Abstract
Background
Updated evidence was required to compare the efficacy and safety of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) inhibitors for patients with hormone receptor-positive and HER2-negative metastatic breast cancer.
Methods
A systematic review and network meta-analysis was conducted utilizing data from randomized controlled trials (RCTs) that contained interventions of CDK4/6 inhibitors or PI3K/AKT/mTOR inhibitors. Progression-free survival (PFS), overall survival (OS), and treatment-related adverse events (TRAEs) were primary outcomes of interest. Pooled hazard ratios (HRs) and odds ratios (ORs) with 95% credible intervals (CrIs) were used to assess the survival outcomes and safety profiles, respectively.
Results
A total of 28 RCTs with 12,129 participants were included. Pooled analysis showed that CDK4/6 inhibitors significantly prolonged PFS than PI3K/AKT/mTOR inhibitors (HR, 0.81; 95% CrI, 0.69–0.94), whereas no significant differences were detected regarding OS. After balancing the treatment lines and metastatic sites, the superiority of CDK4/6 inhibitors only appeared in the visceral and non-visceral subgroups. Among CDK4/6 inhibitors, abemaciclib was significantly better than others in ≥3 grade neutropenia (OR, 0.04; 95% CrI, 0.01–0.15). The incidence of stomatitis and digestive disorders was different among diverse kinds of PI3K/AKT/mTOR inhibitors. Discrepancies appeared regarding TRAEs of hepatotoxicity, diarrhea, and hyperglycemia among different interventions.
Conclusions
CDK4/6 inhibitors showed better efficacy in PFS, but the benefits disappeared when taking treatment line into consideration. Specific and discrepant safety profiles were found in two categories of agents.
Systematic Review Registration
https://www.crd.york.ac.uk/PROSPERO, identifier CRD42022321172.
Keywords: CDK4/6 inhibitors, PI3K/AKT/mTOR inhibitors, hormone receptor-positive, HER2-negative, metastatic breast cancer, network meta-analysis
Introduction
Surpassing lung cancer, breast cancer has become the most common malignancy diagnosed worldwide, with 2.3 million new cases in 2020 (1). According to the status of hormone receptor, including estrogen receptor (ER) and progesterone receptor (PR), and human epidermal growth factor 2 (HER2), breast cancer is categorized into distinctive molecular subtypes, guiding the diagnosis and treatment for decades (2). For hormone receptor-positive/HER2-negative subtype, which accounts for a large proportion of breast cancer, endocrine therapy (ET), including aromatase inhibitors (AIs), selective ER modulators (SERMs), and selective ER down-regulators (SERDs), is the bedrock (3, 4). However, the resistance to ET, either primary or secondary, poses a barrier for the subsequent treatment options (5). The emergence of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) inhibitors has addressed this issue to some extent.
CDK4/6, as a subgroup of serine/threonine kinases, promote cell cycle regulation by phosphorylating retinoblastoma (Rb) protein after interacting with cyclin D and then initiate the cell cycle transition from G1 phase to S phase (6). CDK4/6 overexpression is frequently encountered in hormone receptor-positive breast cancer and the highly selective inhibitors of which combined with ET are standard care of first-line treatment for these patients in advanced-stage (7). Other than CDK4/6 signaling pathway, PI3K/AKT/mTOR (or PAM) signaling pathway was also proved to be a major one in disease recurrence or progression (8). Studies indicated that the PAM pathway was essential for cellular proliferation and metabolism, and the activation of which was found in up to 70% of the breast cancer (9). There also existed elaborate cross-talk between the PAM and estrogen-mediated signaling pathway (10). It is estimated that approximately 40% to 50% of patients with hormone receptor-positive/HER2− breast cancer have aberrant activation of PIK3CA (encoding the p110α isoform of PI3K) (11, 12), which is closely correlated with ET resistance. Currently, pan or selective PI3K/AKT/mTOR inhibitors are promising agents for patients with hormone receptor-positive/HER2− metastatic breast cancer who have progressed after pretreatment with CDK4/6 inhibitors (13).
So far, many clinical trials were designed to compare the efficacy and safety between CDK4/6 inhibitors or PI3K/AKT/mTOR inhibitors and endocrine monotherapy. Nevertheless, there were no direct comparisons between the two categories of inhibitors until now. Our previous network meta-analysis was conducted to work out this problem (14), after which a large number of randomized controlled trials (RCTs) emerged. Therefore, we performed this updated systematic review and network meta-analysis, attempting to replenish the latest survival outcomes and incorporate all the eligible studies.
Methods
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses–Network Meta-Analyses (PRISMA-NMA) checklist (15). The study protocol was consistent with that of one previous systematic review and network meta-analysis conducted by our research group (14). This analysis was registered in PROSPERO (https://www.crd.york.ac.uk/PROSPERO/) with the registration number CRD42022321172.
Search strategy and selection criteria
Two independent authors (Xu and Wang) searched the literature from PubMed, Embase, the Cochrane Library, and ClinicalTrials.gov from 1 February 2020 since the cutoff date of the original article was 31 January 2020, with our last search on 23 November 2021. In addition, the annual conferences of American Society of Clinical Oncology, European Society of Medical Oncology, San Antonio Breast Cancer Symposium, and Chinese Society of Clinical Oncology were replenished for integrity. The main search strings that we used were as follows: “breast cancer”, “HER2”, “hormone receptor”, “metastasis”, “CDK4/6 inhibitors”, “PI3K inhibitors”, “AKT inhibitors”, “mTOR inhibitors”, and “endocrine therapy”, among which diverse concrete agents were searched. The key terms and free terms were combined in every possible form. Full searching strategy was detailed in Supplementary Table 1 . The selection criteria were as previously displayed (14). Phase 2/3 RCTs meeting the following criteria were included (1): involving adults with hormone receptor-positive and HER2-negative metastatic breast cancer (2); participants were treated with regimens containing CDK4/6 inhibitors or PI3K/AKT/mTOR inhibitors; and (3) data regarding survival were available. Studies portrayed as single-arm trials or retrospective analyses, chemotherapy-containing regimens, positive or ambiguous HER2 status, and incomplete survival or follow-up data were excluded.
Data extraction and quality assessment
On the basis of the designed protocol, baseline characteristics including clinical trial name, first author, published time, study phase, trial design, number of participants, lines of previous therapy and metastatic sites were extracted from each RCT. The progression-free survival (PFS) and overall survival (OS) were primary endpoints, whereas treatment-related adverse events (TRAEs) based on the Common Toxicity Criteria for Adverse Events (CTCAE) version 5.0 were the second, which consisted of not only grades 3–5 similar to the original article but also all-grade TRAE data. Other outcomes including time to the first chemotherapy and PFS of different metastatic site subgroups were also collected.
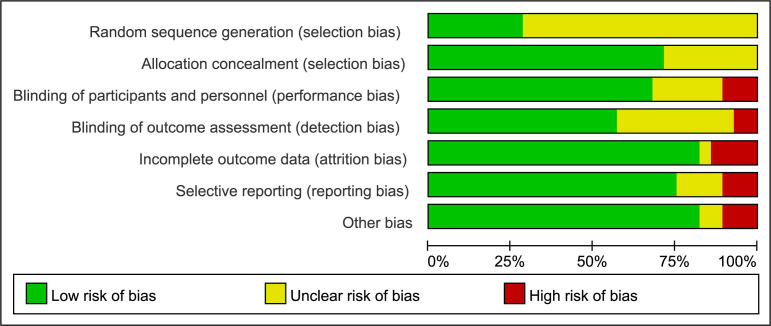
For every included study, the potential risk of bias was assessed by Cochrane Collaboration’s tool (16), including six pre-specified domains: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. All studies were evaluated as high, low, or unclear risk according to the documented methodological quality. Review Manager (version 5.3, Nordic Cochrane Centre) was employed to assess the risk of bias. Two investigators (Xu and Wang) independently contributed to the above process. Discussion was required with a third reviewer (Han) when disagreement existed.
Data synthesis and statistical analysis
This network meta-analysis was performed by R software (version 4.1.1) with “gemtc” package based on Bayesian random effects models. Regarding survival data, hazard ratios (HRs) and 95% confidence intervals (CIs) extracted from each RCT were used to generate mean log HRs and according standard errors that were required for the subsequent analysis. The formulae applied were reported previously by Woods et al. (17). Regarding categorical data, summary odds ratios (ORs) with 95% credible intervals (CrIs) were estimated. Because not all the included studies reported all the endpoints of interest, each individual network plot of different outcomes was generated. In the network plot, each node represented an intervention and every two different nodes were connected with a single line if direct comparisons existed. The studies comparing different agents of the same classification were excluded. Markov chain Monte Carlo (MCMC) approach was utilized to build the network meta-analysis. In brief, the amount of adaptation and simulation iterations was 10,000 and 50,000, respectively, with the thinning interval set as 10. The surface under the cumulative ranking curve (SUCRA) was utilized to assess the relative ranking probabilities of different treatments for each outcome. The interventions were ordered by the SUCRA percentage (range, 0%–100%). A two-sided P-value below 0.05 was regarded as statistically significant.
Results
Study characteristics
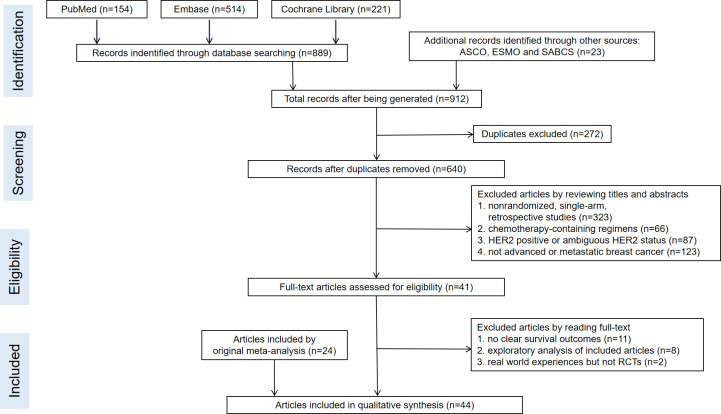
A total of 28 RCTs were included in our study, of which 20 RCTs had been included in the previous review and eight RCTs were newly subsumed. The detailed process of literature screening was shown in Figure 1 . Because this was an updated analysis of previous research (14), we mainly retrieved the newly published articles after 31 January 2020. Meanwhile, we also updated the OS and other data of the original 20 RCTs. The quality assessment of all included studies was illustrated in Figure 2 . In total, 12,129 patients with hormone receptor-positive and HER2-negative advanced breast cancer were included, among which 7,193 and 4,936 patients were in the experimental and control groups, respectively. The basic characteristics of enrolled studies including author, published time, study phase, interventions, the prior treatment lines, and different metastatic sites were listed in Table 1 . The study was dissected into different cohorts if it consisted more than one intervention comparison. Table 2 recorded PFS, OS, time to the first chemotherapy of all enrolled populations, and PFS of different subgroups.
Figure 1.
The flow chart of detailed literature screening process.
Figure 2.
Risk of bias summary.
Table 1.
Baseline characteristics of 28 RCTs.
| Study | Author. Published Time | Phase | Regimen (No. of Patients) | Prior Lines of Therapy (%) | Bone-Only metastasis (%) | Visceral metastasis (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| I-group | C-group | I-group | C-group | I-group | C-group | I-group | C-group | |||
| PALOMA-1/ TRIO-18 (18, 19) |
Richard S. Finn et al. 2015.01/2020.07 |
II | Pabociclib+ Letrozole (84) |
Letrozole (81) |
0(100) | 0(100) | 19 | 15 | 45 | 53 |
| PALOMA-2 (20) | Richard S Finn et al. 2016.11 |
III | Pabociclib+ Letrozole (444) |
Letrozole (222) |
0(100) | 0(100) | 23.2 | 21.6 | 48.2 | 49.5 |
| PALOMA-3 (21–23) |
Robert H Lurie et al., 2015.07/2018.10/2021.06 | III | Pabociclib+ Fulvestrant (347) |
Placebo+ Fulvestrant (174) |
0(21)/1(41)/ 2(37)/≥3(11) |
0(23)/1(48)/ 2(21)/≥3(7) |
NA | NA | 59.4 | 60.3 |
| PALOMA-4 (24) | Binghe Xu et al. 2021.08 |
III | Pabociclib+ Letrozole (169) |
Placebo+ Letrozole (171) |
0(100) | 0(100) | NA | NA | 55.6 | 56.1 |
| PARSIFAL (25) | Antonio L Cussac,et al. 2021.08 | II | Palbociclib+ Fulvestrant (243) |
Palbociclib+ Letrozole (243) |
0(100) | 0(100) | NA | NA | 47.3 | 48.6 |
| FLIPPER (26) | J Albanell et al. 2021.12 |
II | Palbociclib+ Fulvestrant (94) |
Placebo+ Fulvestrant (95) |
0(100) | 0(100) | NA | NA | 60.6 | 60 |
| MONALEESA-2 (27–29) | G N Hortobagyi et al. 2016.11/2018.07/2021.08 |
III | Ribociclib+ Letrozole (334) |
Placebo+ Letrozole (334) |
0(100) | 0(100) | 20.7 | 23.4 | 59 | 58.7 |
| MONALEESA-3 (30–32) | Dennis J. Slamon et al., 2018.08/2020.02/2021.08 | III | Ribociclib+ Fulvestrant (484) |
Placebo+ Fulvestrant (242) |
0(49.2)/ 1(48.8) |
0(53.3)/1(48.0) | 21.3 | 21.1 | 60.5 | 60.3 |
| MONALEESA-7 (33, 34) | Debu Tripathy et al. 2018.05/2019.06 |
III | Ribociclib + TAM/NSAI (335) |
Placebo + TAM/NSAI (337) |
0(100) | 0(100) | 24 | 23 | 58 | 56 |
| MONARCH-2 (35, 36) | George W Sledge Jr, et al. 2017.09/2020.01 |
III | Abemaciclib + Fulvestrant (446) |
Placebo + Fulvestrant (223) |
1(100) | 1(100) | 27.6 | 25.6 | 54.9 | 57.4 |
| MONARCH-3 (37–39) | Stephen Johnston et al., 2017.11/2019.01/2021.06 | III | Abemaciclib + NSAI (328) |
Placebo + NSAI (165) |
0(100) | 0(100) | 21.3 | 23.6 | 52.4 | 53.9 |
| MONARCH plus (a) (40) |
Qingyuan Zhang et al. 2020/08 |
III | Abemaciclib + NSAI (207) |
Placebo + NSAI (99) |
0(100) | 0(100) | NA | NA | 60.9 | 59.6 |
| MONARCH plus (b) (40) |
Qingyuan Zhang et al. 2020/08 |
III | Abemaciclib + Fulvestrant (104) |
Placebo + Fulvestrant (53) |
1(100) | 1(100) | NA | NA | 61.5 | 58.5 |
| next MONARCH(a) (41) |
Erika Hamilton et al. 2021.06 |
II | Abemaciclib 150 mg + Tamoxifen (78) |
Abemaciclib 150 mg (79) |
NA | NA | NA | NA | 61.5 | 62 |
| next MONARC(b) (41) |
Erika Hamilton et al. 2021.06 |
II | Abemaciclib 150 mg (79) |
Abemaciclib200 mg + loperamide (77) |
NA | NA | NA | NA | 62 | 62.3 |
| DAWNA-1 (42) | Binghe Xu et al. 2021.06 |
III | Dalpiciclib + Fulvestrant (241) |
Placebo + Fulvestrant (120) |
1(72.6)/2(27.4) | 1(72.5)/2(27.5) | 17.4 | 15.8 | 58.9 | 62.5 |
| SOLAR-1 (a) (43, 44) | F. André et al. 2019.05/2021.02 |
III | Alpelisib + Fulvestrant (169) |
Placebo + Fulvestrant (172) |
0(52.1)/1(46.7) | 0(51.7)/1(47.7) | 24.9 | 20.3 | 55 | 58.1 |
| SOLAR-1 (b) (43, 44) | F. André et al. 2019.05/2021.03 |
III | Alpelisib + Fulvestrant (115) |
Placebo + Fulvestrant (116) |
0(61.7)/1(36.5) | 0(53.4)/1(45.7) | 22.6 | 19.8 | 57.4 | 63.8 |
| Yen-Shen Lu, et al (45) |
Yen-Shen Lu et al. 2021.01 |
Ib | Alpelisib + Tamoxifen + Goserelin (16) |
Buparlisib + Tamoxifen + Goserelin(13) |
0(100) | 0(100) | 33.3 | 7.7 | NA | NA |
| BELLE-2 (46, 47) | José Baselga et al. 2017.07/2018.11 |
II | Buparlisib + Fulvestrant (576) |
Placebo + Fulvestrant (571) |
0(27)/1(53)/ ≥2(19) |
0(25)/1(53)/ ≥2(22) |
NA | NA | 59 | 59 |
| BELLE-3 (48) | Angelo Di Leo et al. 2018.01 |
III | Buparlisib + Fulvestrant (289) |
Placebo + Fulvestrant (143) |
1(30)/2(57)/≥3(13) | 1(34)/2(53)/ ≥3(13) |
15 | 13 | 73 | 72 |
| SANDPIPER(a) (49) | S Dent et al. 2021.02 |
III | Taselisib + Fulvestrant (417) |
Placebo + Fulvestrant (214) |
NA | NA | NA | NA | NA | NA |
| SANDPIPER(b) (49) | S Dent et al. 2021.02 |
III | Taselisib + Fulvestrant (340) |
Placebo + Fulvestrant (176) |
NA | NA | 20.6 | 18.2 | 59.1 | 58.5 |
| POSEIDON (50) | M Oliveira et al. 2021.09 |
II | Taselisib + Tamoxifen (76) |
Placebo + Tamoxifen (76) |
0(64)/ 1(36) |
0(67)/1(33) | NA | NA | NA | NA |
| FERGI(a) (51) | Ian E Krop et al. 2016.06 |
II | Pictilisib + Fulvestrant (89) |
Placebo + Fulvestrant (79) |
0(27)/1(37)/ 2 (26)/≥3(10) |
0(25)/1(46)/ 2(19)/≥3(10) |
21 | 22 | 57 | 53 |
| FERGI(b) (51) | Ian E Krop et al. 2016.06 |
II | Pictilisib + Fulvestrant (41) |
Placebo + Fulvestrant (20) |
0(12)/1(27)/ 2(20)/≥3(41) |
0(10)/1(35)/ 2(25)/≥3(30) |
17 | 25 | 51 | 50 |
| FAKTION (52) | Robert H Jones et al. 2020.03 |
II | Capivasertib + Fulvestrant (69) |
Placebo + Fulvestrant (71) |
0(13)/1(57)/ ≥2(29) |
0(8)/1(63)/ ≥2(28) |
14 | 11 | 71 | 66 |
| BOLERO-2 (53, 54) | J Baselga et al., 2011.12 M. Piccart, et al., 2014.09 |
III | Everolimus + Exemestane (485) |
Exemestane (239) |
1(16)/2(30)/ ≥3(54) |
1(18)/2(30)/ ≥3(53) |
NA | NA | 56 | 56 |
| TAMRAD (55) | T Bachelot et al. 2021.08 |
II | Everolimus + Tamoxifen (54) |
Tamoxifen (57) |
NA | NA | 25 | 30 | 49 | 57 |
| MANTA(a) (56) | Peter Schmid et al. 2019.11 |
II | Visusertib + Fulvestrant (101) |
Fulvestrant (66) |
0(44)/1(45)/ ≥2(12) |
0(44)/1(41)/ ≥2(15) |
24 | 27 | 63 | 62 |
| MANTA(b) (56) | Peter Schmid et al. 2019.11 |
II | Visusertib + Fulvestrant (95) |
Fulvestrant (66) |
0(47)/1(38)/ ≥2(15) |
0(44)/1(41)/ ≥2(15) |
22 | 27 | 56 | 62 |
| MANTA(c) (56) | Peter Schmid et al. 2019.11 |
II | Everolimus + Fulvestrant (64) |
Fulvestrant (66) |
0(42)/1(39)/ ≥2(19) |
0(44)/1(41)/ ≥2(15) |
17 | 27 | 69 | 62 |
| PrE0102 (57) | Noah Kornblum et al. 2018.06 |
II | Everolimus + Fulvestrant (66) |
Placebo + Fulvestrant (65) |
NA | NA | NA | NA | NA | NA |
| MIRACLE (58) | Ying Fan et al. 2021.08 |
II | Everolimus + Letrozole + OFS (101) |
Letrozole + OFS (98) |
0(100) | 0(100) | NA | NA | 57.4 | 58.2 |
| LEO (59, 60) | Jae Ho Jeong et al. 2020.12/2021.04 |
II | Everolimus + Leuprorelin + Letrozole (92) |
Leuprorelin + Letrozole (45) |
0(49)/1(34)/≥2(17) | 0(58)/1(29)/ ≥2(13) |
5.4 | 13.3 | 60.9 | 60 |
Six RCTs were dissected into different cohorts, wherein SOLAR-1, SANDPIPER, and FERGI were divided according to PIK3CA mutation status, nextMONARCH and MANTA compared different usage and dosage of one specific drug, MONARCH plus was separated by different endocrine agents (NSAI or fulvestrant). RCTs, randomized clinical trials; NA, not available; TAM, tamoxifen; NSAI, non-steroidal aromatase inhibitors; I-group, interventional group; C-group, control group.
Table 2.
Survival outcomes of RCTs.
| Study | Median PFS (HR,95% CI) | Median OS (HR,95% CI) | Time to First Chemotherapy (HR,95% CI) | Visceral Metastasis (HR,95% CI) | Non-Visceral Metastasis (HR,95% CI) | Bone-Only Metastasis (HR,95% CI) | Liver Metastasis (HR,95% CI) |
|---|---|---|---|---|---|---|---|
| PALOMA-1/ TRIO-18 (18, 19) |
20.2 m vs. 10.2 m (0.488, 0.319–0.748) |
37.5 m vs. 34.5 m (0.897, 0.623–1.294) |
0.662 (0.445–0.989) |
NA | NA | NA | NA |
| PALOMA-2 (20) | 24.8 m vs. 14.5 m (0.58, 0.46–0.72) |
NA | NA | 0.6 (0.47–0.85) |
0.50 (0.36–0.70) |
0.36 (0.22–0.59) |
NA |
| PALOMA-3 (21–23) | 9.5 m vs. 4.6 m (0.46, 0.36–0.59) |
34.8 m vs. 28.0 m (0.81, 0.65–0.99) |
NA | 0.45 (0.32–0.63) |
0.36 (0.22–0.60) |
NA | NA |
| PALOMA-4 (24) | 21.5 m vs. 13.9 m (0.68, 0.53–0.87) |
NA | NA | 0.657 (0.467–0.925) |
0.700 (0.488–1.002) |
NA | NA |
| PARSIFAL (25) | 27.9 m vs. 32.8 m (1.13, 0.89–1.45) |
NA vs. NA (1.00, 0.68–1.48) |
NA | 1.27 (0.91–1.77) |
0.97 (0.67–1.40) |
NA | NA |
| FLIPPER (26) | 31.8 m vs. 22.0 m (0.48, 0.37–0.64) |
NA | NA | 0.45 (0.32–0.63) |
0.62 (0.39–0.97) |
1.13 (0.53–2.41) |
0.56 (0.32–0.99) |
| MONALEESA-2 (27–29) | 25.3 m vs. 16.0 m (0.568, 0.457–0.704) |
63.9 m vs. 51.4 m (0.76, 0.63–0.93) |
0.74 (0.61–0.91) |
NA | NA | 0.642 (0.393–1.048) |
NA |
| MONALEESA-3 (30–32) | 37.4 m vs. 28.1 m (0.693, 0.57–0.844) |
53.7 m vs. 41.5 m (0.73, 0.59–0.90) |
0.704 (0.566–0.876) |
0.804 (0.596–1.083) |
NA | NA | NA |
| MONALEESA-7 (33, 34) | 23.8 m vs. 13.0 m (0.55, 0.44–0.69) |
58.7 m vs. 48.0 m (0.763, 0.608–0.956) |
0.694 (0.556–0.867) |
0.698 (0.462–1.054) |
NA | 0.70 (0.41–1.19) |
NA |
| MONARCH-2 (35, 36) | 16.9 m vs. 9.3 m (0.536, 0.445–0.645) |
46.7 m vs. 37.3 m (0.757, 0.606–0.945) |
0.625 (0.501–0.779) |
0.471 (0.371–0.598) |
NA | 0.580 (0.398–0.844) |
NA |
| MONARCH-3 (37–39) | 28.2 m vs. 14.8 m (0.525, 0.415–0.665) |
NA | 0.513 (0.380–0.691) |
0.567 (0.407–0.789) |
NA | 0.471 (0.280–0.793) |
0.449 (0.259–0.777) |
| MONARCH plus (a) (40) |
NA vs. 14.7 m (0.499, 0.346–0.719) |
NA | NA | 0.615 (0.396–0.955) |
0.335 (0.175–0.639) |
NA | 0.385 (0.194–0.763) |
| MONARCH plus (b) (40) |
11.5 m vs. 5.6 m (0.376, 0.240–0.588) |
NA | NA | 0.423 (0.247–0.724) |
0.328 (0.149–0.722) |
NA | 0.513 (0.270–0.974) |
| next MONARCH(a) (41) |
9.1 m vs. 6.5 m (0.805, 0.551–1.177) |
24.2 m vs. 20.8 m (0.620, 0.397–0.969) |
NA | NA | NA | NA | NA |
| next MONARC(b) (41) |
6.5 m vs. 7.4 m (1.045, 0.711–1.535) |
20.8 m vs. 17.0 m (0.956, 0.635–1.438) |
NA | NA | NA | NA | NA |
| DAWNA-1 (42) | 13.6 m vs. 7.7 m (0.45, 0.32–0.64) |
NA | 0.47 (0.32–0.69) |
0.48 (0.33–0.70) |
0.36 (0.20–0.63) |
0.76 (0.31–1.85) |
NA |
| SOLAR-1 (a) (43, 44) | 11.0 m vs. 5.7 m (0.65, 0.50–0.85) |
39.3 m vs. 31.4 m (0.86, 0.64–1.15) |
0.72 (0.54–0.95) |
NA | NA | NA | NA |
| SOLAR-1 (b) (43, 44) | 7.4 m vs. 5.6 m (0.85, 0.58–1.85) |
NA | NA | NA | NA | NA | NA |
| Yen-Shen Lu, et al (45) |
25.2 m vs. 20.6 m (NA, NA–NA) |
NA | NA | NA | NA | NA | NA |
| BELLE-2 (46, 47) | 6.9 m vs. 5.0 m (0.78, 0.67–0.89) |
33.2 m vs. 30.4 m (0.87, 0.74–1.02) |
NA | 0.76 (0.62–0.92) |
0.79 (0.58–1.07) |
0.66 (0.46–0.95) |
NA |
| BELLE-3 (48) | 3.9 m vs. 1.8 m (0.67, 0.53–0.84) |
NA | NA | 0.56 (0.43–0.74) |
0.96 (0.61–1.50) |
1.06 (0.52–2.15) |
NA |
| SANDPIPER(a) (49) | NA | NA | NA | NA | NA | NA | NA |
| SANDPIPER(b) (49) | 9.0 m vs. 5.4 m (0.66, 0.51–0.86) |
NA | NA | 0.74 (0.56–1.00) |
0.72 (0.49–1.04) |
0.58 (0.33–1.01) |
0.73 (0.51–1.04) |
| POSEIDON (50) | 4.8 m vs. 3.2 m (0.63, 0.43–0.93) |
20.9 m vs. 24.4 m (0.97, 0.63–1.5) |
NA | NA | NA | NA | NA |
| FERGI(a) (51) | 6.6 m vs. 5.1 m (0.74, 0.52–1.06) |
NA | NA | 0.74 (0.46–1.18) |
0.70 (0.41–1.27) |
0.57 (0.32–1.02) |
NA |
| FERGI(b) (51) | 5.4 m vs. 10.0 m (1.07, 0.53–2.18) |
NA | NA | NA | NA | NA | NA |
| FAKTION (52) | 10.3 m vs. 4.8 m (0.58, 0.39–0.84) |
26.0 m vs. 20.0 m (0.59, 0.34–1.05) |
NA | NA | NA | NA | NA |
| BOLERO-2 (53, 54) | 10.6 m vs. 4.1 m (0.36, 0.27–0.47) |
31.0 m vs. 26.6 m (0.89, 0.73–1.10) |
NA | 0.47 (0.37–0.80) |
0.41 (0.31–0.55) |
NA | NA |
| TAMRAD (55) | 8.6 m vs. 4.5 m (0.54, 0.36–0.81) |
NA vs. 32.9 m (0.45, 0.24–0.81) |
NA | NA | NA | NA | NA |
| MANTA(a) (56) | 7.6 m vs. 5.4 m (0.88, 0.63–1.24) |
27.1 m vs. 24.4 m (NA, NA–NA) |
NA | NA | NA | NA | NA |
| MANTA(b) (56) | 8.0 m vs. 5.4 m (0.79, 0.55–1.12) |
24.2 m vs. 24.4 m (NA, NA–NA) |
NA | NA | NA | NA | NA |
| MANTA(c) (56) | 12.3 m vs. 5.4 m (0.63, 0.42–0.92) |
NA vs. 24.4 m (NA, NA–NA) |
NA | NA | NA | NA | NA |
| PrE0102 (57) | 10.3 m vs. 5.1 m (0.61, 0.40–0.92) |
31.4 m vs. 28.3 m (1.31, 0.72–2.38) |
NA | NA | NA | NA | NA |
| MIRACLE (58) | 19.4 m vs. 12.9 m (0.64, 0.46–0.89) |
NA | NA | 0.762 (0.503–1.157) |
0.469 (0.270–0.817) |
NA | NA |
| LEO (59, 60) | 18.1 m vs. 13.8 m (0.73, 0.48–1.11) |
48.3 m vs. 50.8 m (NA, NA–NA) |
NA | 0.58 (0.34–0.99) |
1.09 (0.53–2.21) |
NA | NA |
PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; m, months; NA, not available.
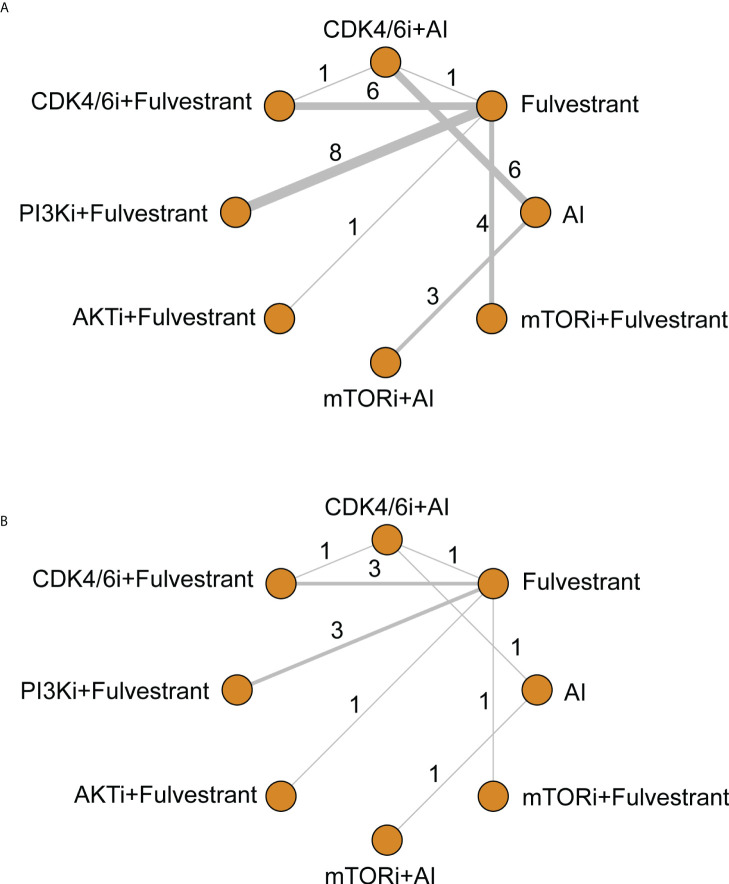
There were 13 different treatment regimens in the 28 RCTs, among which eight interventions were incorporated into the network meta-analysis ( Figure 3 ). One of the interventions was excluded from the network because the corresponding RCT (45) conducted comparisons within PI3K inhibitors (alpelisib and buparlisib), without connections with other interventions in the network. The involved agents were CDK4/6 inhibitors (including palbociclib, ribociclib, abemaciclib, and dalpiciclib), PI3K inhibitors (including alpelisib, buparlisib, pictilisib, and taselisib), AKT inhibitor (capivasertib), and mTOR inhibitors (everolimus and vistusertib). All kinds of AIs, fulvestrant, and tamoxifen were amalgamated into ET in subsequent data analysis.
Figure 3.
Network of comparative interventions for (A) PFS and (B) OS. CDK4/6i, CDK4/6 inhibitors; PI3K, PI3K inhibitors; AKTi, AKT inhibitors; mTORi, mTOR inhibitors; AI, aromatase inhibitors.
Survival outcomes
Intervention arms that reported PFS or OS with available HRs and 95% CIs were utilized for data synthesis. Regarding PFS and OS, there were eight different interventions that formed a network, and the pairwise comparisons of them were shown in Table 3 . In this table, the treatments were sequenced according to the SUCRA values of PFS ( Table 4A ), whereas the SUCRA values of OS were displayed in Table 4B . All varieties of CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors plus ET showed remarkable advantages in PFS compared with fulvestrant monotherapy, with HR and 95% CrI valued as 0.47 (0.32–0.69) for mTORi plus AI, 0.52 (0.40–0.66) for CDK4/6i plus AI, 0.53 (0.46–0.61) for CDK4/6i plus fulvestrant, 0.57 (0.36–0.90) for AKTi plus fulvestrant, 0.72 (0.63–0.83) for PI3Ki plus fulvestrant, and 0.73 (0.58–0.91) for mTORi plus fulvestrant. Compared with AI, significant improvements of PFS were observed in three therapeutic regimens, consisting of mTORi plus AI (HR, 0.51; 95% CrI, 0.41–0.66), CDK4/6i plus AI (HR, 0.56; 95% CrI, 0.48–0.66), and CDK4/6i plus fulvestrant (HR, 0.58; 95% CrI, 0.43–0.77). CDK4/6i plus ET saliently improved the PFS compared with PI3Ki plus fulvestrant (CDK4/6i plus AI: HR, 0.72; 95% CrI, 0.54–0.95; CDK4/6i plus fulvestrant: HR, 0.74; 95% CrI, 0.6–0.8) and mTORi plus fulvestrant (CDK4/6i plus AI: HR, 0.71; 95% CrI, 0.51–0.99; CDK4/6i plus fulvestrant: HR, 0.73; 95% CrI, 0.56–0.94). In addition, mTORi plus AI showed a better PFS than PI3Ki plus fulvestrant (HR, 0.66; 95% CrI, 0.44–0.98). With respect to OS, CDK4/6i plus either AI or fulvestrant significantly prolonged the survival compared with fulvestrant monotherapy (HR, 0.76; 95% CrI, 0.61–0.96; HR, 0.76; 95% CrI, 0.66–0.89, respectively). No significant difference was observed in OS when comparing PI3K/AKT/mTOR inhibitors plus ET with endocrine monotherapy. In general, compared with ET (AI or fulvestrant), forest plots indicated that CDK4/6i significantly prolonged PFS than PAM pathway inhibitors (HR, 0.81; 95% CrI, 0.69–0.94) ( Supplementary Figure 1A ). To be more precise, the diversity seemed to mainly exist between CDK4/6i and PI3Ki (HR, 1.3; 95% CrI, 1.1–1.6) ( Supplementary Figure 1B ). However, the similar difference was not displayed in OS ( Supplementary Figures 1C, D ).
Table 3.
Pairwise comparisons of 8 interventions for PFS and OS (HR, 95% CrI).
| mTORi + AI | 1.00 (0.61, 1.63) | 0.99 (0.57, 1.72) | 1.25 (0.57, 2.80) | 0.86 (0.49, 1.52) | 0.58 (0.25, 1.33) | 0.89 (0.69, 1.17) | 0.76 (0.44, 1.31) |
| 0.91 (0.69, 1.22) |
CDK4/6i + AI | 1.00 (0.78, 1.28) |
1.26 (0.68, 2.44) |
0.87 (0.65, 1.16) |
0.58 (0.30, 1.14) |
0.90 (0.60, 1.35) |
0.76 (0.61, 0.96) |
| 0.89 (0.62, 1.32) |
0.98 (0.77, 1.26) |
CDK4/6i + Fulvestrant | 1.27 (0.70, 2.39) |
0.87 (0.69, 1.10) |
0.58 (0.30, 1.13) |
0.90 (0.56, 1.46) |
0.76 (0.66, 0.89) |
| 0.83 (0.46, 1.50) |
0.91 (0.54, 1.53) |
0.93 (0.57, 1.48) |
AKTi + Fulvestrant |
0.69 (0.36, 1.26) |
0.46 (0.19, 1.09) |
0.71 (0.33, 1.50) |
0.60 (0.33, 1.08) |
|
0.66
(0.44, 0.98) |
0.72
(0.54, 0.95) |
0.74
(0.60, 0.89) |
0.79 (0.50, 1.27) |
PI3Ki + Fulvestrant |
0.67 (0.35, 1.31) |
1.04 (0.63, 1.72) |
0.88 (0.74, 1.05) |
| 0.65 (0.42, 1.01) |
0.71
(0.51, 0.99) |
0.73
(0.56, 0.94) |
0.78 (0.47, 1.30) |
0.99 (0.76, 1.29) |
mTORi + Fulvestrant |
1.55 (0.71, 3.36) |
1.32 (0.69, 2.48) |
|
0.51
(0.41, 0.66) |
0.56
(0.48, 0.66) |
0.58
(0.43, 0.77) |
0.62 (0.36, 1.06) |
0.78 (0.57, 1.08) |
0.79 (0.55, 1.15) |
AI | 0.85 (0.53, 1.35) |
|
0.47
(0.32, 0.69) |
0.52
(0.40, 0.66) |
0.53
(0.46, 0.61) |
0.57
(0.36, 0.90) |
0.72
(0.63, 0.83) |
0.73
(0.58, 0.91) |
0.92 (0.69, 1.22) |
Fulvestrant |
Contrast of PFS (on the lower triangle) and OS (on the upper triangle). The HRs lower than 1 revealed the favorable tendency of column-defining regimens for PFS and row-defining regimens for OS. Significant differences were bolded. HR, hazard ratio; CrI, credible interval; CDK4/6i, CDK4/6 inhibitors; PI3Ki, PI3K inhibitors; AKTi, AKT inhibitors; mTORi, mTOR inhibitors; AI, aromatase inhibitors.
Table 4.
SUCRA values of each combination regimen for PFS (A) and OS (B).
| (A) SUCRA values for PFS | |
|---|---|
| Interventions | SUCRA% |
| mTORi + AI | 88.62 |
| CDK4/6i + AI | 77.94 |
| CDK4/6i + Fulvestrant | 75.5 |
| AKTi + Fulvestrant | 65.66 |
| PI3Ki + Fulvestrant | 38.18 |
| mTORi + Fulvestrant | 36.75 |
| AI | 13.39 |
| Fulvestrant | 3.96 |
| (B) SUCRA values for OS | |
| Interventions | SUCRA% |
| AKTi + Fulvestrant | 85.26 |
| CDK4/6i + Fulvestrant | 67.90 |
| CDK4/6i + AI | 67.19 |
| mTORi + AI | 64.18 |
| AI | 44.65 |
| PI3Ki + Fulvestrant | 41.99 |
| Fulvestrant | 19.15 |
| mTORi + Fulvestrant | 9.67 |
(SUCRA, surface under the cumulative ranking curve).
Subgroup survival analysis
We then stratified the survival data based on prior treatment lines and metastatic sites. Pooled results suggested that there was no significant difference between CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors regarding PFS and OS in both first and second lines. HRs and 95% CrIs between CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors for first-line PFS, second-line PFS, first-line OS, and second-line OS were 1.2 (0.94–1.6), 1.1 (0.87–1.5), 1.0 (0.61–1.8), and 1.1 (0.54–2.0), respectively ( Supplementary Figure 2 ). Subgroups with different metastatic sites were classified as visceral (generally defined as all lesions except breast, skin, soft tissue, lymph node, and bone), bone, and liver metastasis, of which PFS data were collected. In both visceral and non-visceral metastasis subgroups, PI3K inhibitors showed worse curative effects than CDK4/6 inhibitors (HR, 1.3: 95% CrI, 1.1–1.7; HR, 1.6; 95% CrI, 1.1–2.5, respectively) ( Supplementary Figure 3 ). However, we did not discover conspicuous discrepancy in bone and liver metastatic subgroups ( Supplementary Figures 4 , 5 ). In addition, there was no significant difference between PI3K inhibitors and CDK4/6 inhibitors in the time to the first subsequent chemotherapy ( Supplementary Figure 6 ).
Safety
Four kinds of hematological and 13 kinds of non-hematological TRAEs were collected, and they were divided into all grades and grade ≥ 3.
In terms of adverse effects within CDK4/6 inhibitors, pooled analysis demonstrated that abemaciclib was significantly better than others regarding neutropenia of grade ≥3 (OR, 0.035; 95% CrI, 0.0058–0.15) ( Supplementary Figure 7 ). There was no significant difference in other hematological adverse events among groups of CDK4/6 inhibitors, regardless of all grades or grade ≥3. Among PI3K/AKT/mTOR inhibitors, both alpelisib (OR, 0.24; 95% CrI, 0.035–1.0) and burparlisib (OR, 0.27; 95% CrI, 0.052–0.96) tended to exhibit lower risk of all-grade stomatitis than everolimus ( Supplementary Figure 8 ). Compared with capivasertib, vistusertib was more prone to bring nausea (OR, 6.6; 95% CrI, 1.6–27) ( Supplementary Figure 9 ); alpelisib (OR, 3.1; 95% CrI, 1.2–8.3) and vistusertib (OR, 3.6; 95% CrI, 1.1–12) had significantly higher risk than pictilisib regarding anorexia ( Supplementary Figure 10 ).
As for common safety concerns, CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors showed different profiles in hepatotoxicity, gastrointestinal (GI) toxicity, and hyperglycemia. Burparlisib and everolimus remarkably elevated alanine aminotransferase (ALT) concentration (OR, 7.0; 95% CrI, 2.8–16.0; OR, 4.1; 95% CrI, 1.5–13.0, respectively) in all grades compared with palbociclib ( Supplementary Figure 11 ). Burparlisib showed similar tendency in aspartate aminotransferase (AST) elevation ( Supplementary Figure 12 ). In addition, although not reaching statistical significance, dalpiciclib tended to have less hepatotoxicity (OR, 0.37; 95% CrI, 0.13–1.00; OR, 0.40; 95% CrI, 0.14–1.10, respectively) ( Supplementary Figures 11 , 12 ). From Supplementary Figure 13 , we observed that the risk of diarrhea of all grades caused by PI3K/AKT/mTOR inhibitors was significantly higher than that of palbociclib and ribociclib. Compared with palbociclib, all interventions except burparlisib and everolimus significantly increased the risk of diarrhea. Regarding hyperglycemia, compared with CDK4/6 inhibitors, three of four PI3K inhibitors were more likely to induce hyperglycemia of all grades, including alpelisib (OR, 11.0; 95% CrI, 2.3–50.0), burparlisib (OR, 10.0; 95% CrI, 2.9–48.0), and taselisib (OR, 6.5; 95% CrI, 1.6–34.0), whereas pictilisib, capivasertib, and everolimus showed the same trend without salient discrepancy ( Supplementary Figure 14 ).
Discussion
Although the long-term prognosis is favorable for patients with early breast cancer (61), the 5-year survival rate for patients whose disease has progressed or metastasized is still not optimistic (62). For hormone receptor+/HER2− subtype, not all patients are responsive to the first-line ET, and drug resistance and subsequent disease progression may eventually occur in some patients. Many inhibitors involved in the CDK4/6 and PAM signaling pathways are investigated, with great clinical efficacy and acceptable toxicities, confirmed by numerous RCTs (62). Despite the increasing use of these two kinds of agents in clinical practice, there is controversy about their advantages and disadvantages regarding efficacy and safety. Our original meta-analysis was the first pooling analysis that synthesized outcomes of many clinical trials and used indirect comparisons to shed light on the above issue (14). Considering that data of some RCTs continue to mature and many novel studies rise, we updated the previous meta-analysis. Compared with our previous research, the present study integrated much more data regarding both efficacy and toxicity. We also further explored the data of more subgroups and other endpoints.
The survival outcomes of our study showed that compared with endocrine monotherapy, both CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors acquired longer PFS and OS, demonstrated by the SUCRA values. Favorable PFS reached statistically significance nearly in all the combination regimens compared with monotherapy except for PI3K/AKT/mTOR inhibitors plus fulvestrant versus AI. As for the pairwise comparisons between different targeted therapeutic groups, CDK4/6 inhibitors took conspicuous superiority than PI3K/AKT/mTOR inhibitors in short-term PFS but not long-term OS, and the difference might mainly exist between CDK4/6 and PI3K inhibitors. Notably, although mTOR inhibitors plus AI ranked the highest from SUCRA values of PFS, pairwise comparisons did not indicate this treatment strategy was better than CDK4/6 inhibitors plus ET. Our study only suggested the tendency of CDK4/6 inhibitor-containing treatment regimens toward better OS from the view of SUCRA values. Currently, there are still challenges in differentiating the population that can acquire OS benefit from CDK4/6 inhibitors due to the profound heterogeneity of patients (63).
Considering that the timing of treatment with PI3K/AKT/mTOR inhibitors is generally later than CDK4/6 inhibitors, past studies were usually layered by treatment lines (64, 65). We collected information on the lines of previous ET and classified the survival outcomes accordingly to eliminate the potential bias. However, after analyzing the data by this way, we did not observe obvious advantages in PFS and OS of CDK4/6 inhibitors over PI3K and mTOR inhibitors in terms of different treatment lines, which was discordant with our original analysis (14). The discrepancy might be due to the fact that the delineation of the treatment lines in previous meta-analysis was not very refined and clear; meanwhile, the data of new clinical trials became available. Current study findings were also inconsistent with another meta-analysis conducted by Leung et al. that compared CDK4/6 and PI3K/AKT/mTOR inhibitors plus fulvestrant in the setting of second-line treatment, of which the synthetic outcomes supported the superior efficacy of CDK4/6 inhibitors (65). Some of the clinical trials included in the above meta-analysis did not contain patients of second line exclusively. Thus, the indistinct prior treatment lines may partly explain the different results between two meta-analyses because we divided the treatment lines more precisely. Nevertheless, we noted that the efficacy of CDK4/6 inhibitors was superior to other kinds of inhibitors both in first and second lines from the SUCRA values. Another point to be noted was that there only existed data of PI3K inhibitors regarding OS for first-line treatment. Therefore, we could not conclude that CDK4/6 inhibitors were superior to all PAM pathway inhibitors in this situation. In summary, we believe that the overall efficacy of PI3K/AKT/mTOR inhibitors is not inferior to that of CDK4/6 inhibitors, but only because their application is usually in later lines. With respect to the data stratified by metastatic sites and the time to first subsequent chemotherapy, salient advantages of CDK4/6 inhibitors only existed in the visceral and non-visceral subgroups.
As for the safety, CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors each had their own specific profiles, whereas there were also common concerns between the two. Among CDK4/6 inhibitors, previous research studies indicated that abemaciclib was associated with a low rate of neutropenia and a high incidence of GI toxicity (66, 67). Our present analysis confirmed the above findings. The hematologic toxicity of CDK4/6 inhibitors is mainly caused by the inhibiting effect on CDK6 in hematopoietic cells, whereas abemaciclib has a 14-fold higher affinity for CDK4 than CDK6 (68). Moreover, abemaciclib also exerts inhibiting effect on CDK9, which is considered to be related with the increased GI toxicity like diarrhea (69). Within PI3K/AKT/mTOR inhibitors, the incidence of stomatitis, nausea, anorexia, and hepatic toxicity of different agents showed significant differences. Therein, everolimus led to more stomatitis, vistusertib and alpelisib increased the risk of digestive disorders, whereas both burparlisib and everolimus remarkably elevated the ALT/AST levels according to our analysis. In the comparisons of the two categories, PI3K/AKT/mTOR inhibitors generally incurred more diarrhea than palbocilib and ribociclib. In addition, hyperglycemia is reported in nearly all kinds of PI3K/AKT/mTOR inhibitors but only in two CDK4/6 inhibitors (palbociclib and dalpiciclib). Pooled analysis revealed the tendency for PAM inhibitors especially PI3K inhibitors to result in escalated blood glucose level. Hyperglycemia is one of the common on-target side effects of PI3K inhibitors due to the dysregulation of glucose metabolism that warrants prevention, monitoring, and treatment (70, 71). Our original study mainly focused on the severe TRAEs of grade ≥3, whereas current study further supplemented the data regarding all grades and capivasertib-caused toxicity. On the basis of the above results, individualized treatment options could be deliberated.
Inevitably, there existed several limitations. On one hand, the enrolled patients were mainly postmenopausal, and the data for premenopausal women were still insufficient. However, this issue was hard to be fully addressed due to the restriction by the inclusion criteria of original trials. On the other, all data in our meta-analysis were extracted from published literatures without original prospective outcomes, which may cause bias to the present results. In addition, although we have updated the data, some interim results were still from conference abstracts without available full texts. In addition, the heterogeneity among included studies was inevitable although we performed subgroup analyses to minimize it. Nevertheless, the heterogeneity was low from I2 values, which indicated the satisfactory credibility of our study.
However, our investigation is of clinical significance to some extent and could provide clues for the future practice. Although PI3K/AKT/mTOR inhibitors are recommended for later lines of treatment than CDK4/6 inhibitors in hormone receptor+/HER2− metastatic breast cancer patients, the relative equivalent efficacy in different treatment lines provide more reasons for PI3K/AKT/mTOR inhibitors to be used in earlier clinical settings. At present, numerous studies have focused on this aspect and evaluate the efficacy of PI3K/AKT/mTOR inhibitors in the setting of neoadjuvant, adjuvant, and first-line treatments (72–75). Despite the similar efficacy of two kinds of agents, the safety profiles varied on the basis of the current results, which signified that different recommendations could be made to patients accordingly given the different tolerance to TRAEs. In addition, several in vitro and in vivo preclinical studies indicated that the triplet combination strategy of CDK4/6 and PI3K/AKT/mTOR inhibitors with traditional endocrine agents could overcome endocrine resistance and show synergistic effects (76–79). The endocrine resistance remains a difficult problem, of which the mechanism is complicated and not yet clearly defined (5). The combination therapy is expected to restrain and reverse drug resistance and tumor metastasis. As all mentioned above, this study provides further insight into CDK4/6 and PI3K/AKT/mTOR inhibitors, and we anticipate the implementation of large-scale RCTs with head-to-head comparisons to ultimately address the clinical issue.
Conclusions
In conclusion, CDK4/6 inhibitors showed conspicuous superiority than PI3K/AKT/mTOR inhibitors regarding PFS, whereas the superiority no longer existed by balancing the treatment lines. Detailed subgroup analysis suggested the advantages of CDK4/6 inhibitors in the population with visceral and no-visceral metastatic sites. The safety profiles were diverse between two varieties of agents.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
HX, YaW, and YH: protocol development, data extraction, data analysis, and manuscript writing. YuW: data extraction and manuscript writing. JW and BX: protocol development and final manuscript review. All authors contributed to the article and approved the submitted version.
Acknowledgments
We deeply appreciate all authors who performed and patients who participated in the included studies in our systematic review and meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.956464/full#supplementary-material
Full search strategy in (A) PubMed, (B) Embase and (C) the Cochrane Library.
Overall comparisons between CKD4/6 inhibitors or PI3K/AKT/mTOR inhibitors and endocrine therapy for PFS (A, B) and OS (C, D).
Comparisons between CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors when stratified by treatment lines: (A) for first-line PFS, (B) for second-line PFS, (C) for first-line OS and (D) for second-line OS.
PFS comparisons between CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors in visceral (A) and non-visceral (B) metastasis subgroups.
PFS comparisons between CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors in bone-only metastasis subgroup.
PFS comparisons between CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors in liver metastasis subgroup.
Time to first subsequent chemotherapy comparisons between CDK4/6 inhibitors and PI3K inhibitors.
≥3 grade neutropenia comparisons within CDK4/6 inhibitors.
All grade stomatitis comparisons within PI3K/AKT/mTOR inhibitors.
All grade nausea comparisons within PI3K/AKT/mTOR inhibitors.
All grade anorexia comparisons within PI3K/AKT/mTOR inhibitors.
All grade elevated ALT concentrations comparison among all treatments.
All grade elevated AST concentrations comparison among all treatments.
All grade diarrhea comparisons among all treatments.
All grade hyperglycemia comparisons among all treatments.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature (2000) 406(6797):747–52. doi: 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 3. Clarke CA, Keegan TH, Yang J, Press DJ, Kurian AW, Patel AH, et al. Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst (2012) 104(14):1094–101. doi: 10.1093/jnci/djs264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American society of clinical oncology guideline. J Clin Oncol (2016) 34(25):3069–103. doi: 10.1200/JCO.2016.67.1487 [DOI] [PubMed] [Google Scholar]
- 5. Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell (2020) 37(4):496–513. doi: 10.1016/j.ccell.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov (2015) 14(2):130–46. doi: 10.1038/nrd4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Piezzo M, Chiodini P, Riemma M, Cocco S, Caputo R, Cianniello D, et al. Progression-free survival and overall survival of CDK 4/6 inhibitors plus endocrine therapy in metastatic breast cancer: A systematic review and meta-analysis. Int J Mol Sci (2020) 21(17):6400. doi: 10.3390/ijms21176400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mills JN, Rutkovsky AC, Giordano A. Mechanisms of resistance in estrogen receptor positive breast cancer: overcoming resistance to tamoxifen/aromatase inhibitors. Curr Opin Pharmacol (2018) 41:59–65. doi: 10.1016/j.coph.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature (2012) 490(7418):61–70. doi: 10.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller TW, Hennessy BT, González-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest (2010) 120(7):2406–13. doi: 10.1172/JCI41680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goncalves MD, Hopkins BD, Cantley LC. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N Engl J Med (2018) 379(21):2052–62. doi: 10.1056/NEJMra1704560 [DOI] [PubMed] [Google Scholar]
- 12. Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol (2011) 29(33):4452–61. doi: 10.1200/JCO.2010.34.4879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nunnery SE, Mayer IA. Targeting the PI3K/AKT/mTOR pathway in hormone-positive breast cancer. Drugs (2020) 80(16):1685–97. doi: 10.1007/s40265-020-01394-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han Y, Wang J, Wang Z, Xu B. Comparative efficacy and safety of CDK4/6 and PI3K/AKT/mTOR inhibitors in women with hormone receptor-positive, HER2-negative metastatic breast cancer: a systematic review and network meta-analysis. Curr Probl Cancer (2020) 44(6):100606. doi: 10.1016/j.currproblcancer.2020.100606 [DOI] [PubMed] [Google Scholar]
- 15. Hutton B, Catalá-López F, Moher D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med Clin (Barc) (2016) 147(6):262–6. doi: 10.1016/j.medcli.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 16. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj (2019) 366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 17. Woods BS, Hawkins N, Scott DA. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol (2010) 10:54. doi: 10.1186/1471-2288-10-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol (2015) 16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3 [DOI] [PubMed] [Google Scholar]
- 19. Finn RS, Boer K, Bondarenko I, Patel R, Pinter T, Schmidt M, et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res Treat (2020) 183(2):419–28. doi: 10.1007/s10549-020-05755-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med (2016) 375(20):1925–36. doi: 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 21. Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-Receptor-Positive advanced breast cancer. N Engl J Med (2015) 373(3):209–19. doi: 10.1056/NEJMoa1505270 [DOI] [PubMed] [Google Scholar]
- 22. Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med (2018) 379(20):1926–36. doi: 10.1056/NEJMoa1810527 [DOI] [PubMed] [Google Scholar]
- 23. Cristofanilli M, Rugo HS, Im S-A, Slamon DJ, Harbeck N, Bondarenko I, et al. Overall survival (OS) with palbociclib (PAL) + fulvestrant (FUL) in women with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer (ABC): Updated analyses from PALOMA-3. J Clin Oncol (2021) 39(15_suppl):1000. doi: 10.1200/JCO.2021.39.15_suppl.1000 [DOI] [Google Scholar]
- 24. Xu B, Hu X, Li W, Sun T, Shen K, Wang S, et al. 228MO PALOMA-4: Primary results from a phase III trial of palbociclib (PAL) + letrozole (LET) vs placebo (PBO) + LET in Asian postmenopausal women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative (ER+/HER2–) advanced breast cancer (ABC). Ann Oncol (2021) 32:S457. doi: 10.1016/j.annonc.2021.08.511 [DOI] [Google Scholar]
- 25. Llombart-Cussac A, Pérez-García JM, Bellet M, Dalenc F, Gil-Gil M, Ruíz-Borrego M, et al. Fulvestrant-palbociclib vs letrozole-palbociclib as initial therapy for endocrine-sensitive, hormone receptor-positive, ERBB2-negative advanced breast cancer: A randomized clinical trial. JAMA Oncol (2021) 7(12):1791–9. doi: 10.1001/jamaoncol.2021.4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albanell J, Martínez MT, Ramos M, O'Connor M, de la Cruz-Merino L, Santaballa A, et al. Randomized phase II study of fulvestrant plus palbociclib or placebo in endocrine-sensitive, hormone receptor-positive/HER2-advanced breast cancer: GEICAM/2014-12 (FLIPPER). Eur J Cancer (2022) 161:26–37. doi: 10.1016/j.ejca.2021.11.010 [DOI] [PubMed] [Google Scholar]
- 27. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med (2016) 375(18):1738–48. doi: 10.1056/NEJMoa1609709 [DOI] [PubMed] [Google Scholar]
- 28. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol (2018) 29(7):1541–7. doi: 10.1093/annonc/mdy155 [DOI] [PubMed] [Google Scholar]
- 29. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, et al. LBA17 overall survival (OS) results from the phase III MONALEESA-2 (ML-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2–) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib (RIB). Ann Oncol (2021) 32:S1290–S1. doi: 10.1016/j.annonc.2021.08.2090 [DOI] [Google Scholar]
- 30. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol (2018) 36(24):2465–72. doi: 10.1200/JCO.2018.78.9909 [DOI] [PubMed] [Google Scholar]
- 31. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med (2020) 382(6):514–24. doi: 10.1056/NEJMoa1911149 [DOI] [PubMed] [Google Scholar]
- 32. Slamon DJ, Neven P, Chia S, Jerusalem G, De Laurentiis M, Im S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol (2021) 32(8):1015–24. doi: 10.1016/j.annonc.2021.05.353 [DOI] [PubMed] [Google Scholar]
- 33. Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol (2018) 19(7):904–15. doi: 10.1016/S1470-2045(18)30292-4 [DOI] [PubMed] [Google Scholar]
- 34. Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med (2019) 381(4):307–16. doi: 10.1056/NEJMoa1903765 [DOI] [PubMed] [Google Scholar]
- 35. Sledge GW, Jr., Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol (2017) 35(25):2875–84. doi: 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]
- 36. Sledge GW, Jr., Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: A randomized clinical trial. JAMA Oncol (2020) 6(1):116–24. doi: 10.1001/jamaoncol.2019.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol (2017) 35(32):3638–46. doi: 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 38. Johnston S, Martin M, Di Leo A, Im SA, Awada A, Forrester T, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer (2019) 5:5. doi: 10.1038/s41523-018-0097-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnston S, O'Shaughnessy J, Martin M, Huober J, Toi M, Sohn J, et al. Abemaciclib as initial therapy for advanced breast cancer: MONARCH 3 updated results in prognostic subgroups. NPJ Breast Cancer (2021) 7(1):80. doi: 10.1038/s41523-021-00289-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang QY, Sun T, Yin YM, Li HP, Yan M, Tong ZS, et al. MONARCH plus: abemaciclib plus endocrine therapy in women with HR+/HER2- advanced breast cancer: the multinational randomized phase III study. Ther Adv Med Oncol (2020) 12:1758835920963925. doi: 10.1177/1758835920963925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hamilton E, Cortes J, Ozyilkan O, Chen SC, Petrakova K, Manikhas A, et al. nextMONARCH: Abemaciclib monotherapy or combined with tamoxifen for metastatic breast cancer. Clin Breast Cancer (2021) 21(3):181–90.e2. doi: 10.1016/j.clbc.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 42. Xu B, Zhang Q, Zhang P, Hu X, Li W, Tong Z, et al. Dalpiciclib versus placebo plus fulvestrant in HR+/HER2- advanced breast cancer that relapsed or progressed on previous endocrine therapy (DAWNA-1): A multicenter, randomized, phase 3 study. J Clin Oncol (2021) 39(15_suppl):1002. doi: 10.1200/JCO.2021.39.15_suppl.1002 [DOI] [Google Scholar]
- 43. André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med (2019) 380(20):1929–40. doi: 10.1056/NEJMoa1813904 [DOI] [PubMed] [Google Scholar]
- 44. André F, Ciruelos EM, Juric D, Loibl S, Campone M, Mayer IA, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol (2021) 32(2):208–17. doi: 10.1016/j.annonc.2020.11.011 [DOI] [PubMed] [Google Scholar]
- 45. Lu YS, Lee KS, Chao TY, Tseng LM, Chitapanarux I, Chen SC, et al. A phase ib study of alpelisib or buparlisib combined with tamoxifen plus goserelin in premenopausal women with HR-positive HER2-negative advanced breast cancer. Clin Cancer Res (2021) 27(2):408–17. doi: 10.1158/1078-0432.CCR-20-1008 [DOI] [PubMed] [Google Scholar]
- 46. Baselga J, Im SA, Iwata H, Cortés J, De Laurentiis M, Jiang Z, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2017) 18(7):904–16. doi: 10.1016/S1470-2045(17)30376-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Campone M, Im SA, Iwata H, Clemons M, Ito Y, Awada A, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant for postmenopausal, hormone receptor-positive, human epidermal growth factor receptor 2-negative, advanced breast cancer: Overall survival results from BELLE-2. Eur J Cancer (2018) 103:147–54. doi: 10.1016/j.ejca.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 48. Di Leo A, Johnston S, Lee KS, Ciruelos E, Lønning PE, Janni W, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2018) 19(1):87–100. doi: 10.1016/S1470-2045(17)30688-5 [DOI] [PubMed] [Google Scholar]
- 49. Dent S, Cortés J, Im YH, Diéras V, Harbeck N, Krop IE, et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: the SANDPIPER trial. Ann Oncol (2021) 32(2):197–207. doi: 10.1016/j.annonc.2020.10.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oliveira M, Baird RD, Voorthuis RAB, De Boo L, van Rossum AGJ, Garrigos Cubells L, et al. LBA18 POSEIDON randomized phase II trial: Tamoxifen (TAM) + taselisib or placebo (PLA) in patients (pts) with hormone receptor positive (HR+)/HER2- metastatic breast cancer (MBC). Ann Oncol (2021) 32:S1291–S2. doi: 10.1016/j.annonc.2021.08.2091 [DOI] [Google Scholar]
- 51. Krop IE, Mayer IA, Ganju V, Dickler M, Johnston S, Morales S, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol (2016) 17(6):811–21. doi: 10.1016/S1470-2045(16)00106-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jones RH, Casbard A, Carucci M, Cox C, Butler R, Alchami F, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): A multicentre, randomised, controlled, phase 2 trial. Lancet Oncol (2020) 21(3):345–57. doi: 10.1016/S1470-2045(19)30817-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med (2012) 366(6):520–9. doi: 10.1056/NEJMoa1109653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2†. Ann Oncol (2014) 25(12):2357–62. doi: 10.1093/annonc/mdu456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero JM, Freyer G, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol (2012) 30(22):2718–24. doi: 10.1200/JCO.2011.39.0708 [DOI] [PubMed] [Google Scholar]
- 56. Schmid P, Zaiss M, Harper-Wynne C, Ferreira M, Dubey S, Chan S, et al. Fulvestrant plus vistusertib vs fulvestrant plus everolimus vs fulvestrant alone for women with hormone receptor-positive metastatic breast cancer: The MANTA phase 2 randomized clinical trial. JAMA Oncol (2019) 5(11):1556–64. doi: 10.1001/jamaoncol.2019.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kornblum N, Zhao F, Manola J, Klein P, Ramaswamy B, Brufsky A, et al. Randomized phase II trial of fulvestrant plus everolimus or placebo in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer resistant to aromatase inhibitor therapy: Results of PrE0102. J Clin Oncol (2018) 36(16):1556–63. doi: 10.1200/JCO.2017.76.9331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fan Y, Sun T, Shao Z, Zhang Q, Ouyang Q, Tong Z, et al. Effectiveness of adding everolimus to the first-line treatment of advanced breast cancer in premenopausal women who experienced disease progression while receiving selective estrogen receptor modulators: A phase 2 randomized clinical trial. JAMA Oncol (2021) 7(10):e213428. doi: 10.1001/jamaoncol.2021.3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jeong JH, Kim JE, Ahn JH, Jung KH, Koh SJ, Cheon J, et al. Leuprorelin combined with letrozole with/without everolimus in ovarian-suppressed premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer: The LEO study. Eur J Cancer (2021) 144:341–50. doi: 10.1016/j.ejca.2020.11.044 [DOI] [PubMed] [Google Scholar]
- 60. Jeong H, Jeong JH, Kim JE, Ahn JH, Jung KH, Koh SJ, et al. Final results of the randomized phase 2 LEO trial and bone protective effects of everolimus for premenopausal hormone receptor-positive, HER2-negative metastatic breast cancer. Int J Cancer (2021). doi: 10.1002/ijc.33613 [DOI] [PubMed] [Google Scholar]
- 61. Allemani C, Minicozzi P, Berrino F, Bastiaannet E, Gavin A, Galceran J, et al. Predictions of survival up to 10 years after diagnosis for European women with breast cancer in 2000-2002. Int J Cancer (2013) 132(10):2404–12. doi: 10.1002/ijc.27895 [DOI] [PubMed] [Google Scholar]
- 62. Burstein HJ, Somerfield MR, Barton DL, Dorris A, Fallowfield LJ, Jain D, et al. Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline update. J Clin Oncol (2021) 39(35):3959–77. doi: 10.1200/JCO.21.01392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Elfgen C, Bjelic-Radisic V. Targeted therapy in HR+ HER2- metastatic breast cancer: Current clinical trials and their implications for CDK4/6 inhibitor therapy and beyond treatment options. Cancers (Basel) (2021) 13(23):5994. doi: 10.3390/cancers13235994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Petrelli F, Ghidini A, Pedersini R, Cabiddu M, Borgonovo K, Parati MC, et al. Comparative efficacy of palbociclib, ribociclib and abemaciclib for ER+ metastatic breast cancer: an adjusted indirect analysis of randomized controlled trials. Breast Cancer Res Treat (2019) 174(3):597–604. doi: 10.1007/s10549-019-05133-y [DOI] [PubMed] [Google Scholar]
- 65. Leung JH, Leung HWC, Wang SY, Huang SS, Chan ALF. Efficacy and safety of CDK4/6 and PI3K/AKT/mTOR inhibitors as second-line treatment in postmenopausal patients with hormone receptor-positive, HER-2-negative metastatic breast cancer: a network meta-analysis. Expert Opin Drug Saf (2021) 20(8):949–57. doi: 10.1080/14740338.2021.1931116 [DOI] [PubMed] [Google Scholar]
- 66. Desnoyers A, Nadler MB, Kumar V, Saleh R, Amir E. Comparison of treatment-related adverse events of different cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: A network meta-analysis. Cancer Treat Rev (2020) 90:102086. doi: 10.1016/j.ctrv.2020.102086 [DOI] [PubMed] [Google Scholar]
- 67. Onesti CE, Jerusalem G. CDK4/6 inhibitors in breast cancer: differences in toxicity profiles and impact on agent choice. a systematic review and meta-analysis. Expert Rev Anticancer Ther (2021) 21(3):283–98. doi: 10.1080/14737140.2021.1852934 [DOI] [PubMed] [Google Scholar]
- 68. Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs (2014) 32(5):825–37. doi: 10.1007/s10637-014-0120-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marra A, Curigliano G. Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast Cancer (2019) 5:27. doi: 10.1038/s41523-019-0121-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tankova T, Senkus E, Beloyartseva M, Borštnar S, Catrinoiu D, Frolova M, et al. Management strategies for hyperglycemia associated with the α-selective PI3K inhibitor alpelisib for the treatment of breast cancer. Cancers (2022) 14(7):1598. doi: 10.3390/cancers14071598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nunnery SE, Mayer IA. Management of toxicity to isoform α-specific PI3K inhibitors. Ann Oncol (2019) 30(Suppl_10):x21–x6. doi: 10.1093/annonc/mdz440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee JS, Yost SE, Yuan Y. Neoadjuvant treatment for triple negative breast cancer: Recent progresses and challenges. Cancers (Basel) (2020) 12(6):1404. doi: 10.3390/cancers12061404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mayer IA, Prat A, Egle D, Blau S, Fidalgo JAP, Gnant M, et al. A phase II randomized study of neoadjuvant letrozole plus alpelisib for hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer (NEO-ORB). Clin Cancer Res (2019) 25(10):2975–87. doi: 10.1158/1078-0432.CCR-18-3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Royce M, Bachelot T, Villanueva C, Özgüroglu M, Azevedo SJ, Cruz FM, et al. Everolimus plus endocrine therapy for postmenopausal women with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: A clinical trial. JAMA Oncol (2018) 4(7):977–84. doi: 10.1001/jamaoncol.2018.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zardavas D, Tryfonidis K, Goulioti T, Piccart M. Targeted adjuvant therapy in breast cancer. Expert Rev Anticancer Ther (2016) 16(12):1263–75. doi: 10.1080/14737140.2016.1247698 [DOI] [PubMed] [Google Scholar]
- 76. Liu J, Duan Z, Guo W, Zeng L, Wu Y, Chen Y, et al. Targeting the BRD4/FOXO3a/CDK6 axis sensitizes AKT inhibition in luminal breast cancer. Nat Commun (2018) 9(1):5200. doi: 10.1038/s41467-018-07258-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cretella D, Ravelli A, Fumarola C, La Monica S, Digiacomo G, Cavazzoni A, et al. The anti-tumor efficacy of CDK4/6 inhibition is enhanced by the combination with PI3K/AKT/mTOR inhibitors through impairment of glucose metabolism in TNBC cells. J Exp Clin Cancer Res (2018) 37(1):72. doi: 10.1186/s13046-018-0741-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell (2014) 26(1):136–49. doi: 10.1016/j.ccr.2014.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bardia A, Hurvitz SA, DeMichele A, Clark AS, Zelnak A, Yardley DA, et al. Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR(+)/HER2(-) advanced breast cancer after progression on CDK4/6 inhibitors (TRINITI-1). Clin Cancer Res (2021) 27(15):4177–85. doi: 10.1158/1078-0432.CCR-20-2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full search strategy in (A) PubMed, (B) Embase and (C) the Cochrane Library.
Overall comparisons between CKD4/6 inhibitors or PI3K/AKT/mTOR inhibitors and endocrine therapy for PFS (A, B) and OS (C, D).
Comparisons between CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors when stratified by treatment lines: (A) for first-line PFS, (B) for second-line PFS, (C) for first-line OS and (D) for second-line OS.
PFS comparisons between CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors in visceral (A) and non-visceral (B) metastasis subgroups.
PFS comparisons between CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors in bone-only metastasis subgroup.
PFS comparisons between CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors in liver metastasis subgroup.
Time to first subsequent chemotherapy comparisons between CDK4/6 inhibitors and PI3K inhibitors.
≥3 grade neutropenia comparisons within CDK4/6 inhibitors.
All grade stomatitis comparisons within PI3K/AKT/mTOR inhibitors.
All grade nausea comparisons within PI3K/AKT/mTOR inhibitors.
All grade anorexia comparisons within PI3K/AKT/mTOR inhibitors.
All grade elevated ALT concentrations comparison among all treatments.
All grade elevated AST concentrations comparison among all treatments.
All grade diarrhea comparisons among all treatments.
All grade hyperglycemia comparisons among all treatments.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.