Abstract
Objectives:
We sought to determine the safety and efficacy of the oral androgen receptor antagonist enzalutamide in patients with previously treated, recurrent, AR-positive (AR+) ovarian cancer.
Methods:
This was a single-institution phase II study of patients with AR+ ovarian cancer with measurable disease with 1–3 prior lines of chemotherapy; patients were screened for enrollment from 11/2013–7/2018. Following consent, archival tissue was evaluated for AR+. Enrolled patients received daily enzalutamide 160 mg until progression of disease or treatment discontinuation. Adverse events were graded by CTCAE v4.0. Co-primary endpoints were 6-month progression-free survival (PFS6) and overall response rate (ORR) by RECIST 1.1 criteria.
Results:
During the study period, 160 patients were screened and 59 (45 high-grade serous [HGS] and 14 low-grade serous [LGS]) consented to treatment on study. There was 1 confirmed and 1 unconfirmed partial response. The ORR was 1.7% (90% CI: 0.2–100%). The overall PFS6 rate (as binary) was 22% (90% CI: 15.1–100%). The 6-month PFS rate (as time to event) was 19.8% for HGS patients (90% CI: 12.7–100%) and 38.5% (90% CI: 21.7%−100%) for LGS patients. Grade 3 toxicities occurred in 6 patients (one toxicity (Grade 3 rash) was considered a dose-limiting toxicity). One patient died of cardiac arrest after 42 days on treatment of a cardiac arrest not attributed to study drug.
Conclusions:
The study met its primary endpoint, with a PFS6 rate of 22% (n=13); however, the overall response rate was low. Enzalutamide was well tolerated and may be a potential treatment option in select patients.
Keywords: Ovarian cancer, Serous ovarian cancer, Recurrent ovarian cancer, Enzalutamide, Androgen receptor expression
INTRODUCTION
Epithelial carcinoma of the ovary accounts for approximately 90% of ovarian, tubal, and peritoneal cancers, and up to 80% of advanced-stage patients ultimately recur (1, 2). Despite high rates of recurrence, 25–32% of patients with advanced-stage disease will survive 10 years or longer (3, 4). With each recurrence, however, treatment strategies shift, and patients can require multiple lines of therapy as chemoresistance progresses. Therefore, the identification of active targeted agents with good tolerability is important.
Forty-four percent to 90% of epithelial ovarian carcinomas are androgen receptor positive (AR+), representing a potential targetable pathway (5–8). Preclinical data have demonstrated that AR+ ovarian cancer cells show increased cell division when exposed to androgens and that this activity is reversed with androgen inhibition (9). It has been hypothesized that AR+ ovarian tumors may preferentially respond to AR antagonists. A prior phase II study by our group investigated the efficacy of dual anti-androgen and gonadotropin-releasing hormone analog therapy with bicalutamide and goserelin in patients with epithelial ovarian cancer, and found no survival benefit (10). Of note, the study was performed with a less potent, first-generation AR antagonist in an unselected patient population, of whom only 58% were AR+ (11).
Enzalutamide is an orally available, potent, and selective small-molecule second-generation AR antagonist that slows growth and induces cell death in AR-expressing tumor cells. Unlike first-generation AR antagonists such as bicalutamide, enzalutamide works through three mechanisms: by blocking testosterone binding, impeding nuclear translocation of the AR, and inhibiting binding of the AR to DNA in the nucleus (11). Preclinical data have demonstrated that enzalutamide is superior to bicalutamide in both cell line and mouse xenograft models, and it has demonstrated efficacy for androgen blockade in prostate cancer (11, 12). The drug also has a compelling safety and tolerability profile, with only 2–5% of patients in large clinical trials discontinuing enzalutamide secondary to adverse events (13, 14). We sought to determine the safety and efficacy of enzalutamide in patients with previously treated, recurrent, AR+ ovarian cancer.
METHODS
This single-institution, phase II study with safety lead-in was designed to evaluate the activity and safety of enzalutamide 160 mg oral daily treatment in patients with recurrent AR+ epithelial ovarian cancer, with measurable disease, who had undergone 1–3 prior lines of chemotherapy. The primary endpoints were to estimate the proportion of women surviving progression-free for at least 6 months (PFS6) and the proportion of patients who experienced an objective tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria. Secondary endpoints included determining the frequency and severity of adverse events in patients treated with enzalutamide, as assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.0. The study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board, and all patients enrolled in the study provided written informed consent to participate.
Patients
Patients were eligible for enrollment if they were ≥18 years of age, had a histologically confirmed diagnosis of AR+ epithelial ovarian, fallopian tube, or primary peritoneal carcinoma, and had undergone 1–3 prior lines of cytotoxic treatment, including ≥1 platinum-based chemotherapy. Eligible patients had measurable recurrent or persistent disease (as defined by RECIST 1.1 criteria) that had progressed (defined as radiologic and/or clinical progression) on or after last therapy and was not amenable to surgery with potentially curative intent. Patients were required to have a Karnofsky Performance Status (KPS) score of ≥70%. Patients were excluded if they had a condition precluding adequate study drug absorption or were unable to tolerate oral medications, had prior use of an AR antagonist or androgen synthesis inhibitor or had participated in a clinical trial evaluating such agents, had known brain metastases or a history of seizure, had a history of uncontrolled hypertension or clinically significant heart disease, or had persistence of Grade 2 or higher toxic effects of prior therapy by NCI CTCAE version 4.0 (excluding Grade 2 neuropathy or Grade 2 alopecia, which were allowed). For a description of full study eligibility criteria, please see supplemental data.
Screening for AR positivity was performed from archival formalin-fixed paraffin-embedded tumor tissue in a CLIA (Clinical Laboratory Improvement Amendments)-approved laboratory with immunohistochemistry (IHC) using the Ventana androgen antibody (Roche); AR expression ≥5% was required for study entry (Figures 1 and 2). If no archival tissue was available, patients were required to undergo a tumor biopsy for study enrollment consideration. In cases where multiple samples were available, 3 IHCs were performed. If the patient had ≥1 slide with ≥5% AR tumor staining, she was considered eligible for enrollment.
Figure 1:
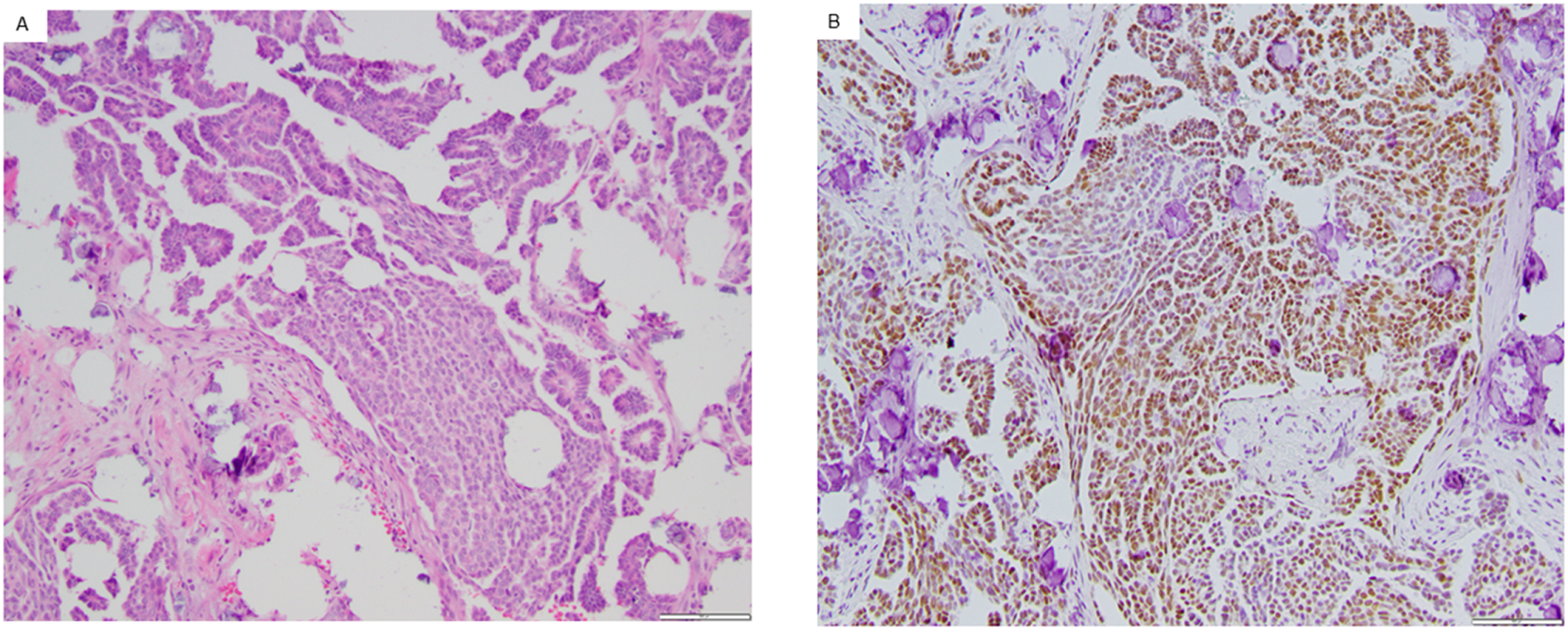
Representative example of an Androgen Receptor positive low-grade serous ovarian carcinoma. Androgen Receptor (AR) expression was assessed in archival (fresh frozen paraffin embedded) or fresh tissue. Pictured is the hematoxylin and eosin stain of a low-grade serous carcinoma (A) as well as AR+ immunohistochemistry (B). Both images obtained with magnification of 100x.
Figure 2:
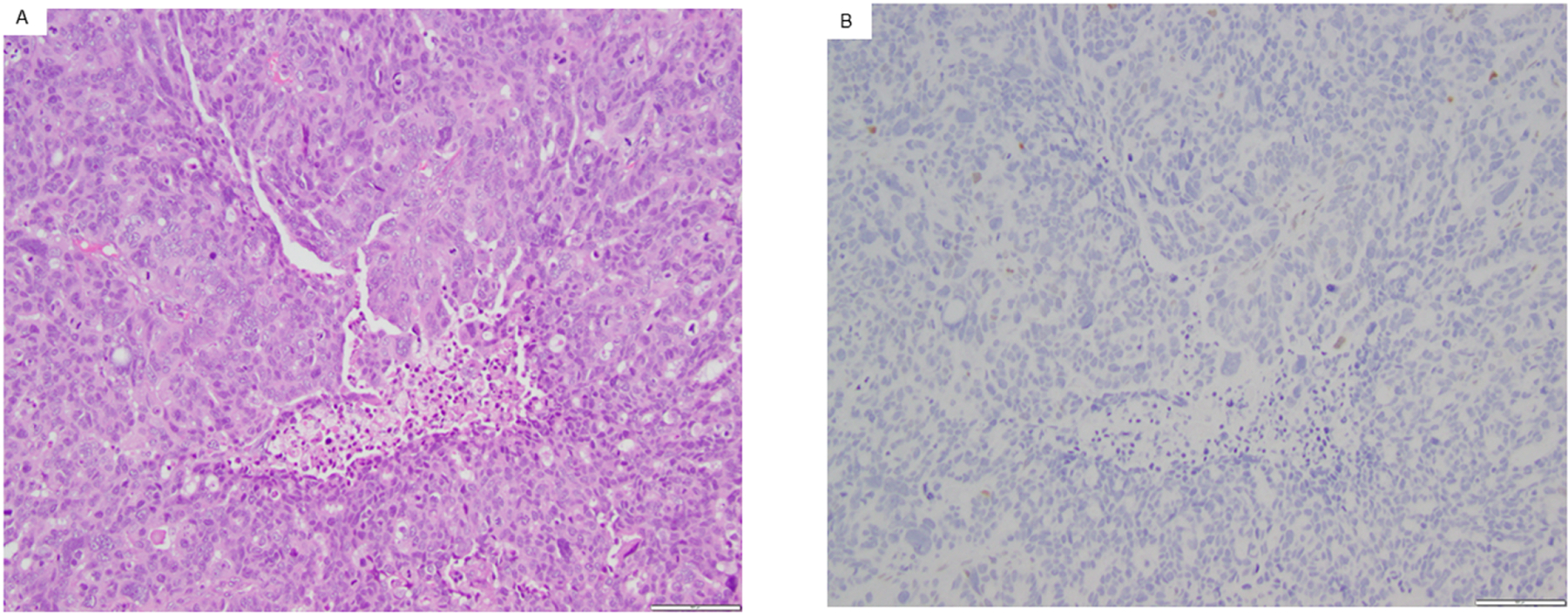
Representative example of an Androgen Receptor negative high-grade serous ovarian carcinoma. Androgen Receptor (AR) expression was assessed in archival (fresh frozen paraffin embedded) or fresh tissue. Pictured is the hematoxylin and eosin stain of a high-grade serous carcinoma (A) as well as AR-immunohistochemistry (B). Both images obtained with magnification of 100x.
Study design
This was a single-arm, open-label, phase II, single-institution study. All patients were enrolled and treated at Memorial Sloan Kettering Cancer Center. To reduce patient risk, a safety lead-in phase was designed and included 15 patients. By applying Bayesian methodology from Gonen et al, a detailed probability table of dose-limiting toxicity (DLT) was provided (details in the protocol; see supplemental data) (15). The probability of a DLT exceeding 0.3 was 66% with 5 DLTs in the safety lead-in cohort. This was chosen as the most appropriate threshold for putting the trial on hold.
After the safety lead-in phase, a two-stage, phase II trial was designed to evaluate the efficacy of enzalutamide by employing a previously reported method by Sill and Yothers (16). PFS6 and overall response rate (ORR) were the co-primary endpoints. We hypothesized that treatment with enzalutamide would result in a PFS6 rate increase from 15% to 30% or an ORR increase from 5% to 20%. The treatment would be considered of clinical interest and worthy of further investigation if either endpoint was met. The first stage would accrue 28 eligible patients. The second stage, which would enroll an additional 31 patients, would open if ≥3 patients experienced a clinical response and/or ≥5 patients did not progress/die at 6 months. The study would be considered positive if there were ≥7 clinical responses and/or ≥13 progression-free survivors among 59 patients. The power of the design was ≥0.9 (ORR and PFS6) with a type 1 error range 0.09–0.1 (detail in the protocol; see supplemental data). The patients in the safety lead-in phase would be included in the first stage.
Patients who died from any cause or were lost to follow-up (including patients who discontinued treatment due to toxicity, or who withdrew consent) within 6 months were considered failures for the PFS6 binary outcome. ORR and PFS6 were reported assuming binomial proportions, with exact binomial confidence intervals (CIs). Overall response was defined by RECIST 1.1 criteria. PFS was also analyzed with time-to-event methodology using treatment start date as time zero, and progression was identified by clinical progression as well as progression of disease according to RECIST 1.1 criteria. Median PFS and PFS rate were summarized using the Kaplan-Meier method. Reported CIs were one-sided 90% CIs. The association of AR expression (on a scale of 0–100%) with ORR and PFS was determined using the two-samples Wilcoxon test (for ORR) and Cox Proportional Hazards model (for PFS).
Study treatment
After determination of eligibility, patients received 160 mg (4 capsules) of enzalutamide by mouth daily and continued with study drug until progression of disease, unacceptable toxicity, or withdrawal from the study. Tumors were assessed every 8 weeks (±1 week) while on treatment, as well as at the end of the study with computed tomography (CT) or magnetic resonance imaging (MRI).
Toxicity assessment
Toxicities were evaluated during physician assessments performed every 2 weeks during the first cycle (28 days) and, thereafter, every 4 weeks during subsequent treatment cycles; toxicities were graded using CTCAE version 4.0 criteria. DLTs were defined as any event consistent with a seizure of any grade, grade ≥3 diarrhea, nausea, or vomiting that did not improve to grade 1 within 14 days of initiating standard of care therapy, grade ≥3 decreased platelet count with associated bleeding, grade ≥3 absolute neutrophil count (ANC) that persisted for 7 or more days or that was associated with fevers (febrile neutropenia), any other grade ≥3 non-hematologic toxicity determined to be related to the study drug. Grade 1 or 2 toxicities were treated with supportive care. Grade ≥3 toxicities considered related to enzalutamide prompted cessation of enzalutamide until the toxicity resolved to grade ≤1. Report of DLTs resulted in the option to withdraw from study or continue study treatment following adequate recovery and dose modification.
RESULTS
Patient characteristics
One hundred sixty-five patients were screened for enrollment between November 2013 and July 2018. Of these 165 patients, 145 had sufficient tissue to test for AR positivity, 87 (60%) were deemed AR+, and 59 consented to treatment.
Baseline demographics and disease characteristics of the study population are presented in Table 1. Median patient age was 64 years (range, 29–87 years). Median body mass index (BMI) was 26 kg/m2 (range, 19.8–56.4 kg/m2). Forty-five patients (76.3%) had high-grade serous (HGS) carcinoma and 14 (23.7%) had low-grade serous (LGS) carcinoma. Most patients had received 2 (54.2%) or 3 (27.1%) prior lines of chemotherapy.
Table 1:
Study population
| Characteristic | N (%) |
|---|---|
| Race | |
| White | 48 (81%) |
| Asian | 5 (9%) |
| Black or African American | 2 (3%) |
| Unknown | 4 (7%) |
| Histology | |
| High-grade serous | 45 (76%) |
| Low-grade serous | 14 (24%) |
| Debulking surgery | |
| Optimal | 53 (90%) |
| Suboptimal | 5 (8%) |
| Unknown | 1 (2%) |
| Prior lines of chemotherapy | |
| 1 line | 11 (19%) |
| 2 lines | 32 (54%) |
| 3 lines | 16 (27%) |
| Prior hormonal therapy | |
| No | 46 (78%) |
| Yes | 13 (22%) |
| Prior radiation therapy | |
| No | 58 (98%) |
| Yes | 1 (2%) |
Primary endpoints and efficacy
The results presented here include an assessment of endpoints (PFS6, ORR) up to the data cut-off date of July 30, 2019. At the time of data cut-off, all patients had discontinued enzalutamide. The most common reasons for discontinuing treatment were disease progression by RECIST (n=50), clinical progression (n=3), adverse events (n=4), withdrawal of consent (n=1), and death (n=1). The median duration of exposure to enzalutamide was 2.1 months (range, 0.4–32.3 months).
After enrollment of the first 28 patients, 7 patients remained progression-free for 6 months, which met criteria for expanding the trial to its second stage for a total enrollment of 59 patients. Among this total cohort, 13 of 59 patients (22.0%; 90% CI: 15.1–100.0%) remained progression-free for ≥6 months. Among the 13 patients with PFS ≥6 months, 5 had 3 prior lines of chemotherapy, 7 had 2 prior lines of chemotherapy, and 1 had 1 prior line of chemotherapy. For the secondary endpoint, considering PFS as time to event outcome, the median PFS was 1.7 months for HGS and 4.6 months for LGS. The PFS rate at 6 months for HGS patients was 19.8% (90%CI: 12.7–100%) and for LGS patients 38.5% (90%CI: 21.7–100%) (Figure 3).
Figure 3:
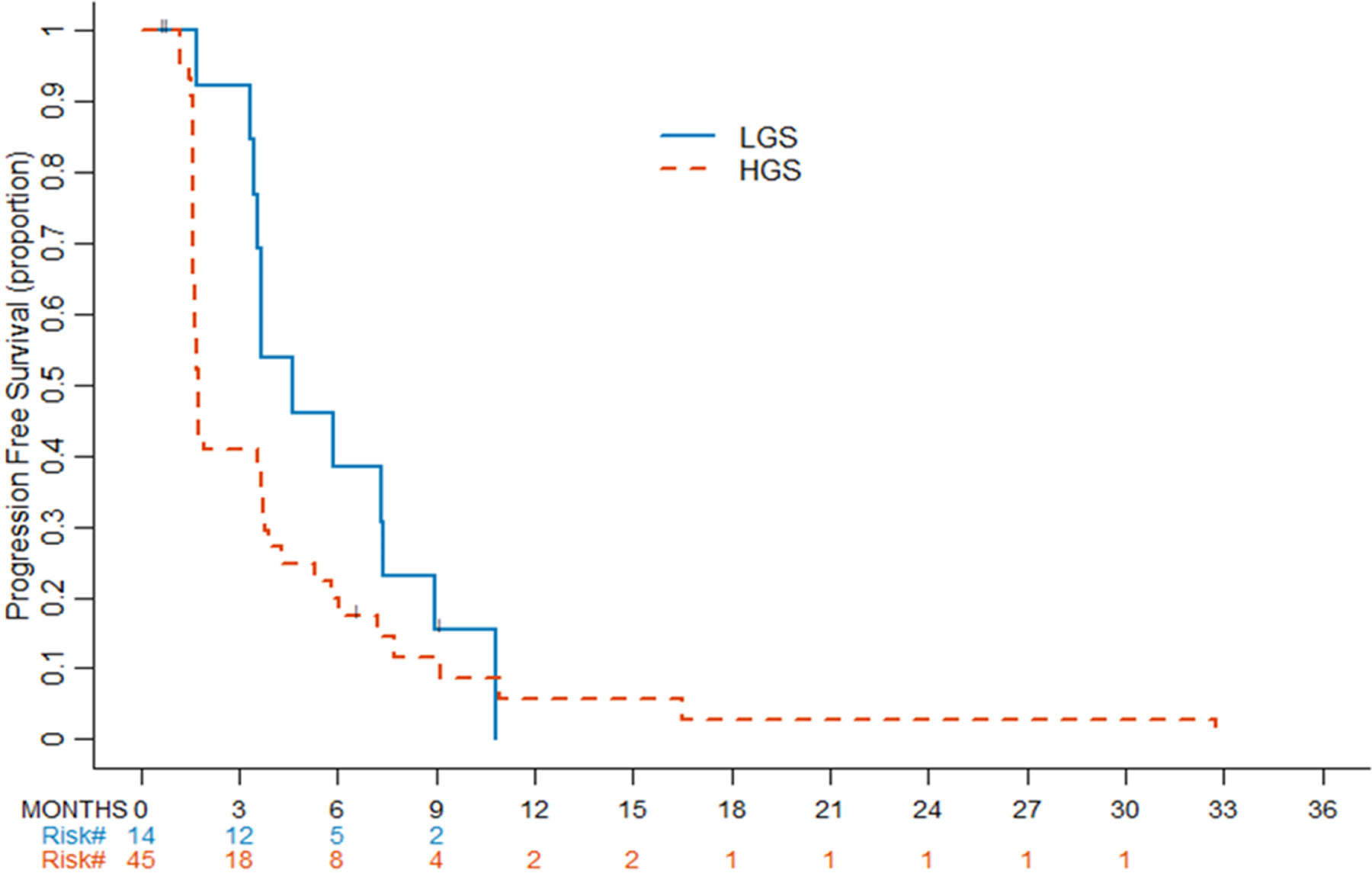
Progression-free survival by histology. Kaplan-Meier curves for progression-free survival by histology. LGS, low-grade serous. HGS, high-grade serous.
Of 59 patients, 1 patient with HGS had a confirmed response by RECIST 1.1, for an ORR of 1.7% (90% CI: 0.2–100%). One other LGS patient had an unconfirmed response, with a partial response by RECIST demonstrated on her initial imaging, followed by clinical progression prior to her second radiographic tumor assessment.
AR positivity and response
Among the study population, median AR expression was 60% (range, 5–99%). There was no significant association between AR expression and PFS (HR, 0.98; 95% CI: 0.92-NR; p=0.674). For the 13 patients with PFS ≥ 6 months the mean level of AR expression was 56% and the median level of AR expression was 70% (range 5–99%). The patient who experienced a partial response by RECIST had 10% AR expression; the patient who experienced an unconfirmed response had 40% AR expression. Level of AR expression by quartile was also examined and median PFS and percentage of patients progression free at 6 months for each quartile reported in Table 2; no difference was found between quartile defined categories by logrank test (p=0.81).
Table 2:
Median PFS and percentage of patients progression free at 6 months by quartile of AR expression.
| % AR Expression | N | Progression/Death | Median PFS (90%CI) | PFS6 (90%CI) | Logrank P value |
|---|---|---|---|---|---|
| <25% | 14 | 12 | 1.7 (1.6-Inf) | 23.1% (5.6–100%) | 0.81 |
| 25–60% | 15 | 14 | 1.9 (1.6-Inf) | 13.3% (2.2–100%) | |
| 60–80% | 13 | 11 | 3.5 (1.6-Inf) | 33.3% (10.3–100%) | |
| >=80% | 17 | 17 | 3.7 (1.6-Inf) | 29.4% (10.7–100%) |
Safety
Grade 3 adverse events were reported in 17 (29%) patients. Events non-attributable to the study drug included 9 episodes of electrolyte disturbances, 7 episodes of lymphopenia, 4 episodes of anemia, 2 episodes of thrombocytopenia, 1 episode of abdominal pain, 1 small bowel obstruction, 1 abdominal infection, 1 colitis, 1 rash, 1 thromboembolic event, 1 episode of hypertension, 1 hip fracture, 1 episode of hearing impairment, and 1 episode of Grade 3 weight loss. In total, 6 (10%) patients experienced Grade 3 toxicities attributed to the study drug, including 2 (3%) with rash, 1 (2%) with fatigue, 1 (2%) with new-onset hypertension, 1 (2%) with anemia, and 1(2%) with transaminase elevation. One of these Grade 3 toxicities, a Grade 3 rash with onset 10–12 days after initiation of enzalutamide, was classified as a dose-limiting toxicity (DLT) that led to study discontinuation. In addition, 2 (3%) patients experienced Grade 4 events non-attributable to the study drug (episodes of neutropenia). One patient died due to a cardiac arrest (Grade 5 event); this was felt to be unrelated to study treatment and occurred after 42 days on treatment. The cardiac arrest occurred before her first scan and the patient was replaced; she was included in PFS6 and ORR analysis.
DISCUSSION
For long-term survivors of epithelial ovarian cancer, many of whom experience multiple recurrences, it is important to have several well-tolerated treatment options that can be leveraged throughout a disease course. This phase II trial investigated the efficacy of enzalutamide, a well-tolerated oral AR antagonist, in recurrent AR+ epithelial ovarian cancer. This study met its primary endpoint, as PFS6 was observed in at least 13 (22%) of 59 patients. More specifically, 19.8% of patients with HGS and 38.5% of patients with LGS met the PFS6 endpoint. Furthermore, enzalutamide was well tolerated in this population; no patients reported a treatment-related grade >3 toxicity and only 6 (10.2%) reported a treatment-associated Grade 3 toxicity. Based on these findings, enzalutamide may be an option that can confer modest PFS benefits, with minimal toxicity, for selected patients with recurrent ovarian cancer.
Enzalutamide has proven to effectively suppress tumor growth in multiple disease sites that also have AR+ phenotypes, such as prostate cancer. In prostate cancer, the AFFIRM randomized, double-blind, placebo-controlled, phase III trial demonstrated a median overall survival (OS) difference of 4.8 months in men with metastatic castration-resistant prostate cancer treated with enzalutamide (13). Subsequently, the PROSPER randomized, double-blind, placebo controlled, phase III trial demonstrated a median OS difference of 10.7 months in men with non-metastatic castration-resistant prostate cancer treated with enzalutamide (21). These trials, among others, led to the FDA approval of enzalutamide in the treatment of castration-resistant prostate cancer (22). In endometrioid endometrial cancer, another AR+ phenotype, emerging data have demonstrated promising efficacy of enzalutamide in combination with chemotherapy. The phase II ENPAC trial investigated ORR and PFS6 in 35 patients with advanced-stage or recurrent endometrioid endometrial cancer (23). Findings demonstrated the safety and promising efficacy of enzalutamide in this setting, with a 71% ORR (95% CI: 54%−85%) and 83% PFS6 rate (95% CI: 66%−92%).
In ovarian cancer, initial trials of AR blockade have reported limited benefit of AR-targeted therapies. Levine et al. investigated dual hormonal blockade with bicalutamide (an oral nonsteroidal anti-androgen) and goserelin (a subcutaneous gonadotropin-releasing hormone analogue), and found that this combination conferred no significant PFS benefit in patients in second or higher remission (10). Similarly, studies on gonadotropin agonists such as letrozole have reported low ORRs (0–15%) and modest clinical benefit rates (up to 26%) in recurrent ovarian cancer (24–26). A phase II trial of anastrozole in platinum-resistant ovarian cancer reported an ORR of 0% and a clinical benefit rate of 27% (27). Studies on flutamide, a non-steroidal drug with anti-androgen properties, failed to demonstrate efficacy in patients with recurrent ovarian cancer (28, 29). More recently, a phase II trial reported the efficacy of abiraterone acetate, a CYP17 inhibitor that targets androgen synthesis, in patients with recurrent epithelial ovarian cancer. This study reported an ORR of 2.4% at 12 weeks, a clinical benefit rate of 26.2% at 12 weeks, and a PFS6 rate of 16.7% (30). Of note, 27.5% of patients on this study had AR-negative disease. and greater than 20% had received 4 or more prior lines of treatment. Our study differed from these trials in three important ways: 1) patients were limited to 3 or fewer prior cytotoxic treatments; 2) all patients had confirmed AR+ disease; 3) we used a second-generation AR antagonist, with greater potency than other agents such as bicalutamide.
Despite these parameters, only 1 of our patients experienced a confirmed partial response (ORR, 1.7%; 90% one-sided CI: 0.2–100%). There are several possible explanations for this low ORR. First, it is possible that targeting the AR pathway may suppress tumor growth but not actively decrease tumor burden. It is also conceivable that tumors readily circumvent the AR signaling pathway, which could potentially explain why there was no observed association between AR expression and PFS. This finding echoes that of other studies on AR blockade in ovarian cancer, which have also demonstrated no correlation between AR expression and outcomes with AR-targeted therapies (10). For example, Banerjee et al. reported no correlation between AR positivity and percent change in sum of target lesions or percent change in CA-125 in patients treated with the AR antagonist abiraterone (30). It is also possible that mechanisms of enzalutamide resistance, such as activation of mutated ARs, may have contributed to the low ORR, as has been demonstrated in patients with prostate cancer treated with abiraterone (31). Future studies may investigate whether dual hormonal blockade improves response rates to enzalutamide, as has been described in breast cancer studies (32).
There are several limitations to this study. Patients with AR+ archival tissue samples were permitted to enroll on trial. It is possible that AR expression had decreased with treatment cycles, and thus, archival tissue may not have been representative of the patient’s current tumor biology (9). Furthermore, although we noted a higher PFS6 rate (38.5%) in patients with LGS cancer, the sample size (n=14) was small, precluding a subset analysis of these patients. It is possible that subsequent investigation of enzalutamide may be most beneficial in low-grade histologies, as these patients can have prolonged disease courses requiring multiple lines of treatment, as well as broad chemoresistance.
In summary, our findings suggest a potential role for enzalutamide, particularly in the AR+ LGS subpopulation. In this scenario, it would be clinically beneficial to have another well-tolerated oral treatment option that can be used in recurrent disease. Further study of enzalutamide, perhaps in combination with other hormonal agents, may be warranted.
Supplementary Material
Highlights.
Enzalutamide was well-tolerated in AR+ recurrent ovarian cancer patients and demonstrated a suitable safety profile.
This study met its primary endpoint, as enzalutamide afforded modest progression-free survival.
The overall response rate to enzalutamide was low, with less than 2% of patients demonstrating radiographic response.
Further study of enzalutamide may be warranted, particularly in patients with AR+ low-grade serous ovarian cancer.
ACKNOWLEDGMENTS
FUNDING
This study was supported in part by the NIH/NCI Cancer Center Support Grant (P30CA008748). This was a single-site investigator-initiated trial, with funding and drug provided by the study sponsor (Pfizer, Astellas). This study was also supported by an American Society of Clinical Oncology Career Development Award (RNG). RNG receives additional funding from The Ovarian Cancer Research Fund Alliance and Cycle for Survival.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
None declared
DISCLOSURES
CAA reports personal fees from Tesaro, personal fees from Immunogen, grants and personal fees from Clovis, grants from Genentech, grants from AbbVie, grants from Astra Zeneca, grants from Astra Zeneca, personal fees from Eisai/Merck, personal fees from Mersana Therapeutics, personal fees from Roche/Genentech, personal fees from Abbvie, personal fees from AstraZeneca/Merck, personal fees from Repare, outside the submitted work. DSC reports personal fees from Bovie Medical Co., personal fees from Verthemia Inc. (now Apyx Medical Corp.), personal fees from C Surgeries, personal fees from Biom ‘Up, non-financial support from Intuitive Surgical Inc., non-financial support from Transenterix, other from DOCS, personal fees from AstraZeneca, outside the submitted work. AI reports personal fees from Mylan, outside the submitted work. REO reports personal fees from Tesaro, personal fees from GlaxoSmithKline , personal fees from Regeneron , other from Genentech USA, personal fees from Seattle Genetics , other from AstraZeneca Pharmaceuticals, outside the submitted work; and is a non-compensated steering committee member for the PRIMA, Moonstone (Tesaro/GSK) and DUO-O (AstraZeneca) studies. REO’s institute receives funding for clinical research from Bayer/Celgene/Juno, Tesaro/GSK, Ludwig Cancer Institute, Abbvie/StemCentrx, Regeneron, TCR2 Therapeutics, Atara Biotherapeutics, MarkerTherapeutics, Syndax Pharmaceuticals, Genmab Therapeutics, Sellas Therapeutics, Genentech, Kite Pharma, Gynecologic Oncology Foundation. DZ reports personal fees from Merck, personal fees from Synlogic Therapeutics, personal fees from Biomed Valley Discoveries, personal fees from Trieza Therapeutics, personal fees from Tesaro, personal fees from Agenus, grants from AstraZeneca, grants from Plexxikon, grants from Genentech, outside the submitted work.
REFERENCES
- 1.Gershenson DM. Low-grade serous carcinoma of the ovary or peritoneum. Ann Oncol 2016;27 Suppl 1:i45–i9. [DOI] [PubMed] [Google Scholar]
- 2.Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29(Suppl 4):iv259. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol 2012;120(3):612–8. [DOI] [PubMed] [Google Scholar]
- 4.Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS. Characteristics of Long-Term Survivors of Epithelial Ovarian Cancer. Obstet Gynecol 2015;126(3):491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chadha S, Rao BR, Slotman BJ, van Vroonhoven CC, van der Kwast TH. An immunohistochemical evaluation of androgen and progesterone receptors in ovarian tumors. Hum Pathol 1993;24(1):90–5. [DOI] [PubMed] [Google Scholar]
- 6.Cardillo MR, Petrangeli E, Aliotta N, Salvatori L, Ravenna L, Chang C, et al. Androgen receptors in ovarian tumors: correlation with oestrogen and progesterone receptors in an immunohistochemical and semiquantitative image analysis study. J Exp Clin Cancer Res 1998;17(2):231–7. [PubMed] [Google Scholar]
- 7.Lee P, Rosen DG, Zhu C, Silva EG, Liu J. Expression of progesterone receptor is a favorable prognostic marker in ovarian cancer. Gynecol Oncol 2005;96(3):671–7. [DOI] [PubMed] [Google Scholar]
- 8.Mizushima T, Miyamoto H. The Role of Androgen Receptor Signaling in Ovarian Cancer. Cells 2019;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elattar A, Warburton KG, Mukhopadhyay A, Freer RM, Shaheen F, Cross P, et al. Androgen receptor expression is a biological marker for androgen sensitivity in high grade serous epithelial ovarian cancer. Gynecol Oncol 2012;124(1):142–7. [DOI] [PubMed] [Google Scholar]
- 10.Levine D, Park K, Juretzka M, Esch J, Hensley M, Aghajanian C, et al. A phase II evaluation of goserelin and bicalutamide in patients with ovarian cancer in second or higher complete clinical disease remission. Cancer 2007;110(11):2448–56. [DOI] [PubMed] [Google Scholar]
- 11.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009;324(5928):787–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice MA, Malhotra SV, Stoyanova T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castration Resistant Prostate Cancer. Front Oncol 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367(13):1187–97. [DOI] [PubMed] [Google Scholar]
- 14.Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O’Shaughnessy J, et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J Clin Oncol 2018;36(9):884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonen M. A Bayesian evaluation of enrolling additional patients at the maximum tolerated dose in Phase I trials. Contemp Clin Trials 2005;26(2):131–40. [DOI] [PubMed] [Google Scholar]
- 16.Sill MW, Rubinstein L, Litwin S, Yothers G. A method for utilizing co-primary efficacy outcome measures to screen regimens for activity in two-stage Phase II clinical trials. Clin Trials 2012;9(4):385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park BY, Grisham RN, den Hollander B, Thapi D, Berman T, de Stanchina E, et al. Tumor Inhibition by Enzalutamide in a Xenograft Model of Ovarian Cancer. Cancer Invest 2016;34(10):517–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhnel R, de Graaff J, Rao BR, Stolk JG. Androgen receptor predominance in human ovarian carcinoma. J Steroid Biochem 1987;26(3):393–7. [DOI] [PubMed] [Google Scholar]
- 19.Evangelou A, Jindal SK, Brown TJ, Letarte M. Down-regulation of transforming growth factor beta receptors by androgen in ovarian cancer cells. Cancer Res 2000;60(4):929–35. [PubMed] [Google Scholar]
- 20.Edmondson RJ, Monaghan JM, Davies BR. The human ovarian surface epithelium is an androgen responsive tissue. Br J Cancer 2002;86(6):879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sternberg CN, Fizazi K, Saad F, Shore ND, De Giorgi U, Penson DF, et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 2020;382(23):2197–206. [DOI] [PubMed] [Google Scholar]
- 22.Beer TM, Tombal B. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371(18):1755–6. [DOI] [PubMed] [Google Scholar]
- 23.Westin SNFB, Yuan Y, et al. , editor ENPAC: Phase II study with safety lead-in of enzalutamide in combination with carboplatin and paclitaxel in advanced/recurrent endometrioid endometrial cancer. . Society of Gynecologic Oncology; 2021. 2021; Virtual. [Google Scholar]
- 24.Smyth JF, Gourley C, Walker G, MacKean MJ, Stevenson A, Williams AR, et al. Antiestrogen therapy is active in selected ovarian cancer cases: the use of letrozole in estrogen receptor-positive patients. Clin Cancer Res 2007;13(12):3617–22. [DOI] [PubMed] [Google Scholar]
- 25.Papadimitriou CA, Markaki S, Siapkaras J, Vlachos G, Efstathiou E, Grimani I, et al. Hormonal therapy with letrozole for relapsed epithelial ovarian cancer. Long-term results of a phase II study. Oncology 2004;66(2):112–7. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez PT, Schmeler KM, Milam MR, Slomovitz BM, Smith JA, Kavanagh JJ, et al. Efficacy of letrozole in the treatment of recurrent platinum- and taxane-resistant high-grade cancer of the ovary or peritoneum. Gynecol Oncol 2008;110(1):56–9. [DOI] [PubMed] [Google Scholar]
- 27.Bonaventura A, O’Connell RL, Mapagu C, Beale PJ, McNally OM, Mileshkin LR, et al. Paragon (ANZGOG-0903): Phase 2 Study of Anastrozole in Women With Estrogen or Progesterone Receptor-Positive Platinum-Resistant or -Refractory Recurrent Ovarian Cancer. Int J Gynecol Cancer 2017;27(5):900–6. [DOI] [PubMed] [Google Scholar]
- 28.Tumolo S, Rao BR, van der Burg ME, Guastalla JP, Renard J, Vermorken JB. Phase II trial of flutamide in advanced ovarian cancer: an EORTC Gynaecological Cancer Cooperative Group study. Eur J Cancer 1994;30A(7):911–4. [DOI] [PubMed] [Google Scholar]
- 29.Vassilomanolakis M, Koumakis G, Barbounis V, Hajichristou H, Tsousis S, Efremidis A. A phase II study of flutamide in ovarian cancer. Oncology 1997;54(3):199–202. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee S, Tovey H, Bowen R, Folkerd E, Kilburn L, McLachlan J, et al. Abiraterone in patients with recurrent epithelial ovarian cancer: principal results of the phase II Cancer of the Ovary Abiraterone (CORAL) trial (CRUK - A16037). Ther Adv Med Oncol 2020;12:1758835920975352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards J, Lim AC, Hay CW, Taylor AE, Wingate A, Nowakowska K, et al. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res 2012;72(9):2176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krop I, Abramson V, Colleoni M, Traina T, Holmes F, Garcia-Estevez L, et al. A Randomized Placebo Controlled Phase II Trial Evaluating Exemestane with or without Enzalutamide in Patients with Hormone Receptor-Positive Breast Cancer. Clin Cancer Res 2020;26(23):6149–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


