Abstract
Dextran has been frequently used during intracoronary imaging, such as in optical coherence tomography, optical frequent domain imaging, and coronary angioscopy. We report a case of dextran-induced anaphylaxis in a 70-year-old male with chronic coronary disease. Upon admission, we performed coronary angiography and coronary angioscopy on the patient. After the intracoronary imaging, the patient's blood pressure suddenly fell to 50 mmHg and a rash appeared on his chest. The patient was diagnosed as having dextran-induced anaphylactic shock. Epinephrine was administered repeatedly, and his blood pressure gradually recovered after administering a total of 6 mg epinephrine. There was no recurrence of the anaphylactic shock, and the patient was discharged 12 days later. The incidence of dextran-induced anaphylactic reactions is extremely low; however, they can be fatal. The possibility of anaphylactic shock induced by dextran should be kept in mind by all cardiovascular interventionalists performing intracoronary imaging.
Learning objective
Dextran has been frequently used during intracoronary imaging. We report on a case of dextran-induced anaphylaxis in a 70-year-old male with chronic coronary disease. While the incidence of dextran-induced anaphylactic reactions is extremely low, it can lead to fatal events. The possibility of anaphylactic shock induced by dextran should be kept in mind by all cardiovascular interventionalists while performing intracoronary imaging.
Keywords: Anaphylaxis, Dextran, Coronary angioscopy
Introduction
Low-molecular-weight dextran (LMWD) has been increasingly used to displace blood during intracoronary imaging examinations, such as optical coherence tomography, optical frequency domain imaging, and coronary angioscopy (CAS). Although the incidence of dextran-induced anaphylactic reactions (DIAR) is extremely low, it can lead to fatal events. We hereby report a case of anaphylactic shock induced by LMWD immediately after an intracoronary imaging examination.
Case report
A 70-year-old male presenting with symptoms of chest discomfort and dyspnea was admitted to our hospital. The patient was treated with a drug-eluting stent implantation at the left anterior descending coronary artery (LAD) for acute myocardial infarction two years previously. The patient had no history of allergy to foods or drugs, including contrast medium. Chest X-ray showed lung congestion and cardiomegaly. No new ischemic changes were observed in his electrocardiogram. The patient's blood tests showed a significant elevation of N-terminal prohormone of B-type natriuretic peptide (11,654 pg/ml), with slight elevations of C-reactive protein (2.252 mg/dl) and troponin T values (0.105 ng/ml), and serum creatinine (1.78 mg/dl) levels. The patient was diagnosed with acute decompensated heart failure (ADHF) with pneumonia and a type 2 myocardial infarction. The administration of ceftriaxone, carperitide, furosemide, and oxygen were initiated. The signs and symptoms of heart failure and pneumonia immediately improved; however, the patient developed an itchy, diffuse erythematous rash over his whole body four days after admission. The patient was diagnosed as having a drug-induced anaphylactic reaction. The candidate drug treatments (ceftriaxone, azosemide, and calcium polystyrene sulfonate) were stopped. Methylprednisolone and an antihistamine were administered for several days, after which the anaphylactic reaction symptoms had completely resolved.
Once the symptoms of ADHF and drug-induced anaphylactic reaction had improved, a transthoracic echocardiography was performed; an abnormal anteroseptal wall motion was observed, which was suggestive of an old anteroseptal myocardial infarction. A modified Simpson's method was used to calculate the ejection fraction to 41%. A coronary angiography (CAG) was performed three weeks after hospital admission. The procedure was undertaken via radial arterial access, whereafter nitrate was administered into the coronary artery after the left coronary artery was engaged; the CAG showed no significant stenosis (Fig. 1). Then, we performed a CAS to evaluate arterial healing and any presence of neoatherosclerosis on the stent that was implanted at the proximal LAD. The CAS demonstrated good neointimal coverage over the stent struts with white neointima and no adhering thrombus (Fig. 1). Several minutes after intracoronary imaging, the patient's blood pressure suddenly dropped to 50 mmHg (Fig. 2); auscultation revealed normal vesicular sound with no rales or crackles, and the electrocardiogram showed no change in the ST segment (Fig. 2). A subsequent CAG was performed to confirm coronary flow and found no occlusion of the coronary arteries, coronary dissection, or air embolism (Fig. 1). Then, a rash appeared on the patient's chest with no sign of bronchospasm, and we diagnosed the patient as having anaphylactic shock induced by dextran (Fig. 3). The patient's blood pressure gradually recovered after repeated epinephrine administration, with a total of 6 mg epinephrine. After an intraaortic balloon pump was inserted, the patient was transferred to the intensive care unit. Subsequent blood tests showed no increase in eosinophils, and no further anaphylaxis reoccurrence; the patient was discharged after 12 days.
Fig. 1.
Findings of coronary angiography (CAG) and angioscopy. (Left panel) CAG shows no re-stenosis at the drug-eluting stent (DES) site of the proximal left anterior descending artery. Coronary angioscopy demonstrates good neointimal coverage over stent struts with white neointima and no adhering thrombus. (Right panel) CAG after dextran-induced anaphylactic shock shows no obstruction of the coronary artery.
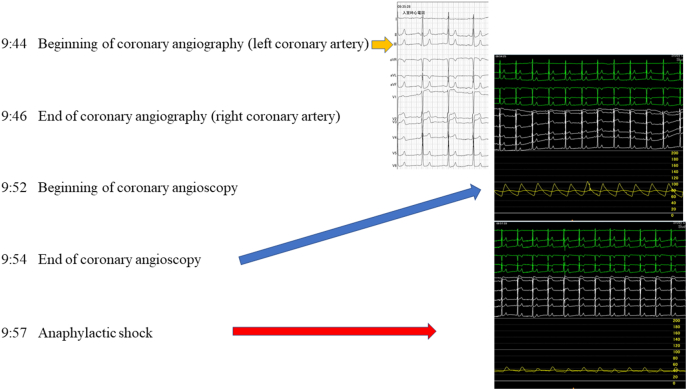
Fig. 2.
Timeline and serial electrocardiogram, and blood pressure during coronary angiography (CAG) and coronary angioscopy (CAS). We performed the CAG and CAS at 9:44 and 9:52, respectively. Anaphylactic shock occurred 3 min subsequent to the CAS. (Right upper panel) Electrogram at the outset of the CAG. (Right mid panel) Electrogram directly after the CAS. (Right lower panel) Electrogram 3 min subsequent to CAS.
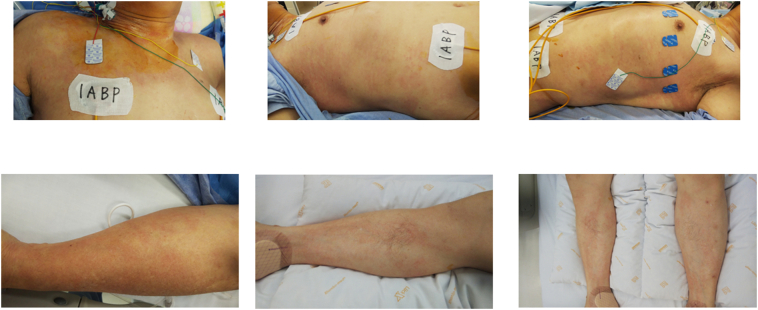
Fig. 3.
Images of skin rash. Skin rash appeared on the patient's chest and limbs after intracoronary imaging.
Discussion
LMWD is considered to be an appropriate flushing agent in place of contrast media for intracoronary imaging, because its viscosity is high enough to remove blood cells [1]. The incidence of DIAR is considered to be extremely low [2]. In this case, anaphylactic shock induced by dextran occurred immediately after a CAG and the patient recovered with the appropriate treatment.
Although it is important to diagnose a DIAR, we cannot deny the possibility of contrast medium-induced anaphylactic shock. However, the patient had previously undergone CAG twice and no allergic reaction was observed; this particular allergic reaction occurred immediately after the administration of dextran. From these findings, we diagnosed the phenomenon as a DIAR, rather than a contrast medium-induced allergic reaction.
Dextrans are highly branched polysaccharide molecules that are produced from the bacterium Leuconostoc mesenteroides and were first produced as colloid plasma volume expanders in 1947 [3]. DIAR are type III immune-complex-mediated anaphylactic reactions that occur when infused dextran molecules bind to endogenous dextran-reactive immunoglobulin (Ig) G antibodies [4]; IgE antibodies are not involved in these type III anaphylactic reactions. Several reports evaluating the incidence of DIAR during major surgery, were published in the 1980s. A retrospective study in Sweden, from 1970 to 1979, showed that the incidence of severe DIAR was 0.013% for dextran 40 and 0.025% for dextran 70. The incidence of fatal DIAR was 0.003% and 0.004%, respectively [2]. Zinderman et al. showed that most patients who have an anaphylactic reaction do so within 5 min of the dextran infusion (60.5%), and all patients developed symptoms within an hour according to the United States Food and Drug Administration reports between 1969 and 2004 [5]. Severe DIAR can be triggered by small or even minute (0.5 to 1.0 ml) volumes of dextran. The anaphylactic reaction induced by dextran appears to depend on both the molecular size and the branching frequency with the anaphylactic potential increasing as the molecular size and branch numbers increase [6]. Globally, the two most frequently used dextrans are dextran 40 and dextran 70, which have molecular weights of 40,000 Da and 70,000 Da respectively, however LMWD 40 is only available in Japan.
The JCS 2018 Guideline on Diagnosis of Chronic Coronary Heart Diseases advised that the routine follow-up CAG was categorized as a Class III recommendation, while the CAS examination, for the detection of vulnerable plaque at follow-up CAG, was a Class IIa recommendation [7]. In this case, the patient had a history of myocardial infarction and was admitted for ADHF with elevated cardiac enzymes. We performed a CAS to detect vulnerable plaque on a stent implanted at the proximal LAD; however, because this patient had a prior anaphylactic reaction to antibiotics during the same hospitalization, the risk of additional anaphylactic reactions might have been lessened by not performing the CAS.
In conclusion, while the incidence of DIAR is extremely low, it can lead to fatal events. The possibility of anaphylactic shock induced by dextran should be kept in mind by all cardiovascular interventionalists on performing intracoronary imaging.
Declaration of competing interest
There is no conflict of interest related to this manuscript.
Footnotes
The work was performed at Hiroshima City Hiroshima Citizens Hospital.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jccase.2022.05.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article.
Findings of coronary angiography
References
- 1.Dai K., Ishihara M., Inoue I., Kawagoe T., Shimatani Y., Kurisu S., et al. Coronary angioscopic findings eight months after sirolimus-eluting stent implantation: a comparison between ST-elevation myocardial infarction and stable angina pectoris. EuroIntervention. 2010;6:251–256. doi: 10.4244/EIJV6I2A90. [DOI] [PubMed] [Google Scholar]
- 2.Ljungstrom K.G., Renck H., Strandberg K., Hedin H., Richter W., Widerlov E. Adverse reactions to dextran in Sweden 1970–1979. Acta Chir Scand. 1983;149:253–262. [PubMed] [Google Scholar]
- 3.Paull J.D. Dextrans. Dev Biol Stand. 1987;67:133–138. [PubMed] [Google Scholar]
- 4.Ljungstrom K.G. Pretreatment with dextran 1 makes dextran therapy safer. J Vasc Surg. 2006;43:1070–1072. doi: 10.1016/j.jvs.2005.11.056. [DOI] [PubMed] [Google Scholar]
- 5.Zinderman C.E., Landow L., Wise R.P. Anaphylactoid reactions to dextran 40 and 70: reports to the United States Food and Drug Administration, 1969 to 2004. J Vasc Surg. 2006;43:1004–1009. doi: 10.1016/j.jvs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Kabat E.A., Turino G.M., Tarrow A.B., Maurer P.H. Studies on the immunochemical basis of allergic reactions to dextran in man. J Clin Invest. 1957;36:1160–1164. doi: 10.1172/JCI103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamagishi M., Tamaki N., Akasaka T., Ikeda T., Ueshima K., Uemura S., et al. JCS 2018 guideline on diagnosis of chronic coronary heart disease. Circ J. 2021;85:402–572. doi: 10.1253/circj.CJ-19-1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Findings of coronary angiography