Abstract
Coenzyme Q10 (CoQ10) is a popular nutritional supplement, an antioxidant and an essential component of the mitochondrial electron transport chain. Several clinical studies have suggested that fatigue can be reduced by antioxidant supplementation. However, the data on this topic has been sparse to date. Hence, we conducted this meta-analysis with the aim of investigating the effectiveness of fatigue reduction via CoQ10 supplementation. More specifically, we searched electronic databases for randomized controlled trials (RCTs) published from the database inception to January 2022. A random effects model was implemented to conduct the meta-analysis among 13 RCTs (with a total of 1,126 participants). As compared with the placebo groups evaluated in each RCT, the CoQ10 group showed a statistically significant reduction in fatigue scores (Hedges’ g = −0.398, 95% confidence interval = −0.641 to −0.155, p = 0.001). The directions of the treatment effects were consistent between the healthy and diseased participants. Compared with the placebo group, the effect of reducing fatigue was statistically significant in the subgroup using the CoQ10-only formulation but not in the subgroup using CoQ10 compounds. The results of our meta-regression demonstrate that increases in the daily dose (coefficient = −0.0017 per mg, p < 0.001) and treatment duration (coefficient = −0.0042 per day, p = 0.007) of CoQ10 supplementation were correlated with greater fatigue reduction. There was only one adverse (gastrointestinal) event in the 602 participants who underwent the CoQ10 intervention. Based on the results of this meta-analysis, we conclude that CoQ10 is an effective and safe supplement for reducing fatigue symptoms.
Systematic Review Registration: https://inplasy.com/inplasy-2022-1-0113/, identifier INPLASY202210113
Keywords: coenzyme Q10, fatigue, clinical trials, meta-analysis, systematic review
1 Introduction
Fatigue is a symptom that occurs in both healthy and diseased individuals (Finsterer and Mahjoub, 2014). This symptom is described as unusual overwhelming tiredness that cannot be explained by physiological exhaustion in the wake of physical or mental efforts and that is not sufficiently recovered by regular rest and sleep (Haß et al., 2019). In the general population, the prevalence of temporary fatigue ranges from 4 to 45%, while that of chronic fatigue (i.e., fatigue lasting for >6 months) ranges from 2 to 11% (Cathébras et al., 1992; Ridsdale et al., 1993; Jason et al., 1999; Cullen et al., 2002). Fatigue is also common in patients with poliomyelitis (Peel et al., 2015) and multiple sclerosis (Sanoobar et al., 2016; Moccia et al., 2019) as well as in cancer patients undergoing chemotherapy (Iwase et al., 2016). The annual total cost of productivity loss due to chronic fatigue syndrome in the United States alone is approximately US$ 9.1 billion, which is roughly equal to US$ 20,000 per resident (Reynolds et al., 2004). Although the etiology of fatigue remains poorly understood (Filler et al., 2014), mitochondrial dysfunction (Filler et al., 2014) and pro-inflammatory status (Haß et al., 2019) may play a role. Fortunately, fatigue can be rigorously measured and is potentially treatable (Finsterer and Mahjoub, 2014).
Coenzyme Q10 (CoQ10) is a popular nutritional supplement and a lipid-soluble antioxidant that is endogenously produced by the human body. It is also an essential component of the mitochondrial electron transport chain (Aaseth et al., 2021). Case-control studies conducted by Maes et al. showed that, as compared with healthy subjects, patients with chronic fatigue syndrome have lower plasma levels of CoQ10 (Maes et al., 2009a; Maes et al., 2009b). A statistically significant inverse relationship has also been found between CoQ10 levels and fatigue severity (Maes et al., 2009a). Thus, CoQ10 supplementation has been successfully applied for reducing fatigue in patients with various conditions, including chronic fatigue syndrome (Castro-Marrero et al., 2015; Castro-Marrero et al., 2016; Fukuda et al., 2016; Castro-Marrero et al., 2021) and fibromyalgia (Cordero et al., 2013; Miyamae et al., 2013; Di Pierro et al., 2017), as well as in healthy subjects (Morikawa et al., 2019; Mizuno et al., 2020). However, inconsistencies in clinical outcomes have been identified across different trials. Therefore, in the current study, we performed a systematic review and meta-analysis to investigate the effects of CoQ10 treatment on fatigue symptoms and syndromes.
2 Materials and Methods
2.1 General Guidelines
We followed the guidelines delineated in the latest version of the PRISMA 2020 guidelines (Supplementary Table S1) to conduct this meta-analysis (Page et al., 2021a). This study, which was registered in INPLASY with the registration number INPLASY202210113 (Tsai and Chang, 2022), did not require ethics review board approval or participant informed consent.
2.2 Database Searches and the Identification of Eligible Manuscripts
Two authors (I-CT and K-VC) conducted independent electronic searches in the PubMed, Embase, Cochrane CENTRAL, Web of Science, and ClinicalTrials.gov databases using the following keywords (“Q10” OR “Q 10” OR “CoQ10” OR “Coenzyme Q10” OR “ubiquinol-10” OR “ubiquinol” OR “ubiquinone”) AND (“fatigue” OR “chronic fatigue syndrome” OR “tiredness”). The search was conducted from the inception of each database (i.e., the earliest record) to the date of the database search (16 January 2022). We note that ubiquinone is the fully oxidized form of CoQ10, and ubiquinol is the reduced form. These two forms are continually interconverted in the body and have similar bioactivities (Mantle and Dybring, 2020). The detailed search strategy for this systematic review and meta-analysis is provided in the Supplementary Material (Supplementary Table S2).
Initially, the two authors responsible for conducting this search screened the identified titles and abstracts for eligibility through a consensus process. The PubMed and EMBASE databases were thoroughly scrutinized for any potentially eligible trials. We also checked the reference lists of an identified review article (Mehrabani et al., 2019) and performed additional manual searches. A third reviewer and study author (P-TT) was consulted for situations in which the two aforementioned authors could not reach a consensus. No language restrictions were applied to this search.
2.3 Inclusion and Exclusion Criteria
The PICO (population, intervention, comparison, outcome) setting of the current meta-analysis was as follows: P: human participants; I: CoQ10 supplementation; C: placebo; and O: changes in fatigue symptom scores.
The following inclusion criteria were used: 1) randomized controlled trials (RCTs) enrolling human participants, 2) RCTs investigating the quantitative evaluation of fatigue symptoms before and after CoQ10 supplementation, 3) placebo-controlled trials (without age or treatment duration limitations), and 4) trials with available data for pre- and post-intervention fatigue assessments or evaluations of changes in fatigue scores. Open-label studies were also included in this meta-analysis as recent studies have found that open-label placebos had similar efficacy to double-blind placebos (Lembo et al., 2021; von Wernsdorff et al., 2021).
The exclusion criteria for this review and meta-analysis were as follows: 1) non-RCTs, 2) studies focusing on athletic muscle exhaustion rather than generalized fatigue, 3) studies lacking a placebo-controlled group, 4) studies lacking quantitative assessments of fatigue, and 5) studies enrolling participants that overlapped with a previously published trial.
2.4 Methodological Quality Appraisal
To investigate the methodological quality of the evaluated studies, we used the Cochrane risk of bias tool for randomized trials (version 2, RoB 2, London, United Kingdom), which consists of six main items for evaluating study quality: the randomization process, intervention adherence, missing outcome data, outcome measurement, selective reporting, and the overall risk of bias (Sterne et al., 2019).
In the intervention adherence section of the RoB 2, there are two options presented for literature assessment: intention-to-treat (intervention assignment) and per-protocol (intervention adherence). In this meta-analysis, we chose the per-protocol evaluation (Sterne et al., 2019) since it fits best with the design of our included studies.
2.5 Primary Outcome (Fatigue Score Change)
The primary outcomes evaluated in this investigation were changes in fatigue scores following CoQ10 supplementation or placebo regimens. We also examined the validity and appropriateness of the fatigue scale used in each trial (Fisk et al., 1994; Chandran et al., 2007; Fukuda et al., 2008). If there was more than one scoring system for fatigue evaluation implemented in a single trial, the index test included in the meta-analysis was decided by author consensus (I-CT and K-VC).
2.6 Secondary Outcome (Treatment-Associated Adverse Event Rates)
The secondary outcome evaluated in this investigation was the treatment-associated adverse event rate. For cells with zero events, zero was replaced by 0.5 to enable calculation (Deeks et al., 2021). The aforementioned outcomes were quantified using odds ratios.
2.7 Data Extraction and Management
Two independent authors (I-CT and K-VC) extracted data from the evaluated studies, including demographic data, study design parameters, details of the administered CoQ10 and placebo regimens, and the primary and secondary outcome values. To avoid misinterpretation, the evaluators paid special attention to the effect direction of the scale used in each trial. In situations where data were unavailable within the published articles, we contacted the corresponding authors to obtain the original data.
Data extraction and conversion as well as merging the results from various study arms using different CoQ10 dosages were processed according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions and the associated medical literature (Hozo et al., 2005; Higgins et al., 2021a; Higgins et al., 2021b). If post-treatment data were available at multiple time points, we extracted the outcome reported at the end of the intervention for statistical analysis. For crossover studies, we included only the first study interval to avoid carry-over effects (Higgins et al., 2021a).
2.8 Statistical Analyses
Because of the heterogeneity of the target populations within the enrolled studies, the current meta-analysis was conducted with a random-effects model (Borenstein et al., 2009) implemented using Comprehensive Meta-Analysis software (version 3, Biostat, Englewood, NJ, United States). A two-tailed p value of less than 0.05 was considered statistically significant.
We used Hedges’ g and 95% confidence intervals (CIs) to quantify the primary study outcomes (i.e., changes in fatigue scores). Hedges’ g values of 0.2, 0.5, and 0.8 were considered small, moderate, and large effect sizes, respectively (Hedges, 1981). Odds ratios (ORs) and their associated 95% CIs were evaluated to investigate secondary outcomes (i.e., treatment-associated adverse event rates).
I 2 and Cochran’s Q statistics were also examined to evaluate the degree of heterogeneity across studies. I 2 values of 25, 50, and 75% were considered low, moderate, and high heterogeneity, respectively (Higgins et al., 2003). We likewise performed subgroup analyses based on disease and CoQ10 formulations. Meta-regression analyses regarding the treatment effects of daily CoQ10 doses as well as evaluations of various treatment durations were conducted to determine whether the fatigue-alleviating effects of CoQ10 correlated with the aforementioned parameters.
To confirm the robustness of this meta-analysis, sensitivity analyses were performed using the one-study removal method to determine whether there was a statistically significant change in the summary effect size after removing a particular trial from the analysis (Deeks et al., 2021).
Potential publication bias was evaluated using guidelines set forth by the Cochrane Handbook for Systematic Reviews of Interventions (Page et al., 2021b). Funnel plots were generated and visually inspected. Egger’s regression tests were implemented when 10 or more datasets were available.
3 Results
3.1 Study Identification and Selection
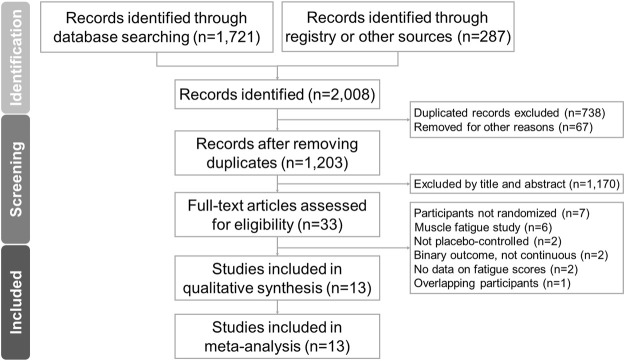
The PRISMA flowchart for the literature search is shown in Figure 1. After removing duplicate articles and excluding non-relevant articles by an examination of titles and abstracts, we ultimately included 13 RCTs in the final analysis (Berman et al., 2004; Lee et al., 2011; Cordero et al., 2013; Lesser et al., 2013; Castro-Marrero et al., 2015; Fukuda et al., 2015; Peel et al., 2015; Sanoobar et al., 2016; Di Pierro et al., 2017; Morikawa et al., 2019; Mizuno et al., 2020; Mousavi et al., 2020; Castro-Marrero et al., 2021). The articles excluded in the final stage and the reasons for exclusion are listed in Supplementary Table S3 (Singh et al., 2003; Langsjoen et al., 2005; Kumar et al., 2007; Mizuno et al., 2008; Gökbel et al., 2010; Cordero et al., 2012a; Cordero et al., 2012b; Fedacko et al., 2013; Gharahdaghi et al., 2013; Miyamae et al., 2013; Castro-Marrero et al., 2016; Fukuda et al., 2016; Iwase et al., 2016; Menon et al., 2017; Langsjoen et al., 2019; Moccia et al., 2019; Negro et al., 2019; Gomez-Centeno et al., 2020; Schweiger et al., 2020; Suzuki et al., 2021). Details of data extraction from included randomized controlled trials are summarized in Supplementary Table S4.
FIGURE 1.
PRISMA 2020 flowchart for the current meta-analysis.
The 13 eligible RCTs encompassed a total of 1,126 participants with a mean age of 49.3 ± 12.6 (standard deviation) years, of whom 25.6% (n = 288) were male. The study duration ranged from four (Mousavi et al., 2020) to 24 weeks (Lesser et al., 2013). Subject diagnoses included chronic fatigue syndrome (Castro-Marrero et al., 2015; Castro-Marrero et al., 2021), fibromyalgia (Cordero et al., 2013; Di Pierro et al., 2017), end-stage heart failure (Berman et al., 2004), obesity (Lee et al., 2011), breast cancer (with patients undergoing chemotherapy) (Lesser et al., 2013), end-stage renal disease (Fukuda et al., 2015), poliomyelitis (Peel et al., 2015), and multiple sclerosis (Sanoobar et al., 2016), and we also evaluated healthy subjects (Morikawa et al., 2019) and healthy individuals with fatigue (Mizuno et al., 2020; Mousavi et al., 2020). The details of the retrieved trials are summarized in Table 1.
TABLE 1.
Summary of the retrieved trials investigating the effect of CoQ10 on reducing fatigue in the enrolled participants.
| First author (year) | Country | Population | Participants (F/M) | Age a | Study design | Allocation concealment | Randomization | Funding/grants/support |
|---|---|---|---|---|---|---|---|---|
| Berman (2004) | Israel | End-stage heart failure | Total: 4/28 b | 54.6 (40-67) b | RCT, double-blind | External party | Not mentioned | N/A |
| Lee (2011) | Korea | Obesity | CoQ10: 15/11
b
Placebo: 15/10 |
42.7±11.3
b
42.5±11.2 |
RCT, double-blind | Not mentioned | Not mentioned | N/A |
| Cordero (2013) | Spain | Fibromyalgia | CoQ10: 10/0
b
,
c
Placebo: 10/0 |
44.3±9.7
b
,
c
55.0±5.0 |
RCT, double-blind | Not mentioned | Not mentioned | N/A |
| Lesser (2013) | USA | Breast cancer under chemotherapy | CoQ10: 122/0
b
Placebo: 114/0 |
52 (31-85)
b
50 (28-72) |
RCT, double-blind | Not mentioned | Stratified block | • National Cancer Institute |
| Castro-Marrero (2015) | Spain | Chronic fatigue syndrome | CoQ10+NADH: 39/0
b
,
c
Placebo: 34/0 |
49.3±7.1 b , c | RCT, double-blind | Not mentioned | Not mentioned | • Vitae Natural Nutrition, S.L. |
| Fukuda (2015) | Japan | End-stage renal disease | CoQ10 mix drink: 17/70
c
Placebo: 15/71 |
55.6±10.0
c
56.2±8.9 |
RCT, double-blind | Coordination center | Stratified | • Asahi Kasei Kuraray Medical Cooperation• Ministry of Education, Culture, Sports, Science and Technology (Japan)• Health Labor Sciences Research Grant (Japan) |
| Peel (2015) | Australia | Poliomyelitis | CoQ10: 39/15
b
,
d
Placebo: 32/17 |
69.9±8.4
b
69.8±8.2 |
RCT, double-blind | Coordination center | Matrix sequence | • Cancer and Polio Research Fund (UK) |
| Sanoobar (2016) | Iran | Multiple sclerosis | CoQ10: 20/2
c
Placebo: 21/2 |
33.1±7.6
c
30.9±7.7 |
RCT, double-blind | Not mentioned | Not mentioned | • Tehran University of Medical Sciences |
| Di Pierro (2017) | Italy | Fibromyalgia | CoQ10: 12/0
b
,
c
Placebo: 10/0 |
52.5±10.4
b
,
c
53.6±7.8 |
RCT, open-label | Coin tossing | Coin tossing | N/A |
| Morikawa (2019) | Japan | Healthy subjects | CoQ10: 16/14
b
Placebo: 16/14 |
40.5±7.3
b
42.9±7.3 |
RCT, double-blind | Not mentioned | Stratified block | • Kaneka, Inc. |
| Mizuno (2020) | Japan | Healthy subjects with fatigue | CoQ10: 28/14
b
,
c
Placebo: 13/7 |
42.1±10.9
b
,
c
41.3±13.4 |
RCT, double-blind | Not mentioned | Stratified | • Kaneka, Inc.• Japan Science and Technology Agency |
| Mousavi (2020) | Iran | Health subjects (nurses) with fatigue | CoQ10: 47/7
c
Placebo: 45/6 |
35.1±8.1
c
35.7±8.9 |
RCT, double-blind | Assignment double-blinded | Permuted block | • Isfahan University of Medical Sciences |
| Castro-Marrero (2021) | Spain | Chronic fatigue syndrome | CoQ10+NADH: 72/0
c
Placebo: 72/0 |
45.38±7.81
c
46.79±6.48 |
RCT, double-blind | Independent investigator | Random number | • Vitae Health Innovation• Vall d’Hebron Hospital Research Institute |
CoQ10, coenzyme Q10; NADH, reduced form of nicotinamide adenine dinucleotide; RCT, randomized controlled trial; UK, United Kingdom; USA, United States of America.
Age is presented as means ± standard deviations or as medians (ranges).
Allocated participants.
Per-protocol participants.
The total sample size of the CoQ10 group was retrieved from data presented in Figure 1 and Table 2 due to inconsistency in Table 1.
Three studies evaluated compounds mixed with CoQ10. More specifically, CoQ10 with a reduced form of nicotinamide adenine dinucleotide (NADH) was evaluated in two of these trials (Castro-Marrero et al., 2015; Castro-Marrero et al., 2021) and CoQ10 in a multi-vitamin nutritional drink was evaluated in the other trial (Fukuda et al., 2015). Ten studies evaluated CoQ10 only (Berman et al., 2004; Lee et al., 2011; Cordero et al., 2013; Lesser et al., 2013; Peel et al., 2015; Sanoobar et al., 2016; Di Pierro et al., 2017; Morikawa et al., 2019; Mizuno et al., 2020; Mousavi et al., 2020). The intervention details, tools for fatigue assessment, adverse events, and study withdrawals are summarized in Table 2.
TABLE 2.
Summary of the CoQ10 interventions administered in the study treatment arms of the retrieved trials.
| First author(year) | Population | Duration | CoQ10 product/manufacturer | Daily CoQ10 dose (per-protocol N) | Control (per-protocol N) | Fatigue outcome measurement (score range) | AE associated with CoQ10 withdrawal |
|---|---|---|---|---|---|---|---|
| Berman (2004) | End-stage heart failure | 3 months | Ultrasome capsules/Herbamed Ltd. (Israel) | 60 mg/day (13) | Matching placebo (14) | Minnesota Living with Heart Failure Questionnaire fatigue score (0-5) | One ultrasome-induced intestinal upset withdrawal |
| Lee (2011) | Obesity | 12 weeks | Ubiquinone/Daewoong Pharmacy (Korea) | 200 mg/day (17) | Matching placebo (19) | Fatigue Severity Scale (9-63) | No |
| Cordero (2013) | Fibromyalgia | 40 days | CoQ10 capsules/Pharma Nord (Denmark) | 300 mg/day (10) | Matching placebo (10) | Fibromyalgia Impact Questionnaire fatigue score (0-10) | No |
| Lesser (2013) | Breast cancer under chemotherapy | 24 weeks | CoQ10/Soft Gel Technologies (USA) |
300 mg/day (78) | Matching placebo (61) | Profile of Mood States fatigue subscale (0-4) | No. All AE-related withdrawals were due to chemotherapy based on the investigators’ review. |
| Castro-Marrero (2015) | Chronic fatigue syndrome | 8 weeks | ReConnect/Vitae Natural Nutrition (Spain) | 200 mg/day with 20 mg/day NADH (39) | Matching placebo (34) | Fatigue Impact Scale (0-160) | No |
| Fukuda (2015) | End-stage renal disease | 12 weeks | AMP01/Asahi Kasei Kuraray Medical Corporation (Japan) | Nutritional drink with 30 mg/day CoQ10 (87) | Matching placebo (86) | Fatigue Scale (0-32) | No. All AE and withdrawals were not associated with the study intervention (which was conducted by a safety monitoring board). AE-associated withdrawal: CoQ10 6/97, placebo 5/99 |
| Peel (2015) | Poliomyelitis | 60 days | CoQ10 capsules/Health World Limited (Australia) | 100 mg/day (54) a | Matching placebo (49) | Multidimensional Assessment of Fatigue (1-50) | No. All AEs were not associated with CoQ10 supplementation. AE: CoQ10 7/54, placebo 5/49. AE-associated withdrawal: CoQ10 5/54, placebo 2/49 |
| Sanoobar (2016) | Multiple sclerosis | 12 weeks | CoQ10 capsules/not mentioned | 500 mg/day (22) | Matching placebo (23) | Fatigue Severity Scale (9-63) | No |
| Di Pierro (2017) | Fibromyalgia | 3 months | DDM Chinone® sachets/Labomar (Italy) | 400 mg/day (12) | Comparable placebo (10) | Functional Assessment of Chronic Illness Therapy (0-44) | No |
| Morikawa (2019) | Healthy subjects | 8 weeks | Uniquinol soft capsules/Kaneka, Inc. (Japan) | 100 mg/day (24) | Matching placebo (28) | Fatigue Visual Analogue Scale (0-10) | No. All AEs were not associated with the treatment. AE: CoQ10 8/30, placebo 6/30 |
| Mizuno (2020) | Healthy individuals with fatigue | 12 weeks | Uniquinol soft capsules/Kaneka, Inc. (Japan) | 150 mg/day (22) 100 mg/day (20) |
Matching placebo (20) | Fatigue Visual Analogue Scale (0-100) | No |
| Mousavi (2020) | Nurses with fatigue | 4 weeks | CoQ10 capsules/Nutri Century (Canada) | 200 mg/day (54) | Matching placebo (51) | Nurses' fatigue scale (21-105) | No |
| Castro-Marrero (2021) | Chronic fatigue syndrome | 8 weeks | ReConnect/Vitae Health Innovation (Spain) | 200 mg/day with 20 mg/day NADH (72) | Matching placebo (72) | Fatigue Impact Scale (0-160) | No. All AEs were not associated with the treatment. AE-associated withdrawal: CoQ10+NADH 8/104, placebo 11/103 |
AE, adverse events; CoQ10, coenzyme Q10; NADH, reduced form of nicotinamide adenine dinucleotide; USA, United States of America
The total number of subjects enrolled in the CoQ10 group was retrieved based on data reported in Figure 1 and Table 2, due to inconsistency in Table 1.
3.2 Methodological Quality of the Included Studies
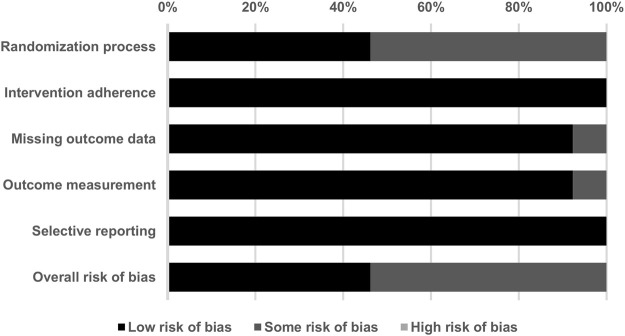
With respect to the overall methodological quality of the included studies, we found that 46.2% of the evaluated studies had a low risk of bias, 53.8% had some risk of bias, and 0% had a high risk of bias (Figure 2). In a detailed assessment, seven studies (Lee et al., 2011; Cordero et al., 2013; Lesser et al., 2013; Castro-Marrero et al., 2015; Sanoobar et al., 2016; Morikawa et al., 2019; Mizuno et al., 2020) were rated as having “some” risk of bias in the randomization process because they did not reveal details of the allocation concealment. One study (Lee et al., 2011) was rated as having “some” risk of bias with regard to missing outcome data, since the study excluded five subjects from the CoQ10 group due to an unchanged level of serum CoQ10 after supplementation. One study (Di Pierro et al., 2017) was rated as having “some” risk of bias in the outcome measurement because it was an open-label study and the participants were aware of the interventions they received. The details of the risk of bias assessment are summarized in Table 3.
FIGURE 2.
Summary of quality assessment for the studies included in the current meta-analysis using version 2 of the Cochrane risk-of-bias tool for randomized trials.
TABLE 3.
Detailed quality assessment of the included studies using the Cochrane risk-of-bias tool, version 2.
| First author (year) | Randomization process | Intervention adherence | Missing outcome data | Outcome measurement | Selective reporting | Overall RoB |
|---|---|---|---|---|---|---|
| Berman (2004) | L | L | L | L | L | L |
| Lee (2011) | S a | L | S b | L | L | S |
| Cordero (2013) | S a | L | L | L | L | S |
| Lesser (2013) | S a | L | L | L | L | S |
| Castro-Marrero (2015) | S a | L | L | L | L | S |
| Fukuda (2015) | L | L | L | L | L | L |
| Peel (2015) | L | L | L | L | L | L |
| Sanoobar (2016) | S a | L | L | L | L | S |
| Di Pierro (2017) | L | L | L | S c | L | L |
| Morikawa (2019) | S a | L | L | L | L | S |
| Mizuno (2020) | S a | L | L | L | L | S |
| Mousavi (2020) | L | L | L | L | L | L |
| Castro-Marrero (2021) | L | L | L | L | L | L |
CoQ10, coenzyme Q10; H, high risk of bias; L, low risk of bias; RoB, risk of bias; S, some risk of bias
These studies didn't provide allocation concealment details.
This study excluded five subjects from the CoQ10 group because their blood levels of CoQ10 failed to increase over the study course.
An open-label study. The participants were aware of the intervention they received.
3.3 Primary Outcome: Effects of CoQ10 Supplementation on Fatigue
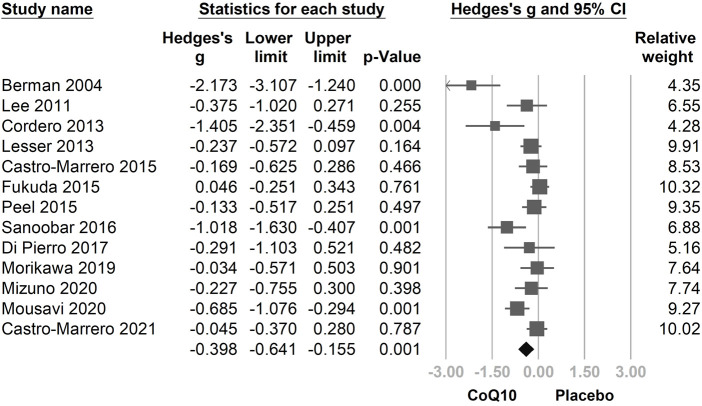
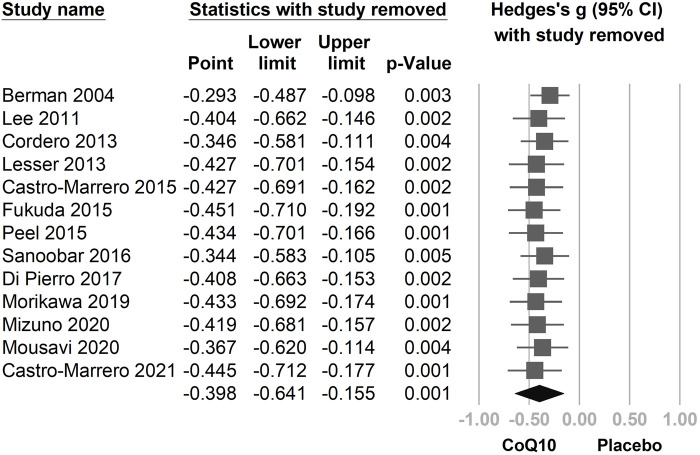
In the combined 13 trials (Figure 3), CoQ10 demonstrated a statistically significant reduction with regard to fatigue symptomology (Hedges’ g = −0.398, 95% CI = −0.641 to −0.155, p = 0.001, I 2 = 69.5%). However, moderate-to-high heterogeneity was observed. A sensitivity analysis was performed using the one-study removal method. The results showed a consistently statistically significant effect of CoQ10 on fatigue reduction. The summary effect sizes did not change the statistical significance of these findings when any of the included studies were removed (Figure 4).
FIGURE 3.
Forest plot of the effects of oral coenzyme Q10 (CoQ10) on fatigue as compared with the placebo. CoQ10 was found to be effective in reducing fatigue. CI, confidence interval.
FIGURE 4.
The results of a sensitivity analysis using the one-study removal method. The main result did not change significantly after removing any one of the included trials. All analyses showed statistically significant effects of CoQ10 in relieving fatigue. CI, confidence interval.
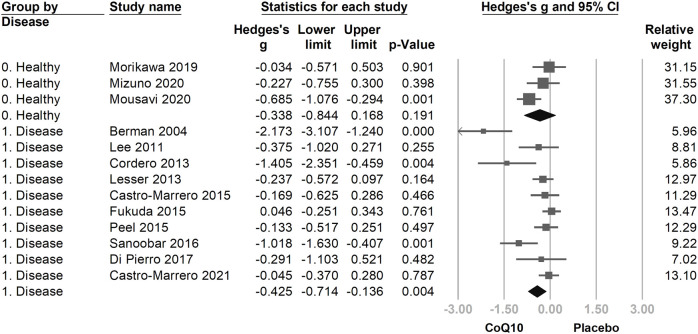
We then divided the included trials into two subgroups: healthy participants (Morikawa et al., 2019; Mizuno et al., 2020; Mousavi et al., 2020) and patients with a fatigue-associated disease (fibromyalgia (Cordero et al., 2013; Di Pierro et al., 2017), chronic fatigue syndrome (Castro-Marrero et al., 2015; Castro-Marrero et al., 2021), heart failure (Berman et al., 2004), obesity (Lee et al., 2011), breast cancer (Lesser et al., 2013), end-stage renal disease (Fukuda et al., 2015), poliomyelitis (Peel et al., 2015), multiple sclerosis (Sanoobar et al., 2016)). The direction of association between the use of CoQ10 and fatigue assessment was consistent in the subgroups of healthy participants (Hedges’ g = −0.351, 95% CI = −0.756 to 0.053, p = 0.089) and in patients with disease (Hedges’ g = −0.433, 95% CI = −0.732 to −0.133, p = 0.005), with overlapping 95% CIs (Figure 5).
FIGURE 5.
The forest plot of subgroup analysis using the participants’ condition as the moderator, including healthy participants and patients with disease. The directions of association between the use of coenzyme Q10 (CoQ10) and fatigue assessment were consistent in the subgroups, with overlapping 95% confidence intervals (CIs).
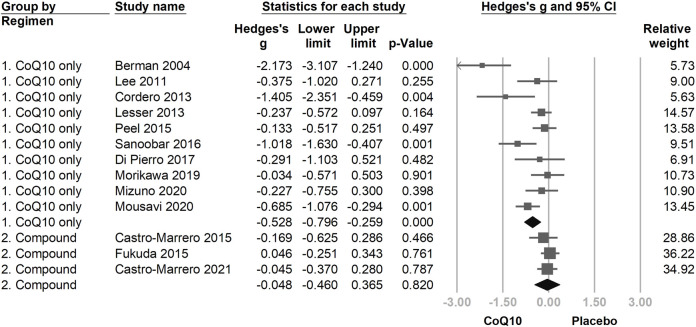
Another subgroup analysis was performed according to differences in the evaluated CoQ10 regimens. The group that used only CoQ10 showed a statistically significant fatigue reduction after supplementation (Hedges’ g = −0.552, 95% CI = −0.863 to −0.241, and p = 0.001). However, the effect of fatigue reduction in the group using CoQ10 compounds (Figure 6) was trivial and statistically insignificant as compared with the placebo group (Hedges’ g = −0.028, 95% CI = −0.225 to 0.170, p = 0.781).
FIGURE 6.
The forest plot of subgroup analysis using the composition of the interventional regimen, including CoQ10 only and CoQ10 compound interventions as the moderators. CoQ10 is effective in reducing fatigue in the CoQ10-only formulation, but not in the mixing compound, which might be associated with the lower dose of CoQ10 used in compound regimens.
Meta-regression was performed to examine whether daily CoQ10 dose and treatment duration could modify effects on fatigue reduction. Both daily doses (coefficient = −0.0017 per mg, p < 0.001) and treatment duration (coefficient = −0.0042 per day, p = 0.007) correlated with increased fatigue reduction (Figures 7, 8). The funnel plot of the 13 included trials showed some asymmetry in effect size distributions (Supplementary Figure S1). Egger’s regression test showed a p-value of 0.008, indicating potential publication bias.
FIGURE 7.
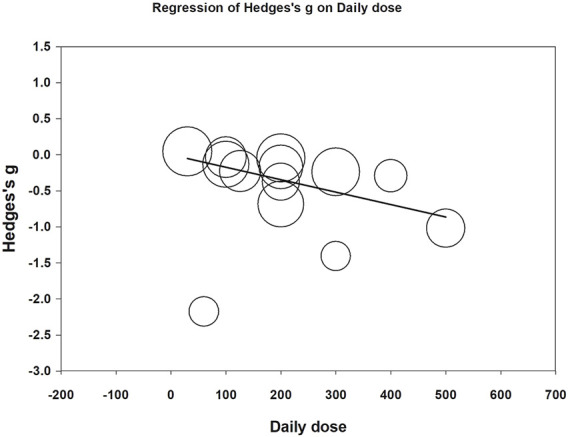
Meta-regression of Hedges’ g on daily dose (mg/day). The coefficient was -0.0017 with a p value <0.001.
FIGURE 8.
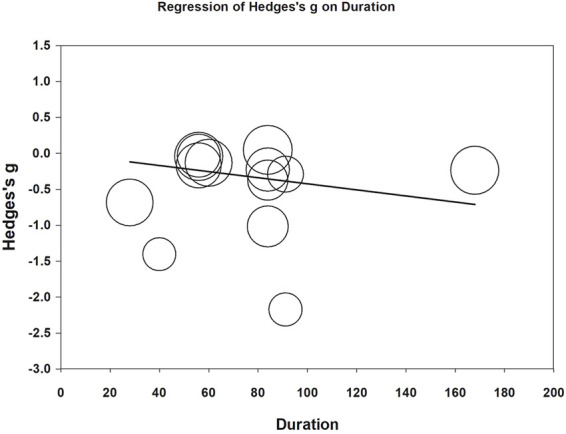
Meta-regression of Hedges’ g on treatment duration (day). The coefficient was -0.0042 with a p value of 0.007.
3.4 Secondary Outcome: Treatment-Associated Adverse Event Rates
Among the 602 participants treated with CoQ10, only one subject presented with an adverse event (i.e., gastrointestinal upset) and was thus withdrawn from the study conducted by Berman et al. (Berman et al., 2004). The meta-analysis of treatment-associated adverse event rates (Supplementary Figure S2) showed no between-group differences (odds ratio [OR] = 1.05, 95% CI = 0.36 to 3.08, p = 0.933, I 2 < 0.01%).
4 Discussion
In this meta-analysis, CoQ10 was shown to statistically significantly reduce fatigue, and statistical significance was maintained within sensitivity analyses. The background condition of the participants did not have a statistically significant impact on the direction of the association between the use of CoQ10 and fatigue reduction. We found that CoQ10-only formulations were effective in relieving fatigue, in contrast to CoQ10 compounds. Moreover, an increase in the daily dose and treatment duration of CoQ10 correlated with a better reduction in fatigue. To our knowledge, our study is the first systematic review or meta-analysis to demonstrate that CoQ10 has a mild-to-moderate effect (Hedges, 1981).
Previous meta-analyses have suggested that CoQ10 supplementation can reduce oxidative stress (Sangsefidi et al., 2020) and inflammatory markers (Fan et al., 2017). And in heart failure patients, it is related to lower mortality and higher exercise capacity as compared with placebo-treated group (Lei and Liu, 2017). In 2019, Mehrabani et al. published a systematic review (Mehrabani et al., 2019) reported that CoQ10 was effective against fatigue in patients with myopathy associated with statin use (Fedacko et al., 2013) as well as in patients with fibromyalgia (Cordero et al., 2012a; Cordero et al., 2013; Miyamae et al., 2013; Di Pierro et al., 2017). However, the aforementioned review did not quantify the fatigue-reducing effect of CoQ10. Thus, our meta-analysis was highly novel in addressing a gap in the current literature.
The causes of fatigue are multifactorial (Manjaly et al., 2019), including inflammatory oxidative injury in neurons (Haß et al., 2019; Manjaly et al., 2019) and mitochondrial dysfunction (Filler et al., 2014) and CoQ10 is likewise known to be involved in pathogenic pathways. First, CoQ10 plays a crucial role in inhibiting oxidation of lipid-containing structures, such as cellular membranes and lipoproteins (Aaseth et al., 2021). For example, in a rat model, CoQ10 was found to protect both the peripheral and central nervous systems after passing through the blood-brain barrier (Belousova et al., 2016). CoQ10 may provide widespread protection to neurons through neural pathways connecting the brain to the muscles (Aaseth et al., 2021). Second, CoQ10 regulates the mitochondrial respiratory chain and is an intensively studied enzyme associated with mitochondrial dysfunction (Filler et al., 2014). To this end, Maes et al. compared patients with depression (Maes et al., 2009b) and chronic fatigue syndrome (Maes et al., 2009a) with healthy volunteers and found that CoQ10 deficiency was positively associated with fatigue, whereas serum CoQ10 levels were inversely correlated with fatigue severity (Maes et al., 2009a; Maes et al., 2009b). The above pathways and clinical observations could explain the fatigue-reducing effect of CoQ10 concluded by our meta-analysis.
In the subgroup analysis evaluating healthy participants separately from patients with disease, the directions of the CoQ10-associated effect sizes in both subgroups were consistent. We found that the subgroup of patients demonstrated statistical significance (p = 0.004) in terms of the summary effect size, while the healthy subgroup did not show statistical significance (p = 0.191); however, this subgroup showed a strong tendency towards positive effects of CoQ10, with demonstrated marginal significance and the upper limit of the 95% CI just crossing zero (Hedges’ g = −0.338, 95% CI = −0.844 to 0.168). This could be related to between-group differences in CoQ10 depletion, which might be more severe in patients with diseases than in healthy participants, thus indicating that CoQ10 was more effective in reducing fatigue in the former population within the evaluated studies. Additionally, the number of studies included in the patient subgroup was greater than in the healthy subgroup, which subsequently increased the statistical power and narrowed the pooled 95% CI to facilitate a statistically significant result.
The fatigue-reducing effects of CoQ10 were not statistically significant in the subgroup treated with CoQ10-compounds. We speculated that this might be associated with the lower CoQ10 dose in the evaluated CoQ10 compound regimens (i.e., 30 mg/day (Fukuda et al., 2015) to 200 mg/day (Castro-Marrero et al., 2015; Castro-Marrero et al., 2021)). In contrast, in the subgroup using CoQ10 only (Berman et al., 2004; Lee et al., 2011; Cordero et al., 2013; Lesser et al., 2013; Peel et al., 2015; Sanoobar et al., 2016; Di Pierro et al., 2017; Morikawa et al., 2019; Mizuno et al., 2020; Mousavi et al., 2020), the daily dose ranged from 60 to 500 mg/day with four studies utilizing 300–500 mg/day (Cordero et al., 2013; Lesser et al., 2013; Sanoobar et al., 2016; Di Pierro et al., 2017). Meta-regression also confirmed that the daily dose of CoQ10 was associated with fatigue reduction (Kirkland, 2022; NOW, 2022).
Moreover, we identified a positive relationship between treatment duration and fatigue reduction. In our included studies, the longest period of CoQ10 supplementation was 6 months. As fatigue is a complicated disorder involving both psychological and physiological pathways, a sufficient period of intervention is needed to restore CoQ10 from chronic depletion status. Our speculation was evident in the results of a prior study (Hershey et al., 2007), which indicated that it takes approximately 3 months for CoQ10 supplementation to take effect in patients with chronic illness and CoQ10 deficiency. In the future, a prospective trial may be necessary to examine when ceiling effects are expected within CoQ10 interventions.
We also tried to explore if the fatigue-reducing effect of CoQ10 is related to the formulation, bioavailability or the patient’s disease. However, no specific pattern can be found. For example, in the three studies (Berman et al., 2004; Cordero et al., 2013; Sanoobar et al., 2016) with the Hedges’ g < −1.0, the formulations and manufacturers of CoQ10 were all different. Furthermore, Cordero et al. (Cordero et al., 2013) studied patients with fibromyalgia and the Hedges’ g was −1.405. Nonetheless, Di Pierro et al. (Di Pierro et al., 2017) studied the same fibromyalgia condition just got an Hedges’ g of −0.291. The effects of the formulation, bioavailability and disease might need more studies to conclude.
Coenzyme Q10 is a well-studied substance with a well-documented safety level of 1,200 mg/day per person (Hidaka et al., 2008). Moreover, evidence from pharmacokinetic studies suggests that exogenous CoQ10 does not influence the biosynthesis of endogenous CoQ10 and is less likely to accumulate in plasma or tissues after the cessation of supplementation (Bhagavan and Chopra, 2007; Miles, 2007). The high safety profile in the latter research was also compatible with our findings that, even at relatively high daily doses of 300–500 mg/day, the adverse event rate in the CoQ10 group was never higher than in the placebo group.
This study has several limitations. First, the fatigue scales used in different trials differed, which may have contributed to heterogeneity. Thus, in this study, we standardized measurements using Hedges’ g, employed a random-effects model to pool the studies, and conducted a subgroup analysis in accordance with the standard practice for addressing heterogeneity suggested by the Cochrane Handbook (Higgins et al., 2021a; Higgins et al., 2021b; Page et al., 2021b; Deeks et al., 2021; Tsai et al., 2021). Second, the daily dose and intervention duration were different in each trial, which may have contributed to variations in the estimated effects. Therefore, meta-regressions were performed to examine whether a linear relationship exists between the aforementioned factors and fatigue reduction. Third, these studies did not follow the participants after the cessation of CoQ10 supplementation to investigate how long the fatigue-reducing effect lasted, which serves as an intriguing topic to explore in future trials.
In conclusion, CoQ10 demonstrated a statistically significant fatigue-alleviating effect as compared with the evaluated placebos. The effect was statistically significantly correlated with daily dose and treatment duration. Future studies are needed to investigate the lasting effects of CoQ10 on fatigue reduction after the discontinuation of supplementation.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
Data curation, I-CT and K-VC; formal analysis I-CT and K-VC; investigation, C-WH, C-HC, P-TT, K-VC; methodology, C-WH, P-TT, K-VC; software, I-CT, P-TT; supervision, P-TT, K-VC; validation, C-WH, C-HC, P-TT, K-VC; writing the original draft, I-CT; writing review and editing, C-WH, C-HC, P-TT, K-VC. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Taiwan University Hospital, Bei-Hu Branch; Ministry of Science and Technology, Taiwan (MOST 106-2314-B-002-180-MY3 and MOST 109-2314-B-002-114-MY3); and the Taiwan Society of Ultrasound in Medicine. APC was funded by the Ministry of Science and Technology of Taiwan and Taiwan Society of Ultrasound in Medicine.
Conflict of Interest
I-CT is the founder of the company InnovaRad Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.883251/full#supplementary-material
References
- Aaseth J., Alexander J., Alehagen U. (2021). Coenzyme Q10 Supplementation - in Ageing and Disease. Mech. Ageing Dev. 197, 111521. 10.1016/j.mad.2021.111521 [DOI] [PubMed] [Google Scholar]
- Belousova M. A., Tokareva O. G., Gorodetskaya E. A., Kalenikova E. I., Medvedev O. S. (2016). Neuroprotective Effectiveness of Intravenous Ubiquinone in Rat Model of Irreversible Cerebral Ischemia. Bull. Exp. Biol. Med. 161, 245–247. 10.1007/s10517-016-3387-1 [DOI] [PubMed] [Google Scholar]
- Berman M., Erman A., Ben-Gal T., Dvir D., Georghiou G. P., Stamler A., et al. (2004). Coenzyme Q10 in Patients with End-Stage Heart Failure Awaiting Cardiac Transplantation: A Randomized, Placebo-Controlled Study. Clin. Cardiol. 27, 295–299. 10.1002/clc.4960270512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagavan H. N., Chopra R. K. (2007). Plasma Coenzyme Q10 Response to Oral Ingestion of Coenzyme Q10 Formulations. Mitochondrion 7 (Suppl. l), S78–S88. 10.1016/j.mito.2007.03.003 [DOI] [PubMed] [Google Scholar]
- Borenstein M., Hedges L. V., Rothstein H. R. (2009). “Fixed-Effect versus Random-Effects Models,” in Introduction to Meta-Analysis. Editors Borenstein M., (Hoboken, NJ, USA: Wiley; ), 77–86. 10.1002/9780470743386.ch13 [DOI] [Google Scholar]
- Castro-Marrero J., Cordero M. D., Segundo M. J., Sáez-Francàs N., Calvo N., Román-Malo L., et al. (2015). Does Oral Coenzyme Q10 Plus NADH Supplementation Improve Fatigue and Biochemical Parameters in Chronic Fatigue Syndrome? Antioxid. Redox Signal 22, 679–685. 10.1089/ars.2014.6181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Marrero J., Sáez-Francàs N., Segundo M. J., Calvo N., Faro M., Aliste L., et al. (2016). Effect of Coenzyme Q10 Plus Nicotinamide Adenine Dinucleotide Supplementation on Maximum Heart Rate After Exercise Testing in Chronic Fatigue Syndrome - A Randomized, Controlled, Double-Blind Trial. Clin. Nutr. 35, 826–834. 10.1016/j.clnu.2015.07.010 [DOI] [PubMed] [Google Scholar]
- Castro-Marrero J., Segundo M. J., Lacasa M., Martinez-Martinez A., Sentañes R. S., Alegre-Martin J. (2021). Effect of Dietary Coenzyme Q10 Plus NADH Supplementation on Fatigue Perception and Health-Related Quality of Life in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 13, 2658. 10.3390/nu13082658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathébras P. J., Robbins J. M., Kirmayer L. J., Hayton B. C. (1992). Fatigue in Primary Care: Prevalence, Psychiatric Comorbidity, Illness Behavior, and Outcome. J. Gen. Intern. Med. 7, 276–286. 10.1007/bf02598083 [DOI] [PubMed] [Google Scholar]
- Chandran V., Bhella S., Schentag C., Gladman D. D. (2007). Functional Assessment of Chronic Illness Therapy-Fatigue Scale Is Valid in Patients with Psoriatic Arthritis. Ann. Rheum. Dis. 66, 936–939. 10.1136/ard.2006.065763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero M. D., Alcocer-Gómez E., de Miguel M., Culic O., Carrión A. M., Alvarez-Suarez J. M., et al. (2013). Can Coenzyme Q10 Improve Clinical and Molecular Parameters in Fibromyalgia? Antioxid. Redox Signal 19, 1356–1361. 10.1089/ars.2013.5260 [DOI] [PubMed] [Google Scholar]
- Cordero M. D., Cano-García F. J., Alcocer-Gómez E., De Miguel M., Sánchez-Alcázar J. A. (2012). Oxidative Stress Correlates with Headache Symptoms in Fibromyalgia: Coenzyme Q₁₀ Effect on Clinical Improvement. PLOS ONE 7, e35677. 10.1371/journal.pone.0035677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero M. D., Santos-García R., Bermejo-Jover D., Sánchez-Domínguez B., Jaramillo-Santos M. R., Bullón P. (2012). Coenzyme Q10 in Salivary Cells Correlate with Blood Cells in Fibromyalgia: Improvement in Clinical and Biochemical Parameter After Oral Treatment. Clin. Biochem. 45, 509–511. 10.1016/j.clinbiochem.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Cullen W., Kearney Y., Bury G. (2002). Prevalence of Fatigue in General Practice. Ir. J. Med. Sci. 171, 10–12. 10.1007/bf03168931 [DOI] [PubMed] [Google Scholar]
- Deeks J. J., Higgins J. P. T., Altman D. G. (2021). Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 https://training.cochrane.org/handbook/current/chapter-10 (Accessed Jan. 16, 2022). Chapter 10: Analysing Data and Undertaking Meta-Analyses. [Google Scholar]
- Di Pierro F., Rossi A., Consensi A., Giacomelli C., Bazzichi L. (2017). Role for a Water-Soluble Form of CoQ10 in Female Subjects Affected by Fibromyalgia. A Preliminary Study. Clin. Exp. Rheumatol. 35 (Suppl. 105), 20–27. [PubMed] [Google Scholar]
- Fan L., Feng Y., Chen G. C., Qin L. Q., Fu C. L., Chen L. H. (2017). Effects of Coenzyme Q10 Supplementation on Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacol. Res. 119, 128–136. 10.1016/j.phrs.2017.01.032 [DOI] [PubMed] [Google Scholar]
- Fedacko J., Pella D., Fedackova P., Hänninen O., Tuomainen P., Jarcuska P., et al. (2013). Coenzyme Q(10) and Selenium in Statin-Associated Myopathy Treatment. Can. J. Physiol. Pharmacol. 91, 165–170. 10.1139/cjpp-2012-0118 [DOI] [PubMed] [Google Scholar]
- Filler K., Lyon D., Bennett J., McCain N., Elswick R., Lukkahatai N., et al. (2014). Association of Mitochondrial Dysfunction and Fatigue: A Review of the Literature. BBA Clin. 1, 12–23. 10.1016/j.bbacli.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J., Mahjoub S. Z. (2014). Fatigue in Healthy and Diseased Individuals. Am. J. Hosp. Palliat. Care 31, 562–575. 10.1177/1049909113494748 [DOI] [PubMed] [Google Scholar]
- Fisk J. D., Ritvo P. G., Ross L., Haase D. A., Marrie T. J., Schlech W. F. (1994). Measuring the Functional Impact of Fatigue: Initial Validation of the Fatigue Impact Scale. Clin. Infect. Dis. 18 (Suppl. 1), S79–S83. 10.1093/clinids/18.supplement_1.s79 [DOI] [PubMed] [Google Scholar]
- Fukuda S., Koyama H., Kondo K., Fujii H., Hirayama Y., Tabata T., et al. (2015). Effects of Nutritional Supplementation on Fatigue, and Autonomic and Immune Dysfunction in Patients with End-Stage Renal Disease: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. PLOS ONE 10, e0119578. 10.1371/journal.pone.0119578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S., Nojima J., Kajimoto O., Yamaguti K., Nakatomi Y., Kuratsune H., et al. (2016). Ubiquinol-10 Supplementation Improves Autonomic Nervous Function and Cognitive Function in Chronic Fatigue Syndrome. Biofactors 42, 431–440. 10.1002/biof.1293 [DOI] [PubMed] [Google Scholar]
- Fukuda S., Takashima S., Iwase M., Yamaguti K., Kuratsune H., Watanabe Y. (2008). Development and Validation of a New Fatigue Scale for Fatigued Subjects with and without Chronic Fatigue Syndrome. Tokyo, Japan: Springer, 89–102. Fatigue Science for Human Health. [Google Scholar]
- Gharahdaghi N., Shabkhiz F., Azarboo E., Keyhanian A. (2013). The Effects of Daily Coenzyme Q10 Supplementation on VO 2max, vVO 2max and Intermittent Exercise Performance in Soccer Players. Life Sci. J. 10, 22–28. [Google Scholar]
- Gökbel H., Gül İ., Belviranl M., Okudan N. (2010). The Effects of Coenzyme Q10 Supplementation on Performance During Repeated Bouts of Supramaximal Exercise in Sedentary Men. J. Strength Cond. Res. 24, 97–102. 10.1519/JSC.0b013e3181a61a50 [DOI] [PubMed] [Google Scholar]
- Gomez-Centeno A., Ramentol M., Gonzalez M. J., Alegre C. (2020). AB0952 Coenzyme Q10, Tryptophan and Magnesium: A Nutritional Supplement in the Treatment of Fibromyalgia Symptoms. Ann. Rheum. Dis. 79, 1773–1774. 10.1136/annrheumdis-2020-eular.5531 [DOI] [Google Scholar]
- Haß U., Herpich C., Norman K. (2019). Anti-Inflammatory Diets and Fatigue. Nutrients 11, 2315. 10.3390/nu11102315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges L. V. (1981). Distribution Theory for Glass's Estimator of Effect Size and Related Estimators. J. Educ. Statistics 6, 107–128. 10.2307/1164588 [DOI] [Google Scholar]
- Hershey A. D., Powers S. W., Vockell A. L., Lecates S. L., Ellinor P. L., Segers A., et al. (2007). Coenzyme Q10 Deficiency and Response to Supplementation in Pediatric and Adolescent Migraine. Headache 47, 73–80. 10.1111/j.1526-4610.2007.00652.x [DOI] [PubMed] [Google Scholar]
- Hidaka T., Fujii K., Funahashi I., Fukutomi N., Hosoe K. (2008). Safety Assessment of Coenzyme Q10 (CoQ10). Biofactors 32, 199–208. 10.1002/biof.5520320124 [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J., Altman D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327, 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T., Eldridge S., Li T. (2021). “Chapter 23: Including Variants on Randomized Trials,” in Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 Available at: https://training.cochrane.org/handbook/current/chapter-23 (Accessed Jan. 16, 2022). [Google Scholar]
- Higgins J. P. T., Li T., Deeks J. J. (2021). “Chapter 6: Choosing Effect Measures and Computing Estimates of Effect,” in Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 Available at: https://training.cochrane.org/handbook/current/chapter-06 (Accessed Jan. 16, 2022). [Google Scholar]
- Hozo S. P., Djulbegovic B., Hozo I. (2005). Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 5, 13. 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase S., Kawaguchi T., Yotsumoto D., Doi T., Miyara K., Odagiri H., et al. (2016). Efficacy and Safety of an Amino Acid Jelly Containing Coenzyme Q10 and L-Carnitine in Controlling Fatigue in Breast Cancer Patients Receiving Chemotherapy: A Multi-Institutional, Randomized, Exploratory Trial (JORTC-CAM01). Support Care Cancer 24, 637–646. 10.1007/s00520-015-2824-4 [DOI] [PubMed] [Google Scholar]
- Jason L. A., Jordan K. M., Richman J. A., Rademaker A. W., Huang C. F., McCready W., et al. (1999). A Community-Based Study of Prolonged Fatigue and Chronic Fatigue. J. Health Psychol. 4, 9–26. 10.1177/135910539900400103 [DOI] [PubMed] [Google Scholar]
- Kirkland (2022). Kirkland Signature Ubiquinol 25Mg + Vitamin E 150 Softgels. Available at: https://bit.ly/3A2n8o6 (Accessed Jan 16, 2022). [Google Scholar]
- Kumar A., Singh R. B., Saxena M., Niaz M. A., Josh S. R., Chattopadhyay P., et al. (2007). Effect of Carni Q-Gel (Ubiquinol and Carnitine) on Cytokines in Patients with Heart Failure in the Tishcon Study. Acta Cardiol. 62, 349–354. 10.2143/ac.62.4.2022278 [DOI] [PubMed] [Google Scholar]
- Langsjoen P. H., Langsjoen J. O., Langsjoen A. M., Lucas L. A. (2005). Treatment of Statin Adverse Effects with Supplemental Coenzyme Q10 and Statin Drug Discontinuation. Biofactors 25, 147–152. 10.1002/biof.5520250116 [DOI] [PubMed] [Google Scholar]
- Langsjoen P. H., Langsjoen J. O., Langsjoen A. M., Rosenfeldt F. (2019). Statin-Associated Cardiomyopathy Responds to Statin Withdrawal and Administration of Coenzyme Q10. Perm. J. 23, 18.257. 10.7812/tpp/18.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. J., Cho W. J., Kim J. K., Lee D. C. (2011). Effects of Coenzyme Q10 on Arterial Stiffness, Metabolic Parameters, and Fatigue in Obese Subjects: A Double-Blind Randomized Controlled Study. J. Med. Food 14, 386–390. 10.1089/jmf.2010.1202 [DOI] [PubMed] [Google Scholar]
- Lei L., Liu Y. (2017). Efficacy of Coenzyme Q10 in Patients with Cardiac Failure: A Meta-Analysis of Clinical Trials. BMC Cardiovasc. Disord. 17, 196. 10.1186/s12872-017-0628-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo A., Kelley J. M., Nee J., Ballou S., Iturrino J., Cheng V., et al. (2021). Open-Label Placebo vs Double-Blind Placebo for Irritable Bowel Syndrome: A Randomized Clinical Trial. Pain 162, 2428–2435. 10.1097/j.pain.0000000000002234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser G. J., Case D., Stark N., Williford S., Giguere J., Garino L. A., et al. (2013). A Randomized, Double-Blind, Placebo-Controlled Study of Oral Coenzyme Q10 to Relieve Self-Reported Treatment-Related Fatigue in Newly Diagnosed Patients with Breast Cancer. J. Support Oncol. 11, 31–42. 10.1016/j.suponc.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. (2009). Coenzyme Q10 Deficiency in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Is Related to Fatigue, Autonomic and Neurocognitive Symptoms and Is Another Risk Factor Explaining the Early Mortality in ME/CFS Due to Cardiovascular Disorder. Neuro Endocrinol. Lett. 30, 470–476. [PubMed] [Google Scholar]
- Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. (2009). Lower Plasma Coenzyme Q10 in Depression: A Marker for Treatment Resistance and Chronic Fatigue in Depression and A Risk Factor to Cardiovascular Disorder in That Illness. Neuro Endocrinol. Lett. 30, 462–469. [PubMed] [Google Scholar]
- Manjaly Z. M., Harrison N. A., Critchley H. D., Do C. T., Stefanics G., Wenderoth N., et al. (2019). Pathophysiological and Cognitive Mechanisms of Fatigue in Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 90, 642–651. 10.1136/jnnp-2018-320050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle D., Dybring A. (2020). Bioavailability of Coenzyme Q10: An Overview of the Absorption Process and Subsequent Metabolism. Antioxidants (Basel) 9, 386. 10.3390/antiox9050386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabani S., Askari G., Miraghajani M., Tavakoly R., Arab A. (2019). Effect of Coenzyme Q10 Supplementation on Fatigue: A Systematic Review of Interventional Studies. Complement. Ther. Med. 43, 181–187. 10.1016/j.ctim.2019.01.022 [DOI] [PubMed] [Google Scholar]
- Menon R., Cribb L., Murphy J., Ashton M. M., Oliver G., Dowling N., et al. (2017). Mitochondrial Modifying Nutrients in Treating Chronic Fatigue Syndrome: A 16-Week Open-Label Pilot Study. Adv. Integr. Med. 4, 109–114. 10.1016/j.aimed.2017.11.001 [DOI] [Google Scholar]
- Miles M. V. (2007). The Uptake and Distribution of Coenzyme Q10. Mitochondrion 7 (Suppl. l), S72–S77. 10.1016/j.mito.2007.02.012 [DOI] [PubMed] [Google Scholar]
- Miyamae T., Seki M., Naga T., Uchino S., Asazuma H., Yoshida T., et al. (2013). Increased Oxidative Stress and Coenzyme Q10 Deficiency in Juvenile Fibromyalgia: Amelioration of Hypercholesterolemia and Fatigue by Ubiquinol-10 Supplementation. Redox Rep. 18, 12–19. 10.1179/1351000212y.0000000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Sasaki A. T., Watanabe K., Watanabe Y. (2020). Ubiquinol-10 Intake Is Effective in Relieving Mild Fatigue in Healthy Individuals. Nutrients 12, 1640. 10.3390/nu12061640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Tanaka M., Nozaki S., Mizuma H., Ataka S., Tahara T., et al. (2008). Antifatigue Effects of Coenzyme Q10 During Physical Fatigue. Nutrition 24, 293–299. 10.1016/j.nut.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Moccia M., Capacchione A., Lanzillo R., Carbone F., Micillo T., Perna F., et al. (2019). Coenzyme Q10 Supplementation Reduces Peripheral Oxidative Stress and Inflammation in Interferon-Β1a-Treated Multiple Sclerosis. Ther. Adv. Neurol. Disord. 12, 1. 10.1177/1756286418819074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H., Sawashita J., Miyakoshi Y., Horie F., Hosoe K. (2019). Reduced Form of Coenzyme Q10 Relieves Daily Life Stress and Improves Sleep Quality in Healthy Subjects with High Stress Sensitivity-A Randomized, Double-Blind, Parallel-Group Study. Jpn. Pharmacol. Ther. 47, 1231–1244. [Google Scholar]
- Mousavi S., Mohammadi V., Foroughi Z. (2020). Effect of Coenzyme Q10 Supplementation on Work-Related Fatigue in Nurses: a Double-Blind, Randomized Placebo-Controlled Study. Fatigue Biomed. Health & Behav. 8, 1–10. 10.1080/21641846.2019.1704374 [DOI] [Google Scholar]
- Negro M., Perna S., Spadaccini D., Castelli L., Calanni L., Barbero M., et al. (2019). Effects of 12 Weeks of Essential Amino Acids (EAA)-Based Multi-Ingredient Nutritional Supplementation on Muscle Mass, Muscle Strength, Muscle Power and Fatigue in Healthy Elderly Subjects: A Randomized Controlled Double-Blind Study. J. Nutr. Health Aging 23, 414–424. 10.1007/s12603-019-1163-4 [DOI] [PubMed] [Google Scholar]
- Now (2022). NOW Supplements, CoQ10 400 Mg, Pharmaceutical Grade, All-Trans Form Produced by Fermentation, 60 Softgels. Available at: https://amzn.to/3nytwi5 (Accessed Jan 16, 2022). [Google Scholar]
- Page M. J., Higgins J. P. T., Sterne J. A. C. (2021). “Chapter 13: Assessing Risk of Bias Due to Missing Results in a Synthesis,” in Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 Available at: https://training.cochrane.org/handbook/current/chapter-13 (Accessed Jan 16, 2022). [Google Scholar]
- Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 372, n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel M. M., Cooke M., Lewis-Peel H. J., Lea R. A., Moyle W. (2015). A Randomized Controlled Trial of Coenzyme Q10 for Fatigue in the Late-Onset Sequelae of Poliomyelitis. Complement. Ther. Med. 23, 789–793. 10.1016/j.ctim.2015.09.002 [DOI] [PubMed] [Google Scholar]
- Reynolds K. J., Vernon S. D., Bouchery E., Reeves W. C. (2004). The Economic Impact of Chronic Fatigue Syndrome. Cost. Eff. Resour. Alloc. 2, 4. 10.1186/1478-7547-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridsdale L., Evans A., Jerrett W., Mandalia S., Osler K., Vora H. (1993). Patients with Fatigue in General Practice: A Prospective Study. BMJ 307, 103–106. 10.1136/bmj.307.6896.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangsefidi Z. S., Yaghoubi F., Hajiahmadi S., Hosseinzadeh M. (2020). The Effect of Coenzyme Q10 Supplementation on Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Food Sci. Nutr. 8, 1766–1776. 10.1002/fsn3.1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanoobar M., Dehghan P., Khalili M., Azimi A., Seifar F. (2016). Coenzyme Q10 as a Treatment for Fatigue and Depression in Multiple Sclerosis Patients: A Double Blind Randomized Clinical Trial. Nutr. Neurosci. 19, 138–143. 10.1179/1476830515y.0000000002 [DOI] [PubMed] [Google Scholar]
- Schweiger V., Secchettin E., Castellani C., Martini A., Mazzocchi E., Picelli A., et al. (2020). Comparison Between Acupuncture and Nutraceutical Treatment with Migratens® in Patients with Fibromyalgia Syndrome: A Prospective Randomized Clinical Trial. Nutrients 12, 821. 10.3390/nu12030821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. B., Neki N. S., Kartikey K., Pella D., Kumar A., Niaz M. A., et al. (2003). Effect of Coenzyme Q10 on Risk of Atherosclerosis in Patients with Recent Myocardial Infarction. Mol. Cell Biochem. 246, 75–82. 10.1007/978-1-4615-0298-2_11 [DOI] [PubMed] [Google Scholar]
- Sterne J. A. C., Savović J., Page M. J., Elbers R. G., Blencowe N. S., Boutron I., et al. (2019). RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 366, l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Nagato S., Sakuraba K., Morio K., Sawaki K. (2021). Short-Term Ubiquinol-10 Supplementation Alleviates Tissue Damage in Muscle and Fatigue Caused by Strenuous Exercise in Male Distance Runners. Int. J. Vitam. Nutr. Res. 91, 261–270. 10.1024/0300-9831/a000627 [DOI] [PubMed] [Google Scholar]
- Tsai I. C., Chang K. V. (2022). INPLASY202210113: Effectiveness of Coenzyme Q10 for Reducing Fatigue: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Available at: https://inplasy.com/inplasy-2022-1-0113/ (Accessed Jan. 23, 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai I. C., Hsu C. W., Chang C. H., Tseng P. T., Chang K. V. (2021). The Effect of Curcumin Differs on Individual Cognitive Domains Across Different Patient Populations: A Systematic Review and Meta-Analysis. Pharm. (Basel) 14, 1235. 10.3390/ph14121235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wernsdorff M., Loef M., Tuschen-Caffier B., Schmidt S. (2021). Effects of Open-Label Placebos in Clinical Trials: A Systematic Review and Meta-Analysis. Sci. Rep. 11, 3855. 10.1038/s41598-021-83148-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.