Abstract
Environmental contamination is widespread and can negatively impact wildlife health. Some contaminants, including heavy metals, have immunosuppressive effects, but prior studies have rarely measured contamination and disease simultaneously, which limits our understanding of how contaminants and pathogens interact to influence wildlife health. Here, we measured mercury concentrations, influenza infection, influenza antibodies and body condition in 749 individuals from 11 species of wild ducks overwintering in California. We found that the odds of prior influenza infection increased more than fivefold across the observed range of blood mercury concentrations, while accounting for species, age, sex and date. Influenza infection prevalence was also higher in species with higher average mercury concentrations. We detected no relationship between influenza infection and body fat content. This positive relationship between influenza prevalence and mercury concentrations in migratory waterfowl suggests that immunotoxic effects of mercury contamination could promote the spread of avian influenza along migratory flyways, especially if influenza has minimal effects on bird health and mobility. More generally, these results show that the effects of environmental contamination could extend beyond the geographical area of contamination itself by altering the prevalence of infectious diseases in highly mobile hosts.
Keywords: avian influenza, body condition, duck, environmental contaminant, mercury, wildlife health
1. Introduction
Environmental pollution is widespread. Many contaminants, such as heavy metals and organic compounds, can persist in ecosystems for years or decades [1], leading to prolonged exposure of wildlife to potential toxicants. Exposure to contaminants can impact wildlife health, behaviour, survival and reproduction [2,3], but animals can detoxify and/or excrete many contaminants, and the physiological effects of contamination vary across species and ecological contexts [2,4]. Understanding how and when contaminants most strongly affect wildlife is important for prioritizing monitoring, managing exposure and implementing mitigation measures.
Infection with pathogens and parasites is one important context in which contaminants might affect wildlife health and fitness [5]. Wildlife naturally experience infection over their lives, but the probability and effects of infection are context dependent. For example, the pathogens that cause chytridiomycosis in amphibians and white-nose syndrome in bats can cause massive die-offs, but in some contexts cause no disease [6,7]. This variation in pathogen impacts stems from differences in pathogen exposure, susceptibility (i.e. probability of infection given exposure) and immune responses across hosts. Exposure to contaminants could influence these pathways via changes to a host's behaviour, immune system and/or energetic state [8], but the relationships between contamination and infection are complex. For instance, contaminants could increase infection prevalence if they have immunosuppressive effects [9–11] (figure 1a,b), but could decrease infection prevalence if contamination reduces host competence [12] or parasite fitness [8] (figure 1d). Therefore, the influence of environmental contaminants on infection prevalence depends on the contaminant–pathogen combination, the host's physiological response to each, and the environmental context.
Figure 1.
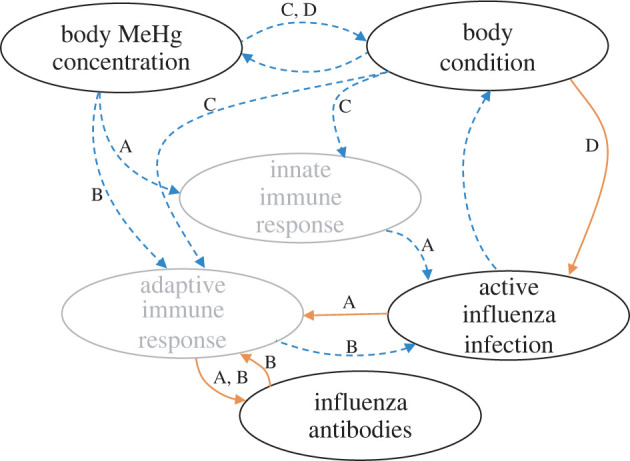
Hypothesized and predicted relationships among mercury concentrations, body condition, active infection and antibody detection. Dashed blue arrows represent negative relationships between variables and solid orange arrows represent positive relationships. Grey ovals are variables that are important mechanistically but were not measured directly in this study. In pathway A, MeHg immunotoxicity compromises the innate immune response to infection, leading to a higher probability of active infection, more antibody production and an increased probability of antibody detection. In B, MeHg immunotoxicity compromises the adaptive immune response, reducing antibody detection but increasing the probability of active infection. In C, MeHg toxicity reduces host body condition, reducing the energy available to mount an immune response and increasing infection probabilities. In pathway D, MeHg toxicity reduces host body condition, altering physiologic conditions for viral replication and decreasing the probabilities of both active infection and antibody detection. Feedbacks complicate these relationships, including disease-induced reductions in body condition following influenza infection and effects of body condition on MeHg concentrations (due to concentrating of body MeHg with mass loss). (Online version in colour.)
In addition to altering infection prevalence, the toxic effects of contaminants can increase disease severity by reducing the amount of energy available to mount an immune response (figure 1c). Body condition metrics (e.g. body mass or fat stores), which represent energy stores and overall health, are useful and common proxies for the severity of disease. However, relationships of body condition with infection or contamination are bidirectional (figure 1) [13]. For example, animals in poor body condition sometimes increase their feeding rates, which can increase their exposure to trophically transmitted pathogens and contaminants. Changes in body size can also concentrate or dilute contaminants in a host's body (figure 1), which could change their toxicity [14]. Body condition is therefore a measurement of overall health that depends on interacting ecological factors including disease and contamination, as well as stress, nutrition and reproductive status [13].
Waterfowl are natural hosts of avian influenza viruses (hereafter influenza) and exposure to environmental contaminants could affect the prevalence and disease severity of this common pathogen. Influenza is endemic in waterfowl populations, but infection prevalence is highest in late summer [15], in juveniles [16] and in dabbling and filter-feeding ducks [17,18]. Antibodies against influenza, which generally last months to over a year [19–21], are more prevalent in adults [16,22]. Influenza is transmitted via the environment and can persist in wetland environments for months or longer [23,24]. Infection occasionally affects body condition, behaviour and/or movement, but low pathogenic avian influenza infection is generally considered asymptomatic in wild birds [25]. However, co-exposure to immunotoxic contaminants could increase infection probabilities and magnify any negative health effects of infection. In addition, highly pathogenic strains of avian influenza viruses, which cause severe disease in poultry and occasionally humans and other mammals [26,27], are increasing in wild waterfowl [28]. Understanding the factors, including contaminant exposure, that influence influenza infection in wild birds is therefore important for informing assessments of risk to wildlife, livestock and human health.
Mercury contamination from natural and anthropogenic sources (e.g. atmospheric deposition from industrial outputs, gold mining [29]) is common in the aquatic habitats inhabited by waterfowl. Bacteria in aquatic soils convert inorganic mercury into methylmercury (MeHg), its toxic form, which is then acquired by animals through feeding and biomagnifies through food webs [30,31]. Animals can sequester and depurate mercury (e.g. via eggs and feathers), but it also bioaccumulates throughout individuals' lifetimes, so mercury concentrations in the internal tissues of wild animals reflect a combination of long-term and recent exposure [31]. Animals that feed at higher trophic levels (e.g. diving ducks as compared to dabbling ducks) tend to have higher mercury concentrations from increased dietary exposure [32]. MeHg exposure can reduce body condition [33,34], reproductive output [4,35] and survival [36]. MeHg can also alter immune function in mammals and birds [37], including compromising both the innate immune response (e.g. inflammation [9,38–41]) and the adaptive immune response (e.g. lymphocyte production [38,42,43], antibody titres [10]). Both innate and adaptive immunity influence the probability and severity of influenza infection [44,45], so mercury contamination could affect the prevalence of influenza infection and its impacts waterfowl populations.
Here, we study relationships among mercury contamination, influenza infection and body condition in 11 species of dabbling and diving ducks in the Pacific Flyway. The Central Valley and San Francisco Bay Estuary of California are hotspots of mercury contamination in North America [4,46] and are important sites for overwintering waterfowl; approximately 60% of the migratory waterfowl in the Pacific Flyway overwinter in this region annually [47,48]. Multiple waterfowl species co-occur at overwintering sites in the region, which enables cross-species influenza transmission and viral reassortment [49,50], and waterfowl occasionally use wetlands and agricultural habitats near and within poultry facilities [51]. This combination of mercury contamination, high waterfowl abundance and intensive poultry production makes northern California an important region for understanding how mercury contamination affects influenza prevalence and wildlife health.
We hypothesized that influenza infection would be positively related to mercury concentrations due to the immunotoxic effects of mercury [9,31] (figure 1), even while accounting for factors that could influence both infection and mercury concentrations, including age [16,31], species [4,17], sex [11,52] and body condition [33,53]. We also hypothesized that influenza infection would have sublethal effects on body condition [25,54], and that these effects would be exacerbated by mercury, especially at high mercury concentrations [55]. We explored these relationships for both active infection (i.e. PCR analysis of cloacal and oropharyngeal swabs) and for detectable antibodies (i.e. ELISA assay of blood samples, hereafter ‘prior infection’). Antibodies to influenza are estimated to last 6 months-1.5 years [19–21] but usually peak within three weeks of infection [19,20]. The strength of the antibody response and the probability of detecting antibodies following infection vary across age classes and species [20,56]. Antibody assay results therefore represent probable prior infection within the last 1.5 years, but most likely within the last six months [57], and depend on individual traits.
2. Methods
(a) . Study system and data collection
Dabbling and diving ducks were collected lethally by scientists during the non-breeding season (October–March) in 2017–2018 and 2018–2019 from two major bays in the San Francisco Bay Estuary of California (table 1). Cloacal and oropharyngeal swabs and a cardiac venipuncture, which supplied blood samples for both antibody and mercury analysis, were taken within 5 min of collection. We determined age (juvenile or adult) and sex using plumage characteristics [58–60]. In the laboratory, we measured body mass, extracted sera for blood mercury analysis, dissected carcasses to extract samples of liver and muscle for mercury analysis [61] and validated field ageing and sexing techniques [62,63]. For more detail, see electronic supplementary material, methods.
Table 1.
Sample sizes and prevalence of detected influenza antibodies or viral RNA in samples from ducks collected in the San Francisco Bay Estuary, California during winters of 2017–2018 and 2018–2019. All samples included in this table also included paired mercury data. Confidence intervals (CI) are derived from 10 000 bootstrapped samples.
| common name | scientific name | antibody sample size (ELISA) | antibody prevalence [95% CI] | active infection sample size (rRT-PCR) | active infection prevalence [95% CI] |
|---|---|---|---|---|---|
| American green-winged teal | Anas crecca carolinensis | 11 | 0.36 [0.09, 0.64] | 89 | 0.08 [0.03, 0.13] |
| American wigeon | Mareca americana | 22 | 0.50 [0.27, 0.68] | 64 | 0.05 [0.00, 0.11] |
| canvasback | Aythya valisineria | 70 | 0.84 [0.74, 0.91] | 65 | 0.05 [0.00, 0.11] |
| cinnamon teal | Anas cyanoptera | 7 | 0.71 [0.29, 1.00] | 15 | 0.13 [0.00, 0.33] |
| gadwall | Mareca strepera | 17 | 0.59 [0.35, 0.82] | 33 | 0.03 [0.00, 0.09] |
| greater scaup | Aythya marila | 24 | 0.83 [0.67, 0.96] | 24 | 0.04 [0.00, 0.12] |
| lesser scaup | Aythya affinis | 86 | 0.77 [0.67, 0.86] | 88 | 0.09 [0.03, 0.16] |
| mallard | Anas platyrhynchos | 50 | 0.80 [0.68, 0.90] | 90 | 0.04 [0.01, 0.09] |
| northern pintail | Anas acuta | 28 | 0.79 [0.64, 0.93] | 61 | 0.08 [0.02, 0.15] |
| northern shoveler | Spatula clypeata | 26 | 0.88 [0.73, 1.00] | 89 | 0.11 [0.04, 0.18] |
| ruddy duck | Oxyura jamaicensis | 96 | 0.65 [0.55, 0.74] | 102 | 0.06 [0.02, 0.11] |
| total/mean | 437 | 0.74 [0.70, 0.78] | 720 | 0.07 [0.05, 0.09] |
(b) . Mercury concentrations
We measured total mercury (THg) concentrations in blood, liver and muscle (electronic supplementary material, methods). We used whole blood THg concentration to represent body MeHg concentration, because 95% of THg in blood is MeHg [31,64], and because studies of blood mercury are common [34], allowing us to compare our results directly to those from other studies. For the 88 birds (12% of data) for which blood mercury values were unavailable, we estimated blood THg concentrations using the strong relationships between mercury concentrations in blood and those in muscle and liver tissue [61] (electronic supplementary material, methods). We repeated analyses without these imputed data and found qualitatively similar results.
(c) . Influenza laboratory analysis
Cloacal and oropharyngeal swabs were tested for the presence of influenza RNA using real-time reverse transcriptase-PCR (rRT-PCR) targeting the matrix gene [65] (electronic supplementary material, methods). We considered a sample to indicate active influenza infection if the cycle threshold (Ct) value was less than or equal to 45 [66]. All rRT-PCR-positive samples tested negative for highly pathogenic H5 clade 2.3.4.4 viruses [66], the only highly pathogenic influenza lineage previously identified in North American wild birds, and thus most likely represent infection with low pathogenic influenza viruses. Sera samples were tested for the presence of influenza A antibodies using commercially available blocking enzyme-linked immunoassay (ELISA; AI MultiS-Screen Avian Influenza Virus Antibody Test Kit; IDEXX Laboratories, Westbrook, Maine, USA). We considered samples with a signal-to-noise ratio less than 0.5 to be positive for the presence of influenza antibodies [45,67].
(d) . Body condition and composition
We used percentage crude fat from a sample of the whole body, as determined by body composition analysis (electronic supplementary material, methods), to measure body condition. For data analysis, we standardized percentage fat within species, age class and/or sex, which accounted for differences in average fat content among groups that were unrelated to condition. To do so, we subtracted the mean and divided by the standard deviation within each group (i.e. z-score scaling). For most species, we standardized percentage fat within three groups: adult male, adult female and juveniles of both sexes; for the two species that had fewer than eight samples within a group (cinnamon teal and greater scaup), percentage fat was scaled across all individuals in the species. Because we used percentage fat (rather than fat mass), our measurement of body condition accounted for body mass.
(e) . Statistical analysis
We first examined relationships between blood mercury concentrations and influenza prevalence at the species level using univariate generalized linear models (GLMs). We modelled antibody prevalence and active infection prevalence separately; each GLM used a logit link function where the response variable was the number of positive and negative samples for each species and the predictor variable was the species' geometric mean blood mercury concentration (log10-transformed).
Next, we studied relationships between influenza infection probabilities, mercury and ecological variables at the individual level. We transformed the date of sampling (hereafter date) to a fraction of the year beginning on 1 July so that it would be continuous throughout the winter. To do so, we converted each date to the day of year, subtracted 365 from any day after 180 (30 June) and divided these numbers by 365. In our dataset, this variable ranged from −0.21 (16 October) to 0.24 (30 March), where 0 was 1 January.
We used GLMs and generalized additive models (GAMs) and AICc-based multi-model inference [68,69] to analyse relationships among influenza infection, mercury contamination, body condition and ecological variables. First, we built a GLM predicting the influenza antibody status of an individual (a binary variable) from the following predictors: species (11 species, a categorical variable), age class (adult or juvenile, categorical), sex (male or female, categorical), blood mercury concentration (log10 µg g−1 wet weight (ww)), percentage crude fat (scaled), date (scaled), date2 and year (2017 or 2018, categorical). The quadratic effect of date was included because influenza prevalence is higher in mid-winter than during fall and spring migration in many ducks [70]. We also included the interaction between age and date to test for age-specific changes in the probability detecting antibodies over the season, as well as pairwise interactions of blood mercury concentration with species, age and sex, to examine differences in each group's response to mercury. The GLM used a logit link.
For model selection, our candidate model set included most model subsets, but included interactions and quadratic effects only when main effects were also included. Model selection was implemented using the MuMIn package in R [71,72]. Because there were many models with similar AICc values, indicating uncertainty in our ability to identify a single best model, we used model averaging on the full candidate set to estimate the overall effect of each variable. We used conditional averaging, which averages parameters from models in which they appear [69]. We report results from the averaged model following guidelines for reporting statistical evidence from Muff et al. [73] and using predictions from the averaged model [74]. We report 85% confidence intervals (CIs), which are consistent with model selection criteria [75], and provide 95% CIs for reference. In addition, we calculated relative variable importance using Akaike weights (w = e−0.5ΔAICc) and an adjusted metric of relative variable importance [76], in which variables with a relative importance greater than 0 have a higher weight than expected based on their inclusion in the candidate set (electronic supplementary material, methods). We also measured relative support for pairs of models in the model set using evidence ratios (i.e. ratios of Akaike weights [69]) and differences in R2 values.
We used the same procedure and the same set of predictor variables to fit, evaluate and average models for active influenza infection status (Ct ≤ 45).
Finally, we used a similar procedure to model body condition, measured using percentage crude fat (scaled, described above). For this model set, we tested both linear models and GAMs, which can model complex nonlinear relationships [77], because avian endogenous reserves can change rapidly and nonlinearly throughout the non-breeding season [78]. We modelled body condition as a normally distributed (Gaussian) variable. Predictors in the LMs were blood mercury concentration, antibody status, active infection status, date, year and the interaction of blood mercury with antibody status, active infection status, age, sex and species. The GAMs included smoothed effects of all continuous variables (mercury, date and the interactions of mercury) instead of their parametric terms, and the same categorical variables as the LMs (electronic supplementary material, methods). We performed model averaging using the model set (LMs or GAMs) with the lowest AICc.
3. Results
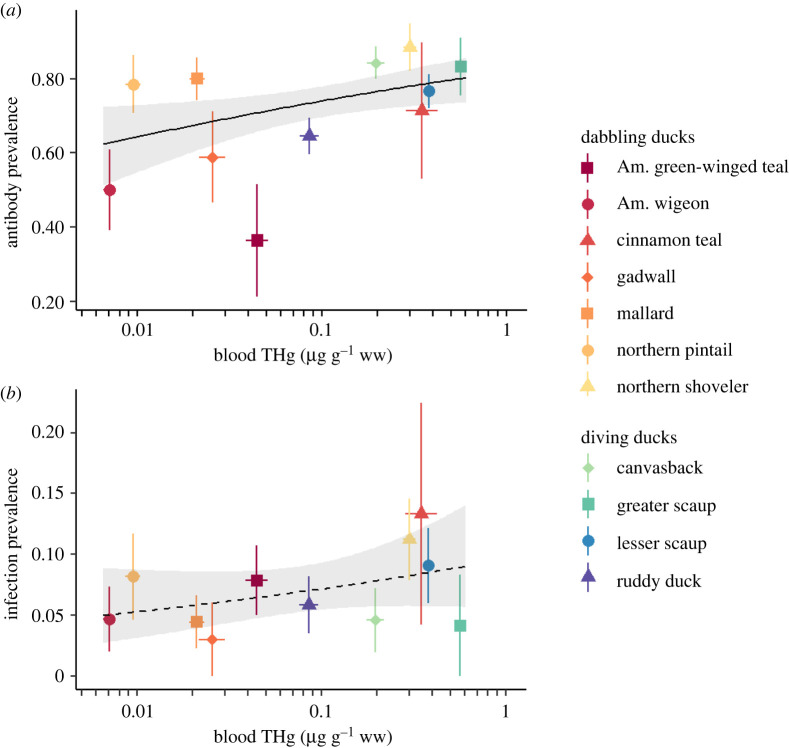
We analysed 437 influenza antibody samples and 720 active influenza infection samples with paired mercury data from 749 individuals across 11 species of dabbling and diving ducks (table 1). Mean blood mercury concentrations ranged from 0.007 µg g−1 ww in American wigeon to 0.566 µg g−1 ww in greater scaup. Antibody prevalence ranged from 0.364 (i.e. 36.4%) in American green-winged teal to 0.885 in northern shoveler. Across species, antibody prevalence increased with blood mercury concentrations (figure 2a). Active influenza infection prevalence ranged from 0.030 in gadwall to 0.133 in cinnamon teal; active infection prevalence was unrelated to average blood mercury concentrations at the species level (figure 2b).
Figure 2.
Species-level relationships between influenza prevalence and average blood mercury concentration. Points and error bars show geometric mean and standard error for each variable in raw data. Black lines show the fitted relationship from univariate GLMs; shaded areas show 95% CIs. Note the log scale of the x-axis. (a) Antibody prevalence increases with blood mercury concentrations (β = 0.198, p = 0.013). (b) There is little evidence that infection prevalence increases with blood mercury concentrations (β = 0.140, p = 0.190). (Online version in colour.)
(a) . Prior infection
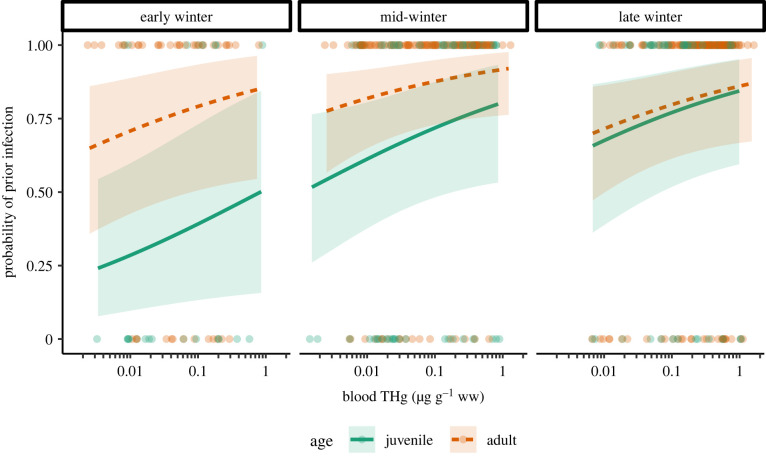
At the individual level, the most important variables predicting prior influenza infection (i.e. antibody detection) were age, date, date2, blood mercury and the interaction between date and age. The averaged model showed a positive effect of blood mercury concentration on antibody status (figure 3; electronic supplementary material, table S1); the evidence for this effect was relatively weak, but its effect size was substantial (odds ratio (OR): 1.723; 85% CI: [1.087, 2.729]; p = 0.089), indicating that the odds of prior infection increased 1.7 times for every 10-fold increase in mercury concentrations, and that the predicted probability of infection increased 1.2- to 3.7-fold across the observed range of mercury concentrations (0.001–1.623 µg g−1 ww), depending on other variables in the model.
Figure 3.
The probability of prior influenza infection increases with blood mercury concentrations. Lines show mean predictions and shaded areas show 85% CIs of predictions in early (16 October–10 December), mid (11 December–2 February) and late (3 February–16 March) winter. Points show raw data (i.e. points at bottom are birds without antibodies; points at top are birds with antibodies). Prediction lines for each panel are based on parameters for 16 October, 1 January and 16 March, respectively, and extend only within the observed range of mercury values for each age class and date. Lines show predicted values for a male northern pintail and all other variables held at their mean values across the dataset, but points for all individuals are shown. For 95% CIs, see electronic supplementary material, figure S2. (Online version in colour.)
The averaged model also provided strong evidence for a positive effect of age on antibody status, such that the odds of prior infection were 2.7 times greater in adults than juveniles (OR: 2.697; 85% CI: [1.564, 4.651]; p = 0.009) (figure 3). For juveniles, the probability of prior infection (i.e. antibody detection) increased over the course of the winter, whereas prior infection probabilities in adults changed relatively little over time and were highest in mid-winter (electronic supplementary material, figure S1). The full model (i.e. containing all predictor variables) had an R2 of 0.15 (electronic supplementary material, table S2).
Most models with substantial support (i.e. low AICc) contained terms for blood mercury and/or species (electronic supplementary material, table S2). The model with the lowest AICc included both, but models without each variable were competitive (ΔAICc < 2). Evidence for the nested model with blood mercury only (i.e. age, date, date2, blood mercury and age × date) was 1.5 times stronger than for the model with species only, but this model explained 3% less of the variance in antibody status (R2 of 0.08 versus 0.11, electronic supplementary material, table S2). Together, these patterns suggest prior infection is positively associated with blood mercury concentrations and that blood mercury concentrations explain a small portion of the variation in antibody detection, both within and across species.
(b) . Active infection
Our ecological predictors explained very little of the variation in active infection status; the full model had an R2 of only 0.08, there was no strong evidence for an effect of any variable in the averaged model, and the intercept-only model was competitive (ΔAICc = 1.490, electronic supplementary material, table S3), indicating that the other parameters were uninformative [75]. The only variables with positive relative importance scores were age and the interaction between age and date (electronic supplementary material, table S4). Nevertheless, the direction of each effect in the averaged model was consistent with our hypotheses and with results from the antibody status models (electronic supplementary material, figure S3): infection probabilities tended to increase with blood mercury concentrations (OR: 1.609; 85% CI: [1.021, 2.536]; p = 0.132); adults were generally less likely to be infected than juveniles (OR: 0.471; 85% CI: [0.220, 1.006]; p = 0.153); and infection probabilities tended to decrease through the winter for juveniles (electronic supplementary material, figure S3).
(c) . Body condition
GAMs performed better than linear models for predicting body condition. Across all GAMs, date was the only variable with a positive relative importance score (electronic supplementary material, table S5); body condition decreased nonlinearly through the winter. The R2 of the full GAM was 0.11. In the averaged model, there was no evidence for an effect of active influenza infection status or influenza antibody status on body condition, and no evidence for an interaction of either with mercury concentration.
4. Discussion
Environmental contaminants can influence wildlife health by mediating the prevalence of pathogens and the severity of disease. Here, we found evidence for a positive relationship between the probability of prior influenza infection and blood mercury concentrations across 11 species of waterfowl wintering in California's San Francisco Bay Estuary. The odds of prior infection (i.e. antibody detection) increased 5.2 times across the observed range of blood mercury concentrations (0.001–1.623 µg g−1 ww) and the odds of active influenza infection increased 5.1 times (0.001–2.023 µg g−1 ww). We found no effect of influenza infection on body fat stores, even in an interaction with mercury. Our results also confirm established age-specific patterns of influenza infection in wild waterfowl, including higher probabilities of prior infection and lower probabilities of active infection in adults as compared to juveniles [21].
The positive relationship between prior infection and mercury concentrations suggests that immunotoxic effects of chronic mercury exposure could increase the probability of influenza infection. Mercury contamination has documented impacts on immune function in multiple avian taxa [9,10,37,42,55], but has rarely been linked to actual infections (but see [11]). Blood mercury concentrations represent a combination of chronic and acute mercury exposure that could affect both long- and short-term infection dynamics [79,80], and our results showed that the positive relationship between influenza infection and mercury concentrations was stronger when measuring antibodies indicative of prior infection than for active infection. This pattern suggests that short-term active infection risk might depend on ecological drivers of influenza exposure (e.g. habitat use, local density or stochasticity [81]), whereas chronic mercury contamination could increase influenza infection risk over the long term by increasing susceptibility to infection upon exposure [42]. Further, while influenza antibody prevalence was related to average mercury concentrations across species, this relationship persisted at the individual level, suggesting that interspecific differences in habitat use, diet and immunology can explain some, but not all, of the relationship between mercury and influenza infection probability. Together, these results provide compelling evidence that the immunotoxic effects of mercury might increase the prevalence of influenza antibodies across waterfowl host species.
Although the probability of detecting antibodies increased through the winter, suggesting that influenza transmission was ongoing, most of the variation in active infection status remained unexplained by our covariates. Even variables known to affect influenza prevalence in wild birds (e.g. species and age [17,49]) were only weakly related to infection status. Influenza prevalence in wild ducks usually peaks in autumn in the Northern Hemisphere [15] and infected birds shed influenza for only 5–11 days [54,82]; this combination of low infection prevalence during winter and the short infectious period produced an infection prevalence of only 7% in our dataset, which limited the statistical power to detect any effects. By contrast, detectable antibodies are estimated to last months to over a year [19–21] and antibody prevalence was 74%. The substantial increase in antibody detection through the winter among juvenile birds implies that infections were occurring but difficult to detect, whereas antibodies left longer lasting evidence of prior infection. Alternatively, individual traits such as age and species could produce an apparent relationship between antibody detection and mercury concentrations; for example, if adults exhibit both longer antibody persistence and higher contaminant concentrations [31,57], the relationship between antibody detection and contaminants could be driven primarily by age. However, our results suggest that antibody–mercury relationships persist even within age and species groups. These results highlight the value of analysing antibody data alongside samples for active infection, especially for pathogens like influenza, where infection is only detectable for a short duration but antibodies last much longer.
Despite the positive relationship between the probability of prior influenza infection and mercury concentrations, we found no evidence that body condition was related to influenza infection, even in combination with mercury. This pattern could indicate that low pathogenic influenza has no effect on bird body condition (and vice versa), or that unmeasured variables (e.g. time since infection) or sampling biases (e.g. contaminant- or infection-induced changes in behaviour and mortality) obscured the true relationship between fat stores and influenza infection [13]. Most prior studies have concluded that low pathogenic influenza infection has no effects on wild waterfowl body condition [25], but at least one field study found that influenza-infected swans fed at reduced rates, suggesting that they had lower energy intake [83]. While reduced energy intake would eventually affect fat stores, behavioural adaptations to short-term infection, such as reducing flight activity (an energetically demanding behaviour [84]), could offset any energy imbalance from infection-induced reduced feeding rates. Further, mercury concentrations in most ducks we studied were below benchmarks for effects of mercury on reproductive success and mortality (less than 1.0 µg g−1 ww blood) [4]. However, sublethal effects of mercury can occur as low as 0.2 µg g−1 ww blood in some birds [4]. For comparison, blood mercury concentrations in our data ranged from 0.001 to 2.02 µg g−1 ww, with a geometric mean of 0.07 µg g−1 ww. Longitudinal or experimental studies that control for infection timing, body condition prior to infection, and behavioural responses to infection could further explore how influenza infection and environmental contamination interact to affect body condition.
Even in the absence of direct effects on wild bird health, the positive relationship between low pathogenic influenza infection and mercury concentrations could have important implications for the epidemiology of influenza viruses and, ultimately, avian health. All 11 species we studied can be medium- or long-distance migrants, some of which breed as far north as Alaska and northern Canada. Migrants can spread viruses over large spatial scales, introduce influenza viruses of diverse origins into resident populations [16,50] and amplify local viral transmission [16]. Immunotoxic effects of mercury could therefore promote the spatial spread of influenza viruses by increasing infection prevalence in migrants, especially if neither infection nor mercury contamination impairs their long-distance movements. While most influenza viruses have minimal impacts on wild waterfowl, highly pathogenic strains, which are an emerging disease threat for wild birds, can cause significant morbidity and mortality [28]. Further, influenza infection can cause mass mortality in poultry [26], so increased susceptibility to highly pathogenic avian influenza in wild birds could pose a significant health risk to both wild and domestic birds as well as economic risk to the agriculture sector.
Beyond its implications for the mercury-influenza system, this study highlights the potential strength of the relationships among contaminants, infection and wildlife health. The increase in antibody detection associated with mercury was as large as some species-specific differences in influenza prevalence [17,18], suggesting that contaminant-induced susceptibility to pathogens could be a major contributor to differences in infection prevalence across species. Moreover, we observed these relationships at relatively low mercury concentrations, consistent with a prior study of bat immune function [38], and suggesting that infection risk might be relatively sensitive to contaminant exposure. In addition, although we found no association between infection and body condition, environmental contaminants might have more significant population impacts when interacting with more virulent and emerging pathogens. The immunotoxic effects of environmental contaminants could also scale up to affect population or community health; infectious diseases spread between individuals, across space and among species, meaning that hosts with minimal contaminant exposure could experience negative impacts of contaminants via increased prevalence of infectious diseases. Understanding when and where these effects appear is particularly important because environmental contaminants can persist in the environment for years after emissions end [1,29].
Acknowledgements
We thank Matt Toney and Brady Fettig for assistance with mercury laboratory analyses. Jeffrey Kohl, Desmond Mackell, Stacy Moskal, Aliya McCarthy, Kelsey Navarre, Luke Hawk, David Nelson, Avi Kertesz, Jillian Cosgrove, Nathan Lashomb, Alex Vidal and Cooper Walton assisted with waterfowl collections, and staff from the Grizzly Island State Wildlife Area and the Suisun Resource Conservation District assisted with field collection logistics. Cliff Feldheim provided logistical and funding support from the California Department of Water Resources. We thank the following duck clubs for access and permission for collections: Cordelia Duck Club, Cygnus, Denverton, Goodyear Duck Club, Grizzly Ranch, Hidden Cove, Island Club, Joice Island Mallard Co., MILCO, Mulberry Land Co., Sprigateal, The Teal Club, Tulle Belle, Tule Meadows and Wings Landing. We thank Cara Love for helpful comments on an earlier version of the manuscript. Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Ethics
Ducks were collected under federal migratory bird permits MB102896–1 and MB57358C-0; California state Scientific Collection Permits SC-003855, 007908 and SC-8090; and USGS Federal Bird Banding Permit 21142. Sampling procedures were reviewed by the Animal Use and Care committee at the USGS Western Ecological Research Center on 11/08/2017 (diving ducks) and through the permit 'Breeding and Wintering Ecology of Waterfowl April 2015' (dabbling ducks).
Data accessibility
Data are available at the US Geological Survey's ScienceBase: https://doi.org/10.5066/P9QC53G9 [85]. Code to reproduce the results are archived at Zenodo: https://doi.org/10.5281/zenodo.6985261 [86].
The data are provided in the electronic supplementary material [87].
Authors' contributions
C.S.T.: formal analysis, methodology, software, validation, visualization, writing—original draft and writing—review and editing; J.T.A.: conceptualization, funding acquisition, methodology, project administration, resources, supervision and writing—review and editing; M.A.H.: investigation and writing—review and editing; J.M.S.: investigation; M.L.C.: conceptualization, funding acquisition, methodology, resources and writing—review and editing; S.E.W.D.L.C.: conceptualization, funding acquisition, methodology, resources and writing—review and editing; W.M.B.: investigation, resources and writing—review and editing; E.J.B.: data curation and writing—review and editing; J.M.E.: resources and supervision; M.P.H.: investigation, methodology and writing—review and editing; E.L.M.: data curation, investigation and writing—review and editing; C.T.O.: data curation, investigation and writing—review and editing; S.H.P.: data curation, investigation, methodology and writing—review and editing; M.P.: investigation and writing—review and editing; A.M.R.: conceptualization, methodology and writing—review and editing; J.D.S.: data curation and writing—review and editing; D.J.P.: conceptualization, funding acquisition, methodology, project administration, resources, supervision and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This study was funded by the US Geological Survey Ecosystem Mission Area's Environmental Health Program (Toxic Substances Hydrology and Contaminant Biology) and Biological Threats & Invasive Species Research Program. Funding was also provided by the California Department of Water Resources and by NIH NIAID grants HHSN272201400008C and HHSN266200700010C to W.M.B.
References
- 1.Webster E, Mackay D, Wania F. 1998. Evaluating environmental persistence. Environ. Toxicol. Chem. 17, 2148-2158. () [DOI] [Google Scholar]
- 2.Saaristo M, et al. 2018. Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proc. R. Soc. B 285, 20181297. ( 10.1098/rspb.2018.1297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith PN, Cobb GP, Godard-Codding C, Hoff D, McMurry ST, Rainwater TR, Reynolds KD. 2007. Contaminant exposure in terrestrial vertebrates. Environ. Pollut. 150, 41-64. ( 10.1016/j.envpol.2007.06.009) [DOI] [PubMed] [Google Scholar]
- 4.Ackerman JT, et al. 2016. Avian mercury exposure and toxicological risk across western North America: a synthesis. Sci. Total Environ. 568, 749-769. ( 10.1016/j.scitotenv.2016.03.071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcogliese DJ, Pietrock M. 2011. Combined effects of parasites and contaminants on animal health: parasites do matter. Trends Parasitol. 27, 123-130. ( 10.1016/j.pt.2010.11.002) [DOI] [PubMed] [Google Scholar]
- 6.Daskin JH, Alford RA. 2012. Context-dependent symbioses and their potential roles in wildlife diseases. Proc. R. Soc. B 279, 1457-1465. ( 10.1098/rspb.2011.2276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoyt JR, et al. 2016. Host persistence or extinction from emerging infectious disease: insights from white-nose syndrome in endemic and invading regions. Proc. R. Soc. B 283, 20152861. ( 10.1098/rspb.2015.2861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez CA, Altizer S, Hall RJ. 2020. Landscape-level toxicant exposure mediates infection impacts on wildlife populations. Biol. Lett. 16, 20200559. ( 10.1098/rsbl.2020.0559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawley DM, Hallinger KK, Cristol DA. 2009. Compromised immune competence in free-living tree swallows exposed to mercury. Ecotoxicology 18, 499-503. ( 10.1007/s10646-009-0307-4) [DOI] [PubMed] [Google Scholar]
- 10.Kenow KP, Hines RK, Meyer MW, Suarez SA, Gray BR. 2007. Effects of methylmercury exposure on the immune function juvenile common loons (Gavia immer). Environ. Toxicol. Chem. 26, 1460-1469. ( 10.1897/06-442r.1) [DOI] [PubMed] [Google Scholar]
- 11.Provencher JF, Gilchrist HG, Mallory ML, Mitchell GW, Forbes MR. 2016. Direct and indirect causes of sex differences in mercury concentrations and parasitic infections in a marine bird. Sci. Total Environ . 551–552, 506-512. ( 10.1016/j.scitotenv.2016.02.055) [DOI] [PubMed] [Google Scholar]
- 12.Arsnoe DM, Ip HS, Owen JC. 2011. Influence of body condition on influenza a virus infection in mallard ducks: experimental infection data. PLoS ONE 6, 1-9. ( 10.1371/journal.pone.0022633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sánchez CA, Becker DJ, Teitelbaum CS, Barriga P, Brown LM, Majewska AA, Hall RJ, Altizer S. 2018. On the relationship between body condition and parasite infection in wildlife: a review and meta-analysis. Ecol. Lett. 21, 1869-1884. ( 10.1111/ele.13160) [DOI] [PubMed] [Google Scholar]
- 14.Hughes KD, de Solla SR, Schummer ML, Petrie SA, White A, Martin PA.. 2019. Rapid increase in contaminant burdens following loss of body condition in Canvasbacks (Aythya valisineria) overwintering on the Lake St. Clair region of the Great Lakes. Ecotoxicol. Environ. Saf. 186, 109736. ( 10.1016/j.ecoenv.2019.109736) [DOI] [PubMed] [Google Scholar]
- 15.Olsen B, Munster VJ, Wallensten A, Waldenström J, Osterhaus ADME, Fouchier RAM. 2006. Global patterns of influenza A virus in wild birds. Science 312, 384-388. ( 10.1126/science.1122438) [DOI] [PubMed] [Google Scholar]
- 16.van Dijk JGB, Hoye BJ, Verhagen JH, Nolet BA, Fouchier RAM, Klaassen M. 2014. Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus. J. Anim. Ecol. 83, 266-275. ( 10.1111/1365-2656.12131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill NJ, Takekawa JY, Cardona CJ, Ackerman JT, Schultz AK, Spragens KA, Boyce WM. 2010. Waterfowl ecology and avian influenza in California: do host traits inform us about viral occurrence? Avian Dis. 54, 426-432. ( 10.1637/8912-043009-Reg.1) [DOI] [PubMed] [Google Scholar]
- 18.Munster VJ, et al. 2007. Spatial, temporal, and species variation in prevalence of influenza a viruses in wild migratory birds. PLoS Pathog. 3, e61. ( 10.1371/journal.ppat.0030061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shriner SA, Root JJ, Ellis JW, Bentler KT, VanDalen KK, Gidlewski T, Bevins SN. 2021. Influenza A virus surveillance, infection and antibody persistence in snow geese (Anser caerulescens). Transbound. Emerg. Dis. 69, 742-752. ( 10.1111/tbed.14044) [DOI] [PubMed] [Google Scholar]
- 20.Fereidouni SR, Grund C, Häuslaigner R, Lange E, Wilking H, Harder TC, Beer M, Starick E. 2010. Dynamics of specific antibody responses induced in mallards after infection by or immunization with low pathogenicity avian influenza viruses. Avian Dis. 54, 79-85. ( 10.1637/9005-073109-Reg.1) [DOI] [PubMed] [Google Scholar]
- 21.Hoye BJ, Munster VJ, Nishiura H, Fouchier RAM, Madsen J, Klaassen M. 2011. Reconstructing an annual cycle of interaction: natural infection and antibody dynamics to avian influenza along a migratory flyway. Oikos 120, 748-755. ( 10.1111/j.1600-0706.2010.18961.x) [DOI] [Google Scholar]
- 22.Hill SC, Manvell RJ, Schulenburg B, Shell W, Wikramaratna PS, Perrins C, Sheldon BC, Brown IH, Pybus OG. 2016. Antibody responses to avian influenza viruses in wild birds broaden with age. Proc. R. Soc. B 283, 20162159. ( 10.1098/rspb.2016.2159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramey AM, et al. 2020. Influenza A viruses remain infectious for more than seven months in northern wetlands of North America. Proc. R. Soc. B 287, 20201680. ( 10.1098/rspb.2020.1680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramey AM, et al. 2022. Evidence for interannual persistence of infectious influenza A viruses in Alaska wetlands. Sci. Total Environ. 803, 150078. ( 10.1016/j.scitotenv.2021.150078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuiken T. 2013. Is low pathogenic avian influenza virus virulent for wild waterbirds? Proc. R. Soc. B 280, 20130990. ( 10.1098/rspb.2013.0990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capua I, Alexander DJ. 2006. Avian influenza infections in birds - a moving target. Influenza Other Respi. Viruses 1, 11-18. ( 10.1111/j.1750-2659.2006.00004.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reperant LA, Rimmelzwaan GF, Kuiken T. 2009. Avian influenza viruses in mammals. OIE Rev. Sci. Tech. 28, 137-159. ( 10.20506/rst.28.1.1876) [DOI] [PubMed] [Google Scholar]
- 28.Ramey AM, et al. 2022. Highly pathogenic avian influenza is an emerging disease threat to wild birds in North America. J. Wildl. Manage. 86, e22171. ( 10.1002/jwmg.22171) [DOI] [Google Scholar]
- 29.Singer MB, Aalto R, James LA, Kilham NE, Higson JL, Ghoshal S. 2013. Enduring legacy of a toxic fan via episodic redistribution of California gold mining debris. Proc. Natl Acad. Sci. USA 110, 18 436-18 441. ( 10.1073/pnas.1302295110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavoie RA, Jardine TD, Chumchal MM, Kidd KA, Campbell LM. 2013. Biomagnification of mercury in aquatic food webs: a worldwide meta-analysis. Environ. Sci. Technol. 47, 13 385-13 394. ( 10.1021/es403103t) [DOI] [PubMed] [Google Scholar]
- 31.Chételat J, Ackerman JT, Eagles-Smith CA, Hebert CE. 2020. Methylmercury exposure in wildlife: a review of the ecological and physiological processes affecting contaminant concentrations and their interpretation. Sci. Total Environ. 711, 135117. ( 10.1016/j.scitotenv.2019.135117) [DOI] [PubMed] [Google Scholar]
- 32.Eagles-Smith CA, Ackerman JT, De La Cruz SEW, Takekawa JY. 2009. Mercury bioaccumulation and risk to three waterbird foraging guilds is influenced by foraging ecology and breeding stage. Environ. Pollut. 157, 1993-2002. ( 10.1016/j.envpol.2009.03.030) [DOI] [PubMed] [Google Scholar]
- 33.Ackerman JT, Hartman CA, Herzog MP. 2019. Mercury contamination in resident and migrant songbirds and potential effects on body condition. Environ. Pollut. 246, 797-810. ( 10.1016/j.envpol.2018.11.060) [DOI] [PubMed] [Google Scholar]
- 34.Carravieri A, et al. 2022. Quantitative meta-analysis reveals no association between mercury contamination and body condition in birds. Biol. Rev. 97, 1253-1271. ( 10.1111/brv.12840) [DOI] [PubMed] [Google Scholar]
- 35.Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, Erwin CA. 2009. Species differences in the sensitivity of avian embryos to methylmercury. Arch. Environ. Contam. Toxicol. 56, 129-138. ( 10.1007/s00244-008-9160-3) [DOI] [PubMed] [Google Scholar]
- 36.Wolfe MF, Schwarzbach S, Sulaiman RA. 1998. Effects of mercury on wildlife: a comprehensive review. Environ. Toxicol. Chem. 17, 146-160. () [DOI] [Google Scholar]
- 37.Whitney MC, Cristol DA. 2017. Impacts of sublethal mercury exposure on birds: a detailed review. In Reviews of environmental contamination and toxicology (ed. de Voogt P), pp. 113-163. Cham, Switzerland: Springer. [DOI] [PubMed] [Google Scholar]
- 38.Becker DJ, et al. 2020. Disentangling interactions among mercury, immunity and infection in a Neotropical bat community. J. Appl. Ecol. 58, 879-889. ( 10.1111/1365-2664.13809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker DJ, Chumchal MM, Bentz AB, Platt SG, Czirják GÁ, Rainwater TR, Altizer S, Streicker DG. 2017. Predictors and immunological correlates of sublethal mercury exposure in vampire bats. R. Soc. Open Sci. 4, 170073. ( 10.1098/rsos.170073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finkelstein ME, Grasman KA, Croll DA, Tershy BR, Keitt BS, Jarman WM, Smith DR. 2007. Contaminant-associated alteration of immune function in black-footed albatross (Phoebastria nigripes), a North Pacific predator. Environ. Toxicol. Chem. 26, 1896-1903. ( 10.1897/06-505R.1) [DOI] [PubMed] [Google Scholar]
- 41.Fallacara DM, Halbrook RS, French JB. 2011. Toxic effects of dietary methylmercury on immune function and hematology in American kestrels (Falco sparverius). Environ. Toxicol. Chem. 30, 1320-1327. ( 10.1002/etc.494) [DOI] [PubMed] [Google Scholar]
- 42.Lewis CA, Cristol DA, Swaddle JP, Varian-Ramos CW, Zwollo P. 2013. Decreased immune response in zebra finches exposed to sublethal doses of mercury. Arch. Environ. Contam. Toxicol. 64, 327-336. ( 10.1007/s00244-012-9830-z) [DOI] [PubMed] [Google Scholar]
- 43.Desforges JPW, Sonne C, Levin M, Siebert U, De Guise S, Dietz R. 2016. Immunotoxic effects of environmental pollutants in marine mammals. Environ. Int. 86, 126-139. ( 10.1016/j.envint.2015.10.007) [DOI] [PubMed] [Google Scholar]
- 44.Kapczynski DR, Pantin Jackwood MJ. 2007. Innate immune responses to avian influenza differ between chickens and ducks. In 15th World Veterinary Poultry Congress, Beijing, China, 13–16 September. [Google Scholar]
- 45.van Dijk JGB, Verhagen JH, Hegemann A, Tolf C, Olofsson J, Järhult JD, Waldenström J. 2020. A comparative study of the innate humoral immune response to avian influenza virus in wild and domestic mallards. Front. Microbiol. 11, 608274. ( 10.3389/fmicb.2020.608274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eagles-Smith CA, et al. 2016. Spatial and temporal patterns of mercury concentrations in freshwater fish across the Western United States and Canada. Sci. Total Environ. 568, 1171-1184. ( 10.1016/j.scitotenv.2016.03.229) [DOI] [PubMed] [Google Scholar]
- 47.Gilmer DS, Miller MR, Bauer RD, LeDonne JR. 1982. California's Central Valley wintering waterfowl: concerns and challenges. In Transactions of the 47th North American wildlife and natural resources conference (ed. Sabol K), pp. 441-452. Washington, DC: Wildlife Management Institute. [Google Scholar]
- 48.Ackerman JT, Herzog MP, Yarris GS, Casazza ML, Burns E, Eadie JM. 2014. Waterfowl ecology and management. In Suisun marsh: ecological history and possible futures (eds Moyle P, Manfree A, Fiedler P), pp. 103-132. Berkeley, CA: University of California Press. [Google Scholar]
- 49.Siembieda JL, Johnson CK, Cardona C, Anchell N. 2010. Influenza A viruses in wild birds of the Pacific Flyway, 2005–2008. Vector-Borne Zoonotic Dis. 10, 793-800. ( 10.1089/vbz.2009.0095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill NJ, Takekawa JY, Ackerman JT, Hobson KA, Herring G, Cardona CJ, Runstadler JA, Boyce WM. 2012. Migration strategy affects avian influenza dynamics in mallards (Anas platyrhynchos). Mol. Ecol. 21, 5986-5999. ( 10.1111/j.1365-294X.2012.05735.x) [DOI] [PubMed] [Google Scholar]
- 51.McDuie F, et al. 2022. Pathways for avian influenza virus spread: GPS reveals wild waterfowl in commercial livestock facilities and connectivity with the natural wetland landscape. Transbound. Emerg. Dis. In press. ( 10.1111/tbed.14445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Dijk JG, Verhagen JH, Wille M, Waldenström J. 2018. Host and virus ecology as determinants of influenza A virus transmission in wild birds. Curr. Opin. Virol. 28, 26-36. ( 10.1016/j.coviro.2017.10.006) [DOI] [PubMed] [Google Scholar]
- 53.Dannemiller NG, Webb CT, Wilson KR, Bentler KT, Mooers NL, Ellis JW, Jeffrey Root J, Franklin AB, Shriner SA. 2017. Impact of body condition on influenza A virus infection dynamics in mallards following a secondary exposure. PLoS ONE 12, e0175757. ( 10.1371/journal.pone.0175757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Latorre-Margalef N, et al. 2009. Effects of influenza A virus infection on migrating mallard ducks. Proc. R. Soc. B 276, 1029-1036. ( 10.1098/rspb.2008.1501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW. 2007. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio 36, 12-18. ( 10.1579/0044-7447(2007)36[12:EOEMOT]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 56.Curran JM, Robertson ID, Ellis TM, Selleck PW, O'Dea MA. 2013. Variation in the responses of wild species of duck, gull, and wader to inoculation with a wild-bird-origin H6N2 low pathogenicity avian influenza virus. Avian Dis. 57, 581-586. ( 10.1637/10458-112712-Reg.1) [DOI] [PubMed] [Google Scholar]
- 57.Tolf C, et al. 2013. Individual variation in influenza A virus infection histories and long-term immune responses in mallards. PLoS ONE 8, e61201. ( 10.1371/journal.pone.0061201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carney SM. 1992. Species, age and sex identification of ducks using wing plumage. Washington, DC: US Department of the Interior, US Fish and Wildlife Service. See https://digitalmedia.fws.gov/digital/collection/document/id/1407/. [Google Scholar]
- 59.Fleskes JP, Yee JL, Yarris GS, Loughman DL. 2016. Increased body mass of ducks wintering in California's Central Valley. J. Wildl. Manage. 80, 679-690. ( 10.1002/jwmg.1053) [DOI] [Google Scholar]
- 60.Hanson HC. 1949. Methods of determining age in Canada geese and other waterfowl. J. Wildl. Manage. 13, 177-183. [Google Scholar]
- 61.Eagles-Smith CA, Ackerman JT, Adelsbach TL, Takekawa JY, Miles AK, Keister RA. 2008. Mercury correlations among six tissues for four waterbird species breeding in San Francisco Bay, California, USA. Environ. Toxicol. Chem. 27, 2136-2153. ( 10.1897/08-038.1) [DOI] [PubMed] [Google Scholar]
- 62.Esler D, Grand J. 1994. Comparison of age determination techniques for female northern pintails and American wigeon in spring. Wildl. Soc. Bull. 22, 260-264. [Google Scholar]
- 63.Hochbaum HA. 1942. Sex and age determination of waterfowl by cloacal examination. Trans. North Am. Wildl. Conf. 7, 299-307. [Google Scholar]
- 64.Rimmer CC, Mcfarland KP, Evers DC, Miller EK, Aubry Y, Busby D, Taylor RJ, Road CH, Gv PQC. 2005. Mercury concentrations in Bicknell's thrush and other insectivorous passerines in Montane forests of Northeastern North America. Ecotoxicology 14, 223-240. ( 10.1007/s10646-004-6270-1) [DOI] [PubMed] [Google Scholar]
- 65.Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. 2002. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 40, 33-44. ( 10.1128/JCM.40.9.3256-3260.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramey AM, et al. 2017. Surveillance for highly pathogenic influenza A viruses in California during 2014–2015 provides insights into viral evolutionary pathways and the spatiotemporal extent of viruses in the Pacific Americas Flyway. Emerg. Microbes Infect. 6, e80. ( 10.1038/emi.2017.66) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown JD, Stallknecht DE, Berghaus RD, Luttrell MP, Velek K, Kistler W, Costa T, Yabsley MJ, Swayne D. 2009. Evaluation of a commercial blocking enzyme-linked immunosorbent assay to detect avian influenza virus antibodies in multiple experimentally infected avian species. Clin. Vaccine Immunol. 16, 824-829. ( 10.1128/CVI.00084-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burnham KP, Anderson DR. 2004. Multimodel inference. Sociol. Methods Res. 33, 261-304. ( 10.1177/0049124104268644) [DOI] [Google Scholar]
- 69.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer-Verlag. [Google Scholar]
- 70.Kent CM, et al. 2022. Spatiotemporal changes in influenza A virus prevalence among wild waterfowl inhabiting the continental United States throughout the annual cycle. Sci. Rep. 12, 13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bartoń K. 2016. MuMIn: multi-model inference. R Package version 1.43.17, See https://rdrr.io/cran/MuMIn.
- 72.R Development Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 73.Muff S, Nilsen EB, O'Hara RB, Nater CR. 2021. Rewriting results sections in the language of evidence. Trends Ecol. Evol. 37, 203-210. ( 10.1016/j.tree.2021.10.009) [DOI] [PubMed] [Google Scholar]
- 74.Banner KM, Higgs MD. 2017. Considerations for assessing model averaging of regression coefficients. Ecol. Appl. 27, 78-93. ( 10.1002/eap.1419) [DOI] [PubMed] [Google Scholar]
- 75.Arnold TW. 2010. Uninformative parameters and model selection using Akaike's information criterion. J. Wildl. Manage. 74, 1175-1178. ( 10.2193/2009-367) [DOI] [Google Scholar]
- 76.Ackerman JT, Hartman CA, Eagles-Smith CA, Herzog MP, Davis J, Ichikawa G, Bonnema A. 2015. Estimating mercury exposure of piscivorous birds and sport fish using prey fish monitoring. Environ. Sci. Technol. 49, 13 596-13 604. ( 10.1021/acs.est.5b02691) [DOI] [PubMed] [Google Scholar]
- 77.Wood SN. 2006. Generalized additive models: an introduction with R. London, UK: Chapman and Hall. [Google Scholar]
- 78.Araújo PM, Viegas I, Rocha AD, Villegas A, Jones JG, Mendonça L, Ramos JA, Masero JA, Alves JA. 2019. Understanding how birds rebuild fat stores during migration: insights from an experimental study. Sci. Rep. 9, 10065. ( 10.1038/s41598-019-46487-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lavoie RA, Baird CJ, King LE, Kyser TK, Friesen VL, Campbell LM. 2014. Contamination of mercury during the wintering period influences concentrations at breeding sites in two migratory piscivorous birds. Environ. Sci. Technol. 48, 13 694-13 702. ( 10.1021/es502746z) [DOI] [PubMed] [Google Scholar]
- 80.Monteiro LR, Furness RW. 2001. Kinetics, dose-response, and excretion of methylmercury in free-living adult Cory's shearwaters. Environ. Sci. Technol. 35, 739-746. ( 10.1021/es000114a) [DOI] [PubMed] [Google Scholar]
- 81.Nallar R, Papp Z, Leighton FA, Epp T, Pasick J, Berhane Y, Lindsay R, Soos C. 2016. Ecological determinants of avian influenza virus, West Nile Virus, and avian paramyxovirus infection and antibody status in blue-winged teal (Anas discors) in the Canadian prairies. J. Wildl. Dis. 52, 33-46. ( 10.7589/2013-07-191) [DOI] [PubMed] [Google Scholar]
- 82.Hénaux V, Samuel MD, Bunck CM. 2010. Model-based evaluation of highly and low pathogenic avian influenza dynamics in wild birds. PLoS ONE 5, e10997. ( 10.1371/journal.pone.0010997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Gils JA, Munster VJ, Radersma R, Liefhebber D, Fouchier RAM, Klaassen M. 2007. Hampered foraging and migratory performance in swans infected with low-pathogenic avian influenza A virus. PLoS ONE 2, e184. ( 10.1371/journal.pone.0000184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Norberg UM. 1996. Energetics of flight. In Avian energetics and nutritional ecology (ed. Carey C), pp. 199-249. Boston, MA: Springer. [Google Scholar]
- 85.Teitelbaum CS, et al. 2022. Data measuring avian influenza infection, mercury concentration, and body condition in wild waterfowl. US Geol. Surv. Data Release. ( 10.5066/P9QC53G9) [DOI]
- 86.Teitelbaum CS et al. 2022. Release of code for ‘Avian influenza antibody prevalence increases with mercury contamination in wild waterfowl’. Zenodo. ( 10.5281/zenodo.6985261) [DOI] [PMC free article] [PubMed]
- 87.Teitelbaum CS, et al. 2022. Avian influenza antibody prevalence increases with mercury contamination in wild waterfowl. Figshare. ( 10.6084/m9.figshare.c.6168165) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Teitelbaum CS, et al. 2022. Data measuring avian influenza infection, mercury concentration, and body condition in wild waterfowl. US Geol. Surv. Data Release. ( 10.5066/P9QC53G9) [DOI]
- Teitelbaum CS et al. 2022. Release of code for ‘Avian influenza antibody prevalence increases with mercury contamination in wild waterfowl’. Zenodo. ( 10.5281/zenodo.6985261) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are available at the US Geological Survey's ScienceBase: https://doi.org/10.5066/P9QC53G9 [85]. Code to reproduce the results are archived at Zenodo: https://doi.org/10.5281/zenodo.6985261 [86].
The data are provided in the electronic supplementary material [87].