Abstract
A patient with follicular lymphoma treated with obinutuzumab and bendamustine experienced prolonged coronavirus disease-2019 (COVID-19). One month after the symptoms transiently improved, the patient experienced exacerbated COVID-19 symptoms. The patient recovered from COVID-19 with remdesivir and dexamethasone and was discharged 77 days after the disease onset. The patient completed a primary series of SARS-CoV-2 vaccinations on day 176, but the anti-spike protein IgG was not detected later. A careful observation to detect any subsequent relapse of COVID-19 symptoms is necessary in immunocompromised patients. Chemotherapy should be based on the disease status and type of lymphoma.
Keywords: immunocompromised patient, prolonged COVID-19, obinutuzumab and bendamustine, follicular lymphoma
Introduction
Numerous studies have shown that coronavirus disease-2019 (COVID-19) can persist or relapse in immunocompromised patients with impaired viral clearance (1). Polymerase chain reaction (PCR) tests have confirmed that severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) persists for more than 100 days in immunocompromised patients (2). Prolonged viral shedding is thought to be induced by both humoral and cellular immunosuppression (3). That hypothesis was based on the fact that persistent positive SARS-CoV-2 PCR test results were observed in patients with lymphoma, where the humoral immune system was impaired by B-cell depleting therapy (2,4), and in a patient infected with the human immunodeficiency virus, who had reduced numbers of CD4-positive T cells (2).
The Centers for Disease Control guidelines suggest that severely immunocompromised patients with SARS-CoV-2 infections be isolated for at least 20 days after symptoms have resolved (5). However, to our knowledge, no definite standards of care exist for managing COVID-19 in immunocompromised patients or for continuing chemotherapy in patients with hematological cancers who have recovered from COVID-19.
We herein report our management of a patient with follicular lymphoma (FL) who experienced prolonged COVID-19. The patient had been treated with obinutuzumab and bendamustine (GB), which cause both B-cell and T-cell depletion. To treat COVID-19, the patient received combination therapy of remdesivir and dexamethasone. A negative SARS-CoV-2 PCR test result was finally achieved on day 169.
Case Report
A 63-year-old woman had a medical history of left breast cancer and had undergone mastectomy and removal of the axillary lymph nodes without adjuvant chemotherapy. One month later, she had been diagnosed with FL, and GB was initiated. Before presenting to our hospital, she had completed four courses of induction therapy with GB for stage IVA FL, with a high tumor burden, according to the Groupe d-Etude des Lymphomes Folliculaires criteria.
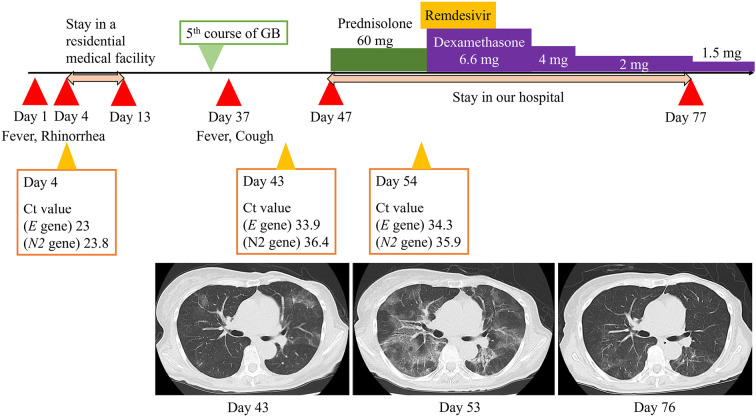
She presented to our hospital with a three-day history of a fever and upper respiratory symptoms. We collected a saliva specimen and conducted PCR with the GeneXpert system (Cepheid, Sunnyvale, USA) for detecting SARS-CoV-2. The E and N2 genes of the virus were detected with cycle threshold (Ct) values of 23 and 23.8, respectively. Consequently, on day 4 of her illness, we diagnosed her with COVID-19. Her symptoms were mild, and she did not require supplemental oxygen; therefore, she was isolated and monitored at a residential medical facility without medication (Fig. 1). Her symptoms resolved within nine days of observation, and she was discharged from the facility. After she had remained asymptomatic for one month, we decided to continue induction therapy for FL. Thus, at one month behind the original schedule, we administered the fifth course of GB in an outpatient setting.
Figure 1.
Clinical course and CT findings of a patient with follicular lymphoma who contracted COVID-19. The patient presented with a three-day history of a fever and rhinorrhea and was diagnosed with COVID-19 (positive SARS-CoV-2 PCR test). She was placed in a residential medical facility on day 4, after which the symptoms resolved, and she was discharged on day 13. After remaining asymptomatic, she underwent a fifth course of GB. The day after completing the fifth course, however, the fever and cough reappeared (day 37). On day 43, a SARS-CoV-2 PCR test was positive, with Ct values of 33.9 (E gene) and 36.4 (N2 gene). CT axial images of the chest. Day 43 CT showed diffuse bilateral ground-glass opacities and infiltrations. On day 47, she was hospitalized and received 60 mg prednisolone for suspected organizing pneumonia, but the fever and respiratory symptoms did not improve. A SARS-CoV-2 PCR test was positive, with Ct values of 34.3 (E gene) and 35.9 (N2 gene). Day 53 CT showed worsening bilateral ground-glass opacities and infiltrations. After a 5-day course of remdesivir and 6.6 mg dexamethasone, day 76 CT showed regression of the bilateral ground-glass opacities and infiltrations. On day 77, she was discharged. CT: computed tomography, Ct: cycle threshold, GB: obinutuzumab and bendamustine
On day 43 after the COVID-19 onset, she was referred to our hospital with a six-day history of a fever and cough. The fifth course of GB had been completed one day before the fever and cough appeared. Her saliva specimen was positive for SARS-CoV-2, with PCR Ct values of 33.9 (E gene) and 36.4 (N2 gene), which were higher than those in the initial test. Chest computed tomography (CT) performed on day 43 showed diffuse bilateral ground-glass opacities and infiltrations (Fig. 1). We considered the amount of replication-competent virus to be quite low, and the chest CT findings led to a diagnosis of organizing pneumonia due to COVID-19.
On day 47 of her illness, we started to monitor the patient in an isolated ward. Her oxygen saturation at a peripheral artery was 93% in room air. Thus, we administered 1 L/min supplemental oxygen to maintain an oxygen saturation above 94% (Fig. 2). A blood analysis showed hypogammaglobulinemia (IgG=818 mg/dL) and lymphopenia (lymphocyte count=376 /μL), which indicated both humoral and cellular immunosuppression. We suspected these effects to be due to her history of repetitive chemotherapy.
Figure 2.
Clinical measurements during treatment of COVID-19 relapse in a patient with follicular lymphoma. On hospital admission (day 47), the patient displayed a fever (blue line) and cough (red bar). To improve blood oxygen (orange line), we administered 1 L/min supplemental oxygen (gray bar). Oral prednisolone (green bar) was administered to treat organizing pneumonia, but the symptoms did not improve. A 5-day course of remdesivir (orange bar) and intravenous dexamethasone (6.6 mg, purple bar) led to clinical improvement. The symptoms did not recur during dexamethasone tapering. On day 77, the patient was discharged with a prescription of 1.5 mg oral dexamethasone daily. SpO2: blood oxygen saturation, BT: body temperature
On day 47, we initiated an oral intake of 60 mg (1 mg/kg/day) prednisolone to protect against the suspected organizing pneumonia. However, the patient showed clinical worsening, with a persistent fever and respiratory symptoms that required 1 L/min supplemental oxygen. On day 53, follow-up CT revealed new bilateral ground-glass opacities and infiltrations (Fig. 1). On day 54, a PCR test of a saliva sample was positive for SARS-CoV-2, with Ct values of 34.3 (E gene) and 35.9 (N2 gene). To check for other viral species related to respiratory diseases, we performed multiplex PCR with the FilmArray Respiratory Panel (bioMérieux, Marcy-l'Etoile, France), which targeted 17 viruses and 3 bacterial species. Those results revealed that only SARS-CoV-2 was present. We considered Cytomegalovirus, Pneumocystis jirovecii, and other fungal infections to be unlikely, as Cytomegalovirus antigen was absent in the blood test, and the serum beta-D-glucan titer, measured using the Fungitec G Test MKII (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan), was low (12.4 pg/mL). Thus, based on the laboratory tests and CT findings, we concluded that her fever and respiratory symptoms were associated with COVID-19.
On day 55, we decided to administer a 5-day course of remdesivir. On day 56, we administered 6.6 mg intravenous dexamethasone, instead of prednisolone. Soon after the initiation of remdesivir and dexamethasone, her fever and respiratory symptoms regressed. We gradually decreased the dexamethasone dose and confirmed that the symptoms did not recur. On day 76, the CT findings improved (Fig. 1). The patient was discharged from our hospital on day 77, with a prescription of 1.5 mg dexamethasone, taken orally daily. The patient remained asymptomatic after discharge.
On day 169, at a regular follow-up evaluation, a PCR test confirmed that she was negative for SARS-CoV-2. We performed the test with a nasopharyngeal sample, as she had insufficient saliva for sampling at that time. On day 176, she completed the primary series of SARS-CoV-2 vaccinations. At 107 days after the vaccinations, we performed a SARS-CoV-2 IgG test, which detects anti-spike protein IgG (LumipulseⓇ; Fujirebio, Tokyo, Japan). We were unable to detect the SARS-CoV-2 anti-spike protein IgG, which indicated that she did not mount an immune response to the vaccinations. Therefore, we deemed it necessary to monitor the patient carefully in order to check for any COVID-19 symptoms in the future.
Discussion
This case was considered to be one of prolonged COVID-19 infection rather than reinfection, based on the clinical course and Ct values.
The combination of a five-day course of remdesivir and dexamethasone resulted in clinical improvement. Previously, remdesivir was shown to be effective for the clinical resolution and clearance of viral infections in patients with impaired humoral immunity (6,7). Remdesivir also reduced the risk of clinical worsening, which would have required invasive mechanical ventilation, in patients that received low-flow oxygen (8). However, the addition of corticosteroids to remdesivir did not reduce the 28-day mortality (7). Dexamethasone was beneficial for reducing the 28-day mortality among patients with COVID-19 who received invasive mechanical ventilation or oxygen, but the benefit was unremarkable among patients without invasive respiratory management (9). Therefore, in our patient, who received low-flow oxygen, clinical improvement was mainly attributed to remdesivir.
COVID-19 vaccinations can benefit patients with hematological cancers, but we must take into account that the vaccination will be less effective in eliciting an immune response than in otherwise healthy individuals (10). One report showed that vaccinations should not be given until at least six months after chemotherapy (11). The SARS-CoV-2 IgG test showed that patients with hematologic malignancies had a lower probability of seroconversion, particularly those who received anti-CD-20 antibody therapy and stem cell transplantations (12). Obinutuzumab is an anti-CD-20 monoclonal antibody that mainly affects B cells. GB is known to induce severe lymphopenia; it impairs both cellular and humoral immunity for about one year (13,14). Our patient received a primary series of vaccinations at six months after finishing the fifth course of GB. However, the SARS-CoV-2 IgG test performed 107 days after the vaccinations indicated no seroconversion. Therefore, the patient should receive booster SARS-CoV-2 vaccinations to prevent COVID-19 recurrence.
In patients with lymphoma, one of the risk factors for COVID-19 mortality is active disease, defined as a partial response or progression, but mortality was not shown to be significantly affected by whether or not patients received chemotherapy (15). COVID-19-related mortality also differs based on the type of lymphoma. For example, an aggressive lymphoma, like diffuse large B-cell lymphoma, had a worse overall survival than FL, which is an indolent type of lymphoma (15). Therefore, for patients who survive COVID-19, we should consider starting chemotherapy based on the disease status and type of lymphoma. Regarding the present patient, we intend to continue observation without chemotherapy, as the FL has been in remission since the fifth course of induction therapy.
The present study had two main limitations. First, we only described a single case; thus, our management of COVID-19 cannot be generalized to all patients with hematological malignancies. Second, we did not perform genome sequencing to verify that the prolonged disease was COVID-19 when the patient was admitted for relapsed symptoms.
Conclusion
As previously reported, patients with hematological malignancies who undergo chemotherapy can experience prolonged COVID-19. These patients require careful observation to detect any subsequent relapse of COVID-19 symptoms due to their lack of an immune response to vaccinations after chemotherapy.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Rhee C, Kanjilal S, Baker M, Klompas M. Duration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectivity: when is it safe to discontinue isolation? Clin Infect Dis 72: 1467-1474, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tarhini H, Recoing A, Bridier-Nahmias A, et al. Long-term severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectiousness among three immunocompromised patients: from prolonged viral shedding to SARS-CoV-2 superinfection. J Infect Dis 223: 1522-1527, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baang JH, Smith C, Mirabelli C, et al. Prolonged severe acute respiratory syndrome coronavirus 2 replication in an immunocompromised patient. J Infect Dis 223: 23-27, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duléry R, Lamure S, Delord M, et al. Prolonged in-hospital stay and higher mortality after COVID-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am J Hematol 96: 933-944, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Ending isolation and precautions for people with COVID-19: interim guidance [Internet]. [cited 2022 Jan 14]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html
- 6. Buckland MS, Galloway JB, Fhogartaigh CN, et al. Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat Commun 11: 6385, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garibaldi BT, Wang K, Robinson ML, et al. Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID-19. JAMA Netw Open 4: e213071, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ansems K, Grundeis F, Dahms K, et al. Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev 8: CD014962, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 25: 693-704, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pagano L, Salmanton-García J, Marchesi F, et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association survey (EPICOVIDEHA). J Hematol Oncol 14: 168, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giesen N, Sprute R, Rüthrich M, et al. 2021 update of the AGIHO guideline on evidence-based management of COVID-19 in patients with cancer regarding diagnostics, viral shedding, vaccination and therapy. Eur J Cancer 147: 154-160, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thakkar A, Pradhan K, Jindal S, et al. Patterns of seroconversion for SARS-CoV2-IgG in patients with malignant disease and association with anticancer therapy. Nat Cancer 2: 392-399, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakajima Y, Ogai A, Furukawa K, et al. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J Infect Chemother 27: 387-389, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gafter-Gvili A, Polliack A. Bendamustine associated immune suppression and infections during therapy of hematological malignancies. Leuk Lymphoma 57: 512-519, 2016. [DOI] [PubMed] [Google Scholar]
- 15. Regalado-Artamendi I, Jiménez-Ubieto A, Hernández-Rivas JÁ, et al. Risk factors and mortality of COVID-19 in patients with lymphoma: a multicenter study. Hemasphere 5: e538, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]