Abstract
We report the case of a patient with metastatic cardiac tumor who presented with chest pain and electrocardiographic changes mimicking acute inferior myocardial infarction. An 84-year-old man who had undergone lung cancer surgery one year earlier was referred to emergency outpatient visit because of chest pain. His 12-lead electrocardiography (ECG) showed ST-segment elevation in the inferior leads with reciprocal ST-segment depression in the precordial and lateral leads, which was initially interpreted as inferior acute myocardial infarction. By emergency coronary angiography, however, there was no significant stenosis or occlusion in the right coronary artery or the left circumflex artery. In echocardiographic examinations after admission, a large mass was found in the area corresponding to the infero-posterior wall of the left ventricle, which had been detected only by positron emission tomography with computed tomography six months earlier. He died one month after admission. Pathological autopsy revealed a tumor of 8 × 5 cm size in the myocardium of the posterior to inferior wall of the left ventricle, and diagnosed as cardiac metastasis from lung cancer. ECG changes with ST-segment elevation, in particular persistent ST-elevation in the absence of Q waves, can be a sign for tumor invasion of the heart.
Learning objective
It is necessary to consider the possibility of myocardial metastasis when a patient with malignancy presents with acute myocardial infarction-like electrocardiography findings. Besides, in this case, positron emission tomography with computed tomography (PET-CT) had detected an abnormal accumulation in the left ventricle earlier than when the tumor was pointed out by echocardiography. Multimodality imaging including PET-CT could help physicians to make the early and accurate diagnosis of metastatic cardiac tumor.
Keywords: Cardiac metastasis, Lung cancer, Acute myocardial infarction
Introduction
Secondary cardiac cancer most frequently originates from primary lung cancer, and cardiac metastases are detected in about 20% of autopsy examinations of patients with lung cancer [1]. However, it is not easy to diagnose cardiac metastasis before death because it is often asymptomatic. In cases with cardiac invasion of the metastatic cancer, abnormalities on electrocardiography (ECG) are occasionally observed, but most of the ECG changes are nonspecific [2]. In the present report, we describe a unique case of cardiac metastasis from squamous cell lung carcinoma who presented with ST-segment elevation in the inferior leads accompanied by reciprocal changes on ECG, just mimicking acute inferior myocardial infarction.
Case report
An 84-year-old man was referred to the emergency department of our hospital because of chest pain at rest from the morning of the day. One year earlier, he had undergone lower lobectomy of the left lung for primary lung cancer (squamous cell carcinoma, stage IB) at our hospital. Six months after the surgery (i.e. six months before the current episode), bone metastases to the right upper arm and left femur were pointed out by 18F-fluorodeoxyglucose positron emission tomography with computed tomography (18F-FDG PET-CT), and radiation therapy was given to these sites. In the PET-CT examination at this time, an abnormal accumulation in the left ventricle was also detected (Fig. 1A). However, he had no chest symptoms and no mass could be found by chest CT or transthoracic echocardiography (Fig. 1B and C). His ECG at that time also presented no specifically abnormal findings including ST-T changes (Fig. 2A).
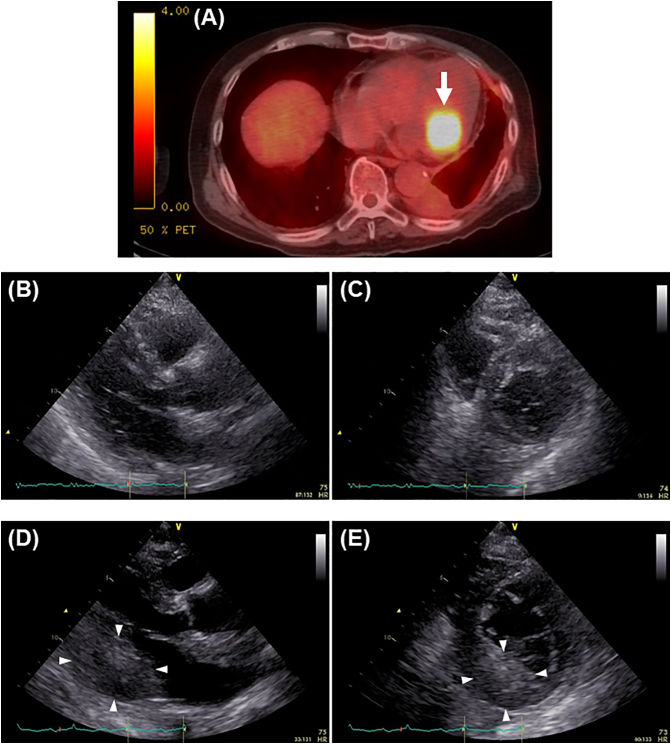
Fig. 1.
(A) An abnormal uptake in the left ventricle (white arrow) detected in 18F-fluorodeoxyglucose positron emission tomography with computed tomography 6 months prior to presentation. (B, C) Parasternal long-axis (B) and short-axis views (C) in transthoracic echocardiography 6 months prior to presentation. (D, E) Parasternal long-axis (D) and short-axis views (E) in transthoracic echocardiography on admission. White arrowheads show a left ventricular mass.
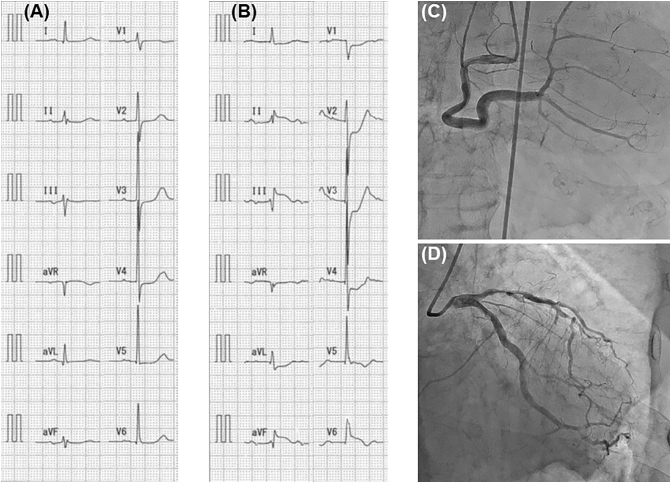
Fig. 2.
(A, B) Recordings of 12-lead electrocardiography 6 months prior to presentation (A) and at the time of emergency outpatient visit (B). (C, D) Angiographic images of right (C) and left (D) coronary arteries during emergency coronary angiography.
On examination at the time of emergency outpatient visit, his blood pressure was 170/82 mm Hg, pulse rate 96 beats/min, and oxygen saturation of 97% on room air. He had no heart murmur and normal breath sounds. As shown in Fig. 2B, 12-lead ECG showed grade I atrioventricular block and evidence of ST-segment elevation in the inferior leads (II, III, and aVF) with reciprocal ST-segment depression in the precordial and lateral leads (aVL and V1-V4). Although portable echocardiography in the emergency room did not show a clear image, regional wall motion abnormality in the infero-posterior wall was suspected. Based on ECG and echocardiographic findings accompanied by chest pain, acute inferior myocardial infarction was strongly suspected, and then the patient underwent emergency coronary angiography. As a result, moderate stenosis was observed in the left anterior descending artery, but there was no significant stenosis or occlusion in the right coronary artery or the left circumflex artery, which was the lesion expected as a culprit for inferior myocardial infarction (Fig. 2C and D).
After coronary angiographic examination, the patient was admitted and transthoracic echocardiography was re-examined. A large mass was found in the area corresponding to the infero-posterior wall of the left ventricle (Fig. 1D and E). There was no significant increase in serum creatinine kinase or creatinine kinase-MB after admission. ECG ST-segment elevation in the inferior leads with coexisting ST-segment depression in the antero-lateral leads did not change after admission. In addition, neither abnormal Q waves nor coronary T waves appeared.
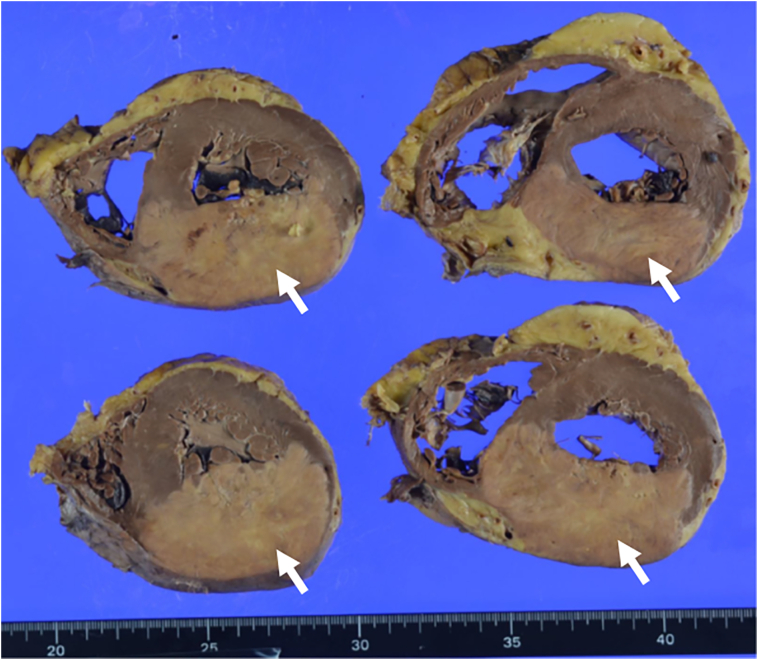
Taken together with the findings of the previous PET-CT, the mass pointed out by echocardiography was determined to be probably a metastatic heart tumor. Because the patient had multiple metastases of primary lung cancer and his general condition was poor, we judged that it would be difficult to perform myocardial biopsy or surgery. He died of respiratory failure one month after admission, and an autopsy was performed. In macroscopic evaluation of the heart, a tumor of 8 × 5 cm size was observed in the myocardium of the posterior to inferior wall of the left ventricle (Fig. 3), and histologically it was squamous cell carcinoma. Other organ metastases were found in the bones (left femur and right humerus), spleen, bilateral adrenal glands, and the lesser curvature of the stomach.
Fig. 3.
Autopsy findings of the heart. White arrows show a tumor metastasis in the cross-section of the left ventricle.
Discussion
Metastatic cardiac tumors are 20 to 40 times more frequent than primary cardiac tumors, that is, most cardiac tumors are metastatic. Bussani et al. [1] showed that, of 7289 patients in whom one or more malignant neoplasms were found, 662 cases (9.1%) of cardiac metastases were ascertained. In their study, the most common cancers among cardiac metastases were lung cancer, followed by leukemia/lymphoma, breast cancer, and mesothelioma. They also reported that about two thirds of all cardiac metastases involved the pericardium (69.4%), one third the epicardium (34.2%) or the myocardium (31.8%), and only 5% the endocardium [1]. It is thought that there are three patterns of metastasis to the heart: direct invasion, lymphatic metastasis pathway, and hematogenous metastatic pathway. In direct invasion and lymphatic metastasis, pericardial effusion and pericardial metastasis are more frequent than in hematogenous metastasis [3]. In this case, pathological autopsy revealed that the neoplastic lesion did not reach the pericardium and was localized in the myocardium, suggesting a high possibility of hematogenous metastasis.
ECG changes in metastasis to the cardiac tissue are not specific for tumor involvement and are occasionally difficult to differentiate from those associated with pre-existing heart disease. Various abnormalities on ECG have been reported to be observed in cases with metastatic cardiac tumors, including ST-T changes (such as ST depression, ST elevation, T wave inversion, and nonspecific ST-T change), low voltage, bradycardia, atrial fibrillation/flutter, bundle branch block, and multiple combined abnormal ECG patterns [2,4]. Cates et al. [4] showed that, in 47 patients who had cardiac metastasis at autopsy, the most frequently observed premortem ECG abnormalities were ST-T wave changes suggestive of myocardial ischemia or injury [19 patients (40%)], but ST elevation was found only in 2 patients (4% of 47 cases of cardiac metastasis). In the present case, ST-segment elevation in leads II, III, and aVF suggesting inferior acute myocardial infarction was observed, consistent with the location of metastatic cardiac tumor. In fact, a previous report described the case of acute myocardial infarction from total occlusion of the coronary artery via invasion or direct compression by a metastatic tumor [5]. In this case, however, there was no significant stenosis or occlusion in the right coronary artery or the left circumflex artery which was the lesion expected as a culprit for inferior myocardial infarction. In addition, no significant increase in serum creatinine kinase was observed during follow-up, in spite of persistent ST-segment elevation in the same leads. Therefore, it was concluded that the abnormal ST-segment elevation in this patient was not a manifestation of acute myocardial infarction. The mechanism of ST-segment elevation in metastatic cardiac tumor cases without coronary artery occlusion is unclear, but transmural myocardial damage by tumor infiltration is thought to be the cause of persistent ST-segment elevation without the development of abnormal Q waves. Suggested mechanisms for such pseudo-infarction ECG patterns are continuous myocardial injury preventing formation of new cardiac cell membrane, stretched adjacent muscle fibers, inflammatory reaction, and ionic transfer of potassium from damaged tissue to the adjacent myocardium producing electropotential differences [6,7].
Interestingly, the ST-segment elevation in the present case was accompanied by reciprocal ST-segment depression, which more mimicked acute myocardial infarction. A recent review article evaluating case reports of metastatic cardiac tumors revealed that only a limited number of cases had reciprocal ST-segment depression in 36 patients with electrocardiographic ST-segment elevation [8]. Cheon et al. [6] have shown that possible mechanisms of coexisting ST-segment depression are pure electrical phenomena with ST-segment elevation, constant compression of the coronary arteries with resulting ischemia, and combined coronary artery disease. In the present case, pure electrical phenomenon was thought to be the most plausible mechanism for persistent ST-segment depression because there was no severe stenosis or occlusion in coronary arteries of the patient.
PET has the advantage of high sensitivity in identifying cancerous tissues at an early stage, and in fact 18F-FDG PET-CT has been reported to be useful for early diagnosis of cardiac metastasis [9]. In the present case, even six months before the tumor was pointed out by echocardiography, abnormal FDG accumulation was observed in the left ventricle, suggesting the possibility of cardiac metastasis. At that point, however, the patient had no chest symptoms and no mass could be found by chest CT or transthoracic echocardiography. Cardiac magnetic resonance imaging (MRI) is known to be a superior modality for evaluating pericardial disease and cardiac masses because of its unmatched capacity for tissue characterization and high spatial resolution. In fact, a case report showed that MRI permitted the diagnosis of cardiac metastasis of malignant melanoma which was not made by echocardiography [10]. Taken together with these findings, MRI scan at the time that 18F-FDG PET-CT showed an abnormal accumulation in the heart might have ensured an earlier diagnosis of metastatic cardiac tumor in this case.
In conclusion, we experienced a rare case of cardiac metastasis of lung cancer precisely mimicking ST-elevation myocardial infarction with reciprocal ST-segment depression. When having found acute myocardial infarction-like ECG changes in a cancer-bearing patient, physicians should be aware of the possibility of cardiac metastasis and multimodality imaging including PET-CT and MRI probably plays a pivotal role in the diagnosis of metastatic cardiac tumor.
Funding
None.
Declaration of competing interest
We have no conflict of interest.
References
- 1.Bussani R., De-Giorgio F., Abbate A., Silvestri F. Cardiac metastases. J Clin Pathol. 2007;60:27–34. doi: 10.1136/jcp.2005.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe S., Watanabe N., Ogura S., Kunikane H., Isobe H., Yamaguchi E., et al. Myocardial metastasis from primary lung cancer: myocardial infarction-like ECG changes and pathologic findings. Jpn J Med. 1991;30:213–218. doi: 10.2169/internalmedicine1962.30.213. [DOI] [PubMed] [Google Scholar]
- 3.Tamura A., Matsubara O., Yoshimura N., Kasuga T., Akagawa S., Aoki N. Cardiac metastasis of lung cancer. A study of metastatic pathways and clinical manifestations. Cancer. 1992;70:437–442. doi: 10.1002/1097-0142(19920715)70:2<437::aid-cncr2820700211>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Cates C.U., Virmani R., Vaughn W.K., Robertson R.M. Electrocardiographic markers of cardiac metastasis. Am Heart J. 1986;112:1297–1303. doi: 10.1016/0002-8703(86)90363-7. [DOI] [PubMed] [Google Scholar]
- 5.Kuramoto M., Okada M., Saeki H., Yoshida Y., Hasegawa S. Acute myocardial infarction due to coronary occlusion caused by a metastatic cardiac tumor arising from squamous cell lung cancer: an evaluation with three-dimensional transthoracic echocardiography. Intern Med. 2022;61:345–350. doi: 10.2169/internalmedicine.7580-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheon D.Y., Park K.H., Hong S.E., Lee S.H., Jang S.H., Park W.J. Metastatic cardiac tumor manifested by persistent ST-segment elevation with coexisting reciprocal changes on electrocardiography. Int Heart J. 2014;55:466–468. doi: 10.1536/ihj.14-050. [DOI] [PubMed] [Google Scholar]
- 7.Demir V., Turan Y., Ede H., Hidayet S., Erkoç M.F. Electrocardiographic changes in right ventricular metastatic cardiac tumor mimicking acute ST elevation myocardial infarction: a case of misdiagnosis. Turk J Emerg Med. 2019;19:33–35. doi: 10.1016/j.tjem.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akgun T., Gulsen K., Cinier G., Pay L., Uslu A., Kup A., et al. Electrocardiographic characteristics of metastatic cardiac tumors presenting with ST-segment elevation. J Electrocardiol. 2020;59:93–99. doi: 10.1016/j.jelectrocard.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Johnson T.R., Becker C.R., Wintersperger B.J., Herzog P., Lenhard M.S., Reiser M.F. Detection of cardiac metastasis by positron-emission tomography-computed tomography. Circulation. 2005;112:e61–e62. doi: 10.1161/CIRCULATIONAHA.104.488775. [DOI] [PubMed] [Google Scholar]
- 10.Mousseaux E., Meunier P., Azancott S., Dubayle P., Gaux J.C. Cardiac metastatic melanoma investigated by magnetic resonance imaging. Magn Reson Imaging. 1998;16:91–95. doi: 10.1016/s0730-725x(97)00200-2. [DOI] [PubMed] [Google Scholar]