Abstract
Objective
Denosumab, an anti-RANKL monoclonal antibody, was reported to improve bone mineral density (BMD) and reduce fracture risk, offering favorable efficacy against postmenopausal osteoporosis. However, some patients have experienced a reduced BMD despite denosumab therapy.
Methods
We performed an observational study to clarify the clinical efficacy of denosumab for osteoporosis in rheumatic disease patients. Serum levels of bone turnover markers and lumber BMD in 100 rheumatic disease patients were examined at baseline and 6 and 12 months after denosumab therapy. The independent influence of changes in the BMD was examined by multiple regression analyses adjusted for patient characteristics and bone turnover markers.
Results
As bone resorption markers, serum levels of N-telopeptide crosslinked of type I collagen (NTx) and tartrate-resistant acid phosphatase isoform 5b were statistically decreased after 12 months. As bone formation markers, serum levels of osteocalcin, procollagen type I N-terminal peptide, and bone alkaline phosphatase were significantly decreased after 12 months. The mean BMD was significantly increased after 12 months. However, in 10 patients, the BMD decreased. A multivariate analysis of factors related to BMD changes highlighted a young age, low prednisolone dosage, and reduction in NTx.
Conclusions
Denosumab increases the BMD to combat osteoporosis in rheumatic disease patients, and potential predictors of a better response to denosumab include a young age, reduction in bone turnover markers, and low-dose glucocorticoid use.
Keywords: denosumab, RANKL, rheumatic diseases, osteoporosis
Introduction
Several rheumatic diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus, systemic sclerosis, dermatomyositis/polymyositis, and vasculitis, are characterized by osteoporosis and fragility fractures (1). Among the risk factors investigated, inflammatory cytokines, glucocorticoid treatment, and reduced physical activity due to tender joints and muscle weakness have been shown to play key roles in favoring low bone mineral density (BMD) values in these diseases (2). Inflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-1, IL-6, and IL-17, are reportedly involved in the upregulated expression of receptor activator of the nuclear factor-κB ligand (RANKL) (3). RANKL is responsible for inducing osteoclastogenesis by binding to receptor activator of the nuclear factor-κB (RANK) on the osteoclast precursor. RANKL is expressed on the surface of activated T-cells (4), and its production by T-cells can directly trigger osteoclastogenesis and bone remodeling. These data demonstrate that immune cells can function as regulators of bone physiology and provide a rationale concerning how inflammation is coupled to local bone destruction.
Denosumab, a fully human monoclonal antibody against for RANKL, has been used to treat osteoporosis. RANKL is essential for the formation, activation, and survival of osteoclasts. Consequently, the drug strongly abrogates bone resorption, increases the BMD, and prevents fragility fractures in postmenopausal osteoporosis (5,6). The pathogenesis of bone loss in patients with rheumatic diseases might be influenced by either the systemic or local inflammatory status. In addition, the pathogenesis might also be influenced based on whether or not the patient is taking glucocorticoids. Therefore, osteoporosis should be managed appropriately. It is thus important to demonstrate therapeutic efficacy of denosumab against osteoporosis for patients with rheumatic diseases.
In the present study, we analyzed the effects of denosumab in patients with rheumatic diseases.
Materials and Methods
Patients
This study was a retrospective observational study that was carried out by the opt-out method. Patients with rheumatic disease who had been diagnosed with osteoporosis and received denosumab for the first time between August 2013 and February 2016 at Toho University Omori Medical Center were enrolled.
Osteoporosis was diagnosed as primary osteoporosis based on the Diagnostic Criteria for Primary Osteoporosis of the Japanese Society for Bone and Mineral Research (JSBMR) (7). This included patients with fragility fractures caused by a low BMD (young adult mean <80% or T score <-1.7), those with between 1 and 4 vertebral fractures from the fourth thoracic to the fourth lumbar vertebra (Th4 to L4), and patients whose BMD was equal to or below either the young adult mean 70% or T score -2.5. According to the JSBMR guidelines for the management of glucocorticoid-induced osteoporosis (8), glucocorticoid-treated patients can be diagnosed with osteoporosis.
A total of 100 patients were enrolled, including 51 patients with rheumatoid arthritis (RA), 14 with systemic lupus erythematosus, 11 with polymyalgia rheumatica, 5 with polymyositis/dermatomyositis, 4 with mixed connective tissue disease, 4 with Sjögren's syndrome, 4 with vasculitis syndrome, 3 with systemic sclerosis, 1 with Behçet's disease, 1 with eosinophilic fasciitis, 1 with seronegative spondyloarthropathy, and 1 with sarcoidosis. No restrictions on glucocorticoid dosage were placed for inclusion in this study.
All patients received denosumab (60 mg) via subcutaneous injection at baseline and 6 and 12 months, supplemented with 610 mg calcium, 400 IU vitamin D, and 30 mg magnesium.
This study was approved by the Ethics Committees at Toho University Omori Medical Center (approval number: 27-147 and 27-265).
Measurement of the BMD
Before starting denosumab therapy and at 6 and 12 months later, the BMD of the lumbar spine (L2-4) was measured by dual-energy X-ray absorptiometry using a Discovery A device (Hologic, Waltham, USA). The BMD was automatically calculated from the bone area (cm2) and bone mineral content (g) and was expressed in g/cm2.
Serum bone turnover markers
Regarding bone resorption markers, the serum levels of the cross-linked N-telopeptide of type I collagen (NTx; Inverness, Princeton, USA; normal range, 14.3-89.0 nmolBCE/L) and tartrate-resistant acid phosphatase isoform 5b (TRACP-5b; DS Pharma Biomedical, Tokyo, Japan; normal range, 120-420 mU/dL) were measured by an enzyme-linked immunosorbent assay. Regarding bone formation markers, the serum levels of procollagen type I N-terminal peptide (PINP; Orion Diagnostica, Espoo, Finland; normal range, 17.1-64.7 ng/mL) and osteocalcin (OC; Mitsubishi Kagaku Bioclinical Laboratories, Tokyo, Japan; normal range, 14.2-54.8 ng/mL) were determined by an immunoradiometric assay. The serum level of bone alkaline phosphatase (BAP; Quidel, San Diego, USA; normal range, 3.8-22.6 μg/L) was measured by an enzyme immunoassay. Fasting morning blood samples were collected from the patients before the initiation of treatment as well as after 6 and 12 months of denosumab therapy.
Adverse events
The safety of denosumab therapy was assessed based on the reports of clinical adverse events, including clinical symptoms/signs, changes in serum chemistry, hematology values, and Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (9).
Statistical analyses
Statistical analyses were performed with the Prism ver. 8.0 (Graphpad Software, San Diego, USA) and StatFlex (ver. 6; ARTEC, Osaka, Japan) software programs. Numerical data were expressed as both the mean±standard error of the mean (SEM) and the median with the interquartile range. Changes during denosumab treatment were assessed with Friedman's test, followed by Dunnett's multiple comparison test. When comparing two groups, the Mann-Whitney U test was applied for numerical data, and Fisher's exact test was used for categorical data. Simple linear regression was used to assess the correlations between the BMD change and patient characteristics or serum bone turnover markers. A stepwise forward multiple regression analysis was used for the multivariate analysis. The level of significance was set at p<0.05.
Results
Patients' characteristics
Table 1 shows the demographic and clinical data of the patients. The mean age of the patients was 67.5±1.2 years old, and the majority of patients were women (92.4%). The mean body mass index (BMI) was 20.8±3.2. The mean BMD of the patients was 0.76±0.10 g/cm2. The usage rate of prednisolone was 68%. The underlying disease was RA in about 50% of the patients. Sixty patients (60.0%) had been treated with daily bisphosphonate prior to denosumab, and 10 (10%) had been treated with teriparatide prior to denosumab.
Table 1.
Demographics and Clinical Data at Baseline of the Study Population.
| n=100 | |
|---|---|
| Age (yr) | 67.5±1.2 |
| Number of male/female | 7/93 |
| Postmenopausal (%) | 86 (92.4) |
| Body mass index (kg/m2) | 20.8±0.32 |
| Prednisolone usage (%) | 68 (68.0) |
| Bone mineral density (g/cm2) | 0.76±0.10 |
| Past history of fracture (%) | 21 (21) |
| Diagnosis, no. (%) | |
| Rheumatoid arthritis | 51 (51) |
| Systemic lupus erythematosus | 14 (14) |
| Polymyalgia rheumatica | 11 (11) |
| Polymyositis/dermyositis | 5 (5) |
| Mixed connected tissue disease | 4 (4) |
| Sjögren’s syndrome | 4 (4) |
| Vasculitis syndrome | 4 (4) |
| Systemic scleroderma | 3 (3) |
| Others | 4 (4) |
| Pre-denosumab treatment, no. (%) | |
| Bisphosphonates | 60 (60) |
| Teriparatide | 10 (10) |
| Vitamin D | 8 (8) |
| Vitamin K | 3 (3) |
| SERM | 3 (3) |
| Bone turnover markers | |
| NTx (nmolBCE/L) | 16.4±0.6 |
| TRACP-5b (mU/dL) | 265.6±17.0 |
| P1NP (µg/L) | 33.5±3.0 |
| BAP (µg/L) | 13.6±1.1 |
| OC (ng/mL) | 7.1±0.5 |
SERM: selective estrogen receptor modulators, NTx: N-telopeptide crosslinked of type I collagen, TRACP-5b: tartrate-resistant acid phosphatase isoform 5b, P1NP: procollagen type I N-terminal peptide, BAP: bone alkaline phosphatase, OC: osteocalcin
BMD
As shown in Fig. 1, the BMD of the patients tended to increase over time (median: 0.76 to 0.79 g/cm2 after 6 months of denosumab treatment), although the difference was not statistically significant after 6 months. However, the BMD had significantly increased after 12 months of treatment (0.76 to 0.79 g/cm2 after 12 months of denosumab treatment) (p<0.05). There was no significant difference in the change in the BMD among diseases or depending on prior treatment (data not shown).
Figure 1.
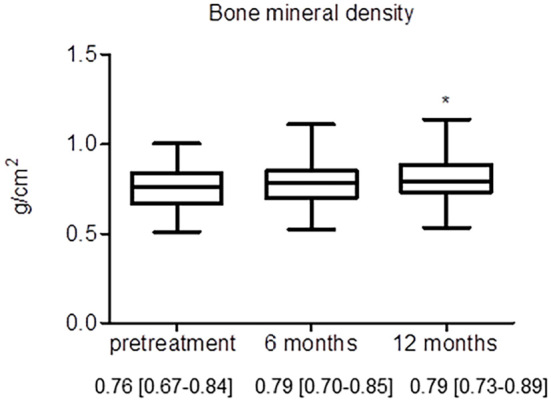
Changes in the BMD during denosumab therapy. Data are expressed as the median with 25th to 75th percentiles. *: p<0.05 versus baseline by Dunnett’s multiple comparison test
Serum bone turnover markers
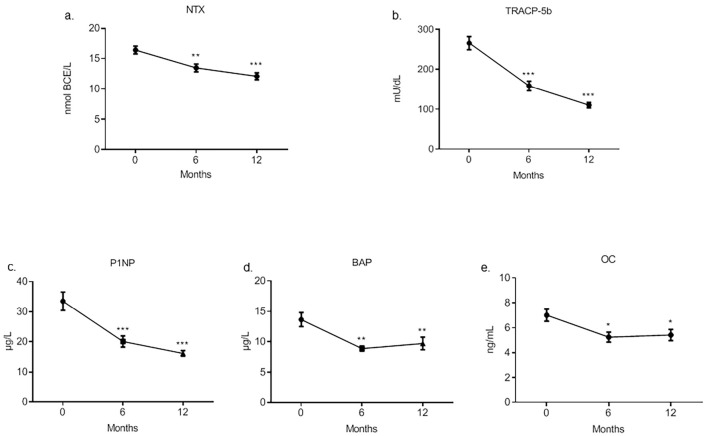
Regarding the serum bone resorption markers, the NTx and TRACP-5b levels had decreased significantly after six months and remained low subsequently (Fig. 2a, b). Regarding the serum bone formation markers, the procollagen type I N-terminal peptide (P1NP), BAP and OC levels had also decreased significantly after six months and remained low (Fig. 2c-e, respectively). There was no significant difference in the change in serum bone resorption or bone formation markers among diseases or depending on prior treatment (data not shown).
Figure 2.
Serum levels of bone turnover markers during denosumab therapy. Serum NTx (a) and TRACP-5b (b) are bone resorption markers, and serum P1NP (c), BAP (d), and OC (e) are bone formation markers. Data are expressed as the mean±SEM. *: p<0.05 versus baseline, **: p<0.001 versus baseline, ***: p<0.0001 versus baseline by Dunnett’s multiple comparison test
Comparing the glucocorticoid-treated and non-glucocorticoid-treated groups
The baseline characteristics were compared between the glucocorticoid-treated (n=68) and non-glucocorticoid-treated (n=32) groups (Table 2). The mean dose of prednisolone was 6.7±0.7 mg/day in the glucocorticoid-treated group. There were no significant differences between these two groups with respect to the age, sex, menopausal status, or BMI. The percentage of patients with systemic lupus erythematosus was significantly higher in the glucocorticoid-treated group than in the non-glucocorticoid-treated group, while the percentage of patients with RA was significantly lower in the glucocorticoid-treated group than in the non-glucocorticoid-treated group. There were no significant differences in the baseline serum levels of NTx, TRACP-5b or P1NP, and BAP between groups; however, the serum OC level was significantly lower in the glucocorticoid-treated group than in the non-glucocorticoid-treated group at baseline. There were no significant differences in the change in bone turnover markers between the glucocorticoid-treated group and the non-glucocorticoid-treated group at 12 months after denosumab treatment.
Table 2.
Comparison between Glucocorticoid Treated Group and Non-glucocorticoid Treated Group at Baseline.
| Patients treated with glucocorticoid (n=68) | Patients not treated with glucocorticoid (n=32) | p value | |
|---|---|---|---|
| Age (yr) | 67.4±1.6 69.5 [63.0-77.0] |
67.8±1.6 68.0 [62.0-75.5] |
0.5450 |
| Number of male/female | 6/62 | 1/31 | 0.4247 |
| Postmenopausal (%) | 56 (90.3) | 30 (96.8) | 0.4161 |
| Body mass index (kg/m2) | 20.8±0.4 20.2 [18.1-22.8] |
20.9±0.5 20.5 [18.8-22.6] |
0.6132 |
| Daily prednisolone-equivalent dose (mg/day) | 6.7±0.7 5.0 [3.3-8.0] | 0 | |
| Bone mineral density (g/cm2) | 0.76±0.11 | 0.75±0.09 | 0.4904 |
| Diagnosis, no. (%) | |||
| Rheumatoid arthritis | 26 (38.3) | 25 (78.2) | 0.0002 |
| Systemic lupus erythematosus | 14 (20.6) | 0 (0) | 0.0041 |
| Polymyalgia rheumatica | 10 (14.7) | 1 (3.1) | 0.1001 |
| Polymyositis/dermyositis | 4 (5.9) | 1 (3.1) | 0.9999 |
| Mixed connected tissue disease | 4 (5.9) | 0 (0) | 0.3029 |
| Sjögren’s syndrome | 2 (2.9) | 2 (6.3) | 0.5910 |
| Vasculitis syndrome | 3 (4.4) | 1 (3.1) | 0.9999 |
| Systemic scleroderma | 2 (2.9) | 1 (3.1) | 0.9999 |
| Others | 3 (4.4) | 1 (3.1) | 0.9999 |
| Pre-denosumab treatment, no. (%) | |||
| Bisphosphonates | 40 (58.8) | 20 (62.5) | 0.8279 |
| Teriparatide | 9 (13.2) | 1 (3.1) | 0.1618 |
| Vitamin D | 4 (5.9) | 4 (12.5) | 0.2637 |
| Vitamin K | 1 (1.4) | 2 (6.3) | 0.2393 |
| SERM | 2 (2.9) | 1 (3.1) | 0.9999 |
| Baseline of bone turnover markers | |||
| NTx (nmolBCE/L) | 16.1±0.8 13.9 [11.9-20.7] |
17.0±1.1 15.9 [13.0-20.3] |
0.3844 |
| TRACP-5b (mU/dL) | 260.5±21.5 231.5 [129.0-336.8] |
276.4±27.7 252.0 [160.0-351.5] |
0.5115 |
| P1NP (µg/L) | 32.0±4.1 25.3 [16.0-37.3] |
36.6±3.7 30.5 [19.2-53.3] |
0.0715 |
| BAP (µg/L) | 13.6±1.6 10.40 [8.4-14.4] |
13.8±1.2 12.1 [9.7-16.3] |
0.1104 |
| OC (ng/mL) | 6.6±0.6 5.3 [3.8-7.7] |
8.0±0.8 7.2 [5.1-9.9] |
0.0258 |
| Change of bone turnover markers (%) | |||
| Δ NTx | -20.9±4.1 -28.1 [-38.6- -8.1] |
-6.7±7.1 -20.3 [-47.4- 18.5] |
0.4671 |
| Δ TRACP-5b | -36.2±6.3 -51.1 [-65.2- -8.7] |
-45.0±11.0 -66.5 [-77.7- -49.2] |
0.0512 |
| Δ P1NP | -28.1±6.3 -39.9 [-62.7- -7.8] |
-31.2±10.7 -44.1 [-69.3- -18.2] |
0.4519 |
| Δ BAP | -8.8±14.7 -24.2 [-42.3- -12.4] |
-25.8±6.3 -30.6 [-52.2- -14.0] |
0.2874 |
| Δ OC | -2.1±10.9 -27.9 [-48.8- 7.8] |
15.0±32.0 -24.8 [-45.1- -7.8] |
0.8164 |
SERM: selective estrogen receptor modulators, NTx: N-telopeptide crosslinked of type I collagen, TRACP-5b: tartrate-resistant acid phosphatase isoform 5b, P1NP: procollagen type I N-terminal peptide, BAP: bone alkaline phosphatase, OC: osteocalcin, Δ: the rate of change during 12 months
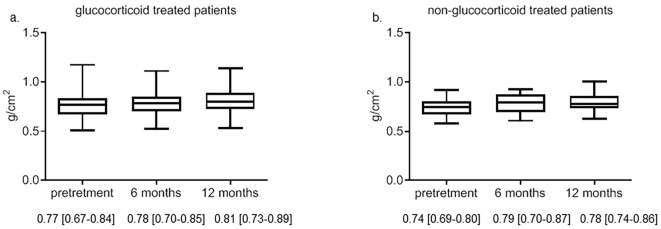
As shown in Fig. 3a, the median BMD was increased at 6 and 12 months after denosumab treatment in the glucocorticoid-treated group compared with the baseline BMD values (0.77 to 0.81 g/cm2 after 12 months of denosumab treatment), although this was not statistically significant. As shown in Fig. 3b, the median BMD was also increased at 6 and 12 months after denosumab treatment in the non-glucocorticoid-treated patients compared with the baseline BMD values (0.74 to 0.78 g/cm2 after 12 months of denosumab treatment), although this was also not statistically significant. The rate of increased BMD at 12 months was not markedly different between the patients with and without glucocorticoid therapy (glucocorticoid-treated group: 5.1%±1.0% and non-glucocorticoid-treated group: 7.0%±0.9%).
Figure 3.
Changes in the BMD during denosumab therapy in patients with or without glucocorticoid administration. The BMD of patients with (a) and without (b) glucocorticoid treatment. Data are expressed as the median with 25th to 75th percentiles.
Comparing the BMD changes by glucocorticoid dose
Based on the JSBMR guidelines for the management of osteoporosis with glucocorticoids, we stratified the glucocorticoid doses (prednisolone equivalent dose: <5 mg/day, 5-7.5 mg/day and ≥7.5 mg/day) and analyzed the differences in the BMD increase. As shown in Fig. 4, the increased rate of BMD from baseline to 12 months tended to decrease with increasing glucocorticoid dose (<5 mg/day: 7.6%±1.9%, 5-7.5 mg/day: 5.8%±1.1% and ≥ 7.5 mg/day: 4.8±1.7%).
Figure 4.
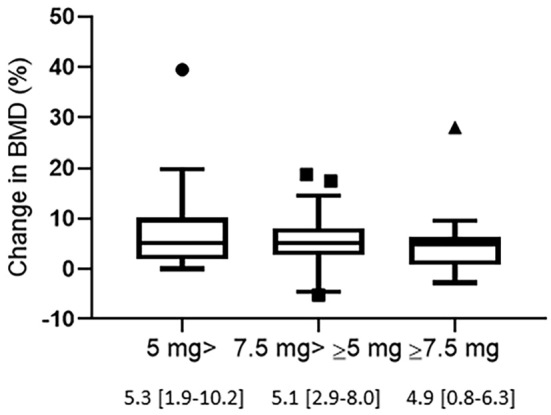
Changes in the BMD from baseline to 12 months in patients treated with denosumab by glucocorticoid dose. Glucocorticoid doses are expressed in mg/day of prednisolone equivalent. Data are expressed as the median with 25th to 75th percentiles.
Comparing RA and non-RA patients
Baseline characteristics were compared between RA patients (n=51) and non-RA patients (n=49) (Table 3). There were no significant differences between these two groups with respect to the age, sex, menopausal status, or BMI. The percentage of prednisolone usage was significantly lower in the RA patients than in the non-RA patients. The daily prednisolone-equivalent dose at baseline was significantly lower in the RA patients than in the non-RA patients. The serum P1NP and BAP levels were significantly higher in the RA patients than in the non-RA patients at baseline. There were no significant differences in the change in bone turnover markers between the RA and non-RA patients after 12 months of denosumab treatment. As shown in Fig. 5, the increased rate of BMD from baseline to 12 months was significantly higher in the RA patients than in the non-RA patients (RA patients: 7.7%±0.9%; non-RA patients: 5.9%±1.1%).
Table 3.
Comparison between RA Patients and Non-RA Patients at Baseline.
| RA patients (n=51) | Non-RA patients (n=49) | p value | |
|---|---|---|---|
| Age (yr) | 69.9±1.3 71.0 [64.0-76.0] |
65.1±2.9 67.0 [59.0-76.0] |
0.1843 |
| Number of male/female | 5/46 | 2/47 | 0.4367 |
| Postmenopausal (%) | 50 (98.0) | 43 (87.8) | 0.0572 |
| Body mass index (kg/m2) | 20.7±0.5 20.0 [18.3-22.6] |
21.0±0.4 20.4 [18.6-23.4] |
0.4888 |
| Prednisolone usage (%) | 26 (51.0) | 42 (85.7) | 0.0002 |
| Daily prednisolone-equivalent dose (mg/day) | 4.6±0.5 5.0 [2.0-5.0] |
8.2±1.2 5.0 [4.0-10.0] |
0.0223 |
| Bone mineral density (g/cm2) | 0.74±0.01 0.74 [0.65-0.84] |
0.77±0.02 0.78 [0.69-0.84] |
0.1638 |
| Pre-denosumab treatment, no. (%) | |||
| Bisphosphonates | 30 (58.9) | 30 (70) | 0.8405 |
| Teriparatide | 4 (10) | 6 (10) | 0.5208 |
| Vitamin D | 6 (88.9) | 2 (0) | 0.2695 |
| Vitamin K | 1 (33.3) | 2 (0) | 0.6136 |
| SERM | 1 (2.2) | 2 (10) | 0.6136 |
| Baseline of bone turnover markers | |||
| NTx (nmolBCE/L) | 16.7±0.9 15.2 [12.5-21.1] |
15.9±0.9 13.3 [11.4-19.5] |
0.2926 |
| TRACP-5b (mU/dL) | 287.5±23.7 258.0 [169.0-368.0] |
194.3±36.3 215.0 [114.0-285.5] |
0.0505 |
| P1NP (µg/L) | 37.5±4.5 27.4 [20.7-46.6] |
29.4±4.0 24.1 [12.7-36.1] |
0.0127 |
| BAP (µg/L) | 16.2±2.0 13.4 [10.4-17.9] |
10.9±0.8 10.1 [8.1-11.7] |
0.0001 |
| OC (ng/mL) | 6.8±0.4 6.1 [4.8-8.0] |
7.3±0.9 5.7 [3.7-8.2] |
0.4093 |
| Change of bone turnover markers (%) | |||
| Δ NTx | -14.6±5.2 -23.5 [-40.8- 7.2] |
-22.5±5.0 -30.6 [-41.7- -6.7] |
0.2327 |
| Δ TRACP-5b | -42.4±7.6 -60.5 [-75.7- -29.4] |
-35.8±8.2 -53.0 [-68.8- -9.9] |
0.3545 |
| Δ P1NP | -38.7±5.2 -43.4 [-66.6- -20.3] |
-19.9±9.4 -41.6 [-67.4- 7.4] |
0.4683 |
| Δ BAP | -25.8±5.0 -31.9 [-45.8- -16.0] |
-2.3±20.0 -19.8 [-36.3- -10.8] |
0.0770 |
| Δ OC | 10.9±22.2 -27.3 [-44.5- 5.8] |
63.5±47.8 -26.0 [-50.5- 4.1] |
0.5658 |
RA: rheumatoid arthritis, SERM: selective estrogen receptor modulators, NTx: N-telopeptide crosslinked of type I collagen, TRACP-5b: tartrate-resistant acid phosphatase isoform 5b, P1NP: procollagen type I N-terminal peptide, BAP: bone alkaline phosphatase, OC: osteocalcin, Δ: the rate of change during 12 months
Figure 5.
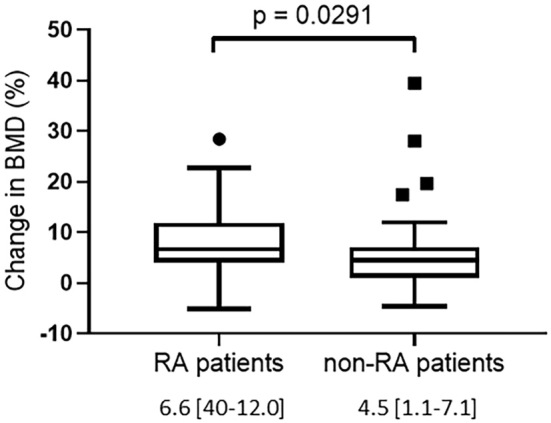
Changes in the BMD from baseline to 12 months in RA and non-RA patients. Data are expressed as the median with 25th to 75th percentiles.
Comparing responders to denosumab and non-responders
In accordance with previous clinical trials (10), we defined non-responders as those with no gain in the BMD (i.e. BMD change < 0%) and responders as those with any gain in the BMD. Specifically, responders to denosumab treatment were defined as patients whose BMD after 12 months had increased compared with baseline (n=90), while non-responders to denosumab treatment were patients whose BMD after 12 months had decreased compared with the baseline or stayed the same (n=10).
The demographics and clinical data at baseline were compared between responders and non-responders (Table 4). There were no significant differences between these two groups with respect to the age, sex, menopausal status, or BMI. There was also no marked difference in the prednisolone rate between the two groups, although the daily prednisolone dosage tended to be higher in the non-responders than in the responders. The percentages of underlying diseases were not significantly different between the two groups, nor were there any marked differences between the groups in the baseline serum levels of NTx, TRACP-5b or P1NP, and OC, although the serum BAP level was significantly higher in the responders than in the non-responders at baseline. The changes in serum TRACP-5b and BAP from baseline to 12 months were significantly lower in the responders than in non-responders (p=0.0454 and 0.0457, respectively). The changes in serum NTx, P1NP and OC from baseline to 12 months were also lower in the responders than in non-responders, although not to a significant degree.
Table 4.
Comparison between Responder Group and Non-responder Group at Baseline.
| Responder group (n=90) | Non-responder group (n=10) | p value | |
|---|---|---|---|
| Age (yr) | 67.3±1.3 69.0 [62.8-76.0] |
69.3±2.9 70.5 [60.5-77.3] |
0.7879 |
| Number of male/female | 6/84 | 1/9 | 0.5333 |
| Postmenopausal (%) | 77 (91.7) | 9 (100) | 0.9999 |
| Body mass index (kg/m2) | 20.8±0.3 20.3 [18.6-22.7] |
20.5±1.0 19.5 [18.0-22.7] |
0.6202 |
| Prednisolone usage (%) | 61 (67.8) | 7 (70.0) | 0.9999 |
| Daily prednisolone-equivalent dose (mg/day) | 6.1±0.6 5.0 [3.0-8.0] |
13.6±5.0 6.0 [5.0-20.0] |
0.0666 |
| Bone mineral density (g/cm2) | 0.75±0.01 0.76 [0.67-0.84] |
0.78±0.04 0.78 [0.67-0.81] |
0.8763 |
| Diagnosis, no. (%) | |||
| Rheumatoid arthritis | 47 (52.2) | 4 (40) | 0.5208 |
| Systemic lupus erythematosus | 13 (14.4) | 1 (10) | 0.9999 |
| Polymyalgia rheumatica | 10 (11.1) | 1 (10) | 0.9999 |
| Polymyositis/dermyositis | 3 (3.3) | 2 (20) | 0.0628 |
| Mixed connected tissue disease | 4 (4.4) | 0 (0) | 0.9999 |
| Sjögren’s syndrome | 4 (4.4) | 0 (0) | 0.9999 |
| Vasculitis syndrome | 3 (3.3) | 1 (10) | 0.3484 |
| Systemic scleroderma | 3 (3.3) | 0 (0) | 0.9999 |
| Others | 3 (3.3) | 1 (10) | 0.3484 |
| Pre-denosumab treatment, no. (%) | |||
| Bisphosphonates | 53 (58.9) | 7 (70) | 0.7357 |
| Teriparatide | 9 (10) | 1 (10) | 0.9999 |
| Vitamin D | 8 (88.9) | 0 (0) | 0.9999 |
| Vitamin K | 3 (33.3) | 0 (0) | 0.9999 |
| SERM | 2 (2.2) | 1 (10) | 0.2735 |
| Baseline of bone turnover markers | |||
| NTx (nmolBCE/L) | 16.3±0.7 14.7 [11.9-19.5] |
17.3±1.8 17.4 [12.8-22.5] |
0.3877 |
| TRACP-5b (mU/dL) | 271.3±18.5 251.0 [156.5-347.8] |
194.3±36.3 189.0 [88.0-278.5] |
0.1572 |
| P1NP (µg/L) | 34.1±3.4 25.4 [17.7-41.8] |
27.9±3.7 28.3 [18.1-39.3] |
0.9465 |
| BAP (µg/L) | 14.1±1.3 11.6 [8.9-15.6] |
9.3±0.7 9.6 [7.2-11.3] |
0.0499 |
| OC (ng/mL) | 7.2±0.5 6.0 [4.0-8.2] |
5.8±1.0 5.6 [3.5-8.1] |
0.5044 |
| Change of bone turnover markers (%) | |||
| Δ NTx | -20.4±3.4 -25.6 [-39.1- -4.6] |
-1.5±18.7 -30.3 [-46.1-61.1] |
0.7736 |
| Δ TRACP-5b | -45.2±4.8 -59.0 [-72.7- -27.5] |
16.5±29.5 10.5 [-64.4- 69.4] |
0.0454 |
| Δ P1NP | -33.1±5.2 -43.4 [-68.2- -15.9] |
8.0±27.5 -21.0 [-62.7- 104.1] |
0.2281 |
| Δ BAP | -15.5±11.3 -27.9 [-44.4- -13.6] |
-4.4±10.7 -13.0 [-29.5- 25.1] |
0.0457 |
| Δ OC | -3.4±13.3 -28.6 [-46.7- 3.3] |
63.5±47.8 -5.7 [-48.6- 189.1] |
0.1914 |
SERM: selective estrogen receptor modulators, NTx: N-telopeptide crosslinked of type I collagen, TRACP-5b: tartrate-resistant acid phosphatase isoform 5b, P1NP: procollagen type I N-terminal peptide, BAP: bone alkaline phosphatase, OC: osteocalcin, Δ: the rate of change during 12 months
Correlations between changes in bone mineral density and patient characteristics
We examined the independent influence of changes in the BMD in our patients with rheumatic diseases by multiple regression analyses adjusted for patient characteristics (age, gender, and BMI), prednisolone dose at baseline, and change in bone turnover markers (NTx, TRACP-5b, P1NP, BAP, and OC) (Table 5). Factors significantly related to an increased BMD according to a univariate analysis were a young age and low prednisolone dosage at baseline. The multivariate model resulted in the final selection of a young age, low prednisolone dosage, and reduction in NTx levels as significantly related factors (Table 5).
Table 5.
Univariate and Multivariate Analysis of Characteristics Associated with Bone Mineral Density Response to Denosumab: Analysis with Change of Bone Metabolism Markers.
| Characteristics | Change of bone mineral density | ||||
|---|---|---|---|---|---|
| Univariate model | Multivariate model | ||||
| β | p value | R2 | β | p value | |
| Age | -0.0031 | 0.00067 | 0.103 | -0.0042 | 0.0006 |
| Female | -0.0132 | 0.76689 | 0.030 | ||
| Body mass index | 0.0047 | 0.17990 | 0.008 | ||
| Prednisolone dose at baseline | -0.0039 | 0.03385 | 0.035 | -0.0113 | 0.0016 |
| Δ NTx | -0.00064 | 0.07768 | 0.022 | -0.0013 | 0.0264 |
| Δ TRACP-5b | 0.00021 | 0.30230 | 0.001 | ||
| Δ P1NP | 0.00012 | 0.2457 | 0.004 | ||
| Δ BAP | 0.00029 | 0.40372 | 0.086 | ||
| Δ OC | 0.00004 | 0.78455 | 0.029 | ||
NTx: N-telopeptide crosslinked of type I collagen, TRACP-5b: tartrate-resistant acid phosphatase isoform 5b, P1NP: procollagen type I N-terminal peptide, BAP: bone alkaline phosphatase, OC: osteocalcin, Δ: the rate of change during 12 months
As shown in Table 6, we also assessed the independent influence of changes in the BMD in our patients with rheumatic diseases by multiple regression analyses adjusted for patient characteristics (age, gender, and BMI), prednisolone dose at baseline, and bone turnover markers at baseline. Factors significantly related to an increased BMD according to a univariate analysis were the NTx and P1NP levels at baseline. The multivariate model resulted in the final selection of the BMI as a significantly related factor (Table 6).
Table 6.
Univariate and Multivariate Analysis of Characteristics Associated with Bone Mineral Density Response to Denosumab: Analysis with Bone Metabolism Markers at Baseline.
| Characteristics | Change of bone mineral density | ||||
|---|---|---|---|---|---|
| Univariate model | Multivariate model | ||||
| β | p value | R2 | β | p value | |
| Age | 0.1041 | 0.07575 | 0.0325 | ||
| Female | 3.072 | 0.26883 | 0.0127 | ||
| Body mass index | 0.3970 | 0.07448 | 0.0328 | 0.476 | 0.02892 |
| Prednisolone dose at baseline | -0.1402 | 0.22493 | 0.0153 | ||
| NTx | 0.2677 | 0.02035 | 0.0554 | ||
| TRACP-5b | 0.005648 | 0.18122 | 0.0185 | ||
| P1NP | 0.04703 | 0.04201 | 0.0437 | ||
| BAP | 0.04707 | 0.45998 | 0.0058 | ||
| OC | 0.2716 | 0.0667 | 0.035 | ||
NTx: N-telopeptide crosslinked of type I collagen, TRACP-5b: tartrate-resistant acid phosphatase isoform 5b, P1NP: procollagen type I N-terminal peptide, BAP: bone alkaline phosphatase, OC: osteocalcin
Adverse events
CTCAE grade 2 minor upper respiratory tract infections occurred in 13 patients, and colitis occurred in 5 patients. Two patients developed eczema (CTCAE grade 1). However, no serious adverse events, including infections and neoplasms, were observed during this study. Two patients experienced asymptomatic hypocalcemia (CTCAE grade 1). No osteonecrosis of the jaw or atypical femoral fracture was observed. The incidence of any infection in the glucocorticoid-treated and non-glucocorticoid-treated groups was 22.0% (15/68) and 9.3% (3/32), respectively.
Fracture incidence
No new vertebral fracture cases were detected by radiography during the first 12 months of denosumab treatment.
Discussion
The present study demonstrated that treatment with denosumab increased the BMD in patients with rheumatic diseases. We found that the factors related to an increased BMD in response to denosumab were a young age, low prednisolone dosage, and high reduction in NTx according to a multivariate analysis.
Previous reports have shown the effects of denosumab in patients with rheumatic diseases. Saag et al. (11) compared the efficacy of denosumab and risedronate in 615 patients with rheumatic disease in a multicenter, randomized, double-blind, active-controlled, double-dummy, non-inferiority study. They found that denosumab had superior efficacy to risedronate at 12 months for increasing the BMD at both lumbar and hip sites. In a recent retrospective study, the mean percentage change in the BMD from baseline to 6 and 12 months in 66 rheumatic diseases patients under glucocorticoid therapy was significantly increased, regardless of the history of anti-osteoporotic drug treatment (12). In this study, we also showed that denosumab was effective at increasing the BMD in patients with rheumatic diseases.
Bone turnover markers are considered useful tools for monitoring the response to treatment. After 12 months of denosumab therapy, most studies in patients with rheumatic diseases showed significant reductions in bone-turnover markers (12-17). We evaluated various bone turnover markers, including serum P1NP, BAP, and OC as bone formation markers and serum NTx and TRACP-5b as bone resorption markers. Both bone formation and bone resorption markers were significantly decreased from baseline to 12 months, regardless of glucocorticoid presence. These results indicate that denosumab increases the BMD via the strong suppression of bone turnover, even in patients with rheumatic diseases, regardless of glucocorticoid treatment. Taken together, these findings suggest that denosumab may be a viable first-line drug as a therapeutic option for patients with glucocorticoid-induced osteoporosis.
Iwamoto et al. (12) reported that 16% of patients with rheumatic diseases under glucocorticoid therapy had a decreased BMD at 12 months after the initiation of denosumab. In our study, 10% of patients with rheumatic diseases had a decreased BMD at 12 months after the initiation of denosumab. Bone turnover markers, including TRAP-5b and BAP, were more markedly decreased in denosumab responders than in non-responders.
We found that young patients who received low-dose glucocorticoid enjoyed an increased effectiveness of denosumab according to a multivariate analysis. As a result, these were considered response-related factors. We need to examine the BMD in order to evaluate the effect of denosumab, especially for patients with correlated factors, such as old age and high-dose glucocorticoids. In addition, the rate of decrease in the NTx level was extracted as independently associated with an increased BMD. Previous studies have reported that early changes in serum NTx levels predict long-term changes in vertebral BMD in elderly women receiving alendronate therapy (18). Among bone turnover markers, changes in NTx specifically may be useful for predicting changes in the BMD among patients with rheumatic diseases after denosumab therapy.
Petranova et al. (15) reported that the fracture risk reduction after 12 months of treatment with denosumab was weaker in glucocorticoid-treated patients than in non-glucocorticoid-treated patients. We further stratified our analysis by the glucocorticoid dose. The results suggested that denosumab treatment decreased the rate of BMD change in a glucocorticoid dose-dependent manner. The rate of BMD change in RA patients was significantly higher than that in non-RA patients. This result may reflect the difference in baseline glucocorticoid dosage.
It was reported that no patients developed antibodies against denosumab with neutralizing activity in previous clinical trials (19). However, patients can rarely but occasionally develop denosumab-binding antibodies. In particular, the frequency of denosumab-binding antibodies in the RA patients was higher than in cancer and postmenopausal osteoporosis patients (20). Antibodies against denosumab may inhibit its effect.
A previous meta-analysis of 11 studies using denosumab to treat postmenopausal women with osteoporosis indicated an increased risk of serious adverse events related to infections (21). However, a recent meta-analysis of denosumab treatment in patients with administered glucocorticoids did not detect a marked difference in the frequency of infections between the denosumab and control groups (22). In the present study, the incidence of infections in glucocorticoid-treated patients tended to be higher than in non-glucocorticoid-treated patients. However, our patients did not develop serious adverse events during the study period. Furthermore, osteonecrosis of the jaw, atypical fracture, and severe symptomatic hypocalcemia did not occur in our study. Thus, denosumab was demonstrated to be a viable option for treating rheumatic disease patients with osteoporosis.
This study however has several limitations. Since it was performed in a single hospital with a retrospective observational design, patient selection bias may have affected the results. Furthermore, we evaluated the efficacy of denosumab over 12 months. However, the long-term efficacy and safety of denosumab, which are required to cement it as a viable treatment for rheumatic disease patients with osteoporosis, remain unclear. Further research will be necessary in the future.
Conclusion
The present study indicated that denosumab was effective for increasing the BMD in osteoporosis in rheumatic disease patients. We found that old age, a low change in bone turnover markers, and high-dose glucocorticoid use were associated with non-response to the therapy. We should closely monitor the BMD of patients with these risk factors.
Author's disclosure of potential Conflicts of Interest (COI).
Shinichi Kawai: Speaking fees, Daiichi Sankyo; Research funding, Daiichi Sankyo. Toshihiro Nanki: Speaking fees, Daiichi Sankyo; Research funding, Daiichi Sankyo.
References
- 1.Maruotti N, Corrado A, Cantatore FP. Osteoporosis and rheumatic diseases. Reumatismo 66: 125-135, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Bultink IE, Vis M, van der Horst-Bruinsma IE, Lems WF. Inflammatory rheumatic disorders and bone. Curr Rheumatol Rep 14: 224-230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schett G. Osteoimmunology in rheumatic diseases. Arthritis Res Ther 11: 210, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402: 304-309, 1999. [DOI] [PubMed] [Google Scholar]
- 5.McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354: 821-831, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Silva I, Branco JC. Denosumab: recent update in postmenopausal osteoporosis. Acta Reumatol Port 37: 302-313, 2012. [PubMed] [Google Scholar]
- 7.Soen S, Fukunaga M, Sugimoto T, et al. Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab 31: 247-257, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Nawata H, Soen S, et al. Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J Bone Miner Metab 32: 337-350, 2014. [DOI] [PubMed] [Google Scholar]
- 9. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. 2017. Common terminology criteria for adverse events (CTCAE) version 5.0 [Internet]. [cited 2017 Nov 27]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- 10.Lewiecki EM. Nonresponders to osteoporosis therapy. J Clin Densitom 6: 307-314, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 6: 445-454, 2018. [DOI] [PubMed] [Google Scholar]
- 12.Iwamoto N, Okamoto M, Tsuji S, et al. Denosumab is effective toward glucocorticoid-induced osteoporosis patients complicated with rheumatic diseases regardless of prior anti-osteoporotic drugs. J Bone Miner Metab 37: 554-562, 2019. [DOI] [PubMed] [Google Scholar]
- 13.Coskun Benlidayi I. Denosumab in the treatment of glucocorticoid-induced osteoporosis. Rheumatol Int 38: 1975-1984, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Dore RK, Cohen SB, Lane NE, et al. Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann Rheum Dis 69: 872-875, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petranova T, Sheytanov I, Monov S, Nestorova R, Rashkov R. Denosumab improves bone mineral density and microarchitecture and reduces bone pain in women with osteoporosis with and without glucocorticoid treatment. Biotechnol Biotechnol Equip 28: 1127-1137, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mok CC, Ho LY, Ma KM. Switching of oral bisphosphonates to denosumab in chronic glucocorticoid users: a 12-month randomized controlled trial. Bone 75: 222-228, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Sawamura M, Komatsuda A, Togashi M, Wakui H, Takahashi N. Effects of denosumab on bone metabolic markers and bone mineral density in patients treated with glucocorticoids. Intern Med 56: 631-636, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenspan SL, Rosen HN, Parker RA. Early changes in serum N-telopeptide and C-telopeptide cross-linked collagen type 1 predict long-term response to alendronate therapy in elderly women. J Clin Endocrinol Metab 85: 3537-3540, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res 33: 190-198, 2018. [DOI] [PubMed] [Google Scholar]
- 20. Adrienne Rothstein. US Food and Drug Administration. MedWatch: the FDA safety information and adverse event reporting program [Internet]. [cited 2021 Jul 10]. Available from: https://courses.washington.edu/bonephys/denosumab/Rothstein%20FDA%20deno%20safety.pdf
- 21.Zhou Z, Chen C, Zhang J, et al. Safety of denosumab in postmenopausal women with osteoporosis or low bone mineral density: a meta-analysis. Int J Clin Exp Pathol 7: 2113-2122, 2014. [PMC free article] [PubMed] [Google Scholar]
- 22.Yanbeiy ZA, Hansen KE. Denosumab in the treatment of glucocorticoid-induced osteoporosis: a systematic review and meta-analysis. Drug Des Devel Ther 13: 2843-2852, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]