particularly berries), and vegetables |
| β-glucan | β-(1–3)-D-glucose and β-(1–4)-D-glucose | - | Lactobacilli, Bifidobacteria, Roseburia hominis, Clostridiaceae (Clostridium orbiscindens and Clostridium spp.), and Ruminococcus spp. | The intake of barley β-glucan in the human diet had resulted in a marked increase of Clostridiaceae, Roseburia hominis, and Ruminococcus spp. and SCFAs including butyric, acetic, propionic, and 2-methyl-propanoic acids [100]. | Seaweed, brewer’s yeast, oats, lentinan (shiitake), barley, and maitake (grifola) |
| Hemicellulose | |||||
| Xyloglucan | β-(1–4)-D-glucose | Alfa-xylose attached at position 6- of β-D-glucose | Clostridia, lactobacilli, and Bifidobacterium (endo-β-glucanase) | The bacterial strains such as Clostridia, lactobacilli, and Bifidobacterium degraded the xyloglucan and generate acetate and propionate. The in vivo and in vitro application of xyloglucan restored the mucosal leakage by reducing the numbers of E. coli [101]. | All vascular plants (i.e. gymnosperms, clumbosses, ferns, horsetails, and angiosperms) and seeds from tamarind and nasturtium. |
| Arabinoxylan | β-(1–4)-D-xylose | 5-0-trans-feruloyl-α-(L-arabinose), L-Arabinose, 5-0-p-coumaroyl-α-Larabinose at position 2- or 3- of D-xylose | Megamonas and Bifidobacterium spp. | Arabinoxylan utilization by ducks had increased the stimulation of Megamonas and Bifidobacterium spp., which further resulted in increased concentration of branch-chain fatty acids (isobutyric acids) and SCFAs (butyrate, acetate, and propionate) [19]. | ryegrass |
| Galactomannan | β-(1,4)-D-mannose | α-D-galactose at position 6- of β-D-mannose | Lactobacillus and Bifidobacterium spp. | The fermentation end products of prebiotic (B-galactomannan) are the acetates, butyrate, and propionates in humans. B-galactomannan ingestion enhanced mucus production which halted the access of S. Enteritidis to the epithelium in chickens [102]. | Guar gum, locust bean gum, fenugreek, and alfalfa |
| Oligosaccharides | |||||
| Inulin | One terminal α-(1,2)-D-glucose and β-(2,1)-D-fructose | - | Lactobacillus and Bifidobacterium spp. | Inulin supplementation had been known to improve host gut inflammatory responses and gut health through promoting Lactobacilli and Bifidobacteria colonization and increasing SCFAs production largely butyrate, acetate, and propionate in broilers which exert antibacterial properties to combat Salmonella infection in chicks [15]. | Rye, wheat, barley, onion, leek, garlic, and banana |
| Resistant starch | α-(1,4)-D-glucose | α-(1,4)-D-glucose and α-(1,6)-D-glucose | Lactobacillus spp. | The provision of resistant starch resulted in increased number of butyrate-producing Bifidobacterium adolescentis, Parabacteroides distasonis, Faecalibacterium prausnitzii, and total SCFAs in the lumen, small intestine, cecum, and colon of pigs [14]. | Oatmeal, brown rice, corn, lentils, bananas, potatoes, yams, pasta, pearl barley, and navy beans |
| Galactooligosaccharides | One terminal β-(1,3)-D-glucose and β-(1,4)-D-galactose | - | Bifidobacterium and Lactobacillus spp. | In vitro studies demonstrated that Galactooligosaccharides supplementation increased acetate, lactate, and butyrate. Lactate and acetate formation is dependable on lactobacillus and Bifidobacterium fermentation. Its relative abundance within the intestinal tract has been observed to reduce the relative attack and adherence of Salmonella in chickens [103]. | Milk, beans, root vegetables, etc. |
| Fructooligosaccharide | Derived from inulin hydrolysis: one terminal α-(1,2)-D-glucose and β-(2,1)-D-fructose, | - | Bifidobacterium and Lactobacillus spp. | The intake of dietary Fructooligosaccharide enhanced the production of SCFAs and reduced the colonization of Salmonella spp., Clostridia perfringens, and E. coli in broiler chickens [104]. | Onion, chicory, garlic, asparagus, banana, artichoke, etc. |
| Gums | |||||
| Gum Guaran (Guar) | β-(1,4)-D-mannose | α-(1,6)-D-galactose | Bacteroidetes | In vitro application of gum, guar increased the production of SCFAs in the feces of mice which indicated reduced growth of Desulfovibrio [105]. | Bean of guar plant, soy, wheat, corn, yeast, dairy, egg, gluten, and sugar |
| Gum Arabic | β-(1,3)-D-galactose | β-D-glucuronic acid, β-D-galactose, α-L-rhamnose, L-arabinose . Branches attached at position 6- of β-(1,3)-D-galactose | Bifidobacterium and Lactobacillus spp. | Gum Arabic fermentation in the large intestine of human resulted in increased Bifidobacterium spp. In a latest study, the in vitro application of gum Arabic has been observed to increase the production of SCFAs and antimicrobial activity against clostridium [106]. | Acacia Senegal (Acacia) |
It is suggested that young birds should be fed on low levels of DFs (less than 1.5%) diets because high levels decrease nutrient digestibility in early life period and increase transit speed of digesta [20]. During growing phase, however, inclusion levels of 2% to 3% DFs are recommended which improve the gizzard size and feed efficiency in poultry species [21]. For example, the quails fed on a 1.5% wheat bran-based diet showed increased relative length of intestinal segments, villi to crypt depth, villi height, and villi thickness [22]. Whereas lignin supplementation in geese decreased villi length but on the contrary, the supplementation of pectin, alfalfa, and rice hulls had a positive effect on villi height [23,24]. Efforts are on to increase fiber inclusion rate in poultry feed by fortification with the extracted non-digestible carbohydrates. A large variety of fortified oligosaccharides and carbohydrate polymers are commercially available in the form of prebiotics which increases the population of beneficial bacteria in the gut [25]. In addition to their natural presence in different foods, DFs-rich ingredients or isolated DFs molecules can be added to the diet by technological means to provide benefits for extra health.
INTERRELATIONSHIP OF MICROBIOTA, DIETARY FIBERS, AND DIGESTION
Feed ingredients differentially affect the bacterial communities and the production of metabolites depending on their particle size, type, and chemical properties. The DFs act as a carrier of feed anti-oxidants (AOXs). Though not reported in chickens yet, the main physiological function of DFs is to convey AOXs across the GIT. Upon reaching the colon, AOXs secrete a fiber matrix to produce an AOX environment and reveal metabolites [26]. The type of fiber reaching the posterior gut is the key in defining the type of bacteria and the metabolites (SCFAs) being produced. Such that supplementation of pigs diet with RS increased SCFAs production by 34% compared with the digestible starch diet [27]. Though corn-soybean meal-based diet increases the concentration of Lactobacillus spp. and SCFAs production in duodenum, jejunum, and ileum in broilers of all age groups yet high DFs diets based on corn-soybean meal-dried distillers grains and wheat bran produce even higher SCFAs compared with low DFs diets (i.e. corn-soybean meal) [28]. So increased production of SCFAs by dietary manipulation with DFs reflects that fiber maintains integrity and diversity of the GIT microbiota which further increases the fermentation of complex fibers and release of energy for the host. Chickens, ducks, and geese do not produce enzymes as hosts to break down DFs such as fructooligosaccharides, xylooligosaccharides, and mannan [29]. In these species, DFs are believed to reach the ceca in undigested form and then undergo microbial fermentation. Culture-based studies showed that different bacterial genera i.e. Clostridium, Bacteroides, and Bacillus synthesize mannanases that can unfold β-1,4 mannopyranoside bonds in mannan products [29]. Metagenomics analysis of ceca in chickens showed over 200 non-starch polysaccharides degrading enzymes, oligosaccharide- and polysaccharide-degrading enzymes, and several pathways related to SCFAs production which highlight the functional properties of the cecal microbiota [30]. At present exogenous enzymes including phytases and carbohydrases such as amylases, xylanases, and β-glucanases are used in broilers to degrade complex carbohydrates into their respective sugars or amino acid components [31]. Bacteria can then ferment them into metabolites (i.e. SCFAs), CO2, H2, branched-chain fatty acids, ammonia, and other carboxylic acids [32]. However, to reduce the use of exogenous enzymes, future studies need to focus on identifying specific intestinal bacteria that promote different enzymatic reactions to support the fermentation of DFs in poultry species.
INTERACTION OF DIETARY FIBERS WITH GUT MUCUS
The entire surface of the chicken GIT is protected by a layer of mucus. Mucus is a glycoprotein and being a part of innate host, immune response is produced by goblet cells. It protects the IECs from bacterial, mechanical, and chemical injuries [33]. It halts the direct interaction of luminal antigens with the epithelium. The DFs have a significant effect on mucus secretion, cell proliferation, and changing luminal environment thus they are involved directly or indirectly with intestinal health [34]. To maintain the mucus layer, however, the genetic makeup of the host and interaction of intestinal microbiota with DFs are important. The goblet cell numbers are used as an indicator of mucus production in monogastric animals [35]. In golden hamsters feeding different sources of DFs i.e. oat bran and rye bran were found to improve goblet cell numbers in the gut, while pectin was also found to intensify the mucus layer via a high-water holding capacity mechanism (dehydration combat mechanism) [36]. Therefore, they are known as a stimulator of goblet cells proliferation. The role of DFs in increasing the mucin2 gene expression, fecal mucin output, and goblet cell numbers has also been reported recently [37]. A lower level of DFs predisposes redundancy of mucus layer and can lead to infectious susceptibility and the reclamation of chronic inflammatory diseases [38]. A higher level of DFs however, can cause loss of endogenous amino acids. For example, DFs have been found to increase the number of mucin-producing goblet cells. Mucus contains a high concentration of mucin (a threonine-rich glycoprotein) that becomes scarce during high mucus production [39].
The IECs are enclosed by a mucus layer, thus keeping the bacteria away from mucosa deterioration [40]. Intestinal microbiota ferments DFs and produces SCFAs and both stimulate mucus production and regulation. The Bacteroides thetaiotaomicron spp. which produces acetate and propionate, also stimulate goblet cell differentiation and expression of mucin-related genes. Moreover, Faecalibacterium prausnitzii spp. uses acetate and can produce butyrate, thus stabilizing the proper physiology of the gut epithelium by averting the overproduction of mucus.
MECHANISMS TO CONTROL INTESTINAL EPITHELIAL CELL POLARITY, ROLE OF DIETARY FIBERS, AND GUT HEALTH
Introduction of intestinal epithelial cell polarity in intestine
The mammalian GIT consists of distinct layers of cells. It requires precise interaction between each layer or cell type to perform different functions i.e. absorption of dietary nutrients and water, discharge of waste products via peristaltic contractile movements, and maintaining a physical barrier against pathogens. The two most remarkable cell populations of the intestine are the muscle cells and epithelial cells. The intestinal epithelium is distributed into two parts; flask-shaped submucosal invaginations- crypts, and finger-like luminal protrusions- villi [41]. The IECs start their growth from Wnt responsive Lgr5+ stem cells (multipotent stem cells), differentiate, and get mature at the base of crypts [42]. While the absorptive Alpi+, Lgr5− enterocytes move up toward the villus until they reach the villus tip where they go through apoptosis and shed themselves into the lumen to balance defensive barrier under different physiological conditions [43] (Figure 1). This process of turnover lasts for two to five days followed by a bone morphogenetic protein (BMP) signaling pathway [44].
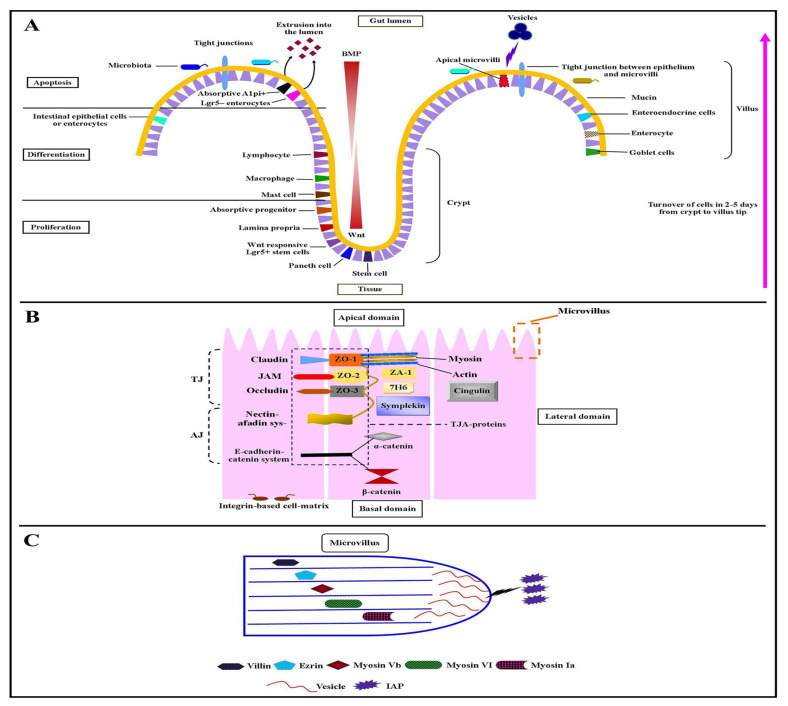
Figure 1.
A depiction of epithelial cell polarity. (A) Turnover of cells in 2–5 days from crypt to villus tip and (B) α-catenin in Figure 1. (C) The box area from panel B describes the single microvillus within its protein components such as villin, ezrin, myosin Vb, myosin-VI, and myosin-Ia, and the microvillus-derived vesicle with alkaline phosphatase enzyme.
IECs are organized as a monolayer of columnar-shaped, polarized epithelial cells (Figure 1A). The surface of IECs is divided into two domains; apical domain—facing to the lumen of the gut, and basolateral domain—facing to the intestinal tissue. The basolateral cell-surface domain is further divided into a basal (face basement membrane) and a lateral domain (face neighboring cells) (Figure 1B). The basal surface domain is a home for integrin-based cell-matrix adhesions, through which the cells are interconnected with the basement membrane. While the lateral surface domain is a house for intercellular adhesions such as E-cadherin-based adherens junctions and claudin-based tight junctions (TJs) [45] (Figure 1B). The intestinal tight junction-complex-associated proteins consist of surface membrane proteins (i.e. claudin, occludin, junctional adhesion molecules, the coxsackievirus and adenovirus receptors proteins), and intracellular proteins (i.e. zona occludens [ZO-1, ZO-2, and ZO-3], cingulin, 7H6, symplekin, and ZA-1) [46]. It has been documented that the TJs and polarized intracellular trafficking machinery are the central players in the IECs’ polarity and establish communications between gut lumen and bodily tissues to maintain immune tolerance with commensal bacteria and control gastrointestinal pathogens [47]. The adherens junction consists of the E-cadherin-catenin system and nectin-afadin system. The partner of nectin that is known as “afadin” directly or indirectly binds with a number of proteins, including zona occludens proteins. Several studies demonstrated that the modulation of selectively induced Bifidobacterium spp. enhanced barrier function and the function of tight junction-associated proteins [48]. The TJs have further joined with the actin filament-based projections of apical plasma membrane known as microvilli (Figure 1B).
Role of apical plasma membrane microvilli and their protein components in intestinal epithelial cell polarity
The apical plasma membrane microvilli and their protein components such as villin, ezrin, myosin-Vb, myosin-VI, and myosin-Ia play important role in IEC’s polarity. Villin is an actin-modifying protein situated in the basic subapical terminal web and the apical plasma membrane microvilli (Figure 1C) [49]. Villin expression in IECs represents induced inflammation and related lesions under inflammatory bowel disease, which highlights the dynamics of IEC’s polarity in gut wound healing, immunopathology, and intestinal epithelial homeostasis. In humans, the depletion of myosin-Vb protein in microvillus due to disease and MYO5B mutations results in inactivation of ezrin protein at apical surface and cause microvillus atrophy in the IECs [50]. The loss of another component of microvilli i.e. myosin-Ia results in tumor development, loss of IECs polarity, and carcinogenesis in mice [51]. The function of myosin-VI and myosin-Ia controls the base-directed movement and microvillus tip of plasma membrane respectively [52]. Both of these myosins regulate the circulation of brush-border enzymes such as intestinal alkaline phosphatase (IAP) and intramicrovillus (microvillus tip and microvillus base). The tips of the apical microvilli produce vesicles that are extruded into the gut lumen [53]. The microvillus-derived vesicles are rich in IAP (Figure 1C). The overexpression of this enzyme under the exposure of IECs to E. coli gives rise to an increased abundance of microvilli-derived vesicles [54].
Role of dietary fibers in controlling intestinal epithelial cell polarity
The DFs such as wheat bran has been used in controlling colonic mucosal proliferation and in preventing proliferative diseases of colon [55]. Thought, specific therapeutic strategies such as DFs, probiotics, and prebiotics supplementation have been used to manage some of the diseases including obesity, type-1 diabetes, and colorectal cancer in mice, and different pathogens causing diseases such as salmonella enteritidis, necrotic enteritis, and clostridium perfringens in broilers [56]. Up to date in poultry, it is not clear yet whether the DFs, probiotics, prebiotics, and the microbial-derived metabolites (i.e. SCFAs) affect polarized intracellular trafficking machinery involving proteins such as villin, ezrin, myosin-Vb, myosin-VI, myosin Ia, and IAP and either their (proteins) expression increase the stimulation of microvilli-derived vesicles, establishing intestinal epithelial homeostasis, wound healing, and tumor suppression or not. There is a need to get insight into the IEC polarity to understand the mechanisms involved in the maintenance of epithelial homeostasis, immune system by commensal bacteria, and balanced communications among gut lumen, body tissues, and gastrointestinal pathogens. Owing to fast differentiation and turnover the IECs have become a focus of the current research. However various factors such as diets, diseases, hormones, and genetics affect IECs turnover and differentiation along crypt-villus axis.
INTERPLAY BETWEEN DIETARY FIBERS AND MICROBIOME GIVES RISE TO METABOLITES (SHORT-CHAIN FATTY ACIDS)
Metabolites are small molecules produced by metabolic reactions, catalyzed by gut enzymes or bacterial fermentation of various foods. Through these small molecules, the gut microbiota communicates and makes a tensile network with the host [57]. The microbial metabolites affect host immune maturation, energy metabolism, immune homeostasis, and mucosal integrity. Variation in the gut microbial metabolites has been illustrated in many studies during salmonellosis, E. coli infection, necrotic enteritis condition, and Campylobacter jejuni infection [58]. Beneficial microbiota produces SCFAs including acetic acid, propionic acid, and butyric acid which have bacteriostatic ability to destroy campylobacteriosis, salmonellosis, and E. coli causing bacteria.
In this context, the composition and particle size of diet not only maintain host health but also modulates the beneficial microbial communities, their richness, and diversity in the digestive tract. The origin, type, and quality of diet modulate the gut microbiota in a time-dependent manner. Long-term dietary regimes particularly plant-based and animal fat or protein-based diets are associated with so-called enterotypes. This dichotomy in the plant-based diet/animal-based diet ratio was also observed in broilers and laying hens arguing that these bacterial communities are driven by a long-term modification in the diet [59].
Gut microbiota can ferment undigested carbohydrates in most parts of the GIT, but in poultry, this process mainly occurs in crop and caecum where bacteria are abundantly populated. The crop and ileum are the main lactic acid-producing repertoires, owing to the presence of a high concentration of Lactobacillus spp., as opposed to caeca where the quantity of butyric, acetic, and propionic acids are higher [60]. Furthermore, Jozefiak et al [6] observed that the cereal-type feed ingredients such as wheat, rye, and triticale determine the quantity of acetic acid in the caecum except for the crop and gizzard. This suggests that the application of DFs is a potential way to influence SCFAs concentration. The SCFAs enhance beneficial microbial populations to regulate endogenous enzymatic activities and produce more energy and carbon for IECs [61], thus contributing to maintaining mucosal integrity, immunity, and health of broilers, geese, and ducks [3]. Therefore it is important to highlight the potential role of specific types and sources of DFs that can be used in manipulating the commensal bacterial populations which specifically induce the synthesis of SCFAs. Similar studies in poultry species are warranted to investigate possible involvement of DFs in early postnatal development and microbially modulated DNA methylation.
MECHANISMS INVOLVED BEHIND THE GENERATION AND TRANSPORTATION OF SHORT-CHAIN FATTY ACIDS AND THEIR ROLE IN MODULATING PATHOPHYSIOLOGICAL CHANGES IN THE GUT
Generation of short-chain fatty acids
Microbiota hydrolyze DFs into oligosaccharides and then produce monosaccharides in the hindgut under anaerobic environment conditions and produce SCFAs. This production of SCFAs consists of enzymatic pathways which are regulated by several bacterial species (Figure 2). The prime pathways for SCFAs generation are the Embden-Meyerhof-Parnas pathway (glycolytic pathway) and pentose phosphate pathway driven by Bifidobacteria which transform monosaccharides into phosphoenolpyruvate (PEP) [62]. The PEP is then converted into alcohols or organic acids (Figure 2A).
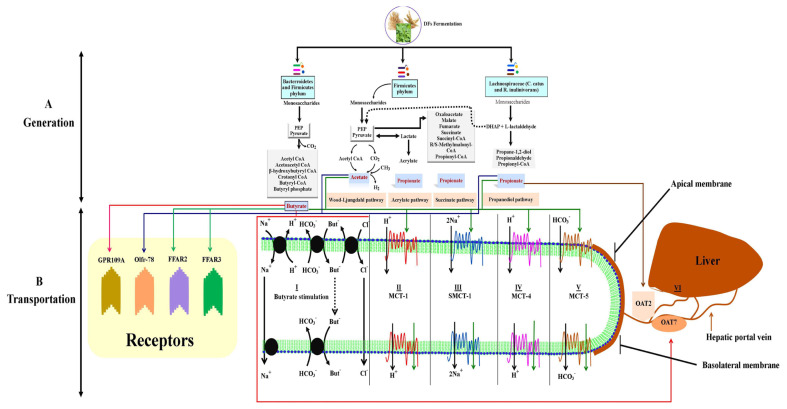
Figure 2.
(A) Gut microbial fermentation and enzymatic pathways involved in acetate, propionate, and butyrate generation. Butyrate is produced either by the precipitation of two molecules of acetyl CoA or by enzyme butyryl- CoA:acetate-CoA-transferase. Acetate is produced either by acetyl CoA or by the Wood-Ljungdahl pathway. Propionate is formed through three pathways namely acrylate, succinate, and propanediol pathways. (B) The proposed transport mechanisms of short-chain fatty acids (SCFAs). I) butyrate stimulation of Na+ and Cl−, II) transportation of SCFAs via monocarboxylate transporter (MCT)-1, III) transportation by sodium-coupled monocarboxylate transporter-1, IV) SCFAs which are not absorbed by colonocytes transported through a basolateral membrane, where MCT-4 transports SCFA anions in an H+-dependent electroneutral manner, V) transportation by MCT-5 is via unknown HCO3− exchanger, and VI) transportation of unabsorbed propionate and butyrate into the liver by organic anion transporter (OAT) 2 and 7 respectively via sinusoidal membrane of liver cells (hepatocytes).
The enzymatic pathways for the synthesis of SCFAs have been previously described. In brief, oxygen-sensitive Wood-Ljungdahl pathway of SCFAs production was detected by radioisotope analysis and in this, propionate production was detected via CO2 fixation pathway and butyrate by the condensation of acetyl-S coenzyme A [63]. The main bacteria engaged in butyrate production were Cytophaga and Flavobacterium belonging to Bacteroidetes phylum (main bacterial spp; Coprococcus species, Clostridium leptum, Faecalibacterium prausnitzii, and Roseburia species belonging to both Bacteroidetes and Firmicutes phyla) [64] (Figure 2A). The Wood-Ljungdahl pathway is carried out by acetate-producing bacteria namely Acetogens (Firmicutes phylum) [65] (Figure 2A). Besides this, there are other pathways such as the fructose-6-phosphate phosphoketolase pathway present in the Bifidobacterium genus, also known as the Bifidobacterium pathway. In this pathway, the Bifidobacterium genus uses monosaccharides to produce SCFAs, particularly acetate. Propionate is produced by the acrylate pathway. Reichardt et al [66] described three pathways used by bacteria for the production of propionates such as succinate pathway, acrylate pathway, and propanediol pathway (Figure 2A). In these pathways, two species of Lachnospiraceae such as C. catus and R. inulinivorans have been seen to switch butyrate to propionate production from different substrates.
Transportation of short-chain fatty acids
The epithelial cells of the colon i.e. colonocytes are mostly studied for the transportation of SCFAs (Figure 2B). The apical membrane gains the SCFAs in two ways i.e. active transport of dissociated SCFA anions, and passive diffusion of undissociated SCFAs. Three mechanisms support the transportation of SCFA anions via the apical membrane of colonocytes. In the first mechanism, the transporter introduces SCFA anions to HCO3− to form SCFA-HCO3− exchange in the vesicle and then secret it into the gut lumen. So this exchange is independent of Na+ transporter and Cl−-HCO3− exchange [67]. The second mechanism involves the members of family of monocarboxylate transporters that increase the chemical reaction of SCFA anions with cations [68]. The monocarboxylate transporter-1 (MCT-1) transports SCFAs in an H+ dependent electroneutral manner in the apical membrane of enterocytes. Besides this, MCT-1 shows high expression in the lymphocytes which denotes its role in SCFA transportation. The third mechanism of SCFA anion transportation is carried by the sodium-coupled monocarboxylate transporter-1 (SMCT-1) [69]. In this, the SCFA anions are introduced to Na+ transporters in a 1:2 stoichiometry to increase water and Cl− absorption (Figure 2B). The SMCT-1 is highly expressed in the large intestine, kidney, and thyroid gland, where it transports butyrate with high affinity as compared to propionate and acetate [70].
The leftover SCFAs, not absorbed by the colonocytes, are transported towards the basolateral membrane. This membrane contains MCT-4 and MCT-5 transporters. The MCT-4 transporter, transports SCFA anions in an H+-dependent electroneutral manner, however, MCT-5 transporter, transports SCFA through HCO3− exchangers [71] (Figure 2B). The transporters of SCFAs have been observed from duodenum, ileum, ceca, colon, lungs, and liver, however, the transporters for the uptake of SCFAs from the blood are mostly undefined. Organic anion transporters such as organic anion transporter 2 and 7 are involved in the transportation of propionate and butyrate respectively through the sinusoidal membrane of liver cells (hepatocytes) [72]. In the liver, the propionate and acetate can be used as substrates for the energy-producing tricarboxylic acid cycle to produce glucose. For better understanding, the transportation and uptake mechanisms for SCFAs in various tissues should be investigated.
Role of short-chain fatty acids in modulating the pathophysiological changes in the gut
It is known that SCFAs play a key role in modulating the gut health of pigs and poultry species (Table 2). SCFAs undergo antimicrobial pathways and enter the membrane of the pathogenic bacteria. The cytoplasmic pH of bacteria is generally neutral while SCFAs exist in associated or dissociated forms [73]. When SCFAs attack, they dissociate into protons and anions and reduce the pH of a bacterial cell, failing to maintain which, the bacterial cell gets destroyed. Antimicrobial activation of formate, acetate, and propionate has been documented in in-vitro studies. The in-vivo studies conducted in humans, pigs, and broilers have also shown antimicrobial effects of SCFAs on pathogens. In this regard, supplementation of piglets’ diet with wheat, improved the production of SCFAs and reduced the incidences/infection of E. coli [74]. Similarly, supplementation of butyralated high-amylose maize starch to broilers challenged with necrotic enteritis resulted in the production of ileal acetate and reduction in caecal pH which ameliorated the negative effects of disease [75]. In another study supplementation of wheat bran and aArabinoxylo-oligosaccharides in broilers promoted butyrate, propionate, and Firmicutes to Proteobacteria production and decreased caecal Enterobacteriaceae [76]. Moreover, the supplementation of chicory roots and sweet lupins increased the abundance of commensal microbiota i.e. Bifidobacterium thermacidophilum subspp. Porcinum, and Megasphaera elsdenii, lactate producers and lactate utilizing butyrate producers respectively and attenuated the abundances of intestinal spirochaete and B. hyodysenteriae [77]. So it is clear that fiber intake helps in improving gut health and decreases the load of pathogenic bacteria, however, it is still to find the best source or combination of fibers that can maintain gut health and eliminate chances of E.coli, Salmonella, and necrotic enteritis type of infections from poultry species. In addition to that how fiber sources decrease the involvement of pathogens and what are specific pathways involved, need further investigations.
Table 2.
Role of short-chain fatty acids in modulating the pathophysiological changes in pigs and poultry species
| Origin | Virulence | Virulent factor | Challenge | Dietary fibers | Pathophysiological effects | Reference |
|---|---|---|---|---|---|---|
| Broilers | Necrotic enteritis and inflammation of the small intestine | Lactose-negative enterobacteria and clostridium perfringens | S. Typhimurium DT110 | Whole wheat and oat hulls | Increased hydrochloric acid secretion and grinding processes in the gizzard, and reduced its pH | [107] |
| Broilers | Necrotic enteritis and inflammation of the small intestine | C. perfringens | Necrotic Enteritis | The acetylated high amylose maize starch and Butyralated high amylose maize starch | Increased short-chain fatty acids (SCFAs) generation and decreased luminal pH | [75] |
| Broilers | Paratyphoid infections, S. Enteritidis, and foodborne diseases | Salmonella enterica and E. coli | Streptomycin resistant S. enterica serotype Enteritidis phage type 4 strain 147 (SE147) | Wheat bran and Arabinoxylo-oligosaccharides | Increased butyric acid, propionic acid, and Firmicutes to Proteobacteria production and decreased caecal Enterobacteriaceae levels | [76] |
| Broilers | Destroy the epithelial mucosal layers and infection of Peyer’s patches of the small intestinal wall | S. Typhimurium | S. Typhimurium invasion genes (invA, B, C, and D) | Salmonella with wheat bran | Decreased the impacts of hilA (a transcriptional activator of Salmonella pathogenicity island I vital for Salmonella) into epithelial cells | [108] |
| Laying hens | Destroy the epithelial mucosal layers and infection of Peyer’s patches of the small intestinal wall | Salmonella | Gavage | Fructooligosaccharide | Decreased the intestinal bacterial populations by increasing the growth of Lactobacillus and Bifidobacterium spp. | [109] |
| Piglets | Post-weaning diarrhea | E. coli and Salmonella | - | Oat hulls | Decreased fecal biogenic amines, cadaverine, and β-phenylethylamine. | [110] |
| Piglets | Post-weaning diarrhea | E. coli | E. coli K88 | Wheat bran | Increased butyric acid and total SCFAs production with reduced intestinal enterobacterial populations particularly challenged Ileal E. coli K88 adhesions. | [74] |
| Piglets | Diarrhea | E. coli | E. coli | Inulin | Increased Lactobacillus: coliform ratio and SCFA concentrations. | [111] |
| Piglets | Intestinal mucosal damage and diarrhea | E. coli | Enterotoxigenic E. coli | 10% Wheat bran fiber and pea fiber | Increase Lactobacillus in ileum and Bifidobacterium populations in colon which further increased colonic goblet cells, peptide trefoil factors, and villous height: crypt depth ratio in the ileum. | [112] |
| Pre-weaned pigs | Salmonella induced diarrhea | S. typhimurium | S. typhimurium798 | Fermentable fiber | Increased SCFAs in the colon and glutamine transport. | [113] |
| Pigs | Swine dysentery (contagious diarrheal disease) and Trichuris suis (whipworm) | Intestinal spirochaete and B. hyodysenteriae | B. hyodysenteriae | Chicory root (fructans) and sweet lupins (galactans) | Increased the abundance of commensal microbiota such as Bifidobacterium thermacidophilum subsp. porcinum and Megasphaera elsdenii, lactate producers and lactate utilizing butyrate producers respectively. | [77] |
| Pigs | Swine dysentery (SD) (contagious diarrheal disease) | Affected large-intestinal microbiota to induce extensive inflammation and necrosis of the epithelial surface of the caecum and colon. | B. hyodysenteriae | Inulin and lupins | Increased the caecal SCFAs with reduced concentration of SD colonization | [114] |
RECEPTORS, TARGET TISSUES, AND FUNCTIONS OF SHORT-CHAIN FATTY ACIDS
The SCFAs regulate the immune system through signaling mechanisms, the understanding of which may help improve immune system and reduce risk of certain diseases. There are two mechanisms by which SCFAs modulate immune cell chemotaxis, cytokines, and reactive oxygen species (ROS) of the host. The first mechanism is the activation of G-protein-coupled receptors (GPCRs) such as free fatty acid receptors (FFAR)-2 and -3 (also known as GPR43 and GPR41 receptors), niacin receptor 1, or GPR109A or hydroxyl-carboxylic acid 2 receptor, and olfactory receptor (Olfr78) [78,79]. Currently, we have reviewed four SCFA-sensing GPCRs which are expressed on IECs, endocrine cells, and leukocytes and play a central role in the regulation of metabolism (Table 3). The second mechanism consists of the direct inhibition of HDACs [80]. To get insight into HDACs inhibition, the mechanisms supporting SCFA-facilitated HDAC inhibition and their immunological consequences are discussed here.
Table 3.
Summary of the currently recognized SCFAs-activated GPCRs including their ligands, expression, and functions
| Receptor | Ligands | Expression in tissues | Expression in cell types | Functions | References |
|---|---|---|---|---|---|
| GPR43 (FFAR2) | Acetate, propionate, butyrate, caproate, and valerate | Intestine, immune cells, murine hemopoietic tissues, and spleen | Endocrine L-cells, colonocytes, enterocytes, eosinophils, basophils, neutrophils, monocytes, dendritic cells, mucosal mast cells, and bone marrow | Anorexigenic effects through the secretion of peptide YY and glucagon-like peptide-1, development or differentiation of immune cells, anti-inflammatory role in reducing the risk of preterm labor induced by pathogens, decreases cyclic adenosine monophosphate (cAMP) levels and increases cytoplasmic calcium concentrations, inhibits NF-KB, and reduces the expression of pro-inflammatory cytokines, IL-6 and IL-1β. | [78,115] |
| GPR41 (FFAR3) | Acetate, propionate, butyrate, caproate, and valerate | Adipose tissues, spleen, intestine, immune cells, and pancreas | Adipocytes, monocytes, enteroendocrine L-cells, neutrophils, monocyte-derived dendritic cells, and peripheral blood mononuclear cells | Inhibits adenylyl cyclase, reduces the levels of cAMP, and stimulates sympathetic activation by acting on the sympathetic ganglion. | [116] |
| GPR109A or HCA2 or NIACR1 | Butyrate and niacin | Immune cells, intestine, and adipose tissues | Dermal dendritic cells, monocytes, macrophages, neutrophils, and adipocytes | Suppresses lipolysis and plasma-free fatty acid levels and regulates the vascular inflammation in atherosclerosis | [86] |
| Olfr78 | Acetate and propionate | Kidney, colon, lungs, heart (autonomic nerves), and prostate | Juxtaglomerular cells, enteroendocrine cells, airway smooth muscle cells, prostate epithelium, and melanocytes | Mediates renin secretion in response to SCFAs and controls blood pressure system | [79,117] |
SCFAs, short-chain fatty acids; GPCRs, G-protein-coupled receptors; IL, interleukin; GPR43, free fatty acid receptor; GPR109A, G-protein-coupled receptor 109A; HCA2, hydroxycarboxylic acid receptor 2; NIACR1, niacin receptor 1; Olfr78, olfactory receptor.
Pro- and anti-inflammatory effects of short-chain fatty acids in immune cells
The host immune system impedes the pathogens by producing inflammatory cytokines. Though, unnecessary secretion of cytokines gives rise to systemic inflammation. SCFAs modify systemic inflammation by modulating the release of immune cell cytokines, ROS, and chemotaxis (Figure 3). Propionate and butyrate activate FFAR2/3 or GPR109A or inhibit HDACs to decline the nitric oxide synthase and tumor necrosis factor-alpha (TNFα) expression in monocytes. Upon tracheal inflammation, SCFAs activate FFAR2 and FFAR3 from the macrophages and neutrophils which lead to the reduction of interleukin-8 (IL-8) in the trachea [81]. Butyrate acts anti-inflammatory factor in macrophages by activating FFAR3 and reducing interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), TNFα, and inducible nitric oxide synthase [82]. In mononuclear cells of humans and mice, acetate had anti-inflammatory effects and it inhibits lipopolysaccharides (LPS)-induced TNFα production through FFAR2 and FFAR3 activation. Treatment of allergic mice with propionate decreased inflammatory mediators, e.g. interleukin-17A (IL-17A), IL-4, and IL-5 through FFAR3 activation pathway [83]. Similarly, propionate was found useful in treating lungs of allergic mice.
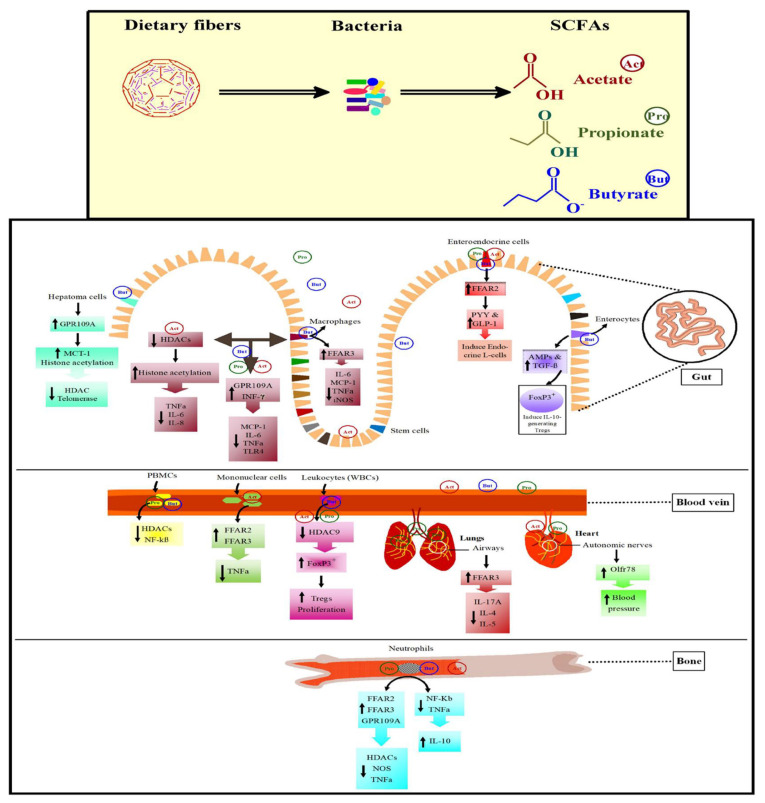
Figure 3.
Synthesis of short-chain fatty acids (SCFAs) serves as an integrative bridge with intestinal epithelium by binding G-protein-coupled receptors (GPCRs) or inhibiting histone deacetylases (HDACs) mechanisms. Dietary fibers are converted into SCFAs such as acetate, propionate, and butyrate by gut microbiota. Then they go through biological processes and modulate pro- and anti-inflammatory immune phenotypes through activating GPCRs and blockading HDACs mechanisms in the gut, blood veins, and bodily tissues including the heart, lungs, and bones. Butyrate is one of the main SCFA that is metabolized by mature enterocytes by anaerobic β-oxidation and provides a maximum of the energy up to 60% to 70%. Butyrate increases the transforming growth factor-beta (TGF-β) and antimicrobial peptides expression in enterocytes [91]. The regulation of TGF-β increases the production of IL-10-generating Tregs in the colon. Acetate increase the expression of FFAR2 and FFAR3 which induce histone acetylation and cause inhibition of HDACs with decreased inflammatory cytokines production such as TNFα, IL-6, and IL-8. Propionate has been used for the treatment of lungs of allergic mice [83] which activates the expression of FFAR3 with reduced inflammatory mediators such as IL-17A, IL-4, and IL-5. FFAR, free fatty acid receptor; IL, interleukin.
Activation of FFAR2 and FFAR3 down-regulate the expression of nuclear factor kappa B (NF-KB) downstream genes and are linked with the regulation of phosphoinositide 3-kinase, mitogen-activated protein kinases, c-Jun N-terminal kinase, extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (p38MAPK), and rapamycin signaling pathways [84]. Upon activation of FFA receptors, acetate regulates p38MAPK and extracellular signal-regulated kinases 1/2 (ERK1/2) signaling pathways thus enhancing the synthesis of cytokines (chemokine (C-X-C motif) ligand1/2 and IL-6). In mice, the knockout of FFA receptors decreased IL-6 synthesis and delay the expression of chemokines and interferon-gamma (INF-γ) [85]. These hallmark studies determine the pro-inflammatory effects of activated FFAR2 and FFAR3.
The expression of GPR109A in macrophages by enhanced INF-γ represents its importance in inflammation and immune regulation system. The activation and regulation of GPR109A blockade the production of MCP-1, IL-6, and TNFα and the expression of TLR4, thus, reducing the chances of atherosclerosis [86]. Butyrate on activating GPR109A acts as an anticancer mediator in human cancer cell lines. In vitro treatment of human hepatoma cells by butyrate decline the activity of telomerase through HDAC blockade. Butyrate as an activator of GPR109A in human colon cancer cells enhances the butyrate transporter MCT-1 expression and further increases apoptosis independent of HDAC inhibition [87]. This suggests that the activation of GPR109A may directly halt colon cancer development or indirectly transport the butyrate to the cell by increasing MCT-1 expression. The enhanced MCT-1 expression in the colon cancer cells is necessary for histone acetylation and also for its anti-tumorigenic function. The intake of whole grain and cereals were coupled with decreased incidence of colorectal cancer predicting the efficient role of SCFAs in cancer treatment [88].
Gut microbial fermentation of polysaccharides undergoes SCFAs production which goes through the portal vein circulation and becomes a part of plasma. A novel receptor of SCFAs “Olfr78” has been observed in blood vessels and autonomic nerves in heart [79] (Figure 3). In a study, antibiotics were used to reduce a load of gut microbiota to notice whether the antibiotics affect blood pressure (BP) in Olfr78−/− mice or not. The results showed a significant increase in BP in Olfr78-deficient mice which suggests that propionate and acetate produced by gut microbiota might be involved in controlling BP. Based on current literature, we can say that SCFAs act as a bridge in maintaining gut health (Figure 3). In poultry species, the role of SCFAs receptors and their efficient participation in inhibiting HDACs has not yet been studied. So further research is required to unveil these processes in poultry.
Short-chain fatty acids (SCFAs)-facilitated histone deacetylases (HDACs) inhibition
Many of the HDACs show expression on smooth vascular muscle cells, immune and endothelial cells. Microflora helps SCFA-facilitated HDAC inhibition and protects immune system. The exact mechanism supporting SCFA-facilitated HDAC inhibition is unclear however it is proposed that two mechanisms are involved i.e. the expression of SMCT-1 transporter and the activation of GPCRs. The SMCT-1 mechanism might cause direct inhibition of HDACs by entering into the cells with the help of transporters. The SMCT-1 promotes butyrate- and propionate-induced barrier of a murine dendritic cell which results in induced HDAC inhibition and DNA acetylation [89]. The GPCRs mechanism involves indirect inhibition of HDACs by SCFAs. For example, the stimulation of FFAR3 in Chinese hamster ovary cell lines inhibited HDACs and caused suppression of histone acetylation [90]. Not only FFAR3 but other receptors of SCFAs may involve in the inhibition of HDACs. SCFA-coupled HDAC inhibition that occurred in the colon was mostly dependent on FFAR2 [91]. Besides this, acetate may have controversial effects in stimulating inflammatory processes in a GPCRs-independent manner by regulating epigenetic modifications. Apart from this, propionate and butyrate may restrain the HDAC activity independent of FFAR3 and FFAR2 [82]. Further research is however required to find how SCFAs inhibit the activation of HDACs directly or indirectly in poultry species.
Immunological consequences of short-chain fatty acids-induced histone deacetylases inhibition
Once SCFA-facilitated HDAC inhibition is developed, it results in a strong anti-inflammatory immune response as briefly described in Figure 3. Previously utilization of butyrate and propionate in the treatment of human peripheral blood mononuclear cells, reduced the production of LPS-induced-TNFα in a similar way to trichostatin A [92]. Similarly, the in-vitro use of acetate for the treatment of human macrophages remarkably diminished the HDAC activity and improved histone acetylation with reduced inflammatory cytokines production such as TNFα, IL-6, and IL-8 [93]. It has been observed that NF-kB has a central role in the stimulation of inflammatory cytokines. This suggests that SCFAs might be involved in the modulation of NF-kB by HDAC inhibition. Similarly, treatment of neutrophils and LPS-activated mononuclear cells by butyrate and propionate decreased the NF-kB activity and TNFα production, and subsequently increased the production of anti-inflammatory cytokine interleukin-10 (IL-10) [94]. These results suggest the prominent role of SCFAs in the production of pro-inflammatory cytokines through inhibition of HDAC activity in humans and rodents. HDAC inhibition resulting from SCFAs is not limited to the cell’s innate immune system but it may also affect the white blood cells especially regulatory T cells (Tregs). The inhibition of HDAC9 in mice promotes the expression of forkhead box P3 (Foxp3) transcription factor, which increases proliferation of Tregs [95]. Similarly, butyrate increases the transforming growth factor-beta (TGF-β) and antimicrobial peptides expression in enterocytes [91]. The regulation of TGF-β increases the production of IL-10-generating Tregs in the colon, suggesting its role in restricting the proliferation of effector T cells. Though RNA-Seq analysis revealed that few birds including Falco cherrug, Falco peregrinus, and Parus humilis express Foxp3 [96] yet it is not known whether the gut-derived microbial SCFAs disturb the Tregs in poultry because the Foxp3 has not yet been identified in chickens and turkeys.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
In conclusion, DFs can promote specific SCFA producing bacteria that may present a novel approach for managing E. coli, necrotic enteritis, and Salmonella in broilers and pigs. Fermentative bacteria mostly target the crop and cecum, whereas impacts of exogenously administered SCFAs may depend on the way of administration and hence diverse from microbially produced metabolites. As an example, oral administration of butyrate may target directly the small intestine and reach the periphery without being consumed by the colonocytes. Tissue-specific impacts of SCFAs have been revealed by propionate, whereby propionate-dependent gluconeogenesis improves metabolic health in the small intestine, whereas hepatic gluconeogenesis is injurious. Considering the expression of SCFA receptors in the blood vessels, small intestine, macrophages, and colonocytes, it can be vital to comprehend SCFA generation.
It is a major challenge to recognize the exact role of imposing opportunities for utilizing SCFAs in host pathophysiology which indispose a good understanding of the mechanisms through which SCFAs exploit their impacts in the animals’ gut, tissues, and organs. Of course, studies with the SCFAs seem to affect health via three main mechanisms; (I) activating GPCRs, (II) increasing histone acetylation and inhibiting HDAC activity, and (III) regulating anti-inflammatory mechanisms because of the first two mechanisms in the tissues and periphery, which can provide new and exciting possibilities for modulating poultry health.
To sum up, DFs can be considered key ancestral com pounds which regulate the macronutrients and preserve host physiology. In brief, we discussed how SCFAs are being generated, transported, and modulated the pro-and anti-inflammatory immune responses against pathogens and improve host physiology and gut health. Finally, screening novel fibers, both extracted and purified from food as well as those synthesized (prebiotics), and defining effective strategies to restore a high amount of fibers aiming at reintroducing the gut microbiome with important omitted SCFAs producing microbes, will be the next question to significantly affect gut microbiota-associated poultry diseases.
ACKNOWLEDGMENTS
We thank teacher Sen Ma (Department of Animal Nutrition and Feed Science, College of Animal Science and Technology, Henan Agricultural University) for his constructive reading and comments.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
This research was supported by the Modern Agro-industry Technology Research System of China (CARS-34) and the Science and Technology Innovation Team of Henan Province High Quality Forage and Animal Health (No.22IRTSTHN 022).
REFERENCES
- 1.Maki JJ, Bobeck EA, Sylte MJ, Looft T. Eggshell and environmental bacteria contribute to the intestinal microbiota of growing chickens. J Anim Sci Biotechnol. 2020;11:60. doi: 10.1186/s40104-020-00459-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14:676–84. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin S, Zhang K, Applegate TJ, et al. Dietary administration of resistant starch improved caecal barrier function by enhancing intestinal morphology and modulating microbiota composition in meat duck. Br J Nutr. 2020;123:172–81. doi: 10.1017/S0007114519002319. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Ma N, Johnston LJ, Ma X. Dietary nutrients mediate intestinal host defense peptide expression. Advan Nutr. 2020;11:92–102. doi: 10.1093/advances/nmz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Zhao L, Liu S, Zhang Z, Wang X, Lin H. Propionate inhibits fat deposition via affecting feed intake and modulating gut microbiota in broilers. Poult Sci. 2021;100:235–45. doi: 10.1016/j.psj.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jozefiak D, Rutkowski A, Kaczmarek S, Jensen BB, Engberg RM, Højberg O. Effect of β-glucanase and xylanase supplementation of barley-and rye-based diets on caecal microbiota of broiler chickens. Br Poult Sci. 2010;51:546–57. doi: 10.1080/00071668.2010.507243. [DOI] [PubMed] [Google Scholar]
- 7.Biasato I, Ferrocino I, Biasibetti E, et al. Modulation of intestinal microbiota, morphology and mucin composition by dietary insect meal inclusion in free-range chickens. BMC Vet Res. 2018;14:383. doi: 10.1186/s12917-018-1690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prakatur I, Miskulin M, Pavic M, et al. Intestinal morphology in broiler chickens supplemented with propolis and bee pollen. Animals. 2019;9:301. doi: 10.3390/ani9060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahmood T, Guo Y. Dietary fiber and chicken microbiome interaction: Where will it lead to? Anim Nutr. 2020;6:1–8. doi: 10.1016/j.aninu.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto Y. Epithelial cells as a transmitter of signals from commensal bacteria and host immune cells. Front Immunol. 2019;10:2057. doi: 10.3389/fimmu.2019.02057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blank B, Schlecht E, Susenbeth A. Effect of dietary fibre on nitrogen retention and fibre associated threonine losses in growing pigs. Arch Anim Nutr. 2012;66:86–101. doi: 10.1080/1745039X.2012.663669. [DOI] [PubMed] [Google Scholar]
- 12.Deehan EC, Duar RM, Armet AM, Perez-Munoz ME, Jin M, Walter J. Modulation of the gastrointestinal microbiome with nondigestible fermentable carbohydrates to improve human health. Microb Spect. 2017;5:5.5.04. doi: 10.1128/microbiolspec.BAD-0019-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellegrini N, Vittadini E, Fogliano V. Designing food structure to slow down digestion in starch-rich products. Curr Opin Food Sci. 2020;32:50–7. doi: 10.1016/j.cofs.2020.01.010. [DOI] [Google Scholar]
- 14.Haenen D, Zhang J, Souza da Silva C, et al. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J Nutr. 2013;143:274–83. doi: 10.3945/jn.112.169672. [DOI] [PubMed] [Google Scholar]
- 15.Song J, Li Q, Everaert N, et al. Effects of inulin supplementation on intestinal barrier function and immunity in specific pathogen-free chickens with Salmonella infection. J Anim Sci. 2020;98:skz396. doi: 10.1093/jas/skz396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang Y, Kumar S, Thippareddi H, Kim WK. Effect of dietary fructooligosaccharide (FOS) supplementation on ileal microbiota in broiler chickens. Poult Sci. 2018;97:3622–34. doi: 10.3382/ps/pey131. [DOI] [PubMed] [Google Scholar]
- 17.Ebrahimi SH, Valizadeh R, Heidarian Miri V. Rumen microbial community of Saanen goats adapted to a high-fiber diet in the Northeast of Iran. Iran J Appl Anim Sci. 2018;8:271–9. [Google Scholar]
- 18.De Maesschalck C, Eeckhaut V, Maertens L, et al. Amorphous cellulose feed supplement alters the broiler caecal microbiome. Poult Sci. 2019;98:3811–7. doi: 10.3382/ps/pez090. [DOI] [PubMed] [Google Scholar]
- 19.Tian D, Xu X, Peng Q, et al. In vitro fermentation of arabinoxylan from oat (Avena sativa L.) by Pekin duck intestinal microbiota. 3 Biotech. 2019;9:54. doi: 10.1007/s13205-019-1571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nursiam I, Ridla M, Hermana W, Nahrowi Effect of fiber source on growth performance and gastrointestinal tract in broiler chickens. In: IOP Conference Series. Earth Environ Sci. 2021;788:012058. doi: 10.1088/1755-1315/788/1/012058. [DOI] [Google Scholar]
- 21.Mateos GG, Jiménez-Moreno E, Serrano MP, Lázaro RP. Poultry response to high levels of dietary fiber sources varying in physical and chemical characteristics. J Appl Poult Res. 2012;21:156–74. doi: 10.3382/japr.2011-00477. [DOI] [Google Scholar]
- 22.Rezaei M, Karimi Torshizi M, Wall H, Ivarsson E. Body growth, intestinal morphology and microflora of quail on diets supplemented with micronised wheat fibre. Br Poult Sci. 2018;59:422–9. doi: 10.1080/00071668.2018.1460461. [DOI] [PubMed] [Google Scholar]
- 23.Chiou PW, Lu T, Hsu J, Yu B. Effect of different sources of fiber on the intestinal morphology of domestic geese. Asian-Australas J Anim Sci. 1996;9:539–50. doi: 10.5713/ajas.1996.539. [DOI] [Google Scholar]
- 24.Teimouri Yansari A. Chemical composition, physical characteristics, rumen degradability of NDF and NDF fractionation in rice straw as an effective fibre in ruminants. Ir J Appl Anim Sci. 2017;7:221–8. [Google Scholar]
- 25.Pourabedin M, Guan L, Zhao X. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome. 2015;3:15. doi: 10.1186/s40168-015-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rehman H, Vahjen W, Kohl-Parisini A, Ijaz A, Zentek J. Influence of fermentable carbohydrates on the intestinal bacteria and enteropathogens in broilers. World’s Poult Sci J. 2009;65:75–90. doi: 10.1017/S0043933909000063. [DOI] [Google Scholar]
- 27.Umu ÖC, Frank JA, Fangel JU, et al. Resistant starch diet induces change in the swine microbiome and a predominance of beneficial bacterial populations. Microbiome. 2015;3:16. doi: 10.1186/s40168-015-0078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu G, Zhao Y, Cao S, et al. Relative bioavailability of selenium yeast for broilers fed a conventional corn–soybean meal diet. J Anim Physiol Anim Nutr. 2020;104:1052–66. doi: 10.1111/jpn.13262. [DOI] [PubMed] [Google Scholar]
- 29.Yamabhai M, Sak-Ubol S, Srila W, Haltrich D. Mannan biotechnology: from biofuels to health. Crit Rev Biotechnol. 2016;36:32–42. doi: 10.3109/07388551.2014.923372. [DOI] [PubMed] [Google Scholar]
- 30.Sergeant MJ, Constantinidou C, Cogan TA, Bedford MR, Penn CW, Pallen MJ. Extensive microbial and functional diversity within the chicken cecal microbiome. PloS One. 2014;9:e91941. doi: 10.1371/journal.pone.0091941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idan F. The role of feed processing and fiber addition on improving the nutrition and growth performance of broilers. Manhattan, KS, USA: Kansas State University; 2019. [Google Scholar]
- 32.De la Fuente G, Yañez-Ruiz DR, Seradj A, Balcells J, Belanche A. Methanogenesis in animals with foregut and hindgut fermentation: a review. Anim Prod Sci. 2019;59:2109–22. doi: 10.1071/AN17701. [DOI] [Google Scholar]
- 33.Duangnumsawang Y, Zentek J, Boroojeni FG. Development and functional properties of intestinal mucus layer in poultry. Front Immunol. 2021;12:745849. doi: 10.3389/fimmu.2021.745849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saqui-Salces M, Huang Z, Vila MF, et al. Modulation of intestinal cell differentiation in growing pigs is dependent on the fiber source in the diet. J Anim Sci. 2017;95:1179–90. doi: 10.2527/jas.2016.0947. [DOI] [PubMed] [Google Scholar]
- 35.Kef S, Arslan S. The effects of different dietary fiber use on the properties of kefir produced with cow’s and goat’s milk. J Food Proc Preserv. 2021;45:e15467. doi: 10.1111/jfpp.15467. [DOI] [Google Scholar]
- 36.McRorie JW, Jr, McKeown NM. Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J Acad Nutr Diet. 2017;117:251–64. doi: 10.1016/j.jand.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 37.Wellington MO, Hamonic K, Krone JEC, Htoo JK, Van Kessel AG, Columbus DA. Effect of dietary fiber and threonine content on intestinal barrier function in pigs challenged with either systemic E. coli lipopolysaccharide or enteric Salmonella Typhimurium. J Anim Sci Biotechnol. 2020;11:38. doi: 10.1186/s40104-020-00444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroeder BO, Birchenough GM, Ståhlman M, et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microb. 2018;23:27–40. doi: 10.1016/j.chom.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang Q, Tan P, Ma N, Ma X. Physiological functions of threonine in animals: beyond nutrition metabolism. Nutrients. 2021;13:2592. doi: 10.3390/nu13082592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansson GC, Johansson MEV. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes. 2010;1:51–4. doi: 10.4161/gmic.1.1.10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin L, Yang H, Li J, et al. Pig models on intestinal development and therapeutics. Amino Acids. 2017;49:2099–106. doi: 10.1007/s00726-017-2497-z. [DOI] [PubMed] [Google Scholar]
- 42.Barker N, Van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 43.Tetteh PW, Basak O, Farin HF, et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell. 2016;18:203–13. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Shahar Y, Abassi Z, Shefer HK, Pollak Y, Bhattacharya U, Sukhotnik I. Accelerated intestinal epithelial cell turnover correlates with stimulated bmp signaling cascade following intestinal ischemia–reperfusion in a rat. Eur J Pediatr Surg. 2020;30:64–70. doi: 10.1055/s-0039-1700550. [DOI] [PubMed] [Google Scholar]
- 45.Giepmans BNG, van IJzendoorn SCD. Epithelial cell–cell junctions and plasma membrane domains. Biochim Biophy Acta (BBA)-Biomembranes. 2009;1788:820–31. doi: 10.1016/j.bbamem.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Hossain Z, Hirata T. Molecular mechanism of intestinal permeability: interaction at tight junctions. Mol Biosyst. 2008;4:1181–5. doi: 10.1039/b800402a. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol. 2014;15:225–42. doi: 10.1038/nrm3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khurana S, George SP. Regulation of cell structure and function by actin-binding proteins: villin’s perspective. FEBS Lett. 2008;582:2128–39. doi: 10.1016/j.febslet.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhekne HS, Pylypenko O, Overeem AW, et al. MYO5B, STX3, and STXBP2 mutations reveal a common disease mechanism that unifies a subset of congenital diarrheal disorders: a mutation update. Hum Mutat. 2018;39:333–44. doi: 10.1002/humu.23386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazzolini R, Dopeso H, Mateo-Lozano S, Arango D. Brush border myosin Ia has tumor suppressor activity in the intestine. Proc Natl Acad Sci USA. 2012;109:1530–5. doi: 10.1073/pnas.1108411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McConnell RE, Tyska MJ. Myosin-1a powers the sliding of apical membrane along microvillar actin bundles. J Cell Biol. 2007;177:671–81. doi: 10.1083/jcb.200701144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McConnell RE, Higginbotham JN, Shifrin DA, et al. The enterocyte microvillus is a vesicle-generating organelle. J Cell Biol. 2009;185:1285–98. doi: 10.1083/jcb.200902147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shifrin DA, Jr, Tyska MJ. Ready…aim…fire into the lumen: a new role for enterocyte microvilli in gut host defense. Gut Microbes. 2012;3:460–2. doi: 10.4161/gmic.21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weerasooriya V, Rennie MJ, Anant S, Alpers DH, Patterson BW, Klein S. Dietary fiber decreases colonic epithelial cell proliferation and protein synthetic rates in human subjects. Am J physiol Endocrinol Metab. 2006;290:E1104–8. doi: 10.1152/ajpendo.00557.2005. [DOI] [PubMed] [Google Scholar]
- 56.Meddings J. The significance of the gut barrier in disease. Gut. 2008;57:438–40. doi: 10.1136/gut.2007.143172. [DOI] [PubMed] [Google Scholar]
- 57.Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:223–37. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 58.Gracia MI, Sánchez J, Millán C, et al. Effect of feed form and whole grain feeding on gastrointestinal weight and the prevalence of campylobacter jejuni in broilers orally infected. PloS One. 2016;11:e0160858. doi: 10.1371/journal.pone.0160858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walugembe M, Hsieh JC, Koszewski NJ, Lamont SJ, Persia ME, Rothschild MF. Effects of dietary fiber on cecal short-chain fatty acid and cecal microbiota of broiler and laying-hen chicks. Poult Sci. 2015;94:2351–9. doi: 10.3382/ps/pev242. [DOI] [PubMed] [Google Scholar]
- 60.Józefiak D, Rutkowski A, Frątczak M, Boros D. The effect of dietary fibre fractions from different cereals and microbial enzyme supplementation on performance, ileal viscosity and short-chain fatty acid concentrations in the caeca of broiler chickens. J Anim Feed Sci. 2004;13:487–96. doi: 10.22358/jafs/67618/2004. [DOI] [Google Scholar]
- 61.Jamroz D, Jakobsen K, Bach Knudsen KE, Wiliczkiewicz A, Orda J. Digestibility and energy value of non-starch polysaccharides in young chickens, ducks and geese, fed diets containing high amounts of barley. Comp Biochem Physiol A Mol Integr Physiol. 2002;131:657–68. doi: 10.1016/s1095-6433(01)00517-7. [DOI] [PubMed] [Google Scholar]
- 62.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Ann Rev Physiol. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 63.Miller TL, Wolin MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol. 1996;62:1589–92. doi: 10.1128/aem.62.5.1589-1592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23:366–84. doi: 10.1017/s0954422410000247. [DOI] [PubMed] [Google Scholar]
- 65.Ragsdale SW, Pierce E. Acetogenesis and the Wood–Ljungdahl pathway of CO2 fixation. Biochim Biophys Acta Proteins Proteom. 2008;1784:1873–98. doi: 10.1016/j.bbapap.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reichardt N, Duncan SH, Young P, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1323–35. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vidyasagar S, Barmeyer C, Geibel J, Binder HJ, Rajendran VM. Role of short-chain fatty acids in colonic HCO3 secretion. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1217–26. doi: 10.1152/ajpgi.00415.2004. [DOI] [PubMed] [Google Scholar]
- 68.Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K. Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol. 2000;279:G775–80. doi: 10.1152/ajpgi.2000.279.4.G775. [DOI] [PubMed] [Google Scholar]
- 69.Takebe K, Nio J, Morimatsu M, et al. Histochemical demonstration of a Na+-coupled transporter for short-chain fatty acids (Slc5a8) in the intestine and kidney of the mouse. Biomed Res. 2005;26:213–21. doi: 10.2220/biomedres.26.213. [DOI] [PubMed] [Google Scholar]
- 70.Ganapathy V, Gopal E, Miyauchi S, Prasad PD. Biological functions of SLC5A8, a candidate tumour suppressor. Biochem Soc Trans. 2005;33:237–40. doi: 10.1042/bst0330237. [DOI] [PubMed] [Google Scholar]
- 71.Halestrap AP, Meredith D. The SLC16 gene family—from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Arch. 2004;447:619–28. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 72.Shin HJ, Anzai N, Enomoto A, et al. Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology. 2007;45:1046–55. doi: 10.1002/hep.21596. [DOI] [PubMed] [Google Scholar]
- 73.Sellin JH. SCFAs: The enigma of weak electrolyte transport in the colon. News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 1999;14:58–64. doi: 10.1152/physiologyonline.1999.14.2.58. [DOI] [PubMed] [Google Scholar]
- 74.Kearney JM, McElhone S. Perceived barriers in trying to eat healthier–results of a pan-EU consumer attitudinal survey. Br J Nutr. 1999;81:S133–S7. doi: 10.1017/S0007114599000987. [DOI] [PubMed] [Google Scholar]
- 75.M’Sadeq SA, Wu S, Swick RA, Choct M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim Nutr. 2015;1:1–11. doi: 10.1016/j.aninu.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vermeulen K, Verspreet J, Courtin CM, et al. Reduced-particle-size wheat bran is efficiently colonized by a lactic acid-producing community and reduces levels of Enterobacteriaceae in the cecal microbiota of broilers. Appl Environ Microbiol. 2018;84:e01343–18. doi: 10.1128/AEM.01343-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu H, Ivarsson E, Dicksved J, Lundh T, Lindberg JE. Inclusion of chicory (Cichorium intybus L.) in pigs’ diets affects the intestinal microenvironment and the gut microbiota. Appl Environ Microbiol. 2012;78:4102–9. doi: 10.1128/AEM.07702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown AJ, Goldsworthy SM, Barnes AA, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–9. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 79.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. 2014;5:202–7. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Advan Immunol. 2014;121:91–119. doi: 10.1016/b978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 81.Halnes I, Baines KJ, Berthon BS, MacDonald-Wicks LK, Gibson PG, Wood LG. Soluble fibre meal challenge reduces airway inflammation and expression of GPR43 and GPR41 in asthma. Nutrients. 2017;9:57. doi: 10.3390/nu9010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohira H, Fujioka Y, Katagiri C, et al. Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J Atheroscler Thromb. 2013;20:425–42. doi: 10.5551/jat.15065. [DOI] [PubMed] [Google Scholar]
- 83.Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–66. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 84.Huang W, Zhou L, Guo H, Xu Y, Xu Y. The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism. 2017;68:20–30. doi: 10.1016/j.metabol.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 85.Kim CH. Microbiota or short-chain fatty acids: which regulates diabetes? Cell Mol Immunol. 2018;15:88–91. doi: 10.1038/cmi.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Digby JE, Martinez F, Jefferson A, et al. Anti-inflammatory effects of nicotinic acid in human monocytes are mediated by GPR109A dependent mechanisms. Arterioscler Thromb Vasc Biol. 2012;32:669–76. doi: 10.1161/ATVBAHA.111.241836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borthakur A, Priyamvada S, Kumar A, et al. A novel nutrient sensing mechanism underlies substrate-induced regulation of monocarboxylate transporter-1. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1126–G33. doi: 10.1152/ajpgi.00308.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh N, Thangaraju M, Prasad PD, et al. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem. 2010;285:27601–8. doi: 10.1074/jbc.M110.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu J, Zhou Z, Hu Y, Dong S. Butyrate-induced GPR41 activation inhibits histone acetylation and cell growth. J Genet Genomics. 2012;39:375–84. doi: 10.1016/j.jgg.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 91.Smith PM, Howitt MR, Panikov N, Michaud M, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Usami M, Kishimoto K, Ohata A, et al. Butyrate and trichostatin A attenuate nuclear factor kappaB activation and tumor necrosis factor alpha secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res (New York, NY) 2008;28:321–8. doi: 10.1016/j.nutres.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 93.Kendrick SF, O’Boyle G, Mann J, et al. Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology. 2010;51:1988–97. doi: 10.1002/hep.23572. [DOI] [PubMed] [Google Scholar]
- 94.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111:2247–52. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lucas JL, Mirshahpanah P, Haas-Stapleton E, Asadullahb K, Zollnerb TM, Numerof RP. Induction of Foxp3+ regulatory T cells with histone deacetylase inhibitors. Cell Immunol. 2009;257:97–104. doi: 10.1016/j.cellimm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 96.Denyer MP, Pinheiro DY, Garden OA, Shepherd AJ. Missed, not missing: phylogenomic evidence for the existence of avian FoxP3. PLoS One. 2016;11:e0150988. doi: 10.1371/journal.pone.0150988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Borrelli L, Coretti L, Dipineto L, et al. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci Rep. 2017;7:16269. doi: 10.1038/s41598-017-16560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Friedman M, Juneja VK. Review of antimicrobial and antioxidative activities of chitosans in food. J Food Prot. 2010;73:1737–61. doi: 10.4315/0362-028X-73.9.1737. [DOI] [PubMed] [Google Scholar]
- 99.Schedle K, Plitzner C, Ettle T, Zhao L, Domig KJ, Windisch W. Effects of insoluble dietary fibre differing in lignin on performance, gut microbiology, and digestibility in weanling piglets. Arch Anim Nutr. 2008;62:141–51. doi: 10.1080/17450390801892617. [DOI] [PubMed] [Google Scholar]
- 100.De Angelis M, Montemurno E, Vannini L, et al. Effect of whole-grain barley on the human fecal microbiota and metabolome. Appl Environ Microbiol. 2015;81:7945–56. doi: 10.1128/AEM.02507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pleşea Condratovici C, Bacarea V, Pique N. Xyloglucan for the treatment of acute gastroenteritis in children: results of a randomized, controlled, clinical trial. Gastroenterol Res Pract. 2016;2016:6874207. doi: 10.1155/2016/6874207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brufau MT, Martín-Venegas R, Guerrero-Zamora AM, et al. Dietary β-galactomannans have beneficial effects on the intestinal morphology of chickens challenged with Salmonella enterica serovar Enteritidis. J Anim Sci. 2015;93:238–46. doi: 10.2527/jas.2014-7219. [DOI] [PubMed] [Google Scholar]
- 103.Azcarate-Peril MA, Butz N, Cadenas MB, et al. An attenuated Salmonella enterica serovar Typhimurium strain and galacto-oligosaccharides accelerate clearance of Salmonella infections in poultry through modifications to the gut microbiome. Appl Environ Microbiol. 2018;84:e02526–17. doi: 10.1128/AEM.02526-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pourabedin M, Zhao X. Prebiotics and gut microbiota in chickens. FEMS Microbiol Lett. 2015;362:fnv122. doi: 10.1093/femsle/fnv122. [DOI] [PubMed] [Google Scholar]
- 105.Fu X, Li R, Zhang T, Li M, Mou H. Study on the ability of partially hydrolyzed guar gum to modulate the gut microbiota and relieve constipation. J Food Biochem. 2019;43:e12715. doi: 10.1111/jfbc.12715. [DOI] [PubMed] [Google Scholar]
- 106.Ahallil H, Abdullah A, Maskat MY, Sarbini SR. AIP Conference Proceedings. AIP Publishing LLC; 2019. Fermentation of gum arabic by gut microbiota using in vitro colon model; p. 050004. [DOI] [Google Scholar]
- 107.Singh Y, Molan AL, Ravindran V. Influence of the method of whole wheat inclusion on performance and caecal microbiota profile of broiler chickens. J Appl Anim Nutr. 2019;7:E4. doi: 10.1017/jan.2019.3. [DOI] [Google Scholar]
- 108.Galán JE, Curtiss R., 3rd Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun. 1990;58:1879–85. doi: 10.1128/iai.58.6.1879-1885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bailey JS, Blankenship LC, Cox NA. Effect of fructooligosaccharide on Salmonella colonization of the chicken intestine. Poult Sci. 1991;70:2433–8. doi: 10.3382/ps.0702433. [DOI] [PubMed] [Google Scholar]
- 110.Kim JC, Mullan BP, Hampson DJ, Pluske JR. Addition of oat hulls to an extruded rice-based diet for weaner pigs ameliorates the incidence of diarrhoea and reduces indices of protein fermentation in the gastrointestinal tract. Br J Nutr. 2008;99:1217–25. doi: 10.1017/S0007114507868462. [DOI] [PubMed] [Google Scholar]
- 111.Halas D, Hansen CF, Hampson DJ, Mullan BP, Wilson RH, Pluske JR. Effect of dietary supplementation with inulin and/or benzoic acid on the incidence and severity of post-weaning diarrhoea in weaner pigs after experimental challenge with enterotoxigenic Escherichia coli. Arch Anim Nutr. 2009;63:267–80. doi: 10.1080/17450390903020414. [DOI] [PubMed] [Google Scholar]
- 112.Chen H, Mao X, He J, et al. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br J Nutr. 2013;110:1837–48. doi: 10.1017/s0007114513001293. [DOI] [PubMed] [Google Scholar]
- 113.Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133:1921–9. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- 114.Hansen CF, Phillips ND, La T, et al. Diets containing inulin but not lupins help to prevent swine dysentery in experimentally challenged pigs. J Anim Sci. 2010;88:3327–36. doi: 10.2527/jas.2009-2719. [DOI] [PubMed] [Google Scholar]
- 115.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406e10. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 117.Pluznick JL, Protzko RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Nat Acad Sci. 2013;110:4410–5. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]