Abstract
Previous studies conducted in other countries showed that neonicotinoid insecticides contaminated environmental waters and reduced aquatic invertebrate abundance. This study analysed neonicotinoid concentrations in estuarine waters of Indramayu Regency, Indonesia, and their potential toxicity to the aquatic environment. Data collection included water sampling and analysis, watershed and paddy field analyses, and literature review. The results showed that the detection frequency of neonicotinoids was 75%, with imidacloprid and thiamethoxam having the highest mean concentrations compared to other compounds. The sample collected in August 2021 from an estuary in the Patrol sub-district contained the highest total neonicotinoid concentration (140.26 ng/L). Five samples (31.25%) contained imidacloprid concentrations that exceeded the chronic benchmark regulated by the Netherlands, thus related regulation and policies are encouraged to be established in Indonesia to prevent potential harmful effect of neonicotinoids to the aquatic environment. There was no significant correlation between the neonicotinoid concentrations and the paddy field and watershed sizes as well as the land use proportion for paddy fields within the watershed. This study is the first to report neonicotinoid contamination in Indonesian estuarine waters.
Keywords: Neonicotinoid contamination, Toxicity, Estuarine waters, Indonesia
Neonicotinoid contamination; Toxicity; Estuarine waters; Indonesia.
1. Introduction
Indonesia, a tropical country located in Southeast Asia, harbours a high biodiversity and is considered one of the 17 megadiverse countries in the world. The country is also rich in vast and abundant arable fertile soils, making it one of the world's centres for agrobiodiversity of plant cultivars (CBD Secretariat, 2022). A wide variety of tropical agricultural products are produced in Indonesia, including rice, which is the major cultivated crop (FAO, 2022) and the main staple food in the Indonesian diet. The high demand for rice has made the country the third largest rice producer worldwide, with a mean annual rice production of 59 million tonnes in the last 10 years (FAO, 2021). In Indonesia, Indramayu Regency (located in West Java Province) is one of the largest rice-producing areas, with a total harvested area of 229 thousand ha and a mean annual rice production of 1.5 million tonnes from two to three planting periods in the last 10 years (BPS Kabupaten Indramayu, 2021).
Rice cultivation activities in Indonesia rely on neonicotinoids to control pests, so as to lead to a successful harvest. A total of 126 insecticide trademarks containing neonicotinoids have received distribution permits from the Ministry of Agriculture of Indonesia for use by the community (Kementan, 2020). Neonicotinoids are a class of systemic insecticides with a chemical structure similar to nicotine and work by attacking the nervous system of insect pests (Buszewski et al., 2019), consisting of acetamiprid, clothianidin, dinotefuran, imidacloprid, nitenpyram, thiacloprid, and thiamethoxam. Despite their utility in eradicating pests in rice cultivation, the application of neonicotinoids, however, also resulted in the pollution of environmental waters surrounding the agricultural lands (Hano et al., 2019; Schaafsma et al., 2019) due to their properties, i.e., high water solubility, low absorbance by soils, resistance to hydrolysis, and having a long half-life (Morrissey et al., 2015). These properties cause the neonicotinoids to be more persistent so that they can easily be transferred from rice fields to the nearby aquatic environment. Previous studies have also confirmed that the presence of neonicotinoids in the aquatic environments reduced the abundance of non-target aquatic invertebrates such as aquatic insects (Van Dijk et al., 2013) and zooplankton (Yamamuro et al., 2019), leading to a population decline in their predators, such as birds (Li et al., 2020), eels, and smelts (Yamamuro et al., 2019), through the food chain system.
To date, most studies on neonicotinoid occurrence in environmental waters were conducted in sub-tropical and temperate zones such as in Europe (e.g., Iancu et al., 2019; Postigo et al., 2021; Sousa et al., 2020), Australia (e.g., Sánchez-Bayo and Hyne, 2014), Canada (e.g., Anderson et al., 2015; Main et al., 2014; Schaafsma et al., 2019; Struger et al., 2017), USA (e.g., Berens et al., 2021; Hladik and Kolpin, 2015), China (e.g., Naumann et al., 2022; Zhang et al., 2019), and Japan (e.g., Hano et al., 2019; Hayashi et al., 2021; Yamamuro et al., 2019), and very few were performed in tropical region (e.g., Bonmatin et al., 2019; Bonmatin et al., 2021; Lamers et al., 2011; Wan et al., 2021). Moreover, very few studies focused on estuarine waters (e.g., Gonzalez-Rey et al., 2015; Hano et al., 2019; Sousa et al., 2020; Yamamoto et al., 2012) where pollutants originating on land will be accumulated before they are transferred to the sea. On the other hand, estuaries provide critical habitats for the survival of many species, such as for feeding grounds, breeding grounds, and nursery locations; thus, the presence of these insecticides in Indonesian estuarine environments may threaten aquatic biodiversity in this country, which can lead to a decrease in higher trophic animal abundance. However, no previous studies have investigated neonicotinoid occurrence in Indonesian estuarine waters, particularly in Indramayu Regency where the farmers apply neonicotinoids by spraying throughout the cultivation period. This insecticide group has not become a parameter in the water quality regulation either, and no regulation regarding water quality thresholds for aquatic life has been established in Indonesia. Therefore, this research was carried out with the aim to analyse the neonicotinoid concentrations in estuarine waters of Indramayu Regency, Indonesia, and its potential toxicity to the aquatic ecosystem, in which the results can serve as a preliminary reference for establishing a regulation or policy brief regarding neonicotinoid pollution in Indonesian environmental waters. To the best of our knowledge, this study is the first to report neonicotinoid contamination in Indonesian estuarine waters and presents new information regarding neonicotinoid pollution in such waters.
2. Materials and methods
2.1. Water sample collection
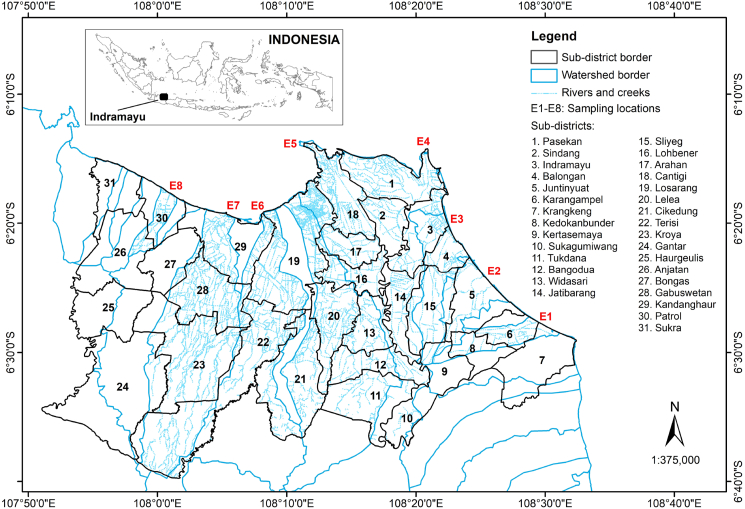
Water samples were collected from eight downstream areas of rivers and creeks that flowed through paddy fields in Indramayu Regency (Table 1, Figure 2) on 9–10 November 2020 (off-season for paddy cultivation during the rainy season) and on 20–24 August 2021 (rice growing season during the dry season). Sample collection was performed using a stainless-steel bucket attached to a rope. The sample collection method followed the methods of Schaafsma et al. (2019) with some modifications. Approximately 200 mL of water sample was collected from each selected location and placed in a triple-rinsed, Nalgene amber high-density polyethylene bottle (DS2085-0016 series from Thermo Scientific, USA). Sample bottles were then placed immediately in a dark container and refrigerated (8 °C) for further analysis in the laboratory.
Table 1.
Coordinates of water sampling locations.
| Location | Area Name | Coordinates |
|---|---|---|
| E1 | Karangampel | 6° 27′ 43.8552″ S, 108° 29′ 12.7032″ E |
| E2 | Juntinyuat | 6° 24′ 2.1846″ S, 108° 25′ 16.5396″ E |
| E3 | Singaraja | 6° 19′ 55.6644″ S, 108° 22′ 24.4302″ E |
| E4 | Pabean Ilir | 6° 14′ 50.8812″ S, 108° 20′ 56.313″ E |
| E5 | Lamarantarung | 6° 13′ 50.0772″ S, 108° 10′ 1.203″ E |
| E6 | Cemara | 6° 19′ 30.4242″ S, 108° 8′ 23.4342″ E |
| E7 | Eretan | 6° 19′ 16.9314″ S, 108° 5′ 19.9962″ E |
| E8 | Patrol | 6° 17′ 46.233″ S, 108° 0′ 56.3322″ E |
Figure 2.
Watershed map of Indramayu Regency used for observing the involvement of sub-district locations with respect to the sampling points.
2.2. Water sample analysis
Analysis of seven neonicotinoid compounds (acetamiprid, clothianidin, dinotefuran, imidacloprid, nitenpyram, thiacloprid, and thiamethoxam) in water samples was conducted at the Limnology Laboratory, Department of Natural Environmental Studies, The University of Tokyo, Japan, on 25 November 2020 and on 15–16 September 2021, following the methods propounded by Yamamuro et al. (2019) with modification. A certified surrogate standard with >97% isotopic purity was purchased from Hayashi Pure Chemicals Industries, Ltd., Japan, and the neonicotinoid standard reagent with >99% compound purity was obtained from Fujifilm Wako Pure Chemical Corporation, Japan. The surrogate standard (10 μg/mL) was diluted 10-fold with methanol to obtain a surrogate concentration of 1 mg/L. Neonicotinoid standard reagent (20 μg/mL) was diluted 2000-fold and 20-fold with methanol to obtain concentrations of 10 μg/L and 1 mg/L, respectively. Diluted neonicotinoid standards were used to prepare standards with concentrations of 1, 3, 10, 30, 100, and 300 μg/L by mixing them with diluted surrogate and methanol.
Water sample (200 mL) from each sampling point were filtered twice using Whatman glass microfiber filter papers (first by GF/D with pore size of 2.7 μm and then by GF/F with pore size of 0.7 μm) to remove the suspensions. Subsequently, 10 μL of surrogate (1 mg/L) was added to the filtered water samples. Solid phase extraction (SPE) cartridges (Inertsep® Pharma FF 3 mL and Inertsep® GC 6 mL, installed together for each sample with Pharma FF being above the GC) were cleaned by passing through 60 mL of methanol and 150 mL of ultrapure water, respectively, at a flow rate of 1 drop/s, using an AQUALoader AL898U from GL Sciences, Japan. The filtered water samples were then passed through the SPE cartridges to capture neonicotinoids with a flow rate of 1 drop/2 s. The SPE cartridges were washed with 30 mL of ultra-pure water to remove the water sample remaining inside the syringe and matrix in the cartridges. The SPE cartridges were then dehydrated by centrifugation for 5 min at 40 rpm. Subsequently, neonicotinoids and surrogates were extracted from the SPE cartridges (with the arrangement of GC being above the Pharma FF) using 7 mL of acetone at a flow rate of 1 drop/3 s using a GL-SPE vacuum manifold from GL Sciences, Japan. The extract was concentrated to dryness using nitrogen gas and a heater (40 °C), and then 200 μL of methanol was added and mixed using a vortex mixer.
Liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis was carried out for 2 μL of eluate containing neonicotinoids by injecting it into a Nexera HPLC system coupled to a LCMS-8030 triple quadrupole mass spectrometer from Shimadzu Corporation, Japan, to measure the concentrations. Neonicotinoids were separated using a Kinetex C18 column (1.7 μm, 2.1 mm × 150 mm) by Phenomenex, Japan, at 40 °C. Mobile phase solvents were 0.1% formic acid in ultrapure water solution (A) and 0.1% formic acid in methanol solution (B) with an initial ratio of 90:10. Separation was performed using a flow rate of 0.2 mL/min with a gradient shifting from 90:10 to 60:40 in 6 min and being held for 6 min, then to 35:65 in 6 min and being held for 3 min, after that to 5:95 in 3 min and being held for 5.1 min, then returned to the initial conditions and kept at equilibration for 4.9 min. The mass spectrometer was operated in multiple reaction monitoring (MRM), and the electrospray ionisation mode selected was reaction monitoring. Neonicotinoid concentrations were calculated using the precursor and fragment ions (m/z) as follows: 203.00 > 129.15 (dinotefuran), 223.00 > 125.90 (acetamiprid), 250.00 > 169.00 (clothianidin), 253.00 > 126.00 (thiacloprid), 256.25 > 209.00 (imidacloprid), 271.00 > 225.00 (nitenpyram), and 291.90 > 211.05 (thiamethoxam). Neonicotinoid standards were also analysed to create a calibration curve for each compound.
For the quality assurance and quality control of the methods used in this study, the tests utilized environmental waters in Japan due to the limitation of the sample's volume from Indonesia, with seven replications for each test. The recovery rate ranged from 89.1% to 101.4% for 10 μg/L of spiking and 93.4%–107.5% for 100 μg/L of spiking. The limit of detection (LOD) and the limit of quantification (LOQ) of each neonicotinoid compound were determined using procedural blanks (ultrapure water) due to high initial concentrations of neonicotinoids in environmental waters from Japan. The LODs ranged between 1.55 ng/L and 1.76 ng/L, while the LOQs ranged from 1.64 ng/L to 1.99 ng/L. The concentrations below the LOQs were stated as ‘non-detected’. The coefficient of variance for 1.5 μg/L of spiking was less than 30% for all compounds.
2.3. Watershed and paddy field mapping
Maps were created using ArcMap 10.8 from Esri with base shape files of regency areas, rivers, watersheds, and paddy fields in Indramayu Regency obtained from the Indonesia Geospatial Portal (https://tanahair.indonesia.go.id). For the watershed map, the watershed base shape file covering the entire Indonesia area was cropped according to the Indramayu Regency area and was then merged with the river shape file which had also been cropped to fit the regency area. Both were combined with the regency shape file which already contained boundaries between sub-districts. The water sampling points were then added to the maps to make it easier to observe the sub-district areas that may have affected the water sample analysis results based on the water sampling locations. The watershed area size was calculated using the Calculate Geometry tool in ArcMap 10.8.
For the paddy field map, the base watershed shape file covering the entire regency area was separated according to the seven selected watersheds. The river and sub-district shape files covering one regency were then cropped according to the watershed area that had been created. A shape file for each sub-district in each watershed was created. The rice field shape file covering one regency area was cropped according to the sub-district area in each watershed that had been created. The paddy field maps in August and November were created by combining the cut watershed shape files, river shape files in the size of related watersheds, and rice fields shape files per sub-district per watershed according to the information on planting season carried out in those two months from local government officials. Sampling points were also included in the maps. The area size of rice fields in each watershed in August and November was calculated using the Calculate Geometry tool in ArcMap 10.8.
A field survey was conducted to confirm the paddy field and watershed maps created by recording the coordinates of field observation points using the Global Position System (GPS), as well as taking photos and notes on land cover conditions around the observed points. Watershed observations were carried out from 28 July to 1 August 2021 in the upstream, middle, and downstream parts of each watershed. The August paddy field map was confirmed through field observations on 20–24 August 2021 along with water sampling activity, and the November paddy field map was checked using photos of rice fields around the sampling locations which were taken during water sampling on 9–10 November 2020. Online communication with local government officials from the Agriculture Office of Indramayu Regency and the Centre for the Protection of Food Crops and Horticulture were also conducted to obtain information on rice planting seasons and insecticide products utilised by farmers in each sub-district in the regency.
2.4. Data analysis
All data were analysed using simple statistics, and Pearson correlation analysis with a significance probability level (1-tailed) of 0.05 was used to observe the correlation between the paddy field and watershed area sizes and the neonicotinoid concentrations in water samples. Statistical analyses were performed using IBM SPSS Statistics Version 26. Neonicotinoid analysis results were compared with the aquatic life benchmarks implemented in some countries (CCME, 2007; Hano et al., 2019; Kreuger et al., 2010; Morrissey et al., 2015; USEPA, 2017) to observe the possible negative impacts of neonicotinoids in water samples on the aquatic environment.
3. Results and discussion
3.1. Neonicotinoid occurrence in estuarine waters
A total of 16 water samples collected from eight estuaries in Indramayu Regency were analysed for their neonicotinoid contents. The data showed that these pollutants were detected in 12 samples (detection frequency of 75%) from all selected estuaries, with a mean concentration of 21.65 ng/L (Table 2). Among all neonicotinoids, imidacloprid and thiamethoxam ranked first and second with respect to the highest occurrence and mean concentrations (Table 2). This information was in accordance with the information from local government officials, stating that imidacloprid and thiamethoxam were the most widely used types by farmers (in 27 sub-districts and 18 sub-districts, respectively) for rice cultivation. These compounds are also the types with the highest number of products in the list of registered insecticide products containing neonicotinoids from the Indonesian government (Kementan, 2020). Surahmat et al. (2016) also confirmed that imidacloprid has mostly been used to eradicate brown plant hoppers (Nilaparvata lugens (Stål)) in the regency. In estuaries of Seto Inland Sea and in rivers and estuaries of Osaka City, Japan, dinotefuran was prominently detected due to its wide application for rice cultivation in the country (Hano et al., 2019; Yamamoto et al., 2012). Meanwhile, in another study in southern Ontario, Canada, imidacloprid was observed across the entire study area due to its broad range of application (Struger et al., 2017).
Table 2.
Total occurrence of neonicotinoids in estuarine waters in November 2020 and August 2021 (n = 16).
| Neonicotinoid | Occurrence (%)∗ | Number of sampling site detected | Mean (ng/L) | Max∗∗ (ng/L) | Sampling location for max | Sampling time for max |
|---|---|---|---|---|---|---|
| Imidacloprid | 75.00 | 8/8 | 8.75 | 35.34 | E8 | August 2021 |
| Thiamethoxam | 62.50 | 8/8 | 7.13 | 65.14 | E8 | August 2021 |
| Dinotefuran | 25.00 | 3/8 | 1.99 | 23.12 | E8 | August 2021 |
| Thiacloprid | 18.75 | 3/8 | 1.77 | 16.66 | E8 | August 2021 |
| Clothianidin | 12.50 | 2/8 | 2.01 | 27.57 | E5 | November 2020 |
| Acetamiprid | - | - | - | - | - | - |
| Nitenpyram | - | - | - | - | - | - |
| Total concentration | 75.00 | 8/8 | 21.65 | 140.26 | E8 | August 2021 |
Percentage of detected samples (>LOQ).
Maximum concentration value.
The third position was occupied by dinotefuran, with an occurrence of 25% and a mean concentration of 1.99 ng/L, followed by thiacloprid and clothianidin with 18.75% and 12.5% of occurrence, respectively. The detected thiacloprid indicated that unregistered products containing this compound might have been used by farmers since no products containing thiacloprid have utilization permit from the central government (Kementan, 2020). It is unclear whether an application of permission for insecticide products containing this compound is currently in progress or not. Acetamiprid and nitenpyram were not found in any sample because acetamiprid is among the least used compounds by the farmers in the sub-districts near the sampling locations, while nitenpyram reacts very rapidly with sunlight and has the highest rate of direct photolysis (DT50 in 9 min) (Todey et al., 2018) compared to other compounds, leading to more rapid dissipation with no neonicotinoid remaining in environmental waters.
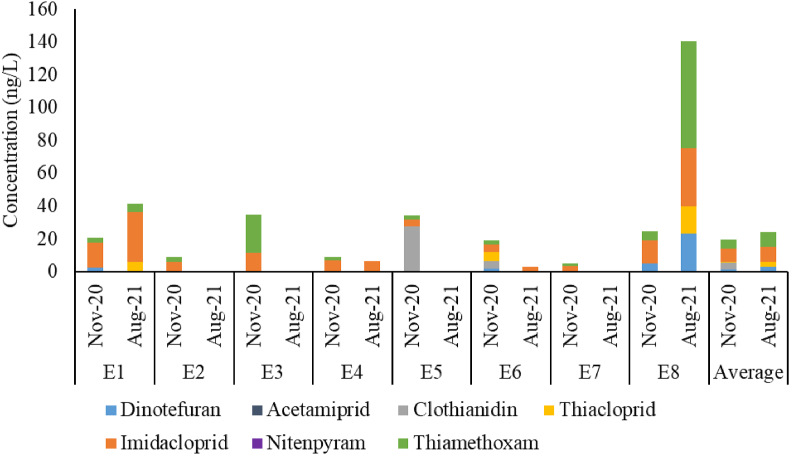
According to Figure 1, average neonicotinoid concentrations detected in all sampling locations increased 1.23-fold in August 2021 compared to November 2020. Average concentrations of all neonicotinoid compounds, except clothianidin, acetamiprid, and nitenpyram, were also higher in August 2021 than those in November 2020. The average imidacloprid, thiamethoxam, dinotefuran, and thiacloprid concentrations increased from 8.17 ng/L, 5.45 ng/L, 1.09 ng/L, and 0.72 ng/L in November 2020 to 9.34 ng/L, 8.81 ng/L, 2.89 ng/L, and 2.83 ng/L in August 2021, respectively. The increased concentrations were correlated to the rice cultivation cycles in Indramayu Regency, which are generally two times a year, according to the information obtained from the local government officials. In November, most farmers in the regency do not cultivate rice due to water shortage. Since the growing season is in August, higher concentrations were detected in the samples collected in August 2021. This also explained the occurrence of maximum concentrations of four compounds in August 2021 (Table 2), particularly in E8 where farmers were cultivating rice and likely using the insecticides in large amounts during the water sample collection. A similar result was reported by Iancu et al. (2019), where the total neonicotinoid levels in surface water samples in Danube River and its tributaries in Romania were much higher in April (planting period, 212 ng/L) than in February (pre-planting period, 16.30 ng/L). In Japan, higher concentrations of neonicotinoids were detected in water samples during their application period in June–September than in other months (Hano et al., 2019). In the rivers, streams, and lakes in Minnesota, USA, neonicotinoid concentrations were also attributed to its application timeline during the growing season (Berens et al., 2021).
Figure 1.
Neonicotinoid concentrations in eight estuaries of Indramayu Regency at two different times (November: the off-season of rice cultivation; August: the rice cultivation season). The absence of bars indicates that the neonicotinoid was not detected (<LOQ).
The results also showed that neonicotinoids could be detected at more sampling points in November 2020 (eight locations) than in August 2021 (four locations) (Figure 1). This may have been because the rainy season occurs in November, causing the release of neonicotinoids stored in soils after the previous rice cultivation season into rivers/creeks through runoff after rainfall events (Sánchez-Bayo and Hyne, 2014; Schaafsma et al., 2019; Zhang et al., 2019). Rainfall also increases the turbidity of water, thus preventing the photodegradation process of neonicotinoids in water (Sánchez-Bayo and Hyne, 2014). In August 2021 (dry season), the neonicotinoids might not have been able to reach the other four sampling points because of the low quantity of pollutants leaching into water bodies due to no rain that caused a low water flow. Intense sunlight exposure in combination with less turbid water also results in a more rapid water photolysis process, which may have reduced the neonicotinoid concentrations along the way to the other four sampling points (Lu et al., 2015).
Figure 1 also illustrates that in November 2020, the highest concentrations of imidacloprid, thiamethoxam, dinotefuran, thiacloprid, and clothianidin were detected in samples from E1 (15.21 ng/L), E3 (23.14 ng/L), E8 (4.82 ng/L), E6 (5.79 ng/L), and E5 (27.57 ng/L), respectively. For August 2021, the sample from E8 (Patrol) contained the highest concentrations of imidacloprid, thiamethoxam, dinotefuran, and thiacloprid (35.34 ng/L, 65.14 ng/L, 23.12 ng/L, and 16.66 ng/L, respectively). These concentrations were higher than those observed in other tropical countries. The highest imidacloprid and thiamethoxam concentrations in the Philippines (3.03 ng/L and 0.15 ng/L, respectively, detected in waters near rice fields, and 5.22 ng/L and 0.20 ng/L in waters near banana plantations and a citrus grove, respectively) were lower than those found in our study (Bonmatin et al., 2021). The mean concentrations of imidacloprid and thiamethoxam in our study (Table 2) were higher than those reported in a river in Vietnam, where the mean concentrations were 0.29 ng/L and 0.23 ng/L, respectively (Wan et al., 2021); however, imidacloprid in the Chieng Khoi watershed in Vietnam, as reported by Lamers et al. (2011), showed much higher concentration (1153 ng/L) than those detected in our study. In water samples from Belize, the maximum concentrations of imidacloprid and thiacloprid (0.014 ng/L and 0.003 ng/L, respectively) were much lower than the concentrations found in our study (Bonmatin et al., 2019).
Neonicotinoid concentrations in our study were generally lower than those observed in sub-tropical and temperate countries. This was probably due to the exposure to intense tropical sunlight that contributes to a higher degree of photolytic degradation of neonicotinoids in water (Lu et al., 2015), and the intense seasonal rainfall in tropical countries in combination with the complex structure of rivers and creeks that lead to a greater dilution of insecticides in environmental water during their transport (Bonmatin et al., 2019). In Spain, the maximum imidacloprid concentration detected in the Llobregat River basin during summer was much higher than those reported in our study (218 ng/L) (Postigo et al., 2021). The maximum concentrations of clothianidin, imidacloprid, and thiamethoxam in river water in Australia (Sánchez-Bayo and Hyne, 2014), Canada (Main et al., 2014), and the USA (Hladik and Kolpin, 2015) were also much higher than those found in our study. In rivers surrounding the Bohai Sea in China, Naumann et al. (2022) observed high concentrations of imidacloprid (104 ng/L), thiamethoxam (99.8 ng/L), and clothianidin (55.2 ng/L), which vastly exceeded those reported in our study. The concentrations of imidacloprid, dinotefuran, and clothianidin in our study were also lower than those reported by Hano et al. (2019) in estuaries of Seto Inland Sea, Japan, where only the thiamethoxam concentration was higher in our study. Similarly, Bonmatin et al. (2019) confirmed that the concentrations of clothianidin, imidacloprid, and thiamethoxam in the environmental waters of Belize, a tropical region in Central America, were below those found in the temperate regions of Europe, Australia, America, and Japan.
Despite the fact that neonicotinoid concentrations in the tropical zone were generally lower than those in other regions, with regard to estuaries, the concentrations of most compounds reported in our study were higher than those in Portugal, where the maximum concentration of imidacloprid in the Arade River estuary was 8 ng/L (Gonzalez-Rey et al., 2015) and the highest concentration of thiamethoxam along the Portuguese coast were 0.34 ng/L; only the thiacloprid concentration detected in our study was lower than that reported in Portugal (32 ng/L) (Sousa et al., 2020). Neonicotinoid concentrations presented in our study were also higher than those reported in the rivers and estuaries of Osaka City, Japan, with maximum imidacloprid, thiamethoxam, and clothianidin concentrations of 25 ng/L, 11 ng/L, and 12 ng/L, respectively, while the dinotefuran concentration in our study was 11-fold lower than that reported for Osaka City (220 ng/L) (Yamamoto et al., 2012).
Neonicotinoids enter nearby environmental waters along with freshwater from rice fields, thus, its concentrations should be expected to decrease closer to the sea due to the dilution with freshwater. However, the contaminants were still detected in water samples, indicating that farmers might have initially used insecticidal products containing large amounts of neonicotinoids. The use of high amounts of neonicotinoids by farmers could result in more neonicotinoids being leached into water bodies, which could cause a lower photo-degradation and biodegradation rates of these neonicotinoids in water, further resulting in neonicotinoids remaining in the sampling sites. A lower photo-degradation may occur because of increased photon absorption competition among neonicotinoid molecules in water and lead to less light energy absorbed per unit molecule (Liang et al., 2019), while higher initial concentrations may result in toxic effects on the microbial cells and disrupt the enzymatic degradation process of the pollutants by degrading microbes (Phugare et al., 2013).
3.2. Contribution of neonicotinoid compounds used by farmers in surrounding sub-districts
To obtain a better understanding of the possible impact of neonicotinoids used by farmers in related sub-districts in Indramayu Regency on the neonicotinoid concentrations detected in water samples, seven watersheds, covering selected estuaries, were identified (Figure 2) using ArcMap 10.8 in combination with field surveys and communication with the local government officials from the Agriculture Office of Indramayu Regency and the Centre for the Protection of Food Crops and Horticulture. These watersheds consist of complex river and creek structures that are connected to each other mainly through artificial irrigation systems, according to field observations (Figure 2). Each watershed also covers some sub-districts (Table 3) where farmers performed paddy cultivation at slightly different times with different insecticide products and applications, according to the information from the local government officials.
Table 3.
Contribution of sub-districts to the sampling locations within the watersheds in Indramayu Regency.
| Sampling location | Contributing sub-district |
|---|---|
| E1 | Karangampel, Krangkeng, Kertasemaya, Juntinyuat, Kedokanbunder |
| E2 | Juntinyuat, Sliyeg, Kertasemaya, Kedokanbunder |
| E3 | Balongan, Indramayu, Sliyeg, Kertasemaya, Jatibarang, Juntinyuat |
| E4 | Indramayu, Pasekan, Sindang |
| E5 | Cantigi, Sindang, Arahan, Lohbener, Pasekan |
| E6 | Kandanghaur, Losarang, Kroya, Cikedung, Gabuswetan, Terisi |
| E7 | Kandanghaur, Gabuswetan, Kroya, Terisi, Gantar, Bongas |
| E8 | Patrol, Anjatan, Bongas |
Among all sampling locations, the water sample collected from Patrol (E8) in August 2021 contained the highest total neonicotinoid concentrations (Figure 1), followed by a sample from Karangampel (E1). E8 is surrounded by Patrol, Anjatan, and Bongas sub-districts (Table 3), where paddy cultivation was still ongoing during the sampling time; thus, insecticide products containing neonicotinoids were still being used by the farmers. A similar situation also occurred in E1; however, the growing season had ended in two of the five sub-districts around it; thus, the neonicotinoid concentrations detected in this site were lower than those in E8. Paddy fields in E8 and E1 were also adjacent to the coastal areas and located very close to the sampling locations compared to the other locations, meaning that a high quantity of pollutants likely reached the sampling locations due to the short distance from the paddy fields to the estuaries. At sampling locations other than E1 and E8, the rice cultivation period in the surrounding sub-districts had ended when the water samples were collected; hence, there was no use of neonicotinoids by farmers during this time and the neonicotinoids present in the sampling locations were only from the previous cultivation periods.
For the samples from November 2020, the highest total concentrations were in a sample from Singaraja (E3), followed by a sample from Lamarantarung (E5) and E8 (Figure 1). In general, farmers in most sub-districts did not cultivate any crops during this month due to water shortages. Nevertheless, farmers in two sub-districts, namely Balongan and Kertasemaya, which are near E3 (Figure 2), performed farming activities for crops other than rice (shallot, long beans, and cucumber) during the water sample collection and used neonicotinoid insecticides. In the case of E5, farmers in two sub-districts (Pasekan and Sindang, Table 3) surrounding the sampling location had started paddy cultivation, meaning that they had been using insecticides. Farmers in the Patrol sub-district, in which E8 was located, were also carrying out rice cultivation during the sampling period.
Figure 1 also indicates that imidacloprid and thiamethoxam were found in all locations due to their wide use by the farmers in the regency, while dinotefuran and thiacloprid were present in the water samples from E1, E6 (Cemara), and E8 only. Dinotefuran was used in three nearby sub-districts, namely Juntinyuat, Kedokanbunder, and Haurgeulis, while thiacloprid was applied in three sub-districts, namely Sukagumiwang, Kandanghaur, and Haurgeulis, according to the information from the local government officials. Juntinyuat, Kedokanbunder, and Sukagumiwang are neighbouring areas of E1, Kandanghaur is adjacent to E6 and E8, and Haurgeulis is on the southern side of E8 (Figure 2). Given the complex structure of rivers and creeks in the regency (Figure 2), it is possible that the pollutants reached the water sampling locations even if the rice fields were not within the related watershed because all watersheds in the regency may be connected through artificial irrigation canal systems. Furthermore, dinotefuran has stable concentrations against water hydrolysis and thiacloprid has the highest resistance against water photolysis (DT50 in 10–63 d) compared to other compounds (Morrissey et al., 2015). This river and creek structure might also be responsible for the existence of clothianidin in E5, in which the related river also passes Indramayu sub-district (Figure 2), where farmers used this neonicotinoid compound. This is also supported by the low hydrolysis rate of clothianidin (DT50 in 1200–5300 d) (Todey et al., 2018) and the low sorption capacity for this compound by soils (Pietrzak et al., 2020). Clothianidin is one of the thiamethoxam metabolites (Liu et al., 2018), thus, its detection in E6 might also be a metabolism result of thiamethoxam applied by the farmers in Kandanghaur, Losarang, Cikedung, and Terisi sub-districts.
3.3. Correlation of the size of paddy fields and watersheds in Indramayu Regency with the neonicotinoid concentrations in water samples
The neonicotinoid level in environmental waters was confirmed to be positively associated with the land use surrounding the sampling locations, such as in the USA (Berens et al., 2021), Japan (Hayashi et al., 2021), Romania (Iancu et al., 2019), and Canada (Struger et al., 2017). Diverging from previous studies which focused more on the correlation of land use types and neonicotinoid concentrations in water samples, this study evaluated the possible influence of the size of paddy fields located within selected watersheds to provide details about the impact of paddy fields on the existence of neonicotinoids in water samples, in which the size of paddy fields was expected to be also positively associated with the number of contaminants in waters, with the assumption that the paddy field size may also have a positive correlation with the pesticide quantity used by the farmers. The size was estimated by correlating paddy fields with the planting season in each sub-district located within the watershed using ArcMap 10.8 and with field observation results (Table 4). Contrary to previous studies, the size of paddy fields in this study showed a non-significant negative correlation with the total neonicotinoid concentrations and with the concentrations of imidacloprid and thiamethoxam (most frequently detected) (Table 5). It was assumed that the neonicotinoid concentrations in water samples might have been more influenced by other factors, especially the actual quantity of insecticides containing neonicotinoids used by farmers in each sub-district, which was not included as a variable in this study.
Table 4.
Comparison of the areas of watersheds and cultivated paddy fields that used neonicotinoids in August and November.
| Sampling point | Watersheds (ha) | Paddy fields (ha) |
Land use proportion for paddy fields (%) |
||
|---|---|---|---|---|---|
| August | November | August | November | ||
| E1 | 4478.71 | 581.79 | 78.30 | 12.99 | 1.75 |
| E2 | 5827.57 | 4270.24 | 27.59 | 73.28 | 0.47 |
| E3 | 8752.15 | 5816.06 | 139.66 | 66.45 | 1.60 |
| E4 & E5∗ | 39433.36 | 13456.57 | 5557.43 | 34.12 | 14.09 |
| E6 | 20837.93 | 2706.86 | 867.27 | 12.99 | 4.16 |
| E7 | 32261.01 | 15314.21 | 13502.01 | 47.47 | 41.85 |
| E8 | 2285.16 | 2079.89 | 1335.92 | 91.02 | 58.46 |
These two sampling locations are within the same watershed.
Table 5.
Pearson correlation between the neonicotinoid concentrations in water samples and the sizes of paddy fields and watersheds as well as the land use proportion for paddy fields (p = 0.05, 1-tailed).
| Neonicotinoids | Paddy field size | Watershed size | Land use proportion for paddy fields |
|---|---|---|---|
| Total | −0.329 | −0.305 | 0.316 |
| Imidacloprid | −0.443 | −0.441 | 0.102 |
| Thiamethoxam | −0.269 | −0.356 | 0.399 |
Another factor that may contribute to the presence of neonicotinoids in environmental waters is the size of related watersheds (Berens et al., 2021), where larger watersheds would result in lower quantities of neonicotinoids in the respective waters due to a higher dilution rate of neonicotinoids that may occur in a wider catchment area (Pietrzak et al., 2020). This study showed similar results, where the neonicotinoid concentrations were negatively correlated with the watershed size (Table 5), meaning that the larger the watershed, the lower the neonicotinoid concentrations would be. However, the correlation was not significant (p > 0.05), presumably because the watershed areas were also within surrounding regencies and cities, which means that neonicotinoids in water samples might have also been influenced by agricultural activities in those areas, and that the overall watershed size covering all areas (which was not measured in this study) might also have had an effect on the neonicotinoid concentrations.
In this study, the correlation between neonicotinoid concentrations and the land use proportion for paddy fields within the related watershed was also examined. The result showed a positive correlation (Table 5), meaning that higher land use proportion for paddy fields in the watershed would produce higher concentration. Since the paddy field size would not exceed the watershed size in where it was located, thus, higher proportion meant that the paddy field size was close to the watershed size, leading to a lower dilution rate of neonicotinoids in water bodies due to more neonicotinoids that might be leached into the waters and causing higher concentrations in the waters. Regarding the correlation results presented in this study, it was also important to note that more reliable correlation may be generated when the same quantities of neonicotinoids are applied by the farmers in the paddy fields in each watershed, which was not considered in this study.
3.4. Potential toxicity of neonicotinoids in water samples
In this study, the neonicotinoid concentrations were compared to the water quality thresholds of neonicotinoids for aquatic life safety that are implemented in other countries since currently Indonesia has not determined the thresholds (Table 6). The concentrations in all samples did not surpass the acute toxicity benchmarks implemented in the mentioned countries; however, about 31.25% of total samples (November 2020: E1, 15.21 ng/L; E3, 11.54 ng/L; and E8, 14.28 ng/L; August 2021: E1, 30.10 ng/L; and E8, 35.34 ng/L) contained imidacloprid above the chronic threshold regulated by the Netherlands. Previous studies reported that the chronic effects of neonicotinoids potentially cause a negative influence on the health of aquatic life, especially to aquatic insects, zooplankton, and crustaceans, such as feeding inhibition, impaired movement, reduced fecundity, reduced body size, immune suppression, and even mortality (Gibbons et al., 2015; Sánchez-Bayo et al., 2016).
Table 6.
Number of samples exceeding water quality benchmarks.
| Neonicotinoid | Guideline source | Level∗∗ | Benchmark∗ (ng/L) | Reference | Total sample |
|---|---|---|---|---|---|
| Imidacloprid | The Netherlands | Acute | 200 | Morrissey et al., (2015) | 0/16 |
| Chronic | 8.3 | Morrissey et al., (2015) | 5/16 | ||
| Canada | Chronic (freshwater) | 230 | CCME 2007 | 0/16 | |
| Chronic (marine water) | 650 | CCME 2007 | 0/16 | ||
| USA | Acute | 385 | USEPA, 2017 | 0/16 | |
| Chronic | 10 | USEPA, 2017 | 0/16 | ||
| Japan | n/a | 1900 | Hano et al., (2019) | 0/16 | |
| Thiamethoxam | Sweden | n/a | 200 | Kreuger et al., (2010) | 0/16 |
| USA | Acute | 17500 | USEPA, 2017 | 0/16 | |
| Chronic | 740 | USEPA, 2017 | 0/16 | ||
| Japan | n/a | 3500 | Hano et al., (2019) | 0/16 | |
| Dinotefuran | USA | Acute | >484150000 | USEPA, 2017 | 0/16 |
| Chronic | >95300000 | USEPA, 2017 | 0/16 | ||
| Japan | n/a | 12000 | Hano et al., (2019) | 0/16 | |
| Thiacloprid | The Netherlands | n/a | 25 | Kreuger et al., (2010) | 0/16 |
| USA | Acute | 18900 | USEPA, 2017 | 0/16 | |
| Chronic | 970 | USEPA, 2017 | 0/16 | ||
| Clothianidin | USA | Acute | 11000 | USEPA, 2017 | 0/16 |
| Chronic | 2100 | USEPA, 2017 | 0/16 | ||
| Japan | n/a | 2800 | Hano et al., (2019) | 0/16 |
Thresholds were applied to freshwater in USA and Japan, freshwater and marine water in Canada, and no information for The Netherlands and Sweden.
n/a indicates no information available.
The presence of neonicotinoids exceeding the regulated benchmark may pose a risk to estuarine ecosystems because affected aquatic organisms potentially play important roles in food chains, organic debris decomposition, and nutrient cycling in the respective ecosystem (Sánchez-Bayo et al., 2016). For example, mayfly nymphs, which are affected by these insecticides, are a food source for a wide range of predators, including fish, crayfishes, amphibians, water beetles, leeches, and dragonflies, and adult mayflies are preyed on by birds and winged insects (Thorp and Rogers, 2014); thus, its decreasing abundance will disrupt the food chain and reduce the population of higher trophic consumers. The mangrove forests in estuaries in Indramayu are inhabited by 97 bird species, including 14 protected rare species and 11 migratory species (Iqbal, 2020), which may also be negatively affected by pollutants indirectly, through the food chain. In addition, the coastal areas of Indramayu Regency are mainly used by the local people as a place for Pacific white shrimp (Litopenaeus vannamei) and black tiger shrimp (Penaeus monodon) farming, which are the largest marine commodities for exports in Indonesia, and Indramayu is one of the largest shrimp producers in this country (KKP, 2021). Thus, the presence of neonicotinoids in estuarine and mangrove areas may become a potential threat to the productivity of shrimps in the future because the latter have similar nervous systems to insects due to their belonging to the same phylum, i.e., Arthropoda (Butcherine et al., 2021). Mud crabs (Scylla serrata), mangrove crustaceans with high economic value, may be similarly impacted. Therefore, it is suggested that preventative actions be taken to prevent potential harmful effects of neonicotinoids on aquatic environments that may occur in the future, considering that Indonesia relies on agriculture but has no regulations on neonicotinoid pollution control in environmental waters.
4. Conclusions
Neonicotinoids were detected in 12 out of 16 samples (75%) from eight estuaries in Indramayu Regency, Indonesia. The highest total concentration was observed in a sample collected in August 2021 from an estuary located in the Patrol sub-district (E8) (140.26 ng/L). Five samples (31.25%) contained imidacloprid above the chronic threshold regulated by the Netherlands. Paddy field and watershed sizes as well as the percentage of land use for paddy fields within the watershed were not significantly correlated with the total concentrations of neonicotinoid, imidacloprid, and thiamethoxam. Given the results of this study, it is suggested to conduct similar study in other environmental waters of Indonesia and in monitoring basis to obtain more complete view on neonicotinoid pollution in Indonesian aquatic environment. Related regulation and policies are also encouraged to be established in Indonesia to prevent potential harmful effect of neonicotinoids to the aquatic environment in this country.
Declarations
Author contribution
Zanne Sandriati Putri: Conceived and designed the experiments; Performed the experiment and field observation; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools and data; Wrote the paper.
Aslan: Performed the field observation; Analyzed and interpreted the data; Contributed analysis tools and data; Funding acquisition.
Armaiki Yusmur: Performed the field observation; Analyzed and interpreted the data; Contributed analysis tools and data.
Masumi Yamamuro: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools and data; Wrote the paper.
Funding statement
This work was supported by Southeast Asian Regional Centre for Tropical Biology [051.1/PSRP/SC/SPK-PNLT/III/2021].
Data availability
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Ms. Toshiko Sato for her assistance during water sample analyses and Ms. Rizqi Nadia Putri for her assistance during water sampling. Cooperation of the Agriculture Office, the Center for the Protection of Food Crops and Horticulture, and the Environment Office of Indramayu Regency is also greatly appreciated.
References
- Anderson J.C., Dubetz C., Palace V.P. Neonicotinoids in the Canadian aquatic environment: a literature review on current use products with a focus on fate, exposure, and biological effects. Sci. Total Environ. 2015;505:409–422. doi: 10.1016/j.scitotenv.2014.09.090. [DOI] [PubMed] [Google Scholar]
- Berens M.J., Capel P.D., Arnold W.A. Neonicotinoid insecticides in surface water, groundwater, and wastewater across land-use gradients and potential effects. Environ. Toxicol. Chem. 2021;40(4):1017–1033. doi: 10.1002/etc.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonmatin J.-M., Noome D.A., Moreno H., Mitchell E.A.D., Glauser G., Soumana O.S., van Lexmond M.B., Sánchez-Bayo F. A survey and risk assessment of neonicotinoids in water, soil and sediments of Belize. Environ. Pollut. 2019;249:949–958. doi: 10.1016/j.envpol.2019.03.099. [DOI] [PubMed] [Google Scholar]
- Bonmatin J.-M., Mitchell E.A.D., Glauser G., Lumawig-Heitzman E., Claveria F., van Lexmond M.B., Taira K., Sánchez-Bayo F. Residues of neonicotinoids in soil, water and people’s hair: a case study from three agricultural regions of the Philippines. Sci. Total Environ. 2021;757(2021) doi: 10.1016/j.scitotenv.2020.143822. [DOI] [PubMed] [Google Scholar]
- BPS (Badan Pusat Statistik) Kabupaten Indramayu . BPS Kabupaten Indramayu; Indramayu, ID: 2021. Indramayu Regency in Figures 2021. (in Indonesian) [Google Scholar]
- Buszewski B., Bukowska M., Ligor M., Staneczko-Baranowska I. A holistic study of neonicotinoids neuroactive insecticides – properties, applications, occurrence, and analysis. Environ. Sci. Pollut. Res. Int. 2019;26:34723–34740. doi: 10.1007/s11356-019-06114-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcherine P., Kelaher B.P., Taylor M.D., Lawson C., Benkendorff K. Acute toxicity, accumulation and sublethal effects of four neonicotinoids on juvenile Black Tiger shrimp (Penaeus monodon) Chemosphere. 2021;275 doi: 10.1016/j.chemosphere.2021.129918. [DOI] [PubMed] [Google Scholar]
- CBD (Convention on Biological Diversity) Secretariat . 2022. Indonesia – Main details, biodiversity facts.https://www.cbd.int/countries/profile/?country=id [Google Scholar]
- CCME (Canadian Council of Ministers of the Environment) 2007. Canadian water quality guidelines: imidacloprid.https://ccme.ca/en/results/121/ch/1,2,3,4,5,6 [Google Scholar]
- FAO (Food and Agriculture Organization) 2021. FAOSTAT. Crops.http://www.fao.org/faostat/en/#data/QC/visualize [Google Scholar]
- FAO (Food and Agriculture Organization) Indonesia. 2022. https://www.fao.org/3/y4632e/y4632e0l.htm#TopOfPage accessed.
- Gibbons D., Morrissey C., Mineau P. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. Int. 2015;22:103–118. doi: 10.1007/s11356-014-3180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey M., Tapie N., Menach K.L., Dévier M.-H., Budzinski H., Bebianno M.J. Occurrence of pharmaceutical compounds and pesticides in aquatic systems. Mar. Pollut. Bull. 2015;96:384–400. doi: 10.1016/j.marpolbul.2015.04.029. [DOI] [PubMed] [Google Scholar]
- Hano T., Ito K., Ohkubo N., Sakaji H., Watanabe A., Takashima K., Sato T., Sugaya T., Matsuki K., Onduka T., Ito M., Somiya R., Mochida K. Occurrence of neonicotinoids and fipronil in estuaries and their potential risks to aquatic invertebrates. Environ. Pollut. 2019;252:205–215. doi: 10.1016/j.envpol.2019.05.067. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Sasaki N., Takazawa M., Inagaki T., Nakamura H., Yamamoto A., Suzuki S. Contamination levels, monthly variations, and predictions of neonicotinoid pesticides in surface waters of Gifu Prefecture in Japan. EMCR. 2021;1:17–27. [Google Scholar]
- Hladik M.L., Kolpin D.W. First national-scale reconnaissance of neonicotinoid insecticides in streams across the USA. Environ. Chem. 2015;13(1):12–20. [Google Scholar]
- Iancu V.-I., Petre J., Galaon T., Radu G.L. Occurrence of neonicotinoid residues in Danube River and tributaries. Rev. Chim. 2019;70(1):313–318. [Google Scholar]
- Iqbal D. 2020. Density of the Karangsong mangroves is back amid the Java North Coast crisis.https://www.mongabay.co.id/2020/03/28/kembali-lebatnya-mangrove-karangsong-ditengah-ancaman-krisis-pesisir-utara-jawa/ (in Indonesian) [Google Scholar]
- Kementan (Kementerian Pertanian) 2020. Pesticide information system.http://pestisida.id/simpes_app/ (in Indonesian) [Google Scholar]
- KKP (Kementerian Kelautan dan Perikanan) KKP; Jakarta, ID: 2021. Ministry of Maritime Affairs and Fisheries performance report 2020. (in Indonesian) [Google Scholar]
- Kreuger J., Graff S., Patring J., Adielsson S. Swedish University of Agricultural Sciences; Uppsala, SE: 2010. Pesticides in Surface Water in Areas with Open Ground and Greenhouse Horticultural Crops in Sweden 2008. [Google Scholar]
- Lamers M., Anyusheva M., La N., Nguyen V.V., Streck T. Pesticide pollution in surface- and groundwater by paddy rice cultivation: a case study from Northern Vietnam. CLEAN. 2011;39(4):356–361. [Google Scholar]
- Li Y., Miao R., Khanna M. Neonicotinoids and decline in bird biodiversity in the United States. Nat. Sustain. 2020;3:1027–1035. [Google Scholar]
- Liang R., Tang F., Wang J., Yue Y. Photo-degradation dynamics of five neonicotinoids: bamboo vinegar as a synergistic agent for improved functional duration. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0223708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Pan X., Yang Q., Ji M., Zhang Z. The dissipation of thiamethoxam and its main metabolite clothianidin during strawberry growth and jam-making process. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-33334-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Challis J.K., Wong C.S. Quantum yields for direct photolysis of neonicotinoid insecticides in water: implications for exposure to nontarget aquatic organisms. Environ. Sci. Technol. Lett. 2015;2:188–192. [Google Scholar]
- Main A.R., Headley J.V., Peru K.M., Michel N.L., Cessna A.J., Morrissey C.A. Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada’s Prairie Pothole region. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0092821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey C.A., Mineau P., Devries J.H., Sanchez-Bayo F., Liess M., Cavallaro M.C., Liber K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ. Int. 2015;74:291–303. doi: 10.1016/j.envint.2014.10.024. [DOI] [PubMed] [Google Scholar]
- Naumann T., Bento C.P.M., Wittmann A., Gandrass J., Tang J., Zhen X., Liu L., Ebinghaus R. Occurrence and ecological risk assessment of neonicotinoids and related insecticides in the Bohai Sea and its surrounding rivers, China. Water Res. 2022;209 doi: 10.1016/j.watres.2021.117912. [DOI] [PubMed] [Google Scholar]
- Phugare S.S., Kalyani D.C., Gaikwad Y.B., Jadhav J.P. Microbial degradation of imidacloprid and toxicological analysis of its biodegradation metabolites in silkworm (Bombyx mori) Chem. Eng. J. 2013;230:27–35. [Google Scholar]
- Pietrzak D., Kania J., Kmiecik E., Malina G., Wątor K. Fate of selected neonicotinoid insecticides in soil-water systems: current state of the art and knowledge gaps. Chemosphere. 2020;255 doi: 10.1016/j.chemosphere.2020.126981. [DOI] [PubMed] [Google Scholar]
- Postigo C., Ginebreda A., Barbieri M.V., Barceló D., Martín-Alonso J., de la Cal A., Boleda M.R., Otero N., Carrey R., Solà V., Queralt E., Isla E., Casanovas A., Frances G., López de Alda M. Investigative monitoring of pesticide and nitrogen pollution sources in a complex multi-stressed catchment: the lower Llobregat River basin case study (Barcelona, Spain) Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142377. [DOI] [PubMed] [Google Scholar]
- Sánchez-Bayo F., Hyne R.V. Detection and analysis of neonicotinoids in river waters – development of a passive sampler for three commonly used insecticides. Chemosphere. 2014;99:143–151. doi: 10.1016/j.chemosphere.2013.10.051. [DOI] [PubMed] [Google Scholar]
- Sánchez-Bayo F., Goka K., Hayasaka D. Contamination of the aquatic environment with neonicotinoids and its implication for ecosystems. Front. Environ. Sci. 2016;4:71. [Google Scholar]
- Schaafsma A.W., Limay-Rios V., Baute T.S., Smith J.L. Neonicotinoid insecticide residues in subsurface drainage and open ditch water around maize fields in southwestern Ontario. PLoS One. 2019;14(4) doi: 10.1371/journal.pone.0214787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa J.C.G., Barbosa M.O., Ribeiro R.L.A.R.L., Ratola N., Pereira M.F.R., Silva A.M.T. Distribution of micropollutants in estuarine and sea water along the Portuguese coast. Mar. Pollut. Bull. 2020;154 doi: 10.1016/j.marpolbul.2020.111120. [DOI] [PubMed] [Google Scholar]
- Struger J., Grabuski J., Cagampan S., Sverko E., McGoldrick D., Marvin C.H. Factors influencing the occurrence and distribution of neonicotinoid insecticides in surface waters of southern Ontario, Canada. Chemosphere. 2017;169:516–523. doi: 10.1016/j.chemosphere.2016.11.036. [DOI] [PubMed] [Google Scholar]
- Surahmat E.C., DadangPrijono D. Susceptibility of the brown plant hopper (Nilaparvata lugens) from six locations in Java Island to three types of insecticides. J. HPT Tropika. 2016;16(1):71–81. (in Indonesian) [Google Scholar]
- Thorp J.H., Rogers D.C. Academic Press; London, UK: 2014. Thorp and Covich’s Freshwater Invertebrates: Ecology and General Biology. [Google Scholar]
- Todey S.A., Fallon A.M., Arnold W.A. Neonicotinoid insecticide hydrolysis and photolysis: rates and residual toxicity. Environ. Toxicol. Chem. 2018;37(11):2797–2809. doi: 10.1002/etc.4256. [DOI] [PubMed] [Google Scholar]
- USEPA (United States Environmental Protection Agency) 2017. Aquatic life benchmarks and ecological risk assessments for registered pesticides.https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/aquatic-life-benchmarks-and-ecological-risk [Google Scholar]
- Van Dijk T.C., Van Staalduinen M.A., Van der Sluijs J.P. Macro-invertebrate decline in surface water polluted with imidacloprid. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Tran T.M., Nguyen V.T., Wang A., Wang J., Kannan K. Neonicotinoids, fipronil, chlorpyrifos, carbendazim, chlorotriazines, chlorophenoxy herbicides, bentazon, and selected pesticide transformation products in surface water and drinking water from northern Vietnam. Sci. Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.141507. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Terao T., Hisatomi H., Kawasaki H., Arakawa R. Evaluation of river pollution of neonicotinoids in Osaka City (Japan) by LC/MS with dopant-assisted photoionisation. J. Environ. Monit. 2012;14:2189–2194. doi: 10.1039/c2em30296a. [DOI] [PubMed] [Google Scholar]
- Yamamuro M., Komuro T., Kamiya H., Kato T., Hasegawa H., Kameda Y. Neonicotinoids disrupt aquatic food webs and decrease fishery yields. Science. 2019;366:620–623. doi: 10.1126/science.aax3442. [DOI] [PubMed] [Google Scholar]
- Zhang C., Tian D., Yi X.H., Zhang T., Ruan J., Wu R., Chen C., Huang M., Ying G.G. Occurrence, distribution and seasonal variation of five neonicotinoid insecticides in surface water and sediment of the Pearl Rivers, South China. Chemosphere. 2019;217:437–446. doi: 10.1016/j.chemosphere.2018.11.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.