Abstract
Leaves from Adhatoda vasica Nees, Acanthaceae (synonym Justicia adhatoda L.) have been widely used in traditional medicine for their beneficial effect in the treatment of respiratory diseases. Vasicine, the main quinazoline alkaloid in A. vasica, has been linked to its medicinal properties. The purpose of this work was to develop and validate a reliable analytical method for the quantification of vasicine in A. vasica leaves and commercially available products. For this purpose, a high-performance liquid chromatography method coupled to diode array detection (HPLC-DAD) was used. After optimization of the extraction process and the HPLC conditions, linearity, precision, accuracy, and specificity were checked. During the validation, six commonly available food supplements and dosage forms were tested using the validated method. The calibration model was found to be linear in the concentration range of 5.125–205 μg/mL. The average vasicine content at different concentration levels was 0.99 g/100 g with an RSD% of 0.05%. The average recovery was found to be 102.3% with an RSD of 4.3%. Additionally, it was confirmed that the validated method was still precise and accurate for quantifying vasicine in other matrices like the tested preparations. In summary, the validated method was suitable for the determination of vasicine in leaves of Adhatoda vasica, as well as for investigating the quality and the prescribed intake of several commercial products.
Keywords: Adhatoda vasica, Leaves, Vasicine, Method development, Method validation, HPLC-DAD
Adhatoda vasicaLeavesVasicineMethod developmentMethod validationHPLC-DAD
1. Introduction
In many countries (India, Pakistan, Nepal, Sri Lanka) herbal formulations containing Adhatoda vasica Nees, Acanthaceae (or the synonym Justicia adhatoda L., or “Malabar Nut Tree” in English) are used, like Kada, Fermiforte, Salus Tuss, Kan Jang, and Spirote, to treat numerous respiratory disorders. Moreover, it is frequently included as an ingredient of several proprietaries, over-the-counter (OTC), and polyherbal products for a variety of respiratory ailments including cough, bronchitis, and asthma [1]. Not surprisingly, the frequent ethnobotanical utilization of A. vasica has resulted in its inclusion in the WHO (World Health Organization) manual “The use of Traditional Medicines in Primary Health Care” [2]. By now, a wide range of phytochemical constituents has been isolated from the leaves of A. vasica, including alkaloids, phenols, tannins, anthraquinones, saponins, steroids, flavonoids, and reducing sugars. However, pharmacologically the most studied chemical component is the bitter quinazoline alkaloid vasicine (Figure 1) which was first isolated by Sen and Ghose in 1924 [[3], [4]]. Consequently, a reliable method for its determination is essential for the quality control of herbal products [5]. Since vasicine is readily oxidized to vasicinone, and the pharmacological effects of these compounds are rather different, it is of utmost importance to apply a method that separates the two compounds. Previously, several methods have been developed and sometimes validated to determine vasicine or the general content of quinazoline alkaloids [1, 6, 7, 8, 9]. However, some previously published studies still showed limitations. Particularly, in the sample preparation described in literature, the extraction was usually followed by an extra alkaloid extraction step. Consequently, this long multistep procedure may lead to compound loss, and also, it is time- and solvent-consuming. Moreover, a central issue in the former methods was the very often small amount of extraction solvent which was not proved to be sufficient for the quantitative extraction of vasicine. Another questionable aspect in some articles was, that though the validation was following the ICH guidelines, it was performed on vasicine standards and not on leaves samples which disregard the whole sample preparation procedure including the extraction. In addition, in some cases, the crude extract was used as a starting point, and again, the extraction itself was never validated. Therefore, the goal of this project was to obtain a method validated according to the ICH guidelines, covering the whole experimental procedure which had to be proved accurate and repeatable for the determination of vasicine in the leaves of A. vasica, and in commercially available products containing A. vasica leaves powder or extract.
Figure 1.
Chemical structure of the pyrroquinazoline alkaloid vasicine.
2. Materials and methods
2.1. General experimental procedures
All reagents used were HPLC or analytical grade unless otherwise stated. Methanol, n-hexane, chloroform, absolute ethanol, acetonitrile, and glacial acetic acid were purchased from Fisher Scientific (Leicestershire, UK). Potassium dihydrogen phosphate for analysis was acquired from Merck Millipore (Darmstadt, Germany). The sterile and pyrogen-free water for HPLC analysis was obtained by a Milli-Q® Integral Water Purification System, Millipore (Bedford MA, USA).
The main equipment used in the method was: heating mantles Isopad Labmaster (Horsham, UK); filters Macherey-Nagel-MN 640 M No.43 with diameter 110 mm (Duren, Germany); Buchi Rotavapor® R-200 (Flawil, Switzerland) with Vacuubrand PC 2001 Vario Vacuum Pump CVC2000 controller (Wertheim, Germany); ultrasonic bath Cleanosonic Branson B3510 Wareham (Massachusetts, USA); syringe filter, Macherey-Nagel polyamide (nylon) Chromafil® AO-45/25 pore size 0.45 μm, diameter 25 mm (Duren, Germany).
2.2. Instrumentation and chromatographic conditions
All analyses were performed on an Agilent Technologies 1260 Infinity HPLC-DAD system (Diegem, Belgium) from the 1200 Infinity Series equipped with degasser, quaternary pump, automatic liquid sampler, thermostatic column compartment, and diode array detector (DAD). Agilent OpenLAB CDS ChemStation edition software was used (version A.01.05). The column used for validation was a Purosphere STAR RP-18 Endcapped (250 × 4.6 mm, 5 μm) (Merck, Darmstadt, Germany). The mobile phase comprised of eluent A: potassium dihydrogen phosphate buffer (pH 3.9; 0.1 M)/acetonitrile/glacial acetic acid (85:15:1), v/v/v; eluent B: acetonitrile/glacial acetic acid (99:1), v/v. MS spectra were obtained with a Thermo Fisher Surveyor LC-MS system equipped with a degasser, a quaternary pump, an autosampler, and a DAD, which was coupled to an LXQ linear ion trap (Thermo Fisher, Waltham, Massachusetts, USA). The LXQ linear ion trap consists of an atmospheric pressure ionization source, ion optics, a mass analyzer, and an ion detection system. The column used was Purosphere STAR RP-18 Endcapped, the flow rate was 0.7 mL/min and the solvent program was as follows: A: water with 0.1% formic acid, B: acetonitrile; 0.0 min - 10% B, 12.0 min–10% B, 17.0 min–50% B, 22.0–50% B, 25.0 min–10% B, 30.0 min–10% B. The injection volume was 10 μL. The spectra were recorded in the (+) ESI TIC mode in the mass range m/z 100.00–500.00. The UV detector was set at 282 nm. All data were acquired and processed using Xcalibur software, version 2.0.
2.3. Plant material and dietary supplements
The first batch of dried leaves A. vasica and the reference standard (vasicine) were provided by the European Pharmacopoeia (Council of Europe, EDQM: code 0000057451; batch 1502–5546/al). The second batch of Adhatoda leaves was collected and identified in Nepal (May 2015). Subsequently, the leaves were air-dried and shipped to the University of Antwerp.
The commercially available food supplements and dosage forms, which included A. vasica powder or extract, were obtained from several online sources. The first group comprised dry leaves powder products and they were referred to as preparation A, B and C. Suggested use for preparation A by the manufacturer was: ½ to 1 teaspoon with warm water, once or twice daily; for preparation B: ¼ to ½ teaspoon with warm water, once or twice daily; and preparation C: ½ to 1 teaspoon two times per day powder with water. The second group contained plant(s) powder(s) or plant extract(s) in tablets or capsules and were referred to as preparation D, which consisted of capsules containing seven plant extracts, where the amount of Adhatoda extract per capsule was 14 mg; preparation E were tablets containing plant powder of seventeen different species, and the amount of A. vasica per capsule was 65 mg; and preparation F, capsules with Adhatoda extract only, where the declared amount of Adhatoda extract was 400 mg per capsule.
The content of water in the A. vasica leaves to powder and dietary supplements were determined in triplicate based on the procedure in the European Pharmacopoeia (Method 2.2.32 Loss On Drying, LOD).
2.4. Method development
The starting point for method development was the monograph “Malabar Nut Tree, Leaf” from the United States Pharmacopeia (USP) where the plant material (2.0 g finely powdered A. vasica leaves) was extracted with 50.0 mL methanol, using reflux for 15 min. Afterward, the extract was cooled down to room temperature and the supernatant was decanted. The process was repeated until the last extract was colorless. The extracts were combined, filtered, and concentrated under a vacuum. Finally, the volume was adjusted to 25.0 mL with methanol. Then, this sample solution was diluted five times with methanol. Before injection, the diluted sample was filtered through a membrane filter of 0.45 μm. These sample preparation steps can be applied both for thin-layer identification tests and for high-pressure liquid chromatography, except for the five-fold dilution step in the HPLC method. The initial instrument conditions for analyzing A. vasica leaves were: Agilent 1260 Infinity HPLC system, Merck LiChrosphere® CN, L10 column (nitrile groups chemically bonded to porous silica particles) (250 × 4.6 mm, 5 μm), mobile phase: buffer solution (pH 2.8)/acetonitrile/tetrahydrofuran (92:5:3), 1.0 mL/min flow rate, 20 μL injection volume and detection set at 280 nm. The buffer solution was made as follows: 1.36 g of anhydrous potassium dihydrogen phosphate was dissolved in 900 mL Milli-Q water, then 2.0 mL of 85% phosphoric acid was added, followed by dilution with water to 1000 mL and finally, the solution was filtered.
During the method optimization, several instrument parameters were selected for investigation: column, mobile phase, the optimal wavelength for UV detection; several extraction procedures, and specific conditions for sample preparation. Three different columns with a length of 250 mm and an internal diameter of 4.6 mm were tested: a Purospher® STAR RP C18 Endcapped (5 μm), a Purospher® STAR RP C8 Endcapped (5 μm), and a Hypersil butyl Genesis C4 (5 μm). Additionally, various compositions of the mobile phase were assessed such as methanol and water (40/60); eluent A: 0.2% diethylamine and formic acid until pH 3, and eluent B: acetonitrile with 0.2% diethylamine; then, a mobile phase consisting of phosphate buffer (pH 3.9; 0.1 M)/acetonitrile/glacial acetic acid (85:15:1), v/v/v [1, 10, 11, 12]. The difference between isocratic and gradient elution was investigated. Detection was performed at 282 nm and the flow rate was set at 0.7 mL/min. Three different extraction procedures were tested. A general extraction (which is referred to as the method described in the USP) was compared to a typical alkaloid extraction and an alkaloid micro-extraction. Similarly, for all three procedures, the extraction was executed on 2.0 g of sample (finely powdered Adhatoda vasica leaves) with 50.0 mL methanol under reflux for 1 h. The supernatant was filtered and a fresh amount of the solvent was added to the remaining powder, the extraction was repeated two more times. The combined methanol extracts were evaporated to dryness. The next steps differed among the several extraction methods and the conditions are summarized in Table 1. Next to the extraction procedure, the initial amount of sample - 2.0 g and 1.0 g, and the solvent composition - pure methanol, 50% methanol, pure water, and methanol/water (40:60) used to make the final dilution of the sample for injection, were investigated.
Table 1.
Different extraction methods For alkaloids and obtained alkaloid fraction through each one of them (amount was calculated as a ratio between the peak area (mAU) and the concentration of the sampe (g/mL) multiplied by 105.
| General extraction | Alkaloid extraction | Alkaloid micro-extraction | |
|---|---|---|---|
| Sample preparation | - Dissolve the evaporated sample in 20.0 mL methanol - Filter through a 0.45 μm filter before injecting |
- Dissolve the evaporated sample in 15.0 mL 5% acetic acid - Perform three times liquid-liquid extraction with 10.0 mL hexane - Prepare an aqueous solution alkaline to pH 9.0 with ammonia - Perform three times liquid-liquid extraction with 10.0 mL chloroform - Evaporate the combined chloroform fractions - Dissolve in 20.0 mL methanol - Filter through a 0.45 μL filter before injecting |
- Dissolve the evaporated sample in 10.0 mL 5% acetic acid - Transfer into a headspace vial, add 8.0 mL of hexane and close the vial - Shake for 15 min in an ultrasonic bath in a horizontal position - Remove the hexane layer and make the aqueous layer alkaline to pH 9.0 with ammonia - Add 8.0 mL of chloroform and shake for 30 min in an ultrasonic bath in a horizontal position - Repeat two more times - Evaporate the combined chloroform fractions - Dissolve in 20.0 mL methanol - Filter through a 0.45 μL filter before injecting |
| Obtained alkaloid fraction | 4.1373 | 3.8364 | 0.3427 |
2.5. Final method
2.5.1. Standard preparation
A standard solution was made by dissolving 10.0 mg vasicine in 10.0 mL pure methanol. The solution was sonicated for 15 min and then, cooled down to room temperature. After completing the extraction procedure, 2.0 mL was taken and diluted to 25.0 mL with methanol/water (40:60).
2.6. Sample preparation
Finely powdered 1.0 g dried leaves (355 μm) were placed into a round bottom flask of 250 mL. Then, 50.0 mL pure methanol was added and 30 min reflux was performed using a heating mantle and a condenser. After cooling down to room temperature, the supernatant was decanted through a filter paper into a new round bottom flask of 250 mL. The reflux procedure was repeated two more times. The combined extracts were concentrated under a vacuum until the volume was less than 25 mL. The concentrated extract was transferred to a 25.0 mL volumetric flask. The 250 mL flask was rinsed with methanol, which was also transferred to the measuring flask and the volume was adjusted with methanol to 25.0 mL. Finally, the methanol solution was diluted five times with methanol/water (40:60). The aqueous solution was filtered through a syringe filter (pore size 0.45 μm) into an HPLC vial ready for analysis. The conditions for the chromatographic analysis are included in Table 2. The content of vasicine is expressed in percentage (equal to g/100 g) by the formula:
where x is the amount of vasicine in the standard solution in μg/mL; Ast is the peak area of vasicine in the standard solution; Ap is the peak are of vasicine in the sample solution; m is the weighted amount of the finely powdered leaves of A. vasica in grams; H is the water content in percentage of the powdered leaves determined by loss on drying.
Table 2.
Summary of the final separation conditions.
| HPLC conditions | |||
|---|---|---|---|
| Instrument: | Agilent Technologies 1260 Infinity | ||
| Column: | Purosphere STAR RP-18 Endcapped (250 × 4.6 mm, 5 μm) | ||
| Mobile phase A: | potassium dihydrogen phosphate buffer (pH 3.9; 0.1 M)/acetonitrile/glacial acetic acid (85:15:1), v/v/v | ||
| Mobile phase B: | Acetonitrile/glacial acetic acid (99:1) | ||
| Gradient: | isocratic elution with a washing step: | ||
| Min | % A | % B | |
| 0 | 100 | 0 | |
| 12 | 100 | 0 | |
| 17 | 50 | 50 | |
| 22 | 50 | 50 | |
| 25 | 100 | 0 | |
| Run time: | 30 min | ||
| Column temperature: | 20 °C | ||
| Flow: | 0.7 mL/min | ||
| Injection volume: | 20 μL | ||
| Detection: | 282 nm | ||
As for the sample preparation from dietary supplements: The content of two capsules/two tablet masses of the dietary supplement and 50 mL methanol were placed in a 250 mL round-bottom flask and the further steps were the same as for the plant powder. The samples were tested in triplicate.
2.7. Method validation
The method was validated on the first batch (1502–5546/al) of dried leaves A. vasica EDQM provided by the European Pharmacopoeia according to the ICH guidelines on the validation of analytical methods (ICH 1994, 1996, 2002) [13, 14, 15]. The calibration model, repeatability, accuracy, and specificity were investigated. For the statistical analysis Excel, 2010 (Microsoft Office Microsoft Corporation, USA) was applied. All results were expressed as percentages, where n represented the number of values. A 5% level of significance was selected.
2.8. Calibration model
A stock solution containing 10.0 mg vasicine in 10.0 mL absolute methanol was prepared (1 mg/mL, as a level of the expected amount). From this solution eight different dilutions were made in a concentration range between 5 μg/mL (as 5% level) and 200 μg/mL (as 200% level), using methanol/water (40:60). Each concentration was injected in duplicate and regression analysis was performed.
2.8.1. Precision
To verify the precision of the injector one sample was injected six times. Consequently, the standard deviation and the relative standard deviation were estimated to evaluate the injection repeatability.
Regarding the repeatability and intermediate precision, six separately prepared samples (1.0 g), which correspond to the 100% level, were analyzed on three successive days. Each sample was injected once. The vasicine standard (concentration between 70 and 80 μg/mL), which was used to estimate the amount of vasicine, was freshly prepared every day and injected at the beginning (in duplicate) and the end of the sequence. The mean, the (relative) standard deviation, and the 95% confidence interval were calculated for each day, and also the overall mean, confidence interval and relative standard deviation were established.
The data, which was collected on three different days, were analyzed by an ANOVA single factor test. To test the homogeneity of variance on the different days, a Cochran test was performed in advance. As long as the ANOVA single factor test calculates the mean squares between groups and within groups, these values were used to calculate the within-day and between-day coefficients of variations.
To check the linearity of the method, which corresponds to the repeatability of different concentration levels, six samples containing half of the amount of dry leaves powder (50% or 0.5 g) and six samples with one and a half the amount (150% or 1.5 g) were prepared and analyzed. Those results were compared to those from the 100% level on the different days and similar calculations were applied for the intermediate precision as well.
Because the EDQM batch had completely been used during the precision experiment, new plant material was used for further analysis, namely, A. vasica leaves collected from Nepal. Extensive preliminary research on the Adhatoda leaves from Nepal was required, which included analysis on different levels (50%, 100%, 150%, in triplicate on the same day) and on different days. Additionally, a second analyst repeated three samples of 100%. For each day and concentration level the average vasicine amount, the standard deviation, the relative standard deviation, and the 95% confidence interval were determined.
2.8.2. Accuracy
To determine the accuracy of the method samples were prepared in triplicate, using the Adhatoda leaves collected in Nepal, and a recovery experiment was performed by the standard addition method. Different amounts of the vasicine standard were added to the 50% level sample (0.5 g) to obtain ±75%, ±100%, and ±125% of the vasicine concentration. The preparation on each level was done in triplicate and the mean recovery percentage of the different concentrations, the relative standard deviation, and the 95% confidence interval were investigated.
2.8.3. Specificity
To unambiguously verify the specificity of the compound of interest, an additional MS analysis was performed. The LC/MS analysis of the standard solution vasicine and solutions of the two samples (both from the EDQM and Nepal) were analyzed on a Surveyor LC system coupled to an LXQ linear ion trap and a Purosphere STAR RP-18 Endcapped column (Merck, Darmstadt, Germany) (the MS data is not included).
2.9. Validation within flexible scope
Physicochemical analysis according to official monographs is developed and validated for a specific analyte in a specific matrix. When such an analyte needs to be determined in a different matrix a validation within a flexible scope (re-validation or validation to a lesser extent) should be completed. Therefore, validation with a flexible scope was performed to analyze vasicine in different matrix, mainly commercial products with the validated method. It was carried out for preparation D regarding precision and accuracy. The methods and materials, as well as the chromatographic parameters and conditions, were the same as those previously discussed in the method validation part. Also, the preparation of the reference and the test solutions was performed in the same manner as before.
3. Results and discussion
3.1. Method development
In general, one of the major difficulties in developing a reliable method for the quantification of vasicine is the lack of baseline separation between vasicine and another quinazoline alkaloid - vasicinone, resulting in peaks overlapping of the compound of interest [1]. Therefore, to avoid merging of peaks and carry-over effects, different chromatographic aspects (e.g. the composition of the mobile phase and the type of elution) were investigated. It was found that the mobile phases containing eluent A: potassium dihydrogen phosphate buffer (pH 3.9; 0.1 M)/acetonitrile/glacial acetic acid (85:15:1), v/v/v, and as an eluent B: acetonitrile/glacial acetic acid (99:1) in combination with Purospher® STAR C18 Endcapped column and isocratic elution gave the best separation. Gradient elution has also been investigated. However, because phosphate buffer can crystallize faster when changing the ratio between eluent A and eluent B, the initial isocratic elution was kept. After performing multiple injections of samples, merging of peaks was observed and an additional washing step was implemented in the existing isocratic elution. The final state of the chromatographic run was as follows: after 12 min all peaks of interest were eluted with 100% eluent A, from 12th to 17th min the mobile phase was changed to 50% eluent B and it was kept for 5 min. At the 22nd min, the mobile phase was restored to 100% A and maintained for 3 min. The optimal wavelength was established at 282 nm. The comparison between the results from the three extraction types is presented in Table 1. It was concluded that the amount of vasicine obtained by alkaloid extraction was lower compared to the content obtained by general extraction. The value from the alkaloid micro-extraction was about ten times less than with the other two methods. Therefore, the alkaloid micro-extraction was considered the least reliable method. With the typical alkaloid extraction peaks were observed in the chromatogram and a better separation of the sample was achieved. However, it was considered rather labor-intensive and time-consuming, and consequently, less convincing in perspective to apply the procedure in routine analysis. Also, it contained a liquid-liquid extraction step which is questionable to ensure the total quantitative transfer of all compounds. The next step was to compare if the typical alkaloid extraction would give the same results as the general extraction. The ratio peak area to concentration was 8.9% lower for the alkaloid extraction than for the general method, and the relative standard deviation was ten times higher for the typical alkaloid extraction (RSD 4.5%) compared to the general (RSD 0.42%). These considerations were the reason to continue the further method optimization using the general extraction procedure. After the extraction procedure, the reflux process was optimized. To establish a quantitative extraction of the sample, different period of extraction time (15 min, 30 min), solvents (methanol, ethanol), and volumes were optimized. The initially described conditions in the USP method included three times reflux for 15 min each. Experiments were carried out to check if three times were sufficient for exhaustive extraction of the sample, and if it was necessary to increase the duration of the reflux (data not shown). Moreover, when ethanol was used as an extraction solvent the detected amount of vasicine was lower compared to the samples extracted with methanol (data not shown). In the end, it was concluded that refluxing three times during 30 min with a volume of 50.0 mL methanol was sufficient for extracting a 1.0 g sample. The method development addressed another challenge, which was the solvent used to prepare the final dilution. Thus fronting, previously observed with other solvents like pure methanol, was considerably improved with a methanol/water (60:40) solvent mixture. As a result, the latter composition was implemented in the final method.
At first, preliminary validation was performed on the general extraction method according to the ICG guidelines. In the beginning, the calibration model was checked, the repeatability of the method was accepted, and the accuracy was evaluated by the standard addition method (data not included). The recovery reached only an average of 69.72% which was far below the acceptable limit of 98.0–102.0%. Unfortunately, the validation procedure revealed a considerable loss of compounds of interest during sample preparation due to degradation or conversion of vasicine [16]. One of the possible explanations is the autooxidation of vasicine to vasicinone that might take place in bright daylight or sunlight [1]. Consequently, all steps in the experimental procedure were performed protected from light.
3.2. Method validation
3.2.1. Loss on drying
The water content of 7.07 ± 0.05% was determined for the batch of powdered leaves used in the validation of the method.
3.3. Calibration model
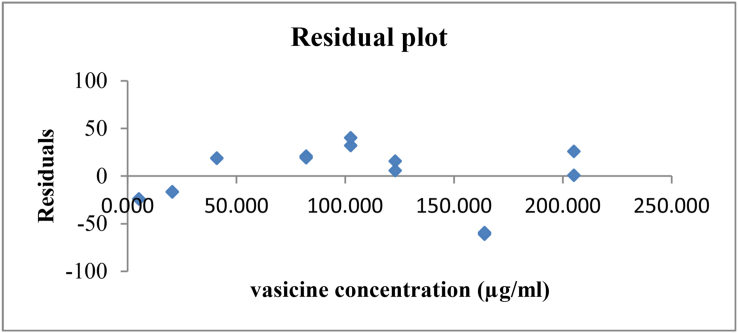
The vasicine standard dilutions were analyzed on eight different concentration levels, ranging from 5% (5.125 μg/ml) to 200% (205.00 μg/ml). A calibration curve was generated, and the equation and determination coefficient (R2) were calculated. The regression line was presented by the equation: y = 49,58 x + 22,40 and the calculated R2 (0.9999) was higher than 0.99, revealing a linear correlation between the concentration of vasicine and the obtained signal. Moreover, a Student's t-test was performed to obtain additional information on the slope of the curve and the intercept. Through this test, the 95% confidence interval on the intercept and the significance of the regression coefficient were studied. Regarding the intercept, the calculated (1.629) value was smaller than the t-critical value (2.145), which implies that the point (0.0) was included in the 95% confidence interval, as a consequence a single point calibration was justified. The t-value on the slope was 409.9 which was larger than the t-critical value of 2.145, therefore the null hypothesis should be rejected and the slope differed from 0. In the residual plot (Figure 2) no trend could be observed, the residuals were randomly scattered and the condition for homoscedasticity was fulfilled. Therefore, the linear model was applicable. The maximum deviation was established at 1.48%, which is lower than the accepted 5% limit.
Figure 2.
Residual plot for vasicine.
3.3.1. Repeatability – intermediate precision
To evaluate the repeatability of the method the analysis was performed six times on the same day, and for intermediate precision – on three different days on different concentration levels, to prove that the uncertainty of the measurement was equal in the whole range of the method. Regarding the intermediate precision, eighteen samples of 1.0 g powdered leaves of A. vasica equally divided over three days were analyzed. The variation within each day and the variation between days were compared. The average vasicine content of the six samples on the first day was (0.95 ± 0.02) g/100 g, for the second day it was (0.95 ± 0.01) g/100 g, and for the third day (1.00 ± 0.02) g/100 g, respectively. The relative standard deviation varied from 0.79% to 2.48%. The variances of the different days were checked using a Cochran test which revealed a value of 0.680 below the critical value of 0.707 (C-value). Therefore the variance can be considered equal. Based on the ANOVA single factor there was a difference between the three days (F = 20.64 ˃ 3.682 (critical F-value)). However, the RSD% between (3.72%) was calculated to be smaller than the RSD% max (= 5.0%), thus the method was considered precise (Table 3). It could be concluded that the eighteen samples analyzed over three different days do not differ significantly from each other in terms of average vasicine concentration.
Table 3.
Summarized results of the statistical analysis to determine the precision and the repeatability of different concentration levels of the method.
| Intermediate precision | Linearity | |||
|---|---|---|---|---|
| Std dev within | 0.017% | 0.020% | ||
| RSD% within | 1.80% | 1.90% | ||
| Std dev between | 0.036% | 0.038% | ||
| RSD% between | 3.72% | 3.70% | ||
| Cochran | 0.680 | C crit = 0.707 | 0.530 | C crit = 0.707 |
| F-test | 20.64 | F crit = 3.68 | 17.25 | F crit = 3.682 |
3.3.2. Repeatability – linearity
The repeatability on three different concentration levels (linearity) was compared. The average vasicine content for each concentration level was calculated: for the 50% level it was (1.01 ± 0.01) g/100 g, for the 100% level (1.00 ± 0.02) g/100 g and for the 150% level (1.06 ± 0.01) g/100 g, respectively. The relative standard deviation varied from 1.37% to 2.48%. In the same manner, as for the intermediate precision, the Cochran value was calculated: 0.530 < 0.707, which allowed us to conclude that the variances of the different concentration levels were considered equal. In the ANOVA single factor for the thirty values of the three different concentration levels the F-value was higher than the critical value (F = 17.25 ˃ 3.682). Based on the statistical analysis, the results from the three different days and levels were significantly different but the RSD between levels (see Table 3) was in the same order as the RSD between days, and smaller than the RSD max which is 2/3∗RSD Horwitz (= 4.0%). There was no concentration-related difference in the results, therefore, the method was considered precise.
In conclusion, the variation of the method for the three different days and concentration levels was accepted as equal. Both for the intermediate precision and the repeatability on different concentration levels the average vasicine amount, standard deviation, and relative standard deviation were calculated. The average vasicine content calculated with the 18 values of the intermediate precision was established at (0.96 ± 0.03%) g/100 g (RSD 3.28%). The average vasicine content calculated with the 30 values obtained through the repeatability test on different concentration levels was (0.99 ± 0.05%) g/100 g (RSD 4.40%). In both cases, the relative standard deviation was less than 5%.
3.4. Additional test on a new batch
The water content for the batch collected in Nepal was evaluated with a loss on drying test and was determined as (7.40 ± 0.03)%. The average vasicine amount for the six samples of the 50% level was estimated at (1.15 ± 0.01) g/100 g; the average vasicine content for the nine samples of the 100% level was calculated as (1.13 ± 0.01) g/100 g, and for the six samples of the 150% the average was (1.12 ± 0.01) g/100 g. In total, the average vasicine content for the 21 values was calculated as (1.14 ± 0.01) g/100 g with a relative standard deviation of 1.23%. The Cochran test revealed: 0.506 < 0.561 and the ANOVA test showed F = 6.443 ˃ 2.848. The calculated RSD% between was 1.0%, which was smaller than the RSD% max, as a result, it was confirmed that there was no significant difference between the different days. When another analyst conducted the method, the same concentration of vasicine was recorded. The result demonstrated that there was no influence by the analyst or by different concentration levels.
3.5. Accuracy of the method
The accuracy was analyzed, using three concentration levels by spiking the sample with vasicine reference standard. On average, 102.34 ± 4.29% of the added vasicine was recovered. The confidence interval was calculated to be 99.04–105.64%, which included 100%, therefore, the method could be considered accurate.
3.5.1. Specificity – selectivity
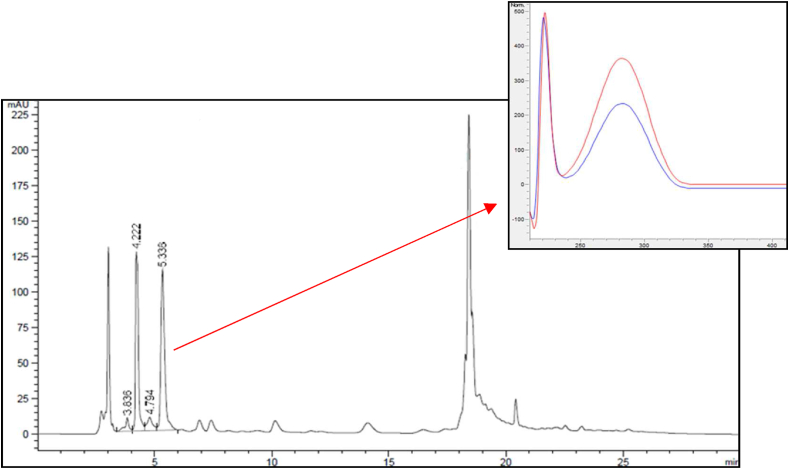
The specificity and selectivity of the quantification method of vasicine were investigated based on its UV spectrum and LC-MS analysis. The peak of vasicine in a test solution, prepared from the A. vasica leaves from Nepal, showed the same UV spectrum, with a UV max at 222 nm and 282 nm, as the peak of vasicine in the reference solution. The presence of vasicine in samples from different extracts was confirmed by a comparison of the chromatogram of the vasicine standard and the extract (Figure 3). Based on their retention time and overlying their UV-spectra, the identity of the peak was confirmed and no co-elution of vasicine and other compounds was detected. The peak resulting from the vasicine standard (m/z 189 [M + H]+) as the peak with a pseudo molecular ion of m/z 189 [M + H]+ was found at the same retention time in the chromatogram for both test samples of USP leaf powder and the leaf powder from Nepal. Therefore, it was concluded that vasicine could be discovered in the composition of the analyzed plant material, and most importantly, unambiguously confirmed that no underlying compounds were present.
Figure 3.
Chromatogram of the reference solution vasicine with a UV maximum at 282 nm (1), the Adhatoda vasica EDQM sample - test solution (2), and the Adhatoda vasica sample from Nepal - test solution (3).
3.6. Dietary supplements
In contrast to the Adhatoda leaves powder, the commercial product such as capsules and tablets, can contain other constituents such as different plant extracts and/or powders, which might influence aspects of the analytical method (i.e. the extraction process, the chromatographic separation). Therefore, it was highly recommended to investigate, if the composition of the commercial products would not affect the performance (precision and accuracy) of the validated method. The so-called “Flexible scope” or “Flexible validation” was carried out for preparation D to analyze the effect of the discussed factors. The standard procedure followed in the precision experiment normally includes analysis of the sample on 3 different concentration levels. In particular, two samples of preparation D on the lowest level and the highest level, together with three samples on the 100% level of the method were analyzed. The calculations were performed according to the ISO 5725-6 guideline and the SFSTP – guide and the mean values, as well as the standard deviation (S) and the relative standard deviation (RSD%), were obtained [17]. The percent vasicine at different concentration levels was calculated: for the 50% level it was 0.114 ± 0.003 (RSD 2.77%), for the 100% level it was 0.116 ± 0.005 (RSD 4.66%) and for the 150% level 0.121 ± 0.001 (RSD 1.23%). The range [xmax-xmin] of the 7 results should fall within the critical range (95% probability level) and the range [xmax-xmin] of the 3 results at the 100% levels should fall within the critical range (95% probability level). The RSD% value (4.80%) from the method validation for repeatability in different concentration levels reported previously was used in calculating the critical range. In the current case, the result could conform because for both groups of samples the critical differences were smaller than the critical ranges (see Table 4). The accuracy was tested on the 100% concentration level in triplicate. To 50% of the sample ±50% standard was added. The obtained recovery values should fall within the accuracy range of the validated method. On average, (104.8 ± 6.0)% of the added vasicine was recovered. The confidence interval was calculated to be (89.9–119.7)%, which included 100% and the method could be considered accurate. To check, if there is a difference between the precision in the recovery values compared to the general precision of the whole validated method, an F-test was performed. In the end, the test F-value was smaller than the F-critical (1.41 < 3.328), which excluded any variation between the precision within the flexible scope and the general validation. Additionally, Figure 4 presents the chromatogram from preparation D with the UV spectrum of vasicine (UV maximum at 282 nm). The spectra from the standard and the vasicine peak in the tested sample were overlaying. Therefore, this proved that the vasicine was present in the tested samples, and most importantly, that the separation of chemical compounds was successful. Regarding preparation E and F, the results followed a similar pattern.
Table 4.
Critical range values and range for the 3 results at 100% level and all 7 results values.
| <x> (3) = | 0.116 | RSD% method | 4.8 |
|---|---|---|---|
| s (3) = | 0.005 | CR 0.95 (3) = | 0.0183 |
| max-min = | 0.0100 | ||
| <x> (7) = | 0.117 | CR 0.95 (7) = | 0.0235 |
| s (7) = | 0.005 | max-min = | 0.0130 |
| RSD% | 3.92 |
Figure 4.
Chromatogram from preparation D and comparison between the UV spectrum of vasicine in the standard solution (red) and test solution (blue).
The validated method was applied for quantifying vasicine in six commercial products. The results of all preparations containing A. vasica leaves powder (A-C) and the summary of the obtained results for the dosage forms: the amount of Adhatoda extract in each test, the amount of vasicine per tablet, and the amount of vasicine in extract in percent for preparation D - E, are included in Table 5.
Table 5.
Vasicine content in three commercial products of Adhatoda Vasica powder, and vasicine content in formulated products: Preparation D – containing Adhatoda Vasica extract in capsules, Preparation E – containing Adhatoda Vasica plant material in tablets, preparation F – containing Adhatoda Vasica extract in capsules.
| result (%) | LOD 2h (%) | Result (%) | |
|---|---|---|---|
|
Preparation A <x>, s, RSD% |
0.333, 0.007, 2.21 | 11.63 | 0.377, 0.008, 2.21 |
|
Preparation B <x>, s, RSD% |
0.778, 0.007, 0.93 | 6.94 | 0.836, 0.008, 0.93 |
|
Preparation C <x>, s, RSD% |
0.470, 0.002, 0.51 | 3.74 | 0.488, 0.002, 0.51 |
| result (%) | result (mg) | Result (%) | |
| Preparation D | vasicine per capsule |
vasicine in extract |
|
| <x>, s, RSD% | 0.093, 0.001, 0.84 | 0.332, 0.002, 0.49 | 2.371, 0.012, 0.49 |
| Preparation E | vasicine per tablet |
vasicine in plant powder |
|
| <x>, s, RSD% | 0.150, 0.002, 1.62 | 0.360, 0.003, 0.71 | 0.554, 0.004, 0.71 |
| Preparation F | vasicine per capsule |
vasicine in extract |
|
| <x>, s, RSD% | 0.134, 0.006, 4.14 | 0.537, 0.008, 1.54 | 0.134, 0.002, 1.54 |
Generally, the analysis showed precise and reproducible results of the vasicine amount within a wide range of contents. However, in the six food supplements that were investigated in this work, a substantial difference in the amount of vasicine (Table 6) could be observed between the leaves powders and the dosage forms, and within the samples of each group (results ranging from 0.13% till 2.37%). Concerning the powders, a possible explanation might be the different origins of the plant material. Many factors like climate condition, time the material was collected, way of drying, transportation, and storage might affect the vasicine content in the final product. Regarding the dosage forms, which contained A. vasica extract (preparation D and F), a limitation factor could be the way of extracting the plant material – the extraction solvent, taking into consideration the effect of bright daylight or sunlight on vasicine stability. Nevertheless, after comparison between the amount of vasicine found in each product (preparation D-F) and the recommended dose by the manufacturer, it was observed that the vasicine intake between the several dosage forms, was approximately in the same range – from 0.996 to 1.074 mg/day (Table 7). However, the amount of vasicine, which was administered in the body with the plant powders was significantly different, ranging between 6.71 to 40.60 mg vasicine. Despite that the values were higher compared to the vasicine intake with the dosage forms, the manufacturer did not provide additional information about the exact amount of vasicine which is absorbed in the body. Therefore, evaluating the quality and the suggested dose for those plant powders can be made after a detailed study of their bioavailability. In summary, based on the results obtained in the course of the analysis, the validated method can be applied for unambiguous quantification of vasicine in plant-derived products which would be used to investigate the quality and the supplement intake prescribed by the manufacturer.
Table 6.
Mean value of vasicine content in Adhatoda Vasica – derived products.
| Preparation | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Vasicine [%] | 0.377 | 0.836 | 0.488 | 2.371 per extract | 0.554 per powder | 0.134 per extract |
| S | 0.008 | 0.008 | 0.002 | 0.012 | 0.004 | 0.002 |
| RSD% | 2.21 | 0.93 | 0.51 | 0.49 | 0.71 | 1.54 |
| Product characteristic | Plant powder | Plant powder | Plant powder | A mix of plant extracts | A mix of plant powders | Plant extract |
Table 7.
Comparison between the vasicine intake for the investigated dosage forms containing extract or powder of Adhatoda Vasica.
| Preparation | Content manufacturer | Dosage Manufacturer |
Results from the analysis (vasicine, mg) | Intake (vasicine, mg) |
|---|---|---|---|---|
| A | leaf powder | 1/2 teaspoon - 1 teaspoon/1–2 times per day | 3.77 mg/1 g powder | 6.71 mg–26.84 mg/day |
| B | leaf powder | 1/4–1/2 teaspoon/1–2 times per day | 8.36 mg/1 g powder | 8.44 mg–33.94 mg/day |
| C | leaf powder | 1/2–1 teaspoon/2 times per day | 4.88 mg/1 g powder | 20.30 mg–40.60 mg/day |
| D | 14 mg extr. | 2-3 caps/day for 2–3 months | 0.332 mg/caps | 0.996 mg/day |
| E | 65 mg powder | 3 tabs/day | 0.360 mg/tab | 1.08 mg/day |
| F | 400 mg extr. | 2 caps/day | 0.537 mg/caps | 1.074 mg/day |
4. Conclusion
In conclusion, a method was successfully optimized for the determination of vasicine in the leaves of A. vasica. The optimization was based on a monograph from the USP and further developed in the research laboratory (NatuRA). In particular, the adaptations of the USP considered instrument parameters like the column and the mobile phase; also considerable efforts were made for finding the optimal extraction conditions and sample preparation. Due to the risk for autooxidation of vasicine (the main quinazoline alkaloid) to vasicinone that can take place in bright daylight or sunlight, all steps in the experimental procedure were performed protected from light. This consideration was necessary because of their completely different pharmacological activity. After establishing the final method, the second objective of the study was to validate the procedure according to the ICH guidelines regarding linearity, precision, accuracy, and specificity. The calibration model was found to be linear in the concentration range of 5.125–205 μg/mL. The intermediate precision was determined on eighteen samples of 1.0 g equally divided over three days. The average vasicine content was calculated to be 0.96 g/100g with a relative standard deviation of 0.03%. The precision at different concentration levels was estimated by testing six samples of 0.5 g and six samples of 1.5 g. Those values were compared with the values for the intermediate precision. Regarding the precision at different concentration levels, the average vasicine content of 0.99 g/100g with an RSD% of 0.05% was obtained. The average recovery was found to be 102.34% with an RSD of 4.29%. Finally, the specificity and the selectivity of the method were investigated by applying UV and MS detection. The latter confirms the good chromatographic separation without the presence of underlying coelution of other compounds and vasicine.
Next, the study aimed to test whether the validated method for quantification of vasicine in plant material, as applicable for the quantitative determination of vasicine in commercially available products. The test was carried out on six commonly available supplements which contained A. vasica leaves powder, or dosage forms (tablets or capsules) containing A. vasica powder or extract. The percentage of vasicine in each one of them was successfully obtained. Additionally, to be able unambiguously to apply the method for investigating vasicine in various matrices, a validation within a flexible scope was required. As a result, it was confirmed that the validated method was still precise and accurate for vasicine quantification in matrices that differ from the one used to perform the validation. The experimental conditions were proved to ensure good baseline separation between vasicine and vasicinone, and no co-elution of other compounds in both chromatograms for leaves powder samples and dosage forms products occurred. Based on the presented results, the established method can be efficiently used for the quantification of vasicine in various commercial products in the future. Consequently, providing a better understanding of the recommended dosage and the quality of the products. Moreover, the validated method can be applied to determine the different amounts of vasicine between the plant parts of A. vasica, or plant parts of A. vasica collected from different geographical locations, and also, the vasicine content in other plants which have been reported to contain the compound in their phytochemical composition.
Declarations
Author contribution statement
Stefaniya Velichkova: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
M. Theunis: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
T. Naessens; K. Foubert: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
L. Pieters: Contributed reagents, materials, analysis tools or data.
Funding statement
Dr Stefaniya Velichkova was supported by Fonds Wetenschappelijk Onderzoek [11T8118N].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The paper is dedicated to the memory of our colleague, Sandra Apers, who left us far too early on 5th February 2017. And special acknowledgment is given to Karen Joosen, a master's student at the University of Antwerp, who was also passionately involved in the method optimization and validation process.
References
- 1.Srivastava S., Verma R., Gupta M., Singh S., Kumar S. HPLC determination of vasicine and vasicinone in Adhatoda vasica with photodiode array detection. J. Liq. Chromatogr. Relat. Technol. 2001;24:153–159. [Google Scholar]
- 2.Singh T.P., Singh O.M., Singh H.B., Rea G., Giardi M.T. Adhatoda vasica Nees: phytochemical and pharmacological profile. Nat. Prod. J. 2011;1:29–39. [Google Scholar]
- 3.Dhankhar S., Kaur R., Ruhil S., Balhara M., Dhankhar S., Chhillar K. A review on Justicia adhatoda: a potential source of natural medicine. Afr. J. Plant Sci. 2011;5:620–627. [Google Scholar]
- 4.Sen J., Ghose T. Alkaloids from leaves of Adhatoda vasica. J. Indian Chem. Soc. 1924;1:315. [Google Scholar]
- 5.Singh A., Kumar S., Reddy J., Rameshkumar K., Kumar B. Screening of tricyclic quinazoline alkaloids in the alkaloidal fraction of Adhatoda beddomei and Adhatoda vasica leaves by high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2015;29:485–496. doi: 10.1002/rcm.7126. [DOI] [PubMed] [Google Scholar]
- 6.Patil S., Ojha R., Kaur G., Nepali K., Aggarwal S., Lal Dhar K. Estimation of seasonal variation of two major pyrrolo [2,1-b] quinazoline alkaloids of Adhatoda vasica by HPLC. Nat. Prod. J. 2013;3:30–34. [Google Scholar]
- 7.Liu L., Zhao T., Cheng X., Wang C., Wang Z. Characterization and determination of trace alkaloids in seeds extracts from Peganum harmala Linn. Using LC-ESI-MS and HPLC. Acta Chromatogr. 2013;25:221–240. [Google Scholar]
- 8.Dhalwal K., Shinde V., Mahadik K. Optimization and validation of reverse phase HPLC and HPTLC method for simultaneous quantification of vasicine and vasicinone in Sida species. J. Med. Plants Res. 2010;4:1289–1296. [Google Scholar]
- 9.Madhukar G., Tamboli E., Rabea P., Ansari S., Abdin M., Sayeed A. Rapid, sensitive, and validated UPLC/Q-TOF-MS method for the quantitative determination of vasicine in Adhatoda vasica and its in vitro culture. Phcog. Mag. 2014;10:198. doi: 10.4103/0973-1296.127375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roja G., Vikrant B., Sandur S., Sharma A., Pushpa K. Accumulation of vasicine and vasicinone in tissue cultures of Adhatoda vasica and evaluation of the free radical-scavenging activities of the various crude extracts. Food Chem. 2011;126:1033–1038. [Google Scholar]
- 11.Dhooghe L., Mesia K., Kohtala E., Tona L., Pieters L., Vlietinck A., Apers S. Development and validation of an HPLC-method for the determination of alkaloids in the stem bark extract of Nauclea pobeguinii. Talanta. 2008;76:462–468. doi: 10.1016/j.talanta.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Sujata B., Mamta P., Priyanka K., Sonam S. Review & future perspectives of using vasicine, and related compounds. Indo Global J. Pharmaceut. Sci. 2011;1:85–98. [Google Scholar]
- 13.ICH Expert Working Group . 1994. ICH Harmonized Tripartite Guideline: Note for Guidance on Toxicokinetics. [Google Scholar]
- 14.ICH harmonized tripartite guideline . 1996. ICH Guideline for Good Clinical Practice E6 (R1) [PubMed] [Google Scholar]
- 15.ICH expert working group ICH Guideline Q1D bracketing and matrixing designs for stability testing of new drug substances and products. Int Conf Harmon. 2003;68:2339–2340. [PubMed] [Google Scholar]
- 16.Joosen K., Apers S. 2016. Elaboration of a Monograph for Adhatoda vasica Nees in the European Pharmacopoeia : Development and Validation of an Assay for the Determination of Vasicine. [Google Scholar]
- 17.ANSES . 2015. Pôle Recherche et Référence, Guide de validation des méthodes d’analyses 67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.