ABSTRACT
Background
Increasing unsaturated fat intake is beneficial for cardiovascular health, but the type of unsaturated fat to recommend remains equivocal.
Objectives
We investigated the effects of an 8-week diet intervention that was rich in either cottonseed oil (CSO; PUFA rich) or olive oil (OO; MUFA rich) on blood lipids in hypercholesterolemic adults.
Methods
Forty-three men and women with hypercholesterolemia (53 ± 10 years; BMI, 27.6 ± 4.8 kg/m2) completed this randomized parallel clinical trial consisting of an 8-week partial outpatient feeding intervention. Participants were given meals and snacks accounting for ∼60% of their daily energy needs, with 30% of energy needs from either CSO (n = 21) or OO (n = 22). At pre- and postdiet intervention visits, participants consumed a high-SFA meal (35% of total energy needs; 70% of energy from fat). The primary outcomes of fasting cholesterol profiles and secondary outcomes of postprandial blood lipids and glycemic markers were assessed over a 5-hour period.
Results
There were greater reductions from baseline to week 8 in fasting serum total cholesterol (TC; −17.0 ± 3.94 mg/dL compared with −2.18 ± 3.72 mg/dL, respectively; P = 0.008), LDL cholesterol (−19.7 ± 3.94 mg/dL compared with −5.72 ± 4.23 mg/dL, respectively; P = 0.018), non–HDL cholesterol (−20.8 mg/dL ± 4.00 compared with −6.61 ± 4.01 mg/dL, respectively; P = 0.014), and apoB (−11.8 mg/dL ± 2.37 compared with −3.10 ± 2.99 mg/dL, respectively; P = 0.05), in CSO compared with OO. There were also visit effects from baseline to week 8 for increases in HDL cholesterol (CSO, 56.5 ± 2.79 mg/dL to 60.2 ± 3.35 mg/dL, respectively; OO: 59.7 ± 2.63 mg/dL to 64.1 ± 2.24 mg/dL, respectively; P < 0.001), and decreases in the TC:HDL-cholesterol ratio (CSO, 4.30 ± 0.27 mg/dL to 3.78 ± 0.27 mg/dL, respectively; OO, 3.94 ± 0.16 mg/dL to 3.57 ± 0.11 mg/dL, respectively; P < 0.001), regardless of group assignment. In response to the high-SFA meal, there were differences in postprandial plasma glucose (P = 0.003) and triglyceride (P = 0.004) responses and a trend in nonesterified fatty acids (P = 0.11) between groups, showing protection in the postprandial state from an occasional high-SFA fat meal with CSO, but not OO, diet enrichment.
Conclusions
CSO, but not OO, diet enrichment caused substantial improvements in fasting and postprandial blood lipids and postprandial glycemia in hypercholesterolemic adults. This trial was registered at clinicaltrials.gov as NCT04397055.
Keywords: hypercholesterolemia, LDL cholesterol, HDL cholesterol, cottonseed oil, olive oil, stearoyl-CoA desaturase-1, SCD1, high-fat diet
Introduction
Cardiovascular disease (CVD) is the primary cause of death, representing an estimated 17.9 million deaths globally in 2019 (1). A common underlying cause for CVD is the inflammatory condition of atherosclerosis. As an early process of atherosclerosis is the infiltration of the arterial wall by cholesterol, elevated blood lipids (hypercholesterolemia) have been shown to be an independent and primary risk factor for the development of CVD (2). In 2015–2018, the National Health and Nutrition Examination Survey showed that 11.4% of adults had high total cholesterol (TC) and 17.2% had low HDL cholesterol (3), demonstrating that unfavorable blood lipids are a relatively prevalent condition among US adults.
Nutrient intake and diet quality have significant impacts on the development of CVD risks. Dietary fatty acid composition is one component of the diet that has been shown to modulate fasting and postprandial blood lipids. It is well accepted that high SFA intake has unfavorable effects on blood lipid profiles, including measures of cholesterol, triglycerides (TG), and related lipoproteins (4). While the strength of this effect may be dependent on the type and amount of SFA consumed (5), higher unsaturated fatty acid intake has been shown by meta-analysis to have favorable impacts on blood lipids (6). Additionally, increasing evidence suggests that PUFA intake has a stronger effect on lowering blood lipids compared to MUFAs (7, 8). This may be due in part to the regulatory roles that PUFAs elicit on the expression of genes related to the lipid and cholesterol metabolisms (9, 10). Importantly, we eat whole foods, rather than individual fatty acids, so studying the impact of specific PUFA- and MUFA-rich whole-food sources is necessary.
Cottonseed oil (CSO) is a rich dietary source of PUFAs, especially the omega-6 (ω-6) PUFA linoleic acid (18:2n–6), which has been in the food supply and consumed by Americans for over 100 years. Previous studies have shown that CSO-rich diets can improve cholesterol profiles in healthy, young adults (11, 12). In addition to a high PUFA content, CSO contains a cyclopropyl fatty acid, dihydrosterulic acid (DHSA), a known inhibitor of the lipogenic enzyme stearoyl-CoA desaturase-1 (SCD1) (13). The combination of the DHSA-mediated inhibition of SCD1 and the regulation of lipogenic and cholesterogenic gene expression by the high PUFA content of CSO make it a potentially ideal nutritional therapeutic to target cardiovascular health. To date, the cardiovascular benefits of CSO enrichment in the diet have only been shown in healthy adults over a single week. Olive oil (OO) is a rich source of MUFAs (14) that has gained popularity with consumers as a primary component of the Mediterranean diet (15, 16) due to a plethora of substantiated health claims (17). OO is generally considered heart healthy by consumers (18), but it has been shown in a meta-analysis to be less effective at lowering blood lipids compared to other plant oils (8). The purpose of this study was to examine the impact of a diet enriched with either CSO or OO for an 8-week period on our primary outcomes of fasting cholesterol profiles and secondary outcomes of postprandial blood lipids following a high-SFA meal challenge in adults with hypercholesterolemia. We hypothesized that all measures of fasting and postprandial blood lipids would decrease (and HDL cholesterol would increase) following the CSO treatment compared to the OO treatment.
Methods
Study design
This study was a single-blinded, randomized, parallel-design clinical trial. Subjects were randomly assigned to 1 of 2 groups, either the CSO group or OO group, with an allocation ratio of 1:1, using balanced blocks stratified by TC (desirable through “borderline undesirable” TCs ≥ 180 mg/dL or “undesirable” TCs ≥ 240), LDL cholesterol (desirable through “borderline undesirable” LDL cholesterol ≥ 110 mg/dL or “undesirable” LDL cholesterol ≥ 160), and BMI (healthy through overweight BMIs of 18.5–29.9 kg/m2 or obese BMIs ≥30 kg/m2). Participants were blinded to group assignment. Data collection occurred from May 2018 to June 2021, when the goal of 20 participants per group was obtained. This study included an 8-week diet intervention and 4 study visits: a screening visit, prediet intervention visit (V1), middiet intervention visit (V2), and a postdiet intervention visit (V3; Supplemental Figure 1). In addition, there were weekly nontesting visits for the purpose of picking up study food and returning containers and paperwork from the previous week. The Institutional Review Board from the University of Georgia approved the study, and written informed consent was obtained from all participants prior to beginning study procedures. This trial was registered at clinicaltrials.gov as NCT04397055 on 30 April 2020.
Participants
Fifty-three sedentary adults between the ages of 30 and 75 years with hypercholesterolemia or elevated blood lipids and BMIs > 18.5 kg/m2 were recruited for the study. To be included, an individual's blood lipids at the screening visit had to be “borderline undesirable” or “at risk” in 2 blood lipid measures or “undesirable” in 1 measure. “Borderline undesirable” or “at risk” measures were defined as a TC ≥ 180 mg/dL, LDL cholesterol ≥ 110 mg/dL, HDL cholesterol < 50 mg/dL, or TG ≥ 130 mg/dL, while “undesirable” measures were defined as a TC ≥ 240 mg/dL, LDL cholesterol ≥ 160 mg/dL, HDL cholesterol < 40 mg/dL, or TG ≥ 200 mg/dL (19–22).
To rule out individuals with familial hypercholesterolemia, participants with LDL cholesterol levels greater than the 95th percentile or HDL cholesterol levels lower than the 20th percentile were excluded. Other exclusion criteria included women on hormone replacement therapy for <2 years, regular exercise (>3 h/week), weight gain or loss >5% of body weight in the past 3 months, plans to begin a weight loss or exercise regimen during the study, allergies to study foods, a medically prescribed diet, a history of medical or surgical events that could affect digestion or swallowing, gastrointestinal surgery conditions or disorders, chronic or metabolic diseases (including atherosclerosis, previous myocardial infarction or stroke, cancer, diabetes, moderate to severe asthma, chronic lung disease, and kidney disease), or fasting blood glucose levels > 126 mg/dL or blood pressure > 180/120 mmHg. Individuals with medication use affecting their digestion and absorption or metabolism (e.g., thyroid medications), lipid-lowering medications, medications for diabetes, steroid or hormone therapies, or attention-deficit disorder or attention-deficit hyperactivity disorder were excluded. Finally, individuals with fish oil or calciumfloroboron supplement use within 3 months of their participation, excessive alcohol use [>3 drinks/d (42 g ethanol/d) for men; >2 drinks/d (28 g ethanol/d) for women], or tobacco or nicotine use were also excluded.
Screening visit
Participants reported to the Human Nutrition Lab (HNL) following an overnight fast of 8–12 hours and abstaining from exercise and alcohol for at least 24 hours. Anthropometrics were measured, including height, weight, waist and hip circumference, and blood pressure, and a fasting blood draw was performed to assess blood lipids and glucose. The validated Alcohol Use Disorders Identification Test (23) was used to assess alcohol consumption habits and confirm habitual consumption of <3 drinks/d in men or 2 drinks/d in women. The resting metabolic rate (RMR; kilocalories/day) was measured using indirect calorimetry (TrueOne 2400, Parvo Medics) under standard conditions (24), as described previously (25, 26). A participant's RMR was multiplied by an average activity factor of 1.65 to estimate daily energy needs (27). Individuals that qualified for the study were then randomized into 1 of the 2 diet groups (CSO or OO) by a researcher who was not involved in data collection or analysis, using a random-number generator. Participants were contacted and instructed to complete a 2-day food diary, including 1 weekday and 1 weekend day, that was turned in at V1 (28).
Prediet intervention visit
The night before V1, participants consumed a lead-in dinner and a snack (provided by research personnel) that contained 50% or total energy from carbohydrate, 15% of energy from protein, and 35% of energy from fat. The same fasting procedures from the screening visit were repeated for V1. Upon arrival at the HNL at 07:00 hours, height, weight, hip-waist circumference, blood pressure, and body composition were measured. Body composition was assessed using the Bod Pod (Cosmed USA, Inc.). Two questionnaires were administered: 1) the Perceived Stress Scale (PSS) (29); and 2) the International Physical Activity Questionnaire (IPAQ) (30). Physical activity was assessed by calculating total metabolic equivalent task (MET) min/wk from the IPAQ responses. An intravenous catheter was then placed in the antecubital vein, and fasting blood samples were collected 5 minutes later. The line was kept patent with a saline solution.
After fasting measures were taken, a high-fat, liquid meal challenge rich in SFAs was administered. The nutrient content from this meal was designed to provide 35% of the participant's total estimated daily energy needs (determined from the RMR at screening), as described previously (25). The meal was made from an original, milk-chocolate, ready-to-drink shake (Ensure, Abbott Laboratories, Inc.), unsalted butter, red palm oil, coconut oil, soy lecithin granules, and powdered chocolate drink mix. Briefly, this meal had 5.0% energy from protein, 25.0% from carbohydrate, and 69.5% from fat. SFAs, MUFAs, and PUFAs contributed 46.9%, 15.7%, and 6.9% of energy from fat, respectively. Participants had 10 minutes to drink the meal. To ensure the entire meal was consumed, 4 oz of water were used to rinse out the container, which was then also consumed. Following the meal challenge, blood was drawn at 30, 60, 90, 120, 150, 180, 240, and 300 minutes (5 hours) postprandially. Participants were also given 4 oz of water every hour.
Eight-week dietary intervention
The day following V1, participants began the 8-week OO- or CSO-enriched diet intervention. The OO used was California Olive Ranch Destination series Everyday Extra Virgin Olive Oil. The CSO was Chef's Pride. A fatty acid analysis of a sample of each test oil (Supplemental Table 1) showed expected differences in the fatty acid compositions between the 2 oils; however, other components that may differ between the 2 oils, such as the phenolic content, were not measured, nor was an analysis of fatty acid compositions done when different batches or lot numbers were used.
By design, this partial outpatient feeding trial provided participants with most, but not all, of their required daily energy needs, thus allowing them to maintain some of their usual diet. Before leaving the HNL after V1, participants were provided a 1-week supply of daily meals and snacks that corresponded to their assigned diet group. Researchers, who were dietetic interns, also counseled participants on their estimated energy needs, including how much energy (kilocalories) they had each day to fill with foods of their own choosing to maintain energy balance. The provided diets were identical between groups, with the only difference being the type of oil used in the preparation of the foods (CSO compared with OO). Foods were prepared in the research kitchen, with all ingredients weighed to the 0.01 g. A 7-day rotating menu of meals was used, providing 2 meals (breakfast and a lunch or dinner entrée) and snacks daily. Therefore, participants typically ate only 1 meal of their own choosing each day. Examples of meals provided include soups, sautéed vegetable medleys, pasta with sauce, turkey meatloaf and mashed potatoes, breakfast muffins, and brownies or cookies as desserts. These meals were selected based on the successful incorporation of oils into them. Five days per week, a breakfast shake was provided as 1 of the 2 meals. Participants were instructed to prepare this liquid meal by mixing provided, individually portioned, instant meal shake mix with a milk of their choice and a designated amount of the assigned oil provided. The rest of the meals were preportioned by weighing ingredients to 0.01 g, and were then individually packaged in microwave-safe containers by research personnel and frozen. Participants were instructed on safe reheating practices of the meals.
For the provided diets, participants were instructed to consume all of the provided foods and follow their normal dietary patterns for any meals not provided. Along with being randomly assigned a diet treatment (CSO or OO), participants were nonrandomly assigned to a kilocalorie tier. The tier assignment was dependent on the participant's estimated energy requirement, determined from their RMR at screening (Table 1). The provided foods accounted for about 60% of the estimated energy needs of the participants, and allowed each tier to provide about 30% of the estimated daily energy needs from the assigned oil (CSO or OO).
TABLE 1.
Nutrient breakdown of provided intervention foods for each kilocalorie tier1
| Cottonseed Oil | Olive Oil | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention Tiers, kcal | <1600 | 1600–2299 | 2300–3000 | >3000 | <1600 | 1600–2299 | 2300–3000 | >3000 |
| Energy, kcal | 1090 | 1402 | 1,678 | 2,107 | 1090 | 1402 | 1678 | 2107 |
| Energy from protein, % | 7.2 | 6.7 | 6.5 | 6.8 | 7.2 | 6.7 | 6.5 | 6.8 |
| Protein, g | 19.1 | 23.0 | 26.8 | 35.2 | 19.1 | 23.0 | 26.8 | 35.2 |
| Energy from carbohydrates, % | 36.9 | 36.3 | 36.3 | 39.0 | 36.9 | 36.3 | 36.3 | 39.0 |
| Carbohydrates, g | 98.0 | 124.0 | 148.5 | 200.6 | 98.0 | 124.0 | 148.5 | 200.6 |
| Fiber, g | 3.2 | 4.1 | 5.1 | 7.9 | 3.2 | 4.1 | 5.1 | 7.9 |
| Sugar, g | 51.3 | 64.8 | 76.5 | 101.5 | 51.3 | 64.8 | 76.5 | 101.5 |
| Energy from fat, % | 55.9 | 57.0 | 57.2 | 54.1 | 55.9 | 57.0 | 57.2 | 54.1 |
| Total fat, g | 65.5 | 85.9 | 103.1 | 122.7 | 65.5 | 85.9 | 103.1 | 122.7 |
| Saturated fat, g | 15.3 | 20.0 | 24.0 | 28.7 | 12.3 | 16.0 | 19.3 | 23.2 |
| Trans fat, g | 0.17 | 0.22 | 0.27 | 0.38 | 0.17 | 0.22 | 0.27 | 0.38 |
| Monounsaturated fat, g | 13.3 | 17.4 | 20.8 | 24.7 | 44.8 | 58.8 | 70.6 | 83.5 |
| Polyunsaturated fat, g | 36.8 | 48.4 | 58.1 | 68.8 | 8.3 | 10.9 | 13.0 | 15.5 |
| Omega-3 fatty acid, g | 0.28 | 0.36 | 0.43 | 0.51 | 0.60 | 0.79 | 0.95 | 1.13 |
| Omega-6 fatty acid, g | 36.6 | 48.0 | 57.6 | 68.3 | 7.8 | 10.1 | 12.1 | 14.4 |
| Cholesterol, mg | 57.5 | 75.2 | 89.8 | 121.0 | 57.5 | 75.2 | 89.8 | 121.0 |
| Total fat from intervention oil, % | 88.2 | 87.3 | 87.3 | 85.6 | 88.2 | 87.3 | 87.3 | 85.6 |
| Fat from intervention oil, g | 57.8 | 75.0 | 90.0 | 105.0 | 57.8 | 75.0 | 90.0 | 105.0 |
Daily nutrients were delivered through the provided study foods within each treatment and energy tier. Participants were assigned to a kilocalorie tier based on their estimated energy requirements from a resting metabolic rate measurement at the screening visit. Energy tiers are named for the range of total energy requirements of the participants that were assigned to that tier. Energy (kcal) in the first row is the amount of energy actually provided each day from the diet intervention foods. The only difference between treatments was from the different treatment oil used (cottonseed oil compared with olive oil).
Weekly responsibilities
Participants completed a daily Meal Compliance Checklist where they checked off meals consumed each day. Meal compliance checklists were submitted to research personnel once per week for analysis. Participants consuming less than 75% of provided foods were deemed noncompliant, and were to be removed from the final data analysis. In addition to compliance records, participants were asked to keep food diaries once per week, alternating between weekdays and weekend days. Daily nutrient intakes from the food diaries were assessed using the Food Processor SQL software (ESHA Research; version 10.12.0).
Weekly visits to the HNL
Participants returned to the HNL once per week to pick up weekly supplies of meals. During these visits, participants returned meal compliance checklists and food diaries. Participants were also asked to fill out the IPAQ questionnaire in 2-week intervals during the intervention.
Middiet intervention visit
Four weeks after V1, participants reported to the HNL following an 8- to 12-hour fast and 24 hours without exercise and alcohol for V2. The same lead-in meal provided at V1 was consumed for dinner the night before V2. Anthropometrics, PSS, IPAQ, and a fasting blood draw were repeated as in V1. No SFA-rich meal challenge was performed at V2.
Postdiet intervention visit
Four weeks after v2, participants returned for V3. The same lead-in procedures from V1 and V2 were repeated. Participants then completed the exact same study procedures and measurements that took place at V1, including anthropometrics, questionnaires, the SFA meal challenge, and blood draws.
Blood lipid analysis
All blood samples were drawn into K2 EDTA-coated vacutainers (Becton, Dickinson, and Company), and immediately placed on ice. For the lipid panel at all 3 testing visits (V1–V3), a portion of the fasting blood sample was drawn into a serum separator clot activator vacutainer (Greiner Bio-One North America Inc.) and kept at room temperature for 30 minutes before centrifugation for 15 minutes at 3000 rpm at 4°C. The serum was transferred into a transport tube and kept at 4°C until the advanced blood lipid panel was performed (Quest Diagnostics). The lipid panel was the primary outcome and included TC, HDL cholesterol, TG, LDL cholesterol, the LDL particle number (LDL-P), LDL small, LDL medium, HDL large, and apoB through spectrophotometry and ion mobility lipoprotein fractionation.
The remainder of the fasting blood sample and all postprandial samples were centrifuged as described above. The plasma was aliquoted and stored at −80°C until further analysis. A sample analysis of secondary outcomes included TGs, nonesterified fatty acids (NEFAs), glucose, and insulin. Postprandial responses of plasma TGs, NEFAs, and glucose were measured by enzyme-based calorimetric assays (Wako Chemicals USA, Inc.). Glucose was measured using a colorimetric glucose oxidase and peroxidase method (glucose oxidase, G2133; peroxidase, P8250; Sigma Aldrich). Insulin was measured by radioimmunoassay (MilliporeSigma).
Statistical analyses
The SAS version 9.2 statistical package (SAS Institute Inc.) was used for all data analyses. All results are reported as means ± SEMs unless otherwise noted. Statistical significance was set at P < 0.05 for all measures. A sample size of 38 (19/group) was estimated to detect a change in TC of 3.72 mg/dL between groups, with a pooled SD of 25.26 mg/dL (Cohen's D of 0.14) in healthy males using G*power 3.19.7, assuming at least 80% power and an α of 0.05 based on the previous CSO study by Polley et al. (11). Sample-size calculations were run in the same manner to detect at least a 10% change between groups based on results for LDL cholesterol, TG, and the postprandial TG AUC from Polley et al. (11), resulting in estimated sample sizes of 30, 36, and 32, respectively (Cohen's Ds of 0.17, 0.15, and 0.16, respectively). To ensure appropriate power was reached, we used the TC sample-size estimation for this trial since it was the most conservative estimate. The decision to utilize per-protocol analyses was made a priori. An unpaired t-test was used to compare differences in compliance and changes from baseline, fasting biochemical markers between groups. A 2-factor [treatment × visit (time)] repeated-measures ANOVAs using PROC MIXED was used to determine between- and within-group differences for fasting biochemical data, anthropometrics, perceived stress, total MET minutes, and self-reported intake. An intervention average was calculated for the total MET minutes and self-reported intake. Intervention averages were compared to baseline averages. For all time-course (meal challenge) data, a 3-factor (treatment × visit × time point) repeated-measures ANOVA using PROC MIXD was used to determine differences between and within groups. Subjects were modeled as random effects, while treatment, visit, and time point were fixed effects, in both ANOVA models, with no covariates. Additionally, the AUC was calculated for all postprandial measures and analyzed using the same 2-factor repeated-measures ANOVA as described for fasting biochemical data. When significance was found, post hoc analyses were done using Tukey's test. Continuous variables were examined for normality using the Shapiro-Wilk test, and an appropriate transformation was applied to nonnormal data before analysis. No such transformations were required in the current analysis.
Results
Participants
Fifty-three participants were randomly assigned to the intervention (CSO, n = 26; OO, n = 27); however, 10 participants did not start or complete the intervention and were not included in the final analysis (Figure 1). Therefore, 43 participants completed the intervention (12 women and 9 men in the CSO group; 15 women and 7 men in the OO group) and were included in this per-protocol analysis of primary and secondary outcomes. One of the 42 participants did not complete the meal challenge; thus, only their fasting data are included. Participant characteristics at baseline are presented in Table 2. For body weight and BMI, there was a significant main effect of visit (both P values < 0.001), but not of treatment (P = 0.98 and P = 0.77, respectively), and there was no treatment × visit interaction (P = 0.99 and P = 0.98, respectively). Post hoc analyses revealed that this visit effect was driven by increases in body weight and BMI from pre- to midintervention (P = 0.03 and P = 0.04, respectively) and postintervention (P < 0.001 and P < 0.001, respectively), regardless of group assignment. There were no other main or interaction effects in any other measure of anthropometrics, perceived stress, or reported physical activity.
FIGURE 1.
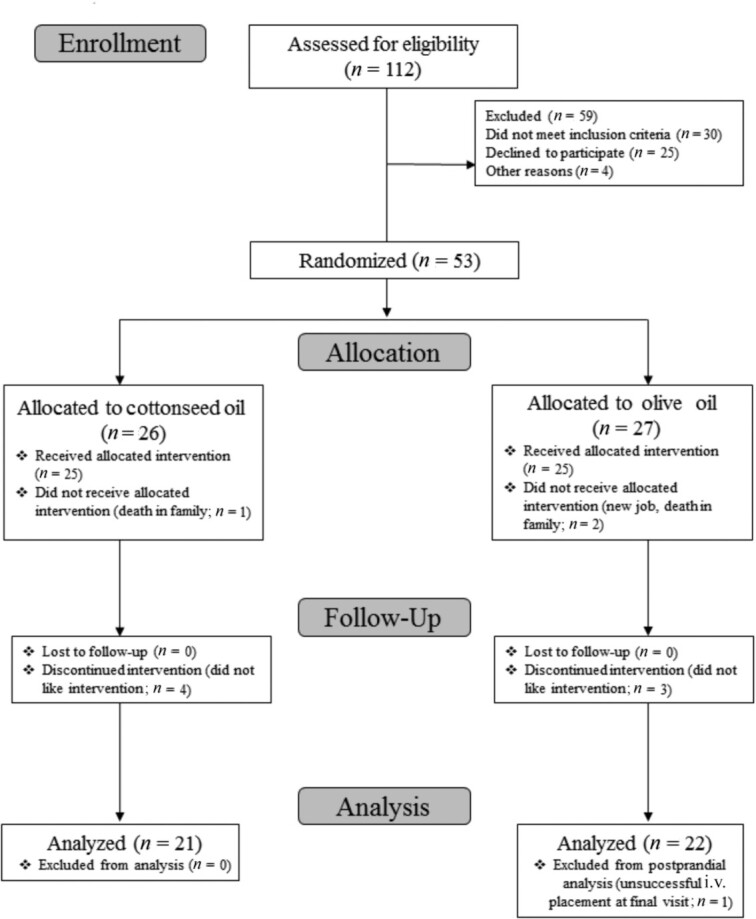
CONSORT flow diagram selection of participants.
TABLE 2.
Characteristics of adults with hypercholesterolemia at pre-, mid-, and postintervention visits in cottonseed oil or olive oil intervention groups1
| Cottonseed Oil, n = 21 | Olive Oil, n = 22 | |||||
|---|---|---|---|---|---|---|
| Characteristic | Week 0 | Week 4 | Week 8 | Week 0 | Week 4 | Week 8 |
| Female, % | 57 | — | — | 68 | — | — |
| Age, y | 53 ± 2 | — | — | 54 ± 2 | — | — |
| Height, cm | 169.1 ± 2.2 | 168.9 ± 2.2 | 169.0 ± 2.2 | 168.3 ± 1.8 | 168.3 ± 1.8 | 168.3 ± 1.9 |
| Weight,2 kg | 78.6 ± 3.6 | 79.1 ± 3.5 | 79.6 ± 3.5 | 78.7 ± 3.5 | 79.2 ± 3.6 | 79.7 ± 3.6 |
| BMI,2 kg/m2 | 27.3 ± 0.9 | 27.5 ± 0.9 | 27.7 ± 0.9 | 27.7 ± 1.2 | 27.9 ± 1.2 | 28.1 ± 1.2 |
| Waist circumference, cm | 91.1 ± 3.1 | 93.6 ± 3.0 | 92.3 ± 3.2 | 90.9 ± 3.0 | 91.1 ± 3.1 | 91.3 ± 3.2 |
| Hip circumference, cm | 107.7 ± 1.4 | 108.3 ± 1.3 | 107.9 ± 1.4 | 109.9 ± 2.3 | 109.8 ± 2.3 | 110.4 ± 2.3 |
| Waist-to-hip ratio | 0.85 ± 0.02 | 0.86 ± 0.02 | 0.85 ± 0.02 | 0.83 ± 0.02 | 0.83 ± 0.02 | 0.83 ± 0.02 |
| Systolic blood pressure, mmHg | 122 ± 3 | 125 ± 2 | 124 ± 3 | 127 ± 3 | 122 ± 3 | 122 ± 3 |
| Diastolic blood pressure, mmHg | 77 ± 2 | 80 ± 2 | 79 ± 2 | 80 ± 2 | 79 ± 3 | 78 ± 2 |
| Body fat, % | 31.0 ± 2.1 | — | 32.1 ± 1.9 | 33.6 ± 2.6 | — | 34.0 ± 2.6 |
| Total MET, min/wk | 1759 ± 293 | 1420 ± 275 | 2129 ± 570 | 1129 ± 214 | 1363 ± 405 | 1563 ± 349 |
| Perceived Stress Scale | 11 ± 1 | 11 ± 1 | 12 ± 2 | 10 ± 1 | 9 ± 1 | 11 ± 1 |
All values are means ± SEMs. Anthropometrics were analyzed with a 2-way (treatment × visit) repeated-measures ANOVA. Week 0 is the preintervention visit, week 4 is the midintervention visit, and week 8 is the postintervention visit. MET, metabolic equivalent task.
Indicates a significant effect of the visit at a P value < 0.001.
On average, participants in the CSO and OO groups reported consuming 91% ± 2% and 92% ± 1% of provided foods, respectively, and compliance was not different between groups. No participant reported poor compliance (defined as <75% of study products consumed throughout intervention). A baseline value for self-reported intake was calculated as an average of the 2 food records collected before the start of the intervention. An intervention average was calculated as an average of all food diaries collected during the intervention. There were no significant differences between groups at baseline for any of the analyzed nutrients (Table 3). There were significant visit effects for the percentages of energy from protein (P < 0.001), carbohydrate (P < 0.001), fat (P < 0.001), and alcohol (P = 0.007), as well as grams of grams of fat (P < 0.001), alcohol (P = 0.24), and fiber (P < 0.001), with decreases in all measures except fat across both groups.
TABLE 3.
Self-reported daily nutrient intake for diets enriched with cottonseed oil or olive oil in adults with hypercholesterolemia1
| Cottonseed Oil, n = 21 | Olive Oil, n = 22 | P Values | |||||
|---|---|---|---|---|---|---|---|
| Nutrient | Baseline | Intervention | Baseline | Intervention | Treatment | Visit | Treatment × Visit |
| Energy, kcal | 2075 ± 187 | 2450 ± 110 | 2539 ± 191 | 2731 ± 283 | 0.11 | 0.09 | 0.56 |
| Energy from protein, % | 15.6 ± 1.01 | 10.8 ± 0.27 | 14.6 ± 0.73 | 10.9 ± 0.36 | 0.51 | <0.001 | 0.33 |
| Protein, g | 74.7 ± 4.99 | 65.7 ± 5.19 | 86.2 ± 5.86 | 78.3 ± 13.8 | 0.20 | 0.25 | 0.94 |
| Energy from carbohydrate, % | 46.0 ± 2.16 | 39.2 ± 0.76 | 45.7 ± 2.08 | 38.9 ± 0.97 | 0.88 | <0.001 | 0.99 |
| Carbohydrate, g | 239 ± 29.7 | 234 ± 11.6 | 283 ± 27.2 | 240 ± 12.5 | 0.29 | 0.21 | 0.30 |
| Fiber, g | 19.8 ± 2.11 | 14.7 ± 1.12 | 22.0 ± 2.18 | 13.1 ± 0.65 | 0.87 | <0.001 | 0.20 |
| Sugar, g | 93.0 ± 14.9 | 105 ± 5.46 | 106 ± 14.3 | 109 ± 8.10 | 0.49 | 0.40 | 0.56 |
| Energy from fat, % | 35.5 ± 1.85 | 49.0 ± 0.79 | 37.2 ± 1.52 | 48.0 ± 0.82 | 0.82 | <0.001 | 0.15 |
| Fat, g | 77.9 ± 7.36 | 128 ± 4.11 | 103 ± 8.96 | 146 ± 21.5 | 0.12 | <0.001 | 0.77 |
| Saturated fat, g | 26.3 ± 2.32 | 33.9 ± 1.30 | 33.9 ± 3.74 | 35.9 ± 6.18 | 0.25 | 0.17 | 0.42 |
| Trans fat, g | 0.9 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.67 | 0.32 | 0.21 |
| Monounsaturated fat, g | 32.7 ± 3.90 | 31.4 ± 1.772 | 41.4 ± 4.45 | 86.3 ± 11.83 | <0.001 | 0.001 | <0.001 |
| Polyunsaturated fat, g | 18.0 ± 2.27 | 61.9 ± 1.722,3 | 26.7 ± 2.16 | 23.3 ± 3.48 | <0.001 | <0.001 | <0.001 |
| Omega-3 fatty acid, g | 1.9 ± 0.3 | 1.0 ± 0.1 | 3.0 ± 0.5 | 1.6 ± 0.2 | 0.01 | <0.001 | 0.39 |
| Omega-6 fatty acid, g | 16.1 ± 2.08 | 60.9 ± 1.682,3 | 23.7 ± 1.82 | 21.7 ± 3.29 | <0.001 | <0.001 | <0.001 |
| Energy from alcohol, % | 2.8 ± 1.0 | 1.0 ± 0.4 | 2.5 ± 0.9 | 2.1 ± 0.7 | 0.69 | 0.007 | 0.06 |
| Alcohol, g | 9.1 ± 3.5 | 4.4 ± 2.0 | 10 ± 4.1 | 9.4 ± 3.1 | 0.48 | 0.02 | 0.12 |
All values are means ± SEMs (n = 43). Baseline values represent averages of the 2 food diaries before the intervention. Intervention values represent an average of all food diaries kept during the 8-week intervention. Main and interaction effects were analyzed using a 2-way (treatment x time) repeated-measures ANOVA. CSO, cottonseed oil; OO, olive oil.
Significant difference between CSO compared with OO during the intervention at a P value < 0.05.
Significant difference between intervention compared with baseline within a group at a P value < 0.05.
For fatty acid composition, there was a treatment × visit interaction (P < 0.001) for an increase in MUFA intake in the OO group compared to the CSO group. Conversely, there was a treatment × visit interaction (P < 0.001) for increases in total PUFA and ω-6 in the CSO group compared with the OO group. Finally, there was a main effect of treatment (P = 0.013) for ω-3 fatty acids, showing greater intake in OO compared with CSO regardless of the visit, and a main effect of visit (P < 0.001), showing a decrease in ω-3 intake from baseline to intervention, regardless of the group. There were no significant main or interaction effects for self-reported intakes of protein, carbohydrate, saturated fat, trans fat, or sugar.
Fasting biochemical markers
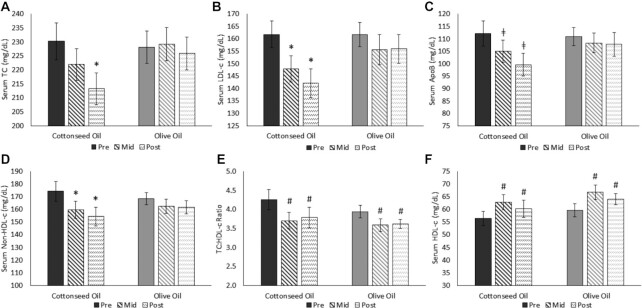
Fasting blood lipids are presented in Figures 2 and 3. There were no differences between groups for any outcome at baseline. For the intervention, there was no effect of treatment (P = 0.42), but there was a significant effect of visit (P = 0.002) and a treatment × visit interaction (P = 0.027) for TC. The interaction effect was driven by a decrease in TC from pre- to postintervention in the CSO group (P < 0.001), with no change in the OO group (P = 0.97). Similarly, for LDL cholesterol, there was no treatment effect (P = 0.31), but there was a visit effect (P < 0.001) and a treatment × visit interaction (P = 0.04) that was driven by reductions in CSO from baseline to midintervention (P = 0.009) and postintervention (P < 0.001), with no change in OO. Likewise, for non–HDL cholesterol, there was no treatment effect (P = 0.76), but there was a visit effect (P < 0.001) and a treatment × visit interaction (P = 0.04). The interaction was driven by reductions in CSO at both the midintervention (P = 0.04) and postintervention (P < 0.001) visits, with no change in OO.
FIGURE 2.
Serum fasting (A) TC, (B) LDL cholesterol, (C) apoB, (D) non–HDL cholesterol, (E) TC:HDL-cholesterol ratio, and (F) HDL cholesterol from pre-, mid-, and postdiet intervention visits in adults with hypercholesterolemia (CSO, n = 21; OO, n = 22). Data were analyzed using a 2-way (treatment × visit) repeated-measures ANOVA. *Significant treatment × visit interaction (P < 0.001) and a difference from baseline at a P value < 0.05. ǂA trend for a treatment × visit interaction (P = 0.09) and a difference from baseline at a P value < 0.01. #Significant visit effect and a difference from baseline, regardless of group assignment, at a P value < 0.05. All values are presented as means ± SEMs. Preintervention visits were at week 0, midintervention visits at week 4, and postintervention visits at week 8. CSO, cottonseed oil; Mid, midintervention; OO, olive oil; Pre, preintervention; Post, postintervention; TC, total cholesterol.
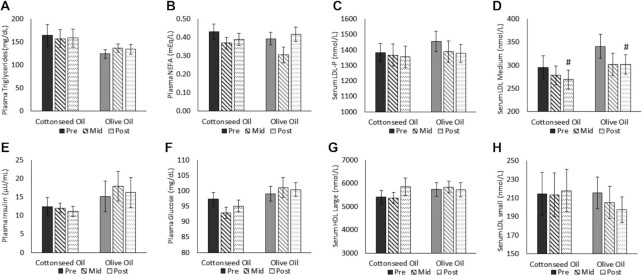
FIGURE 3.
Fasting biochemical markers of (A) plasma triglycerides, (B) plasma NEFAs, (C) serum LDL-P, (D) serum LDL medium, (E) plasma insulin, (F) plasma glucose, (G) serum HDL large, and (H) serum LDL small, from pre-, mid-, and postdiet intervention visits in adults with hypercholesterolemia (CSO, n = 21; OO, n = 22). Data were analyzed using a 2-way (treatment × visit) repeated-measures ANOVA. #Significant visit effect and a difference from baseline, regardless of group assignment, at a P value < 0.05. All values are presented as means ± SEMs. Preintervention visits were at week 0, midintervention visits at week 4, and postintervention visits at week 8. LDL-P, LDL particle number; Mid, midintervention; NEFA, nonesterified fatty acid; Pre, preintervention; Post, postintervention.
For apoB, there was no effect of treatment (P = 0.52), but there was a main effect of visit (P < 0.001), which was for reductions at both the midintervention (P = 0.04) and postintervention (P < 0.001) visits. There was also a trend for a treatment × visit interaction (P = 0.09), driven by a reduction in CSO from pre- to postintervention (P < 0.001), with no change in OO. There were visit effects for HDL cholesterol (P < 0.001), the TC:HDL-cholesterol ratio (P < 0.001), and LDL medium (P = 0.04), but not for treatment effects (P = 0.33, P = 0.46, and P = 0.21, respectively) or interactions (P = 0.92, P = 0.38, and P = 0.04, respectively). The visit effects were for increases for HDL cholesterol (midintervention, P < 0.001; postintervention, P < 0.001) and decreases in the TC:HDL-cholesterol ratio (midintervention, P < 0.001; postintervention, P < 0.001) and LDL medium (postintervention, P = 0.049), regardless of the intervention group. Finally, there were no significant main or interaction effects in fasting TG, NEFA, LDL-P, LDL small, LDL medium, HDL large, insulin, or glucose.
Change in fasting biochemical markers between groups
The changes from pre- to postintervention for fasting biochemical markers are presented in Table 4 as both absolute changes and percent changes. For the absolute changes, the decreases in TC (P = 0.008), LDL cholesterol (P = 0.018), non–HDL cholesterol (P = 0.014), and apoB (P = 0.05) were greater in the CSO group compared to the OO group from pre- to postintervention. There were no differences between groups for the changes in HDL cholesterol, TG, NEFA, the TC:HDL-cholesterol ratio, LDL small, LDL medium, HDL large, LDL-P, fasting insulin, or glucose. To examine the magnitude, we also calculated the mean difference in changes between groups. This is also presented in Table 4 with 95% CIs, and mirrors the significance mentioned above.
TABLE 4.
Change from pre-to postdiet intervention in fasting biochemical markers of adults with hypercholesterolemia in cottonseed oil or olive oil groups1
| Mean Change | |||||||
|---|---|---|---|---|---|---|---|
| CSO | OO | Percent Change | |||||
| Measure | Baseline | Change2 | Baseline | Change 2 | CSO vs OO difference | CSO | OO |
| Total cholesterol,3 mg/dL | 230 ± 6.57 | −17.0 ± 3.94 | 228 ± 5.81 | −2.18 ± 3.72 | −14.8 (−25.2 to −4.43) | −7.38 ± 1.77 | −0.96 ± 1.77 |
| LDL cholesterol,3 mg/dL | 162 ± 5.41 | −19.7 ± 3.94 | 162 ± 4.90 | −5.72 ± 4.23 | −13.9 (−25.0 to −2.88) | −12.16 ± 2.52 | −3.54 ± 2.72 |
| HDL cholesterol, mg/dL | 56.5 ± 2.79 | 3.76 ± 1.71 | 59.7 ± 2.63 | 4.43 ± 1.28 | −0.67 (−4.75 to 3.41) | 6.66 ± 3.42 | 7.43 ± 2.62 |
| Triglyceride, mg/dL | 169 ± 22.9 | −6.01 ± 9.74 | 131 ± 10.8 | 2.87 ± 9.86 | −8.87 (−35.4 to 17.6) | −3.55 ± 6.70 | 2.19 ± 7.84 |
| NEFA, mEq/L | 0.43 ± 0.04 | −0.04 ± 0.04 | 0.39 ± 0.04 | 0.02 ± 0.03 | −0.07 (−0.16 to 0.03) | −4.67 ± 8.44 | 6.07 ± 8.33 |
| TC:HDL-cholesterol ratio | 4.30 ± 0.27 | −0.51 ± 0.10 | 3.94 ± 0.16 | −0.36 ± 0.13 | −0.15 (−0.46 to 0.16) | −11.9 ± 2.88 | −9.10 ± 3.04 |
| Non–HDL cholesterol,3 mg/dL | 175 ± 7.81 | −20.8 ± 4.00 | 169 ± 4.95 | −6.61 ± 4.01 | −14.1 (−3.30 to −25.0) | −11.9 ± 2.45 | −3.93 ± 2.54 |
| LDL-P, nmol/L | 1380 ± 57.4 | −26.9 ± 50.0 | 1460 ± 67.3 | −77.3 ± 68.6 | 50.3 (−112 to 212) | −1.95 ± 3.79 | −5.31 ± 4.64 |
| LDL small, nmol/L | 215 ± 24.0 | 3.43 ± 11.2 | 216 ± 18.2 | −18.3 ± 15.6 | 21.71 (−15.1 to 58.5) | 1.60 ± 6.19 | −8.48 ± 7.01 |
| LDL medium, nmol/L | 296 ± 24.8 | −23.1 ± 15.9 | 341 ± 24.5 | −39.3 ± 19.5 | 16.2 (−31.9 to 64.3) | −7.83 ± 5.33 | −11.5 ± 5.49 |
| HDL large, nmol/L | 5410 ± 377 | 443 ± 295 | 5740 ± 293 | −7.23 ± 316 | 450 (−377 to 1280) | 8.18 ± 5.86 | −0.13 ± 5.61 |
| ApoB,3 mg/dL | 112 ± 4.48 | −11.7 ± 2.37 | 111 ± 3.86 | −3.1 ± 2.99 | −8.65 (−15.4 to −1.35) | −10.5 ± 2.24 | −2.79 ± 2.82 |
| Insulin, μU/mL | 12.4 ± 2.45 | −1.26 ± 1.47 | 15.2 ± 4.19 | 1.05 ± 0.64 | −2.31 (−5.37 to 0.75) | −10.1 ± 11.0 | 6.94 ± 10.0 |
| Glucose, mg/dL | 97.3 ± 2.22 | −2.23 ± 2.02 | 99.1 ± 2.46 | 1.33 ± 3.48 | −3.56 (−11.3 to 4.14) | −2.29 ± 2.10 | 1.34 ± 3.53 |
Changes from baseline values (mean change, percent change) indicate changes from the pre- to postintervention visit (baseline to week 8). Those values are presented as means ± SEMs. The difference in change values from pre- to postintervention visits for CSO (n = 21) compared with OO (n = 22) are presented as mean differences with 95% CIs. All markers are measured in serum, except triglycerides, NEFAs, insulin, and glucose, which were measured in plasma. CSO, cottonseed oil; LDL-P, LDL particle number; NEFA, nonesterified fatty acid; OO, olive oil; TC, total cholesterol.
Change values were compared using unpaired t-tests between groups.
Significant difference between groups (P < 0.05).
Postprandial biochemical markers
The meal responses for TG, NEFA, insulin, and glucose are presented in Figure 4. For postprandial TGs, there was no effect of treatment (P = 0.45) but there were significant effects of visit (P = 0.02), time point (P < 0.001), and a treatment × visit interaction (P = 0.004). The interaction was driven by higher postprandial TGs at the postintervention visit compared with baseline in OO (P = 0.002; Figure 4A, B), with no difference in CSO. This effect, however, was not observed when examining AUC data (CSO preintervention, 213 ± 27.3 mg/dL·5h and postintervention, 213 ± 24.9 mg/dL·5h; OO preintervention, 179 ± 15.9 mg/dL·5h and postintervention, 194 ± 14.6 mg/dL·5h; P = 0.22). For NEFAs (Figure 4C, D), there was no treatment effect (P = 0.98), but there were significant effects of time point (P < 0.001) and visit (P = 0.003) and a trend for the treatment × visit interaction (P = 0.11). The interaction trend was driven by a reduction in postprandial NEFA from pre- to postintervention in CSO (P = 0.008), and no change in OO (P = 0.68). For the AUC, there was a visit effect (P = 0.05) for NEFA (CSO preintervention, 0.45 ± 0.03 mg/dL·5h and postintervention, 0.41 ± 0.03 mg/dL·5h; OO preintervention, 0.44 ± 0.03 and postintervention, 0.42 ± 0.04 mEq/L·5h) showing a decrease from the pre- to postintervention visits, regardless of treatment. There were no main or interaction effects for insulin (Figure 4E, F). Finally, there were also no main effects of treatment or visit for glucose (P = 0.35 and P = 0.76, respectively); however, there was a treatment × visit interaction (P = 0.003; Figure 4G, H) driven by a trend for an increase in OO from the pre- to postintervention visits (P = 0.09), and a nonsignificant decrease in CSO from pre- to postintervention (P = 0.20). There was also a significant treatment × visit interaction for the glucose AUC (CSO preintervention, 99.7 ± 2.50 mg/dL·5h and postintervention, 97.5 ± 2.68 mg/dL·5h; OO preintervention, 99.7 ± 2.52 mg/dL·5h and postintervention, 103 ± 2.67 mg/dL·5h; P = 0.028), again showing an increase in OO compared to CSO after the intervention.
FIGURE 4.
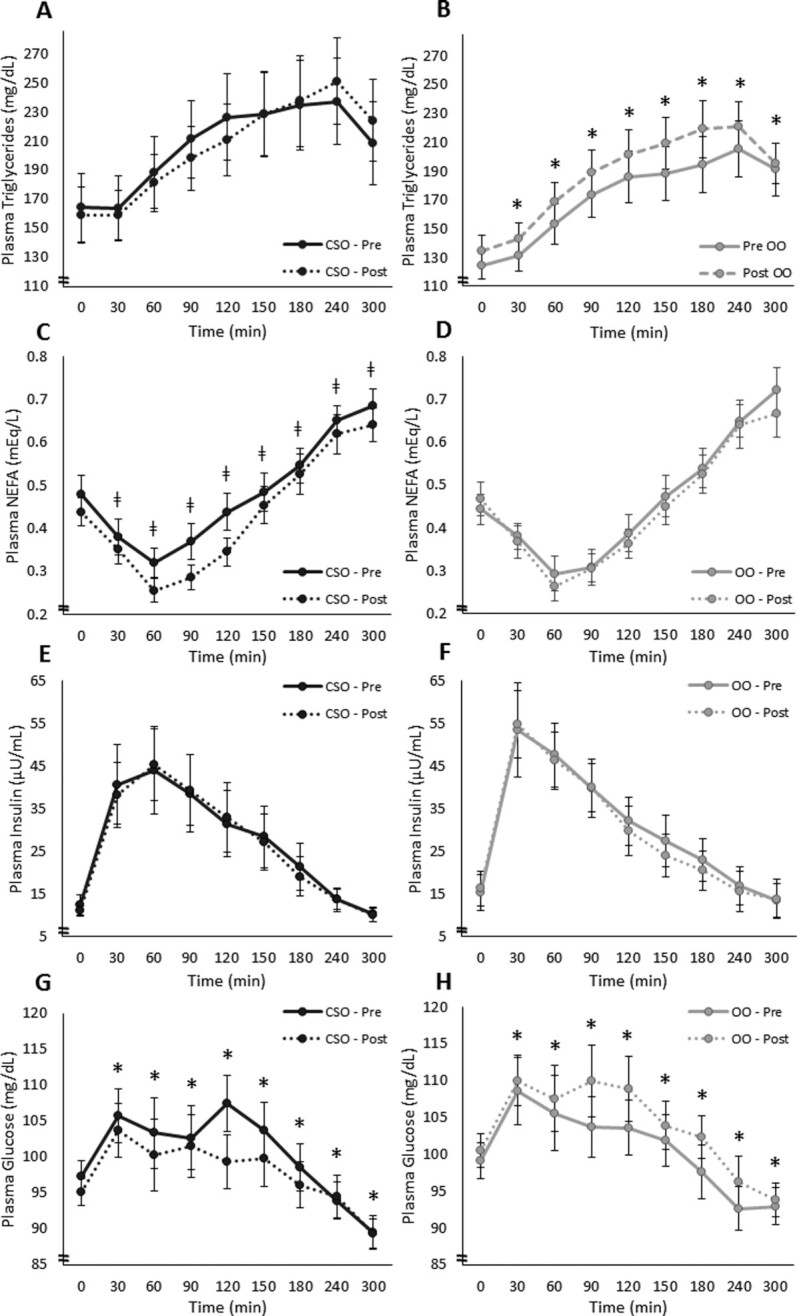
Time course for (A, B) plasma TGs, (C, D) NEFAs, (E, F) insulin, and (G, H) glucose for each treatment at pre- and postintervention visits in adults with hypercholesterolemia (CSO, n = 21; OO, n = 21). Participants consumed a high–saturated fat breakfast meal immediately after time 0. *Significant treatment × visit interaction and a difference between the pre- and postintervention meal responses within a group (P < 0.05). ǂTrend for treatment × visit interaction and a difference between the pre- and postintervention meal responses within a group (P = 0.10). All values are presented as means ± SEMs. Preintervention visits were at week 0 and postintervention visits were at week 8. CSO, cottonseed oil; NEFA, nonesterified fatty acid; OO, olive oil; Post, postintervention; Pre, preintervention; TG, triglyceride.
Discussion
For the first time, we have shown that consumption of a diet enriched with CSO (high in PUFA) for 8 weeks resulted in significant improvements in fasting TC, LDL cholesterol, HDL cholesterol, the TC:HDL-cholesterol ratio, non–HDL cholesterol, and apoB in adults with hypercholesterolemia. CSO diet enrichment also suppressed postprandial NEFA and glucose following the intervention. The only changes in the OO diet (high in MUFA) were improvements in fasting HDL cholesterol and the TC:HDL-cholesterol ratio and a worsening of the postprandial TG and glucose response to a SFA-rich meal. Together, this broadly provides additional evidence on the comparative effects of dietary oils rich in PUFA compared with MUFA for blood lipid control, and specific evidence that CSO has a greater effect than OO for blood lipid improvements in an at-risk population.
The suppression of fasting TC and LDL cholesterol in the CSO group amounted to 7.4% and 12.2% reductions, respectively. These findings are clinically meaningful because every 1.0% reduction in LDL cholesterol has been estimated to reduce the risk of coronary artery disease (CAD) by 1.2% to 2.0% (31, 32) resulting in an estimated 14.6% to 21.4% reduction in the CAD risk in the CSO group.
Additionally, of the CSO participants in our study, the reductions in TC and LDL cholesterol led to a reduction in at least 1 diagnostic category (e.g., “high” to “borderline high”) in 57% of participants. The CSO diet also lowered LDL cholesterol in a similar magnitude to bile acid sequesterants, fibrates, and nicotinic acid. While not as effective as statins, our magnitude of change in LDL cholesterol is similar to that of an extremely low dose of pravastatin, without the adverse side effects commonly reported with these pharmacologic, lipid-lowering therapies, especially statins (33, 34). Finally, apoB is a major structural protein found on atherogenic lipoproteins (35), and is gaining recognition as a potentially more useful marker for predicting the CVD risk than LDL cholesterol (36). By conclusion of a meta-analysis, reductions of 10 mg/dL in apoB reduced the risk of coronary heart disease (CHD) and the overall CVD risk by 9% and 6%, respectively (37). Based on this, the magnitude of reduction in apoB in our CSO group would correspond to 10% and 7% reductions in the CHD and CVD risks, respectively. Therefore, the reductions in TC, LDL cholesterol, and apoB demonstrate the clinical significance of CSO's superior lipid-lowering effect, and the subsequent lowering of the chronic disease risk, compared to OO. These effects were observed despite reductions during the intervention in intakes of other nutrients known to improve lipid profiles, such as fiber and slight weight gain, which would be expected to have detrimental effects on blood lipid profiles.
It is well established that the replacement of SFA with unsaturated fats reduces blood lipids and the risk of CVD (6, 38–41). This was well demonstrated in the Dietary Intervention and Vascular function study, which observed similar improvements in fasting blood lipids when replacing SFAs for unsaturated fats, but no differences between MUFAs and PUFAs, for these outcomes (41). The direct comparison on the effects of MUFAs compared with PUFAs, rather than comparing MUFAs or PUFAs to SFA controls, on blood lipids and the CVD risk is less well understood. This is especially true for CSO compared with OO. High MUFA sources, including OO, often lack the robust lipid-lowering effects regularly observed by high PUFA sources (8, 42, 43). Conversely, trials have shown stronger lipid-lowering effects from high PUFA intake, although they usually have little to no effect on HDL cholesterol (44). While data comparing MUFA and PUFA diets have not been directly analyzed in a meta-analysis, individual trials exist comparing the 2 unsaturated fats. PUFA-rich oils, including corn, soybean, and sunflower oils, have been shown to reduce TC, LDL cholesterol, non–HLD cholesterol, and VLDL cholesterol to a greater degree than MUFA-rich oils, such as OO, rice bran oil, and modified high oleic oils (45–49). From this small body of literature, it appears that consumption of PUFA-rich oils tends to generate greater reductions in blood lipids than consumption of MUFAs.
Not only do PUFAs seem to be superior to MUFAs for their lipid-lowering effects, but CSO appears to have benefits beyond other high-PUFA food sources. In animal models, there are larger improvements in the lipid metabolism following CSO consumption, all compared to high-PUFA treatments, including corn, soybean, and safflower oils (13, 50–55). Additionally, CSO improves glucose tolerance and hepatic lipid accumulation compared to safflower oil in mice (13). This evidence is limited, as there are no human studies comparing CSO to other high-PUFA foods or diets; however, 2 previous studies in humans, both 1 week in duration in healthy, young adults, showed similar lipid-lowering effects to what we have observed presently with CSO consumption (11, 12). These combined data from multiple studies show that CSO, rich in ω-6 PUFAs, has a consistent effect of improving blood lipids and appears to be more effective than other high-PUFA food sources. However, as mentioned above, since these data originate in animal models, clinical trials are needed.
Certain properties of CSO may explain its superior effects on the lipid metabolism. CSO is a unique oil, in that it is high in PUFAs, as well as SFAs (23% SFAs, 20% MUFAs, and 57% PUFAs; see Supplemental Table 1). The high SFA content of CSO makes its lipid-lowering properties somewhat surprising (52). There are multiple potential mechanisms to explain the lowering of blood lipids by CSO. The first is transcriptional regulation of the lipid and cholesterol metabolisms by the high PUFA content of the oil (9, 10). The second was presented when Paton et al. (13) confirmed the presence of trace amounts of DHSA in CSO and confirmed that CSO had a more pronounced effect on the lipid metabolism than a safflower oil–enriched diet (high ω-6 oil devoid of DHSA). DHSA is of note because it is a cyclopropyl intermediate in the synthesis of sterulic acid, which is a known inhibitor of the hepatic lipogenic enzyme, SCD1, potentially mitigating hepatic lipid accumulation in response to excessive dietary fat (13), which could support improvements in the cholesterol metabolism. More specifically, SCD1 catalyzes the desaturation of a range of SFAs to endogenously synthesize MUFAs, promoting the storage of lipid rather than the oxidation of it (56). Paton et al. (13) was able to prove this mechanism in a mouse model with the use of desaturation indices, liver lipid quantification, and expression of SCD1 mRNA. Polley et al. (11) confirmed the possibility of this mechanism in humans using a similar desaturation index, while also having observed a cholesterol-lowering effect following CSO consumption. It is also plausible that the 2-pronged mechanism (SCD1 inhibition by trace DHSA and blood lipid metabolism regulation by PUFAs) is necessary to produce the same observed changes in blood lipids observed in the present trial. This mechanism remains to be confirmed in humans. The effects of CSO may also be a result of other components of its nonsaponifiable portion, including tocopherols and beta-sitosterol (12, 57), as both may modify blood lipid control by modifying expression of regulatory proteins in the metabolism of cholesterol. Lastly, it is important to recognize that CSO as a whole may be required to exert the observed changes in the blood lipid metabolism rather than a singled-out nutrient.
Since adults spend most waking hours in the postprandial state, it is now recognized as a critical period in which disease development can be exacerbated (58, 59). In the current trial, we utilized a high-fat, SFA-rich meal challenge at pre- and postintervention visits, rather than a high-fat meal rich in the oil of the assigned intervention group (CSO or OO). This unique study design allowed us to determine the chronic effects of daily CSO or OO consumption, and their potential protective effects against an occasional meal high in SFAs. Only the CSO group experienced postprandial improvements in NEFAs and glucose following the intervention. Insulin signaling regulates both NEFAs and glucose by the delayed activation of lipoprotein lipase and the immediate translocation of glucose transporter type 4 (GLUT4), respectively (60, 61). The improvements of NEFAs and glucose in CSO suggest protection from lipotoxicity of the high-SFA meal challenge and improved insulin sensitivity, further improving the participants’ abilities to handle an occasional high-SFA meal. While we did not expect outcomes to improve with OO, we were somewhat surprised with the worsening of postprandial TG and glucose following OO treatment. A small weight gain of 1 kg was observed in the trial, regardless of group. Theoretically, this could lead to slight detriments in terms of lipid control; however, the differences between groups for the postprandial outcomes suggest that CSO offered protection from changes that could be due to weight gain, while OO did not. This appears to contradict other studies where OO is used as part of Mediterranean diet studies (62, 63), so more work on the isolated effects of OO consumption is warranted.
This study is not without limitations. We chose a relatively high dose of each oil to match the doses used in previous short-term CSO studies. While high-fat diets, such as the ketogenic diet, are quite popular, the dose used here affects the generalizability to individuals following a lower-fat diet. Another limitation was the decision to compare PUFAs with MUFAs rather than with a true control group that had no intervention. This design allowed us to first detect within-group differences of the intervention diets, and then to compare the magnitude of change directly between the diets. Since OO is a popular “healthy oil” choice among consumers (15, 16) and CSO is less familiar, this design sheds light on the physiologic responses to diets enriched in each oil. The lack of change in most outcomes for the OO group, and the lack of change in known dietary components as seen in the self-reported intake analyses, strengthens our findings that it was CSO, and not some other confounding variable of participating in a dietary intervention study, that improved outcomes. However, only the fatty acid composition of each oil was measured in this trial, so other compounds that can vary in oils, such as the phenolic content, potentially limit our ability to determine what is driving our observed effects on blood lipids. Another limitation may be that we only controlled dinner the night before each testing visit rather than using a multiday, lead-in diet. This was intentional to isolate the effects of the intervention and to have baseline measures closely reflect the participants’’ usual intakes rather than manipulated baseline levels. Additionally, we only used a 2-day baseline food diary rather than a longer diary in order to reduce the participant burden, but we acknowledge this is a relatively short period of time and may have caused our intake analysis to be less sensitive than a longer food record. This trial was also single blinded rather than double blinded, limiting the strength of the design. Finally, the measurements of dietary intake, physical activity, stress, and compliance were all self-reported, which contains some degree of under- or overreporting, and we did not have direct measures or biomarkers to assess compliance.
In conclusion, we have shown that a CSO-enriched diet reduced fasting blood lipids, including TC, LDL cholesterol, apoB, postprandial NEFAs, and glucose, and improved HDL cholesterol in adults with hypercholesterolemia. The results of this study are clinically meaningful, because the magnitude of reduction in LDL cholesterol by CSO (12.2%) could correspond to a 14.6% to 21.4% reduction in the CAD risk. Additionally, the reductions in postprandial NEFAs and the glucose response provide further protection in the fed state. This study shows that these sources of MUFAs and PUFAs have different responses with respect to the blood lipid metabolism and glycemic control. Furthermore, this study provides evidence that CSO in particular may be a beneficial oil to incorporate into the diet for adults with hypercholesterolemia. In addition to its cholesterol-lowering effects, CSO is a practical cooking oil with a neutral flavor and a high smoke point, meaning it is easily incorporated into common foods. It is also easily accessible to consumers; is already commonly found in food items such as salad dressings, condiments, and packaged goods (crackers, chips); and is currently used by many restaurants due to the aforementioned high smoke point, which is good for frying. Cotton is primarily known as a textiles crop; thus, its ability to contribute to the food supply highlights the utility of this US crop. Future studies should investigate the effects of enriching the diet with different high-PUFA oils to see whether CSO is superior to other PUFA-rich oils. Future studies should also examine CSO diet enrichment at lower doses, over different durations, and in various populations. Finally, additional studies should also investigate other markers of CVD risk, including inflammation, to fully characterize CSO's effects on CVD risks.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows – JAC, CMP: conceived the project, provided study oversight, and provided essential reagents and materials; JAC, ARS: developed the overall research plan; JAC, MCP: wrote the manuscript; ARS, MCP: conducted the research; MCP: analyzed the data; and all authors: read and approved the final manuscript.
Notes
This study was funded by National Cottonseed Products Association (AWD00009110) and Cotton Incorporated (AWD00012904).
Author disclosures: The authors report no conflicts of interest.
The funders had no role in the design, implementation, analysis, and interpretation of the data.
Supplemental Figure 1 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: CAD, coronary artery disease; CHD, coronary heart disease; CSO, cottonseed oil; CVD, cardiovascular disease; DHSA, dihydrosterulic acid; HNL, Human Nutrition Lab; IPAQ, International Physical Activity Questionnaire; LDL-P, LDL particle number; MET, metabolic equivalent task; NEFA, nonesterified fatty acids; OO, olive oil; PSS, Perceived Stress Scale; RMR, resting metabolic rate; SCD1, stearoyl-CoA desaturase-1; TC, total cholesterol; TG, triglycerides; V1, prediet intervention visit; V2, middiet intervention visit; V3, postdiet intervention visit.
Contributor Information
M Catherine Prater, Department of Nutritional Sciences, University of Georgia, Athens, GA, USA.
Alexis R Scheurell, Department of Nutritional Sciences, University of Georgia, Athens, GA, USA.
Chad M Paton, Department of Nutritional Sciences, University of Georgia, Athens, GA, USA; Department of Food Science and Technology, University of Georgia, Athens, GA, USA.
Jamie A Cooper, Department of Nutritional Sciences, University of Georgia, Athens, GA, USA.
Data Availability
Data generated and analyzed during this study are available from the corresponding author upon reasonable request.
References
- 1. WHO . Cardiovascular diseases (CVDs) [Internet]. Geneva, Switzerland: WHO; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). [Google Scholar]
- 2. Kopin L, Lowenstein C. Dyslipidemia. Ann Intern Med. 2017;167(11):ITC81–96. [DOI] [PubMed] [Google Scholar]
- 3. Carroll MD, Fryar CD. Total and high-density lipoprotein cholesterol in adults: United States, 2015–2018. NCHS Data Brief, no 363. Hyattsville, MD: National Center for Health Statistics. 2020. [Google Scholar]
- 4. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fatty acids and risk of coronary heart disease: Modulation by replacement nutrients. Curr Atheroscler Rep. 2010;12(6):384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mensink RP, Effects of saturated fatty acids on serum lipids and lipoproteins: A systematic review and regression analysis. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 6. Hooper L, Martin N, Jimoh OF, Kirk C, Foster E, Abdelhamid AS. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2020;5(5):CD011737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014;13(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghobadi S, Hassanzadeh-Rostami Z, Mohammadian F, Nikfetrat A, Ghasemifard N, Raeisi Dehkordi H, Faghih S. Comparison of blood lipid-lowering effects of olive oil and other plant oils: A systematic review and meta-analysis of 27 randomized placebo-controlled clinical trials. Crit Rev Food Sci Nutr. 2019;59(13):2110–24. [DOI] [PubMed] [Google Scholar]
- 9. Jump DB. Fatty acid regulation of gene transcription. Crit Rev Clin Lab Sci. 2004;41(1):41–78. [DOI] [PubMed] [Google Scholar]
- 10. Sampath H, Ntambi JM. Polyunsaturated fatty acid regulation of gene expression. Nutr Rev. 2004;62(9):333–9. [DOI] [PubMed] [Google Scholar]
- 11. Polley KR, Oswell NJ, Pegg RB, Paton CM, Cooper JA. A 5-day high-fat diet rich in cottonseed oil improves cholesterol profiles and triglycerides compared to olive oil in healthy men. Nutr Res. 2018;60:43–53. [DOI] [PubMed] [Google Scholar]
- 12. Davis KE, Prasad C, Imrhan V. Consumption of a diet rich in cottonseed oil (CSO) lowers total and LDL cholesterol in normo-cholesterolemic subjects. Nutrients. 2012;4(7):602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paton CM, Vaughan RA, Alpergin ESS, Assadi-Porter F, Dowd MK. Dihydrosterculic acid from cottonseed oil suppresses desaturase activity and improves liver metabolomic profiles of high-fat–fed mice. Nutr Res. 2017;45:52–62. [DOI] [PubMed] [Google Scholar]
- 14. Alves AQ, da Silva Jr VA, Góes AJS, Silva MS, de Oliveira GG, Bastos I, de Castro Neto AG, Alves AJ. The fatty acid composition of vegetable oils and their potential use in wound care. Adv Skin Wound Care. 2019;32:1–8. [DOI] [PubMed] [Google Scholar]
- 15. Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92(5):1189–96. [DOI] [PubMed] [Google Scholar]
- 16. International Olive Council . Olive oil production and consumption up by 1 million tonnes in the last 25 years. IOC News; Verona, Italy: 2018. https://www.internationaloliveoil.org/1071-olive-oil-production-and-consumption-up-by-1-million-tonnes-in-the-last-25-years/#. [Google Scholar]
- 17. Lombardi A, Carlucci D, Cavallo C, De Gennaro B, Del Giudice T, Giannoccaro G, Paparella A, Roselli L, Vecchio R, Cicia G. Do consumers understand health claims on extra-virgin olive oil?. Food Res Int. 2021;143:110267. [DOI] [PubMed] [Google Scholar]
- 18. Pichierri M, Peluso AM, Pino G, Guido G. Health claims’ text clarity, perceived healthiness of extra-virgin olive oil, and arousal: An experiment using facereader. Trends Food Sci Technol. 2021;116:1186–94. [Google Scholar]
- 19. Mayo Clinic Staff. High cholesterol: Diagnosis [Internet]. Clinic MayoMayo Foundation for Medical Education and Research; 2021. https://www.mayoclinic.org/diseases-conditions/high-blood-cholesterol/diagnosis-treatment/drc-20350806. [Google Scholar]
- 20. National Institutes of Health: National Heart, Lung and Blood Institute . Cholesterol levels: What you need to know. NIH MedlinePlus [Internet]. Bethesda, MD: 2020; https://medlineplus.gov/cholesterollevelswhatyouneedtoknow.html. [Google Scholar]
- 21. Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196(2):696–703. [DOI] [PubMed] [Google Scholar]
- 22. Jeong SM, Choi S, Kim K, Kim SM, Lee G, Park SY, Kim YY, Son JS, Yun JM, Park SM. Effect of change in total cholesterol levels on cardiovascular disease among young adults. J Am Heart Assoc. 2018;7(12):e008819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): Validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56(4):423–32. [DOI] [PubMed] [Google Scholar]
- 24. Cooper JA, Watras AC, O'Brien MJ, Luke A, Dobratz JR, Earthman CP, Schoeller DA. Assessing validity and reliability of resting metabolic rate in six gas analysis systems. J Am Diet Assoc. 2009;109(1):128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stevenson JL, Miller MK, Skillman HE, Paton CM, Cooper JA. A PUFA-rich diet improves fat oxidation following saturated fat-rich meal. Eur J Nutr. 2017;56(5):1845–57. [DOI] [PubMed] [Google Scholar]
- 26. JB Weir. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trumbo P, Schlicker S, Yates A, Poos M, Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. J AM Diet Assoc. 2002;102(11):1621–30. [DOI] [PubMed] [Google Scholar]
- 28. Gersovitz M, Madden JP, Smiciklas-Wright H. Validity of the 24-hr. dietary recall and seven-day record for group comparisons. J Am Diet Assoc. 1978;73(1):48–55. [PubMed] [Google Scholar]
- 29. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 30. Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire short form (IPAQ-SF): A systematic review. Int J Behav Nutr Phys Act. 2011;8(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R, Participants SW. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clinic Proceedings;. Elsevier: 2003:965–78. [DOI] [PubMed] [Google Scholar]
- 32. McRorie JW Jr. Evidence-based approach to fiber supplements and clinically meaningful health benefits, part 1: What to look for and how to recommend an effective fiber therapy. Nutr Today. 2015;50(2):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmitz G, Langmann T. Pharmacogenomics of cholesterol-lowering therapy. Vasc Pharmacol. 2006;44(2):75–89. [DOI] [PubMed] [Google Scholar]
- 34. Oni-Orisan A, Hoffmann TJ, Ranatunga D, Medina MW, Jorgenson E, Schaefer C, Krauss RM, Iribarren C, Risch N. Characterization of statin low-density lipoprotein cholesterol dose-response using electronic health records in a large population-based cohort. Circ Genom Precis Med. 2018;11:e002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shapiro MD, Fazio S. Apolipoprotein B-containing lipoproteins and atherosclerotic cardiovascular disease. F1000Res. 2017;6:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carr SS, Hooper AJ, Sullivan DR, Burnett JR. Non-HDL-cholesterol and apolipoprotein B compared with LDL-cholesterol in atherosclerotic cardiovascular disease risk assessment. Pathology. 2019;51(2):148–54. [DOI] [PubMed] [Google Scholar]
- 37. Robinson JG, Wang S, Jacobson TA. Meta-analysis of comparison of effectiveness of lowering apolipoprotein B versus low-density lipoprotein cholesterol and nonhigh-density lipoprotein cholesterol for cardiovascular risk reduction in randomized trials. Am J Cardiol. 2012;110(10):1468–76. [DOI] [PubMed] [Google Scholar]
- 38. U.S. Department of Agriculture and U.S. Department of Halth and Human Services. Dietary guidelines for Americans, 2020–2025. 9th ed. 2020. Washington D.C.Available atDietaryGuidelines.gov. [Google Scholar]
- 39. Schwingshackl L, Bogensberger B, Benčič A, Knüppel S, Boeing H, Hoffmann G. Effects of oils and solid fats on blood lipids: A systematic review and network meta-analysis. J Lipid Res. 2018;59(9):1771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun Y, Neelakantan N, Wu Y, Lote-Oke R, Pan A, van Dam RM. Palm oil consumption increases LDL cholesterol compared with vegetable oils low in saturated fat in a meta-analysis of clinical trials. J Nutr. 2015;145(7):1549–58. [DOI] [PubMed] [Google Scholar]
- 41. Vafeiadou K, Weech M, Altowaijri H, Todd S, Yaqoob P, Jackson KG, Lovegrove JA. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: Results from the randomized, controlled Dietary Intervention and Vascular Function (DIVAS) study. Am J Clin Nutr. 2015;102(1):40–8. [DOI] [PubMed] [Google Scholar]
- 42. Baum SJ, Kris-Etherton PM, Willett WC, Lichtenstein AH, Rudel LL, Maki KC, Whelan J, Ramsden CE, Block RC. Fatty acids in cardiovascular health and disease: A comprehensive update. J Clin Lipidol. 2012;6(3):216–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids. 2010;45(10):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abdelhamid AS, Martin N, Bridges C, Brainard JS, Wang X, Brown TJ, Hanson S, Jimoh OF, Ajabnoor SM, Deane KH. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;7(7):CD012345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maki K, Lawless A, Kelley K, Kaden V, Geiger C, Palacios O, Dicklin M. Corn oil intake favorably impacts lipoprotein cholesterol, apolipoprotein and lipoprotein particle levels compared with extra-virgin olive oil. Eur J Clin Nutr. 2017;71(1):33–8. [DOI] [PubMed] [Google Scholar]
- 46. Wagner K-H, Tomasch R, Elmadfa I. Impact of diets containing corn oil or olive/sunflower oil mixture on the human plasma and lipoprotein lipid metabolism. Eur J Nutr. 2001;40(4):161–7. [DOI] [PubMed] [Google Scholar]
- 47. Utarwuthipong T, Komindr S, Pakpeankitvatana V, Songchitsomboon S, Thongmuang N. Small dense low-density lipoprotein concentration and oxidative susceptibility changes after consumption of soybean oil, rice bran oil, palm oil and mixed rice bran/palm oil in hypercholesterolaemic women. J Int Med Res. 2009;37(1):96–104. [DOI] [PubMed] [Google Scholar]
- 48. Binkoski AE, Kris-Etherton PM, Wilson TA, Mountain ML, Nicolosi RJ. Balance of unsaturated fatty acids is important to a cholesterol-lowering diet: Comparison of mid-oleic sunflower oil and olive oil on cardiovascular disease risk factors. J Am Diet Assoc. 2005;105(7):1080–6. [DOI] [PubMed] [Google Scholar]
- 49. Baer DJ, Henderson T, Gebauer SK. Consumption of high-oleic soybean oil improves lipid and lipoprotein profile in humans compared to a palm oil blend: A randomized controlled trial. Lipids. 2021;56(3):313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hampden KA. Rober EF, Stewart G. Effects of feeding whole kernel cottonseed on growth, reproduction and biochemical parameters. Fed. Proc. 1984;42:1323. [Google Scholar]
- 51. Edwards M, Radcliffe J. A comparison of the effect of cottonseed oil and corn oil on lipid status in the rat. Biochem Arch. 1995;11:103–9. [Google Scholar]
- 52. Radcliffe J, King C, Czajka-Narins D, Imrhan V. Serum and liver lipids in rats fed diets containing corn oil, cottonseed oil, or a mixture of corn and cottonseed oils. Plant Foods Hum Nutr. 2001;56(1):51–60. [DOI] [PubMed] [Google Scholar]
- 53. Radcliffe J, Czajka-Narins D, Imrhan V. Fatty acid composition of serum, adipose tissue, and liver in rats fed diets containing corn oil or cottonseed oil. Plant Foods Hum Nutr. 2004;59(2):73–7. [DOI] [PubMed] [Google Scholar]
- 54. Radcliffe J, Czajka-Narins D. Lipids and tocopherols in serum and liver of female rats fed diets containing corn oil or cottonseed oil. Plant Foods Hum Nutr. 2006;61(1):33–6. [DOI] [PubMed] [Google Scholar]
- 55. Yang A, Qi M, Wang X, Wang S, Sun L, Qi D, Zhu L, Duan Y, Gao X, Rajput SAet al. Refined cottonseed oil as a replacement for soybean oil in broiler diet. Food Sci Nutr. 2019;7:1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab. 2009;297(1):E28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gutfinger T, Letan A. Studies of unsaponifiables in several vegetable oils. Lipids. 1974;9(9):658–63. [Google Scholar]
- 58. Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, Ooi TC, Perez-Martinez P, Bilianou H, Anagnostopoulou K, Panotopoulos G. Assessment and clinical relevance of non-fasting and postprandial triglycerides: An expert panel statement. Curr Vasc Pharmacol. 2011;9:258–70. [DOI] [PubMed] [Google Scholar]
- 59. Kolovou GD, Watts GF, Mikhailidis DP, Pérez-Martínez P, Mora S, Bilianou H, Panotopoulos G, Katsiki N, Ooi TC, Lopez-Miranda J. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profiles: Executive summary of a 2019 expert panel statement. Curr Vasc Pharmacol. 2019;17(5):538–40. [DOI] [PubMed] [Google Scholar]
- 60. Jelic K, Hallgreen CE, Colding-Jørgensen M. A model of NEFA dynamics with focus on the postprandial state. Ann Biomed Eng. 2009;37(9):1897–909. [DOI] [PubMed] [Google Scholar]
- 61. Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13(6):383–96. [DOI] [PubMed] [Google Scholar]
- 62. Gomez-Delgado F, Alcala-Diaz JF, Leon-Acuña A, Lopez-Moreno J, Delgado-Lista J, Gomez-Marin B, Roncero-Ramos I, Yubero-Serrano EM, Rangel-Zuñiga OA, Vals-Delgado C. Apolipoprotein E genetic variants interact with Mediterranean diet to modulate postprandial hypertriglyceridemia in coronary heart disease patients: CORDIOPREV study. Eur J Clin Invest. 2019;49(8):e13146. [DOI] [PubMed] [Google Scholar]
- 63. Gomez-Marin B, Gomez-Delgado F, Lopez-Moreno J, Alcala-Diaz JF, Jimenez-Lucena R, Torres-Peña JD, Garcia-Rios A, Ortiz-Morales AM, Yubero-Serrano EM, Del Mar Malagon Met al. Long-term consumption of a Mediterranean diet improves postprandial lipemia in patients with type 2 diabetes: The Cordioprev randomized trial. Am J Clin Nutr. 2018;108(5):963–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated and analyzed during this study are available from the corresponding author upon reasonable request.