Abstract
Poultry production was long plagued by coccidiosis, and the development of alternative therapies will make practical sense. In this work, 2 battery experiments were designed. In battery experiment 1, the best effect of 7 anticoccidial herbs (Sophora japonica Linn, Citrus aurantium L, leaf of Acer palmatum, bark of Magnolia officinalis, fruit peel of Punica granatum L., Eclipta prostrata L., and Piper sarmentosum Roxb.) against Eimeria tenella infection of 21-day-old male Chinese Guangxi yellow-feathered chickens were screened out by clinic indexes (bloody feces scores, cecal lesion scores, oocysts output, relative weight gain rate, and survival rate). According to the results from battery experiment 1 and other literature research, we selected 2 monomers which were extracted from fruit peel of Punica granatum L. for further battery experiment 2 which were similar with battery experiment 1. Clinic results showed that Punicalagin had better anticoccidial effect than Ellagic acid. The anticoccidial mechanism exploration results of Elisa, antioxidant test, and pathological observation showed that Punicalagin reduced the cecal inflammation, improved the expression of immunoglobulin in cecal tissue, improved cecal integrity, and restored its REDOX state. Results of 16S rRNA sequencing analysis showed that Punicalagin also maintained the fecal flora health during E. tenella infection through insignificantly increasing the proportion of Lactobacillus and Faecalibacterium as well as significantly reducing the proportion of pathogenic bacteria, Escherichia–Shigella. RNA-Seq analysis results suggested that Punicalagin may play a role in controlling E. tenella infection by interaction with cytochrome P450 family enzymes. Overall, Punicalagin has promising potential as an alternative therapy for chicken Eimeria tenella infection.
Key words: Eimeria tenella, anticoccidial herb, fruit peel of Punica granatum L., Punicalagin
INTRODUCTION
As is known to all, poultry production is plagued by various infectious diseases such as coccidiosis, avian influenza, new-castle disease, and other problems (Quiroz-Castaneda and Dantan-Gonzalez, 2015). The characteristic of domestic fowl coccidiosis is an infectious protozoa disease which caused by intestinal parasite Eimeria. Seven species of Eimeria are notorious as infecting chickens (McDonald and Shirley, 2009). E. tenella was recognized as one of the most pathogenic specie in poultry (Joyner and Long, 2008) and it mainly affects the cecal tissue of chicken. E. tenella causes hemorrhagic enteritis, bloody diarrhea, susceptible weight gains, and gives rise to significant economic losses in this industry all over the world. Though, virtuous husbandry can help to reducing the risk of infection and control the disease (McDonald and Shirley, 2009). During practice, the use of alone or the combination of several drugs has significant effect against disease. However, the problems are increasing due to prolonged drug usage such as drug resistant and their residue in meet and egg (Peek and Landman, 2003). Vaccine is also another preventive approach for controlling the disease, but in practice, many practical problems have been exposed during vaccination, such as the difficulty in keeping the vaccine, the shortcomings of the outbreak of coccidiosis on condition that using incorrectly, and also their high cost (Blake et al., 2017). For these reasons, alternative strategies were needed for safer control to coccidiosis.
Actually, research about alternative strategies, especially for medicinal plants and herbal monomer as natural herbal remedies on controlling coccidiosis, has never been stopped. In the past 10 years, few studies were screened out for detecting the anticoccidial efficacy of medicinal herbs. Though, nowadays a lot of researches have been studied to perceive the anticoccidial mechanism of whole plants or extracts and active components of specific herb, which showed many plants having been found to have anticoccidial effect (Quiroz-Castañeda and Dantán-González, 2015; Muthamilselvan et al., 2016).Therefore, comparative trials were needed to determine whether these herbal remedies are reliable or worthy for possible approach to control this infection. On this basis, we also strived for find out the active monomers of an effective herb.
An herb has certain anticoccidial effects, which is probably due to its one or more functional monomers. The extraction of different herbal monomers involves the use of solvents of different polarity. It is very high costs (time cost, labor and material resources, more experimental animals) to do such operations in the early stage of the experiment. Therefore, a simple, convenient, and feasible preliminary method about screening the effect herb was to feed the herb powder to target animal directly. Then, literature research and analysis were conducted on the most effective herb. Finally, the potential monomers from this herb were selected for a second animal experiment to screen out the most effective monomer.
In this work, 2 battery experiments were designed. In battery experiment 1, we investigated the comparative anticoccidial effect of 7 different herb powders (flower bud of Sophora japonica Linn, Citrus aurantium L, leaf of Acer palmatum, bark of Magnolia officinalis, fruit peel of Punica granatum L., Eclipta prostrata L., and Piper sarmentosum Roxb.) which were 4% mixed in feed. Their anticoccidial effect was compared by clinical parameters (the survival rate, change of body weight, caecal lesion score, and the counts of oocysts in feces) during E. tenella infection. In battery experiment 2, the anticoccidial activity of 2 main active monomers (Ellagic acid and Punicalagin) from fruit peel of Punica granatum L. was compared by the clinical parameters, cecal histopathological observation, cecal protein indexes (IL-1β, IL-2, IL-6, IL-10, IL-21, TNF-α, IgG, IgM, IgA, sIgA, claudin-1, occuludin, ZO-1, SOD, CAT, and GPx), and antioxidant indices of cecal tissue (T-AOC, MDA) during E. tenella infection. Finally, 16S rRNA sequencing analysis of fecal microbiome and RNA-Seq analysis of cecal tissue were also used to study the anticoccidial mechanisms of Punicalagin which was the screened effective herbal monomer against E. tenella.
MATERIALS AND METHODS
Herbs and Medicines
Seven herbs (flower bud of Sophora japonica Linn, Citrus aurantium L, leaf of Acer palmatum, bark of Magnolia officinalis, fruit peel of Punica granatum L., Eclipta prostrata L., and Piper sarmentosum Roxb.) were purchased from Guangxi Taihua Pharmaceutical Co., Ltd., Nanning, Guangxi province, China. These 7 herbs were grounded into powder and passed through a 400-mesh sieve and stored at room temperature until use. Diclazuril, a commercial chemical anticoccidial drug, was purchased from Bolai Pharmacy Co., Ltd. (20190736345), Jiujiang, Jiangxi province, China. Ellagic acid (95%, CAS: 476-66-4) and Punicalagin (95%, CAS: 65995-63-3) were purchased from Shaanxi Liu Jiahui Biotechnology Co., Ltd., China.
Animals and Parasites
One-day-old male Chinese Guangxi yellow-feathered chickens were obtained from Fu Feng Poultry Company, Nanning, Guangxi province, China, and fed in the non-Eimeria conditions (unlimited feed and water supplies) in the animal house of Guangxi University. The animal experiments were strictly carried out according to the Guangxi University Animal Care and Use Committee guidelines. The information of the diet was shown in Table 1.
Table 1.
Basal diet information.1
| Composition | % |
|---|---|
| Crude protein | ≥17.0 |
| Crude fiber | ≤25.0 |
| Crude ash | ≤30.0 |
| Total phosphorus | ≥0.3 |
| Calcium | 0.6–1.5 |
| Sodium chloride | 0.3–0.8 |
| Water | ≤13.0 |
| Methionine | 0.2–0.7 |
Main raw materials of feed: corn, wheat bran, fish meal, soybean meal, methionine, vitamin A, vitamin D3, vitamin E, vitamin B12, calcium hydrogen phosphate, calcium carbonate, ferrous sulfate, copper sulfate, zinc sulfate, manganese sulfate, potassium sulfate, sodium selenite, sodium chloride, and edible oil.
Guangxi strain of E. tenella was separated and purified from feces of a coccidiosis outbreak chicken farm in 2017, Nanning, Guangxi province, China. Then the oocysts were propagated, isolated, sporulated, and storage at 4°C according to the standard procedures (Raether et al., 1995).
For inoculation, the oocysts in potassium dichromate were washed with distilled water for 2 times and then adjusted to 10,000 oocysts per mL of distilled water by improved McMaster method (Haug et al., 2006).
Battery Experimental Protocol
As shown in Table 2, 2 animal battery experiments were conducted.
Table 2.
Design of battery experiments.
| Groups | E. tenella infection | Intervention | Drug type | Intervention methods |
|---|---|---|---|---|
| Experiment 1 | ||||
| G1 | - | - | - | - |
| G2 | + | - | - | - |
| G3 | + | Diclazuril | Chemical synthetic drugs | Given from the day of E. tenella inoculation, 0.15 mL/L in water |
| G4 | + | flower bud of Sophora japonica Linn | Natural or herbal medicine | Given before one day of E. tenella inoculation, grounded into powders, 4% in feed |
| G5 | + | Citrus aurantium L. | ||
| G6 | + | leaf of Acer palmatum | ||
| G7 | + | bark of Magnolia officinalis | ||
| G8 | + | fruit peel of Punica granatum L. | ||
| G9 | + | Eclipta prostrata L. | ||
| G10 | + | Piper sarmentosum Roxb. | ||
| Experiment 2 | ||||
| C | - | - | - | - |
| E | + | - | - | - |
| D | + | Diclazuril | Chemical synthetic drugs | Given from the day of E. tenella inoculation, 0.15 mL/L in water |
| Ea | + | Ellagic acid | Main active monomers of fruit peel of Punica granatum L. | Given before one day of E. tenella inoculation, 0.4% in feed |
| P | + | Punicalagin | ||
In experiment 1, 240 chickens were randomly divided into 10 groups with 24 birds in each group. Each group was divided into 3 cages with 8 chickens in each cage. Groups were healthy control group (G1), E. tenella infected and un-intervened group (G2), and E. tenella infected and Diclazuril intervened (0.15 mL/L in water) group (G3). Other groups, group 4 to group 10 (G4–G10) were E. tenella infected and herb intervened (4% in feed of 7 different kinds of herbs: Sophora japonica Linn, Citrus aurantium L, leaf of Acer palmatum, bark of Magnolia officinalis, fruit peel of Punica granatum L., Eclipta prostrata L., and Piper sarmentosum Roxb.) groups, relatively. Except for the G1, all the birds at 21 days-old in other 9 groups were orally administered with 10,000 infective oocysts. Diclazuril was given at the day of E. tenella inoculation but herbs were given before 1 d of E. tenella inoculation (at 20 days-old). At 8 d postinfection (PI), birds were euthanized for the purpose of cecum collection and lesion scoring.
In experiment 2, 120 chickens were randomly divided into 5 groups with 24 birds in each group. Each group was divided into 3 cages with 8 chickens in each cage. They were healthy control group (C), E. tenella infected and un-intervened group (E), E. tenella infected and Diclazuril intervened (0.15 mL/L in water) group (D), E. tenella infected and Ellagic acid intervened (0.4% in feed) group (Ea), and E. tenella infected and Punicalagin intervened (0.4% in feed) group (P). Except for the C group, all the birds at 21 days-old in other 4 groups were orally administered with 10,000 infective oocysts. Diclazuril was given at the day of E. tenella inoculation but Ellagic acid and Punicalagin were given before 1 d of E. tenella inoculation (at 20 days-old). At day 8 PI, birds were euthanized for the purpose of cecum collection and lesion scoring.
Clinical Parameters
At 8 d PI, birds were euthanized by cervical dislocation for cecal lesion scoring and cecal tissue collection. During the whole experiment, the death number of chickens was recorded. Each chicken in each group was weighed before E. tenella inoculation and at the end (8 d PI) of the trial.
Each chicken was individually weighed for the first time (W1) at 21 days-old. Whereafter, each chicken was individually weighed again (W2) at the end of the animal experiment. Relative weight gain = (W2 - W1) / W1 × 100%.
Cecal lesion scores were recorded from 0 (normal) to 4 (severe) according to the macroscopical appearance of the intestine (Johnson and Reid, 1970). Compare the total average cecal lesion scores of each group.
At 5, 5.5, 6, 6.5, 7, 7.5, and 8 d PI, bloody feces score were recorded from 0 (normal) to 4 (severe): zero is the normal status, and less than 25%, 26–50%, 51–75%, or over 75% bloody feces in total feces (Youn and Noh, 2001). Compare the total average bloody feces scores of each group.
Fresh fecal samples were collected at 6.5, 7, 7.5, and 8 d PI for counting the oocysts excretion according to the improved method of McMaster (Haug et al., 2006). Briefly, take 1 g of feces and add with saturated sodium chloride aqueous solution till the volume of 30 mL then mix thoroughly. Transfer the liquid through a pipette into the McMaster chambers, then count and calculate the average number (N) of the oocysts in each chamber. Weight of feces (Wf) represents the total weight of collected feces in every indicated time. Since the volume of each McMaster chamber is 0.15 ml, so the oocysts excretion value = (30 / 0.15) × N / Wf. Compare the total average oocysts in per gram feces (OPG) value of each group. Besides, oocyst decrease ratio was calculated as: (OEui – OEdi) / OEui × 100%. In the calculation method above, the total average OPG of E. tenella infected and un-intervened groups was marked as OEui, and the total average OPG of E. tenella infected and drug intervened groups was marked as OEdi, respectively.
Histopathological Observation
After obtained the cecal lesion scores, other cecal samples of battery experiment 2 were fixed with neutral buffered formalin (10%) which was 20 times larger than that of the sample volume at room temperature for 18 h, then remove the liquid and replace it with the same formalin. After 48 h, samples were dehydrated, embedded with paraffin, and then cut into 4 μm thick cross sections, and then stained with hematoxylin and eosin for histopathological examination.
Elisa Assay of Target Protein Expression in the Cecal Tissue
After counted the cecal lesion scores in battery experiment 2, cecal tissues were collected and carefully rinsed with PBS. Analysis of SOD, GSH-Px, CAT, IL-1β, IL-2, IL-6, IL-10, IL-21, TNF-α, ZO-1, claudin-1, occuludin, IgA, IgG, IgM, and sIgA in cecal samples were performed according to commercially Elisa kit manual (BYabscience Biotechnelogy Co., Ltd., China).
Antioxidant Capacity Assay of Cecal Tissue
After counted the cecal lesion scores in battery experiment 2, cecal T-AOC and MDA were assessed by chemical detection method which were performed according to commercially kit manual (BYabscience Biotechnelogy Co., Ltd., China).
16S rRNA Sequencing of Fresh Feces
To obtain the fresh feces samples, a new container of feces in each cage was replaced in battery experiment 2 at 3 h before the birds were euthanized. After obtained the last bloody feces score, fresh feces from each independent cage were thoroughly mixed, and 20 small pieces from different positions of these mixed fresh feces were re-mixed and transferred to a tube. Finally, 9 tubes of re-mixed feces samples from 3 groups (C, E, and P group, 3 cages of each group) were used for 16S rRNA sequencing.
After finishing the DNA extraction and DNA integrity verification by using E.Z.N.A. soil DNA Kit (Omega Bio-Tek, Norcross, GA), high-throughput 16Sr DNA sequencing was done by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). TransStart Fastpfu DNA Polymerase was used for PCR and PCR instrument was ABI GeneAmp 9700. The NEXTFLEX Rapid DNA-SEQ Kit was used for library construction, and the Miseq PE300 platform of Illumina was used for sequencing. Data optimization was done by fastp (https://github.com/OpenGene/fastp, version 0.20.0) software for quality control of the original sequencing sequence. FLASH (http://www.cbcb.umd.edu/software/flash, version 1.2.7) software was use for splicing. All data analysis operations in this section were performed on the cloud platform (super computer platform of Majorbio Bio-Pharm Technology Co., Ltd. https://cloud.majorbio.com). Comparison methods between groups were: E vs C, P vs E.
RNA-Seq Assay of Cecal Tissue
In battery experiment 2, total 9 cecal samples (C, E, and P group, 3 cecal samples in each group) were used for RNA-Seq assay.
Total RNA was extracted separately by a Total RNA Isolation System (Promega) according to the manufacturer's protocol. The quality of the RNA samples was examined using the Agilent 2100 Bioanalyzer. Library construction and Illumina sequencing was performed by Novogene China. An RNA-seq analysis was performed according to the protocol recommended by the manufacturer (Illumina Inc., San Diego, CA) (Qiao et al., 2016; Liu et al., 2019).
RNA-Seq reads were mapped to the reference genome of Gallus gallus (http://www.ebi.ac.uk/ena/data/view/GCA_000002315.5) for data analysis. Differentially expressed genes (DEG) were identified by edgeR package based on Genes with 2 criterions: |log2(fold change)| > 0.58499 (|fold change| > 1.50003) and P < 0.05. Comparison method between groups were: E vs C, P vs E.
Quantitative Real-Time PCR
To ensure the accuracy of RNA-seq data, 8 DEGs were randomly selected for quantitative real-time PCR (RT-PCR). The tested samples were the same samples of RNA-Seq assay as mentioned above. RT-PCR assay was carried out as described previously with some modifications (Park et al., 2019). The cDNA for qRT-PCR was synthesized using a QuantiTect Reverse Transcription Kit [Sangon Biotech (Shanghai) Co., Ltd.]. QRT-PCR was performed using specific primer pairs and iQTM SYBR Green supermix [Sangon Biotech (Shanghai) Co., Ltd.]. The primers were designed with the Primer-BLAST (NCBI) and shown in Table 3. The amplification and detection of PCR products were performed using CFX Connect Real-Time System (Bio-Rad). The thermal cycling conditions were as follows. After activation of the polymerase and a DNA denaturation step at 95°C, 40 amplification cycles were performed with a denaturation step at 95°C followed by an annealing and extension step at 55°C. 18S rRNA was selected as the housekeeping gene. All experiments were performed in triplicate with at least 3 independent experiments.
Table 3.
Primer sequences used for quantitative real-time PCR.
| Gene name | Primer sequence (5′->3′) | NCBI-Protein ID | Products (base pairs) |
|---|---|---|---|
| HSD11B1b | TGACTTCTTCAACGGGGACG TTGCAGAGTAGGGAGCAACG |
XP_417988 | 179 |
| CDH2 | AGCCCACGGAGTTTGTAGTG ATGCTGGTTCAGGGGTTAGC |
NP_001001615 | 87 |
| CYP2C45 | GATGCCTGAGGGACCAACTC GGGCCCATATTTCTCAGCGA |
NP_001001752 | 106 |
| IL-18 | GCTGGAATGCGATGCCTTTT TGGACGAACCACAAGCAACT |
NP_989939 | 85 |
| TNC | GATTAGACGCAGGGACGGAG CCAGGTGAGCTGGAAACCAT |
NP_990787 | 158 |
| CYP2J19 | CAACATTGTGCCGTTGGGAG CTCCTGTACTGGCCGTTCTC |
XP_422553 | 183 |
| PIPOX | GACTTCATTCTGGACCGGCA GAAGCGGCTGATAGCGAATG |
XP_001235036 | 165 |
| LOC776719 | CATCGTGCAGGTGTTTTGGG GCACAATTTCGGCCAGATCC |
XP_004948457 | 200 |
| 18S rRNA | AACAGCAGGCACACGTTAGT CCTTCGCTGAGGAAACGGAG |
NP_001264098.2 | 115 |
Data Statistical Analysis
Statistical analysis was performed using SPSS (version 16.0, SPSS Inc., Chicago, IL). The Differences of cecal lesion scores, bloody feces scores, oocysts in per gram feces value, Elisa values of target protein expression, antioxidant values of cecal tissue and quantitative real-time PCR values between groups were analyzed using 1-way analysis of variance followed by post hoc analysis using Tukey test. Differences were considered significant at P < 0.05.
RESULTS
Fruit Peel of Punica granatum L. had the Strongest Anticoccidial Effect in 7 Selected Herbs
The clinical results of battery experiment 1 were shown in Table 4. Chickens in G2 appeared typical clinical symptoms of E. tenella infection such as bloody diarrhea, cecal lesion, large number of oocysts in feces, and weight lost. All the intervened groups had alleviated clinical symptoms. Taken all these clinical indicators together, especially for the OPG value, an important indicator, the intervention of fruit peel of Punica granatum L. (G8) had better effects. Therefore, we decided to choose fruit peel of Punica granatum L. for deeper research.
Table 4.
Battery experiment 1: anticoccidial effects of different herbs in chickens infected with E. tenella.
| Groups | Bloody feces scores | Cecal lesion scores | Oocysts in per gram (OPG) (×105) | Oocyst decrease ratio (%) | Survival rate (%) | Relative weight gain rate (%) |
|---|---|---|---|---|---|---|
| G1 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | - | 100.00 | 100.00 |
| G2 | 3.57 ± 0.69e | 3.33 ± 0.47c | 3.55 ± 1.01d | 0.00 | 61.11 | 42.00 |
| G3 | 1.43 ± 0.09b | 1.92 ± 0.61b | 0.18 ± 0.13ab | 94.93 | 94.44 | 80.00 |
| G4 | 2.75 ± 0.20d | 2.33 ± 0.47bc | 1.36 ± 0.77c | 61.69 | 72.22 | 73.70 |
| G5 | 2.83 ± 0.24d | 2.33 ± 0.47bc | 0.71 ± 0.12 abc | 80.00 | 72.22 | 58.78 |
| G6 | 2.23 ± 0.29cd | 2.00 ± 0.82b | 0.96 ± 0.23bc | 72.96 | 77.78 | 47.83 |
| G7 | 2.25 ± 0.74cd | 2.33 ± 0.47bc | 0.75 ± 0.54 abc | 78.87 | 88.89 | 78.42 |
| G8 | 1.90 ± 0.15bc | 1.83 ± 0.37b | 0.51 ± 0.35abc | 85.63 | 88.89 | 75.00 |
| G9 | 2.33 ± 0.26cd | 2.00 ± 0.58b | 1.03 ± 0.54bc | 70.99 | 83.33 | 72.96 |
| G10 | 1.93 ± 0.21bc | 1.67 ± 0.75b | 1.09 ± 0.62c | 69.30 | 83.33 | 70.94 |
Note: values in a column with different superscripts (a–e) were significantly different (P < 0.05).
The Use of Punicalagin Had Better Clinical Indexes and Pathological Changes Than Ellagic Acid
After literature research, we selected 2 monomers (Ellagic acid and Punicalagin, the 2 most abundant in fruit peel of Punica granatum L.) for another animal experiment (battery experiment 2), and the clinical results was shown in Table 5. Taken these clinical indicators together, the intervention of Punicalagin (P group) had better effects than Ellagic acid (Ea group).
Table 5.
Battery experiment 2: anticoccidial effects of main active monomers of fruit peel of punica granatum l. in chickens infected with E. tenella.
| Groups | Bloody feces scores | Cecal lesion scores | Oocysts in per gram (OPG) (×105) | Oocyst decrease ratio (%) | Relative weight gain rate (%) | Survival rate (%) |
|---|---|---|---|---|---|---|
| C | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | - | 100.00 | 100.00 |
| E | 3.61 ± 0.86d | 3.46 ± 0.52d | 3.08 ± 0.82e | 0 | 48.45 | 70.21 |
| D | 1.34 ± 0.12b | 1.38 ± 0.37b | 0.15 ± 0.23b | 95.13 | 83.51 | 96.64 |
| Ea | 2.50 ± 0.57c | 2.83 ± 0.47c | 1.18 ± 0.56d | 61.69 | 61.84 | 76.82 |
| P | 1.65 ± 0.27bc | 1.67 ± 0.45b | 0.39 ± 0.38c | 87.34 | 76.44 | 90.22 |
Note: values in a column with different superscripts (a–e) were significantly different (P < 0.05).
Compared to the C group (Figure 1A), the infection of E. tenella (E group, Figure 1B) caused severe hemorrhage, structural damage of cecum mucosa, a large number of immature oocysts in the epithelial cells and lamina propria, and oocysts which intermixed with the intestinal contents. Figure 1C showed that D group had almost no pathological lesions and parasites. As shown in Figure 1D, there was hardly pathological difference between Ea group and E group. As compared to the group C, the intervention of Punicalagin (Figure 1E) had also no obvious pathological changes, but there were still some oocysts in mucosal layers and intestinal contents. These were consistent with the clinical results.
Figure 1.
Pathological observation of chickens in battery experiment 2. (A) Group C. (B) Group E. (C) Group D. (D) Group Ea. (E) Group P.
Punicalagin Showed Better Anticoccidial Efficacy Than Ellagic Acid on the Protein Indexes and Antioxidant Capacity
As shown in Figures 2A–2F, the treatment of Punicalagin significantly reversed the changes of inflammatory indexes (IL-1β, IL-2, IL-6, IL-10, IL-21, and TNF-α) by E. tenella. Moreover, there was no significant difference in other inflammatory indexes except IL-6 between the P group and the D group, so did they (IgG, IgM, and IgA, Figures 2G–2I). The infection of E. tenella significantly reduced the concentration of sIgA (Figure 2J) in cecum, and the intervention of Punicalagin completely reversed this decreasing trend (no significant difference between group C). In terms of tight junction protein (claudin-1, occuludin and ZO-1, Figures 2K–2M), there was no significant difference between group P and group C, indicating the good integrity of cecal mucosa of P group. The indexes of peroxidase (SOD, CAT, and GPx, Figures 2N–2P) and REDOX state (T-AOC and MDA, Figures 2Q and 2R) in P group were also excellent and mutually confirmed. In addition, there was no significant difference between Ea and P group in these results.
Figure 2.
Detection of protein expression and antioxidant activity of chicken ceca tissue in battery experiment 2. (A–P) The expression of proteins IL-1β, IL-2, IL-6, IL-10, IL-21, TNF-α, IgG, IgM, IgA, sIgA, Claudin-1, Occludin, ZO-1, SOD, CAT, and GPx were detected by Elisa. (Q, R) antioxidant capacities (T-AOC and MDA) were determined by chemical method.
Combined with all the data of battery experiment 2, both Ellagic acid and Punicalagin have the potential to further develop their anticoccidial applications, but Punicalagin's prospects may be brighter.
The Intervention of Punicalagin Significantly Changed the Diversity and Composition of Fecal Microflora
The raw 16Sr DNA data reported in this work have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2017) in BIG Data Center (Nucleic Acids Res 2019), Beijing Institute of Genomics (BIG), Chinese Academy of Sciences, under accession numbers CRA004508 that is publicly accessible at https://ngdc.cncb.ac.cn/gsa/browse/CRA004508.
To determine whether intervention of Punicalagin changed the microflora, we performed high-throughput gene sequencing of 16Sr DNA in the fecal bacteria DNA of chickens infected with E. tenella. As compared to the C group, the Shannon index of E group was significantly decreased. However, despite the improvement, the Shannon index in P group was not significantly higher than that in E group (Figure 3A). Figure 3B showed that at genus level and as compared to the control, the microbial diversity of fecal samples was decreased by the infection of E. tenella, and the intervention of Punicalagin restored this deterioration to a certain extent. After the infection of E. tenella, the proportion of Lactobacillus was downregulated and the Escherichia–Shigella was significantly increased as compared to the control. Also, the proportion of other microbiota was also significantly reduced (Figure 3C, Faecalibacterium, unclassified_f_Lachnospiraceae, Blautia, Candidatus_Arthromitus, Lachnospiraceae_NK4A136_group, Lachnoclostridium, norank_f_norank_o_Clostridia_UCG-014, Eisenbergiella, Ruminococcus_torques_group, norank_f_Eubacterium_coprostanoligenes_group, norank_f_Ruminococcaceae and Acinetobacter). As shown in Figure 3D, after the treatment of Punicalagin, the proportion of Lactobacillus was upregulated (P = 0.09938) and the Escherichia–Shigella as well as Romboutsia was significantly downregulated as compared to E group. Also, the proportion of Candidatus_Arthromitus, Kurthia, Acinetobacter, and Aerococcus was significantly upregulated.
Figure 3.
Results of 16S rRNA sequencing of fresh feces in battery experiment 2. (A) Shannon index assay of each group. (B) Microbial diversity detection on genus level. (C) Relative abundance (%) genus level, group C vs group E, one or more "*" indicates significant difference. (D) relative abundance (%) genus level, group E vs group P, one or more "*" indicates significant difference.
RNA-Seq Data Analysis
The raw RNA-Seq data reported in this work have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2017) in BIG Data Center (Nucleic Acids Res 2019), Beijing Institute of Genomics (BIG), Chinese Academy of Sciences, under accession numbers CRA004507 that is publicly accessible at https://ngdc.cncb.ac.cn/gsa/browse/CRA004507.
Identified by adjusted |log2(fold change)| > 0.58499 (|fold change| > 1.50003) and P value < 0.05, 13537 DEGs of E group with 3656 and 2473 genes were up- and downregulated, respectively, compared to the control (Figure 4A). The 13537 DEGs were processed by Gene Ontology (GO) enrichment analysis. As shown in the Figure 4C, the top 30 enriched GO terms of each category were listed. Ten GO terms were enriched in biological process, including positive regulation of immune system process, lipid catabolic process, regulation of immune response, inflammatory response, positive regulation of immune response, cytokine production, regulation of cytokine production, activation of immune response, immune response-regulating signaling pathway, and immune response-activating signal transduction. Ten GO terms were enriched in cellular component, including extracellular matrix, ruffle, actin filament, extracellular matrix component, extracellular organelle, peroxisome, microbody, proteinaceous extracellular matrix, cell surface, and basement membrane. In the category of molecular function, 10 GO terms (growth factor binding, monocarboxylic acid binding, fatty acid binding, endopeptidase activity, phosphatidylinositol phospholipase C activity, phospholipase C activity, actin binding, extracellular matrix binding, glycosaminoglycan binding, and cell adhesion molecule binding) were significantly enriched. Within the 30 enriched GO terms, there were 6 search results containing the keyword “immune” and 5 results containing “extracellular.” Thus, E. tenella may have significant effects on the immune system of infected birds. As shown in Figure 4E, the 13537 were mapped to the KEGG database, and the top 10 enriched KEGG pathways were: NOD-like receptor signaling pathway, metabolism of xenobiotics by cytochrome P450, carbon metabolism, cytokine–cytokine receptor interaction, pentose and glucuronate interconversions, citrate cycle (TCA cycle), valine, leucine and isoleucine degradation, drug metabolism—cytochrome P450, peroxisome, and AGE–RAGE signaling pathway in diabetic complications.
Figure 4.
Results of RNA-Seq of cecal tissue in battery experiment 2. (A) Differential expression level of group E and group C identified by |log2(fold change)| >0.58499 (|foldchange| >1.50003) and P < 0.05. (B) differential expression level of group P and group E identified by |log2(fold change)| >0.58499 (|foldchange| >1.50003) and P < 0.05. (C) Significantly enriched GO terms of differentially expressed genes, group E vs group C, BP, biological process; CC, cellular component; MF, molecular function. (D) Significantly enriched GO terms of differentially expressed genes, group P vs group E, BP, biological process; CC, cellular component; MF, molecular function. (E) Significantly enriched KEGG pathways of differentially expressed genes, group E vs group C. (F) Significantly enriched KEGG pathways of differentially expressed genes, group P vs group E.
By the same method of analysis (|fold change| > 1.50003 and P value < 0.05), 19005 DEGs of P group with 475 and 312 genes were up- and downregulated, respectively, compared to the E group (Figure 4B). The 19005 DEGs were also processed by GO enrichment analysis. As shown in the Figure 4D, the top 30 enriched GO terms of each category were listed. Ten GO terms were enriched in biological process, including negative regulation of locomotion, epithelial cell differentiation, negative regulation of cellular component movement, steroid metabolic process, urogenital system development, digestive tract development, cytokine metabolic process, ameboidal-type cell migration, digestive tract morphogenesis, and regulation of peptide secretion. Ten GO terms were enriched in cellular component, including microvillus, external side of plasma membrane, cell surface, actin-based cell projection, basolateral plasma membrane, basal part of cell, side of membrane, lateral plasma membrane, extracellular vesicle, and apical part of cell. In the category of molecular function, 10 GO terms (carboxylic acid binding, organic acid binding, receptor regulator activity, glycosaminoglycan binding, receptor ligand activity, glucuronosyltransferase activity, cell adhesion molecule binding, chemorepellent activity, cytokine activity, and RNA-directed DNA polymerase activity) were significantly enriched. Within the 30 enriched GO terms, 2 terms (“epithelial cell differentiation” and “microvillus”) suggested that the treatment of P improved the intestinal health and integrity. As shown in Figure 4F, the 19005 were mapped to the KEGG database, and the 4 significantly enriched KEGG pathways were: steroid hormone biosynthesis, cell adhesion molecules (CAM), linoleic acid metabolism, and cytokine–cytokine receptor interaction.
In Figure 5A, the infection of E. tenella significantly downregulated the mRNA expression of enzymes (CYP2CJ, CYP3A4) which catalyze the Linoleate to 9,10-12,13-Diepoxyoctadecanoate in linoleic acid metabolism pathway as compared to the control. However, the intervention of Punicalagin significantly reverse this trend (Figure 5B). In the pathway of arachidonic acid metabolism (Figures 5C and 5D), the mRNA expression of [EC:1.14.14.1], CYP2, [EC:1.14.99.1] also significantly reversed by the intervention of Punicalagin. As compared to the control, the infection of E. tenella significantly upregulated the mRNA expression of many proteins which involved in the cytokine–cytokine receptor interaction pathway (Figure 5E). After the intervention of Punicalagin, mRNA expression of CCR8, CCL19, CCL21, CCR5 IL-22, TNFSF15, CD40LG, SF8, TNFSF4, TNFSF13B, IL1R1, IL1RAP, and IL18 were significantly downregulated (Figure 5F). In the pathway of cell adhesion molecules, the infection of E. tenella significantly upregulated the mRNA expression of many proteins which included CLDN (Figure 5G). As compared to the E group, mRNA expressions of CLDN, OCLN, CDHE, PTPRF, and CDH3 were also significantly upregulated in P group (Figure 5H). In the pathway of steroid hormone biosynthesis, the mRNA expression of [EC:1.1.1.146], [EC:2.4.1.17], and [EC:1.14.14.1] was also reversed by the intervention of Punicalagin (Figure 5I and 5J).
Figure 5.
Significantly enriched KEGG pathways. (A, C, E, G, I) Group E vs Group C. (B, D, F, H, J) Group P vs Group E.
RT-PCR Validation
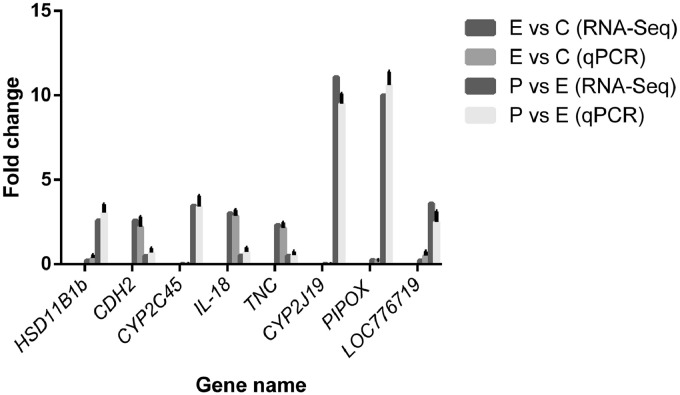
The RT-PCR results of 8 DEGs were highly similar to that of the RNA-Seq (Figure 6), indicating that the RNA-Seq analysis in this work was highly accurate and reliable.
Figure 6.
Validation of RNA-Seq data by quantitative real-time PCR. The data represent the means ± standard deviation of the results.
DISCUSSION
In the battery experiment 1, 7 herbs (Sophora japonica Linn, Citrus aurantium L, leaf of Acer palmatum, bark of Magnolia officinalis, fruit peel of Punica granatum L., Eclipta prostrata L., and Piper sarmentosum Roxb.) which were easily available in China were selected to test and compare their anticoccidial effect. Flower bud of Sophora japonica Linn (in G4) can be used for the treatment of hemorrhagic related diseases of intestine, such as hemorrhoids and hematochezia (; He et al., 2016). Citrus aurantium L. (in G5) can relieve the spasm of small intestinal and enhance the contractile rhythm of gastrointestinal tract and gallbladder (He et al., 2018). The leaf of Acer palmatum (in G6) can inhibit oxidative stress, improve intestinal barrier function, and reduce inflammation in mouse acute colitis model (Kim et al., 2018; Oh et al., 2018b). According to the ancient book shennong materia medica, bark of Magnolia officinalis (in G7) has effects on large intestine meridians and treating with parasites. Lee's pharmacological study also showed that bark of Magnolia officinalis can be used as an analgesic (Lee et al., 2011). It even worked on chicken Eimeria maxima infection with growth-promoting and antioxidant effects (Oh et al., 2018a). The fruit peel of Punica granatum L (in G8) have traditionally used to treat ordinary diarrhea and dysentery. The chemical composition and pharmacological effects of it have been well studied now, such as Casuarinin, Corilagin, Granatin-A, Tellimagrandin-I, Ellagic acid Punicalagin (Rahimi et al., 2012), anti-inflammatory and antibacterial activities (Shaygannia et al., 2016), and so on. Eclipta prostrata L. (in G9), a traditional herbal medicine, has long been used in Asia and South America for the therapy of hemorrhagic diseases (hemoptysis, hematemesis, hematuria, epistaxis and uterine bleeding) (Feng et al., 2019). The last herb is Piper sarmentosum Roxb. (in G10). Research showed that this herb also had anticoccidial effect (Memon et al., 2021). After consulting the searching results of major medical, veterinary related database (PubMed, Web of Science, Science Citation Index, and Google Scholar), this was the first comparative study of anticoccidial effects of different herbal powders in nearly a decade. Traditionally, extracting herbs with different solvents and feeding chickens in corresponding groups resulted in a significant increase experimental cost as well as just hard to succeed. Here, as a preliminary experiment with the ultimate goal of obtaining active anticoccidial monomers, we fed chickens with the ground herbal powders and according to all clinical indexes, especially for the OPG value which is an important indicator (since Eimeria spreads through the infectious oocyst, controlling the oocyst shedding in feces means basically controlling the source of infection), the fruit peel of Punica granatum L. had better anticoccidial activity and research prospects among these 7 herbs. In fact, there were several reports on the anticoccidial effect of fruit peel of Punica granatum L. (Dkhil, 2013; Amer et al., 2015; Ahad et al., 2018; Mohammed, et al., 2021; Khorrami et al., 2022). These were also the reasons why we chose this herb for further experimental work.
Ellagic acid and Punicalagin were the 2 monomers with the highest content in fruit peel of Punica granatum L. (Hernandez-Corroto et al., 2019). Therefore, these 2 monomers were recruited in this work. Study had shown that Ellagic acid in combination with Lactobacillus brevis 23017 had positive effects on coccidiosis infection (Yang et al., 2022a). However, there was no report about Punicalagin on Eimeria infection. In the clinical data of battery Experiment 2, Punicalagin had a better effect than Ellagic acid. Results of inflammatory indexes (IL-1β, IL-2, IL-6, IL-10, IL-21 and TNF-α) showed that Punicalagin has a better inflammation inhibition effect than Ellagic acid under the infection of E. tenella. These results were mutually verified with histopathological changes. The cecal immunoglobulin indexes indicated that the infection of E. tenella caused immunosuppression of the host tissue, and all the treatments moderated this trend. Intervention of Punicalagin restored the sIgA expression to normal, indicating that the organism had a better ability to cope with secondary infection at this time. The expression of intestinal tight junction proteins indicated that the intestinal integrity of group P was very high and the lesion degree was slight. The results were mutually verified with clinical data (cecal lesion scores and OPG) and histopathological changes. Study have shown that the infection of Eimeria is closely related to the change of REDOX state of the organism (Abdelhady et al., 2021).Research also showed that antioxidant therapy had potential benefits in treating coccidial infections (Naidoo et al., 2008). In this work, both antioxidant Ellagic acid (Kilic et al., 2014) and Punicalagin (Aguilar-Zarate et al., 2017) showed resistance to coccidiosis infection, and the data of antioxidant indexes were mutually confirmed with all the experimental results above. Here, we first reported that Punicalagin was an effective anticoccidial monomer in fruit peel of Punica granatum L.
After infected with E. tenella, the proportion of non-pathogenic bacteria, including Lactobacillus and Faecalibacterium declined, and the pathogenic bacteria Escherichia-Shigella increased as compared to the control. This trend was consistent with previous report (Zhou et al., 2020). Studies have shown that the intestinal healthy can be contributed by the proportion of Lactobacillus, and Faecalibacterium had anti-inflammatory properties in humans and murine models (Oakley et al., 2014; Yang et al., 2014). The intervention of Punicalagin insignificantly increased the proportion of these 2 microorganisms and significantly reduced the proportion of pathogenic bacteria, Escherichia–Shigella, suggesting a better integrity and intestinal health in group P. Adding the arginine to the feed improved the growth performance data of Chinese yellow-feathered chickens, and also the proportion of Candidatus_Arthromitus in ileum was increased (Ruan et al., 2020). Here, our data showed similar results. Acinetobacter and Aerococcus were pathogens (Rasmussen, 2016; Yang et al., 2022b) and were significantly increased by Punicalagin. This phenomenon needs further study. Although Kurthia was still poorly understood, recent reports suggest that Kurthia gibsonii is a novel opportunistic pathogen in poultry (Lozica et al., 2022). Here, the treatment of Punicalagin significantly enhanced the proportion of Kurthia. This may be an entry point to study Kurthia. Human study suggested that Romboutsia may play a key role in maintaining the host health (Mangifesta et al., 2018). However, in this work, the proportion of Romboutsia was significantly reduced after the intervention of Punicalagin. This result may support the role of Romboutsia, which needs further study.
Arachidonic acid is synthesized from linoleic acid and studded in biological cell membrane, and both of these 2 acids have many important biological activities (Meng et al., 2021). In this work, the expression of multi-CYP family genes in linoleic/Arachidonic acid metabolism pathways was disordered by E. tenella infection, and this trend was restored by Punicalagin. These results may indicate that CYP family is the entry point for the target study of Punicalagin intervention in E. tenella infection. After the intervention of Punicalagin, the mRNA expression of CCR8, CCL19, CCL21, CCR5 IL-22, TNFSF15, CD40LG, SF8, TNFSF4, TNFSF13B, IL1R1, IL1RAP, and IL18 were significantly downregulated, meanwhile the mRNA expressions of CLDN, OCLN, CDHE, PTPRF, CDH3 were significantly upregulated. These results suggest that chickens in group P had less inflammation and better intestinal integrity than that in group E, in other words, were consistent with Elisa and pathological observation.
Research have shown that Steroid hormone biosynthesis pathway is closely related to Cytochrome P450 oxidoreductase (Ghayee and Auchus, 2007). Here, the disordered expression of genes which involved in Steroid hormone biosynthesis pathway caused by E. tenella infection was restored by Punicalagin to a certain extent. We hypothesized that the biosynthesis disturbances caused by E. tenella infection of some steroid hormone (Testosterone glucuronide; Estrone glucuronide; Adrenosterone; Cortisone; 3alpha, 20alpha, 21-Trihydroxy-5beta-pregnan-11-one; 16alpha-Hydroxydehydroepiandrosterone) which are related to sexual maturation was restored by Punicalagin. And this effect was closely related to the interaction of Punicalagin and Cytochrome P450 family enzymes. Of course, if this is the case, E. tenella infection can also cause sexual maturation disorders in chickens.
CONCLUSION
In this work, according to the clinic indexes (bloody feces scores, cecal lesion scores, OPG, relative weight gain rate, and survival rate), fruit peel of Punica granatum L. was the best anticoccidial herb in total 7 tested herbs (Sophora japonica Linn, Citrus aurantium L, leaf of Acer palmatum, bark of Magnolia officinalis, fruit peel of Punica granatum L., Eclipta prostrata L. and Piper sarmentosum Roxb.). Subsequently, according to the literature research, we selected 2 monomers which were extracted from fruit peel of Punica granatum L. for further experiments. In another animal experiment, clinic results showed that Punicalagin had better anticoccidial effect than Ellagic acid. The results of Elisa, antioxidant test and pathological observation showed that Punicalagin reduced the cecal inflammation caused by coccidia, improved the expression of immunoglobulin in cecal tissue, improved cecal integrity and restored cecal REDOX state. Punicalagin also maintained the fecal flora health of E. tenella infected chickens through insignificantly increased the proportion Lactobacillus and Faecalibacterium as well as significantly reduced the proportion of pathogenic bacteria, Escherichia-Shigella. RNA-Seq analysis results suggested that Punicalagin may play a role in controlling E. tenella infection by interaction with Cytochrome P450 family enzymes. These results suggest that Punicalagin has potential as an alternative therapy for chicken clinical E. tenella infection.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All experimental protocols on animals associated with this study were reviewed and approved by the Guangxi University Animal Care and Use Committee, Nanning, China. The specific code for ethics approval is Gxu-2019-180.
ACKNOWLEDGMENTS
This work was supported by: Key Research and Development Project of Guangxi (19245037), Major R&D Project of Wuming District Nanning China (20210111) and TCM Industrial Pioneers (GuiNongKeMeng 202211). Yunqiao Yang and Xieying Ding contributed equally to this work and share the co-first author. Data supporting the conclusions of this article are included within the article. The datasets generated during and/or analyzed during the present study are available from the corresponding author upon reasonable request. The raw 16Sr DNA data reported in this work have been deposited at https://ngdc.cncb.ac.cn/gsa/browse/CRA004508. The raw RNA-Seq data reported in this work have been deposited at https://ngdc.cncb.ac.cn/gsa/browse/CRA004507.
DISCLOSURES
The authors declare that they have no conflict of interest.
REFERENCES
- Abdelhady A.Y., El-Safty S.A., Hashim M., Ibrahim M.A., Mohammed F.F., Elbaz A.M., Abdel-Moneim A.M.E. Comparative evaluation of single or combined anticoccidials on performance, antioxidant status, immune response, and intestinal architecture of broiler chickens challenged with mixed Eimeria species. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Zarate P., Wong-Paz J.E., Michel M., Buenrostro-Figueroa J., Diaz H.R., Ascacio J.A., Contreras-Esquivel J.C., Gutierrez-Sanchez G., Aguilar C.N. Characterisation of pomegranate-husk polyphenols and semi-preparative fractionation of punicalagin. Phytochem. Anal. 2017;28:433–438. doi: 10.1002/pca.2691. [DOI] [PubMed] [Google Scholar]
- Ahad S., Tanveer S., Malik T.A., Nawchoo I.A. Anticoccidial activity of fruit peel of Punica granatum L. Microb. Pathog. 2018;116:78–83. doi: 10.1016/j.micpath.2018.01.015. [DOI] [PubMed] [Google Scholar]
- Amer O.S.O., Dkhil M.A., Hikal W.M., Al-Quraishy S. Antioxidant and anti-inflammatory activities of pomegranate (Punica granatum) on Eimeria papillata-induced infection in mice. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/219670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.P., Pastor-Fernandez I., Nolan M.J., Tomley F.M. Recombinant anticoccidial vaccines - a cup half full? Infect. Genet. Evol. 2017;55:358–365. doi: 10.1016/j.meegid.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Dkhil M.A. Anti-coccidial, anthelmintic and antioxidant activities of pomegranate (Punica granatum) peel extract. Parasitol. Res. 2013;112:2639–2646. doi: 10.1007/s00436-013-3430-3. [DOI] [PubMed] [Google Scholar]
- Feng L., Zhai Y.Y., Xu J., Yao W.F., Cao Y.D., Cheng F.F., Bao B.H., Zhang L. A review on traditional uses, phytochemistry and pharmacology of Eclipta prostrata (L.) L. J. Ethnopharmacol. 2019;245 doi: 10.1016/j.jep.2019.112109. [DOI] [PubMed] [Google Scholar]
- Ghayee H.K., Auchus R.J. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev. Endocr. Metab. Disord. 2007;8:289–300. doi: 10.1007/s11154-007-9052-2. [DOI] [PubMed] [Google Scholar]
- Haug A., Williams R.B., Larsen S. Counting coccidial oocysts in chicken faeces: a comparative study of a standard McMaster technique and a new rapid method. Vet. Parasitol. 2006;136:233–242. doi: 10.1016/j.vetpar.2005.11.024. [DOI] [PubMed] [Google Scholar]
- He X.R., Bai Y.J., Zhao Z.F., Wang X.X., Fang J.C., Huang L.H., Zeng M., Zhang Q., Zhang Y.J., Zheng X.H. Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L.: a review. J. Ethnopharmacol. 2016;187:160–182. doi: 10.1016/j.jep.2016.04.014. [DOI] [PubMed] [Google Scholar]
- He W., Li Y., Liu M., Yu H., Chen Q., Chen Y., Ruan J., Ding Z., Zhang Y., Wang T. Citrus aurantium L. and Its flavonoids regulate TNBS-induced inflammatory bowel disease through anti-inflammation and suppressing isolated jejunum contraction. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19103057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Corroto E., Marina M.L., Garcia M.C. Extraction and identification by high resolution mass spectrometry of bioactive substances in different extracts obtained from pomegranate peel. J. Chromatogr. A. 2019;1594:82–92. doi: 10.1016/j.chroma.2019.02.018. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Joyner L.P., Long P.L. The specific characters of the Eimeria, with special reference to the coccidia of the fowl. Avian Pathol. 2008;3:145–157. doi: 10.1080/03079457409353827. [DOI] [PubMed] [Google Scholar]
- Khorrami P., Gholami-Ahangaran M., Moghtadaei-Khorasgani E. The efficacy of pomegranate peel extract on Eimeria shedding and growth indices in experimental coccidiosis in broiler chickens. Vet. Med. Sci. 2022;8:635–641. doi: 10.1002/vms3.714. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kilic I., Yesiloglu Y., Bayrak Y. Spectroscopic studies on the antioxidant activity of ellagic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014;130:447–452. doi: 10.1016/j.saa.2014.04.052. [DOI] [PubMed] [Google Scholar]
- Kim K.Y., Oh T.W., Do H.J., Yang J.H., Yang I.J., Jeon Y.H., Go Y.H., Ahn S.C., Ma J.Y., Park K.I. Acer palmatum thumb. Ethanol extract alleviates interleukin-6-induced barrier dysfunction and dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation. J. Immunol. Res. 2018;2018 doi: 10.1155/2018/5718396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Lee Y.M., Lee C.K., Jung J.K., Han S.B., Hong J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011;130:157–176. doi: 10.1016/j.pharmthera.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Liu G.F., Su H.Z., Sun H.Y., Lu G.T., Tang J.L. Competitive control of endoglucanase gene engXCA expression in the plant pathogen Xanthomonas campestris by the global transcriptional regulators HpaR1 and Clp. Mol. Plant. Pathol. 2019;20:51–68. doi: 10.1111/mpp.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozica L., Maljkovic M.M., Mazic M., Gottstein A. Kurthia gibsonii, a novel opportunistic pathogen in poultry. Avian Pathol. 2022;51:26–33. doi: 10.1080/03079457.2021.1993132. [DOI] [PubMed] [Google Scholar]
- Mangifesta M., Mancabelli L., Milani C., Gaiani F., de'Angelis N., de'Angelis G.L., van Sinderen D., Ventura M., Turroni F. Mucosal microbiota of intestinal polyps reveals putative biomarkers of colorectal cancer. Sci. Rep. 2018;8:13974. doi: 10.1038/s41598-018-32413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald V., Shirley M.W. Past and future: vaccination against Eimeria. Parasitology. 2009;136:1477–1489. doi: 10.1017/S0031182009006349. [DOI] [PubMed] [Google Scholar]
- Memon F.U., Yang Y., Soliman A.M., Lv F., Rajput N., Zhang G., Baig M.B., Wang Y., Si H. Dietary supplementation with Piper sarmentosum extract on gut health of chickens infected with Eimeria tenella. Trop. Anim. Health Prod. 2021;53:497. doi: 10.1007/s11250-021-02934-6. [DOI] [PubMed] [Google Scholar]
- Meng J., Ma N., Liu H., Liu J., Liu J., Wang J., He X., Zhao X. Untargeted and targeted metabolomics profiling reveals the underlying pathogenesis and abnormal arachidonic acid metabolism in laying hens with fatty liver hemorrhagic syndrome. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed L.S., Sallam E.A., El Basuni S.S., Eldiarby A.S., Soliman M.M., Aboelenin S.M., Shehata S.F. Ameliorative effect of neem leaf and pomegranate peel extracts in coccidial infections in New Zealand and V-Line rabbits: performance, intestinal health, oocyst shedding, carcass traits, and effect on economic measures. Animals-Basel. 2021;11:2441. doi: 10.3390/ani11082441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthamilselvan T., Kuo T.F., Wu Y.C., Yang W.C. Herbal remedies for coccidiosis control: a review of plants, compounds, and anticoccidial actions. Evid. Based Complement. Altern. Med. 2016;2016:1–19. doi: 10.1155/2016/2657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo V., Mcgaw L.J., Bisschop S.P.R., Duncan N., Eloff J.N. The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens. Vet. Parasitol. 2008;153:214–219. doi: 10.1016/j.vetpar.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Oakley B.B., Buhr R.J., Ritz C.W., Kiepper B.H., Berrang M.E., Seal B.S., Cox N.A. Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Vet. Res. 2014;10:282. doi: 10.1186/s12917-014-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S., Gadde U.D., Bravo D., Lillehoj E.P., Lillehoj H.S. Growth-promoting and antioxidant effects of magnolia bark extract in chickens uninfected or co-infected with Clostridium perfringens and Eimeria maxima as an experimental model of necrotic enteritis. Curr. Dev. Nutr. 2018;2:nzy009. doi: 10.1093/cdn/nzy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh T.W., Do H.J., Kim K.Y., Park K.I., Ma J.Y. Leaves of Acer palmatum thumb. Rescues N-ethyl-N-nitrosourea (ENU)-Induced retinal degeneration in mice. Phytomedicine. 2018;42:51–55. doi: 10.1016/j.phymed.2018.03.026. [DOI] [PubMed] [Google Scholar]
- Park J.Y., Jo S.K., Park K.M., Yu H., Bai J., Ryu S., Chang P.S. Transcriptomic analysis of Staphylococcus aureus under the stress condition of antibacterial erythorbyl laurate by RNA sequencing. Food Control. 2019;96:1–8. [Google Scholar]
- Peek H.W., Landman W.J.M. Resistance to anticoccidial drugs of Dutch avian Eimeria spp. field isolates originating from 1996, 1999 and 2001. Avian Pathol. 2003;32:391–401. doi: 10.1080/0307945031000121149. [DOI] [PubMed] [Google Scholar]
- Qiao Q., Huang Y.Y., Qi J., Qu M.Z., Jiang C., Lin P.C., Li R.H., Song L.R., Yonezawa T., Hasegawa M., Crabbe M.J.C., Chen F., Zhang T.C., Zhong Y. The genome and transcriptome of Trichormus sp NMC-1: insights into adaptation to extreme environments on the Qinghai-Tibet Plateau. Sci. Rep. 2016;6:29404. doi: 10.1038/srep29404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz-Castañeda R.E., Dantán-González E. Control of avian coccidiosis: future and present natural alternatives. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/430610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raether W., Hofmann J., Uphoff M. In: Guidelines on Techniques in Coccidiosis Research. Eckert J., Braun R., Coudert P., Shirley MW, editors. Office for Official Publications of the European Communities; Luxembourg: 1995. Cultivation and cryopreservation; pp. 79–84. (Eds.) [Google Scholar]
- Rahimi H.R., Arastoo M., Ostad S.N. A comprehensive review of Punica granatum (Pomegranate) properties in toxicological, pharmacological, cellular and molecular biology researches. Iran. J. Pharm. Res. 2012;11:385–400. [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M. Aerococcus: an increasingly acknowledged human pathogen. Clin. Microbiol. Infect. 2016;22:22–27. doi: 10.1016/j.cmi.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Ruan D., Fouad A.M., Fan Q.L., Huo X.H., Kuang Z.X., Wang H., Guo C.Y., Deng Y.F., Zhang C., Zhang J.H., Jiang S.Q. Dietary L-arginine supplementation enhances growth performance, intestinal antioxidative capacity, immunity and modulates gut microbiota in yellow-feathered chickens. Poult. Sci. 2020;99:6935–6945. doi: 10.1016/j.psj.2020.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaygannia E., Bahmani M., Zamanzad B., Rafieian-Kopaei M. A review study on Punica granatum L. J. Evid. Based Integr. 2016;21:221–227. doi: 10.1177/2156587215598039. [DOI] [PubMed] [Google Scholar]
- Yang K.M., Jiang Z.Y., Zheng C.T., Wang L., Yang X.F. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 2014;92:1496–1503. doi: 10.2527/jas.2013-6619. [DOI] [PubMed] [Google Scholar]
- Yang X.L., Pan X.H., Jia Z.P., Bai B.R., Zhi W.J., Chen H., Ma C.L., Ma D.X. Oral administration of Lactobacillus brevis 23017 combined with ellagic acid attenuates intestinal inflammatory injury caused by Eimeria infection by activating the Nrf2/HO-1 antioxidant pathway. Vet. Res. 2022;53:21. doi: 10.1186/s13567-022-01042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Chen L.A., Yang C., Gu Y., Cao R., Zhong K. Portable ultrasonic humidifier exacerbates indoor bioaerosol risks by raising bacterial concentrations and fueling pathogenic genera. Indoor Air. 2022;32:e12964. doi: 10.1111/ina.12964. [DOI] [PubMed] [Google Scholar]
- Youn H.J., Noh J.W. Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Vet. Parasitol. 2001;96:257–263. doi: 10.1016/s0304-4017(01)00385-5. [DOI] [PubMed] [Google Scholar]
- Zhou B.H., Jia L.S., Wei S.S., Ding H.Y., Yang J.Y., Wang H.W. Effects of Eimeria tenella infection on the barrier damage and microbiota diversity of chicken cecum. Poult. Sci. 2020;99:1297–1305. doi: 10.1016/j.psj.2019.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]