Key Points
Question
What are the effects of high-dose vs low-dose dexamethasone on 60-day time to all-cause mortality, and oxygenation strategies vs standard oxygen support on 28-day time to fulfilling invasive mechanical ventilation (IMV) criteria in patients with COVID-19 and severe acute hypoxemic respiratory failure (AHRF)?
Findings
In this randomized clinical trial among 546 patients with COVID-19 and severe AHRF, no difference was observed in 60-day mortality according to dexamethasone dose or in 28-day cumulative need for IMV according to oxygenation strategy.
Meaning
These findings suggest that in patients with COVID-19 and AHRF, high-dose dexamethasone or different oxygenation strategies did not significantly modify 60-day mortality or 28-day requirement for IMV criteria.
This randomized clinical trial assesses high-dose dexamethasone vs standard care and 3 oxygenation strategies for intensive care unit (ICU) patients with severe COVID-19 acute hypoxemic respiratory failure.
Abstract
Importance
The benefit of high-dose dexamethasone and oxygenation strategies vs standard of care for patients with severe acute hypoxemic respiratory failure (AHRF) caused by COVID-19 pneumonia is debated.
Objectives
To assess the benefit of high-dose dexamethasone compared with standard of care dexamethasone, and to assess the benefit of high-flow nasal oxygen (HFNo2) or continuous positive airway pressure (CPAP) compared with oxygen support standard of care (o2SC).
Design, Setting, and Participants
This multicenter, placebo-controlled randomized clinical trial was conducted in 19 intensive care units (ICUs) in France from April 2020 to January 2021. Eligible patients were consecutive ICU-admitted adults with COVID-19 AHRF. Randomization used a 2 × 3 factorial design for dexamethasone and oxygenation strategies; patients not eligible for at least 1 oxygenation strategy and/or already receiving invasive mechanical ventilation (IMV) were only randomized for dexamethasone. All patients were followed-up for 60 days. Data were analyzed from May 26 to July 31, 2021.
Interventions
Patients received standard dexamethasone (dexamethasone-phosphate 6 mg/d for 10 days [or placebo prior to RECOVERY trial results communication]) or high-dose dexamethasone (dexamethasone-phosphate 20 mg/d on days 1-5 then 10 mg/d on days 6-10). Those not requiring IMV were additionally randomized to o2SC, CPAP, or HFNo2.
Main Outcomes and Measures
The main outcomes were time to all-cause mortality, assessed at day 60, for the dexamethasone interventions, and time to IMV requirement, assessed at day 28, for the oxygenation interventions. Differences between intervention groups were calculated using proportional Cox models and expressed as hazard ratios (HRs).
Results
Among 841 screened patients, 546 patients (median [IQR] age, 67.4 [59.3-73.1] years; 414 [75.8%] men) were randomized between standard dexamethasone (276 patients, including 37 patients who received placebo) or high-dose dexamethasone (270 patients). Of these, 333 patients were randomized among o2SC (109 patients, including 56 receiving standard dexamethasone), CPAP (109 patients, including 57 receiving standard dexamethasone), and HFNo2 (115 patients, including 56 receiving standard dexamethasone). There was no difference in 60-day mortality between standard and high-dose dexamethasone groups (HR, 0.96 [95% CI, 0.69-1.33]; P = .79). There was no significant difference for the cumulative incidence of IMV criteria at day 28 among o2 support groups (o2SC vs CPAP: HR, 1.08 [95% CI, 0.71-1.63]; o2SC vs HFNo2: HR, 1.04 [95% CI, 0.69-1.55]) or 60-day mortality (o2SC vs CPAP: HR, 0.97 [95% CI, 0.58-1.61; o2SC vs HFNo2: HR, 0.89 [95% CI, 0.53-1.47]). Interactions between interventions were not significant.
Conclusions and Relevance
In this randomized clinical trial among ICU patients with COVID-19–related AHRF, high-dose dexamethasone did not significantly improve 60-day survival. The oxygenation strategies in patients who were not initially receiving IMV did not significantly modify 28-day risk of IMV requirement.
Trial Registration
ClinicalTrials.gov Identifier: NCT04344730; EudraCT: 2020-001457-43.
Introduction
While acute hypoxemic respiratory failure (AHRF) is the main manifestation of severe COVID-19, the most appropriate noninvasive respiratory support (NIRS) and the appropriate timing of invasive mechanical ventilation (IMV) remain to be defined.1,2,3,4 The advantages of high-flow nasal oxygen therapy (HFNo2)5 and continuous positive airway pressure (CPAP)6 in the management of COVID-19–related AHRF, with additional specificities related to the pandemic context, are still debated.7,8
When the RECOVERY trial showed that dexamethasone 6 mg/d for 10 days reduced 28-day mortality in patients with the most severe COVID-19,9 low-dose corticosteroids became a standard of care. A meta-analysis of randomized clinical trials (RCTs) in patients with severe COVID-19 showed that corticosteroids treatment was associated with lower all-cause mortality vs usual care or placebo.10 An RCT by Villar et al described the benefit of dexamethasone 20 mg/d in acute respiratory distress syndrome,11 but a study among patients with severe COVID-19 by Munch et al12 found no difference between dexamethasone 12 mg/d and 6 mg/d for 28-day mortality (−5.2%; P = .10) or days alive without life support at day 28. Therefore, use of high-dose dexamethasone for COVID-19–related AHRF was deemed worthy of further investigation. We report the results of the COVIDICUS trial that tested the benefit of high-dose dexamethasone, compared with standard of care, and of NIRS strategies based on CPAP or HFNo2 in intensive care unit (ICU) patients with COVID-19 AHRF.
Methods
The trial protocol for this RCT was approved by the institutional review board of the Comité de Protection des Personnes Ile-de-France-XI and the French Health Authorities, in initial and amended versions, as provided in Supplement 1 and the eMethods in Supplement 2. This study was conducted in accordance with Helsinki Declaration.13 Consents were obtained in adherence with the French law for emergency inclusion, with signed informed consent obtained from conscious patients and an emergency consent procedure with the patient’s legal guardian or relatives implemented for those unable to consent. An independent data safety monitoring board (DSMB) reviewed the trial data. This study is reported following the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Trial Design and Goal
This multicenter RCT tested 2 interventions, high-dose dexamethasone vs standard of care dexamethasone, and CPAP or HFNo2 vs standard of care o2 (o2SC) support. For patients not receiving IMV eligible to any oxygenation strategies, both interventions were assessed using a 2 × 3 factorial design (Figure 1). Patients receiving IMV at randomization or for whom any 1 oxygenation strategy was contraindicated were randomized with a 1:1 ratio for the dexamethasone interventions only, resulting in 2 other treatment groups: standard of care dexamethasone and high-dose dexamethasone.
Figure 1. Patient Recruitment Flowchart.
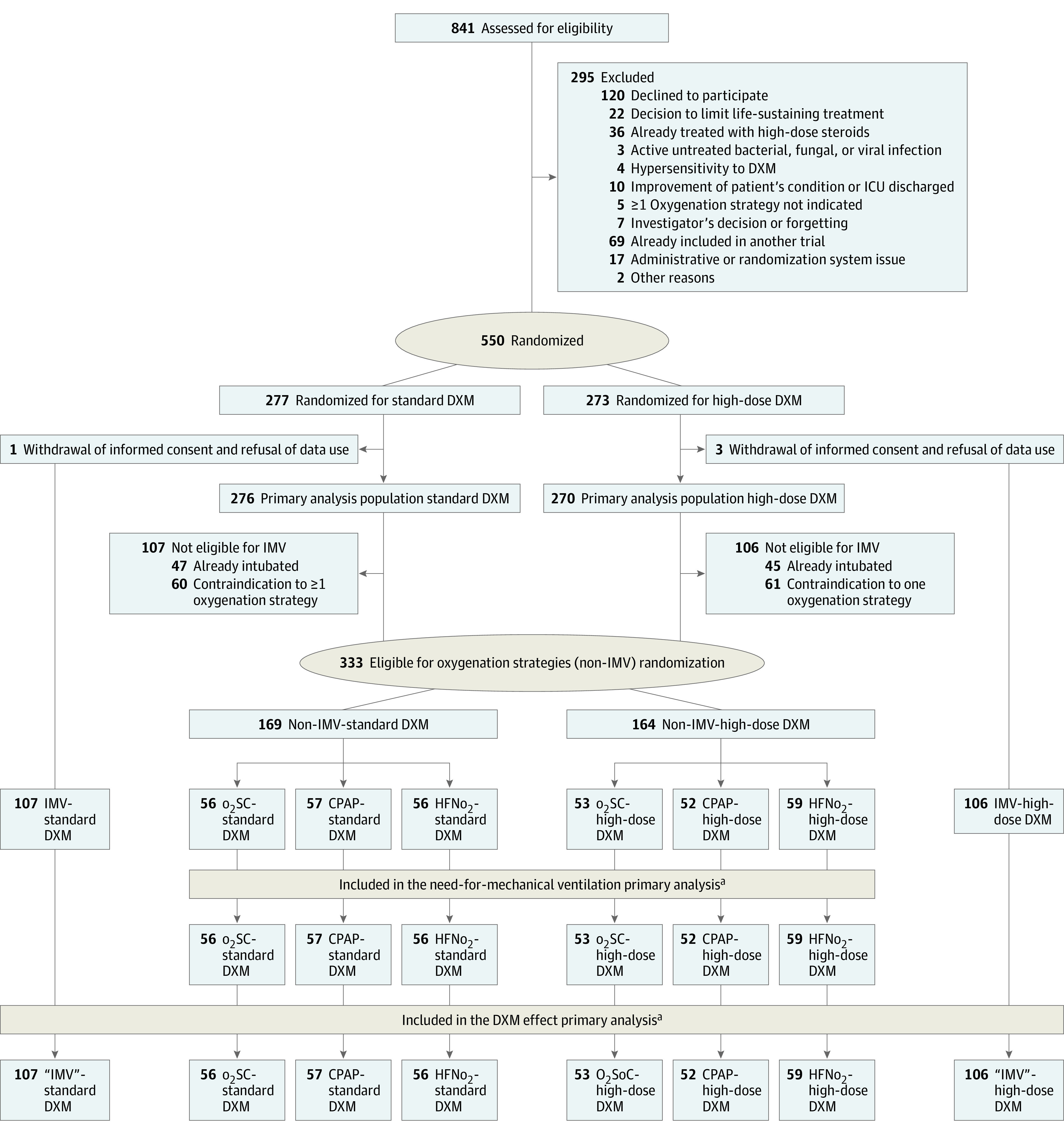
aConsent withdrawal occurred before the date of day 60 of follow-up in no patients receiving standard of care oxygen (o2SC), 8 patients receiving continuous positive airway pressure (CPAP), and 1 patient receiving high-flow nasal oxygen (HFNo2) in the non–invasive mechanical ventilation (IMV) group. In patients with IMV or contraindication to any 1 o2 support strategy, 3 patients withdrew consent. These patients were censored at the date of consent withdrawal.
DXM indicates dexamethasone; ICU, intensive care unit.
Changes in Standard of Care for Dexamethasone
The initial version of the trial investigated the efficacy of high-dose corticosteroid therapy compared with placebo. After the publication of results from the RECOVERY trial,9 the French Health Authorities recommended modifying the standard of care for administering low-dose dexamethasone (6 mg/d) to patients with COVID-19 who were hypoxemic (eMethods in Supplement 2).14 The amended protocol was approved on September 17, 2020 (eFigure 1 in Supplement 2).
Patients
Study participation was proposed to all consecutive patients with COVID-19 admitted to participating ICUs. Eligible patients were adults aged at least 18 years admitted to an ICU within the last 48 hours for confirmed or highly suspected COVID-19, with AHRF (defined as arterial partial pressure of oxygen, [Pao2] <70 mm Hg, transcutaneous oxygen saturation as measured by pulse oximetry [Spo2] <90% on room air, tachypnea with >30 breaths/min, labored breathing, respiratory distress, or need for o2 flow ≥6 L/min), and who could receive any available treatment targeting COVID-19. Those with ongoing IMV at inclusion or with anatomical factors precluding the use of nasal cannula, hypercapnia indicating noninvasive ventilation (Paco2 ≥50 mm Hg), or intolerance at admission to any of the oxygenation strategies, ie, the IMV population, were only eligible to the dexamethasone randomization. The main exclusion criteria were decision to limit life-sustaining treatment, corticosteroid therapy of 0.5 mg/kg/d or more of prednisone equivalent for 3 weeks or longer; active untreated bacterial, fungal, or parasitic infection; and hypersensitivity to dexamethasone.
Randomization
In patients eligible for the 3 o2 support strategies (the non-IMV population), randomization used a factorial design with a 1:1:1 ratio across oxygenation groups, and a 1:1 ratio across the dexamethasone intervention. The IMV population was only randomized 1:1 for the dexamethasone interventions. Randomization was centralized and stratified by center (eMethods in Supplement 2).
Trial Interventions and Blinding Procedures
In France, dexamethasone is administered as dexamethasone-phosphate, thus patients administered with dexamethasone-phosphate 20 mg/d actually received dexamethasone 16.6 mg/d. Initially, the standard dexamethasone group received a nondexamethasone placebo. From the amendment implementation, the standard of care moved to an intravenous administration of dexamethasone-phosphate 6 mg/d on days 1 to 10 to all patients. In addition, all patients received an additional infusion of placebo if they were allocated to standard dexamethasone or of dexamethasone-phosphate 14 mg/d on days 1 to 5, then 4 mg/d on days 6 to 10 if allocated to high-dose dexamethasone. A 7-day treatment with hydrocortisone or fludrocortisone was allowed for septic shock that fulfilled predefined criteria.
Regarding oxygenation strategies (eTable 1 in Supplement 2), patients allocated to CPAP received periods of CPAP in addition to the standard o2 treatment (eMethods in Supplement 2). For HFNo2, gas flow was delivered at 30 L/min and increased up to 60 L/min, based on clinical response. In all groups, o2 flow or inspired o2 fraction (Fio2) were adjusted for a targeted Spo2 of at least 92%. Oxygenation was pursued until death, fulfillment of endotracheal intubation criteria, or predefined cessation criteria (eMethods in Supplement 2).
Study Assessments
Participants were assessed daily in the ICU and at predefined time points after ICU discharge up to day 60 (SD, 14) days. Safety data were collected until day 28. At days 1 and 7, nasopharyngeal swabs were obtained for SARS-CoV-2 detection; if possible, subglottic samples (bronchoalveolar lavage, plugged telescopic catheter, or tracheal aspiration) were also collected.
For high-dose dexamethasone evaluation, the primary end point was time-to-death from all causes up to day 60. For oxygenation strategies evaluation, the primary end point was time to IMV criteria fulfillment within the first 28 days after randomization, based on the fulfillment of previously described IMV criteria5: worsening respiratory failure, hemodynamic instability, and neurological status deterioration (eMethods in Supplement 2).
Prespecified secondary end points were health care–associated infection at day 28, number of IMV-free days alive at day 28, and ICU and hospital lengths of stay (LOS). For dexamethasone interventions, additional end points were the change in Sequential Organ Failure Assessment (SOFA) score and change in viral load, and the number of days alive without kidney replacement therapy at day 28. For o2 supply interventions, additional end points included overall survival at day 60, severe hypoxemia (Spo2 <80%) within the 2 minutes following induction of tracheal intubation, and cardiac arrest within the hour following tracheal intubation. The 28-day cumulative incidence of actual IMV was added at the request of the DSMB. Viral load was determined by real-time semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) (eMethods in Supplement 2).
Statistical Analysis
Regarding the dexamethasone interventions, the 60-day cumulative incidence of all-cause death was assumed at 60%.15,16,17 Thus, a sample size of 550 participants (275 per group) would achieve 80.1% power at an α = .05 significance level to detect a hazard ratio (HR) of 0.75 for a survivor proportion of 0.40 in the control group (2-sided log-rank test). In the non-IMV population, 2 comparisons of each intervention (CPAP or HFNo2) against the o2SC group (with an assumed incidence at 80% for IMV criteria fulfillment at day 28) were designed, with an expected similar benefit of each experimental arm of HR, 0.65. A 2-sided log-rank test with an overall sample size of 220 participants (110 per group) would achieve 80.0% power to detect such an effect, using an adjusted type I error rate of 0.025, given the multiple comparisons. Therefore, 330 patients overall were required to evaluate the o2 support strategies (ie, the non-IMV population).
All analyses were based on the intention-to-treat principle. Summary statistics used frequencies and percentages for categorical variables or medians and IQRs for continuous or discrete variables. Three bayesian interim analyses were presented to the DSMB during the study (eTable 2 in Supplement 2).
The primary end points were analyzed using survival methods assuming noninformative right-censoring of data at days 28 or 60. Survival curves were estimated in each randomization group using the Kaplan-Meier method then compared using the log-rank test. Cox models stratified on the patient populations (IMV and non-IMV) quantified the effect size by HR with 95% CIs. Proportional hazards assumptions were assessed using Grambsch and Therneau statistics.18 Subsets by treatment interactions were tested by Gail and Simon statistics. Period effect (ie, before vs after the protocol amendment for standard dexamethasone) was tested using fixed covariate in the regression models. Secondary planned analyses of primary outcomes were performed on the as-treated populations, defined as patients analyzed in the group of treatment actually received at randomization. The changes in RT-PCR results and SOFA scores were modeled and compared using linear mixed models. The proportions of health care–associated infections at days 28 and 60 were compared using a Fisher exact test. The number of days alive without IMV or kidney replacement therapy and ICU and hospital LOS were compared using a Wilcoxon rank sum test.
We conducted 3 sensitivity analyses of the primary end point. First, we assessed whether the change in the standard dexamethasone group affected the 60-day mortality or 28-day need for IMV. Second, we explored the impact of the IMV population heterogeneity on the dexamethasone effect, as no patients from that population were actually receiving IMV. Third, we investigated for potential center effect in the assessment of CPAP and HFNo2 effects, due to some imbalance in treatment adherence, using frailty models. Because of the potential for type I error owing to multiple comparisons, findings of analyses of other end points than the primary end point should be interpreted as exploratory.
All analyses were conducted blinded to treatment assignment. Statistical analyses were performed using R software version 4.0.3 (R Project for Statistical Computing). All tests were 2-sided, with P = .05 denoting statistical significance. Data were analyzed from May 26 to July 31, 2021.
Results
Patients
From April 10 to September 17, 2020, 73 patients were randomized between placebo (37 patients) and high-dose dexamethasone (36 patients), including 53 patients in the non-IMV population (o2SC: 15 patients; CPAP: 20 patients; HFNo2: 18 patients). Thereafter, 473 patients were randomly allocated between standard dexamethasone (239 patients) or high-dose dexamethasone (234 patients), including 280 in the non-IMV population (o2SC: 94 patients; CPAP: 89 patients; HFNo2, 97 patients). Four patients eventually withdrew their participation consent and declined the use of their data and thus were excluded from the analysis. Therefore, the primary analysis dealt with 546 patients (median [IQR] age, 67.4 [59.3-73.1] years; 414 [75.8%] men) enrolled in 19 ICUs (Figure 1). The DSMB recommended to continue the study to completion. Main baseline characteristics of patients according to dexamethasone groups are in Table 1 and according to the oxygen support strategy are in Table 2.
Table 1. Baseline Characteristics of Patients According to the Dexamethasone Arm.
| Variables | No. (%) | Standardized mean difference | |
|---|---|---|---|
| Standard of care dexamethasone (n = 276) | High-dose dexamethasone (n = 270) | ||
| Age, median (IQR), y | 66.3 (58.9-73.8) | 68.1 (60.1-72.9) | 0.015 |
| Sex | |||
| Women | 79 (28.6) | 53 (19.6) | 0.211 |
| Men | 197 (71.4) | 217 (80.4) | |
| BMIa | |||
| Median (IQR) | 29.4 (26.0-33.7) | 28.6 (25.5-32.0) | 0.184 |
| 25-30 | 94 (34.1) | 98 (36.3) | 0.183 |
| >30 | 114 (41.3) | 110 (40.7) | |
| Comorbidities | |||
| Any | 227 (82.2) | 214 (79.3) | 0.076 |
| Cancer | 28 (10.1) | 33 (12.2) | 0.130 |
| Solid organ transplantation | 8 (2.9) | 3 (1.1) | 0.128 |
| Diabetes | 108 (39.1) | 94 (34.8) | 0.092 |
| Hypertension | 160 (58.0) | 143 (53.0) | 0.101 |
| Dexamethasone administration prior to the inclusionb | |||
| Any | 33 (11.9) | 40 (14.8) | 0.084 |
| Duration, median (IQR), d | 1 (1-2) | 2 (1-3) | 0.511 |
| Oxygenation ventilation status | |||
| IMV | 48 (17.4) | 50 (18.5) | 0.170 |
| o2 standard of care | 64 (23.2) | 61 (22.6) | |
| CPAP | 59 (21.4) | 55 (20.4) | |
| HFNo2 | 101 (36.6) | 98 (36.3) | |
| Noninvasive ventilation | 4 (1.4) | 6 (2.2) | |
| COVID-19–specific treatment | |||
| Any | 182 (65.9) | 168 (62.2) | 0.073 |
| Remdesivir | 46 (16.7) | 47 (17.4) | 0.020 |
| Lopinavir/ritonavir | 6 (2.2) | 6 (2.2) | 0.003 |
| Hydroxychloroquine | 4 (1.4) | 2 (0.7) | 0.068 |
| Tocilizumab | 1 (0.4) | 3 (1.1) | 0.088 |
| Hydrocortisone HS | 1 (0.4) | 3 (1.1) | 0.088 |
| Prednisone/prednisolonec | 2 (0.7) | 4 (1.5) | 0.072 |
| Clinical status at baseline | |||
| Time since symptoms onset, median (IQR), dd | 9 (7-11) | 9 (6-11) | 0.101 |
| Time since ICU admission, median (IQR), d | 1 (0-1) | 0 (0-1) | 0.148 |
| Vasopressor use | 22 (8.0) | 29 (10.7) | 0.099 |
| SOFA score, median (IQR) | 3 (2-4) | 3 (2-4) | 0.060 |
| Positive results on first PCR teste | 253 (91.7) | 237 (87.8) | 0.031 |
| Biochemistry data, median (IQR)f | |||
| White blood cells, /μL | 8000 (5700-10 700) | 8200 (6000-11 300) | 0.086 |
| Lymphocytes, /μL | 600 (400-900) | 700 (400-1000) | 0.118 |
| Platelets, ×103/μL | 236 000 (175 000-307 000) | 234 000 (175 000-302 000) | 0.032 |
| Creatinine, mg/dL | 0.84 (0.67-1.09) | 0.86 (0.70-1.15) | 0.137 |
| C-reactive protein, mg/dL | 12.6 (8.5-18.6) | 13.6 (7.1-20.5) | 0.035 |
| D-dimers, μg/mL | 1030 (573-1948) | 804 (496-1516) | 0.278 |
| Troponin, ng/mL | 20 (10-110) | 20 (10-370) | 0.006 |
| Ferritin, ng/mL | 915 (518-1801) | 1240 (642-1946) | 0.031 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HFNo2, high-flow nasal oxygen; HS, hydrocortisone hemisuccinate; ICU, intensive care unit; IMV, invasive mechanical ventilation; PCR, polymerase chain reaction; OFA, sequential organ failure assessment.
SI conversion factors: To convert white blood cells and lymphocytes to ×109/L, multiply by 0.001; platelets to ×109/L, multiply by 1; creatinine to micromoles per liter, multiply by 88.4; C-reactive protein to milligrams per deciliter, multiply by 10; D-dimer to nanomoles per L, multiply by 5.476; troponin to micrograms per liter, multiply by 1; and ferritin to nanograms per liter, multiply by 1.
Data missing for 19 participants.
Among 73 patients. Of note, 3 patients (2 in the standard dexamethasone group, and 1 in the high-dose dexamethasone group) received corticosteroids for at least 10 days (for 33, 14 and 12 days, respectively). The median (IQR) dose of dexamethasone-phosphate received before inclusion was 6 (6-6) mg/d, with only 2 patients who received 20 mg (1 in standard dexamethasone group, for 4 days, and 1 in the high-dose dexamethasone group, for 6 days).
Long-term treatment with prednisone/prednisolone <0.5 mg/kg/d.
Missing data for 10 patients.
Patients with negative PCR results at randomization had positive PCR results just before.
Missing data for 20 patients for white blood cell count, 152 patients for lymphocytes, 18 patients for platelets, 13 patients for creatinine, 152 patients for C-reactive protein, 200 patients for D-dimers, 224 patients for troponin, and 304 patients for ferritin.
Table 2. Baseline Characteristics of Patients Without Invasive Mechanical Ventilation at Randomization and Eligible for Any Oxygen Support Strategy, According to the Oxygen Support Provided.
| Characteristic | Patients, No. (%) | SMD | |||
|---|---|---|---|---|---|
| Standard care o2 (n = 109) | CPAP (n = 109) | HFNo2 (n = 115) | CPAP vs standard | HFNo2 vs standard | |
| Age, median (IQR), y | 67.4 (60.8-72.3) | 69.1 (59.4-76.3) | 66.8 (58.9-71.9) | 0.109 | 0.123 |
| Sex | |||||
| Women | 25 (22.9) | 32 (29.4) | 24 (20.9) | 0.147 | 0.050 |
| Men | 84 (77.1) | 77 (70.6) | 91 (79.1) | ||
| BMIa | |||||
| Median (IQR) | 29 (26-31.8) | 28.7 (24.5-32.9) | 28.7 (26.0-32.9) | 0.027 | 0.177 |
| 25-30 | 41 (38.7) | 33 (31.4) | 44 (39.3) | 0.503 | 0.258 |
| >30 | 44 (41.4) | 40 (38.0) | 46 (41.0) | 0.221 | 0.082 |
| Comorbidities | |||||
| Any | 95 (87.2) | 84 (77.1) | 88 (76.5) | 0.266 | 0.278 |
| Cancer | 15 (13.8) | 8 (7.3) | 17 (14.8) | 0.102 | 0.042 |
| Solid organ transplant | 4 (3.7) | 2 (1.8) | 4 (3.5) | 0.112 | 0.010 |
| Diabetes | 39 (35.8) | 38 (34.9) | 37 (32.2) | 0.019 | 0.076 |
| Hypertension | 64 (58.7) | 63 (57.8) | 58 (50.4) | 0.019 | 0.166 |
| Oxygenation status, median (IQR) | |||||
| Respiratory rate, breaths/min | 24 (20-29) | 26 (21-30) | 24 (20-28) | 0.216 | 0.070 |
| Gas flow rate, L/minb | 15 (15-15) | 20 (15-30) | 50 (40-60) | 0.075 | 1.104 |
| Fio2, % | NA | 66 (48-91) | 70 (50-97) | 0.206 | 0.056 |
| Spo2, % | 94 (92-96) | 95 (93-97) | 94 (92-97) | 0.322 | 0.060 |
| COVID-19–specific treatment | |||||
| Any | 74 (67.9) | 55 (50.5) | 72 (62.6) | 0.360 | 0.111 |
| Remdesivir | 18 (16.5) | 31 (28.4) | 15 (13.0) | 0.289 | 0.098 |
| Lopinavir/ritonavir | 1 (1.0) | 2 (2.0) | 0 | 0.079 | 0.136 |
| Hydroxychloroquine | 0 | 0 | 0 | NA | NA |
| Tocilizumab | 0 | 0 | 0 | NA | NA |
| Hydrocortisone HS | 0 | 0 | 0 | NA | NA |
| Prednisone/prednisolonec | 1 (1.0) | 0 | 2 (1.9) | 0.079 | 0.136 |
| Clinical status | |||||
| Time since symptoms onset, median (IQR), d | 8 (7-11) | 8 (6-11) | 9 (7-11) | 0.159 | 0.100 |
| Time since ICU admission, median (IQR), d | 0 (0-1) | 0 (0-1) | 0 (0-1) | 0.081 | 0.083 |
| Vasopressor use | 0 | 3 | 0 | 0.238 | NA |
| SOFA, median (IQR) | 2 (2-4) | 2 (2-3) | 2 (2-4) | 0.142 | 0.013 |
| Positive result on first PCR testd | 98 (96.1) | 100 (96.2) | 103 (94.5) | 0.004 | 0.075 |
| Biochemistry data, median (IQR)e | |||||
| White blood cells, /μL | 7300 (5700-10 500) | 8200 (5700-11 200) | 7700 (5800-10 000) | 0.106 | 0.124 |
| Lymphocytes, /μL | 800 (600-1100) | 700 (400-900) | 600 (400-900) | 0.168 | 0.171 |
| Platelets, ×103/μL | 240 000 (185 000-292 000) | 246 000 (178 000-332 000) | 219 000 (169 000-265 000) | 0.153 | 0.253 |
| Creatinine, mg/dL) | 0.85 (0.70-115) | 0.80 (0.64-1.05) | 0.90 (0.75-1.10) | 0.001 | 0.034 |
| C-reactive protein, mg/dL | 12.9 (7.0-18.9) | 13.4 (8.1-19.4) | 13.8 (8.2-19.0) | 0.066 | 0.049 |
| D-Dimers, μg/mL | 798 (483-1252) | 1022 (547-2576) | 900 (490-1596) | 0.308 | 0.043 |
| Troponin, ng/mL | 10 (10-60) | 10 (10-70) | 10 (10-90) | 0.037 | 0.137 |
| Ferritin, ng/mL | 1280 (739-2618) | 891 (494-1846) | 1234 (755-1801) | 0.218 | 0.117 |
| Dexamethasone group | 0.018 | 0.054 | |||
| Standard of care | 56 (51.4) | 57 (52.3) | 56 (48.7) | NA | NA |
| High-dose | 53 (48.6) | 52 (47.7) | 59 (51.3) | NA | NA |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CPAP, continuous positive airway pressure; Fio2, fraction of inspired oxygen; HS, hemisuccinate; HFNo2, high-flow nasal oxygen; ICU, intensive care unit; NA, not applicable; PCR, polymerase chain reaction; SMD, standardized mean difference; SOFA, sequential organ failure assessment; Spo2, oxygen saturation as measured by pulse oximetry.
SI conversion factors: To convert white blood cells and lymphocytes to ×109/L, multiply by 0.001; platelets to ×109/L, multiply by 1; creatinine to micromoles per liter, multiply by 88.4; C-reactive protein to milligrams per deciliter, multiply by 10; D-dimer to nanomoles per L, multiply by 5.476; troponin to micrograms per liter, multiply by 1; and ferritin to nanograms per liter, multiply by 1.
Missing data for 10 patients.
Number of liters of o2 per minute for the standard o2 group.
Long-term treatment with prednisone or prednisolone of less than 0.5 mg/kg/d.
Patients with negative PCR at randomization had a positive PCR just before.
Missing data for 14 patients for white blood cell count, 108 patients for lymphocytes, 18 patients for platelets, 13 patients for creatinine, 140 patients for C-reactive protein, 104 patients for D-dimers, 109 patients for troponin, and 147 patients for ferritin.
Treatment and Interventions
Overall, 276 patients were allocated to the standard dexamethasone group and 270 patients were allocated to the high-dose dexamethasone group, including 213 patients from the IMV population solely randomized for dexamethasone interventions, while 333 patients from the non-IMV population were additionally randomized for the oxygenation interventions (Figure 1). Among 546 patients, 541 (99.1%) were administered at least 1 day of study drugs (dexamethasone-phosphate or placebo [standard dexamethasone]: 273 patients [98.9%]; high-dose dexamethasone: 268 patients [99.3%]). Adherence was unbalanced between allocated oxygenation groups: 77 patients in the o2SC group (70.6%) were adherent (29 patients received HFNo2); 89 patients in the CPAP group (81.7%) were adherent (12 patients received HFNo2); and 110 patients in the HFNo2 group (95.7%) were adherent. Nonadherence in oxygenation supply varied from 0.3% up to 27% across centers.
Primary Outcomes
Median (IQR) follow-up was 60 (27-69) days. A total of 43 patients (7.9%) were discharged from the hospital prior to their 60-day follow-up (IMV population: 13 patients; non-IMV population: 30 patients).
Dexamethasone Interventions
Dexamethasone-phosphate was administered for a median (IQR) of 9 (6-10) days in both groups. Overall, 144 patients died within 60 days after randomization (standard dexamethasone: 74 patients [26.8%]; high-dose dexamethasone: 70 patients [25.9%]; absolute risk difference, −0.8% [95% CI, –8.3 to 6.5]; HR, 0.96 [95% CI, 0.69 to 1.33]; P = .79) (Figure 2 and Table 3). No evidence of any violation of the proportional hazards assumption was found. No significant interaction with the randomization strata was observed in hazard of death (non-IMV: HR, 0.88 [95% CI, 0.58 to 1.29] vs IMV population: HR, 1.08 [95% CI, 0.64 to 1.83]; P for interaction = .55) (eFigure 2 in Supplement 2). The analysis on the as-treated population did not markedly affect the results (HR, 0.95 [95% CI, 0.69 to 1.32]; P = .77).
Figure 2. Primary End Points of Both Interventions.
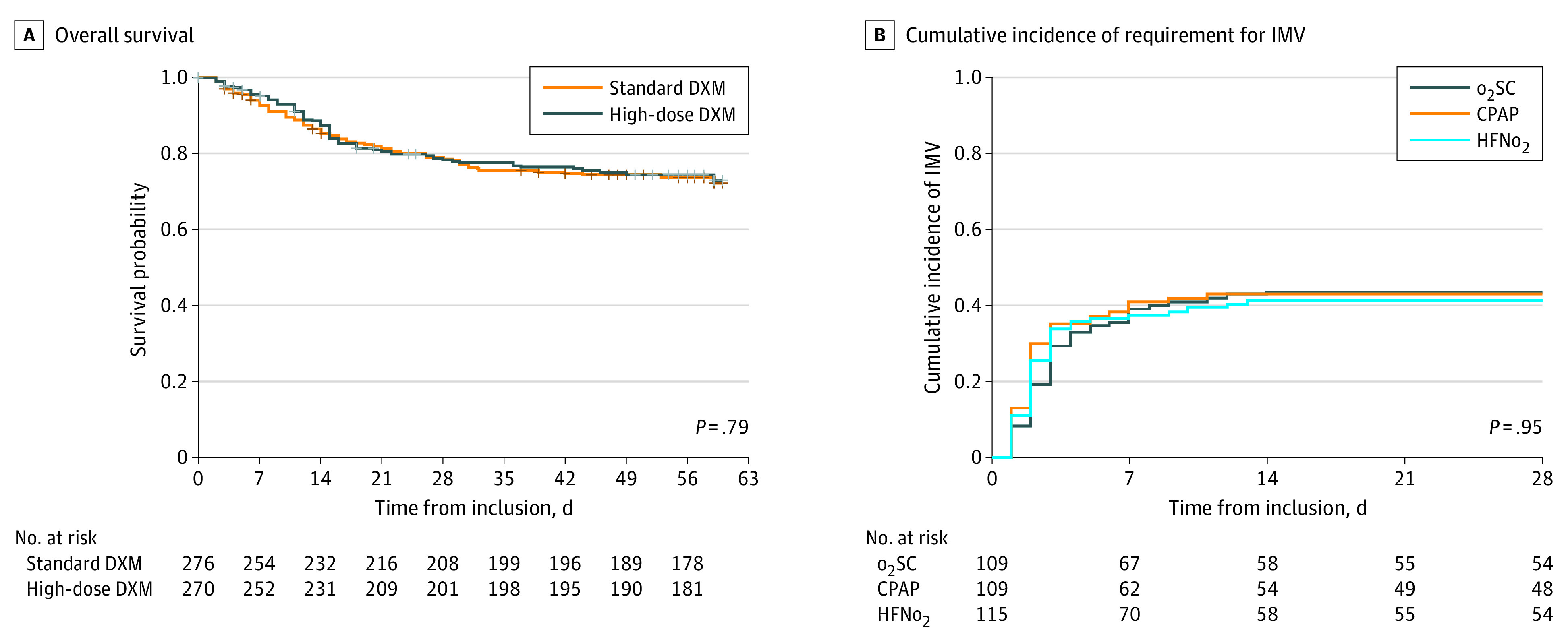
A. Standard dexamethasone (DXM) included 37 patients who received placebo DXM then dexamethasone-phosphate 6 mg/d for days 1 to 10; high-dose DXM was dexamethasone-phosphate 20 mg/d for days 1 to 5, then 10 mg/d for days 6 to 10. CPAP indicates continuous positive airway pressure; HFNo2, high-flow nasal oxygen; and o2SC, oxygen standard of care.
Table 3. Outcomes of Patients According to the Dexamethasone Group in the Overall Study Sample .
| Outcome | Standard of care dexamethasone (n = 276) | High-dose dexamethasone (n = 270) | Estimate (95% CI) | |
|---|---|---|---|---|
| Difference | Hazard ratioa | |||
| Overall survival at 60 d, Kaplan-Meier estimate, % (95% CI) | 72.2 (66.2 to 82.6) | 73.0 (67.8 to 76.5) | 0.8 (–6.8 to 8.4) | 0.96 (0.69 to 1.33) |
| Secondary end points | ||||
| Viral load evolution, mean slope (SE) | 0.31 (0.08) | 0.46 (0.06) | 0.15 (–0.05 to 0.35) | NC |
| HAI at 28 d, No. (%) | 75 (27.2) | 81 (30.0) | 2.8 (–4.8 to 10.4) | 1.10 (0.85 to 1.44) |
| Alive free of IMV at 28 d, median (IQR), d | 28 (6 to 28) | 28 (9 to 28) | 1.0 (–2.9 to 0.9) | NC |
| Alive free of KRT at day 28, median (IQR), d | 28 (14 to 28) | 28 (16 to 28) | 0.8 (–2.4 to 0.8) | NC |
| LOS, median (IQR), d | ||||
| ICU | 9 (5 to 15) | 8 (5 to 15) | 0.1 (–3.0 to 2.7) | NC |
| Hospital | 15 (10 to 24) | 16 (11 to 27) | 1.3 (–4.4 to 1.8) | NC |
| SOFA evolution: mean slope (SE) | 0.10 (0.01) | 0.09 (0.01) | –0.01 (–0.05 to 0.03) | NC |
| ≥1 Adverse event, No. (%) | 208 (75.4) | 202 (74.8) | –0.6 (–7.8 to 6.7) | 0.99 (0.90 to 1.09) |
Abbreviations: HAI, health care–associated infection; ICU, intensive care unit; IMV, invasive mechanical ventilation; KRT, kidney replacement therapy; LOS, length of stay; NC, not calculated; VAP, ventilator-associated pneumonia.
Hazard ratios were stratified on the IMV strata.
Oxygen Support Intervention
In the non-IMV population, the 28-day cumulative incidence of IMV criteria fulfillment was 41.4% (95% CI, 32.0% to 50.4%) for o2SC, 43.0% (95% CI, 33.3% to 52.2%) for CPAP (cause-specific HR, 1.08 [95% CI, 0.71 to 1.63]; P = .71), and 43.8% (95% CI, 34.5% to 52.6%) for HFNo2 (cause-specific HR, 1.04 [95% CI, 0.69 to 1.55]; P = .85), with no significant difference between groups (Figure 2; eTable 3 in Supplement 2). Proportional hazards assumption was checked either for the CPAP effect or for HFNo2, and no significant interaction with the dexamethasone interventions was observed, neither with CPAP, nor HFNo2 (eFigure 3 in Supplement 2). Results in the as-treated population were not significantly modified (CPAP vs o2SC: HR, 1.39 [95% CI, 0.88-2.19]; P = .15; HFNo2 vs o2SC: HR, 0.98 [95% CI, 0.64-1.50]; P = .93.
Secondary Outcomes
Secondary outcomes regarding the effects of dexamethasone and of oxygenation strategies are presented in Table 3, eTable 3, eFigure 4, and eFigure 5 in Supplement 2. Overall, none of the interventions elicited any significant differences in secondary end points vs standard of care.
In post hoc analysis, the estimated effect of dexamethasone on 60-day mortality was not mediated by the type of control received by patients (high-dose dexamethasone vs standard dexamethasone/placebo: HR, 0.99 [95% CI, 0.47 to 2.07]; high-dose dexamethasone vs standard dexamethasone/dexamethasone-phosphate 6 mg/d: HR, 0.95 [95% CI, 0.66 to 1.36]; Gail and Simon P = .92). Similarly, the type of population either actually with IMV (HR, 1.18 [95% CI, 0.64 to 2.16]) or without IMV (HR, 0.88 [95% CI, 0.59 to 1.30]) did not significantly modify these findings (Gail and Simon P = .73). There was no heterogeneity across the oxygenation groups (eFigure 6 in Supplement 2). The as-treated population yielded similar results (eTable 4 in Supplement 2).
In the non-IMV population, there was no period × treatment interaction for the time to need for IMV against the o2SC group for the CPAP or the HFNo2 groups. Similarly, the 28-day cumulative incidence of time to fulfillment of IMV criteria was not significantly affected by any potential center effect (eTable 5 in Supplement 2).
By contrast, there was some heterogeneity across centers either on the 60-day survival (eFigure 7 and eFigure 8 in Supplement 2). However, there was no heterogeneity across centers in the dexamethasone effect on 60-day survival (eFigure 7 in Supplement 2) or in the CPAP and HFNo2 effect on the need for IMV (eFigure 8 in Supplement 2).
Safety Data
The prevalence of adverse events was not significantly different across intervention groups (Table 3; eTable 6 and eTable 7 in Supplement 2). There were no clinically or statistically significant differences between arms, including no significant difference in the rates of infectious and noninfectious complications of dexamethasone-phosphate treatment.
Discussion
The COVIDICUS randomized clinical trial showed neither any benefit of high-dose dexamethasone on 60-day survival compared with standard of care for patients with COVID-19 and severe AHRF, nor any significant benefit of HFNo2 or CPAP compared with standard o2 therapy regarding the IMV criteria fulfillment within 28 days after ICU admission. Our trial had several strengths, such as its multicenter and placebo-controlled design, a sealed randomization to the assigned strategy, a well-defined study protocol that included prespecified criteria for intubation, a prolonged follow-up, and a very low rate of protocol violations for dexamethasone administration.
Randomized clinical trials in patients with COVID-19 have shown that dexamethasone 6 mg/d improves 28-day survival9,19 and dexamethasone 6 mg/d for 10 days became a standard of care for patients with COVID-19 and AHRF. Therefore, we applied it as standard care in this study. The benefits of high-dose dexamethasone in patients with COVID-19 and AHRF remain uncertain. One open-labeled randomized study compared dexamethasone 16 mg/d on days 1 to 5 then 8 mg/d on days 6 to 10 with 6 mg/d for days 1 to 10.20 The trial was prematurely halted after 98 patients were enrolled without any effects observed on ventilator-free days at day 28 or mortality; however, the successful discontinuation from mechanical ventilation was more frequent in the high-dose group. Another open-label RCT with 200 patients with o2 support tested the same dexamethasone intervention as our study.21 High-dose dexamethasone did not impact 28-day mortality or time to recovery. A recent blinded RCT in 1000 patients who were severely hypoxemic with COVID-19 showed no statistically significant difference in days alive without life support at day 28 with dexamethasone 12 mg/d (dexamethasone-phosphate 14.4 mg/d) vs dexamethasone 6 mg/d (dexamethasone-phosphate 7.2 mg/d), and no significant differences in 28-day and 90-day mortality.12 In this study, high-dose dexamethasone also did not improve 60-day survival, regardless of a patient’s IMV status. The rate of infectious and noninfectious complications was comparable between dexamethasone strategies. The population enrolled in both trials was similar, although the 28-day mortality was 9% higher in the study by Munch et al.12 We used dexamethasone-phosphate 20 mg/d (equivalent to dexamethasone 16.6 mg/d) compared with 12 mg/d of dexamethasone in the study by Munch et al.12 The standard of care was similar, except that all our patients were in ICUs and fewer patients received remdesivir. Although the study by Munch et al12 was blinded, its robustness is weakened by drawbacks, such as the inclusion of 55 patients with decisions to limit life-sustaining treatment at randomization or the lack of administration of the assigned intervention to 75 patients (7.6%).
Before the COVID-19 pandemic, NIRS were associated with decreased rates of endotracheal intubation and death in patients with AHRF, as shown in a recent large meta-analysis.3 However, the observed association was no longer significant after excluding patients who were hypercapnic and patients with mean Pao2:Fio2 ratio of less than 200 mm Hg. Nonrandomized studies have suggested that HFNo21,2,3,4,5,6,7,8,9,10,11,12,14,15,16,17,18,19,20,21,22,23,24,25 and CPAP26,27 may improve oxygenation and decrease the likelihood of requiring IMV.28,29 Only few randomized studies have investigated the benefit of NIRS strategies in patients with COVID-19.30,31,32 In a small-size RCT, helmet noninvasive ventilation and HFNo2 yielded similar results in terms of number of days free of respiratory support at day 28, although the rate of endotracheal intubation was significantly decreased with helmet noninvasive ventilation.30 In a trial that compared 22 patients treated with HFNo2 vs standard o2, HNFo2 improved the Pao2:Fio2 ratio and reduced ICU LOS.32
The RECOVERY-RS33 RCT included 1272 inpatients among 3 o2 support strategies: 29.9% received CPAP, 32.8% received HFNo2, and 37.3% received standard o2 therapy. Compared with standard o2 therapy, CPAP, but not HFNo2, reduced the composite outcome of intubation or death at day 30, without significant impact on mortality. Safety events occurred more frequently in the CPAP group (130 events among 380 patients [34.2%]) than in the HFNo2 group (86 events among 417 patients [20.6%]) or the standard o2 therapy group (66 events among 475 patients [13.9%]; P < .001). Of note, findings of the RECOVER-RS study33 cannot be extrapolated to the treatment of patients who have been systematically admitted to the ICU. Ultimately, the investigators in the RECOVERY-RS study33 could choose to enroll in 1 of the 2 tested strategies, and the decision to intubate was left to physician’s discretion instead of adhering to predefined criteria.
Limitations
Our study has some limitations, such as the lack of adherence to the allocated o2 strategy for 57 patients (17%). However, the as-treated analysis provided similar results. We also used as primary end point the fulfillment of criteria for starting IMV, as previously defined,5 but 58 patients that reached the primary end point and fulfilled criteria but they were not intubated. However, neither time-to-IMV criteria fulfillment nor time-to-actual intubation differed among groups.
In addition, CPAP treatment with a face mask is more burdensome than HFNo2, and centers were less experienced with CPAP than with HFNo2; this was illustrated by range of nonadherence in oxygenation supply, from 0.3% up to 27%, across centers. Therefore, signs of failure might have been identified earlier among patients with CPAP vs HFNo2. Nevertheless, results were not modified by analyses assessing center effect or in the as-treated population. The use of awake prone positioning was neither standardized nor recorded; it might have been more frequently used in patients in the o2SC or HFNo2 groups. Additionally, our study was powered to detect large benefits of the experimental arms across the controls and, notably, not to detect the reported effect observed in the RECOVERY-RS trial (ie, an 8% difference in the intubation rate at day 28 with CPAP). Such an analysis would have required enrollment of 585 patients per group for 80% power; nevertheless, no retrospective observed power calculations were performed, given their reported misleading results.34
Overall, we lack strong evidence on the efficiency of 1 NIRS strategy over the others in ICU patients with severe AHRF. The use of HFNo2 or CPAP is only suggested, with low-grade recommendations. Our study supports the use of standard o2 treatment over CPAP or HFNo2 for hospitals managing the COVID-19 crisis. Our findings also support refraining from broadly deploying CPAP or HFNo2 oxygen strategies outside ICUs. To our knowledge, this was the first RCT that investigated fully randomly assigned oxygenation strategies in patients admitted and carefully surveyed in ICUs. The impact of NIRS implementation upstream of ICU admission during COVID-19–related AHRF, compared with later use that starts in the ICU, requires further research.
Other more general limitations should be acknowledged. First, all participating centers were in France, which raises questions about the general applicability of these findings. Second, this study spanned from April 2020 to January 2021, ie, a time period during which the treatment of patients with severe COVID-19 changed greatly, especially for concomitant treatments and supportive care.35 This was highlighted by the need for the change in the control group, owing to reported benefit of low doses of dexamethasone; of note, no evidence of any interaction between the type of control was detected. Additionally, an interaction between both types of interventions cannot be excluded, given the limited power of interaction tests.
Conclusions
In this RCT among ICU patients with COVID-19–related severe AHRF, no significant difference in 60-day survival was observed in patients treated with high-dose dexamethasone compared with standard of care. Standard o2, CPAP via face mask, or HFNo2 as primary oxygenation mode had no significant impact on the 28-day risk of IMV requirement.
Trial Protocol, Statistical Analysis Plan, and Ethical Approvals
eMethods.
eAppendix. Adherence to Trial Interventions
eTable 1. Dexamethasone Treatment and Oxygen Support Strategies in the COVIDICUS Trial
eTable 2. Report of the Blinded Bayesian Interim Analyses
eTable 3. Outcomes of Patients According to Oxygenation Strategy in the Non-IMV Group
eTable 4. Effects of Dexamethasone Treatment and Oxygen Support Strategies on the Main End Points Based on the As-Treated Population of the COVIDICUS Trial
eTable 5. Effect of the Oxygen Support Strategies on the Main End Point Based on the Intent-to-Treat Population of the COVIDICUS Trial, Handling the Potential Center Effect Using a Frailty Model
eTable 6. Serious Adverse Events and Other Adverse Events at Day 28 in the Overall Study Sample, According to Dexamethasone Group
eTable 7. Serious Adverse Events and Other Adverse Events at Day 28 Among Non-IMV Population, According to the Oxygenation Strategy Group
eFigure 1. Cumulative Inclusions in the COVIDICUS Study
eFigure 2. Overall Survival of Patients According to Dexamethasone Group
eFigure 3. Cumulative Incidence of IMV Criterion Fulfillment in Patients Without IMV and Eligible for Oxygen Support Strategies at Randomization (Non-IMV Population) According to the Oxygen Supplementation Strategy
eFigure 4. Sequential Organ Failure Assessment (SOFA) Score Course Over Time According to the Dexamethasone Randomization
eFigure 5. Number of Cycles of the Polymerase Chain Reaction Over Time According to the Dexamethasone Group
eFigure 6. Search for Dexamethasone Effect According to Oxygenation Group Interaction in the Effect of Dexamethasone on 60-Day Survival
eFigure 7. Search for Center Effect on Mortality at Day 60 in the Effect of Dexamethasone on 60-Day Survival
eFigure 8. Search for Center Effect on Time to Need for IMV and in the Effect of CPAP and HFNO on Time to Need for IMV
Members of the COVIDICUS Study Group
Data Sharing Statement
References
- 1.Radovanovic D, Santus P, Coppola S, et al. Characteristics, outcomes and global trends of respiratory support in patients hospitalized with COVID-19 pneumonia: a scoping review. Minerva Anestesiol. 2021;87(8):915-926. doi: 10.23736/S0375-9393.21.15486-0 [DOI] [PubMed] [Google Scholar]
- 2.Carteaux G, Millán-Guilarte T, De Prost N, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med. 2016;44(2):282-290. doi: 10.1097/CCM.0000000000001379 [DOI] [PubMed] [Google Scholar]
- 3.Ferreyro BL, Angriman F, Munshi L, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. 2020;324(1):57-67. doi: 10.1001/jama.2020.9524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang BJ, Koh Y, Lim CM, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41(4):623-632. doi: 10.1007/s00134-015-3693-5 [DOI] [PubMed] [Google Scholar]
- 5.Frat JP, Thille AW, Mercat A, et al. ; FLORALI Study Group; REVA Network . High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185-2196. doi: 10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 6.Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: A randomized controlled trial. JAMA. 2000;284(18):2352-2360. doi: 10.1001/jama.284.18.2352 [DOI] [PubMed] [Google Scholar]
- 7.Carteaux G, Pons M, Morin F, et al. Continuous positive airway pressure for respiratory support during COVID-19 pandemic: a frugal approach from bench to bedside. Ann Intensive Care. 2021;11(1):38. doi: 10.1186/s13613-021-00828-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupuis C, Bouadma L, de Montmollin E, et al. Association between early invasive mechanical ventilation and day-60 mortality in acute hypoxemic respiratory failure related to coronavirus disease–2019 pneumonia. Crit Care Explor. 2021;3(1):e0329. doi: 10.1097/CCE.0000000000000329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Updated January 28, 2020. Accessed May 25, 2022. https://apps.who.int/iris/bitstream/handle/10665/330854/WHO-nCoV-Clinical-2020.2-eng.pdf?sequence=1&isAllowed=y
- 11.Villar J, Ferrando C, Martínez D, et al. ; Dexamethasone in ARDS Network . Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267-276. doi: 10.1016/S2213-2600(19)30417-5 [DOI] [PubMed] [Google Scholar]
- 12.Munch MW, Myatra SN, Vijayaraghavan BKT, et al. ; COVID STEROID 2 Trial Group . Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID STEROID 2 randomized trial. JAMA. 2021;326(18):1807-1817. doi: 10.1001/jama.2021.18295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 14.Orkin AM, Gill PJ, Ghersi D, et al. ; CONSERVE Group . Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances: the CONSERVE 2021 statement. JAMA. 2021;326(3):257-265. doi: 10.1001/jama.2021.9941 [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Hayek SS, Wang W, et al. ; STOP-COVID Investigators . Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436-1447. doi: 10.1001/jamainternmed.2020.3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853-862. doi: 10.1016/S2213-2600(20)30316-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 19.Wagner C, Griesel M, Mikolajewska A, et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;8(8):CD014963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maskin LP, Bonelli I, Olarte GL, et al. High- versus low-dose dexamethasone for the treatment of COVID-19-related acute respiratory distress syndrome: a multicenter, randomized open-label clinical trial. J Intensive Care Med. 2022;37(4):491-499. doi: 10.1101/2021.09.15.21263597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taboada M, Rodríguez N, Varela PM, et al. Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: an open-label, randomised clinical trial. Eur Respir J. 2021;2102518. doi: 10.1183/13993003.02518-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnet N, Martin O, Boubaya M, et al. High flow nasal oxygen therapy to avoid invasive mechanical ventilation in SARS-CoV-2 pneumonia: a retrospective study. Ann Intensive Care. 2021;11(1):37. doi: 10.1186/s13613-021-00825-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demoule A, Vieillard Baron A, Darmon M, et al. High-flow nasal cannula in critically ill patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039-1042. doi: 10.1164/rccm.202005-2007LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen CK, Stempek S, Liesching T, Lei Y, Dargin J. Characteristics and outcomes of patients receiving high flow nasal cannula therapy prior to mechanical ventilation in COVID-19 respiratory failure: a prospective observational study. Int J Crit Illn Inj Sci. 2021;11(2):56-60. doi: 10.4103/IJCIIS.IJCIIS_181_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mellado-Artigas R, Ferreyro BL, Angriman F, et al. ; COVID-19 Spanish ICU Network . High-flow nasal oxygen in patients with COVID-19-associated acute respiratory failure. Crit Care. 2021;25(1):58. doi: 10.1186/s13054-021-03469-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashish A, Unsworth A, Martindale J, et al. CPAP management of COVID-19 respiratory failure: a first quantitative analysis from an inpatient service evaluation. BMJ Open Respir Res. 2020;7(1):e000692. doi: 10.1136/bmjresp-2020-000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oranger M, Gonzalez-Bermejo J, Dacosta-Noble P, et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two-period retrospective case-control study. Eur Respir J. 2020;56(2):2001692. doi: 10.1183/13993003.01692-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco C, Facciolongo N, Tonelli R, et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J. 2020;56(5):2002130. doi: 10.1183/13993003.02130-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sykes DL, Crooks MG, Thu Thu K, et al. Outcomes and characteristics of COVID-19 patients treated with continuous positive airway pressure/high-flow nasal oxygen outside the intensive care setting. ERJ Open Res. 2021;7(4):00318-2021. doi: 10.1183/23120541.00318-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grieco DL, Menga LS, Cesarano M, et al. ; COVID-ICU Gemelli Study Group . Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. 2021;325(17):1731-1743. doi: 10.1001/jama.2021.4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perkins GD, Ji C, Connolly BA, et al. An adaptive randomized controlled trial of non-invasive respiratory strategies in acute respiratory failure patients with COVID-19. medRxiv. Preprint posed online August 4, 2021. doi: 10.1101/2021.08.02.21261379 [DOI]
- 32.Teng XB, Shen Y, Han MF, Yang G, Zha L, Shi JF. The value of high-flow nasal cannula oxygen therapy in treating novel coronavirus pneumonia. Eur J Clin Invest. 2021;51(3):e13435. doi: 10.1111/eci.13435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perkins GD, Ji C, Connolly BA, et al. ; RECOVERY-RS Collaborators . Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. 2022;327(6):546-558. doi: 10.1001/jama.2022.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoenig JM, Heisey DM. The abuse of power. Am Stat. 2001;55(1):19-24. doi: 10.1198/000313001300339897 [DOI] [Google Scholar]
- 35.Azoulay E, de Waele J, Ferrer R, et al. International variation in the management of severe COVID-19 patients. Crit Care. 2020;24(1):486. doi: 10.1186/s13054-020-03194-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol, Statistical Analysis Plan, and Ethical Approvals
eMethods.
eAppendix. Adherence to Trial Interventions
eTable 1. Dexamethasone Treatment and Oxygen Support Strategies in the COVIDICUS Trial
eTable 2. Report of the Blinded Bayesian Interim Analyses
eTable 3. Outcomes of Patients According to Oxygenation Strategy in the Non-IMV Group
eTable 4. Effects of Dexamethasone Treatment and Oxygen Support Strategies on the Main End Points Based on the As-Treated Population of the COVIDICUS Trial
eTable 5. Effect of the Oxygen Support Strategies on the Main End Point Based on the Intent-to-Treat Population of the COVIDICUS Trial, Handling the Potential Center Effect Using a Frailty Model
eTable 6. Serious Adverse Events and Other Adverse Events at Day 28 in the Overall Study Sample, According to Dexamethasone Group
eTable 7. Serious Adverse Events and Other Adverse Events at Day 28 Among Non-IMV Population, According to the Oxygenation Strategy Group
eFigure 1. Cumulative Inclusions in the COVIDICUS Study
eFigure 2. Overall Survival of Patients According to Dexamethasone Group
eFigure 3. Cumulative Incidence of IMV Criterion Fulfillment in Patients Without IMV and Eligible for Oxygen Support Strategies at Randomization (Non-IMV Population) According to the Oxygen Supplementation Strategy
eFigure 4. Sequential Organ Failure Assessment (SOFA) Score Course Over Time According to the Dexamethasone Randomization
eFigure 5. Number of Cycles of the Polymerase Chain Reaction Over Time According to the Dexamethasone Group
eFigure 6. Search for Dexamethasone Effect According to Oxygenation Group Interaction in the Effect of Dexamethasone on 60-Day Survival
eFigure 7. Search for Center Effect on Mortality at Day 60 in the Effect of Dexamethasone on 60-Day Survival
eFigure 8. Search for Center Effect on Time to Need for IMV and in the Effect of CPAP and HFNO on Time to Need for IMV
Members of the COVIDICUS Study Group
Data Sharing Statement


