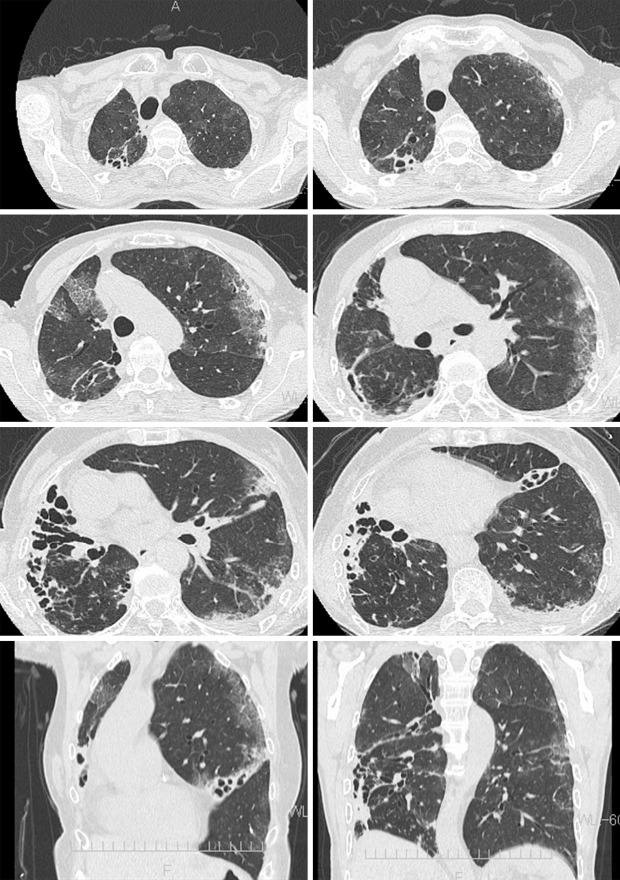
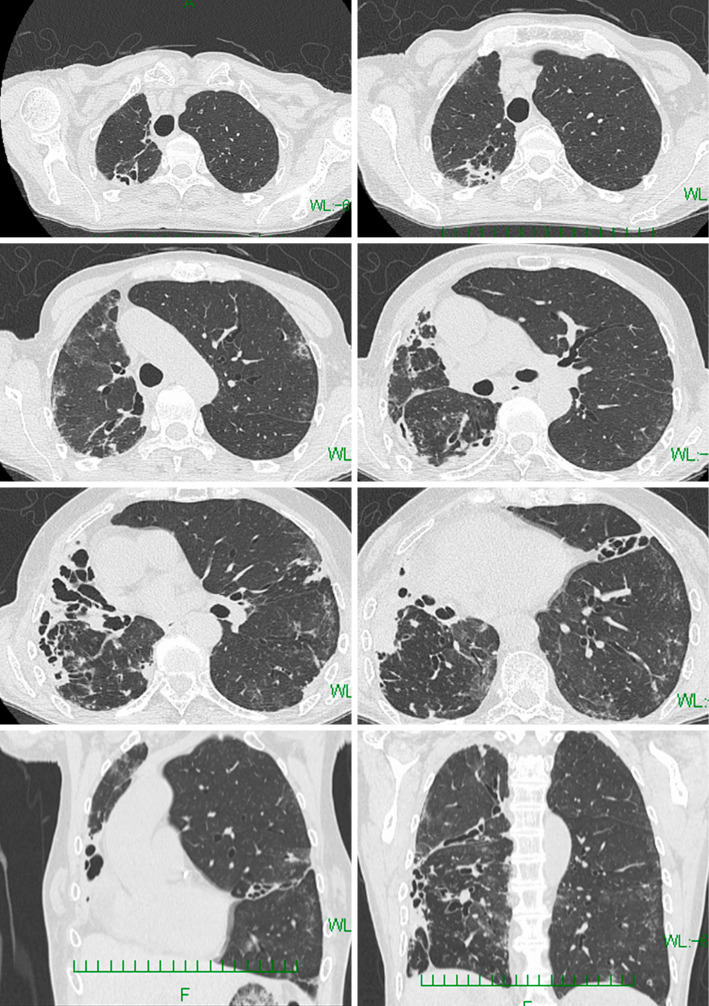
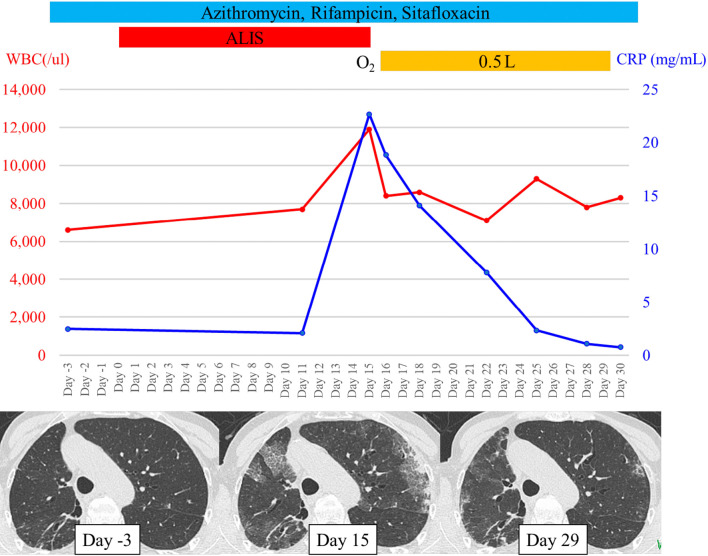
A 78-year-old Japanese woman was prescribed a daily regimen of amikacin liposome inhalation suspension (ALIS) with oral azithromycin, rifampicin, and sitafloxacin for refractory pulmonary mycobacteriosis with Mycobacterium avium complex (MAC) that was resistant to clarithromycin (minimum inhibitory concentration ≥128 μg/mL). She had no history of aminoglycoside antibiotics use prior to the ALIS administration. Two weeks later, she developed a fever (38.0°C) and worsening dyspnea, and her oxyhemoglobin saturation was 96% on room air. Chest computed tomography (CT) revealed new bilateral patchy ground-glass attenuations (Picture 1). Laboratory findings revealed peripheral leukocytosis (11,900 /μL), elevated C-reactive protein levels (18.83 mg/dL) and Krebs von den Lungen-6 (1,158 U/mL). Polymerase chain reaction (FilmArrayⓇ, bioMérieux Japan, Tokyo) tests on nasal swab for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and 19 other pathogens were all negative. The clinical, laboratory, and computed tomography findings (Picture 2) improved 14 days after ALIS cessation (Picture 3). ALIS has been used for refractory pulmonary MAC infection since March 2021 in Japan. Hypersensitivity pneumonitis as an adverse effect was reported in 3.1% of patients in a phase 3 study (1); however, the chest imaging findings have never been reported. This is the first report showing chest imaging findings of ALIS-induced pneumonitis.
Picture 1.
Picture 2.
Picture 3.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Griffith DE, Eagle G, Thomson R, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med 198: 1559-1569, 2018. [DOI] [PubMed] [Google Scholar]