Abstract
A 69-year-old man with advanced non-small-cell lung cancer was treated with pembrolizumab for 4 months. Three months after pembrolizumab was discontinued, computed tomography showed enlargement of the pancreatic head, with hypoattenuating areas in the pancreatic head to body. On endoscopic ultrasonography, the entire pancreatic parenchyma was hypoechoic. Endoscopic retrograde cholangiopancreatography showed narrowing of the main pancreatic duct at the pancreatic head. Endoscopic ultrasound-guided fine-needle aspiration showed inflammatory cell infiltration in the stroma but no neoplastic lesions. CD8-positve T cells were dominant over CD4-positive T cells in the infiltrating lymphocytes, and the patient was diagnosed with pembrolizumab-induced pancreatitis.
Keywords: pembrolizumab, pancreatitis, immune-related adverse event, EUS-FNA
Introduction
Immune checkpoint inhibitors (ICIs), which are monoclonal antibodies against programmed cell death 1 (PD-1), programmed cell death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen 4 (CTLA-4), have been used in recent years for the treatment of various malignancies, including advanced non-small-cell lung cancer, malignant melanoma, Hodgkin lymphoma, renal cell carcinoma, squamous cell carcinoma of the head and neck, and gastric cancer (1). Unlike conventional cytotoxic anticancer agents, ICIs maintain T-cell activation by inhibiting PD-1, PD-L1, and CTLA-4, which suppress T-cell activation, and are expected to have an antitumor effect on the patient's own immune system (1).
Although ICI therapy has a strong anti-cancer effect in some patients, adverse events different from those of conventional cytotoxic anti-cancer agents may occur. These adverse events, called immune-related adverse events (irAEs), develop when autologous organs are damaged by T-cells activated by the release of the immune tolerance brake by ICIs. Although the incidence of irAEs is lower than that of the adverse events caused by cytotoxic anticancer agents (2,3), they can cause a variety of immune-related symptoms in organs but are rarely fatal (4). The most frequently reported irAEs include inflammatory dermatitis, pneumonitis, colitis, hepatitis, and hyperthyroidism (4). In contrast, the incidence of pancreatitis as an irAE tends to be low, although the rate varies among reports, ranging from 0.4% to 15% in patients using anti-PD-1 antibodies (5-8), and its characteristics have not been clarified.
We herein report a case of pancreatitis diagnosed by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) that was considered to be an irAE induced by pembrolizumab, an anti-PD-1 antibody.
Case Report
A 69-year-old man was diagnosed with advanced non-small-cell lung cancer with pleural metastasis at Hiroshima University Hospital and treated with carboplatin, pemetrexed sodium hydrate, and pembrolizumab for about 9 months. He was then treated with pembrolizumab alone for four months, which was suspended due to good disease control. Three months after the discontinuation of pembrolizumab, computed tomography (CT) showed enlargement of the pancreatic head with delayed enhancement compared to before pembrolizumab administration, along with hypoattenuating areas in the pancreatic body (Fig. 1, 2). There were no symptoms of abdominal pain or back pain.
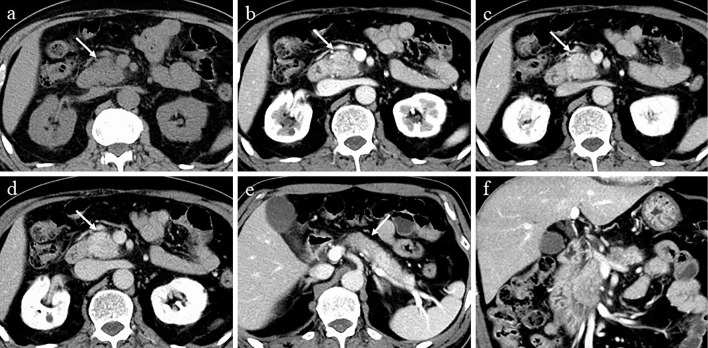
Figure 1.
CT at the time of the diagnosis. a-d: CT showing localized enlargement and delayed enhancement in the head of the pancreas. a: Unenhanced phase, b: parenchyma phase, c: portal phase, d: equilibrium phase. e: CT showing hypoattenuating areas in the body of the pancreas in the parenchyma phase (arrow). f: Coronal section in the parenchyma phase. CT: computed tomography
Figure 2.
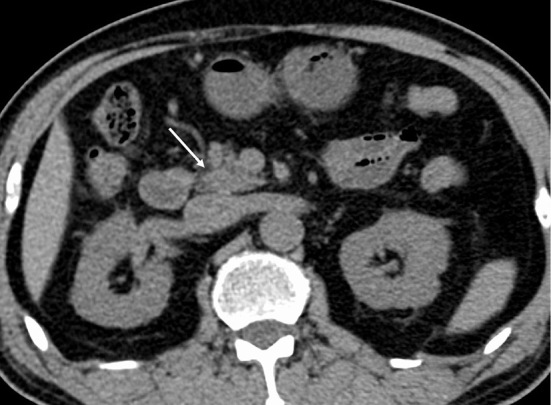
Computed tomography before the administration of pembrolizumab. There was no enlargement of the head (arrow).
Laboratory findings showed no increase in serum levels of pancreatic enzymes (pancreatic amylase, 50 IU/L; lipase, 50 IU/L) or C-relative protein (0.05 mg/dL); however, there was an increase in carbohydrate antigen 19-9 (CA19-9) (148 U/mL). Serum IgG4 level was normal (22 mg/dL). Magnetic resonance imaging (MRI) showed enlargement of the pancreatic head, with a hypointense signal on T1-weighted imaging, hyperintense signal on T2-weighted imaging, and strong hyperintense signal on diffusion-weighted imaging. Magnetic resonance cholangiopancreatography showed multiple stenoses in the main pancreatic duct (MPD) and stenosis of the intrapancreatic bile duct (Fig. 3). Endoscopic ultrasonography (EUS) revealed that the entire pancreatic parenchyma was hypoechoic, and scattered hyperechoic foci and stranding were observed in the enlarged pancreatic head (Fig. 4). Endoscopic retrograde cholangiopancreatography (ERCP) showed narrowing of the MPD at the pancreatic head, an inconsistent caliber of the MPD at the pancreatic tail, and stenosis of the intrapancreatic bile duct (Fig. 5). Pancreatic juice cytology, bile cytology, and brushing cytology of the stenosed bile duct were all negative.
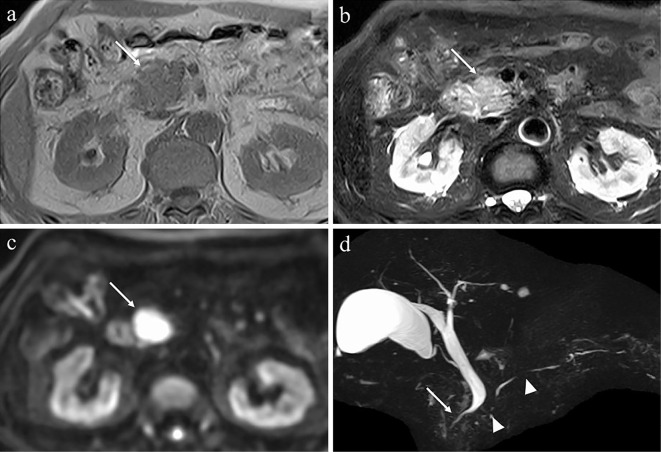
Figure 3.
MRI findings. MRI showing enlargement of the head of pancreas (arrows) with a low signal on T1-weighted imaging (a), faint high signal on T2-weighted imaging (b), and strong high signal on diffusion-weighted imaging (c). Magnetic resonance cholangiopancreatography showing multiple stenoses in the main pancreatic duct (arrowheads) and stenosis of the intrapancreatic bile duct (d) (arrow). MRI: magnetic resonance imaging
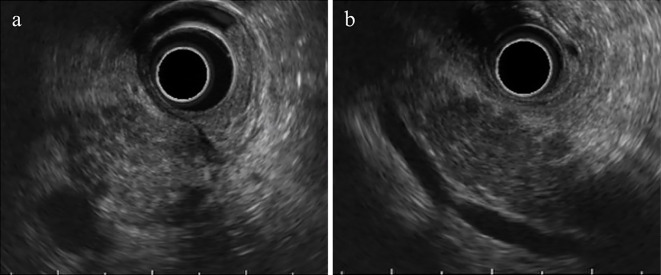
Figure 4.
Endoscopic ultrasonography. a: Scattered hyperechoic foci and stranding were observed in the enlarged head of the pancreas. b: The entire pancreatic parenchyma was hypoechoic.
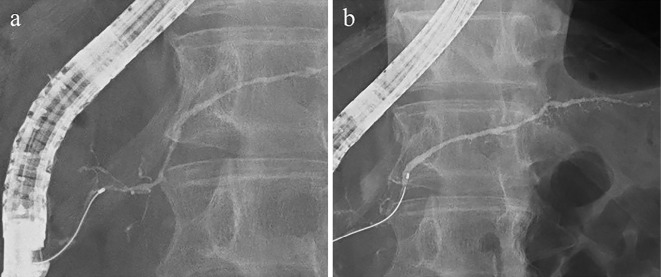
Figure 5.
ERCP. ERCP showing narrowing of the MPD at the head of the pancreas (a), caliber of the MPD, and dilatation of the branched pancreatic duct at the tail of the pancreas (b). ERCP: endoscopic retrograde cholangiopancreatography, MPD: main pancreatic duct
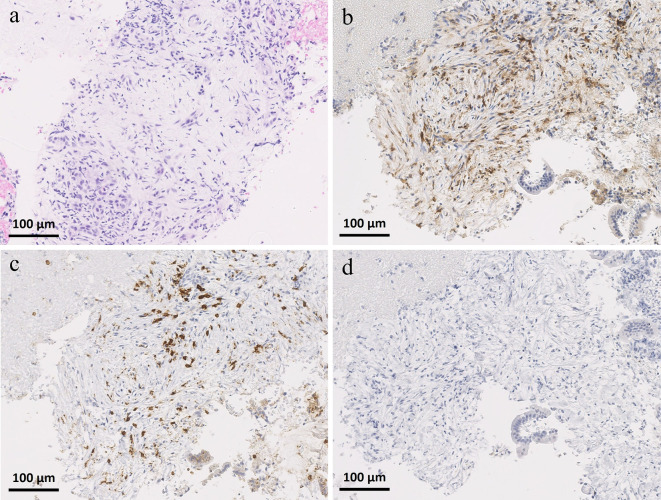
Based on the clinical history, pembrolizumab-induced pancreatitis was deemed highly probable, but pancreatic tumor and mass-forming pancreatitis including autoimmune pancreatitis (AIP) could not be ruled out. Therefore, EUS-FNA was performed using a 22-G FNA needle (Expect™; Boston Scientific, Marlborough, USA) for the histopathological diagnosis. A histopathological examination showed inflammatory cell infiltration with fibrosis in the stroma, and there were no findings characteristics of AIP, such as IgG4-positive plasma cell infiltration, storiform fibrosis, or obliterative phlebitis. CD8-positve T cells were dominant over CD4-positive T cells in the infiltrating lymphocytes (Fig. 6). Based on the pathological findings of CD8-positive T cell-dominant lymphocyte infiltration, the patient was diagnosed with pembrolizumab-induced localized pancreatitis.
Figure 6.
Histopathological findings of specimens obtained by endoscopic ultrasound-guided fine-needle aspiration. a: Hematoxylin and Eosin staining, b: CD4 staining, c: CD8 staining, d: IgG4 staining (original magnification ×200). A histopathological examination showing inflammatory cell infiltration with fibrosis in the stroma, and CD8-positive T cells appear to be dominant over CD4-positive T cells in the infiltrating lymphocytes. There was no IgG4-positive plasma cell infiltration.
As the case was asymptomatic, steroids were not administered, and the patient was carefully followed up. Four months later, CT showed that the pancreatic enlargement had improved (Fig. 7), and CA19-9 had normalized (1.5 U/mL). The lung cancer had progressed, as shown by CT; therefore, docetaxel and ramucirumab combination therapy was introduced, and the treatment was ongoing at the time of the study.
Figure 7.
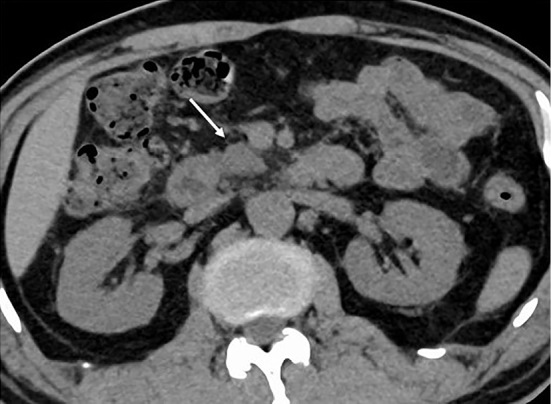
Computed tomography, four months following the diagnosis of pancreatitis. The enlargement of the head of the pancreas had improved (arrow).
Discussion
Although the frequency of ICI-induced pancreatitis is lower than that of other irAEs, with its clinical characteristics still unclear, several case reports have been published (8-17). Table shows the clinical characteristics of the 10 cases of ICI-induced pancreatitis that have been reported. The types of cancer were non-small-cell lung cancer (in four cases), malignant melanoma (in three), renal cell carcinoma (in two), and cancer of unknown primary (in one), and the ICIs administered were pembrolizumab (in six) and nivolumab (in four). The period from the introduction of ICIs to the onset of pancreatitis varied widely, ranging from 18 days to 16 months, and no consistent trend was observed. While nine of these cases developed pancreatitis during treatment with ICIs, the present case developed pancreatitis three months after the discontinuation of pembrolizumab. Nivolumab reportedly continues to bind to T lymphocytes for more than 20 weeks after the last infusion and remains effective (18). No similar studies on the immunokinetics of pembrolizumab have yet been reported, but it is necessary to consider the potential development of pancreatitis not only during administration but also after the discontinuation of ICIs.
Table.
Clinical Features of Anti-PD-1 Antibody-induced Pancreatitis.
| Case | Age Sex | Type of cancer | Anti-PD-1 antibody | Period from ICI introduction to onset | Clinical symptoms | Elevation in pancreatic enzyme | Imaging features of the pancreas | Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (8) | 65 M | Melanoma | P | 3 months | Anorexia, weight loss | Positive | CT | Localized enlargement (body-tail) |
Prednisolone | Improved |
| 2 (9) | 74 F | RCC | N | 4 months | Abdominal pain | Positive | CT | Diffuse enlargement | Prednisolone | Died |
| 3 (10) | 57 M | Melanoma | P | 3 cycles | No symptoms | N/A | PET | Diffuse FDG uptake | N/A | N/A |
| 4 (11) | 66 F | NSCLC | N | 18 days | Vomiting, back pain | Positive | CT MRI PET |
No abnormalities No abnormalities No FDG uptakes |
Prednisolone | Improved |
| 5 (12) | 70 M | NSCLC | P | 14 months | No symptoms | Positive | CT PET |
Diffuse enlargement Localized FDG uptake |
Prednisolone | Improved |
| 6 (13) | 72 M | NSCLC | N | N/A | No symptoms | Positive | CT PET |
Diffuse enlargement Diffuse FDG uptake |
Cessation of ICI | Improved |
| 7 (14) | 70 F | RCC | N | 6 months | N/A | Positive | MRI EUS ERCP |
Diffuse enlargement Hypoechoic enlargement Skipped narrowing of the MPD |
Cessation of ICI | Improved |
| 8 (15) | 43 M | Melanoma | P | 8 months | Abdominal fullness | Negative | CT | Diffuse enlargement | PD | Improved |
| 9 (16) | 65 M | NSCLC | P | 2 months | Abdominal tenderness | Positive | CT | Diffuse enlargement | Limit oral intake | Improved |
| 10 (17) | 62 M | Cancer of unknown primary | P | 9 months | Epigastric pain | Positive | CT | Diffuse enlargement fluid collection around the pancreas | Prednisolone | Died |
| This case | 69 M | NSCLC | P | 16 months | No symptoms | Negative | CT MRI EUS ERCP |
Localized enlargement Stricture of the lower CBD Localized enlargement Skipped narrowing of the MPD |
Limit oral intake, intravenous hydration | Improved |
M: male, F: female, NSCLC: non-small cell lung cancer, RCC: renal cell carcinoma, PD-1: programmed cell death 1, ICI: immune checkpoint inhibitor, P: pembrolizumab, N: nivolumab, N/A: not available, CT: computed tomography, PET: positron emission tomography, MRI: magnetic resonance imaging, EUS: endoscopic ultrasonography, ERCP: endoscopic retrograde cholangiopancreatography, CBD: common bile duct, MPD: main pancreatic duct, FDG: 18F-fluorodeoxyglucose, PD: pancreaticoduodenectomy
In this case, the patient was asymptomatic and had normal serum levels of pancreatic enzymes, and the diagnosis of pancreatitis was informed by the localized enlargement of the pancreas on CT. Although there have been some reports on ICI-induced pancreatitis with symptoms characteristics of pancreatitis, such as upper abdominal pain and back pain (9,11), the frequency of symptomatic pancreatitis corresponding to Common Terminology Criteria for Adverse Events (CTCAE) grade 3 has been reported to be less than 1-2% (8). Following the introduction of ICIs, in order to diagnose pancreatitis as early as possible, serum levels of pancreatic enzymes should be monitored regularly, and the presence or absence of findings in keeping with pancreatitis should be evaluated, regardless of symptoms, when performing imaging examinations to assess the primary lesion, in addition to paying attention to abdominal symptoms.
Although diffuse or localized pancreatic enlargement and an increased fluorodeoxyglucose uptake on positron emission tomography (PET)-CT have been reported as imaging findings of ICI-induced pancreatitis (19), the characteristic findings have not been clarified. In the list of previously reported cases shown in Table, eight cases had diffuse pancreatic enlargement, and only two cases - including the present case - had localized pancreatic enlargement. Although the cause of the differences in the lesion extent is unknown, it is necessary to recognize that ICI-induced pancreatitis often presents with diffuse pancreatic enlargement but can exhibit varying degrees of enlargement. In this case, there were some findings similar to those in AIP, such as decreased echogenicity of the parenchyma, with scattered hyperechoic foci and stranding on EUS along with narrowing of the main pancreatic duct and intrapancreatic bile duct on ERCP. EUS and ERCP findings in ICI-induced pancreatitis have never been reported, and whether or not the findings in this case are characteristic is unknown. In the future, it would be desirable to examine the imaging findings in cases from multiple centers.
In the present case, EUS-FNA was performed to rule out differential diagnoses of pancreatic tumors, including pancreatic cancer and AIP. As a pathological finding of irAEs, infiltration of CD8-positive dominant T cells has been reported in biopsy specimens of irAE cholangitis and gastritis (20,21). Infiltration of CD8-dominant T cells has also been observed in autopsy cases of ICI-induced pancreatitis (9). In this case, infiltration of CD8-positive dominant T cells was observed in the stroma of the pancreatic tissue collected by EUS-FNA, and a diagnosis of pembrolizumab-induced irAE was made. EUS-FNA should be considered when ICI-induced pancreatitis is suspected, because the treatment for pancreatitis as an irAE may differ greatly from that for ordinary pancreatitis and pancreatic tumors.
Generally, immunosuppressive drugs such as steroids are administered for the treatment of irAEs. However, according to the National Comprehensive Cancer Network guidelines, ICI administration can be continued if the pancreatitis is asymptomatic and the serum amylase or lipase level is less than two to five times the upper limit of normal, and administration of steroids can be a choice of treatment for moderate to severe cases (22). Steroid therapy can induce life-threatening adverse effects, such as severe infections, so extreme caution should be exercised when steroids are administered to patients with cancer. Therefore, for ICI-induced pancreatitis as well as normal acute pancreatitis, it is necessary to first consider conservative treatment, including fasting and a large amount of fluid infusion. Although administration of steroids was also initially considered in this case, we decided that steroid administration should be avoided unless necessary, and conservative treatment should be the first choice. In most reported cases, the administration of steroids or discontinuation of ICIs improved pancreatic enlargement and elevation of pancreatic enzymes. However, there have been reports of fatal cases of ICI-induced pancreatitis that rapidly worsened or recurred despite steroid administration (9,17). It is important to carefully select the treatment according to the pathological condition.
We reported a case of pancreatitis that was considered a pembrolizumab-induced irAE. Although ICI-induced pancreatitis is rare, the number of reported cases is expected to increase with the increased use of ICIs, such as pembrolizumab. When upper abdominal pain or elevated pancreatic enzymes are observed after the introduction of ICIs, it is important to carry out diagnostic and therapeutic interventions via appropriate imaging and histological examinations, with ICI-induced pancreatitis in mind.
Informed consent was obtained from the patient included in this study.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Sharon E, Streicher H, Goncalves P, Chen HX. Immune checkpoint inhibitors in clinical trials. Chin J Cancer 33: 434-444, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387: 1540-1550, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389: 255-265, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 36: 1714-1768, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 Study. J Clin Oncol 38: 1-10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 372: 311-319, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372: 2509-2520, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 60: 190-209, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Sakai A, Shiomi H, et al. An autopsy case of severe acute pancreatitis induced by administration of pazopanib following nivolumab. Pancreatology 21: 21-24, 2021. [DOI] [PubMed] [Google Scholar]
- 10.Alabed YZ, Aghayev A, Sakellis C, Van den Abbeele AD. Pancreatitis secondary to anti-programmed death receptor 1 immunotherapy diagnosed by FDG PET/CT. Clin Nucl Med 40: e528-e529, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: a case report. Lung Cancer 99: 148-150, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Kakuwa T, Hashimoto M, Izumi A, Naka G, Takeda Y, Sugiyama H. Pembrolizumab-related pancreatitis with elevation of pancreatic tumour markers. Respir Case Rep 8: e00525, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito H, Ono K. Nivolumab-induced pancreatitis: an immune-related adverse event. Radiology 293: 521, 2019. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, Sakai A, Kobayashi T, et al. Nivolumab-related pancreatitis with autoimmune pancreatitis-like imaging features. J Gastroenterol Hepatol 34: 1274, 2019. [DOI] [PubMed] [Google Scholar]
- 15.Rawson RV, Robbins E, Kapoor R, Scolyer RA, Long GV. Recurrent bowel obstruction: unusual presentation of pembrolizumab-induced pancreatitis in annular pancreas. Eur J Cancer 82: 167-170, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka S, Asakawa R, Komuta K, et al. A case of simultaneous occurrence of hepatitis and pancreatitis during the combination immunochemotherapy for non-small cell lung carcinoma. Respir Med Case Rep 31: 101266, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueno M, Tsuji Y, Yokoyama T, et al. A case of fatal immune checkpoint inhibitor-related pancreatitis. Intern Med 60: 3905-3911, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osa A, Uenami T, Koyama S, et al. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight 3: e59125, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widmann G, Nguyen VA, Plaickner J, Jaschke W. Imaging features of toxicities by immune checkpoint inhibitors in cancer therapy. Curr Radiol Rep 5: 59, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshikawa Y, Imamura M, Yamaoka K, et al. A case with life-threatening secondary sclerosing cholangitis caused by nivolumab. Clin J Gastroenterol 14: 283-287, 2021. [DOI] [PubMed] [Google Scholar]
- 21.Fujii M, Ozato T, Mizukawa S, et al. A rare case of immunotherapy-induced cholangitis and gastritis. Clin J Gastroenterol 13: 1083-1090, 2020. [DOI] [PubMed] [Google Scholar]
- 22.NCCN Clinical Practice Guidelines in Oncology - Management of Immunotherapy-Related Toxicities. Version 2.2021 [Internet]. [cited 2021 Mar 26]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf.