Highlights
-
•
Individuals with MDD spent more time in a DMN-FPN CAP (CAP 3) relative to HCs.
-
•
MDD diagnosis was linked to more transitions between DMN and DMN-FPN CAPs.
-
•
CSA was not associated with any CAP metrics.
-
•
Higher rumination was associated with increased time in a DMN-FPN CAP.
-
•
Higher rumination was related to greater transitions between DMN and DMN-FPN CAPs.
Keywords: Depression, Early life stress, Co-activation pattern analysis, Resting state fMRI, Rumination, Brain network dynamics
Abstract
Early life stress (ELS) and major depressive disorder (MDD) share neural network abnormalities. However, it is unclear how ELS and MDD may separately and/or jointly relate to brain networks, and whether neural differences exist between depressed individuals with vs without ELS. Moreover, prior work evaluated static versus dynamic network properties, a critical gap considering brain networks show changes in coordinated activity over time. Seventy-one unmedicated females with and without childhood sexual abuse (CSA) histories and/or MDD completed a resting state scan and a stress task in which cortisol and affective ratings were collected. Recurring functional network co-activation patterns (CAPs) were examined and time in CAP (number of times each CAP is expressed) and transition frequencies (transitioning between different CAPs) were computed. The effects of MDD and CSA on CAP metrics were examined and CAP metrics were correlated with depression and stress-related variables. Results showed that MDD, but not CSA, related to CAP metrics. Specifically, individuals with MDD (N = 35) relative to HCs (N = 36), spent more time in a posterior default mode (DMN)-frontoparietal network (FPN) CAP and transitioned more frequently between posterior DMN-FPN and prototypical DMN CAPs. Across groups, more time spent in a posterior DMN-FPN CAP and greater DMN-FPN and prototypical DMN CAP transition frequencies were linked to higher rumination. Imbalances between the DMN and the FPN appear central to MDD and might contribute to MDD-related cognitive dysfunction, including rumination. Unexpectedly, CSA did not modulate such dysfunctions, a finding that needs to be replicated by future studies with larger sample sizes.
1. Introduction
Childhood sexual abuse (CSA) is a strong risk factor for Major Depressive Disorder (MDD), with some meta-analyses showing that CSA may be more strongly linked to MDD than other forms of early life stress (ELS) (Li et al., 2016, Munzer et al., 2016). Both MDD and ELS more broadly (including exposure to CSA) have been independently characterized by disrupted resting state functional connectivity (RSFC) of several large-scale neural networks, including the default mode network (DMN), the salience network (SN), and the frontoparietal network (FPN) (Fadel et al., 2021, Kaiser et al., 2015, Manoliu et al., 2014, Philip et al., 2013, Philip et al., 2014, Yu et al., 2019). However, it is unclear whether ELS and MDD may have overlapping or distinct influences on these neural circuits. The DMN is involved in internally focused cognition and has core hubs in the medial prefrontal cortex and posterior cingulate cortex (Greicius et al., 2003, Vanhaudenhuyse et al., 2011). The FPN is typically anticorrelated with the DMN, has core hubs in the dorsolateral prefrontal cortex and posterior parietal cortex, and supports external awareness or goal-directed attention (Fox et al., 2005, Greicius et al., 2003, Seeley et al., 2007, Vanhaudenhuyse et al., 2011). The salience network (SN), which includes the anterior insula and dorsal anterior cingulate cortex, is involved in directing attention toward salient stimuli and also mediates DMN-FPN interactions (Seeley et al., 2007, Sridharan et al., 2008). ELS has been linked to more chronic and treatment-resistant courses of MDD (Nelson et al., 2017), which raises the possibility that MDD development within the context of ELS may represent a different phenotype with unique functional neural network abnormalities compared to those who develop MDD without an ELS history.
Without accounting for ELS histories, individuals with MDD have been characterized by DMN functional hyperconnectivity, but FPN functional hypoconnectivity (Kaiser et al., 2015). MDD has also been associated with between-network functional abnormalities, including hyperconnectivity between the DMN and FPN (Kaiser et al., 2015). Although less frequently reported, SN abnormalities have been implicated in MDD, including within the context of regulating DMN-FPN interactions (Hamilton et al., 2011, Manoliu et al., 2014, Yu et al., 2019). Consistent with findings in MDD, ELS has also been associated with hypoconnectivity of the FPN (Philip et al., 2014). However, unlike MDD, ELS has been linked to DMN and DMN-FPN hypoconnectivity (Demir-Lira et al., 2016, Hoffmann et al., 2018, Philip et al., 2013, Philip et al., 2014). That said, most of these RSFC studies on ELS were conducted in healthy control (HC) samples and thus were unable to examine separable versus interacting effects of MDD and ELS on RSFC.
Few studies have included HC and MDD groups, both with and without ELS histories, allowing for proper examination of ELS versus MDD-related effects on RSFC. One study found that among those with ELS exposure, greater depressive symptom severity was associated with reduced SN RSFC (Fadel et al., 2021). However, among those without ELS exposure, depressive symptom severity was associated with increased SN RSFC (Fadel et al., 2021). Another study probing frontostriatal networks found that ELS and MDD were associated with different frontostriatal network aberrations (Fan et al., 2021). Critically, these neurobiological differences may have important implications for treatment. If those with MDD and an ELS history represent a distinct neurobiological phenotype of MDD, it is possible that different treatment interventions are warranted.
A possible limitation among studies on MDD and ELS is that most studies probed “static” functional brain networks – i.e., the average functional connectivity of neural networks across an entire resting state scan. However, functional interactions among brain regions change over time (Bolton et al., 2020a, Lurie et al., 2020). Dynamic network analytical approaches capture changes in coordinated activity between brain regions over time (Bolton et al., 2020a, Lurie et al., 2020). Also, evidence suggests that dynamic network functioning may be a more sensitive marker of MDD than static network abnormalities (Yan et al., 2020). Among the most commonly used dynamic network approach is the sliding window method, in which changes in RSFC are examined over short time windows across the scan and quantify the extent to which functional connectivity fluctuates over time.
Studies in MDD using the sliding window approach have yielded inconsistent findings, with studies reporting increased or decreased FC variability within and between the DMN, SN, and FPN (Han et al., 2020, Kaiser et al., 2016, Long et al., 2020, Pang et al., 2018, Wang et al., 2020, Wei et al., 2017, Wise et al., 2017, Zhang et al., 2020). ELS has also been linked to increased DMN and FPN RSFC variability within a sample of psychiatrically healthy participants (Huang et al., 2021). A study using the sample investigated in the current report probed amygdala-PFC dynamic RSFC and found that greater severity of ELS was related to increased amygdala-PFC RSFC variability, even when controlling for MDD diagnosis (Kaiser et al., 2018).
Other studies have incorporated a co-activation pattern (CAP) analytical approach, which eliminates the use of arbitrary sliding windows that risks the loss of important information about brain dynamics, and clusters fMRI data into distinctive patterns of brain co-activation. The CAP analysis approach allows for examination of several dynamic metrics, including the amount of time a participant spends in a particular coactivation pattern and the number of times participants transition from one to another coactivation pattern. To date, CAP studies have shown that individuals with MDD spend more time in coactivation patterns involving DMN, FPN, and SN regions as well as transition more frequently between coactivation patterns involving core regions of these networks (Hou et al., 2021, Kaiser et al., 2019). With respect to ELS, the one study applying a CAP analysis, did not uncover associations between ELS and time spent in a certain CAP or transitioning between different coactivation patterns (Iadipaolo et al., 2018). To date, no study has applied a dynamic network analytic approach to HC and MDD groups, both with and without ELS histories, to test the unique versus interacting effects of MDD and ELS on dynamic resting state functional coactivation pattern properties.
In addition to relating whole-brain dynamic brain network properties to MDD diagnosis and CSA exposure, we examined whether CAP metrics were also linked to cognitive and emotional processes relevant to MDD and/or CSA, including rumination and stress reactivity measured by stress-induced cortisol output and subjective negative affective ratings in response to stress. While rumination has been strongly linked to MDD (Nolen-Hoeksema, 1991), abnormal cortisol output in response to stress has been associated with both MDD and ELS exposure (Bunea et al., 2017, Zorn et al., 2017). A study conducted in a largely medicated sample of adolescents with varying degrees of depressive symptomology found that spending more time in, and transitioning more frequently between, coactivation patterns involving DMN, FPN, and SN regions were linked to a greater tendency to ruminate (Kaiser et al., 2019). Additionally, another study related amygdala RSFC variability to cortisol output in response to psychosocial stress (Kaiser et al., 2018). However, no studies have linked stress-induced cortisol responses to whole-brain CAP metrics. Based on initial evidence from the literature, we expected that MDD and CSA would have both unique and interacting influences on CAPs, especially those patterns involving the DMN, FPN, and SN. We further hypothesized that aberrant CAPs involving these networks, particularly the DMN, and transitions between DMN and FPN CAPs, would be linked to higher trait rumination as well as an enhanced cortisol and negative affective responses to stress.
2. Materials and methods
2.1. Participants
Females between the ages of 20–45 were recruited from the greater Boston area to participate if they fit into one of four groups based on an initial screening conducted over the phone: a) HCs with no history of CSA or MDD [HC group]; b) HCs with a CSA history, but no history of any psychiatric disorders, a possible marker of resilience [CSA/RES group]; c) individuals with MDD, but no CSA history [MDD group]; and d) individuals with MDD and a CSA history [MDD/CSA group]. All females were right-handed and were free of psychotropic medications for at least two weeks prior to the scan (six weeks if they had taken fluoxetine and 6 months for neuroleptics). Individuals in the HC and CSA/RES groups had to be free of any lifetime DSM-IV diagnosis and had no first-degree relatives with mood disorders or psychosis. Participants in the MDD and MDD/CSA groups had to have a DSM-IV diagnosis of MDD. Secondary anxiety disorder diagnoses were allowed in these two patient groups, but all other diagnoses, including current alcohol and substance abuse/dependence, were exclusionary. Participants in the CSA/RES and MDD/CSA groups had to endorse at least one incident of contact sexual abuse between the ages of 5 to 14. A total of 71 unmedicated individuals (n = 22 HC, n = 14 CSA/RES, n = 18 MDD, n = 17 MDD/CSA) met all study inclusion criteria, passed all MRI quality control criteria, and were included in all the analyses (see Supplementary Table S1). Demographic and clinical characteristics are summarized in Table 1.
Table 1.
Sample Demographics and Clinical Characteristics.
| Characteristics | HC (n = 22) | CSA/RES (n = 14) | MDD (n = 18) | MDD/CSA (n = 17) | F/χ2 | p |
|---|---|---|---|---|---|---|
| Age (years) | 26.45 (7.16) | 25.14 (5.66) | 24.94 (5.42) | 29.47 (6.61) | 1.82 | 0.152 |
| Race (% White) | 72.7 | 57.1 | 61.1 | 43.8 | 3.30 | 0.347 |
| Education (%) | 5.60 | 0.470 | ||||
| post-secondary years: | ||||||
| 0–2 year(s) | 36.4 | 50.0 | 27.8 | 47.1 | ||
| 4 years | 27.3 | 28.6 | 50.0 | 41.2 | ||
| <4 years | 36.4 | 21.4 | 22.2 | 11.8 | ||
| Income (%) | 0.43 | 0.935 | ||||
| <$50,000 | 61.9 | 64.3 | 61.1 | 70.6 | ||
| > $50,000 | 38.1 | 35.7 | 38.9 | 29.4 | ||
| BDI-II score | 0.83 (1.15)a,b | 2.54 (3.36)c,d | 27.44 (7.68)a,c | 25.52 (8.21)b,d | 110.53 | <0.001 |
| RRS score | 28.29 (6.81)a,b | 30.89 (6.50)c,d | 59.78 (11.42)a,c | 59.63 (8.62)b,d | 69.75 | <0.001 |
| # of MDEs (%) | 0.38 | 0.536 | ||||
| Single Episode | 27.8 | 18.8 | ||||
| Recurrent Episodes | 72.2 | 81.3 | ||||
| Comorbidities (%) | ||||||
| Current Anxiety Dx | 27.8 | 52.9 | 2.31 | 0.129 | ||
| Past Anxiety Dx | 38.9 | 64.7 | 2.33 | 0.127 | ||
| Past Substance Abuse | 11.1 | 23.5 | 0.95 | 0.330 | ||
| TAI-Upsetting | 3.36 (1.08) | 4.06 (1.12) | 3.05 | 0.092 | ||
| TAI-Impact on Life | 2.86 (0.77) | 3.81 (0.40) | 18.80 | <0.001 | ||
| Motion outliers (%) | 3.84 (3.52) | 4.80 (4.69) | 3.60 (3.35) | 4.29 (3.27) | 0.33 | 0.802 |
| Mean FD (motion) | 0.08 (0.03) | 0.10 (0.04) | 0.10 (0.04) | 0.10 (0.04) | 1.51 | 0.220 |
Means with standard deviations in parentheses. Note: HC: Healthy control without childhood sexual abuse (CSA), CSA/RES: healthy control with a CSA history, MDD: Major Depressive Disorder without CSA, MDD/CSA: MDD with CSA exposure. BDI: Beck Depression Inventory. RRS: Ruminative Response Scale. MDEs: major depressive episodes. Dx: diagnosis. TAI-Upsetting: Trauma Antecedents Interview: How upsetting was the sexual abuse? TAI-Impact on Life: How much of an impact did the sexual abuse had on the person’s life? FD: Framewise Displacement. Variables of race, education, income, single versus recurrent MDEs, % with current anxiety disorders, % with past anxiety disorders, % with past substance use disorders were tested using chi square, as indicated by a χ2. Group differences in age, BDI-II scores, RRS scores, TAI sexual abuse severity, % of motion outliers and mean FD were tested via one-way ANOVAs, as indicated by F.
MDD > HC, p < 0.05.
MDD/CSA > HC, p < 0.05.
MDD > CSA/RES, p < 0.05.
MDD/CSA > CSA/RES, p < 0.05.
2.2. Procedures
The study was approved by the Mass General Brigham Institutional Review Board. Participants provided written informed consent and completed three separate study sessions (i.e., conducted on different days) within the context of a larger study involving a pharmaceutical manipulation (see Supplementary Table S2/Methods), which did not significantly impact the current findings (see Supplementary Results). Participants first underwent a structured clinical diagnostic interview as well as an interview assessing for incidences of ELS, both conducted by experienced doctoral and masters level clinicians. After the clinical interview session, eligible participants were invited to participate in a second session involving an MRI scan that included resting state fMRI and completing self-report questionnaires. Most participants (n = 64) also completed a third session involving exposure to an acute psychosocial stressor.
2.3. Clinical diagnostic interview, trauma interview, and self-report questionnaires
Participants were administered the Structured Clinical Diagnostic Interview for the DSM-IV-TR, which assesses lifetime history of DSM-IV psychiatric disorders (First et al. 2002). Participants were also administered the Trauma Antecedents Interview (TAI), a clinician-administered interview that assesses the age of onset, severity, and duration of CSA and other forms of ELS including exposure to peer aggression, parent conflict and domestic violence, as well as parental verbal and physical abuse (Herman et al., 1989). For each ELS experience endorsed, participants were rated by clinicians on how upsetting the experience was for them on a scale of 1 (Not at all) to 5 (Extremely). Additionally, for each early life stressor, participants were rated on how much the experience impacted their life on a scale from 1 (None) to 4 (Great). Participants also completed self-report questionnaires including the Beck Depression Inventory-II (Beck et al., 1996) and Ruminative Response Scale (Nolen-Hoeksema and Morrow, 1991) to assess depressive symptom severity and rumination, respectively.
2.4. fMRI acquisition
Structural and resting state fMRI scans were collected on a Siemens Tim Trio 3 Tesla MR scanner using a 32-channel head coil at the McLean Imaging Center. A T1-weighted anatomical scan was collected with the following parameters: TR = 2200 ms, TE = 4.27 ms, flip angle = 7 degrees, 144 slices, field of view = 216 mm, matrix = 192 × 192, voxel size = 1.2 × 1.2 × 1.2 mm3. A 6-minute, eyes-open, resting state fMRI scan also was acquired with the following parameters: TR = 3000 ms, TE = 30 ms, flip angle = 85 degrees, 47 slices, field of view = 216 mm, matrix = 72 × 72, voxel size 3 × 3 × 3 mm3, total duration = 6.2 min, total volumes = 124.
2.5. fMRI preprocessing
The first six seconds of resting state fMRI data were removed to allow for magnetic field stabilization. The data were preprocessed using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12), including slice time correction, realignment to the mean image, normalization to Montreal Neurological Institute (MNI) space and resampling to 2 × 2 × 2 mm3 voxels, as well as smoothing with a 6 mm full width at half maximum Gaussian kernel. The artifact detection tool box (https://www.nitrc.org/projects/artifact_detect/) was used to identify motion-related outlier data points for subsequent censoring (below), with outlier volumes being defined as those exceeding a global mean intensity of three standard deviations away from the mean intensity across the resting state scan, or a composite threshold of 0.5 mm framewise displacement. Participants with >15 % of volumes marked as outliers were removed from all analyses (n = 1 HC, n = 1 HC/RES, n = 1 CSA/MDD).
Additional preprocessing steps were conducted using the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012), starting with the estimation of physiological noise from white matter and cerebrospinal fluid using the CompCor method (Behzadi et al., 2007). The three translational and three rotational summarizing motion parameters extracted from realignment, one composite motion parameter summarizing each participant’s maximum scan-to-scan movement, outlier images, and the CompCor-derived regressors, were modeled in a first-level hemodynamic response function-weighted general linear model and regressed out from the data. Finally, a high pass 0.01 Hz filter was applied.
2.6. Maastricht acute stress test (MAST)
At the third session, participants underwent the Maastricht Acute Stress Test (MAST), which combines the social evaluative threat elements of the Trier Psychosocial Stress Test and physical stress elements of the Cold Pressure Test (Smeets et al., 2012). The MAST was scheduled to begin between 12 and 1 p.m. to control for diurnal cortisol fluctuations. It starts with a 5-minute introduction phase followed by a 10-minute phase in five trials of a cold pressure exposure that involved participants immersing their left hand in 1–3 °C ice water for a 60- to 90-second duration controlled by a computer (which introduced uncontrollability). In-between cold pressure exposure trials, four blocks (each block duration varying between 45 and 90 s, which introduced unpredictability) of mental arithmetic were administered, in which participants were asked to subtract 17 serially from 2043. Throughout the MAST, participants were monitored by two stern experimenters (one male, one female), who instructed participants to begin the mental subtraction again at 2043 when errors were made (which introduced social evaluation). Participants were also told that, if their performance failed to meet the expected criteria, they would have to repeat the MAST (although later in the experiment, participants were debriefed and told that they would not have to repeat the MAST). Cortisol saliva samples were collected at five time points: 1) −102 min before the MAST (following a 10-minute rest period upon arrival at the laboratory); 2) +12 min post-MAST onset; 3) +28 min post-MAST onset; 4) +38 min post-MAST onset; and 5) +80 min post-MAST onset. Visual Analogue Mood Scale (VAMS) ratings measuring friendly versus hostile, relaxed versus tense, and happy versus sad feelings on a 0–100 scale (Folstein and Luria, 1973), were collected at the same five time points as the cortisol samples.
2.7. Analyses
2.7.1. Co-activation pattern (CAP) analysis
A whole-brain seed-free voxel-wise CAP analysis was conducted using the TbCAPs Toolbox, which is a voxel-wise rather than seed-based region of interest approach that uses all data volumes and has been used in prior studies (Bolton et al., 2020b, Liu et al., 2013). The whole-brain analysis was restricted to gray matter using a template derived from the DPARSF toolbox (Yan and Zang, 2010). The optimal number of CAPs was determined using consensus clustering which has been successfully applied in prior fMRI research (Monti et al., 2003, Zöller et al., 2019). Consensus clustering involves running k-means clustering over several folds, each time on a randomly selected subsample of the data, without replacement. For each fold, each fMRI volume is assigned to a given cluster, and the clustering assignments for each fold and for each fMRI volume are summarized in a consensus matrix. An optimal cluster contains fMRI volume pairs that are consistently clustered together (yielding a consensus value closer to 1) or clustered separately (i.e., a consensus value closer to 0). A suboptimal clustering solution consists of fMRI volume pairs with intermediate consensus values, indicating that across the folds, the fMRI volumes were not consistently clustered together or separately. The quality of the consensus clustering can be evaluated quantitatively by first computing the cumulative distribution of consensus values across all pairs of fMRI volumes. Then, the proportion of ambiguously clustered pairs (PAC) is computed (Șenbabaoğlu, et al., 2015), with lower PAC values indicating more consistent clustering of fMRI volume pairs across folds.
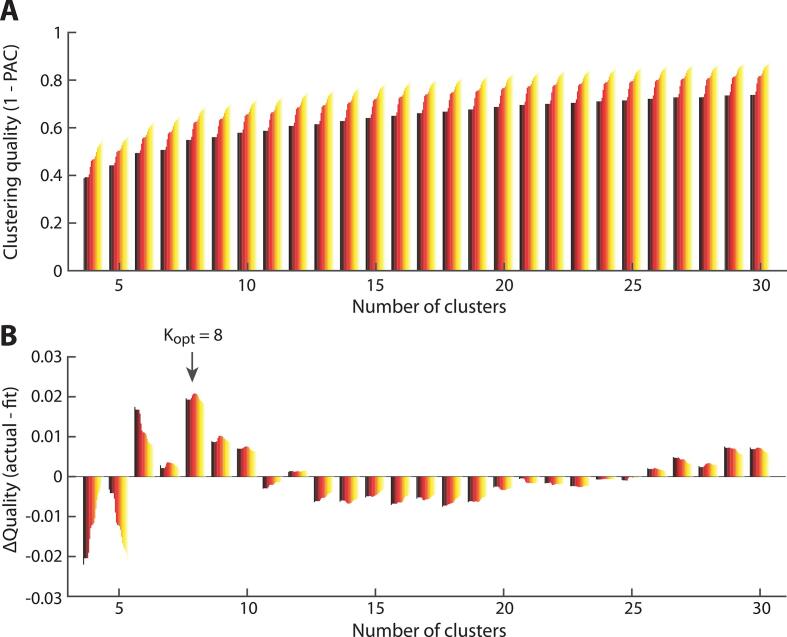
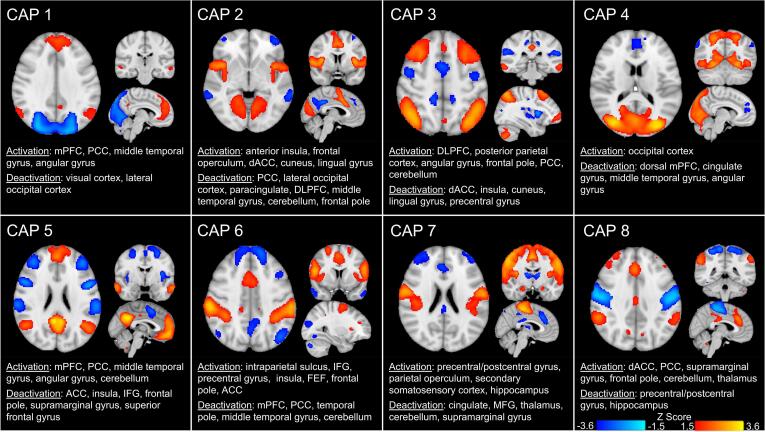
With respect to this dataset, we performed k-means clustering using the cosine distance, and random initialization of the algorithm. We ran consensus clustering for k values of 2–30. For each k, consensus clustering was run over 50 folds, each on a random selection of 80 % of the data. The optimal PAC was achieved for k = 8, which had the highest fit score (0.621) compared to the next best choice of a k = 6, which had a fit score of 0.560 (see Fig. 1). Moreover, an optimal k = 8 is also aligned with several other whole-brain CAP studies involving HC or MDD samples (Kaiser et al., 2019, Kaiser et al., 2022, Janes et al., 2020). Accordingly, we proceeded with k = 8 for subsequent analyses, running k-means clustering 100 times to avoid local minima and ensure the stability of the 8 CAPs. See Fig. 2 for a description of the 8 CAPs, which included an: (1) anterior DMN CAP, (2) SN CAP, (3) posterior DMN-FPN CAP, (4) visual system CAP, (5) prototypical DMN CAP, (6) dorsal attention network CAP, (7) somatosensory network CAP, and (8) a CAP involving prefrontal and posterior cingulate cortex regions. We computed the following CAP metrics: 1) time in CAP (total number of volumes the participant spent in a CAP throughout the scan), and 2) CAP transition frequency (how many times a specific CAP to CAP transition occurred).
Fig. 1.
Consensus clustering yields K = 8 as an optimal number of clusters. (A) Clustering quality (1 minus the percentage of ambiguously clustered frames, or PAC) across candidate cluster numbers. The color gradient for a given cluster number denotes the assessment of clustering quality with a more or less strict definition of “ambiguous clustering” (darker shades denote a more lenient one). Quality gradually increases until it reaches a plateau for large numbers of clusters. (B) When fitting an exponential function to the data and subtracting it from the actual clustering quality values, the positive-valued peaks denote cluster numbers for which the rise in quality measure exceeds that expected from the trend alone (see Bolton et al., 2020b). Kopt = 8 was selected for further analyses, as it was the global optimum within the investigated range.
Fig. 2.
Eight Co-activation Patterns (CAPs). The eight CAPs along with the corresponding brain region activations and deactivations for each CAP. Activations appear in warm colors and deactivations appear in cold colors. Activations and deactivations for each Z-scored CAP were thresholded at 1.5 ≤ Z ≤ 3.6. The eight CAPs included: 1) CAP 1 involving activations in anterior default mode network (DMN) regions, 2) CAP 2 involving activations in salience (SN) regions, 3) CAP 3 involving activations in posterior DMN and frontoparietal (FPN) regions, 4) CAP 4 involving activations in visual system network regions, 5) CAP 5 involving activations in prototypical DMN regions, 6) CAP 6 involving activations in dorsal attention network (DAN) regions, 7) CAP 7 involving activations in somatosensory network regions, and 8) CAP 8 involving prefrontal cortex, anterior cingulate cortex and posterior cingulate cortex regions.
2.7.2. Cortisol and VAMS negative affective responses to MAST
Changes in stress-related cortisol output were captured by calculating the area under the curve with respect to increase (AUCI) on log-transformed cortisol data and using participant-specific cortisol sample timing information (Pruessner et al., 2003). The three VAMS items were summed for each time point, to create a composite negative affect (NA) score at each time point. Then, the maximum post-stress onset NA response was found for each participant. This maximum stress-related VAMS NA response was used in the group-level statistical analyses.
2.7.3. Group-level analyses
Group level analyses were conducted in SPSS. One-way ANOVAs were conducted to examine group differences in age, depressive symptom severity, trait rumination, sexual abuse severity, and motion-related fMRI metrics. Chi square tests were conducted to examine group differences in race, education, income, single versus recurrent episodes of MDD, number of participants with a current or past anxiety disorder, and number of participants with a past substance use disorder. Next, eight CSA (present/absent) × MDD (present/absent) ANCOVAs were conducted on time in CAP for each of the 8 CAPs, controlling for percentage of motion outlier volumes and mean framewise displacement (FD). Given that abnormal interactions between the DMN and FPN have been linked to ELS exposure and MDD, we examined potential group differences in transition frequencies between CAPs involving DMN and FPN regions with a series of ANCOVAs. Moreover, to rule out the possibility of missing important differences in CAP metrics not captured by the above categorical analyses, CSA severity scores were correlated with CAPs metrics, controlling for motion measures. The combined scores from the two TAI questions (i.e., how upsetting was the CSA exposure and how much of an impact did it have on one’s life) was used to assess CSA severity. Additionally, CAPs showing group differences in time spent in CAP, and transition frequencies, were correlated with relevant clinical and stress measures, including BDI-II scores, RRS scores, and cortisol AUCI, controlling for all motion-related variables. Partial correlations were also conducted between significant CAP metrics and maximum self-reported NA responses to stress controlling for pre-stress NA levels and motion measures. All statistical tests were corrected for multiple comparisons using False Discovery Rate (FDR) < 0.05, conducted in R.
3. Results
3.1. Participant characteristics
There were no significant group differences in age, race distribution, education level, or income (Table 1). With respect to clinical characteristics, the MDD and MDD/CSA groups endorsed higher BDI-II and RRS scores than the HC and CSA/RES groups. There were no significant differences between the two HC groups or between the two MDD groups on BDI-II or RRS scores. All group comparisons were Bonferroni-corrected for multiple comparisons. There were no significant differences between the two MDD groups with respect to number of participants experiencing single versus recurrent MDD episodes and number of participants with current or past anxiety disorders as well as with past substance use disorders. With respect to sexual abuse severity, there were no significant differences between the two CSA groups with respect to how upsetting the sexual abuse was. However, the MDD/CSA group reported that the sexual abuse had a greater impact on their life than the CSA/RES group. The Supplemental Results provide statistics on other forms of abuse experienced by the groups. Regarding fMRI motion metrics, the groups also did not differ in number of fMRI motion-related outlier volumes or mean FD. With respect to timing of the peak NA response to stress, 78.1 % of participants had their peak NA response during the VAMS that was collected 28 min after the onset of the stressor. The groups did not differ in the number of participants showing a peak NA response 28 min post-stressor onset compared to less typical NA peak times, including 12-minutes post-stressor onset and 38-minutes post-stressor onset, χ2(3) = 1.58, p = 0.67.
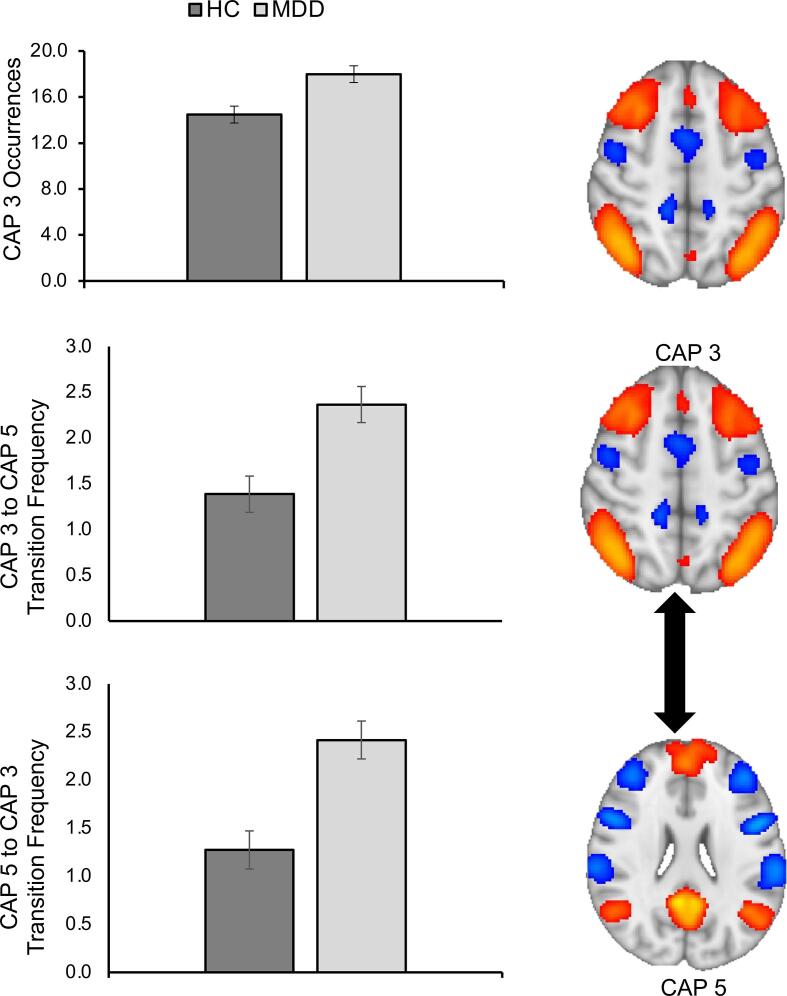
3.2. Effect of MDD and CSA on time spent in CAP
The MDD × CSA ANCOVA’s on time in CAP showed a significant main effect of MDD on time spent in CAP 3 (posterior DMN-FPN), F(1,65) = 11.39, FDR-corrected p = 0.024, ηp2 = 0.149. Compared to HCs (n = 36, HC and CSA/RES groups combined), those with MDD (n = 35; MDD and MDD/CSA groups combined) spent more time in a posterior DMN-FPN CAP (See Fig. 3, see also Supplemental Fig. S1). There were no other significant main effects of MDD on time spent in any of the other CAPs, all F’s (1,65) < 7.50, all FDR-corrected p’s > 0.11. Additionally, there were no significant main effects of CSA (n = 31, CSA/RES and MDD/CSA groups combined versus n = 40, HC and MDD groups combined) or interactions between CSA and MDD on time spent in any of the CAPs, all F’s (1,65) < 3.50, all FDR-corrected p’s > 0.40 (see Supplemental Table S3). We also conducted supplementary analyses involving re-grouping participants based on a broader definition of childhood maltreatment, incorporating experiences of verbal and physical abuse, and found no significant effects of childhood maltreatment or interactions between childhood maltreatment and MDD, all FDR-corrected p’s > 0.35 (see Supplementary Results). Finally, supplementary dimensional analyses were also conducted across the sample based on maximum severity ratings derived from several ELS experiences assessed by the Trauma Antecedents Interview, including peer aggression, parent conflict and domestic violence, parental verbal and physical abuse, as well as sexual abuse. These dimensional analyses also did not demonstrate any significant associations between time spent in any of the CAPs with ELS severity or interactions between MDD diagnostic status and ELS severity, all FDR-corrected p ‘s > 0.25 (see Supplementary Results and Supplementary Table S4). For additional supplementary analyses on other CAP metrics requested by a reviewer that were not a part of the main hypothesis driven analyses, see Supplementary Results.
Fig. 3.
Individuals with MDD spend more time in CAP 3 (posterior DMN-FPN) and transition more frequently between CAP 3 and CAP 5. Top graph: Individuals with major depressive disorder (MDD) compared to healthy controls (HCs) spent more time in coactivation pattern (CAP) 3 consisting of posterior default mode network (DMN) and frontoparietal (FPN) network regions. Middle and bottom graphs: Relative to healthy controls (HCs), individuals with major depressive disorder (MDD) transitioned more frequently from CAP 3 (posterior DMN-FPN) to CAP 5 (prototypical DMN) and from CAP 5 to CAP 3. All error bars reflect the standard error of the mean.
3.3. Follow-up analyses on effect of MDD on frequency of transitions involving CAP 1 (anterior DMN), CAP 3 (posterior DMN-FPN), and CAP 5 (prototypical DMN)
We also examined possible group differences in transition frequencies between CAP 3 (posterior DMN-FPN) and CAP 1 (anterior DMN) as well as between CAP 3 (posterior DMN-FPN) and CAP 5 (prototypical DMN). Four ANCOVAs were conducted, examining the effects of MDD, CSA, and CSA × MDD interactions on CAP 1 → CAP 3, CAP 3 → CAP 1, CAP 3 → CAP5, and CAP 5 → CAP 3 transition frequencies. With respect to transitions involving CAP 1 and CAP 3, there were no significant effects of MDD, CSA, or an MDD × CSA interaction, all FDR corrected ps > 0.60. There was a main effect of MDD on CAP 3 → CAP5, and CAP 5 → CAP 3 transition frequencies, showing that individuals with MDD exhibited a higher frequency of transitioning from CAP 3 → CAP 5 (F(1,65) = 12.10, FDR-corrected p = 0.005, ηp2 = 0.157) and from CAP 5 → CAP 3 (F (1,65) = 14.34, FDR-corrected p = 0.004, ηp2 = 0.181), relative to HCs (see Fig. 3, also Supplementary Table S5, Supplementary Results). There was no significant main effect of CSA or a CSA × MDD interaction on CAP 3 → CAP5, and CAP 5 → CAP 3 transition frequencies, all FDR-corrected p’s > 0.60. This same pattern of results was found when re-grouping participants based on broader definitions of childhood maltreatment as well as dimensional analyses characterizing the entire sample on severity of several ELS experiences (see Supplemental Results; see also Supplementary Table S6 on statistics involving all other CAP transitions requested by a reviewer and not included in the a priori analyses).
3.4. Follow-up correlations between time spent in CAP 3 (posterior DMN-FPN), CAP 3 to CAP 5 (prototypical DMN) (and vice versa) transitions with depressive symptom severity, sexual abuse severity, and stress reactivity measures
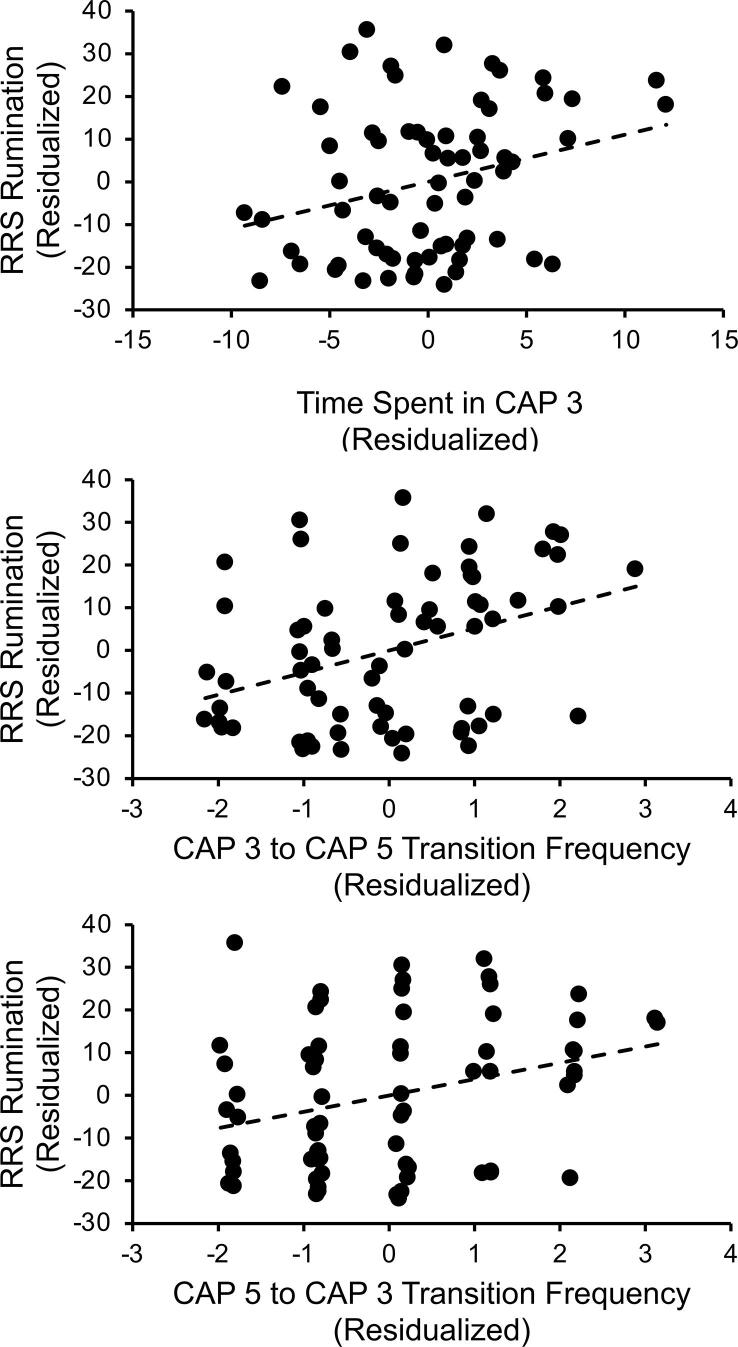
Depressive symptom severity and stress-related measures were correlated with time spent in CAP 3 (posterior DMN-FPN) and transition frequencies between CAP3 and CAP 5 (prototypical DMN). To further examine the effects of CSA potentially not captured by the above categorical analyses, we also conducted partial correlations between CSA severity and all tested CAPs metrics across the two CSA groups. None of these CAP metrics were associated with depressive symptom severity (BDI-II scores, analyzed within the MDD groups combined only), all FDR-corrected ps > 0.70. With respect to CSA severity, there was a significant association between sexual abuse severity scores and transitioning from CAP 3 to CAP 5, with greater sexual abuse severity being associated with transitioning more frequently from CAP 3 to CAP5, partial r(26) = 0.54, FDR-corrected p = 0.030. However, when accounting for MDD diagnosis, this correlation no longer was significant, r(25) = 0.32, p = 0.099. There were no other associations between CSA severity and other CAP metrics, all FDR-corrected ps > 0.90. With regard to trait rumination, which was examined across groups, higher RRS scores were linked to more time spent in CAP 3 (posterior DMN-FPN), partial r(64) = 0.28, FDR-corrected p = 0.024 (corrected for 3 tests conducted between CAP metrics and trait rumination scores), and greater probability of transitioning from CAP 3 (posterior DMN-FPN) → CAP 5 (prototypical DMN), partial r(64) = 0.37, FDR-corrected, p = 0.006, and from CAP 5 (prototypical DMN) → CAP 3 (posterior DMN-FPN), r(64) = 0.30, FDR-corrected p = 0.023 (see Fig. 4). Regarding stress reactivity measures, AUCI cortisol and stress-induced NA were not significantly related to time spent in CAP 3, CAP3 → CAP 5 transition frequencies, or CAP 5 → CAP 3 transition frequencies, all FDR-corrected ps > 0.08.
Fig. 4.
Higher levels of rumination are associated with CAP dynamic metrics. Across the groups, higher levels of rumination partially correlated with spending more time in CAP 3 (posterior DMN-FPN) and transitioning more frequently between CAP 3 and CAP 5 (prototypical DMN), when controlling for fMRI motion metrics. RRS Scores and time in CAP are residualized for motion variables in the graphs.
4. Discussion
The primary aim of this study was to probe effects of MDD and CSA exposure on resting state brain dynamics, using a data-driven CAP analysis approach. Our preliminary findings showed that MDD, but not CSA exposure, or interactions between MDD and CSA, was related to resting state brain dynamics. Specifically, individuals with MDD spent more time in a CAP consisting of posterior DMN and FPN regions compared to HCs. Additionally, those with MDD, relative to HCs, transitioned more frequently between a posterior DMN-FPN CAP and a prototypical DMN CAP. Furthermore, more time spent in the posterior DMN-FPN CAP and greater number of transitions between the prototypical DMN and posterior DMN-FPN CAPs were significantly associated with higher levels of rumination across the groups.
Our findings are consistent with a large body of evidence specifying MDD as a disorder involving imbalances between the DMN and FPN (Hamilton et al., 2011, Kaiser et al., 2015). Studies applying static functional brain network approaches have shown that individuals with MDD are characterized by hyperconnectivity between the DMN and FPN, and greater DMN dominance over FPN at rest compared to healthy controls (Hamilton et al., 2011, Kaiser et al., 2015). Higher levels of rumination have also been consistently linked to DMN-FPN hyperconnectivity and greater DMN relative to FPN activity (Hamilton et al., 2011, Lydon-Staley et al., 2019). A recent study incorporating a CAP approach in an independent sample of depressed adolescents found strikingly similar results to ours (Kaiser et al., 2019). Specifically, higher depressive symptom severity and rumination were linked to more time spent in a CAP involving DMN, FPN, and SN regions as well as to more frequent transitions between this DMN-FPN-SN CAP and a more prototypical DMN CAP (Kaiser et al., 2019). Here, we expanded on this work by evaluating an adult sample as well as examining possible associations between brain network dynamics and CSA. Additionally, we used a potentially more sensitive voxel-wise CAP approach compared to the ROI-based parcellation approach used in the prior study. The DMN is central to internally focused attention, whereas the FPN is critical for top-down regulation of emotion (e.g., Vanhaudenhuyse et al., 2011). Thus, our finding that individuals with MDD spend more time in an DMN-FPN CAP as well transition more frequently between DMN-FPN and prototypical DMN CAPs, may reflect difficulties in re-directing attention toward goal-relevant behaviors and regulating negative emotions. Together, our findings indicate that disrupted DMN-FPN functional interactions are likely important biomarkers of MDD associated with cognitive difficulties, including attentional control problems and perseverative negative thinking.
Surprisingly, we did not find that CSA was associated with resting state CAP metrics, which is inconsistent with prior static and sliding window dynamic RSFC studies demonstrating that ELS is associated with RSFC abnormalities (e.g., Fadel et al., 2021, Kaiser et al., 2018). There were no CAP markers that differentiated the MDD groups with and without CSA, nor were there potential CAP markers of resilience that differentiated the CSA/RES and MDD/CSA groups. Moreover, CSA severity was not significantly associated with any of the CAP metrics when accounting for MDD diagnosis. Additional supplementary analyses re-categorizing participants based on a spectrum of childhood abuse experiences as well as dimensional analyses across the entire sample examining a broader array of ELS experiences (peer aggression, parent conflict and domestic violence, as well as verbal, physical, and sexual abuse), also failed to find ELS associations with CAP metrics. A recent CAP study in youth also did not observe any ELS relationships with brain dynamic measures (Iadipaolo et al., 2018). Together, these studies suggest that these resting state brain co-activation pattern metrics may not be sensitive neurobiological markers of ELS. However, it is possible that our small sample size may have precluded us from identifying links between ELS and CAP metrics. Thus, this work should be considered preliminary and needs to be replicated in larger samples.
Our study has several strengths: first, to the best of our knowledge, this is the first study examining MDD and CSA relationships with resting state brain dynamics of large-scale neural networks in a sample of HCs and individuals with MDD, both with and without CSA. This study design allowed us to examine distinct as well as interacting influences of MDD and CSA on resting state dynamics. Second, the full sample was free of psychotropic medications, which eliminates medication effects as a potential confounding factor. Third, the current study implemented a state-of-the-art CAP analysis which allow for a more nuanced investigation of brain dynamics. Despite the strengths, there are some limitations, including the relatively small sample size, and the fact that only females were included in light of sex differences in neurobiological responses to stress (Mazurka et al., 2018, Ordaz and Luna, 2012). Accordingly, it is unknown if the current findings generalize to males. The study was also cross-sectional, thus causal relationships cannot be established. Finally, while the CAP analysis approach has been found to capture important differences in resting state brain functioning amongst those with mental health disorders compared to healthy controls (e.g., Kaiser et al., 2019), some studies have shown that CAPs may not represent the non-stationarity of resting state activity or transient spatially distinct functional network states (Liégeois et al., 2017, Matsui et al., 2022). Thus, interpreting CAPs as signifying dynamic brain network “states” should be avoided. Nevertheless, the current study provides critical evidence of MDD-related abnormal DMN-FPN dynamic interactions, which were linked to rumination, and additionally lays the groundwork for future larger longitudinal studies examining ELS and MDD-related influences on large-scale neural network modulations.
CRediT authorship contribution statement
Emily L. Belleau: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Thomas A.W. Bolton: Formal analysis, Visualization, Writing – review & editing. Roselinde H. Kaiser: Writing – review & editing. Rachel Clegg: Investigation, Writing – review & editing. Emilia Cárdenas: Investigation, Writing – review & editing. Franziska Goer: Investigation, Writing – review & editing. Pia Pechtel: Writing – review & editing. Miranda Beltzer: Investigation, Writing – review & editing. Gordana Vitaliano: Writing – review & editing. David P. Olson: Writing – review & editing. Martin H. Teicher: Writing – review & editing. Diego A. Pizzagalli: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Over the past 3 years, Dr. Pizzagalli has received consulting fees from Albright Stonebridge Group, Boehringer Ingelheim, Compass Pathways, Engrail Therapeutics, Neumora Therapeutics (formerly BlackThorn Therapeutics), Neurocrine Biosciences, Neuroscience Software, Otsuka, Sunovion, and Takeda; he has received honoraria from the Psychonomic Society (for editorial work) and from Alkermes; he has received research funding from the Brain and Behavior Research Foundation, the Dana Foundation, Millennium Pharmaceuticals, and NIMH; he has received stock options from Compass Pathways, Engrail Therapeutics, Neumora Therapeutics, and Neuroscience Software. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. All other authors have no conflicts of interest or relevant disclosures.
Acknowledgments
Acknowledgments
The authors would like to thank Laurie A. Scott, A.M., Nancy Hall Brooks, M.Ed., Madeline Alexander, Ph.D., and Mei Hua-Hall, Ph.D. for conducting the participant clinical interviews.
Funding
This work was supported by NIMH (DAP, Grant No R01 MH095809). ELB is supported by NIMH 1K23MH122668 and the Klingenstein Third Generation Foundation. RHK is partially supported by NIMH 5R01MH117131. PP is supported by a Wellcome Trust Clinical Research Career Development Fellowship.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103164.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Beck A.T., Steer R.A., Ball R., Ranieri W.F. Comparison of beck depression inventories-IA and -II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T.A.W., Morgenroth E., Preti M.G., Van De Ville D. Tapping into multi-faceted human behavior and psychopathology using fMRI brain dynamics. Trends Neurosci. 2020;43:667–680. doi: 10.1016/j.tins.2020.06.005. [DOI] [PubMed] [Google Scholar]
- Bolton T.A.W., Tuleasca C., Wotruba D., Rey G., Dhanis H., Gauthier B., Delavari F., Morgenroth E., Gaviria J., Blondiaux E., Smigielski L., Van De Ville D. TbCAPs: A toolbox for co-activation pattern analysis. Neuroimage. 2020;211 doi: 10.1016/j.neuroimage.2020.116621. [DOI] [PubMed] [Google Scholar]
- Bunea, I.M., Szentágotai-Tătar, A., Miu, A.C., 2017. Early-life adversity and cortisol responses to social stress: A meta-analysis. Transl. Psychiatry 1274. [DOI] [PMC free article] [PubMed]
- Demir-Lira Ö.E., Voss J.L., O’Neil J.T., Briggs-Gowan M.J., Wakschlag L.S., Booth J.R. Early-life stress exposure associated with altered prefrontal resting-state fMRI connectivity in young children. Dev. Cogn. Neurosci. 2016;19:107–114. doi: 10.1016/j.dcn.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel E., Boeker H., Gaertner M., Richter A., Kleim B., Seifritz E., Grimm S., Wade-Bohleber L.M. Differential alterations in resting state functional connectivity associated with depressive symptoms and early life adversity. Brain Sci. 2021;11:591. doi: 10.3390/brainsci11050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Liu W., Xia J., Li S., Gao F., Zhu J., Han Y., Zhou H., Liao H., Yi J., Tan C., Zhu X. Childhood trauma is associated with elevated anhedonia and altered core reward circuitry in major depression patients and controls. Hum. Brain Mapp. 2021;42:286–297. doi: 10.1002/hbm.25222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M., Spitzer, R., Gibbon, M., Williams, J. Structured Clinical Interview for DSM-IVTR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP). (New York: Biometrics Research, New York State Psychiatric Institute, 2002).
- Folstein M.F., Luria R. Reliability, validity, and clinical application of the visual analogue mood scale. Psychol. Med. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol. Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Cui Q., Wang X., Li L., Li D., He Z., Chen H. Resting state functional network switching rate is differently altered in bipolar disorder and major depressive disorder. Hum. Brain Mapp. 2020;41:3295–3304. doi: 10.1002/hbm.25017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.L., Perry J.C., Van der Kolk B.A. Childhood trauma in borderline personality disorder. Am. J. Psychiatry. 1989;146:490–495. doi: 10.1176/ajp.146.4.490. [DOI] [PubMed] [Google Scholar]
- Hoffmann F., Viding E., Puetz V.B., Gerin M.I., Sethi A., Rankin G., McCrory E.J. Evidence for depressogenic spontaneous thoughts and altered resting-state connectivity in adolescents with a maltreatment history. J. Am. Acad. Child Adolesc. Psychiatry. 2018;57:687–695. doi: 10.1016/j.jaac.2018.05.020. [DOI] [PubMed] [Google Scholar]
- Hou Z., Kong Y., Yin Y., Zhang Y., Yuan Y. Identification of first-episode unmedicated major depressive disorder using pretreatment features of dominant coactivation patterns. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;104 doi: 10.1016/j.pnpbp.2020.110038. [DOI] [PubMed] [Google Scholar]
- Huang D., Liu Z., Cao H., Yang J., Wu Z., Long Y. Childhood trauma is linked to decreased temporal stability of functional brain networks in young adults. J. Affect. Disord. 2021;290:23–30. doi: 10.1016/j.jad.2021.04.061. [DOI] [PubMed] [Google Scholar]
- Iadipaolo A.S., Marusak H.A., Paulisin S.M., Sala-Hamrick K., Crespo L.M., Elrahal F., Peters C., Brown S., Rabinak C. Distinct neural correlates of trait resilience within core neurocognitive networks in at-risk children and adolescents. NeuroImage Clin. 2018;20:24–34. doi: 10.1016/j.nicl.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes A.C., Peechatka A.L., Frederick B.B., Kaiser R.H. Dynamic functioning of transient resting-state co-activation networks in the Human Connectome Project. Hum. Brain Mapp. 2020;41:373–387. doi: 10.1002/hbm.24808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Clegg R., Goer F., Pechtel P., Beltzer M., Vitaliano G., Olson D.P., Teicher M.H., Pizzagalli D.A. Childhood stress, grown-up brain networks: Corticolimbic correlates of threat-related early life stress and adult stress response. Psychol. Med. 2018;48:1157–1166. doi: 10.1017/S0033291717002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Chase H.W., Phillips M.L., Deckersbach T., Parsey R., Fava M., McGrath P., Weissman M., Oquendo M.A., McInnis M.G., Carmody T., Cooper C.M., Trivedi M.H., Pizzagalli D.A. Dynamic resting-state network biomarkers of antidepressant treatment response. Biol. Psychiatry. 2022 doi: 10.1016/j.biopsych.2022.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, R.H., Andrews-Hanna, J.R., Wager, T.D., Pizzagalli, D.A., 2015. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603-611. [DOI] [PMC free article] [PubMed]
- Kaiser R.H., Whitfield-Gabrieli S., Dillon D.G., Goer F., Beltzer M., Minkel J., Smoski M., Dichter G., Pizzagalli D.A. Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacology. 2016;41:1822–1830. doi: 10.1038/npp.2015.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Kang M.S., LewY V.D., Feen J., Aguirre B., Clegg R., Goer F., Esposito E., Auerbach R.P., Hutchison R.W., Pizzagalli D.A. Abnormal frontoinsular-default network dynamics in adolescent depression and rumination: A preliminary resting-state co-activation pattern analysis. Neuropsychopharmacology. 2019;44:1604–1612. doi: 10.1038/s41386-019-0399-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Arcy D., Meng X. Maltreatment in childhood substantially increases the risk of adult depression and anxiety in prospective cohort studies: Systemic review, meta-analysis, and proportional attributable fractions. Psychol. Med. 2016;46:717–730. doi: 10.1017/S0033291715002743. [DOI] [PubMed] [Google Scholar]
- Liégeois R., Laumann T.O., Snyder A., Zhou J., Yeo B.T.T. Interpreting temporal fluctuations in resting-state functional connectivity MRI. NeuroImage. 2017;163:437–455. doi: 10.1016/j.neuroimage.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Liu, X., Chang, C., Duyn, J.H., 2013, Decomposition of spontaneous into distinct fMRI co-activation patterns. Front. Sys. Neurosci. 7, Article 101. [DOI] [PMC free article] [PubMed]
- Long Y., Cao H., Yan C., Chen X., Li L., Castellanos F.X., Bai T., Bo Q., Chen G., Chen N., Chen W., Cheng C., Cheng Y., Cui X., Duan J., Fang Y., Gong Q., Guo W., Hou Z., Hu L., Kuang L., Li F., Li K., Li T., Liu Y., Luo Q., Meng H., Peng D., Qiu H., Qiu J., Shen Y., Shi Y., Si T., Wang C., Wang F., Wang K., Wang L., Wang X., Wang Y., Wu X., Wu X., Xie C., Xie G., Xie H., Xie P., Xu X., Yang H., Yang J., Yao J., Yao S., Yin Y., Yuan Y., Zhang A., Zhang H., Zhang K., Zhang L., Zhang Z., Zhou R., Zhou Y., Zhu J., Zou C., Zang Y., Zhao J., Kin-yuen Chan C., Pu W., Liu Z. Altered resting-state dynamic functional brain networks in major depressive disorder: findings from the REST-meta-MDD consortium. NeuroImage Clin. 2020;26 doi: 10.1016/j.nicl.2020.102163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie D.J., Kessler D., Bassett D.S., Betzel R.F., Breakspear M., Kheilholz S., Kucyi A., Liégeois R., Lindquist M.A., McIntosh A.R., Poldrack R.A., Shine J.M., Thompson W.H., Bielczyk N.Z., Douw L., Kraft D., Miller R.L., Muthuraman M., Pasquini L., Razi A., Vidaurre D., Xie H., Calhoun V.D. Questions and controversies in the study of time-varying functional connectivity in resting fMRI. Netw. Neurosci. 2020;4:30–69. doi: 10.1162/netn_a_00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon-Staley D.M., Kuehner C., Zamoscik V., Huffziger S., Kirsch P., Bassett D.S. Repetitive negative thinking in daily life and functional connectivity among default mode, fronto-parietal, and salience networks. Transl. Psychiatry. 2019;9:234. doi: 10.1038/s41398-019-0560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu, A., Meng, C., Brandl, F., Doll A., Tahmasian, M., Scherr, M., Schwerthöffer, D., Zimmer, C., Förstl, H., Bäuml, J., Riedl, V., Wohlschläger, A.M., Sorg, C., 2014. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci. 7, 930. [DOI] [PMC free article] [PubMed]
- Matsui T., Pham T.Q., Jimura K., Chikazoe J. On co-activation pattern analysis and non-stationarity of resting brain activity. NeuroImage. 2022;249 doi: 10.1016/j.neuroimage.2022.118904. [DOI] [PubMed] [Google Scholar]
- Mazurka R., Wynne-Edwards K.E., Harkness K.L. Sex Differences in the cortisol response to the Trier Social Stress Test in depressed and nondepressed adolescents. Clin. Psychol. Sci. 2018;6:301–314. [Google Scholar]
- Monti S., Tomayo P., Mesirov J., Golub T. Consensus clustering: A resampling-based method for class discovery and visualization of gene expression microarray data. Mach Learn. 2003;52:91–118. [Google Scholar]
- Munzer A., Fegert J.M., Goldbeck L. Psychological symptoms of sexually victimized children and adolescents compared with other maltreatment subtypes. J. Child Sex. Abus. 2016;25:326–346. doi: 10.1080/10538712.2016.1137667. [DOI] [PubMed] [Google Scholar]
- Nelson J., Klumparendt A., Doebler P., Ehring T. Childhood maltreatment and characteristics of adult depression: Meta-analysis. Br. J. Psychiatry. 2017;210:96–104. doi: 10.1192/bjp.bp.115.180752. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J. Abnorm. Psychol. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta Earthquake. J. Pers. Soc. Psychol. 1991;61:115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Ordaz S., Luna B. Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrinology. 2012;37:1135–1157. doi: 10.1016/j.psyneuen.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y., Chen H., Wang Y., Long Z., He Z., Zhang H., Liao W., Cui Q., Chen H. Transdiagnostic and diagnosis-specific dynamic functional connectivity anchored in the right anterior insula in major depressive disorder and bipolar depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;85:7–15. doi: 10.1016/j.pnpbp.2018.03.020. [DOI] [PubMed] [Google Scholar]
- Philip N.S., Sweet L.H., Tyrka A.R., Price L.H., Bloom R.F., Carpenter L.L. Decreased default network connectivity is associated with early life stress in medication-free healthy adults. Eur. Neuropsychopharmacol. 2013;23:24–32. doi: 10.1016/j.euroneuro.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip N.S., Valentine T.R., Sweet L.H., Tyrka A.R., Price L.H., Carpenter L.L. Early life stress impacts dorsolateral prefrontal cortex functional connectivity in healthy adults: Informing future studies of antidepressant treatments. J. Psychiatr. Res. 2014;52:63–69. doi: 10.1016/j.jpsychires.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J.C., Kirschbaum C., Meinlschmid G., Hellhammer D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Șenbabaoğlu Y., Michailidis G., Li J.Z. Critical limitations of consensus clustering in class discovery. Sci. Rep. 2015;4:6207. doi: 10.1038/srep06207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T., Cornelisse S., Quaedflieg C.W.E.M., Meyer T., Jelicic M., Merckelbach H. Introducing the Maastricht Acute Stress Test (MAST): A quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology. 2012;37:1998–2008. doi: 10.1016/j.psyneuen.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaudenhuyse A., Demertzi A., Schabus M., Noirhomme Q., Bredart S., Boly M. Two distinct neuronal networks mediate the awareness of environment and of self. J. Cogn. Neurosci. 2011;23:570–578. doi: 10.1162/jocn.2010.21488. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang Y., Huang H., Jia Y., Zheng S., Zhong S., Chen G., Huang L., Huang R. Abnormal dynamic functional network connectivity in unmedicated bipolar and major depressive disorders based on the triple-network model. Psychol. Med. 2020;50:465–474. doi: 10.1017/S003329171900028X. [DOI] [PubMed] [Google Scholar]
- Wei M., Qin J., Yan R., Bi K., Liu C., Yao Z., Lu Q. Abnormal dynamic community structure of the salience network in depression: Abnormal salience network in depression. J. Magn. Reson. Imaging. 2017;45:1135–1143. doi: 10.1002/jmri.25429. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wise T., Marwood L., Perkins A.M., Herane-Vives A., Joules R., Lythgoe D.J., Luh W.-M., William S.C.R., Young A.H., Cleare A.J., Arnone D. Instability of default mode network connectivity in major depression: A two-sample confirmation study. Transl. Psychiatry. 2017;7:e1105. doi: 10.1038/tp.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Xu X., Liu M., Zheng K., Liu J., Li J., Wei L., Zhang B., Lu H., Li B. Quantitative identification of major depression based on resting-state dynamic functional connectivity: A machine learning approach. Front. Neurosci. 2020;14:191. doi: 10.3389/fnins.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.-G., Zang Y.F. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Linn K.A., Shinohara R.T., Oathes D.J., Cook P.A., Duprat R., Moore T.M., Oquendo M.A., Phillips M.L., McInnis M., Fava M., Trvedi M.H., McGrath P., Parsey R., Weissman M.M., Sheline Y.I. Childhood trauma history is linked to abnormal brain connectivity in major depression. Proc. Natl. Acad. Sci. 2019;116:8582–8590. doi: 10.1073/pnas.1900801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Palaniyappan L., Wu Y., Cong E., Wu C., Ding L., Jin F., Qiu M., Huang Y., Wu Y., Wang J., Ying S., Peng D. The concurrent disturbance of dynamic functional and structural brain connectome in major depressive disorder: The prefronto-insular pathway. J. Affect. Disord. 2020;274:1084–1090. doi: 10.1016/j.jad.2020.05.148. [DOI] [PubMed] [Google Scholar]
- Zöller D.M., Bolton T.A.W., Karahanoglu F.I., Eliez S., Schaer M., Van De Ville D. Robust recovery of temporal overlap between network activity using transient-informed spatio-temporal regression. IEEE Trans. Med. Imaging. 2019;38:291–302. doi: 10.1109/TMI.2018.2863944. [DOI] [PubMed] [Google Scholar]
- Zorn J.V., Schür R.R., Boks M.P., Kahn R.S., Joëls M., Vinkers C.H. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology. 2017;77:25–36. doi: 10.1016/j.psyneuen.2016.11.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.